Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.111212
Revised: August 1, 2025
Accepted: August 25, 2025
Published online: October 15, 2025
Processing time: 112 Days and 8 Hours
Acute and extreme insulin resistance with persistent hyperglycemia requiring excessively high doses of insulin before rapidly resolving is rare and has been referred to as transient and extreme insulin resistance (TEIR). The underlying pathophysiology and optimal management of TEIR are poorly understood, and previous reports of TEIR in the literature are sparse. This report is the first de
A 62-year-old male developed cardiogenic shock and was placed on veno-arterial extracorporeal membrane oxygenation following percutaneous coronary in
Onset of TEIR did not seem to correlate with end-organ hypoperfusion or va
Core Tip: This paper presents the first report of transient and extreme insulin resistance (TEIR) in a patient receiving mechanical circulatory support (MCS). TEIR is an exceedingly rare condition that occurs in critically ill patients and carries with it a high mortality rate and uncertain pathophysiology. Due to the implementation of MCS and vasoactive medication, this case is uniquely positioned to provide an observation on some of the previously proposed mechanisms of TEIR (tissue hypoperfusion and vasoactive drugs).
- Citation: Butler PW, Légaré JF, White CW. Transient extreme insulin resistance in a patient requiring extracorporeal membrane oxygenation for cardiogenic shock: A case report. World J Diabetes 2025; 16(10): 111212
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/111212.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.111212
Insulin resistance is common in critically ill patients; however, acute and extreme insulin resistance with persistent hyperglycemia requiring excessively high doses of insulin before rapidly resolving is rare[1,2]. This clinical scenario has been described as transient and extreme insulin resistance (TEIR), with extreme insulin resistance being defined as a patient requiring more than 3 units/kg/day of insulin[3]. The underlying pathophysiology and optimal management of TEIR are poorly understood, and previous reports of TEIR in the literature are sparse[4,5]. This report is the first description of TEIR in a patient requiring veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for post-myocardial infarction cardiogenic shock.
The patient presented with chest pain.
The patient presented to an emergency department with an inferior-posterior ST-elevation myocardial infarction. He was initially treated with thrombolytic therapy and subsequently transferred to a tertiary care center for rescue percutaneous coronary intervention. Shortly after arrival he deteriorated, developing a ventricular fibrillatory arrest lasting 20 minutes before a return of spontaneous circulation with cardiopulmonary resuscitation. He was brought to the cardiac catheterization lab where his left ventricular ejection fraction was found to be 30%, and an emergency rescue percutaneous coronary intervention of the culprit right coronary artery occlusion was performed successfully.
Following revascularization, he was transferred to the coronary care unit (CCU) where he developed progressive cardiogenic shock with tachycardia, hypoxemia, pulmonary edema, and escalating vasopressor requirements (no
The patient was a 62-year-old male, weighing 117 kg, with a history of ischemic heart disease (coronary artery bypass grafting 8 years prior), type 2 diabetes mellitus (diagnosed 8 years prior, A1C near time of admission was 7.1%). To manage his diabetes, he was prescribed Empagliflozin, Gliclazide, and Janumet; however, medication compliance was questionable. He was not taking insulin at home.
His medical history also included hyperlipidemia, obstructive sleep apnea, nasal papilloma, and chronic tobacco and marijuana use. Family history was unknown.
Upon admission to the ICU the patient presented with stable vitals on ECMO support (Temperature 36.0 °C, heart rate 93, sinus rhythm, blood pressure 110/65, mean arterial pressure 65, SpO2 100%). Pedal pulses were absent to feet bilaterally.
On arrival to the ICU initial laboratory tests revealed potassium 3.3 mmol/L, sodium 144 mmol/L, chloride 101 mmol/L, creatinine 297 μmol/L, urea 11.7 mmol/L, creatine kinase 2892 U/L, and troponin 11800 ng/L. Hemoglobin was 154 g/L and platelets 226 × 109/L. Arterial blood gases revealed pH 7.24, pCO2 38 mmHg, pO2 249 mmHg, bicarbonate 16 mmol/L, and lactate 10.8 mmol/L. Further workup revealed international normalized ratio 1.2, and patrial thr
On post-admission day 2: Laboratory examinations revealed potassium 3.5 mmol/L, sodium 139 mmol/L, chloride 100 mmol/L, creatinine 351 μmol/L, and urea 13.8 mmol/L. Arterial blood gases showed pH 7.4, pCO2 33 mmHg, pO2 86 mmHg, bicarbonate 20 mmol/L, lactate 5.6 mmol/L.
