Published online Jan 15, 2025. doi: 10.4239/wjd.v16.i1.99745
Revised: September 21, 2024
Accepted: October 24, 2024
Published online: January 15, 2025
Processing time: 115 Days and 18.7 Hours
Skin wounds are highly common in diabetic patients, and with increasing types of pathogenic bacteria and antibiotic resistance, wounds and infections in diabetic patients are difficult to treat and heal.
To explore the effects of betaine ointment (BO) in promoting the healing of skin wounds and reducing the inflammation and apoptosis of skin cells in microbially infected diabetic mice.
By detecting the minimum inhibitory concentrations (MICs) of betaine and plant monomer components such as psoralen, we prepared BO with betaine as the main ingredient, blended it with traditional Chinese medicines such as gromwell root and psoralen, and evaluated its antibacterial effects and safety in vitro and in vivo. The skin infection wound models of ordinary mice and diabetic mice were constructed, and the OTC drugs mupirocin ointment and Zicao ointment were used as controls to evaluate the antibacterial effects in vivo and the anti-inflammatory and anti-apoptotic effects of BO.
The MICs of betaine against microorganisms such as Staphylococcus aureus (S. aureus), Candida albicans and Cryptococcus neoformans ranged from 4 to 32 μg/mL. Gromwell root and psoralea, both of which contain antimicrobial components, mixed to prepare BO with MICs ranging from 16 to 64 μg/mL, which is 32-256 times lower than those of Zicao ointment, although the MIC is greater than that of betaine. After 15 days of treatment with BO for USA300-infected ordinary mice, the wound scab removal rates were 83.3%, while those of mupirocin ointment and Zicao ointment were 66.7% and 0%, respectively, and the differences were statistically significant. In diabetic mice, the wound scab removal rate of BO and mupirolacin ointment was 80.0%, but BO reduced wound inflammation and the apoptosis of skin cells and facilitated wound healing.
The ointment prepared by mixing betaine and traditional Chinese medicine can effectively inhibit common skin microorganisms and has a strong effect on the skin wounds of sensitive or drug-resistant S. aureus-infected ordinary mice and diabetic mice.
Core Tip: Antibiotic ointments are commonly used to treat wounds in diabetic patients, but the antibacterial spectrum is narrow, resistance occurs easily or does not effectively inhibit drug-resistant bacteria, and wound healing is slow. The focus of this study was to determine whether some natural Chinese medicines and betaine could enhance the antimicrobial, anti-inflammatory, anti-apoptotic and accelerated wound healing effects of the ointment. Therefore, we generated a novel betaine ointment (BO) and evaluated its effects and safety. We found that BO could reduce the colonization of pathogens, inflammation and apoptosis and contribute to the proliferation of granulomas, which has good prospects for application.
- Citation: Xu WY, Dai YY, Yang SX, Chen H, Huang YQ, Luo PP, Wei ZH. Betaine combined with traditional Chinese medicine ointment to treat skin wounds in microbially infected diabetic mice. World J Diabetes 2025; 16(1): 99745
- URL: https://www.wjgnet.com/1948-9358/full/v16/i1/99745.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i1.99745
Guidelines for the management of skin and soft tissue infections (SSTIs) published jointly by the World Association of Emergency Surgery and the European Society for Surgical Infection in 2018[1] state that SSTIs include a variety of pathologic conditions involving the skin and potentially subcutaneous tissues, fascia, or muscle and that they are infections caused by bacteria or fungi directly damaging the skin tissue or invading the skin soft tissue due to trauma. If not treated promptly, it may cause local suppurative infection of tissues and organs or even systemic infection, and in severe cases, it may lead to patient death[2]. The main reasons for the difficulty in curing infections and healing wounds include immunodeficiency diseases, chronic diseases (such as diabetes mellitus)[3], and the emergence of drug-resistant strains of bacteria such as methicillin-resistant Staphylococcus aureus (S. aureus)[4].
The incidence of skin defects in patients with diabetes mellitus is 2.94%, and diabetic patients have decreased immune function[5,6]. The emergence of vascular disease easily causes blood circulation stagnation[7], and hyperglycemia impedes the production of some inflammatory factors[8] and other problems and can prolong the healing time of wounds. The incidence of diabetic wounds is increasing every year and has become one of the greatest global health problems in the 21st century[9]. The corresponding changes in the pathogenic bacteria of infections as well as drug-resistant variations have also made clinical treatment more difficult[10]; therefore, there is an urgent need to develop new methods for the treatment of wounds infected by drug-resistant microorganisms and for the promotion of wound healing in patients with diabetes mellitus.
Some herbal components have become important raw materials for novel drug development because of their significant antibacterial effects, high safety and low susceptibility to drug resistance[11,12]; thus, the development of bacteriostatic drugs with Chinese medicines has improved application and development prospects. The Chinese medicines frankincense and myrrh disinfect and regenerate muscle; Rehmannia glutinosa clears heat and cools blood; Angelica sinensis nourishes blood, activates blood circulation and relieves pain; Angelica dahurica and divaricate saposhniovia root dispels wind and relieves pain; Huanglian, rhubarb and amur cork-tree bark have had the efficacy of clearing heat, removing toxins and promoting anti-inflammatory effects since ancient times; Psoralen[13] warms the kidneys, helps yang, and helps breathing to calm asthma; and cinnamon[14] can be anti-inflammatory, anti-allergy and anti-viral. Gromwell root is also known as Zicao in China. As a traditional Chinese medicine with a long history of medicinal use, the main active ingredients of Zicao are shikonin and its derivatives, such as acetylshikonin and β,β-dimethylacrylshikonin. Zicao is able to act as an antioxidant, anti-inflammatory, antithrombotic, heat remover and detoxifying agent, decreases redness and swelling, activates the metabolic function of epidermal cells and repairs wound[15], and its antibacterial and anti-inflammatory effects also increase its advantages in the treatment of skin wound infections. Fatty oil is traditionally used as an extraction solvent for Zicao because of its hydrophobicity, and Zicao has been used for wound healing in the form of an oil-based ointment containing the active ingredients[16]. Betaine is a natural product extracted from the roots, stems, or fruits of a variety of plants, such as beets, goji berries, and cotton[17]. In addition to its antitumor and blood pressure-lowering effects[18,19], betaine plays a role in reducing oxidative stress and the inflammatory response of wounds[20-22]. Betaine also has good moisturizing, cleaning and protective functions for the skin and has recently been used in the production of skin care products. We found that some psoralen, cinnamon, and gromwell root components and betaine can inhibit the growth of many bacteria and fungi; therefore, in this study, we combined these Chinese medicines, which have complementary efficacies with betaine, to make a betaine ointment (BO) that can both be antimicrobial and promote the healing of wounds. BO was compared with Zicao ointment and mupirocin ointment to explore its therapeutic value for the treatment of skin wounds in diabetic mice infected with drug-resistant microorganisms.
Mupirocin ointment (approval number: H10930064, batch number: 20230316) was purchased from Sino-United States Tianjin Shijiazhuang Pharmaceutical Co. Ltd. Zicao ointment (batch number: 23042802) was purchased from Hengxiantang Health Industry Company. Betaine, i.e., octadecyldimethyl betaine (model: BS16), was purchased from Huainan Huajun New Material Technology Co. Ltd. Gromwell root, psoralen and cinnamon compounds were purchased from Chengdu Herbpurify Co. Ltd., and the CAS numbers are shown in Table 1.
| Compound | CAS |
| Deoxyshikonin | 43043-74-9 |
| Shikonin | 517-89-5 |
| Lithospermoside | 63492-69-3 |
| β,β-Dimethylacrylshikonin | 24502-79-2 |
| β,β-Dimethylacrylalkannin | 34539-65-6 |
| Lithospermic acid | 28831-65-4 |
| β-Acetoxyisovalerylshikonin | 69091-17-4 |
| Isobutylshikonin | 52438-12-7 |
| Acetylshikonin | 24502-78-1 |
| Isobavachalcone | 20784-50-3 |
| Bavachin | 19879-32-4 |
| Isobavachin | 31524-62-6 |
| Bavachinin | 19879-30-2 |
| 4'-O-Methylbavachalcone | 20784-60-5 |
| Psoralen | 66-97-7 |
| Corylin | 53947-92-5 |
| Psoralidin | 18642-23-4 |
| Astragalin | 480-10-4 |
| Methoxsalen | 298-81-7 |
| Bakuchiol | 10309-37-2 |
| Angelicin | 523-50-2 |
| Neobavaisoflavone | 41060-15-5 |
| Cinnamaldehyde | 104-55-2 |
| Cinnamic acid | 621-82-9 |
| Cinnamon alcohol | 104-54-1 |
| Cinnamyl acetate | 103-54-8 |
| Trans-cinnamic acid | 140-10-3 |
| p-Hydroxy-cinnamic acid | 7400-08-0 |
| 2-Methoxycinnamic acid | 6099-03-2 |
| Methyl 4-hydroxycinnamate | 3943-97-3 |
| 3,4,5-Trimethoxycinnamic acid | 90-50-6 |
An ordinary incubator, centrifuge, enzyme-labeled instrument, electronic balance, UV spectrophotometer, biosafety cabinet, vortex oscillator, homogenizer, blood glucose meter (Roche Vitality), blood glucose test strips, EP tubes, tips, and centrifuge tubes were used.
