Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.111337
Revised: July 28, 2025
Accepted: September 1, 2025
Published online: October 15, 2025
Processing time: 110 Days and 0.7 Hours
Type 1 diabetes mellitus (T1DM) is an autoimmune condition whose prevalence is prominent in children and adolescents, resulting in insulin deficiency with a potential for long-term complications induced by glucotoxicity. As an autoim
Core Tip: Progressive resistance training protocols in pediatric diabetes not only enhance muscular performance but also offer secondary health benefits. Although the effects of strength training on glycemic control outcomes remain variable there is a trend toward improvement. Furthermore, it can act as a foundational stimulus to encourage greater overall physical activity, which may further support glycemic regulation. Additional benefits include increased strength, improved posture, and enhanced motor control.
- Citation: Martin-Rivera F, Alarcón-Gómez J, Rodrigo-Mallorca D, Saez-Berlanga A, Gargallo P, Chulvi-Medrano I. Impact of resistance exercise in type 1 diabetes pediatric patients. World J Diabetes 2025; 16(10): 111337
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/111337.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.111337
The prevalence of type 1 diabetes mellitus (T1DM) has been increasing since the 20th century. For instance, Onkamo et al[1] reported that the global incidence was projected to rise by approximately 3% per year. T1DM constituting a public health problem with increasing projection[2].
T1DM is a metabolic disorder characterized by a complete cessation of insulin secretion by autoimmune-mediated lysis of pancreatic beta cells that leads a chronic hyperglycemia besides changes in the metabolism of lipids and proteins[3]. During long term diabetes is associated with complications, including an increased risk of cardiovascular disease, nephropathy, and neuropathy[4], associated with exercise-induced hypoglycemia and hyperglycemia.
Overall, T1DM has a profound impact on patients' health and quality of life in children[5]. For this reason, organizations such as the American Diabetes Association (ADA) include physical activity as a fundamental component of the therapeutic approach to counteract these complications[6].
Physical exercise as change in lifestyle in T1DM is considered of critical importance—particularly within the pediatric population. The ADA emphasizes that structured physical activity, including resistance training, yields significant metabolic and psychological benefits in children with T1DM[7]. At the same time, it highlights the need for caution due to the potential risks associated with exercise-induced hypoglycemia and hyperglycemia.
Moreover, emphasis is placed on the fact that regular physical activity does not increase the risk of severe hy