On post-admission day 3: Laboratory examinations revealed potassium increased to 6.3 mmol/L and creatinine increased to 420 μmol/L.
On post-admission day 4: Laboratory examinations revealed potassium 5.4 mmol/L, urea 12.1 mmol/L and creatinine 348 μmol/L. Potassium values normalized by post-admission day 6 (3.8 mmol/L).
Percutaneous coronary angiography revealed a totally occluded right coronary artery and an ejection fraction of 30%. Chest X-ray on admission to the ICU showed central lines in good positions and clear lungs.
Inferior STEMI requiring rescue percutaneous coronary intervention of right coronary artery, cardiogenic shock requiring ECMO, acute kidney failure requiring temporary dialysis.
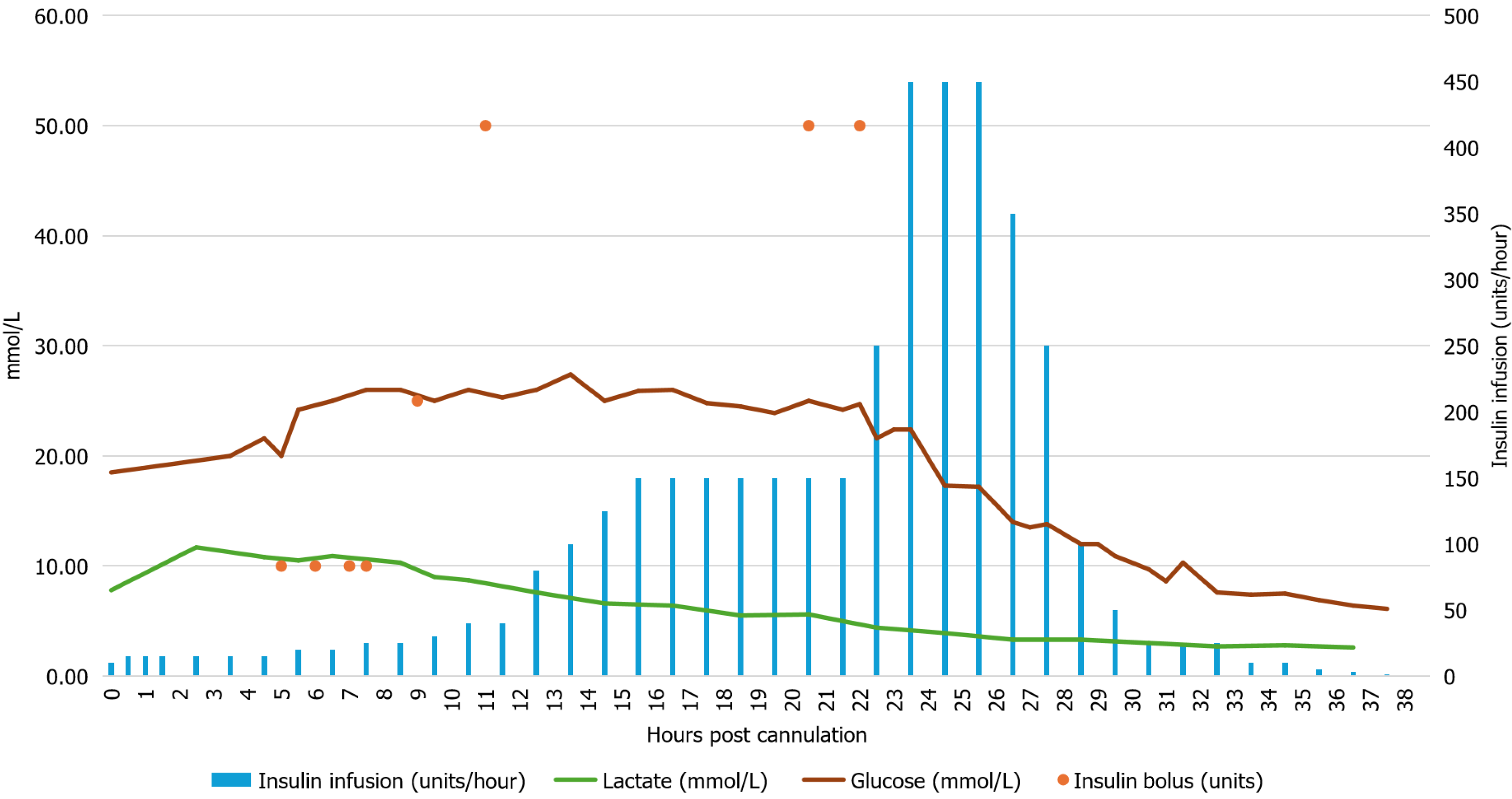
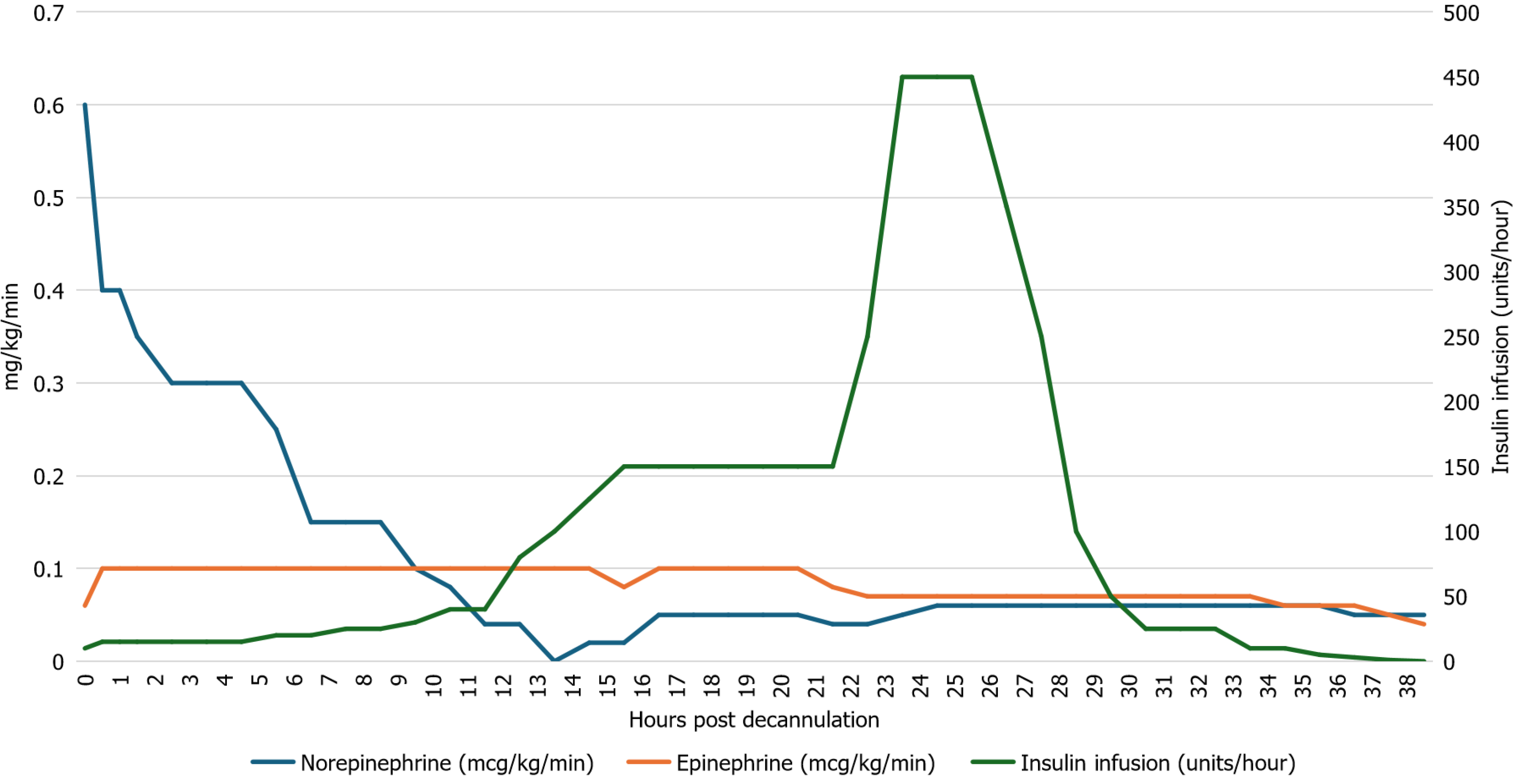
Initial glucose on presentation to hospital was 26 mmol/L, it had declined to 18.5 mmol/L at the time of VA-ECMO cannulation at which point an intravenous Humulin R infusion was started at a rate of 10 units/hour. Over the 24 hours following VA-ECMO cannulation, despite escalating insulin infusion rates and repeated boluses (totaling 215 units), the patient's glucose levels remained markedly elevated, peaking at 27 mmol/L (Figure 1). During this period the patient’s epinephrine infusion ranged from 0.06-0.1 mcg/kg/min, and norepinephrine infusion was weaned down from 0.6 μg/kg/min to 0.06 μg/kg/min (Figure 2). Dopamine infusion (at 3 μg/kg/min) was started 22 hours after initiation of ECMO to assist in weaning off epinephrine infusion. Regular electrolyte monitoring was performed, and potassium, calcium, and magnesium were replaced as