S. aureus strains (including the standard sensitive Newman strain and standard methicillin-resistant USA300 strain), Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, Candida albicans, Bacillus subtilis, Proteus mirabilis, Moraxella catarrhalis, Cryptococcus neoformans, Pseudomonas tropica, Staphylococcus hemolyticus and Enterobacter cloacae were provided by the Drug-resistant Microbial Infection Prevention and Control Research Center at Youjiang Medical University of Nationalities.
The following materials were used: Nutrient broth medium, Biosharp Biologicals, CAS: BS1002; phosphate buffer solution (PBS), Biosharp, Lot: BL601A; Schar's liquid medium, Haber Biologicals, CAS: HB0379; sodium sulfide (Na2S), Nanjing Chemical Reagent Co. Ltd., CAS: 1313-84-4; dexamethasone sodium phosphate injection, Tatsunobu Pharmaceuticals Co. Ltd., CAS: 1313-84-4; and dexamethasone sodium phosphate injection, Cinncin Pharmaceuticals Co. Ltd., CAS: 18883-66-4.
SPF grade BALB/c female mice and SPF grade C57BL/6 male mice were used. All were 4–5-week-old, weighed 18-20 g, and purchased from Changsha Tianqin Biotechnology Co. Ltd.
(1) Composition of traditional Chinese medicine: 10 g of gromwell root, 10 g of psoralen, 1 g of cinnamon, 1 g of frankincense, 1 g of myrrh, 1 g of rehmannia glutinosa, 1 g of angelica sinensis, 10 g of angelica dahurica, 1 g of divaricate saposhniovia root, 1 g of huanglian, 1 g of rhubarb, 1 g of amur cork-tree bark and 1 g of peppermint; (2) All traditional Chinese medicines are ground into powder and sieved coarsely through a 100-mesh sieve to ensure good skin contact; (3) Water was added at a ratio of 1:20 (g: mL), boiled three times at a constant temperature, and filtered through 100-mesh sieve mesh for each half hour of boiling; (4) After standing for 12 hours, the supernatant in the cup was filtered once more, and the resulting liquid was divided into freeze-dried samples; (5) The gelatin was soaked for 5 minutes to allow for expansion and then heated in a water bath to dissolve it into a gelatin slurry (concentration of 0.01 g/mL); (6) The betaine mixture, gelatin slurry and lyophilized powder were coarsely extracted at a ratio of 10:2:1 and then weighed and mixed; and (7) Camellia oil and beeswax were weighed in proportion (mixture obtained in the previous step: Camellia oil: beeswax ratio = 13:2:1), mixed, heated to 60-80 °C, added to the mixture, stirred well, and then poured into a prepared container to cool to obtain BO.
(1) Psoralen, cinnamon, and gromwell root compounds (4 mg/mL), betaine, and BO (32 mg/mL) were prepared; (2) For the minimum inhibitory concentrations (MICs) plate preparation, 173.6 μL of culture medium and 6.4 μL of drugs were added to the first well, which was subsequently diluted to the 10th well; for the 11th well, only culture medium was added, and the 12th well was used as a control for adding bacteria without drugs; (3) To prepare the bacterial solution, the strains growing in the logarithmic phase on the solid plate were removed, and a bacterial suspension was made with the corresponding medium. The adjusted concentrations of Helicobacter pylori (H. pylori), other bacteria and fungi were 1 × 107 CFU/mL, 1 × 106 CFU/mL and 1 × 103 CFU/mL, respectively; (4) The bacterial mixture was inoculated as follows: 10 μL was added to wells 1-10 (concentrations of the bacterial solution = 1 × 102 CFU/mL for fungi, 1 × 106 CFU/mL for H. pylori, and 1 × 105 CFU/mL for other bacteria). H. pylori was cultured for 72 hours, the fungi were cultured for 48 hours, and the other strains were cultured for 24 hours to evaluate the results; and (5) The lowest concentration of drug that completely inhibited the growth of bacteria in small wells was considered the MIC. The results are meaningful when there is significant growth of bacteria in the 12th well but not in the 11th well. The experiment was repeated 3 times.
(1) Ges-1 cell suspensions were prepared, and the concentration was adjusted to 2 × 104; (2) A total of 100 μL of cell suspension was inoculated into each well of a 96-well plate, and 3 replicate wells were generated; (3) The samples were cultivated in a 37 °C incubator for 24 hours; (4) An experimental group and a control group were established by adding the same volume of BO, Zicao ointment or PBS solution at working concentrations of 256 μg/mL, 128 μg/mL, 64 μg/mL, 32 μg/mL, 16 μg/mL, 8 μg/mL, or 0 μg/mL, respectively, and a pure medium group was established; (5) The mixture was incubated in an incubator at 37 °C for 24 hours; (6) A total of 10 μL of MTT was added to each well, mixed, and incubated for 4 hours; and (7) The absorbance at 490 nm was determined, and the survival rate was calculated according to the following formula: Cell survival rate = [(As-Ab)]/[(Ac-Ab)] × 100%. For the wells containing cell culture medium, drugs, and MTT, Ac was used for the wells containing cell culture medium, MTT, and no drugs, and Ab was used for the wells containing no cells or drugs, only culture medium and MTT. Survival curves were established on the basis of survival rates.
Groups of mice: The mice were divided into unwounded and wounded groups (infected with the Newman strain and the USA300 strain, respectively). The wounded groups included the mupirocin ointment group, the Zicao ointment group and the BO group, with 6 mice in each group and 7 groups total. The mice in the unwounded group were subjected to BO on the back skin to observe the effect on the skin surface; the mice in the mupirocin ointment group (positive control), Zicao ointment group (negative control), and BO group (experimental group) were treated with each ointment after wounding and infection, and the ointment was administered twice a day with an interval of 8 hours. The mice in the unwounded group were treated with each ointment after wounding and infection and were treated with each ointment twice a day with an interval of 8 hours.
Immunosuppressant injection: Mice were given intraperitoneal injections of dexamethasone (1 mg/5 mL) before the wound infection experiments at a dose of 0.1 mL per injection, once every two days, for a total of three injections after the wound infection. After infection, dexamethasone was injected 3 times at the same dose in the same manner.
Wound and infection experiments and specific processing operations: For animal hair removal, a disposable cotton swab dipped in 4.0% Na2S was applied to the backs of the mice and were allowed to stand for 3-5 minutes until the hair on the backs of the mice fell off; next, a cotton swab was used to remove the hair, and the area was subsequently washed with saline. One day after hair removal, the state of the mice was observed, and the wound and infection experiments were carried out while the mice were in good condition. The mice in the wound group were anesthetized via intraperitoneal injection of avertin (0.2 mL/10 g); after the mice were anesthetized, 1 round wound 0.5-0.8 cm in diameter was cut at the spine of the positive back of each mouse with sterilized scissors, and the excess blood in the wound was handled with sterile cotton balls. A 1 × 109 CFU/mL S. aureus bacterial mixture was prepared, and the bacterial mixture was applied to the wound to establish an infection model. No wound or infection treatment was performed in the unwounded group, and the same injection of dexamethasone and subsequent dehairing were carried out.
Diabetic mouse model construction: (1) For the preparation of citrate buffer, 21 mg/mL citric acid solution (liquid A) and 29.4 mg/mL trisodium citrate solution (liquid B) were mixed at a ratio of 1:1.32, and a pH meter was used to determine and adjust the pH value to 4.2-4.5 for the 0.1 mol/L sodium citrate buffer; and (2) Streptozotocin (STZ) was administered as follows: STZ was prepared with citrate buffer and incubated on ice away from light. STZ was administered to mice by intraperitoneal injection at a dose of 75 mg/kg after 10 hours of fasting for 3 consecutive days, maintaining a 90-minute state of fasting but not water fasting after each intraperitoneal injection. On the end of the 9th day, blood was taken from the tail vein of the mice after 10 hours of fasting, the fasting blood glucose (FBG) was measured with a Roche glucose meter, and the mice with an FBG concentration ≥ 11.1 mmol/L were considered successfully constructed for the diabetes mellitus model.
