Published online Jan 15, 2025. doi: 10.4239/wjd.v16.i1.99496
Revised: October 11, 2024
Accepted: November 4, 2024
Published online: January 15, 2025
Processing time: 129 Days and 9.8 Hours
Use of immunomodulating agents to prevent the progression of autoimmune β-cell damage leading to type 1 diabetes mellitus (T1DM) is an interesting area for research. These include non-specific anti-inflammatory agents, immunologic vaccination and anti-inflammatory agents targeting specific immune cells or cytokines. Teplizumab is an anti-CD3-molecule that binds to and leads to the disappearance of the CD3/TCR complex and rendering the T cell anergic to its target antigen. Preclinical and clinical trials have demonstrated its efficacy in reducing the decline in serum C-peptide levels and the need for insulin therapy if used early in the disease process of T1DM. The benefits have been apparent as early as six months to as long as seven years after therapy. It has recently been approved by the Food and Drug Administration to delay the onset of clinical (stage 3) type 1 diabetes in children above 8 years of age. In their recent meta-analysis published in the World Journal of Diabetes, Ma et al found that those in the teplizumab treatment group have a greater likelihood of reduction in insulin use, change in C-peptide response, and better glycemic control compared to the control group with a good safety profile. However, all the included randomized control trials have been conducted in high-income countries. High cost of therapy and unknown utility of the molecule in stage 3 disease limit its widespread use.
Core Tip: Teplizumab is an anti-CD3 immunomodulator that can render the T cell anergic to its target antigen and thus protect against autoimmune beta cell destruction. It is approved for use in individuals aged above 8 years in early pre-clinical stages of type 1 diabetes mellitus to prevent progression to clinical stage (stage 3). C-peptide decline is halted and the need for insulin therapy can be delayed even after discontinuation of this therapeutic molecule. Adverse effects are mostly mild. The high cost and lack of availability limit its applicability in low- and middle-income countries.
- Citation: Mondal S, Pappachan JM. Current perspectives and the future of disease-modifying therapies in type 1 diabetes. World J Diabetes 2025; 16(1): 99496
- URL: https://www.wjgnet.com/1948-9358/full/v16/i1/99496.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i1.99496
Type 1 diabetes mellitus (T1DM) is a crippling chronic disease without an option for pharmacological cure currently. The disease results from the autoimmune destruction of pancreatic β-cells causing absolute insulin deficiency that disrupts the human glucose metabolism. Although pancreatic transplantation is the only curative treatment option available at present for T1DM, various pharmacotherapeutic and immunomodulating agents were tested in experimental models and clinical settings over the past few decades with variable success rates as disease modifying approaches[1,2]. It is important to understand the pathobiology and the potential therapeutic targets for cure of T1DM to have an up-to-date knowledge in managing the disease in our clinical practice. While several agents have been tried, the older immunosuppressive therapies were found to exhibit only temporary effects and had high toxicity on the liver, kidney or haematological system along with the risks of long-term immune suppression like infections. Teplizumab is an anti-CD3 monoclonal antibody which has the ability to reduce the number and activity of activated autoreactive T-cells that can destroy pancreatic β cells. Following the positive results of many preclinical and clinical studies, teplizumab has received approval from the Food and Drug Administration (FDA) in 2022 to postpone the onset of clinically overt or stage 3 type 1 diabetes[3]. However, the different randomized control trials (RCTs) with teplizumab have used different duration, doses and regimen of teplizumab with heterogeneity in results and lack of clarity regarding adverse effects. Also, some RCTs with teplizumab have been published very recently. This made it necessary for a meta-analysis including the results of more recent RCTs. A meta-analysis by Ma et al[4] in the recent issue of World Journal of Diabetes is such an attempt examining the role of teplizumab as a disease modifying agent in patients with T1DM. We elaborate the disease modifying approaches against T1DM in this article.