However, some results reported showed that an intermittent and continuous aerobic exercises are associated with a lowering of glycemia in male adolescents with T1DM, but after resistance training intervention the glycemia remained stable without significant alterations. These findings hold important implications related to clinical exercise advice and disease management in adolescents with T1DM[11].
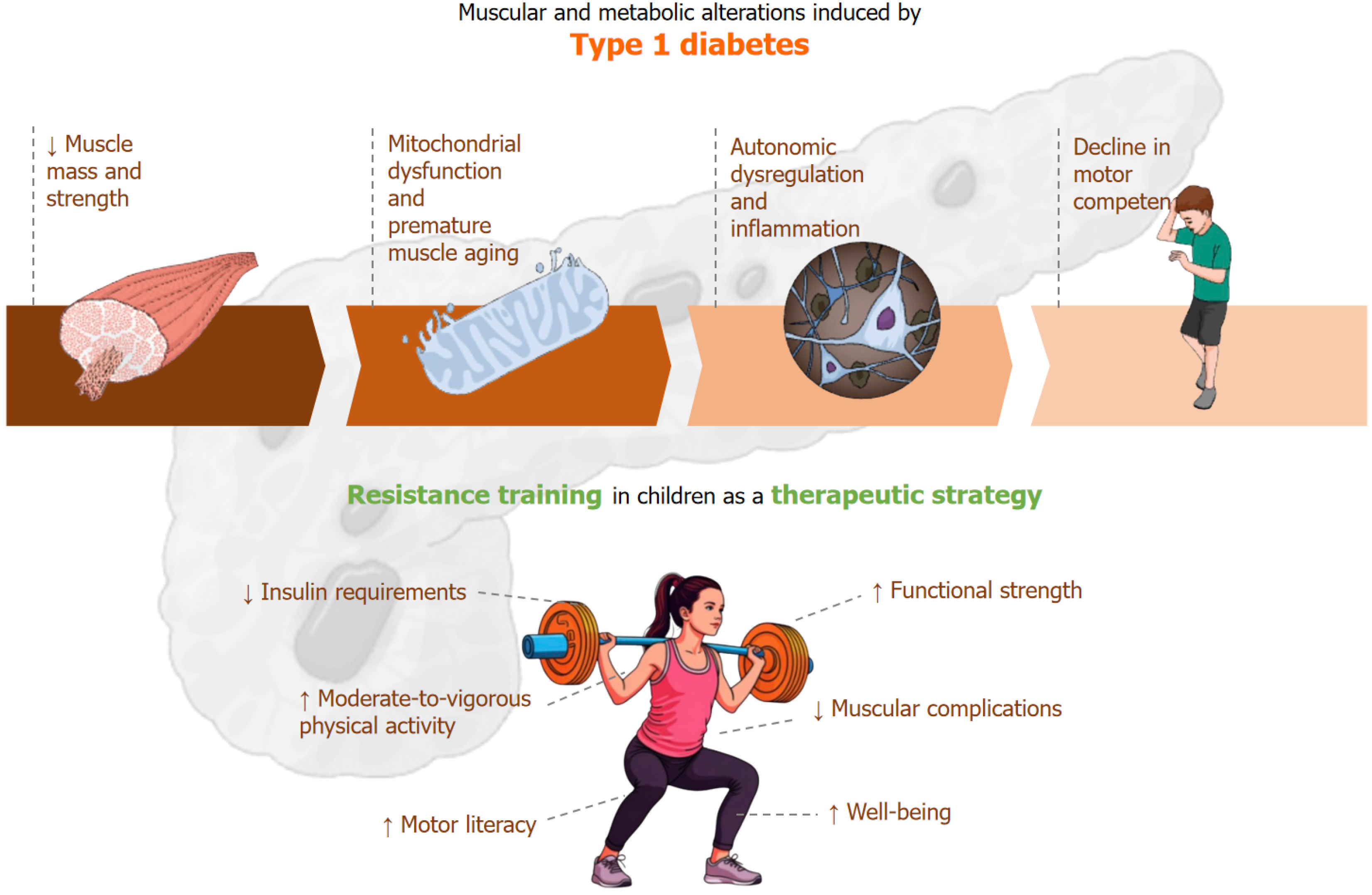
Although cardiovascular training has traditionally received greater importance emphasis in the management of this metabolic condition, it is well established that T1DM can impair both the response to and the capacity for aerobic exercise[12]. This impairment is primarily attributed to autonomic nervous system dysregulation at rest and during exercise[12], as well as to mitochondrial dysfunction, which directly affects skeletal muscle tissue[13]. In pediatric populations, additional disruptions have been reported in inflammatory, oxidative, and metabolic responses[14].
On the other hand, it is well established that T1DM induces alterations in otherwise healthy skeletal muscle tissue, leading to reductions in muscle mass and strength, as well as dysregulation in glucose, lipid, and protein metabolism[13].
These alterations are also evident in pediatric populations, although to a lesser extent when adequate glycemic control is achieved. This physiological disturbance has clinical implications, particularly in terms of force-generating capacity, which is consistently reported to be lower in individuals with T1DM compared to their healthy peers. This documented phenomenon has led to the hypothesis of accelerated muscle aging induced by T1DM[13], a finding that has also been corroborated in pediatric populations[15].
If this strength deficit emerges in the early stages, it tends to progressively worsen over time, becoming particularly severe with the onset of neuropathy[13]. For this reason, the implementation of combined or concurrent exercise (aerobic plus resistance training) has been investigated, as it may reduce the risk of developing cardiovascular risk factors, improve glycemic control, lower insulin requirements, enhance physical fitness, and improve quality of life in individuals with diabetes[16]. For example, the positive effects of concurrent training in the glycemic control has been reported when patients uses concurrent training, because when strength training is added to aerobic training the effective in reducing IG fluctuations is greater that where compared in isolate aerobic o resistance training alone suggesting that the combination activate the metabolism and the anabolic adaptation that improve the glycemic control (i.e., glucose levels) and the muscle health[17].
Eventually, it has been reported that an isolated supervised progressive resistance training two-times a week in children with T1DM must last at least 32 weeks to get a significant decrease in blood glucose level glycated hemoglobin (HbA1C). In addition, exercise-induced increase in adiponectin improves insulin sensitivity[18]. This suggests that long-term isolated resistance training can have substantial metabolic benefits.
