Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.110174
Revised: June 21, 2025
Accepted: August 22, 2025
Published online: October 15, 2025
Processing time: 138 Days and 3.7 Hours
Current evidence suggests that commonly used antidiabetic drugs have varying effects on cancer risk. Some antidiabetics offer protective effects against cancer, whereas others may increase risk in specific populations.
To comprehensively compare the effects of different antidiabetic drugs on the risk of various cancers in patients with type 2 diabetes mellitus (T2DM) through a systematic review and network meta-analysis.
Four databases (PubMed, EMBASE, Cochrane Library, and Web of Science) were searched from their inception until April 11, 2025. Published randomized con
A total of 13535 articles were identified. After applying the inclusion and exclusion criteria, 87 high-quality studies involving 216106 patients and 26 different drugs across seven classes were included in this study. Indirect evidence from network meta-analysis revealed some heterogeneity; however, this did not affect the reliability of the results. The results indicated that antidiabetic drugs did not increase the overall risk of cancer compared with placebo. In contrast, some antidiabetic medications demonstrated a more pronounced advantage in reducing cancer risk, such as dipeptidyl peptidase-4 inhibitors for thyroid and rectal cancers; sodium-glucose co-transporter type 2 inhibitors for lung and bronchial cancers; sulfonylureas for gastric and colon cancers; biguanides for pancreatic cancer; insulin for bladder cancer; glucagon-like peptide-1 receptor agonists for prostate, uterine, hepatocellular, renal, and hematologic cancers; and thiazolidinediones for breast cancer.
Antidiabetic drugs reduce cancer risk in patients with T2DM. However, given the limitations in the number and quality of the included studies, our conclusions should be interpreted with caution. More large-scale, high-quality clinical trials are required to validate our findings towards the optimization of comprehensive cancer management strategies for patients with T2DM.
Core Tip: Diabetes has been linked to an increased risk of various cancers. Relevant research indicates that commonly used antidiabetic drugs have varying impacts on cancer risk; some provide protective effects, whereas others may increase the risk in specific populations. We conducted a network meta-analysis to compare the effects of different anti-diabetic drugs on the risk of various cancers in patients with type 2 diabetes mellitus.
- Citation: An XD, Duan LY, Zhang YH, Jia QY, Zhang YM, Qiao Y. Association between antidiabetic drugs and cancer risk in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. World J Diabetes 2025; 16(10): 110174
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/110174.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.110174
Type 2 diabetes mellitus (T2DM) is one of the most common chronic metabolic diseases worldwide, and its incidence is increasing. This disease not only imposes a significant burden on individual health but also poses a major challenge to public health and medical resources. Moreover, owing to environmental factors, lifestyle habits, and population aging, the prevalence of diabetes and the incidence of cancers, such as renal and bladder cancer, are concurrently increasing[1-5]. Studies have shown that diabetes is associated with an increased risk of various cancers, possibly through chronic inflammation, insulin resistance, metabolic dysregulation, and immune system alterations[6-9]. In addition, abnormal blood glucose levels have been linked to increased cancer risk and reduced survival rates[10].
Studies have indicated that commonly used antidiabetic drugs have varying effects on cancer risk: Some offer protective effects, whereas others may increase cancer risk in specific populations[11,12]. For example, metformin has antitumor properties[11,13,14]; however, some studies have reported no reduction in cancer incidence following its use[15]. Thiazolidinediones have been associated with a reduced risk of lung cancer[16-18], although other studies have suggested that they may increase pancreatic cancer risk[18]. Some studies suggest that certain sodium-glucose cotransporter 2 inhibitors (SGLT-2i) may increase the risk of bladder and breast cancers[11,19], whereas others report no such increase or even suggest that they have anticancer effects[18,20-23]. Glucagon-like peptide-1 receptor agonists (GLP-1RA) have diverse effects on cancer progression, including inhibition or no significant effect[24,25]. Dipeptidyl peptidase-4 inhibitors (DPP-4i) have not been linked to a significant increase in cancer risk, including pancreatic cancer, and may even be associated with a reduced risk of colorectal cancer[11,26]. Among sulfonylureas, glibenclamide may significantly increase cancer risk but may also protect against specific cancers[17,18]. Insulin and its analogs, such as detemir and glargine, have not been shown to significantly increase cancer risk[18].
Many systematic reviews and meta-analyses have compared the cancer risks associated with various antidiabetic drugs, but their conclusions are inconsistent. For example, the administration of metformin, thiazolidinediones, sulfonylureas, insulin, or DPP-4i was not significantly associated with the risk of prostate cancer, while GLP-1RA significantly reduced this risk, and SGLT2i showed no significant association[17]. The use of antidiabetic drugs has been variably associated with the risk of hepatocellular carcinoma and colorectal cancer, with metformin and thiazolidinediones being the exceptions[27,28]. Additionally, insulin, sulfonylureas, and α-glucosidase inhibitors may increase the risk of lung cancer[29,30].
Therefore, this study aimed to comprehensively compare the positive and negative effects of different antidiabetic drugs on various cancer risks through a systematic review and network meta-analysis. With this study, we aim to shed more light on the use of different antidiabetic drugs in T2DM treatment and their association with reduced cancer risk.
This study employed a systematic review and network meta-analysis to evaluate the most recently published high-quality clinical studies and to comprehensively compare the associations between different glucose-lowering medications and cancer risk in patients with T2DM. The study protocol was registered in PROSPERO (CRD42024534491), with detailed information available online at https://www.crd.york.ac.uk/prospero/.
Four databases (PubMed, Web of Science, Cochrane Central Register of Controlled Trials, and EMBASE) were searched for randomized controlled trials (RCTs) of different antidiabetic drugs for the treatment of T2DM from database inception until April 11, 2025. In addition, the references of published articles and relevant systematic reviews were comprehensively reviewed to identify and include additional include peer-reviewed published literature. Only articles published in English were included in the analysis. There were no restrictions on publication date or status. Search keywords included but were not limited to “Cancer” and “T2DM”. Notably, for some large-scale clinical studies (with study duration and sample size meeting our inclusion criteria), even if cancer-related descriptions were not explicitly mentioned in the title or abstract, their eligibility for inclusion were assessed by reviewing the full text and examining publicly available data (such as information released on the ClinicalTrials website). Detailed search strategies for each database are provided in the Supplementary material.