T1DM accounts for up to 90% of childhood-onset diabetes and its incidence is increasing by 3 to 5 fold per year globally, and almost doubling the disease prevalence every 10-15 years in some Western countries[5]. The annual incidence is 0.3% among children, with a higher risk observed among siblings, with as high as 50% risk for monozygotic twins. Both genetic and environmental factors contribute to increased susceptibility to T1DM. Genetic factors like high-risk human leukocyte antigen (HLA) alleles and other gene loci that increase susceptibility to T1DM have been identified. Environmental risk factors that have been implicated including different viral infections like rubella, enterovirus, Coxsackie, rotavirus or even coronavirus disease 2019, as also different vaccines, immunotherapeutic agents and some dietary factors like bovine milk or low vitamin D intake, but the extent to which these factors contribute to the pathogenesis is yet unknown. T1DM progresses in several stages. In stage 1, the patients are euglycemic but bear multiple anti-islet cell autoantibodies. Stage 2 is characterised by progression to dysglycemia (impaired glucose tolerance) in the presence of two or more anti-islet autoantibodies. Progression to stage 3 occurs when the glycemic parameters get deranged and fulfil the criteria for the diagnosis of diabetes[6,7]. Even after patients enter stage 4, for a few months to years, few remaining functional beta cells may maintain adequate insulin secretion and some patients might show an improvement in glycemic status with reduced insulin requirements, termed the “honeymoon” phase of T1DM[8]. However, by the end of three to five years, there is almost total failure of beta cells leading to the lifelong need for exogenous insulin for glycemic control and prevention of acute complications like diabetic ketoacidosis and of macrovascular and microvascular complications and mortality.
The pathogenesis of T1DM involves the gradual immune mediated destruction of the insulin secretory β-cells within the islets of Langerhans. The autoimmune T-cells are the chief players behind this. The nature of β-cell destruction is chronic rather than acute, with a more rapid rate in children than in adults[9].
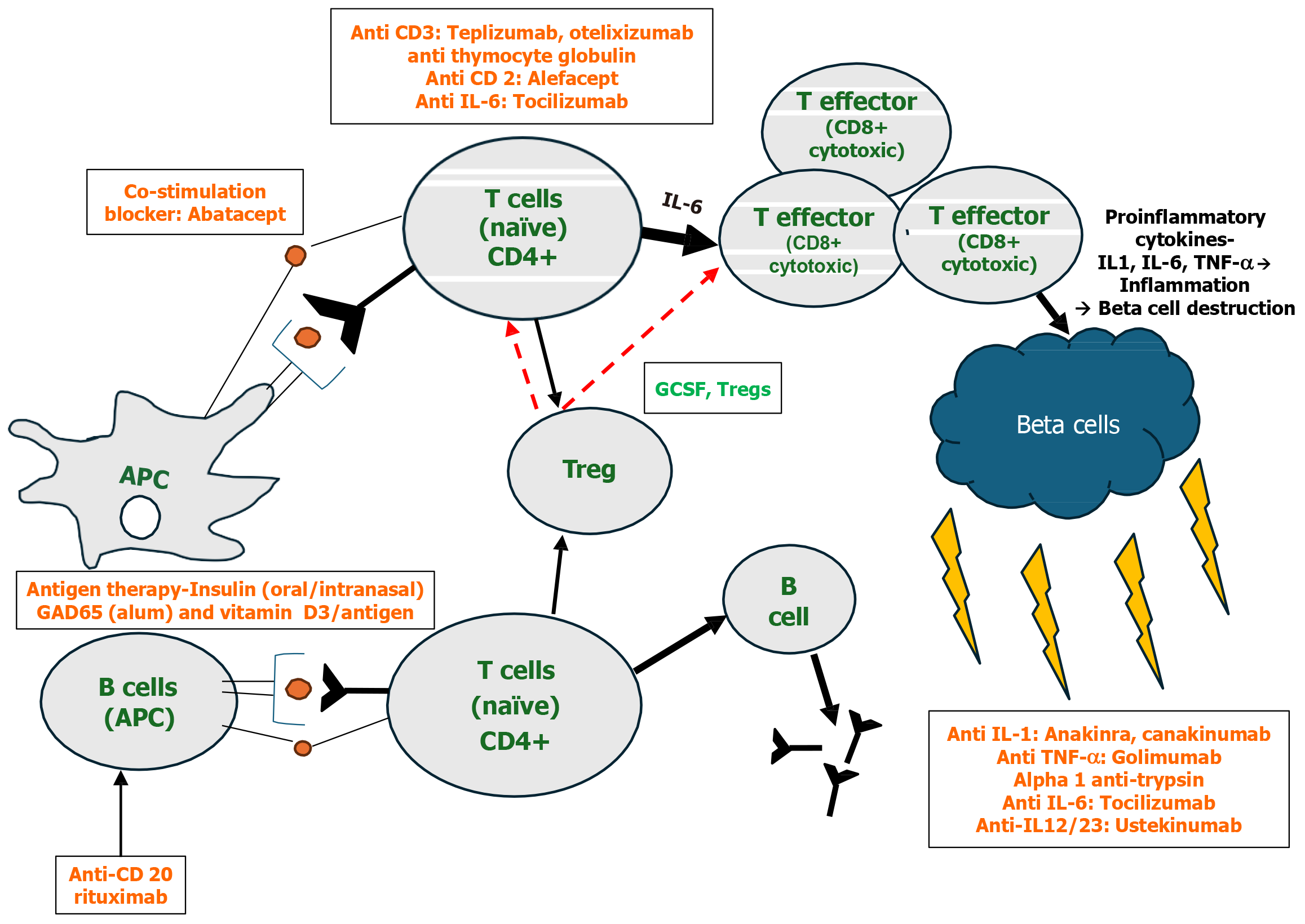
The first step to the destructive process is initiated by the antigen-presenting cells like the macrophages and dendritic cells which presents the β-cell-derived peptides, which are autoantigens. These then migrate to peri-pancreatic lymph nodes and activate the CD4+ helper T cells (Th)[6], enabling their differentiation into pro-inflammatory Th1 cells. Activated Th1 and Th17 cells produce cytokines like interferon γ (IFN-γ), tumor-necrosis-factor-α (TNF-α), interleukin (IL)-1 and IL-2, which then inhibit Th2 cell polarisation. The Th2 cells are responsible for the protection of islet cells. Additionally, the Th1 cells lead to the recruitment and activation