Construction of a diabetic mouse skin soft tissue infection model: The experimental mice were divided into the unwounded group and the wounded group (infected with the USA300 strain), and the wounded group was further divided into the PBS group, the mupirocin ointment group, and the BO group, with 5 animals in each group, for a total of 4 groups. The drug administration method was the same as constructing the common mouse skin soft tissue infection model.
Treatment of infected wounds and detection of in vivo treatment effects: (1) On the 1st day after wounding, wound healing and suppuration in each group were observed and recorded. The corresponding drugs were applied at the wound location for treatment on the 2nd day after wounding, the drugs were administered for 15 days, and the wound healing situation was observed and recorded every day; (2) Mice were sacrificed on the 16th day, and the skin wound tissues were removed to generate pathological sections (formaldehyde-fixed), ground and coated with culture plates to count the number of colonies; and (3) The wound healing and scab removal times of each group of mice were recorded; the bacterial colonization of the skin wound tissues after dissection was determined; the recovery, inflammation and apoptosis of the skin tissues were observed through pathological sections; and HE staining, TUNEL immunohistochemistry, and fluorescence detection of immune factors and apoptotic factors were performed. The samples were submitted to Wuhan Servicebio Technology Co. Ltd. for testing.
All values are expressed as the means ± SD. SPSS 26.0 software was used for statistical analysis via one-way ANOVA, and differences were considered statistically significant when P < 0.05. Graphs were generated via GraphPad Prism 8 software.
The compounds of gromwell root include shikonin, lithospermoside, β,β-dimethylacrylalkannin, acetylshikonin, and deoxyshikonin, which have better antibacterial effects against Candida albicans and Cryptococcus neoformans, with MICs of 4-32 μg/mL; the compounds of psoralen include isobavachalcone, bavachin, neobavaisoflavone, isobavachin, bokuchiol and methoxsalen, which have better antibacterial effects on S. aureus, Candida albicans, and Cryptococcus neoformans, with MICs of 1-64 μg/mL; and the cinnamon compounds include cinnamaldehyde, cinnamic acid, trans-cinnamic acid, o-methoxycinnamic acid, 2-methoxycinnamic acid, and methyl 4-hydroxycinnamate, which have better inhibitory effects on S. aureus, Candida albicans, and Cryptococcus neoformans, with MICs of 64-128 μg/mL. These findings indicate that there are many broad-spectrum antibacterial components in gromwell root, psoralin and cinnamon (Tables 2, 3, and 4). The MIC of betaine was 4-32 μg/mL, and the addition of betaine improved the antimicrobial effect (Table 5).
| Compound | Klebsiella pneumoniae | Pseudomonas aeruginosa | Acinetobacter baumannii | Staphylococcus aureus | Candida albicans | Cryptococcus neoformans | Helicobacter pylori |
| Shiconin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | 2 |
| Lithospermoside | > 128 | > 128 | > 128 | > 128 | 8 | 4 | > 128 |
| β,β-Dimethylacrylshikonin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | 32 |
| β,β-Dimethylacrylalkannin | > 128 | > 128 | > 128 | > 128 | 32 | 8 | 32 |
| Lithospermic acid | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 |
| β-Acetoxyisovalerylshikonin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | 8 |
| Isobutylshikonin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | 8 |
| Acetylshikonin | > 128 | > 128 | > 128 | > 128 | > 128 | 8 | 4 |
| Deoxyshikonin | > 128 | > 128 | > 128 | > 128 | > 128 | 8 | 2 |
| Compound | Klebsiella pneumoniae | Pseudomonas aeruginosa | Acinetobacter baumannii | Staphylococcus aureus | Cryptococcus neoformans | Candida albicans | Helicobacter pylori |
| Isobavachalcone | > 128 | > 128 | > 128 | 8 | 1 | 8 | 8 |
| Bavachin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | 8 |
| Bavachinin | > 128 | > 128 | > 128 | 16 | 32 | > 128 | 16 |
| 4'-O-Methylbavachalcone | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | 8 |
| Neobavaisoflavone | > 128 | > 128 | > 128 | 64 | 32 | > 128 | 16 |
| Isobavachin | > 128 | > 128 | > 128 | 16 | 32 | > 128 | > 128 |
| Bakuchiol | > 128 | > 128 | > 128 | 32 | 16 | > 128 | > 128 |
| Methoxsalen | > 128 | > 128 | > 128 | 64 | > 128 | > 128 | > 128 |
| Angelicin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 |
| Psoralen | > 128 | > 128 | > 128 | 128 | > 128 | > 128 | > 128 |
| Corylin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 |
| Psoralidin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 |
| Astragalin | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 |
| Compound | Staphylococcus aureus | Pseudomonas aeruginosa | Klebsiella pneumoniae | Acinetobacter baumannii | Candida albicans | Cryptococcus neoformans | Helicobacter pylori |
| Cinnamaldehyde | 64 | > 128 | > 128 | > 128 | 128 | 64 | 8 |
| Cinnamic acid | > 128 | > 128 | > 128 | > 128 | > 128 | 128 | 128 |
| Cinnamon alcohol | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | 128 |
| Cinnamyl acetate | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 |
| Trans-cinnamic acid | > 128 | > 128 | > 128 | > 128 | 128 | 64 | 128 |
| p-Hydroxy-cinnamic acid | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | 128 |
| 2-Methoxycinnamic acid | > 128 | > 128 | > 128 | > 128 | > 128 | 128 | > 128 |
| Methyl 4-hydroxycinnamate | > 128 | > 128 | > 128 | > 128 | > 128 | 128 | > 128 |
| Ethyl 4-methoxycinnamate | > 128 | > 128 | > 128 | > 128 | > 128 | 128 | 16 |
| 3,4,5-Trimethoxycinnamic acid | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 |
| Strain | MIC (μg/mL) |
| Bacillus subtilis | 4 |
| Newman | 4 |
| USA300 | 8 |
| Candida albicans | 16 |
| Cryptococcus neoformans | 16 |
| Candida tropicalis | 32 |
| Escherichia coli | > 1024 |
| Staphylococcus haemolyticus | > 1024 |
| Pseudomonas aeruginosa | > 1024 |
| Proteus mirabilis | > 1024 |
| Acinetobacter baumannii | > 1024 |
| Klebsiella pneumoniae | > 1024 |
| Morganella morganii subsp. morganii | > 1024 |
| Enterobacter hormaechei | > 1024 |
The MIC of BO against common skin-infecting microorganisms, such as S. aureus, Candida albicans, and Cryptococcus neoformans, was 16-64 μg/mL, whereas the MICs of traditional Zicao ointment were all > 2048 μg/mL, significantly increasing the antimicrobial effect (Table 6).