Participants: Adults (aged ≥ 18 years) with T2DM.
Interventions: The intervention group received various antidiabetic medications, including biguanides, sulfonylureas, thiazolidinediones, DPP-4i, SGLT-2i, GLP-1RA, and insulin.
Controls: The control group received a placebo or a single alternative antidiabetic drug.
Outcomes: Sufficient data available for meta-analysis, including at least one target outcome (at least one cancer event), such as any cancer, lung cancer, bronchial cancer, thyroid cancer, gastric cancer, pancreatic cancer, rectal cancer, colon cancer, bladder cancer, prostate cancer, uterine cancer, breast cancer, hepatocellular cancer, renal cancer, or hematological cancer.
Study design: RCTs with a minimum intervention duration of 1 year (or 52 weeks) and at least 100 participants.
Study types: Systematic reviews, reviews, trial protocols, basic science studies, conference abstracts, and other non-original RCTs.
Language: Non-English publications.
EndNote 20 was used to remove duplicates by comparing references based on fields, such as title and year. Two reviewers independently screened titles and abstracts to identify potentially eligible studies. Full-text articles were assessed to determine their final inclusion.
Following study selection, the following data were extracted from the included studies using Microsoft Excel (V 16.95.1): Study characteristics, study title, first author, year of publication, clinical trial registration number, study design, duration of intervention, number of participants, intervention medications, age (inclusion criteria), sex, body mass index (inclusion criteria), glycated hemoglobin, and glycated hemoglobin (inclusion criteria), and whether cancer was designated as a predefined outcome. Efficacy outcomes: Incidence of any cancer, lung cancer, bronchial cancer, thyroid cancer, gastric cancer, pancreatic cancer, rectal cancer, colon cancer, bladder cancer, prostate cancer, uterine cancer, breast cancer, hepatocellular cancer, renal cancer, and hematological cancer. Discrepancies in data extraction were resolved through discussions with a third investigator (Duan LY) until a consensus was reached.
When incomplete data were encountered, the ClinicalTrials website was first searched for relevant information or the authors were contacted directly to obtain the missing data.
A network meta-analysis of RCTs was conducted using a Bayesian random-effects model in Stata 17.0. The “network” command suite was used for data processing. In the evidence network, interventions were represented as nodes, with larger nodes indicating a greater number of patients receiving interventions. The lines between the nodes represent direct comparisons between the two interventions, and the thickness of the lines reflects the number of studies included. When the evidence network contained closed loops, the node-splitting method was used to evaluate the local and global inconsistencies by assessing the agreement between direct and indirect comparisons. A P-value > 0.05 indicated no statistically significant difference, suggesting consistency between direct and indirect evidence and justifying the use of a consistency model for analysis. A network of indirect evidence was used for the different medications and calculated the surface under the cumulative ranking curve (SUCRA). A larger SUCRA indicates a better intervention effect, allowing for comparison and ranking of treatment efficacy. Forest plots were generated to compare the different drugs with the placebo.
The methodological quality of the included studies was assessed using the Cochrane risk-of-bias tool. These included evaluations across the following domains: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other sources of bias. Studies were classified as having low, high, or unclear risk of bias. Two reviewers (An XD and Zhang YH) independently performed all assessments. Any disagreements were resolved through discussion with a third reviewer (Jia QY). Furthermore, confidence in network was used to assess the certainty of evidence across six key domains: Within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and inconsistency. Confidence in network meta-analysis uncertainty was evaluated by comparing potential effect modifiers between studies and provided both direct and indirect evidence for each comparison.
To assess the robustness of the study findings, a sensitivity analysis was conducted by systematically removing one study at a time to evaluate its impact on the overall results. Funnel plots and other statistical methods, including Egger’s test, were used to evaluate publication bias and to detect asymmetry. If publication bias was detected, appropriate corrections and adjustments were made, such as using the trim-and-fill method.
In this study, we analyzed the associations between different antidiabetic medications and cancer risk in individuals with T2DM. After integrating and analyzing data from the included studies, 87 Large-scale RCTs involving 216106 patients were included (Figure 1). These studies were conducted in various countries and regions between 2005 and 2022. The 26 included drugs encompassed seven categories, including biguanides (metformin); sulfonylureas (glibenclamide, glimepiride, and glipizide); thiazolidinediones (pioglitazone and rosiglitazone); DPP-4i (alogliptin, linagliptin, oma
We included high-quality RCTs and independently evaluated the quality of each study. Consistency checks across various outcome indicators were consistent (P > 0.05), allowing us to use a consistency model for analysis (Supplementary material). We assessed the consistency between the direct and indirect evidence. We observed significant heterogeneity in the indirect evidence, which is a risk of bias that cannot be completely avoided in network meta-analyses, although it did not affect the stability or reliability of the results. Additionally, we found no significant evidence of funnel plot asymmetry, suggesting a lack of publication bias.