of autoreactive cells like the CD8+ cytotoxic T-lymphocytes (CTL). The activated CTLs lead to destruction of β cells due to release of perforin, granzyme in the granules as well as the effect of the cytokines and caspase-dependent apoptosis. The Th1 cells also stimulate the B cells which produce autoantibodies against β cells. These antibodies include islet cell antibodies, glutamic-acid-decarboxylase antibodies (GADA), insulin autoantibodies (IAA), zinc transporter 8 antibodies and islet tyrosine phosphatase 2 antibodies which are all considered biomarkers of T1DM. The Th1 cells also participate in enhancement of the antigen presenting, co-stimulatory and effector functioning mechanisms of the antigen presenting cells (APCs). The natural killer cells contribute to β-cell destruction by an antibody-dependent cellular cytotoxicity mechanism. There is also downregulation of regulatory T cells (Treg) that inhibit the autoreactive lymphocytes, leading to increased rate of progression of T1DM[10]. These immune cells together infiltrate the islets (insulitis) leading to the progressive loss of the β cells[6].
The mainstay of therapy in T1DM remain insulin, either in the form of multiple daily subcutaneous injections or continuous subcutaneous infusion. However, despite adequate compliance to insulin, a high proportion of individuals with T1DM struggle to reach the recommended glycaemic targets leading to an elevated risk of complications and mortality. From the early days since the discovery of pathogenesis of T1DM, there has been a lot of interest in the development of immunotherapy targeting beta-cell destruction. Immunotherapeutic agents administered during the initial pre-symptomatic stages of T1DM can suppress or prevent the immunity-mediated β-cell destruction and, by reducing the pro-inflammatory cytokines, can improve the ability of residual β-cells to secrete insulin[10]. Thus, the aims of these agents have been either to prevent T1DM (i.e. to avoid overt symptomatic onset of the disease) or once diagnosed, to reverse it (commonly termed intervention).
While many of these agents did reduce the rate of decline in C-peptide levels within the first few years after disease onset, most of the older agents did not have prolonged effects after discontinuing the drug, implying the requirement of continuous treatment to prevent progressive β-cell loss. This led to increased risk for adverse effects on the liver, kidney or haematological system, and prolonged immuno-suppression. Thus, the aim was to have newer agents which do not require continuous immune suppression[11]. Till date, no proven safe and effective immunotherapy that prevents T1DM or reverses the disease is available for routine use. In research settings, however, several potentially promising candidates do exist that can delay the onset of disease. Based on their mechanism, the immunotherapeutic agents used in T1DM may be classified as follows.