The metabolic improvements can be attributed not only to the increased metabolic demand induced by exercise, but also to the fact that muscle contractions during physical activity enhance cell membrane permeability, exerting an insulin-like effect that promotes greater sensitivity to exogenous insulin[19].
Therefore, incorporating strength training during the pediatric stages in children with diabetes serves a dual purpose and is of great importance to maintain adequate strength levels. Firstly, it helps to mitigate functional decline, and secondly, it promotes the achievement of moderate-to-vigorous physical activity (MVPA) by enhancing motor com
Consequently, it is important to further investigate the effects of resistance strength training on the health and quality of life of children with T1DM, as there is evidence suggesting that it helps to support glycemic control, as well as maintain and improve skeletal muscle morphology and function favoring an increase of MVPA.
Collectively, these findings suggest that strength training may serve as a safe and effective approach for pediatric patients with T1DM. Given the importance given the interest in increasing the health and quality of life in the pediatric population with T1DM, a comprehensive and exploratory minireview is warranted to critically evaluate its safety, efficacy, and benefits. Our primary research question is: Is resistance training beneficial for glycemic control (HbA1c or glycemia levels) and health in children with T1DM?
This minireview synthesizes current evidence on strength training in children and pre-adolescents with T1DM populations.
The primary research question was “Is resistance training beneficial for health in children with T1DM?” Inclusion criteria for papers included in this minireview were: (1) To be < 18 years old; (2) with diagnosis of T1DM; (3) Intervention consisted in isolation, supervised or unsupervised with resistance training in any kind of form (calisthenics, barbells, dumbbells, medicine balls etc.,); and (4) At least 6-weeks of intervention. We excluded papers if they: (1) Involve animals; (2) Included dietary intervention; (3) Acute interventions; and (4) Include concurrent training.
The main outcome that was contemplated was glycemic control (HbA1c or glycemic status). The secondary was the impact on skeletal muscle (strength, hypertrophy) as well as functionality (e.g., jump) and motor competence (functional tests), and the occurrence of adverse events (hypoglycemia).
Two reviewers independently conducted an individualized search strategy for PubMed, Scopus and Web of Science database using descriptors related to diabetes mellitus type 1, strength training, and children. Two reviewers in
In Table 1 has been summarized the main information extracted for the retrieved articles.
| Ref. | Sample (n) | Resistance training intervention | Control | Periodization | Primary outcome: Glycemic control | Main results | Secondary outcomes |
| Petschnig et al[18], 2020 | n = 21. Allocated in 2 groups: (1) Strength training n = 11; 11.00 (0.8) years; with 2.63 (1.85) years of duration of diabetic history; and (2) Control group n = 10; 11.30 (0.7) years with 2.80 (2.07) of duration of diabetic history. Diagnosed at least in the last 6 months. No regular sport more often than once per week and sports lessons in school not more than twice a week | 8 exercises for main muscle groups in a circuit; 25-40 seconds per exercise at 30% RM; 30-40 seconds resting; 2-times | No exercise | 32 weeks; twice a week | HbA1c; glycemia before after each session | CG HbA1c; Baseline: 7.84 (1.38); At 17 weeks: 8.26 (1.26); At 32 weeks: 8.72 (1.33). ST HbA1c; Baseline: 8.75 (1.37); At 17 weeks: 8.55 (1.33); At 32 weeks: 8.75 (1.37). Glycemia before and after each session showed a significant reduction after each session 26.5 (4.4%). No adverse effects reported | Adiponectin; strength tests: (1) Seated bench press; (2) Seated bench pull; and (3) Seated leg press. Echocardiogram left common carotid artery |
| Särnblad et al[11], 2021 | n = 8. 17.5 (0.8) years; 8.1 (4.8) years of diabetes duration | 2 blocks; Interspersed 2 minutes; Each block included 3 sets until muscular failure at 70% 1 RM with 2 minutes of resting in lying leg press, lying leg curl and seated leg extension | Aerobic continuous; aerobic intermittent | Acute | Glycemia | Baseline: 11.8 (3.8) mmol/L; 5 minutes before exercise: 11.6 (3.8) mmol /L; exercise start: 11.5 (3.2) mmol/L; changes 0-45 min exercise: -1.0 (1.4) mmol/L; at end of recovery: 11.6 (3.9) mmol/L | Lactate; saturation of O2 |
| Ramalho et al[22], 2006 | n = 6; 19.8 ± 5.1 | 8 exercises in machine: Chest press, bicep curl, triceps extension, lower back, abdominals, leg press, leg curl and leg extension, 3 sets, 8-12 repetitions, 60%-80% 1 RM (60 seconds of rest) | Aerobic exercise | 12 weeks; 3 days per week | HbA1c; glycemia; insulin dosage | There was no change in the parameters evaluated, only a reduction in insulin dosage after exercise. 0.95 (0.34) vs 0.79 (0.28) | Anthropometry |
The aim of this mini-review was to examine the effects of isolated resistance training on glycemic control in children with T1DM. The analyzed data showed a trend agreed with previous data of resistance training in adults, the RT let manage the glycemic control and increase strength fitness[22,23]. Additionally, other potential benefits can be inferred by the adult’s studies and pediatric resistance training studies (Figure 1). The findings reveal a clear paucity of research in this area. Despite methodological heterogeneity and limitations related to small sample sizes, the available studies demonstrate consistent and clinically relevant physiological patterns.