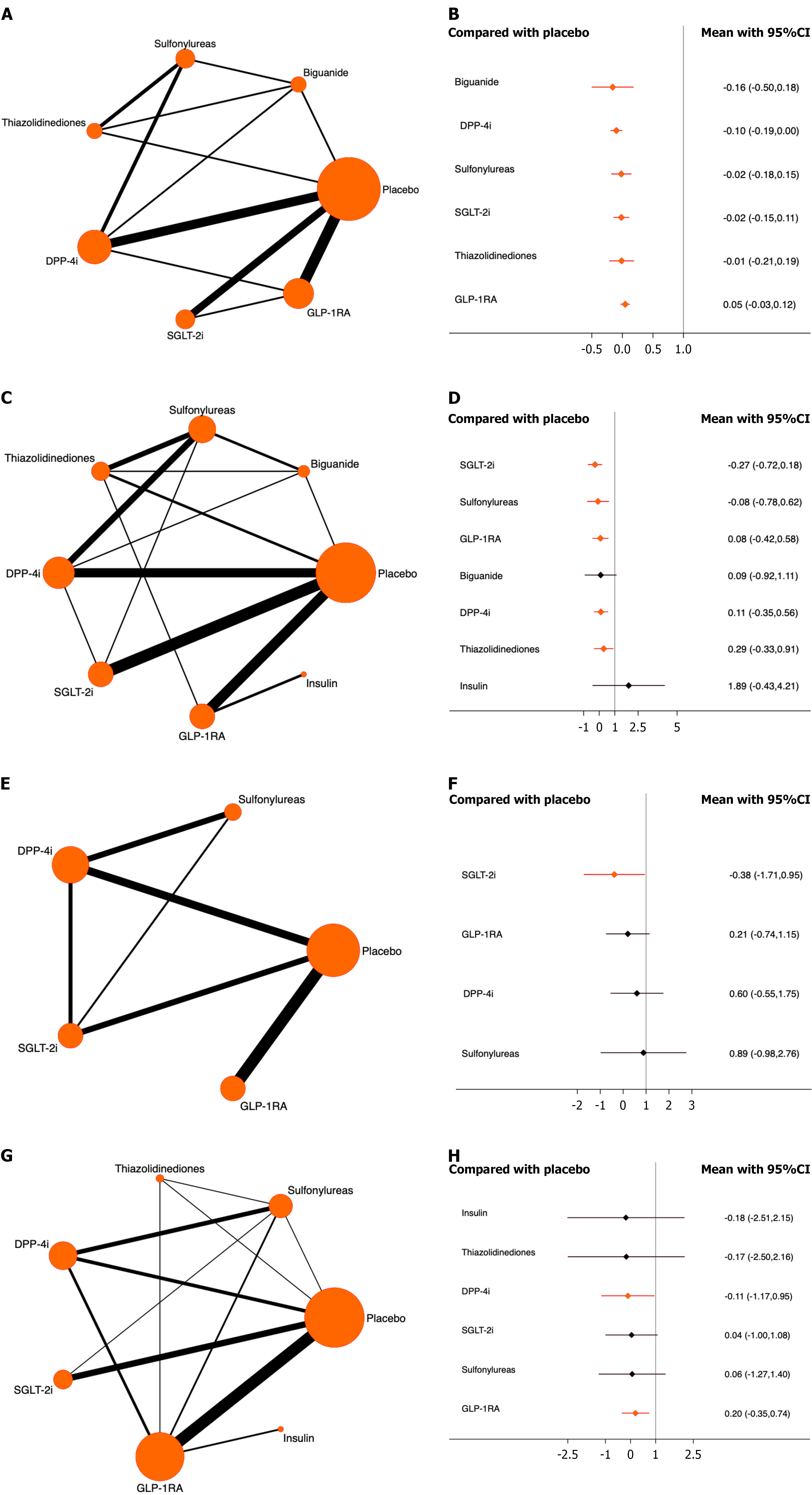
Any cancer: A total of 22 studies involving 126343 patients were included, of whom 6731 experienced cancer events. The results showed that compared with placebo, all antidiabetic medications significantly reduced the risk of cancer. Among them, DPP-4i ranked the highest [odds ratio (OR) = -0.10, 95% confidence interval (CI): -0.19 to 0.00, SUCRA = 80.3%, χ2 = 3.98]. The overall quality of evidence was high (Figure 2A and B; Supplementary material).
Lung cancer: A total of 37 studies involving 166431 patients were included, of whom 297 had lung cancer. Compared with placebo, all antidiabetic drugs, except biguanides and insulin, significantly reduced the risk of lung cancer. SGLT-2i ranked highest (OR = -0.27, 95%CI: -0.72 to 0.18, SUCRA = 84.2%, χ2 = 4.78). The overall quality of the evidence was high (Figure 2C and D; Supplementary material).
Bronchial cancer: Of the 19 studies that included 140492 participants, 43 developed bronchial cancer. The results indicated that only SGLT-2i (OR = -0.38, 95%CI: -1.71 to 0.95, SUCRA = 82.5%, χ2 = 0.65) significantly reduced the risk compared to placebo. The overall quality of the evidence was high (Figure 2E and F; Supplementary material).
Thyroid cancer: A total of 34 studies were included, involving 149757 patients, of whom 85 patients had thyroid cancer. Only DPP-4i and GLP-1RA significantly reduced the risk of cancer compared with placebo, with DPP-4i ranking highest (OR = -0.11, 95%CI: -1.17 to 0.95, SUCRA = 57.1%, χ2 = 6.97). The overall quality of the evidence was high (Figure 2G and H; Supplementary material).