Cyclosporine was the earliest agent to be tried with encouraging results and was found to prevent further loss of C-peptide secretion and improve metabolic function in new-onset T1DM[12]. However, cyclosporine did not maintain an euglycemic state if initiated after the onset of overt diabetes, and patients rapidly lost C-peptide reserve with discontinuation of the drug. The prolonged use would be associated with an increased risk for toxicity, particularly nephrotoxicity and concern about malignancies. Subsequent studies used agents such as prednisone and azathioprine, both of which however demonstrated relatively minimal effect in disease progression.
IL-1 blockers: Anakinra and Canakinumab failed to show significant C-peptide preservation in phase 2 studies in stage 3 T1DM[13].
TNF-α blockers: Etanercept improved C-peptide and glycated hemoglobin A1c (HbA1c) in a small, pilot study in child
IL-12/23 antagonist: A pilot trial of Ustekinumab in Patients with New-onset Type 1DM (USTID) is currently undergoing in Canada[15].
IL-6 blockers: IL-6 promotes the pathogenic Th17 cells in T1DM. Tocilizumab reduced T cell IL-6 receptor signaling, but failed to show delay in the loss of residual β cell functioning in newly diagnosed type 1 diabetes[16].
Targeting the T cells: Several agents can interfere with T-cell signaling or activation and reduce the number of patho
T cell signaling: Abatacept, a CTL-associated-antigen 4 immunoglobulin binds to CD80/86, which is a costimulatory signal necessary for activation of naïve T lymphocytes but not for effector memory T cells. Monthly infusions of 10 mg/kg intravenous abatacept for 2 years has demonstrated efficacy in delaying C-peptide decline by 9.6 months. Further studies on using it earlier, in stage 1 T1DM are underway[18].
Anti CD3 therapy: A humanized monoclonal antibody binding to the ε chain of the CD3 molecule on T cells is tepli
Anti CD2 therapy: An anti-CD2 fusion protein is alefaceft which can block T-cell activation and induce the apoptosis of memory Teff lymphocytes. In the T1DAL study on children and adults with stage 3 T1DM, despite inadequate enrolment, after one year, there was a non-significant trend towards C-peptide preservation, lesser insulin requirement and reduced hypoglycemia. Notably, the benefits were sustained at 15 months after drug discontinuation[19].
Anti-thymocyte globulin: Low-dose anti-thymocyte globulin (ATG) (2.5 mg/kg total dose, given as 0.5 mg/kg on day 1 and 2 mg/kg on day 2) has been used in combination with granulocyte colony-stimulating-factor and can help in the recovery of Tregs. It has showed a trend toward C-peptide preservation[20,21]. The higher dose of 6.5 mg/kg ATG given as monotherapy however was not seen to preserve β-cell function, though C-peptide was preserved in the older participants only.
Low dose IL-2: IL-2, in higher doses promotes Teff activity, but its lower dose promotes Treg activity. A pilot study conducted in 2012 did show the combination of IL-2 with rapamycin to enhance the activity and survival of Tregs[22]. However, there were significant adverse effects, including a transient decrease in C-peptide for which the study was terminated early.
Treg infusion: A direct method to increase Tregs, safety of this approach was demonstrated in a small phase 1 dose-finding study in 2015. However, combination of polyclonal Treg Adoptive Immunotherapy with IL-2 in the TILT study led to transient impairment in C-peptide[23].
Although T1DM is chiefly a T cell-mediated autoimmune disease, antigen-presenting B lymphocytes also play important pathogenic roles.
Targeting B cells: Rituximab is an anti-CD20 monoclonal antibody which leads to B-cell depletion and delays C-peptide loss in stage 3 disease. A study with the B cell-depleting agent Rituximab, showed that 4 weekly infusions of rituximab, could preserve C-peptide for 8.2 months[24]. However, none of the participant could be rendered insulin free. On longer-term follow-up, there persisted a constant decline in C-peptide. Rituximab has been found to suppress IAAs, but no effects could be seen on GAD-antibody, islet tyrosine phosphatase 2 antibody and zinc transporter 8 antibodies[25]. The combination of rituximab with T regulatory cells or therapy targeting CD4+ T cells might lead to better response[26]. A notable side effect of rituximab was the reactivation of asymptomatic polyomavirus infections.
Block major histocompatibility complex function: Methyldopa has been found to interfere with antigen presentation via the DQ8 major histocompatibility complex class II alleles, which is a high-risk HLA allele seen in up to 50%-60% of people with T1DM and is being planned for trials in relatives at risk of TIDM[27].