required. Potassium values dropped during the high rates of insulin infusion and required repeated replacement. Glucose concentration was checked every 30-60 minutes, and insulin requirements reached a maximum of 450 units/hour roughly 24 hours after VA-ECMO cannulation (Figure 1). At this point glucose levels began declining and the frequency of glucose measurement was intensified. Over the next four hours a rapid de-escalation of the insulin infusion dose (from 450 units/hour to 350 units/hour to 250 units/hour to 100 units/hour to 50 units/hour) was required with infusion rates decreasing from 450 units/hour to 50 units/hour. By 36-hours post-cannulation, a normal blood glucose (6.9 mmol/L) was achieved on 5 units/hour of insulin, which was weaned shortly afterwards. The patient did experience some rebound hypoglycemia, lowest reading 3.3 mmol/L.
Two days after ECMO initiation, an improvement in cardiac function was observed. His epinephrine infusion was weaned and repeat echocardiography demonstrated a left ventricular ejection fraction of 45% with moderate right ventricular dysfunction. He subsequently underwent successful ECMO decannulation, following which he developed hyperkalemia. The hyperkalemia was managed with a dose of sodium polystyrene sulfonate (30 g) and hemodialysis, which was performed the same day as ECMO decannulation and the day after. The patient continued to recover and was successfully discharged from hospital 11 days after initial hospital admission.