| Strain | MIC (μg/mL) | |
| BO | Zicao | |
| Newman | 16 | > 2048 |
| USA300 | 32 | > 2048 |
| Candida albicans | 64 | > 2048 |
| Cryptococcus neoformans | 64 | > 2048 |
| Candida tropicalis | 64 | > 2048 |
| Staphylococcus haemolyticus | > 512 | > 2048 |
| Pseudomonas aeruginosa | > 512 | > 2048 |
| Proteus mirabilis | > 512 | > 2048 |
| Acinetobacter baumannii | > 512 | > 2048 |
| Klebsiella pneumoniae | > 512 | > 2048 |
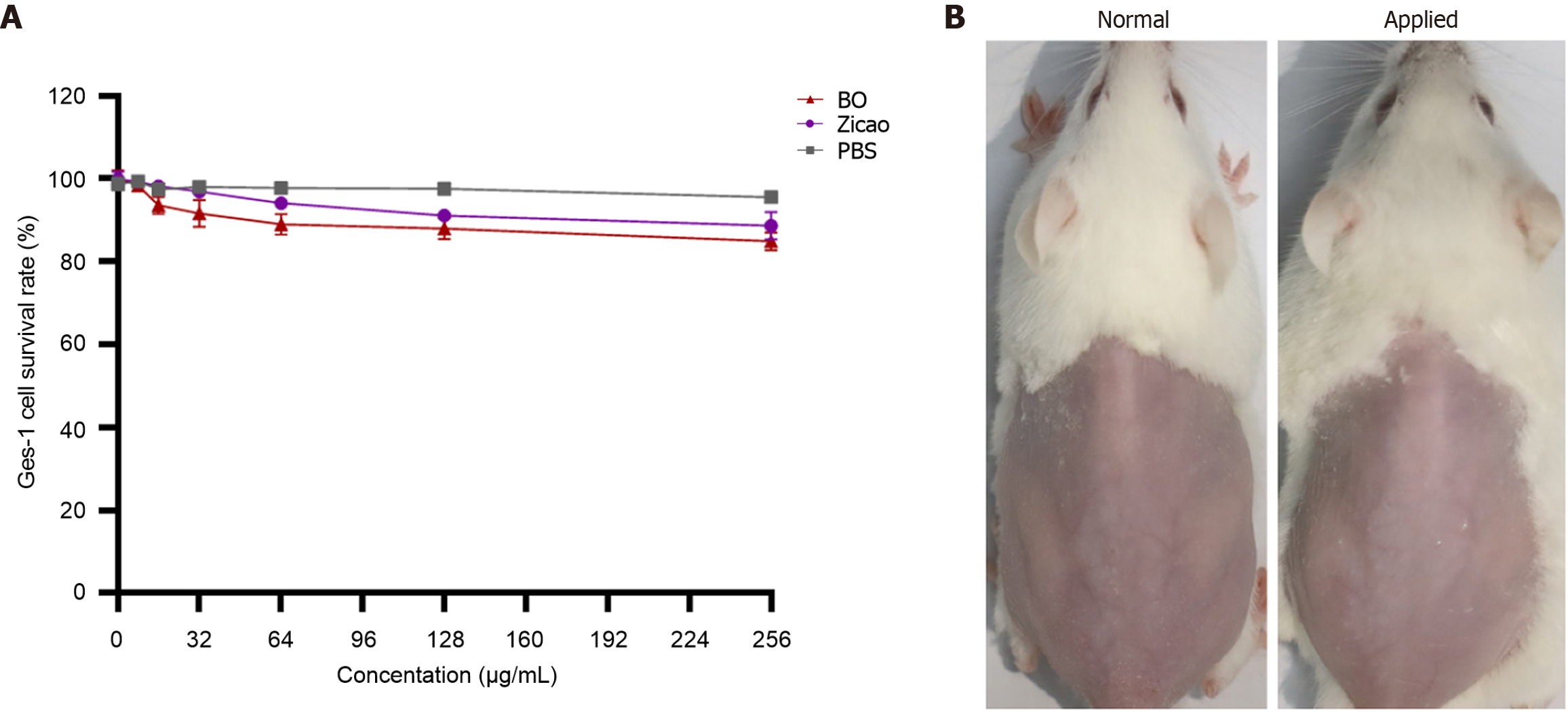
BO at a concentration of 1-16 times the MIC had low toxicity and high safety for Ges-1 cells (Figure 1A). In addition, the surface skin growth of the mice after coating was normal, and there was no allergic phenomenon such as redness, swelling or burning, which indicates safety (Figure 1B).
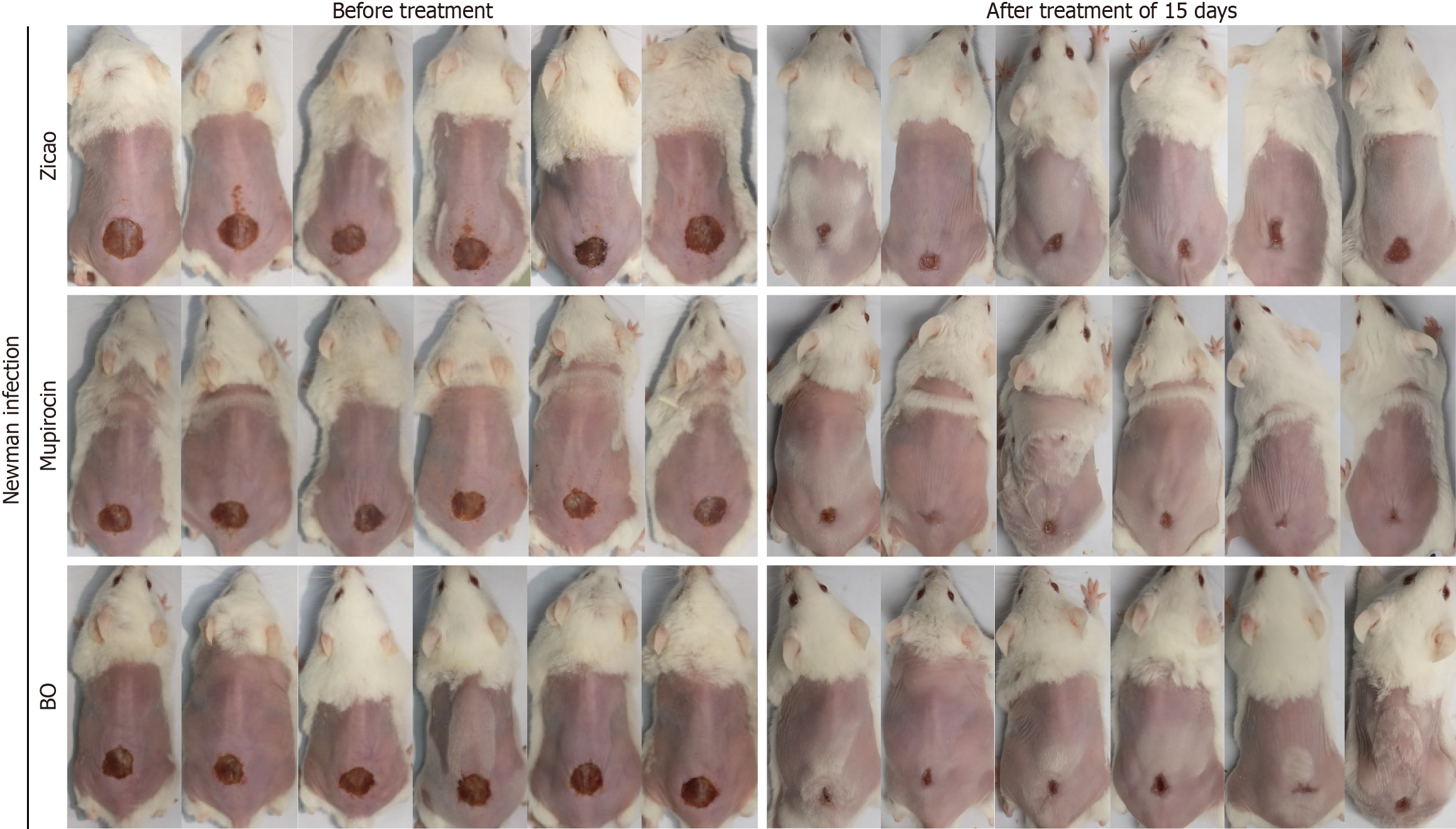
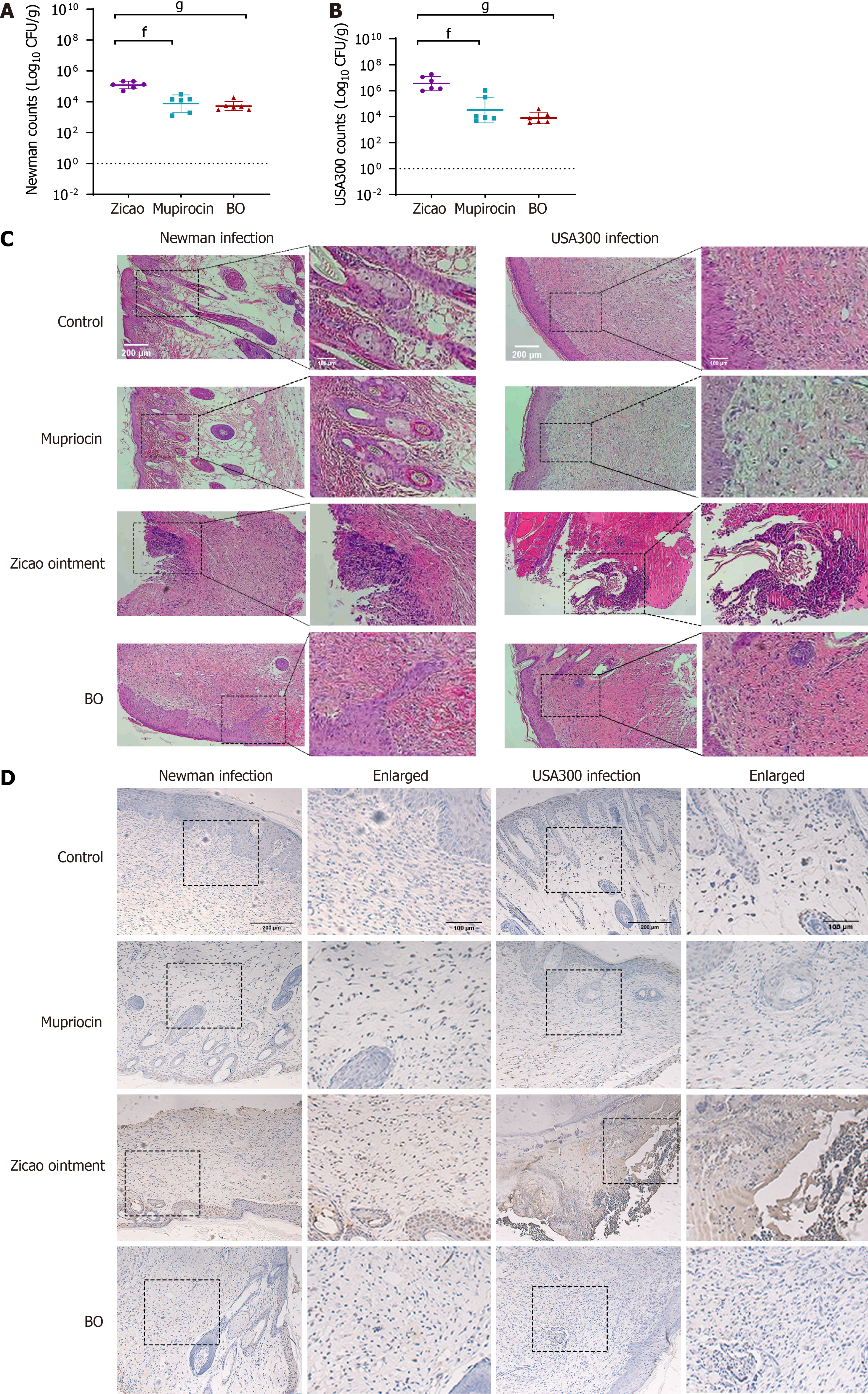
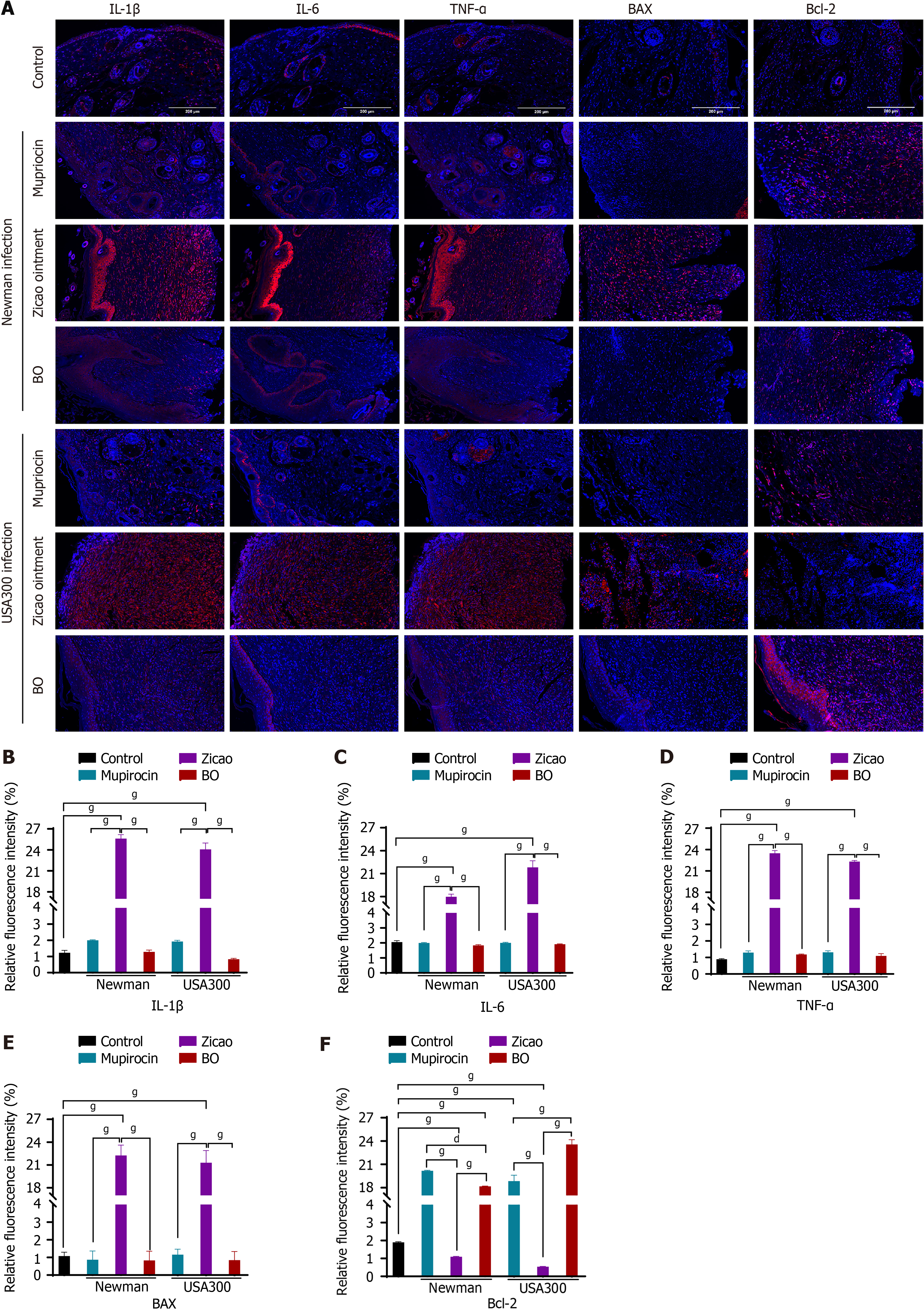
(1) Wound healing: Among the sensitive Newman strain-infected mice, those in the mupirocin ointment group presented the best wound healing conditions, followed by those in the BO group, but both groups experienced the same scab removal, which occurred earlier than that in the Zicao ointment group. Among the drug-resistant USA300 strain-infected mice, the highest rate of scab removal was observed in the BO group of mice, with a wound healing scab removal rate of 83.3% after 15 days of treatment. Compared with 66.7% and 0% for mupirocin ointment and Zicao ointment, respectively, the difference was statistically significant. Wound healing is shown in Figure 2, and the time of scab removal is shown in Table 7; (2) Bacterial colonization of skin wound tissues: For the mice infected with the sensitive Newman strain, the BO group had the lowest bacterial colonization and the best antibacterial effect, which was comparable to that of the mupirocin ointment group and significantly better than that of the Zicao ointment group; for the mice infected with the drug-resistant USA300 strain, the BO group had the lowest bacterial colonization, and the difference from the other two groups was