Insulin and GAD65 antibodies are markers of immune response directed against these self-proteins. Insulin as an antigen has been tried in stage 1 T1DM while GAD65 has been tested in both stage 1 and stage 3 disease[28,29]. Although well tolerated, these approaches have not been very effective. Trials using oral insulin in primary prevention of T1DM, prior to the development of islet autoimmunity, are currently underway. The protective immune response depends on multiple factors like the route of administration and the use of an altered antigen like insulin B-chain and an immunodominant B[9,23] peptide instead of the intact insulin molecule. The diabetes prevention trial-type 1 diabetes found a significant delay in progression to diabetes in a subgroup of participants having high IAA levels only with oral but not parenteral insulin[30]. However, results were not similar in the type 1 diabetes Trial Net study and the potential value of oral insulin remains uncertain. Intra-lymphatic treatment with GAD-alum along with oral vitamin D supplementation demonstrated good metabolic and immunologic outcomes in recent onset T1DM[31].
Beta cell support: Agents like metformin, the glucagon-like peptide 1 agonists such as liraglutide, hydroxychloroquine and even proton-pump-inhibitors such as omeprazole have been tried as adjunctive therapies in T1DM to offer beta cell support but have yielded inconclusive or negative results[32].
Since multiple mechanisms are involved in the pathogenesis of T1DM, combination therapies to prevent or reverse the disease seem to be an interesting option. Examples include an induction component using drugs targeting pro-inflammatory cytokines and T- and B-lymphocytes, and a maintenance component involving antigens to induce immune tolerance to the pancreatic β-cells. Also, combination therapy with drugs that can both stabilize and maintain β-cell survival and function should be considered, an example being verapamil, which demonstrated partial preservation of C-peptide in a phase 2 trial in adults with T1DM. A drug combination that can stop or delay the progressive autoimmune process as well as improve the residual β-cell function is liraglutide along with anti-IL-21[33]. IL-21 activates and recruits the CD8+ T lymphocytes from lymph nodes to the pancreatic islets leading to β-cell destruction. In a recently conducted phase II trial, combined use of liraglutide and IL-21 inhibitor in recently diagnosed T1DM patients with residual β-cell function highlighted that the combination could preserve both fasting and postprandial endogenous insulin, with a non-significant decrease in the hypoglycemic episodes[33].
Parallel to immunotherapy, regenerative therapy using mesenchymal stem cells is also being developed with promising results due to their immunomodulatory properties[34]. However, clinical trials are limited, with small number of patients and short follow-up times. Additionally, there is lack of consensus regarding standardized stem cell processing, transplantation protocols and dosage. Figure 1 shows the therapeutic targets and experimental agents for patients with T1DM.
Teplizumab is a humanized monoclonal antibody belonging to the immunoglobulin G1 subclass, which binds to the ε chain of the CD3 molecule on T cells. Two Leu à Ala substitutions in the Fc region minimized its Fc-receptor binding and rendered it non-activating, resulting in up to 1000-fold reduction in the degree of T-cell activation, T-cell proliferation, and cytokine release compared with OKT3[35]. It attenuates activated autoreactive T-cells that destroy β cells. There is also evidence to suggest that teplizumab induces Tregs activity further augmenting immune tolerance[36].
Several preclinical studies have shown that treatment with anti-CD3 mAb could induce autoimmune tolerance in diabetic mice, and this response persisted long after the discontinuation of antibody treatment[37]. One of the first human trials with hOKT3 gamma1 had reported significantly improved C-peptide response. A series of human clinical trials demonstrated that teplizumab could delay C-peptide decline and preserve β-cell function and up to 5% of the patients became insulin independent[35,38-41]. A phase 3 follow-up trial of at-risk participants having impaired glucose tolerance and two or more T1DM autoantibodies also showed that teplizumab could delay the onset of T1DM by 48.4 months and C-peptide levels were improved even after a follow-up period of 7 years[38]. This has been followed by multiple RCTs comparing teplizumab to placebo in new onset T1DM, showing delay in the decline of C-peptide, improvement in the C-peptide response and reduced need for insulin initiation starting from few months after initiation and lasting up to five to seven years as seen in the long-term follow-up studies. Adverse reactions were mostly well tolerated and included mostly mild ones like vomiting, rash, chills with few episodes of more severe reaction like cytokine release syndrome and Epstein-Barr virus reactivation, lymphopenia, neutropenia, thrombocytopenia, elevated liver enzymes and reduced platelet counts. Following the promising results, in November 2022, teplizumab received FDA approval as the first medication to delay onset of stage 3 T1DM in individuals aged above eight years[42].