This case illustrates an episode of TEIR following myocardial infarction and cardiogenic shock requiring VA-ECMO cannulation, with a peak insulin infusion rate of 450 units/hour. The patient received 4300 units (33 units/kg) of insulin over the 24-hour period of peak insulin resistance. Subsequently, there was a rapid reversal of insulin resistance that coincided with a mean lactate (during the 12-hour peak of insulin infusions) of 4.85 mmol/L and moderate dose of catecholamine infusion support. The patient then recovered sufficiently from myocardial dysfunction to facilitate ECMO decannulation; however, he subsequently required transient hemodialysis for acute hyperkalemia possibly related to reperfusion of subclinical ischemia in the cannulated leg. The patient was eventually discharged neurologically intact. To the best of our knowledge, this is the first case report documenting TEIR in a patient requiring VA-ECMO for post-myocardial infarction cardiogenic shock.
Previous case reports of patients presenting with TEIR in the context of adverse cardiac events are summarized in Table 1. Myocardial infarction and subsequent cardiac arrest were the most common presentations in this group of patients. In our case and two of the previously reported cases, patients required dialysis for their acute kidney injury. As in our case, two of the case reports also employed TTM. The duration of insulin infusion varied considerably among reported cases, ranging from 25 hours up to 26 days. Interestingly, all but one of the case reports observed a resolution of the extreme insulin resistance within 24 hours of the peak insulin infusion rate (Table 1). We observed that the insulin infusion was able to be weaned from a peak of 450 units/hour to 0 units/hour within 12 hours (Figure 1).