even more obvious. Compared with the other two ointments, BO strongly inhibited the drug-resistant strains (Figure 3A and B); and (3) Degree of inflammation and apoptosis: The skin surface of normal mice was intact, and no inflammation or edema occurred. The skin wound surface of the mice in the Zicao ointment group was incomplete, and there was a severe inflammatory reaction, with high levels of inflammatory cell infiltration and aggregation and severe apoptosis. The wound surfaces of the mice in the mupirocin ointment group and the BO group were relatively intact, with no obvious inflammatory cell infiltration, and there was no difference from those of the normal skin tissues Figure 3C and D). Therefore, BO can reduce the apoptosis of skin tissue cells and promote the healing of wounded tissues with an effect comparable to that of mupirocin ointment, and the effect is more remarkable in the case of infection with the drug-resistant USA300 strain. The results of the fluorescence staining of inflammatory and apoptotic factors revealed that the levels of the inflammatory factors IL-1β, IL-6, and TNF-α were greatly reduced in the skin of the BO and mupirocin ointment groups, whereas Zicao ointment did not have anti-inflammatory effects.
The apoptosis-related factors BAX and Bcl-2 were not expressed in normal tissues, and the proapoptotic factor BAX was abundantly expressed in the skin tissues of the wounds of the mice in the Zicao ointment group; however, the apoptosis-inhibiting factor Bcl-2 was weakly expressed, whereas BAX was not expressed in the skin tissues of the mupirocin group or the BO group, and Bcl-2 was more strongly expressed than in normal tissues (Figure 4). The skin was wounded to create skin tissue cells expressing inflammatory factors and apoptosis-promoting factors in large quantities, whereas BO reduced inflammation in skin wounds, inhibited the expression of inflammatory factors and BAX, and promoted the expression of Bcl-2, indicating that it plays good anti-inflammatory and antiapoptotic roles in promoting wound healing.