To date, only two studies have investigated the long-term metabolic effects of resistance training on HbA1C, yielding contradictory results. Petschnig et al[18] reported an improvement in HbA1c after 17 weeks of training, which, however, returned to baseline levels by week 32. In contrast, Ramalho et al[22] found no significant changes in this parameter. Nevertheless, both studies reported noteworthy metabolic adaptations. Petschnig et al[18] observed an approximate 25% reduction in post-exercise blood glucose, suggesting an acute enhancement in peripheral glucose uptake—likely mediated by insulin-independent mechanisms, particularly the contraction-induced translocation of GLUT-4. Although this acute effect is transient, sustained training adherence could lead to cumulative benefits over time, potentially contributing to long-term HbA1c reduction.
Additionally, Ramalho et al[22] reported a significant reduction of approximately 20.25% in daily insulin dosage, indicating improved insulin sensitivity. Conversely, the study by Ramalho et al[22] did not report significant changes in HbA1c or fasting blood glucose.
A third study assessed the acute glycemic response to resistance exercise, analyzing blood glucose kinetics. This investigation confirmed that high-intensity resistance training (70% 1RM to failure) induces a moderate decrease in blood glucose during exercise (-1.0 ± 1.4 mmol/L), with no significant changes observed during the post-exercise recovery phase[11].
In an overall perspective these results show similar trends have been observed in adolescent populations, as reported in previous research’s as for example by Saki et al[24]. In their study, three groups were compared: A T1DM control group, a healthy control group, and a T1DM group undergoing concurrent training. The training protocol consisted of REs followed by aerobic training, performed three-times per week over a 12-week period. The results demonstrated improvements in glycemic control as well as enhancements in heart rate variability, suggesting favorable metabolic and autonomic adaptations in response to the intervention.
Likewise, Farinha et al[25] reported improved glycemic control (before-after intervention) in a cohort of young adults with T1DM (mean age 22 years) who underwent a 10-week resistance training program, performed three-times per week. However, the study found no significant differences between training modalities—resistance training, high-intensity interval training, and concurrent training—when analyzing the effect over time. Specifically, there were no significant changes in HbA1C, lipid profile, fasting glucose levels, or body composition across groups. Nevertheless, longer-term studies are needed to confirm the findings reported by Farinha et al[25].
Following a 12-week concurrent training program (combining resistance and aerobic exercise). However, a clinically relevant finding was the significant reduction in the required dose of exogenous insulin, which decreased from 0.95 to 0.79 U/kg/day. This outcome may indicate improved insulin sensitivity, although the study did not include direct assessments of insulin sensitivity or secretion. Furthermore, the relatively short duration of the intervention and the absence of an individualized progression strategy may have limited its potential impact on long-term glycemic markers such as HbA1c[24].
In most studies, secondary outcomes have been included, highlighting additional benefits beyond those related to metabolic control. reported a particularly notable increase in muscle strength, especially in the lower limbs or anthropometry[22].
However, it is important to emphasize that strength improvements during the pediatric age are of paramount importance. Participation in strength training programs during adolescence is associated with multiple Benefit, greater self-efficacy and enjoyment of exercise, both of which are strong predictors of higher physical activity levels in adulthood[26].