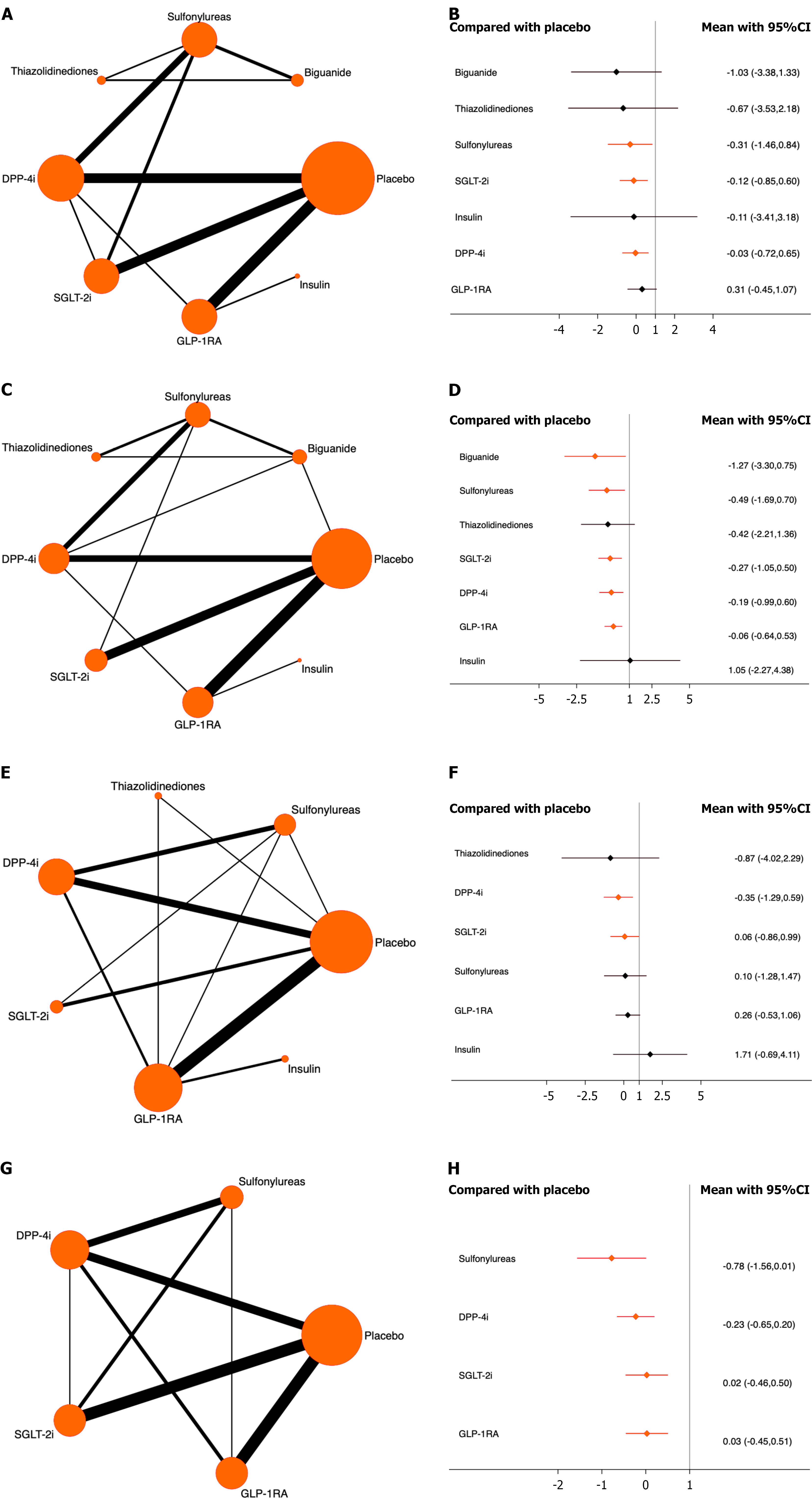
Gastric cancer: Thirty studies with 161997 patients were included, of whom 103 patients developed gastric cancer. Compared with placebo, sulfonylureas, SGLT-2i, and DPP-4i significantly reduced the risk of gastric cancer. Sulfonylureas ranked highest (OR = -0.31, 95%CI: -1.46 to 0.84, SUCRA = 56.8%, χ2 = 2.06). The overall quality of the evidence was high (Figure 3A and B; Supplementary material).
Pancreatic cancer: A total of 31 studies involving 165210 patients were included, with 176 cases of pancreatic cancer. Compared with placebo, all antidiabetic drugs, except thiazolidinediones and insulin, significantly reduced the risk. Biguanides ranked highest (OR = -1.27, 95%CI: -3.30 to 0.75, SUCRA = 84.2%, χ2 = 7.60). The overall quality of the evidence was high (Figure 3C and D; Supplementary material).
Rectal cancer: Twenty-five studies with a total of 152793 patients were included, of whom 74 developed rectal cancer. Only DPP-4i and SGLT-2i significantly reduced the risk, compared with placebo. DPP-4i ranked highest (OR = -0.35, 95%CI: -1.29 to 0.59, SUCRA = 73.9%, χ2 = 3.82). The overall quality of the evidence was high (Figure 3E and F; Supplementary material).
Colon cancer: A total of 39 studies with 170345 participants were included, and 243 patients had colon cancer. All included antidiabetic medications significantly reduced the risk compared with placebo. Sulfonylureas ranked highest (OR = -0.78, 95%CI: -1.56 to 0.01, SUCRA = 95.9%, χ2 = 1.74). The overall quality of the evidence was high (Figure 3G and H; Supplementary material).