| Ref. | Myocardial infarction | Concurrent conditions | Therapeutic hypothermia | Peak insulin infusion rate | Peak insulin daily dose | Peak to resolution time | Duration of insulin infusion | Rebound hypoglycemia | Vasopressors | Peak lactate | Mechanical circulatory support | Outcome |
| Current paper | STEMI | Cardiac arrest (20 minutes), metabolic acidosis, acute kidney injury (dialysis) | Yes (34 ℃) | 450 units/hour | 4300 units | 13 hours | 38 hours | No | Epinephrine Norepinephrine dopamine | 10.8 mmol/L | Veno-arterial extracorporeal membrane oxygenation | Survived |
| Wei et al[5] | NSTEMI | Cardiac arrest (6 minutes), sepsis, mixed acidosis, pneumonia, acute respiratory failure, AKI (dialysis) | Yes (no temperature reported) | 960 units/hour | 18 224 units | 8 hours | 11 days | Yes | Norepinephrine | 19 mmol/L | No | Deceased (recurrent sepsis) |
| July et al[14] | STEMI | Cardiac arrest (unknown duration). acute kidney injury (renal replacement therapy), metabolic acidosis | Yes (32 ℃) | 280 units/hour | Not reported | 24 hours | 5 days | No | No | 1.9 mmol/L | No | Deceased (anoxic brain damage) |
| Oo et al[6] | NSTEMI | Diabetic ketoacidosis, acute on chronic kidney injury | No | 120 units/hour | 2400 units | > 5 days | 26 days | No | No | Not reported | No | Survived |
| Surani et al[16] | None | Cardiac arrest (unknown duration) | No | 832 units/hour | > 15 000 units | 6 hours | 25 hours | No | Norepinephrine | Not reported | No | Not reported |
| Yokoyama et al[9] | NSTEMI | Diabetic ketoacidosis, rhabdomyolysis, acute kidney injury, pneumonia, disseminated intravascular coagulation. | No (however, body temperature was 35 ℃ on arrival) | 10 000 units/hour | 91 580 units (over 25 hours) | 6 hours | 25 hours | Yes | Dopamine | Not reported | No | Survived |
The role that myocardial dysfunction plays in TEIR remains poorly understood. Oo et al[6] reported a case of TEIR in a patient who suffered a myocardial infarction; however, in their case the insulin resistance improved following coronary revascularization (coronary artery bypass grafting). In the present report, insulin resistance persisted and worsened following revascularization of the culprit coronary artery. The interpretation of this is complicated and further confounded by the cardiogenic shock and initiation of VA-ECMO shortly following revascularization.
The potential role of VA-ECMO in the pathophysiology of TEIR has not been discussed. One of the proposed mechanisms of TEIR is rooted in an overactivation of the inflammatory system[5]. The exposure of circulating blood to the ECMO circuit causes a response similar to that of the Systemic Inflammatory Response Syndrome[7]. This results in a cascade of proinflammatory, coagulation, and immunological changes, which may play an important role in TEIR. Two key cytokines that have been demonstrated to play a role in insulin resistance are interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α), both of which become elevated during ECMO[7,8]. The possibility that VA-ECMO may have contributed to the pathophysiology of TEIR in this case warrants consideration. However, if increased IL-6 and TNF-α levels induced by ECMO initiation was the principal driver of the insulin resistance, then it would be expected to see the insulin resistance continue until after ECMO decannulation. We observed that TEIR was resolved while the patient was still supported with VA-ECMO, suggesting that mechanical circulatory support was unlikely to be the primary cause of insulin resistance in this case.
This case also provides a unique perspective on the role of hemodynamics and end organ perfusion on the pathophysiology of TEIR. It has been proposed that tissue hypoperfusion in shock states may contribute to insulin resistance, by decreasing the delivery of insulin to the peripheral tissues[9]. In our case, we were able to restore end organ oxygen delivery following VA-ECMO cannulation as evidenced by a normal central venous oxygen saturation; however, TEIR developed over the next 24 hours and did not correlate with the severity of end organ hypoperfusion. Lactate, which is a product of anaerobic glycolysis, increases during episodes of tissue hypoxia[10]. Interestingly, the peak lactate concentration was observed 24 hours prior to the onset of TEIR, and the mean lactate level during the 12 hours period of peak insulin requirement was 4.8 (Figure 1). This suggests that inadequate tissue perfusion may not have been the primary driver of insulin resistance in this case.