The cumulative evidence analyzed in a recent meta-analysis, indicates that the positive effects of resistance training have been consistently demonstrated when implemented concurrently aerobic or cardiovascular exercise, in HbA1c (SMD = -0.63) and in reduction in daily insulin needs (SMD = -0.79). As is well established, adopting an active lifestyle that includes resistance training during the pediatric stage facilitates the maintenance of this habit into adulthood. In this context, extensive evidence supports the beneficial effects of resistance training on glycemic control in individuals with T1DM during adulthood[27].
Moreover, concurrent training appears to help reduce a commonly perceived barrier to exercise in individuals with T1DM: The risk of hypoglycemia. The risk of hypoglycemia associated with resistance training in children and adolescents does not appear to be a major concern. For instance, data from Särnblad et al[11] showed that blood glucose levels remained stable in a sample of eight participants with a mean age of 17.5 (± 0.8) years during a resistance training session consisting of two rounds of three lower-body exercises (lying leg press, lying leg curl, and seated leg extension), each performed for three sets to muscular failure at 70% of 1RM. This glycemic stability was not observed during intermittent or continuous aerobic exercise, where a reduction of approximately 5 mmol/L in blood glucose was recorded[11]. In another similar paper, Nazari et al[28] in a group of 20 T1DM children [11.22 (1.90) years] concluded that in a concurrent exercise intervention during 16 weeks leaded a reduction in HbA1c [7.98 (1.02) to 7.83 (1.00)] and concomitantly the cortisol and anxiety was lower and the quality of life was improved.
Finally, it is important to note that despite the numerous reported benefits, certain comorbidities associated with T1DM must be carefully considered through appropriate pre-exercise screening. Conditions such as proliferative retinopathy or nephropathy represent clinical scenarios in which resistance training is generally not recommended due to the potential risks involved[29].
For future research, it is worth considering that the lack of improvement in HbA1c may be partly attributed to compensatory behaviors aimed at minimizing the risk of hypoglycemia. These may include increased carbohydrate intake around the time of exercise and/or reductions in insulin dosage, which should be closely monitored and controlled in study.
Isolated resistance training has been shown to improve glycemic control in pediatric patients with T1DM. However, the benefits appear to be even greater when resistance training is combined with other forms of exercise, such as aerobic training, in what is known as concurrent training. This combination not only enhances insulin sensitivity and metabolic regulation but also promotes spontaneous physical activity, which plays a crucial role in maintaining stable glycemic levels over time. Implementing resistance training at an early age may improve adherence to physical activity routines and foster long-term engagement in healthy behaviors. As a result, it increases the likelihood of sustained glycemic monitoring and control throughout the patient’s life.
| 1. | Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 533] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2437] [Cited by in RCA: 2603] [Article Influence: 867.7] [Reference Citation Analysis (18)] |
| 3. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1594] [Article Influence: 531.3] [Reference Citation Analysis (70)] |
| 4. | Gimenez-Perez G, Vlacho B, Navas E, Mata-Cases M, Real J, Cos X, Franch-Nadal J, Mauricio D. Comorbid autoimmune diseases and burden of diabetes-related complications in patients with type 1 diabetes from a Mediterranean area. Diabetes Res Clin Pract. 2022;191:110031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Hesketh KD, Wake MA, Cameron FJ. Health-related quality of life and metabolic control in children with type 1 diabetes: a prospective cohort study. Diabetes Care. 2004;27:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1747] [Article Influence: 174.7] [Reference Citation Analysis (1)] |
| 7. | American Diabetes Association Professional Practice Committee. 14. Children and Adolescents: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S283-S305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 8. | Herbst A, Bachran R, Kapellen T, Holl RW. Effects of regular physical activity on control of glycemia in pediatric patients with type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2006;160:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Newton KH, Wiltshire EJ, Elley CR. Pedometers and text messaging to increase physical activity: randomized controlled trial of adolescents with type 1 diabetes. Diabetes Care. 2009;32:813-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Donat Ergin B, Ergin I, Gökşen D. Fear of Hypoglycemia and Longer Disease Duration Associated with Physical Activity Avoidance in Children and Adolescents with Type 1 Diabetes Mellitus. J Clin Res Pediatr Endocrinol. 