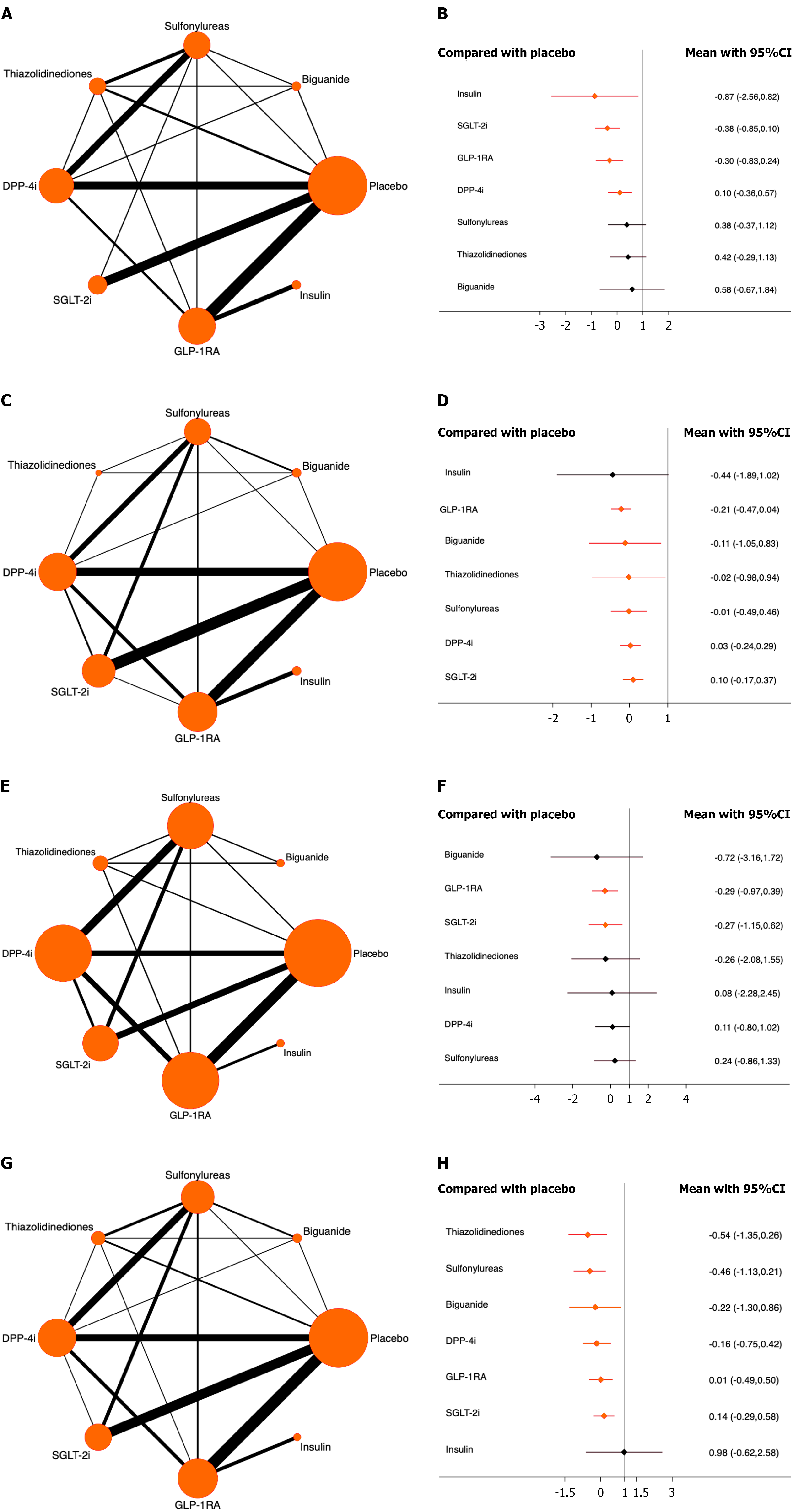
Bladder cancer: Forty studies involving 175500 patients were included, among whom 261 patients developed bladder cancer. The results showed that insulin, SGLT-2i, GLP-1RA, and DPP-4i significantly reduced the risk of bladder cancer compared with placebo. Among these, insulin ranked highest (OR = -0.87, 95%CI: -2.56 to 0.82, SUCRA = 84.4%, χ2 = 6.26). The overall quality of the included studies was high (Figure 4A and B, Supplementary material).
Prostate cancer: A total of 55 studies involving 183497 patients were included, and 760 patients developed prostate cancer. The results indicated that all antidiabetic drugs included in the analysis except for insulin significantly reduced the risk of prostate cancer compared with the placebo. Among them, GLP-1RA ranked highest (OR = -0.21, 95%CI: -0.47 to 0.04, SUCRA = 74.2%, χ2 = 7.59). The overall quality of the studies was high (Figure 4C and D; Supplementary mate
Uterine cancer: A total of 32 studies involving 137166 patients were included, and 80 patients developed endometrial cancer. The results demonstrated that both GLP-1RA and SGLT-2i significantly reduced the risk of endometrial cancer compared to the placebo. Among them, GLP-1RA ranked highest (OR = -0.29, 95%CI: -0.97 to 0.39, SUCRA = 62.9%, χ2 = 1.72). The overall quality of the studies was high (Figure 4E and F; Supplementary material).
Breast cancer: A total of 57 studies involving 191966 patients were included, and 254 developed breast cancer. The results showed that, compared with placebo, all antidiabetic drugs included in the analysis except for insulin significantly reduced the risk of breast cancer. Among them, thiazolidinediones ranked highest (OR = -0.54, 95%CI: -1.35 to 0.26, SUCRA = 83.7%, χ2 = 7.46). The overall quality of the included studies was high (Figure 4G and H; Supplementary material).
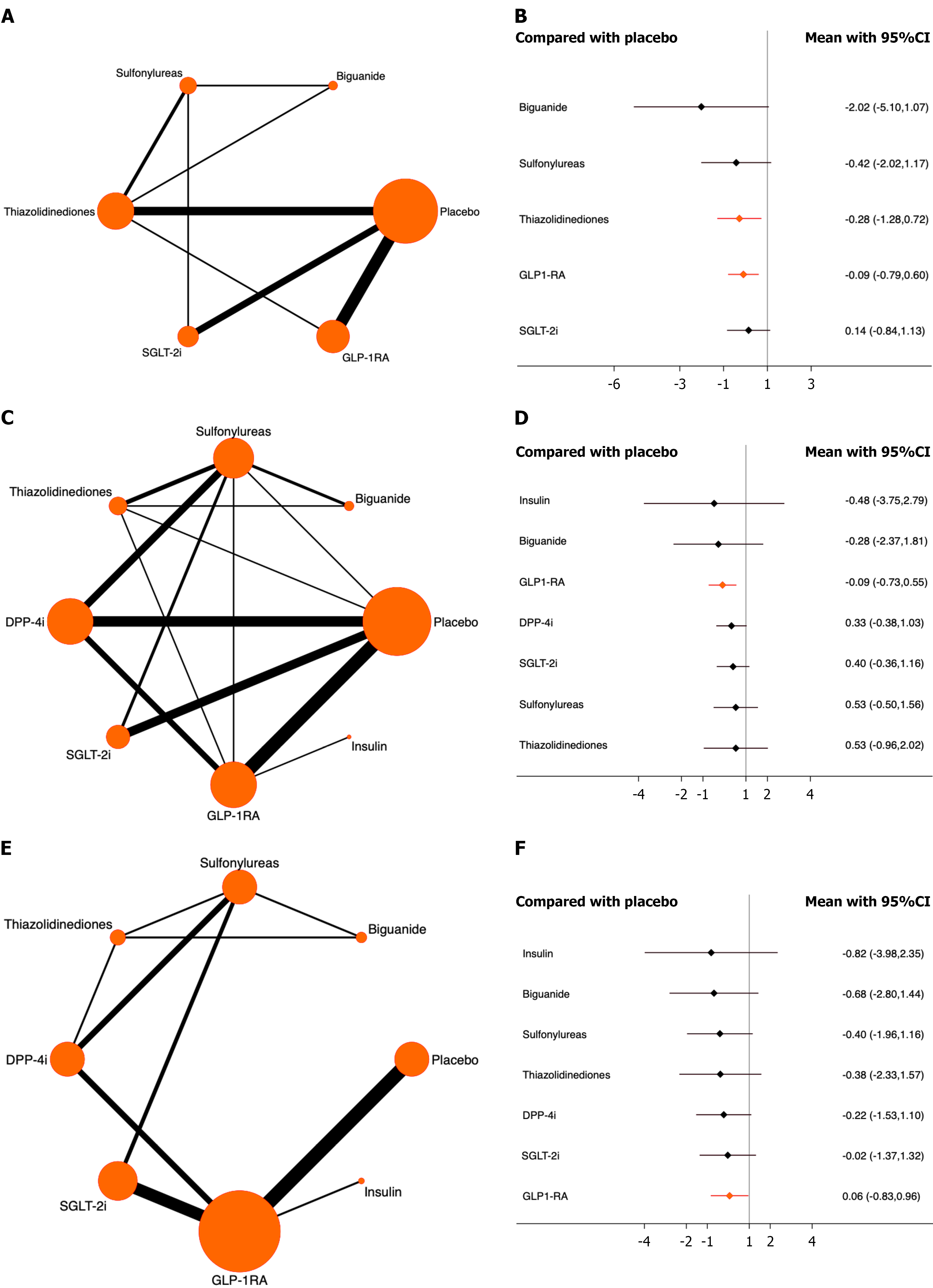
Hepatocellular cancer: Twenty studies involving 154776 patients were included, and 90 patients developed hepatocellular cancer. The results demonstrated that thiazolidinediones and GLP-1RA both significantly reduced the risk of hepatocellular cancer compared with placebo. Among them, thiazolidinediones ranked highest (OR = -0.28, 95%CI: -1.28 to 0.72, SUCRA = 88.0%, χ2 = 2.17). The overall quality of the studies was high (Figure 5A and B; Supplementary mate
Renal cancer: A total of 35 studies involving 169333 patients were included, and 111 patients developed renal cancer. The results demonstrated that only GLP-1RA significantly reduced the risk of renal cancer compared with placebo (OR = -0.09, 95%CI: -0.73 to 0.55, SUCRA = 67.5%, χ2 = 2.34). The overall quality of the studies was high (Figure 5C and D; Supplementary material).
Hematologic cancer: A total of 24 studies involving 150318 patients were included, and 80 patients developed hematologic cancer. The results demonstrated that only GLP-1RA significantly reduced the risk of hematologic cancer compared with placebo (OR = 0.06, 95%CI: -0.83 to 0.96, SUCRA = 31.5%, χ2 = 0.18). The overall quality of the studies was high (Figure 5E and F; Supplementary material).