It has been demonstrated previously that exogenous norepinephrine decreases insulin sensitivity, likely via action on the liver and skeletal muscle[11]. Similarly, epinephrine has been shown to block the inhibitory effect of insulin on hepatic glucose production and impair tissue sensitivity to insulin through β-adrenergic mechanisms[12]. We observed that the TEIR subsided before the intravenous norepinephrine and epinephrine doses were weaned (Figure 2), suggesting that exogenous norepinephrine and epinephrine were not likely the principal cause of insulin resistance in this case.
Another possibility to be reviewed is an antibody to the insulin receptor or an insulin autoantibody. Insulin autoantibodies, when present, can produce a similar phenomenon that was seen in this case, resulting in extreme insulin resistance, and rapid resolution of symptoms[13]. The rapid resolution is within the context of being treated with an immunosuppressive therapy such as mycophenolate mofetil[13]. While insulin auto-antibodies were not screened for during this patient’s admission, the acute timeline and rapid resolution without the use of an immunosuppressive agent makes this diagnosis unlikely.
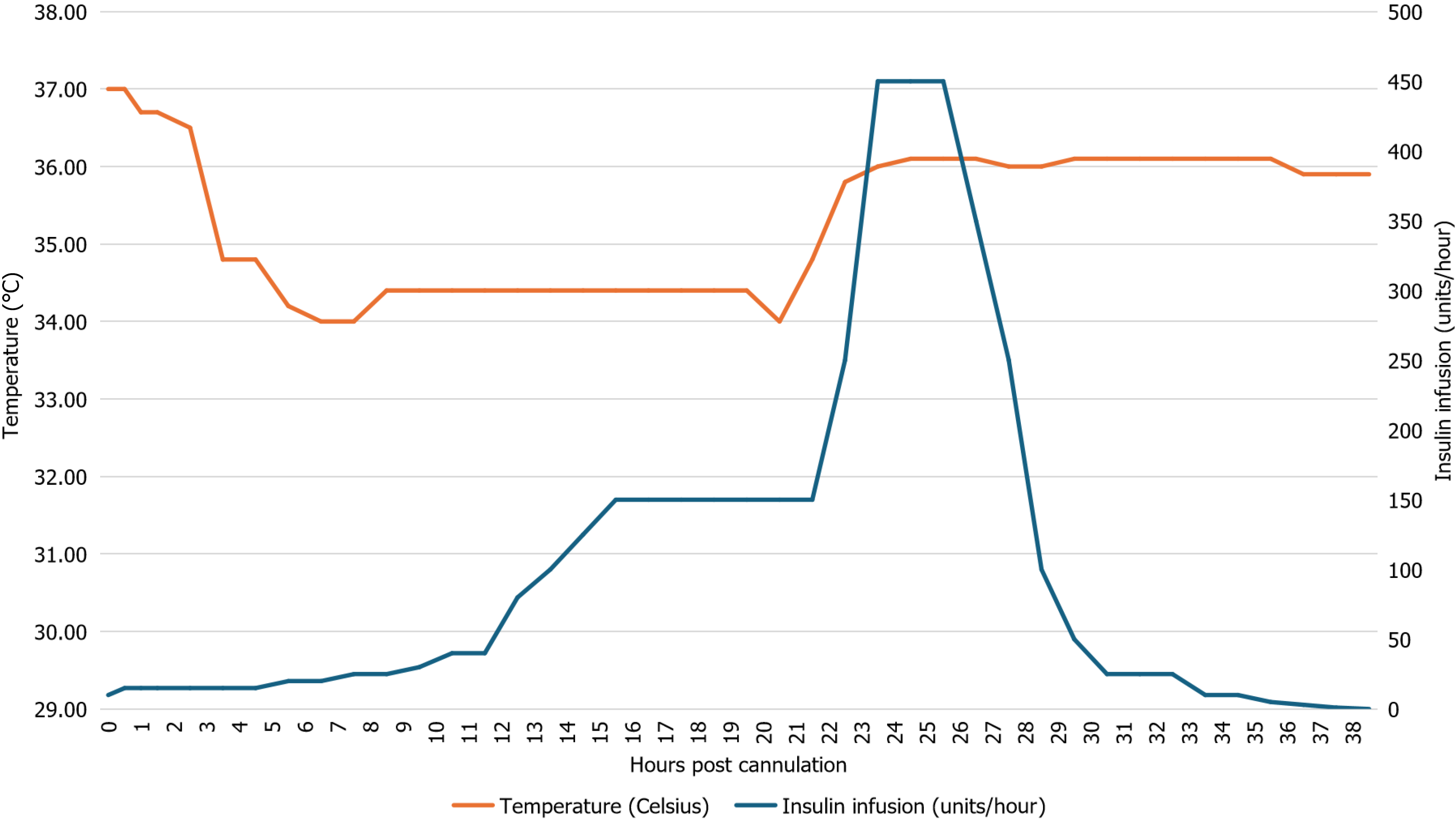
TTM was utilized as a part of the post-resuscitation care to minimize the risk of neurologic injury in this case. Severe insulin resistance during TTM has been previously reported[14-16]. July et al[14] documented a case of TEIR in a patient receiving TTM, and described a clear correlation between onset of TEIR and initiation of therapeutic hypothermia. Interestingly, they also observed a resolution of TEIR once the patient was rewarmed from approximately 32 to approximately 34 °C[14]. Patient’s temperature and insulin infusion rate in our case are displayed in Figure 3. The insulin infusion rate rose rapidly 6 hours after the patient was cooled to 34 °C. This trend continued until 3 hours after the core body temperature normalized (36.1 °C), at which point the insulin requirements declined quickly. However, any correlation between therapeutic hypothermia and insulin resistance in this case must be interpreted with caution due to the presence of multiple confounding factors.