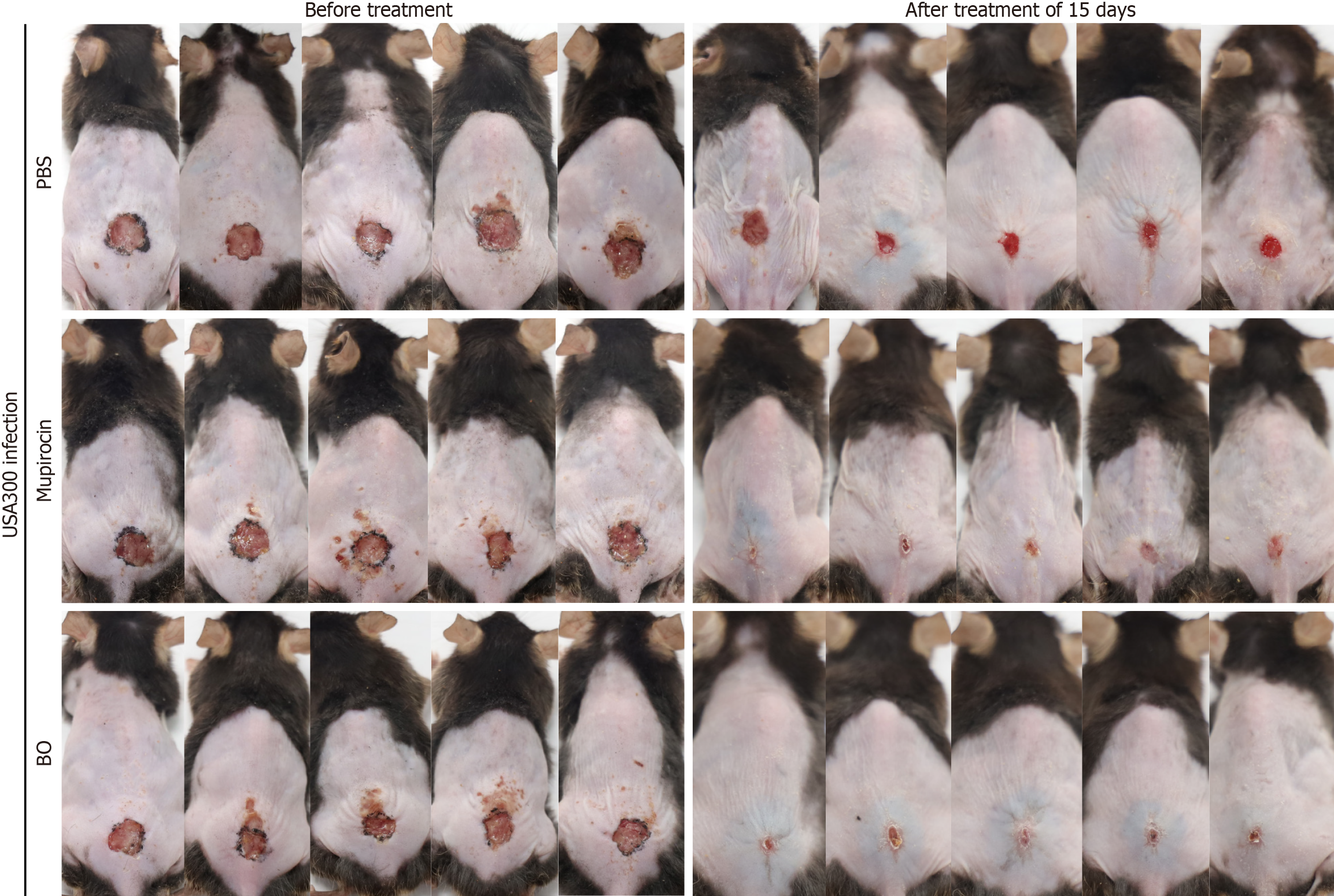
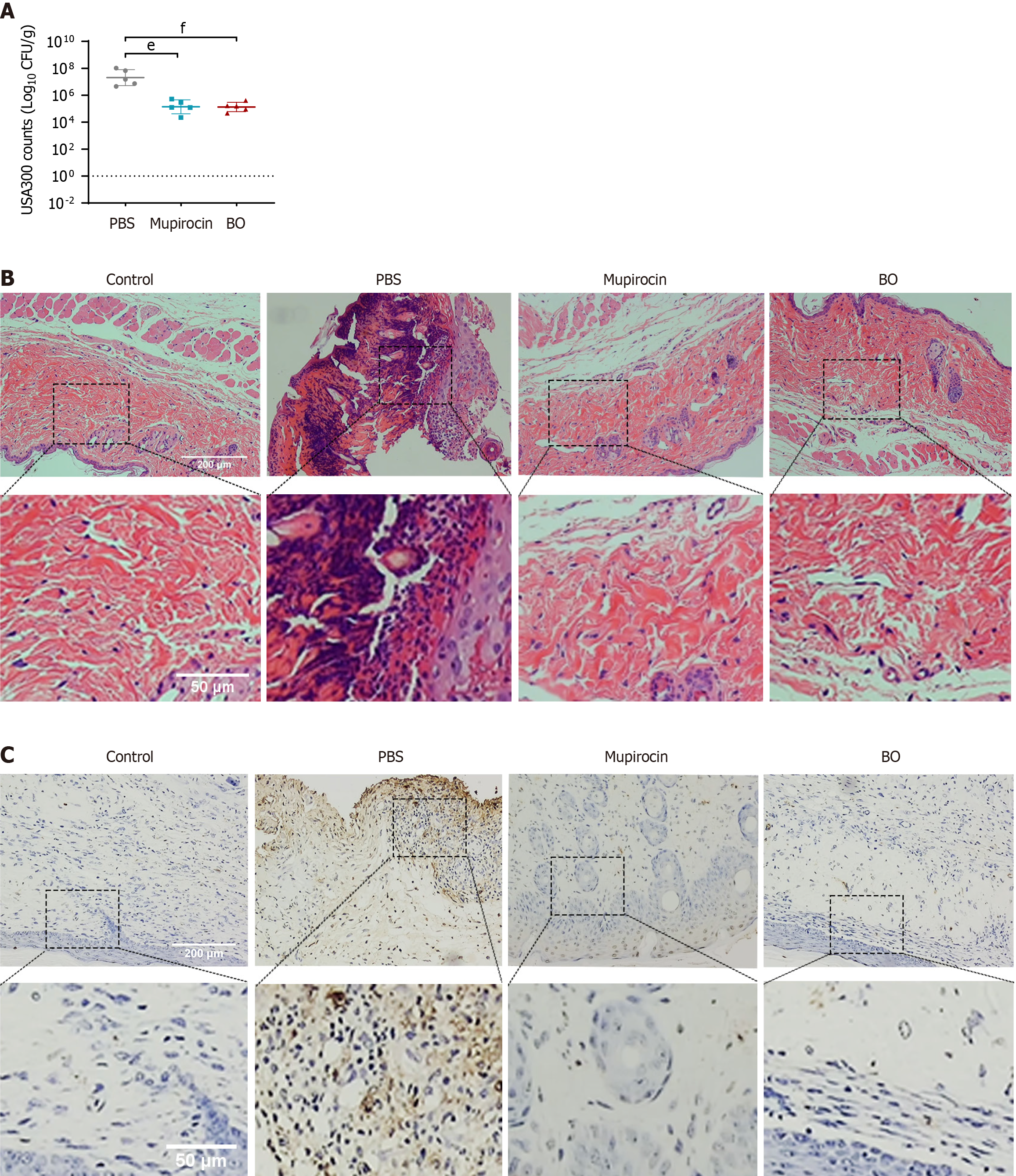
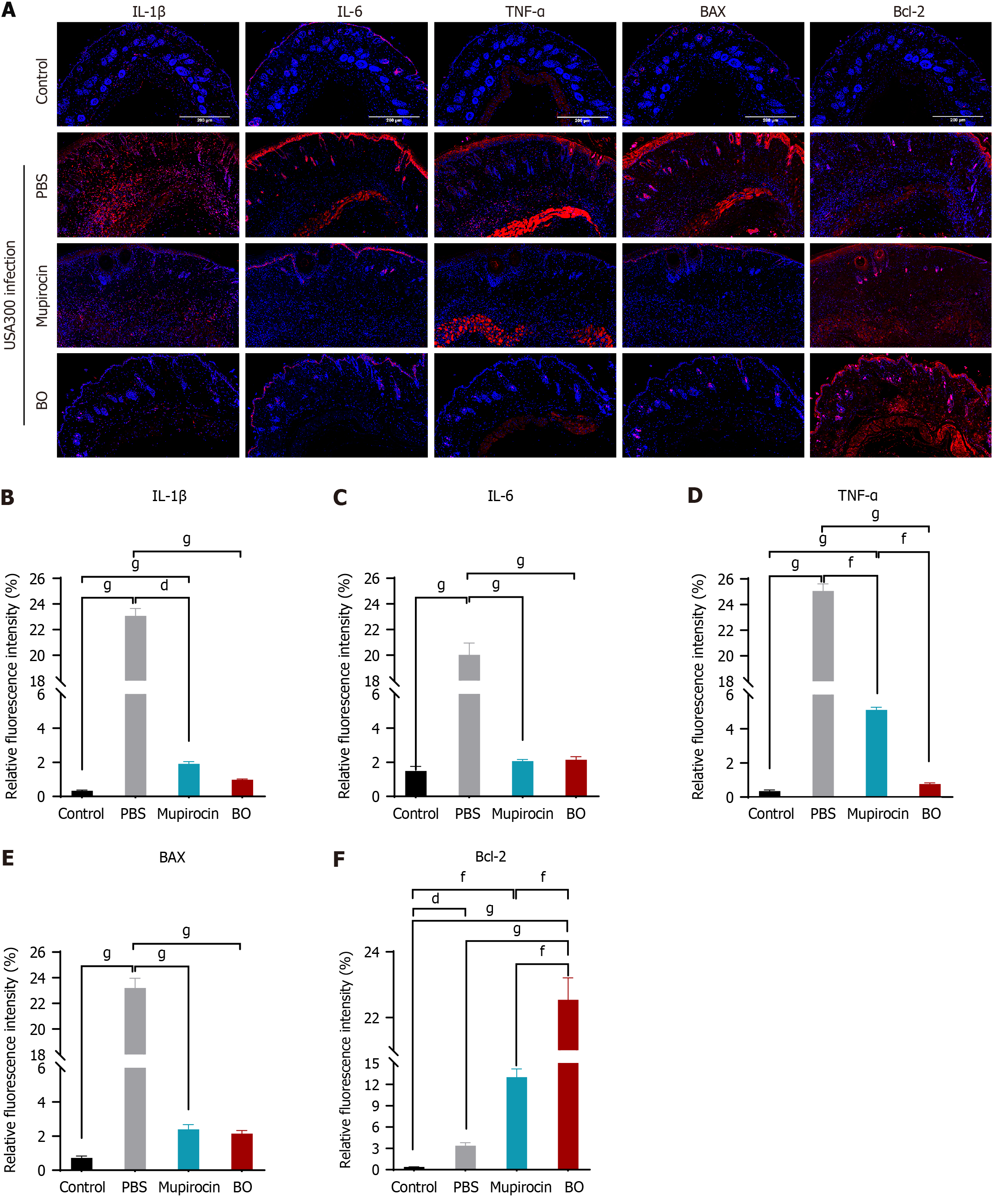
The total number of scabs removed after treatment for 15 days was the same in the BO group and the mupirocin group of diabetic mice, and the rate of scab removal was 80.0% in both groups. The difference was statistically significant, and the speed of wound healing was comparable (Table 8 and Figure 5). The ointment treatment reduced the amount of skin tissue strains colonizing the wounds of the mice, and the two ointments were equally effective (Figure 6A). The results of HE and TUNEL staining of the skin tissues revealed that the wounds of the mice in the BO group recovered more completely without obvious inflammatory cell infiltration or apoptosis of the skin cells, which were not different from those of the normal skin tissues. Skin tissues in the mupirocin group exhibited mild apoptosis (Figure 6B and C). The results of the analysis of inflammatory and apoptotic factors (Figure 7) verified the efficacy of BO, the expression of inflammatory factors was lower than that in the mupirocin ointment group, and the expression of antiapoptotic factors was greater. Therefore, BO can significantly reduce inflammation, decrease apoptosis in diabetic wounds and promote wound healing more effectively than mupirocin ointment.
The skin is the first immune barrier of an organism, and skin disease caused by skin infection has become a common and frequent disease. Subcutaneous tissue is not protected after skin defects, which can cause wound contamination in a short period of time, and if the wound contamination is severe or bacterial virulence is strong, bacterial infection can easily occur, which can then lead to tissue necrosis and systemic infections. While chronic diabetic wounds are among the most serious chronic complications in diabetic patients[23], the problem of delayed wound healing in diabetic patients is becoming increasingly apparent worldwide due to the lack of effective treatment strategies. Antimicrobial topicals and antibiotics for the treatment of skin infections are readily available along with increasing demand. Although some antibacterial ointment preparation techniques are well established, their efficiency in exerting antimicrobial activity and promoting wound healing and scab removal is not comprehensive enough, and the antibiotic resistance situation is serious[24,25], which presents great challenges for clinical treatment. To enable patients with skin infections to receive timely and effective treatment before further deterioration and to prevent pathogenic bacterial infections in daily life, drugs with broad-spectrum antimicrobial effects and toxicity that are not easy to resist are needed for daily use and to promote wound healing. Plant-derived herbs have natural components, many of which have been shown to have good antimicrobial effects, and ointments made from Chinese medicines have been widely used in wound repair[26,27], with many clinical reports of their efficacy.