To the best of our knowledge, this is the first systematic review and network meta-analysis based on high-quality, long-term RCTs, comparing seven classes of antidiabetic drugs (26 different agents) in terms of their association with cancer risk in patients with T2DM. First, we found that most antidiabetic drugs significantly reduced the risk of various cancers without increasing their incidence. In contrast, some drugs significantly reduced the risk of cancer, except in cases of any cancer and colon cancer, such as DPP-4i for any cancer, thyroid cancer, and rectal cancer; SGLT-2i for lung and bronchial cancers; sulfonylureas for gastric and colon cancers; biguanides for pancreatic cancer; insulin for bladder cancer; GLP-1RA for prostate, uterine, hepatocellular, renal, and hematologic cancers; and thiazolidinediones for breast cancer. Our findings are consistent with those of previous meta-analyses[27], though not entirely aligned[29,30]. This study provides valuable and high-quality evidence for cancer management in patients with T2DM (Table 1).
| Classes of antidiabetic drugs | Biguanide | Sulfonylureas | Thiazolidinediones | DPP-4i | SGLT-2i | GLP-1RA | Insulin |
| Any cancer | I | I | I | I | I | I | NA |
| Lung cancer | II | I | I | I | I | I | II |
| Bronchial cancer | NA | II | NA | II | I | II | NA |
| Thyroid cancer | NA | II | II | I | II | I | II |
| Gastric cancer | II | I | II | I | I | II | II |
| Pancreatic cancer | I | I | II | I | I | I | II |
| Rectal cancer | NA | II | II | I | I | II | II |
| Colon cancer | NA | I | NA | I | I | I | NA |
| Bladder cancer | II | II | II | I | I | I | I |
| Prostate cancer | I | I | I | I | I | I | II |
| Uterine cancer | II | II | II | II | I | I | II |
| Breast cancer | I | I | I | I | I | I | II |
| Hepatocellular cancer | II | II | I | NA | II | I | NA |
| Renal cancer | II | II | II | II | II | I | II |
| Hematologic cancer | II | II | II | II | II | I | II |
The complex interplay between diabetes and cancer involves shared molecular pathways such as insulin signaling, AMP-activated protein kinase, and the mammalian target of the rapamycin pathway[31]. For example, metformin activates AMP-activated protein kinase, a key regulator of cellular energy metabolism, and inhibits the AKT serine/threonine kinase/mammalian target of the rapamycin pathway, thereby reducing cancer cell growth and proliferation[32,33]. As insulin secretagogues, sulfonylureas may promote tumor development through elevated insulin and insulin-like growth factor levels[34]. SGLT2i reduces tumor invasion and metastasis, thereby suppressing cancer progression and proliferation[35]. DPP-4i may reduce cancer risk by inhibiting inflammation and oxidative stress, with the potential to suppress tumor growth in cancer patients[36]. GLP-1RA may decrease cancer risk by inhibiting cell proliferation and inducing apoptosis[37]. Thiazolidinediones may reduce cancer risk by alleviating inflammation and oxidative stress[38]. Insulin may act on intestinal insulin receptors to regulate the gut barrier function, potentially delaying the progression of nonalcoholic steatohepatitis-associated hepatocellular carcinoma[39]. However, research on these antidiabetic drugs in different cancers is limited and insufficiently explored[40]. Current mechanistic studies have suggested that antidiabetic medications possess potential anticancer properties. However, the underlying mechanism remains unclear. Further in-depth research is required to fully confirm these findings.