2023;15:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Särnblad S, Ponsot E, Leprêtre PM, Kadi F. Acute effects of aerobic continuous, intermittent, and resistance exercise on glycemia in adolescents males with type 1 diabetes. Pediatr Diabetes. 2021;22:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Samora M, Grotle AK, Stone AJ. Altered Cardiovascular Responses to Exercise in Type 1 Diabetes. Exerc Sport Sci Rev. 2023;51:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Monaco CMF, Gingrich MA, Hawke TJ. Considering Type 1 Diabetes as a Form of Accelerated Muscle Aging. Exerc Sport Sci Rev. 2019;47:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Rosa JS, Oliver SR, Flores RL, Ngo J, Milne GL, Zaldivar FP, Galassetti PR. Altered inflammatory, oxidative, and metabolic responses to exercise in pediatric obesity and type 1 diabetes. Pediatr Diabetes. 2011;12:464-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Travis C, Srivastava PS, Hawke TJ, Kalaitzoglou E. Diabetic Bone Disease and Diabetic Myopathy: Manifestations of the Impaired Muscle-Bone Unit in Type 1 Diabetes. J Diabetes Res. 2022;2022:2650342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 16. | Salem MA, AboElAsrar MA, Elbarbary NS, ElHilaly RA, Refaat YM. Is exercise a therapeutic tool for improvement of cardiovascular risk factors in adolescents with type 1 diabetes mellitus? A randomised controlled trial. Diabetol Metab Syndr. 2010;2:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Minnock D, Annibalini G, Le Roux CW, Contarelli S, Krause M, Saltarelli R, Valli G, Stocchi V, Barbieri E, De Vito G. Effects of acute aerobic, resistance and combined exercises on 24-h glucose variability and skeletal muscle signalling responses in type 1 diabetics. Eur J Appl Physiol. 2020;120:2677-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Petschnig R, Wagner T, Robubi A, Baron R. Effect of Strength Training on Glycemic Control and Adiponectin in Diabetic Children. Med Sci Sports Exerc. 2020;52:2172-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | de Abreu de Lima V, de Menezes FJ Júnior, da Rocha Celli L, França SN, Cordeiro GR, Mascarenhas LPG, Leite N. Effects of resistance training on the glycemic control of people with type 1 diabetes: a systematic review and meta-analysis. Arch Endocrinol Metab. 2022;66:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Behringer M, Vom Heede A, Matthews M, Mester J. Effects of strength training on motor performance skills in children and adolescents: a meta-analysis. Pediatr Exerc Sci. 2011;23:186-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Cuenca-García M, Jago R, Shield JP, Burren CP. How does physical activity and fitness influence glycaemic control in young people with Type 1 diabetes? Diabet Med. 2012;29:e369-e376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Ramalho AC, de Lourdes Lima M, Nunes F, Cambuí Z, Barbosa C, Andrade A, Viana A, Martins M, Abrantes V, Aragão C, Temístocles M. The effect of resistance versus aerobic training on metabolic control in patients with type-1 diabetes mellitus. Diabetes Res Clin Pract. 2006;72:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Siegelaar SE, de Galan BE. Resistance Training: a Strong Case for People With Type 1 Diabetes? J Clin Endocrinol Metab. 2023;108:e491-e492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Saki H, Nazem F, Fariba F, Sheikhsharbafan R. A High intensity Interval training (running and swimming) and resistance training intervention on heart rate variability and the selected biochemical factors in boys with type 1 diabetes. Diabetes Res Clin Pract. 2023;204:110915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Farinha JB, Ramis TR, Vieira AF, Macedo RCO, Rodrigues-Krause J, Boeno FP, Schroeder HT, Müller CH, Boff W, Krause M, De Bittencourt PIH Jr, Reischak-Oliveira A. Glycemic, inflammatory and oxidative stress responses to different high-intensity training protocols in type 1 diabetes: A randomized clinical trial. J Diabetes Complications. 2018;32:1124-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Faigenbaum AD, Ratamess NA, Kang J, Bush JA, Rial Rebullido T. May the Force Be with Youth: Foundational Strength for Lifelong Development. Curr Sports Med Rep. 2023;22:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Riddell MC, Peters AL. Exercise in adults with type 1 diabetes mellitus. Nat Rev Endocrinol. 2023;19:98-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Nazari M, Shabani R, Dalili S. The effect of concurrent resistance-aerobic training on serum cortisol level, anxiety, and quality of life in pediatric type 1 diabetes. J Pediatr Endocrinol Metab. 2020;33:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Adolfsson P, Riddell MC, Taplin CE, Davis EA, Fournier PA, Annan F, Scaramuzza AE, Hasnani D, Hofer SE. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr Diabetes. 2018;19 Suppl 27:205-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/