Normal skin wound healing is a complex process divided into a coagulation phase, an inflammatory phase, a proliferative phase, and a tissue remodeling phase, during which a series of reactions occur, including the inflammatory response, granulation tissue formation, regenerative epithelialization, angiogenesis and matrix remodeling[28,29]. In this study, a variety of Chinese medicinal ingredients with antibacterial and anti-inflammatory efficacy and betaine were combined to prepare BO, which was verified to have stronger and more comprehensive efficacy from multiple perspectives. While evaluating wound recovery, in addition to the intuitive wound healing phenomenon, the inflammatory infiltration phenomenon and the expression of inflammatory factors in the tissues directly reflect the degree of inflammation in the wound. In addition, the role of apoptosis in wound healing should not be ignored. Apoptosis is involved in the process of transforming granulation tissue to scar tissue, and it may also be the reason for the reduction or even disappearance of inflammatory cells in granulation tissue[30,31]. Abnormal apoptosis plays a crucial role in wound healing, and excessive apoptosis slows healing. BAX is a common proapoptotic factor, while Bcl-2 significantly inhibits apoptosis by maintaining the permeability of the mitochondrial membrane; thus, Bcl-2 and BAX play antagonistic roles in apoptosis[32,33], which means that BAX expression decreases when Bcl-2 expression is increased. During the inflammatory phase of skin wounds, the number of inflammatory cells within the wound increases, and BAX expression increases, promoting apoptosis and elimination of inflammatory cells, whereas Bcl-2 expression decreases. BO treatment alleviated the inflammatory infiltration phenomenon in skin wounds, reduced the expression of inflammatory factors and proapoptotic factors, and increased the expression of antiapoptotic factors, indicating that BO promotes wound healing.
The results of in vivo and in vivo antibacterial experiments show that BO is able to exert bacteriostatic effects against a wide range of microorganisms, reduce the amount of colonization of strains of drug-resistant S. aureus-infected wounds, and have a significant effect on antidrug-resistant bacteria. The results of the cytotoxicity and dermatotoxicity experiments revealed that BO has no side effects on normal cells or skin tissues and that its composition is natural and low in cost. However, owing to the possible species differences in the physical conditions of humans and mice, the variability of the environment for clinical use, such as the diversity of infective strains and drug resistance, the presence of disease complications in patients and the possible irritation of drugs on the wounds. There are many unknowns concerning the human health effects of long-term use. We still need to validate the efficacy and safety of BO in human diabetic wound treatment through clinical trials. In this work, the healing effect of BO on diabetic wounds was verified mainly in terms of reducing the amount of pathogenic bacteria colonized and reducing inflammation and apoptosis, which promotes the proliferation of wound granulomas and has good prospects for application, and the efficacy and mechanism of the other aspects need to be further researched. The mechanism underlying the anti-inflammatory and antiapoptotic effects may be related to the promotion of diabetic wound healing through the regulation of the PI3K/Akt, NF-κB and other signaling pathways[34]. It is still necessary to search for the action targets of each component of the ointment through network pharmacology, molecular docking and interactions and to carry out subsequent experiments to validate and obtain more comprehensive research results to provide basic research and clinical management of diabetic wound treatment in a new direction.