Our findings were based on currently available high-quality, long-term RCTs, although some relevant data were missing. However, we must acknowledge the presence of indirect evidence with a relatively high risk of bias in the included studies, which is a potential limitation inherent to network meta-analyses. Additionally, we found no significant evidence of asymmetry in the funnel plots. Taken together, our results demonstrate good reliability.
A previous study[41] conducted network meta-analysis to compare the effects of different incretin-based therapies on gastrointestinal cancers, which indicated that incretin therapies were not associated with an increased risk of digestive system cancers in patients with T2DM. Moreover, several meta-analyses have independently evaluated the association between specific antidiabetic drugs, including metformin, thiazolidinediones, sulfonylureas, insulin, and DPP-4i, and cancer risk in this population[17,27-30]. However, no comprehensive systematic review or network meta-analysis has compared the association between various antidiabetic drugs and the risk of multiple types of cancer, which was the aim of our study.
When comparing our results with those of existing meta-analyses, some differences emerged. For instance, previous studies found no significant association between drugs such as metformin and thiazolidinediones and the risk of prostate cancer, whereas GLP-1RA significantly reduced the risk of prostate cancer[17]. Moreover, SGLT-2i was not significantly associated with prostate cancer risk[17], and insulin, sulfonylureas, and α-glucosidase inhibitors were reported to potentially increase lung cancer risk[29,30]. In contrast, our study found that all the antidiabetic drugs included in the analysis, except insulin, significantly reduced the risk of prostate cancer, whereas all drugs except biguanide and insulin significantly reduced the risk of lung cancer.
These discrepancies may stem from differences in the included studies. Our meta-analysis was limited to RCTs, excluded observational studies, and included only trials with a follow-up period of at least 1 year and a sample size of no fewer than 100 cases. Therefore, our results should be interpreted on the basis of the characteristics of the included studies. Our findings offer high-quality evidence to help clinicians make informed choices regarding antidiabetic treatments that reduce, or at least do not increase, cancer risk in patients with T2DM.
Although this study yields important findings, several issues require further investigation. First, owing to the heterogeneity of the included studies and limitations of the available data, we cannot rule out the possibility of potential confounding factors and bias. Second, because our analysis relied primarily on published literature, publication bias may have been present; however, we attempted to minimize bias by searching multiple databases and conducting sensitivity analyses. More importantly, given the generally low incidence of cancer events and the fact that none of the included studies designated cancer as a primary outcome and only 20.69% (18/87) pre-specified cancer as an outcome or adverse event, the robustness and reliability of SUCRA rankings may be compromised. Furthermore, we must acknowledge that SUCRA values represent rankings based on currently available data and do not directly equate to clinical superiority. Therefore, our findings should be interpreted in conjunction with the effect estimates and their corresponding confidence intervals.
Overall, our study strengthens the literature and compares the differential effects of various antidiabetic drugs on cancer risk in patients with T2DM. However, given the limitations of the quantity and quality of the included studies, our conclusions should be interpreted with caution. More large-scale, high-quality clinical studies are required to validate our findings and further optimize comprehensive cancer management strategies for patients with T2DM.
| 1. | Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 2. | Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6:e6-e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Xu X, Wu J, Mao Y, Zhu Y, Hu Z, Xu X, Lin Y, Chen H, Zheng X, Qin J, Xie L. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS One. 2013;8:e58079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Gong IY, Cheung MC, Read S, Na Y, Lega IC, Lipscombe LL. Association between diabetes and haematological malignancies: a population-based study. Diabetologia. 2021;64:540-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Zhou XH, Qiao Q, Zethelius B, Pyörälä K, Söderberg S, Pajak A, Stehouwer CD, Heine RJ, Jousilahti P, Ruotolo G, Nilsson PM, Calori G, Tuomilehto J; DECODE Study Group. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53:1867-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Cuttica CM, Briata IM, DeCensi A. Novel Treatments for Obesity: Implications for Cancer Prevention and Treatment. Nutrients. 2023;15:3737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Garstka MA, Kedzierski L, Maj T. Diabetes can impact cellular immunity in solid tumors. Trends Immunol. 2025;46:295-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Cao J, Yan W, Ma X, Huang H, Yan H. Insulin-like Growth Factor 2 mRNA-Binding Protein 2-a Potential Link Between Type 2 Diabetes Mellitus and Cancer. J Clin Endocrinol Metab. 2021;106:2807-2818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 553] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Søndergaard CS, Esquivel PN, Dalamaga M, Magkos F. Use of Antihyperglycemic Drugs and Risk of Cancer in Patients with Diabetes. Curr Oncol Rep. 2023;25:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 12. | Lin A, Ding Y, Li Z, Jiang A, Liu Z, Wong HZH, Cheng Q, Zhang J, Luo P. Glucagon-like peptide 1 receptor agonists and cancer risk: advancing precision medicine through mechanistic understanding and clinical evidence. Biomark Res. 2025;13:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 13. | Yu OHY, Suissa S. Metformin and Cancer: Solutions to a Real-World Evidence Failure. Diabetes Care. 2023;46:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | O'Connor L, Bailey-Whyte M, Bhattacharya M, Butera G, Hardell KNL, Seidenberg AB, Castle PE, Loomans-Kropp HA. Association of metformin use and cancer incidence: a systematic review and meta-analysis. J Natl Cancer Inst. 2024;116:518-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 15. | Mesquita LA, Spiazzi BF, Piccoli GF, Nogara DA, da Natividade GR, Garbin HI, Wayerbacher LF, Wiercinski VM, Baggio VA, Zingano CP, Schwartsmann G, Lopes G, Petrie JR, Colpani V, Gerchman F. Does metformin reduce the risk of cancer in obesity and diabetes? A systematic review and meta-analysis. Diabetes Obes Metab. 