Transient extreme insulin resistance may develop in a variety of clinical scenarios and necessitates close monitoring and high doses of insulin to manage hyperglycemia. Once insulin resistance begins to resolve, monitoring must be intensified, and rapid de-escalation of infusion rates are required. In this case, the onset of TEIR did not seem to correlate with the timing of end-organ hypoperfusion or vasoactive drug dosing. While there is a possible correlation between therapeutic hypothermia and the severity of insulin resistance in this case, multiple confounding factors were present. More research is needed to better characterize the pathophysiology of TEIR and identify optimal management strategies.
| 1. | Saberi F, Heyland D, Lam M, Rapson D, Jeejeebhoy K. Prevalence, incidence, and clinical resolution of insulin resistance in critically ill patients: an observational study. JPEN J Parenter Enteral Nutr. 2008;32:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Vidger AJ, Czosnowski QA. Outcomes and adverse effects of extremely high dose insulin infusions in ICU patients. J Crit Care. 2021;63:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Ovalle F. Clinical approach to the patient with diabetes mellitus and very high insulin requirements. Diabetes Res Clin Pract. 2010;90:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Illuri VD, Layden BT, Aleppo G. Extreme Insulin Resistance in Critically Ill Patient With Sepsis. Clin Diabetes. 2016;34:158-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Wei XY, Shen HN. Transient extreme insulin resistance in a critically ill patient: A case report. World J Clin Cases. 2025;13:100889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Oo YH, Karam JG, Resta CA. Extreme insulin resistance in a patient with diabetes ketoacidosis and acute myocardial infarction. Case Rep Endocrinol. 2013;2013:520904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20:387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 564] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 8. | Krogh-Madsen R, Plomgaard P, Møller K, Mittendorfer B, Pedersen BK. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab. 2006;291:E108-E114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Yokoyama H, Wasada T, Shimizu Y, Yoshino H, Hasumi S, Omori Y. Transient extreme insulin resistance in shock during diabetic ketoacidosis. Endocrinol Jpn. 1992;39:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Fuller BM, Dellinger RP. Lactate as a hemodynamic marker in the critically ill. Curr Opin Crit Care. 2012;18:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Khoury N, McGill JB. Reduction in insulin sensitivity following administration of the clinically used low-dose pressor, norepinephrine. Diabetes Metab Res Rev. 2011;27:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980;65:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 383] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Jerkins T, Bell DSH. Development of Exogenous Insulin Antibody Syndrome in a Patient with Newly Diagnosed Type 1 Diabetes Successfully Treated with Oral Immunosuppressive Monotherapy. Diabetes Ther. 2021;12:2795-2799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | July M, Santhanam P, Gabi J, Khthir RA. Severe Insulin Resistance in the Setting of Therapeutic Hypothermia. Mars J Med. 2017;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4038] [Cited by in RCA: 3835] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 16. | Surani SR, Ratnani I, Guntupalli B, Bopparaju S. Severe insulin resistance treatment with intravenous chromium in septic shock patient. World J Diabetes. 2012;3:170-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/