In summary, we combined these Chinese medicines, which have complementary efficacies with betaine, to make a BO that can both be antimicrobial and promote the healing of wounds. The MIC assay and model construction and treatment proved that it has a good effect of inhibiting bacteria and promoting diabetic wound healing. In vivo and in vitro safety assessment evidenced that it has few side effects and high safety, which makes it an ointment with high application potential and provides a new direction for clinical diabetic wound treatment.
The authors would like to thank Youjiang Medical University for Nationalities for their help with the research.
| 1. | Sartelli M, Guirao X, Hardcastle TC, Kluger Y, Boermeester MA, Raşa K, Ansaloni L, Coccolini F, Montravers P, Abu-Zidan FM, Bartoletti M, Bassetti M, Ben-Ishay O, Biffl WL, Chiara O, Chiarugi M, Coimbra R, De Rosa FG, De Simone B, Di Saverio S, Giannella M, Gkiokas G, Khokha V, Labricciosa FM, Leppäniemi A, Litvin A, Moore EE, Negoi I, Pagani L, Peghin M, Picetti E, Pintar T, Pupelis G, Rubio-Perez I, Sakakushev B, Segovia-Lohse H, Sganga G, Shelat V, Sugrue M, Tarasconi A, Tranà C, Ulrych J, Viale P, Catena F. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018;13:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 2. | Barbier F, Timsit JF. Risk stratification for multidrug-resistant bacteria in patients with skin and soft tissue infection. Curr Opin Infect Dis. 2020;33:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Guan H, Dong W, Lu Y, Jiang M, Zhang D, Aobuliaximu Y, Dong J, Niu Y, Liu Y, Guan B, Tang J, Lu S. Distribution and Antibiotic Resistance Patterns of Pathogenic Bacteria in Patients With Chronic Cutaneous Wounds in China. Front Med (Lausanne). 2021;8:609584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Lakhundi S, Zhang K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin Microbiol Rev. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 1015] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 5. | Aitcheson SM, Frentiu FD, Hurn SE, Edwards K, Murray RZ. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 6. | Okizaki S, Ito Y, Hosono K, Oba K, Ohkubo H, Amano H, Shichiri M, Majima M. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed Pharmacother. 2015;70:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, Woo K, Boeni T, Ayello EA, Kirsner RS. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J Am Acad Dermatol. 2014;70:1.e1-18; quiz 19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 8. | Au SC, Tang SM, Rong SS, Chen LJ, Yam JC. Association between hyperglycemia and retinopathy of prematurity: a systemic review and meta-analysis. Sci Rep. 2015;5:9091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Zhang LW, Ruan MH, Liu JL, He CH, Yu JR. Progress on research and development in diabetes mellitus. Yi Chuan. 2022;44:824-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Rong XZ, Zhang T, Liu LZ. [Distribution and drug resistance analysis of bacteria in different wound infections]. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:82-83, 89. [PubMed] |
| 11. | Huang X, Wang P, Li T, Tian X, Guo W, Xu B, Huang G, Cai D, Zhou F, Zhang H, Lei H. Self-Assemblies Based on Traditional Medicine Berberine and Cinnamic Acid for Adhesion-Induced Inhibition Multidrug-Resistant Staphylococcus aureus. ACS Appl Mater Interfaces. 2020;12:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Si L, Li P, Liu X, Luo L. Chinese herb medicine against Sortase A catalyzed transformations, a key role in gram-positive bacterial infection progress. J Enzyme Inhib Med Chem. 2016;31:184-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Chen L, Chen S, Sun P, Liu X, Zhan Z, Wang J. Psoralea corylifolia L.: a comprehensive review of its botany, traditional uses, phytochemistry, pharmacology, toxicology, quality control and pharmacokinetics. Chin Med. 2023;18:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 14. | Lee JH, Kwak HJ, Shin D, Seo HJ, Park SJ, Hong BH, Shin MS, Kim SH, Kang KS. Mitigation of Gastric Damage Using Cinnamomum cassia Extract: Network Pharmacological Analysis of Active Compounds and Protection Effects in Rats. Plants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Salehi B, Sharopov F, Boyunegmez Tumer T, Ozleyen A, Rodríguez-Pérez C, Ezzat SM, Azzini E, Hosseinabadi T, Butnariu M, Sarac I, Bostan C, Acharya K, Sen S, Nur Kasapoglu K, Daşkaya-Dikmen C, Özçelik B, Baghalpour N, Sharifi-Rad J, Valere Tsouh Fokou P, Cho WC, Martins N. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Chen Chen T, Yu SC, Hsu CM, Tsai FJ, Tsai Y. A water-based topical Chinese traditional medicine (Zicao) for wound healing developed using 2-hydroxypropyl-β-cyclodextrin. Colloids Surf B Biointerfaces. 2018;165:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Arumugam MK, Paal MC, Donohue TM Jr, Ganesan M, Osna NA, Kharbanda KK. Beneficial Effects of Betaine: A Comprehensive Review. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 18. | Zhao G, He F, Wu C, Li P, Li N, Deng J, Zhu G, Ren W, Peng Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front Immunol. 2018;9:1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 19. | Rosas-Rodríguez JA, Valenzuela-Soto EM. The glycine betaine role in neurodegenerative, cardiovascular, hepatic, and renal diseases: Insights into disease and dysfunction networks. Life Sci. 2021;285:119943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Xia Y, Chen S, Zhu G, Huang R, Yin Y, Ren W. Betaine Inhibits Interleukin-1β Production and Release: Potential Mechanisms. Front Immunol. 2018;9:2670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Yajun W, Jin C, Zhengrong G, Chao F, Yan H, Weizong W, Xiaoqun L, Qirong Z, Huiwen C, Hao Z, Jiawei G, Xinchen Z, Shihao S, Sicheng W, Xiao C, Jiacan S. Betaine Attenuates Osteoarthritis by Inhibiting Osteoclastogenesis and Angiogenesis in Subchondral Bone. Front Pharmacol. 2021;12:723988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Vesković M, Labudović-Borović M, Mladenović D, Jadžić J, Jorgačević B, Vukićević D, Vučević D, Radosavljević T. Effect of Betaine Supplementation on Liver Tissue and Ultrastructural Changes in Methionine-Choline-Deficient Diet-Induced NAFLD. Microsc Microanal. 2020;26:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4587] [Article Influence: 573.4] [Reference Citation Analysis (7)] |
| 24. | Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N; WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2062] [Cited by in RCA: 4275] [Article Influence: 534.4] [Reference Citation Analysis (1)] |
| 25. | Huemer M, Mairpady Shambat S, Brugger SD, Zinkernagel AS. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020;21:e51034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 506] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 26. | Li FL, Wang GC, Wu BQ. Clinical application of traditional Chinese medicine powder in the treatment of acute and chronic wounds. Int Wound J. 2023;20:799-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Zhang X, Yang L, Zhou S, Li Y, Shen Y, Lu S, Zhou J, Liu Y. Proteomics and transcriptomics explore the effect of mixture of herbal extract on diabetic wound healing process. Phytomedicine. 2023;116:154892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 28. | Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99:665-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1871] [Article Influence: 267.3] [Reference Citation Analysis (0)] |
| 29. | Knoedler S, Broichhausen S, Guo R, Dai R, Knoedler L, Kauke-Navarro M, Diatta F, Pomahac B, Machens HG, Jiang D, Rinkevich Y. Fibroblasts - the cellular choreographers of wound healing. Front Immunol. 2023;14:1233800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 30. | Anderton H, Alqudah S. Cell death in skin function, inflammation, and disease. Biochem J. 2022;479:1621-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 31. | Justynski O, Bridges K, Krause W, Forni MF, Phan QM, Sandoval-Schaefer T, Carter K, King DE, Hsia HC, Gazes MI, Vyce SD, Driskell RR, Miller-Jensen K, Horsley V. Apoptosis recognition receptors regulate skin tissue repair in mice. Elife. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Bhan S, Mitra R, Arya AK, Pandey HP, Tripathi K. A study on evaluation of apoptosis and expression of bcl-2-related marker in wound healing of streptozotocin-induced diabetic rats. ISRN Dermatol. 2013;2013:739054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Cui YF, Xia GW, Fu XB, Yang H, Peng RY, Zhang Y, Gu QY, Gao YB, Cui XM, Hu WH. Relationship between expression of Bax and Bcl-2 proteins and apoptosis in radiation compound wound healing of rats. Chin J Traumatol. 2003;6:135-138. [PubMed] |
| 34. | Zhou X, Guo Y, Yang K, Liu P, Wang J. The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. J Ethnopharmacol. 2022;282:114662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/