2024;26:1929-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Tatsch JM, Furman DP, Nobre RM, Wurzer KM, da Silva LC, Picheth GF, Ramos EA, Acco A, Klassen G. Dulaglutide as a demethylating agent to improve the outcome of breast cancer. Epigenomics. 2023;15:1309-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Cui H, Wang Y, Yang S, He G, Jiang Z, Gang X, Wang G. Antidiabetic medications and the risk of prostate cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Pharmacol Res. 2022;177:106094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 18. | Dankner R, Roth J. More recent, better designed studies have weakened links between antidiabetes medications and cancer risk. Diabet Med. 2020;37:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Andrianu K, Works D, Christiansen A, Enke C, Chaiken L, Baine M. Exploring the Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Genitourinary Toxicities in Prostate Cancer Patients Undergoing Radiation Therapy: A Case Study and Discussion. Pract Radiat Oncol. 2024;14:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Spiazzi BF, Naibo RA, Wayerbacher LF, Piccoli GF, Farenzena LP, Londero TM, da Natividade GR, Zoldan M, Degobi NAH, Niches M, Lopes G, Boyko EJ, Utzschneider KM, Colpani V, Gerchman F. Sodium-glucose cotransporter-2 inhibitors and cancer outcomes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2023;198:110621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 21. | Knura M, Garczorz W, Borek A, Drzymała F, Rachwał K, George K, Francuz T. The Influence of Anti-Diabetic Drugs on Prostate Cancer. Cancers (Basel). 2021;13:1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 22. | Camilli M, Viscovo M, Maggio L, Bonanni A, Torre I, Pellegrino C, Lamendola P, Tinti L, Teofili L, Hohaus S, Lanza GA, Ferdinandy P, Varga Z, Crea F, Lombardo A, Minotti G. Sodium-glucose cotransporter 2 inhibitors and the cancer patient: from diabetes to cardioprotection and beyond. Basic Res Cardiol. 2025;120:241-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Dabour MS, George MY, Daniel MR, Blaes AH, Zordoky BN. The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024;6:159-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 24. | Ibrahim SS, Ibrahim RS, Arabi B, Brockmueller A, Shakibaei M, Büsselberg D. The effect of GLP-1R agonists on the medical triad of obesity, diabetes, and cancer. Cancer Metastasis Rev. 2024;43:1297-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 25. | Figlioli G, Piovani D, Peppas S, Pugliese N, Hassan C, Repici A, Lleo A, Aghemo A, Bonovas S. Glucagon-like peptide-1 receptor agonists and risk of gastrointestinal cancers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2024;208:107401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Abd El Aziz M, Cahyadi O, Meier JJ, Schmidt WE, Nauck MA. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: a meta-analysis based on cardiovascular outcomes trials. Diabetes Obes Metab. 2020;22:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 27. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881-91; quiz 892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 28. | Singh S, Singh H, Singh PP, Murad MH, Limburg PJ. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22:2258-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 29. | Wu Y, Liu HB, Shi XF, Song Y. Conventional hypoglycaemic agents and the risk of lung cancer in patients with diabetes: a meta-analysis. PLoS One. 2014;9:e99577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Wu L, Zhu J, Prokop LJ, Murad MH. Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies. Sci Rep. 2015;5:10147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Hijazi MA, Gessner A, El-Najjar N. Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp. Cancers (Basel). 2023;15:3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 32. | Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60:1639-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 33. | Nagasawa M, Hirano K, Sirato I, Koide H. [Phosphate transport by renal brush border membrane in chronic renal failure]. Nihon Jinzo Gakkai Shi. 1987;29:687-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 34. | Pasello G, Urso L, Conte P, Favaretto A. Effects of sulfonylureas on tumor growth: a review of the literature. Oncologist. 2013;18:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Basak D, Gamez D, Deb S. SGLT2 Inhibitors as Potential Anticancer Agents. Biomedicines. 2023;11:1867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 36. | Almagthali AG, Alkhaldi EH, Alzahrani AS, Alghamdi AK, Alghamdi WY, Kabel AM. Dipeptidyl peptidase-4 inhibitors: Anti-diabetic drugs with potential effects on cancer. Diabetes Metab Syndr. 2019;13:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Fareed A, Hussain A. The Expanding Role of GLP-1: From Diabetes Management to Cancer Treatment. Clin Med Insights Endocrinol Diabetes. 2023;16:11795514231213566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 38. | Blanquicett C, Roman J, Hart CM. Thiazolidinediones as anti-cancer agents. Cancer Ther. 2008;6:25-34. [PubMed] |
| 39. | Soeda K, Sasako T, Enooku K, Kubota N, Kobayashi N, Ikushima YM, Awazawa M, Bouchi R, Toda G, Yamada T, Nakatsuka T, Tateishi R, Kakiuchi M, Yamamoto S, Tatsuno K, Atarashi K, Suda W, Honda K, Aburatani H, Yamauchi T, Fujishiro M, Noda T, Koike K, Kadowaki T, Ueki K. Gut insulin action protects from hepatocarcinogenesis in diabetic mice comorbid with nonalcoholic steatohepatitis. Nat Commun. 2023;14:6584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 40. | Dhas Y, Biswas N, M R D, Jones LD, Ashili S. Repurposing metabolic regulators: antidiabetic drugs as anticancer agents. Mol Biomed. 2024;5:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 41. | Chai S, Yu S, Yang Z, Wu S, Gao L, Wang H, Zhang Y, Zhan S, Ji L, Sun F. Effect of incretin-based therapies on cancers of digestive system among 101 595 patients with type 2 diabetes mellitus: a systematic review and network meta-analysis combining 84 trials with a median duration of 30 weeks. BMJ Open Diabetes Res Care. 2019;7:e000728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/