A summary of the currently available immunotherapies T1DM, their putative mechanisms of actions and the significant clinical trial findings are depicted in Table 1[43-51].
| Mechanism | Agents | Significant findings from landmark trials | |
| Agents causing generalised immunosuppression | Cyclosporine; Azathioprine; Prednisolone | Studies showed preservation of beta cells with cyclosporin; No differences in glycemic control, freedom from insulin or insulin dosage. High risk of organ toxicity | |
| Agents targeting specific cytokines | Interleukin-1 blockers | Anakinra | No significant improvement in AUC C-peptide at 9 months; Some improvement in insulin sensitivity in type 1 diabetes with insulin resistance; High number of injection site reactions[13] |
| Canakinumab | No significant improvement in AUC C-peptide at 9 months; Safe, well tolerated | ||
| TNF-α blockers | Etanercept | Slower C-peptide decline and lower insulin requirement; Small study[14] | |
| Interleukin-12/23 antagonist | Ustekinumab | Marwaha et al[15]: 90 mg maintenance dose reduced proinsulin-specific IFN-γ and IL-17A-producing T cells | |
| Interleukin-6 blockers | Tocilizumab | EXTEND trial[16]: Reduced T cell IL-6R signaling but did not modulate CD4+ T cell phenotypes or slow loss of residual β cell function in newly diagnosed type 1 diabetes | |
| Agents targeting specific immune cells | Targeting the T cells | ||
| Anti CD3 | Teplizumab | Delay in the decline of C-peptide, improvement in the C-peptide response and reduced need for insulin initiation starting from few months after initiation lasting up to seven years[38]; Approved for use in children above 8 years to postpone the onset of stage 3 T1DM[42] | |
| Otelixizumab | DEFEND-1,2[43,44]: No difference in 2 hours MMTT AUC C-peptide at 12 months; Reactivation of EBV | ||
| Anti thymocyte globulin (ATG) | ATG + G-CSF | TriialNet: Slowed decline of C-peptide and reduced HbA1c in new-onset T1D[21] | |
| Anti CD2 | Alefacept | TIDAL[45]: Significantly higher stimulated AUC C-peptide and lower insulin use in treated group | |
| Co-stimulation blocker | Abatacept | TrialNet[46,47]: Significantly higher stimulated C-peptide 2 hour AUC in treated group at the end of treatment and 1-year post treatment | |
| IL-2 | Aldesleukin +/- rapamycin | Hartemann et al[48]: Dose dependent increase in the proportion of Tregs in the treatment group | |
| Treg infusion | Bluestone et al[49]: Subset (25% peak) of adoptively transferred T-regs still in circulation at 1 year; C-peptide preservation in those receiving lower dose | ||
| Target antigen presenting B cells | |||
| Anti-CD20 | Rituximab | TrialNet: Higher AUC-C peptide, lower HbA1c and insulin need at 1 year; No differences in decline of C-peptide at 30 months[25] | |
| Immunologic vaccination/antigen therapy | Insulin | Oral Insulin | Pre-POINT[50]: Increased Tregs in those who received a higher dose of oral insulin (62.5 mg) |
| GAD-65 Ab | Alum-GAD 65+/- oral vitamin D | DiAPREV-IT1, DiAPREV-IT2[28,51]: Decline in total and cytotoxic T cells; No change in AUC C-peptide, oral glucose tolerance tests and HbA1c | |
Ma et al[4] in the recent issue of World Journal of Diabetes has conducted a meta-analysis and systematic review to determine the efficacy and safety of teplizumab in T1DM based on eight RCTs. The outcomes they assessed included reduction in insulin use, improvement in C-peptide response and HbA1c level along with the side-effect profile. After applying inclusion-exclusion criteria, results from 1361 participants with T1DM and 547 controls were included. Those in the teplizumab group had a greater reduction in insulin use (OR: 4.13, 95%CI: 1.72-9.90), change in the C-peptide response, (OR: 2.49, 95%CI: 1.62-3.81) as well as change in HbA1c level, (OR: 1.75, 95%CI: 1.03-2.98). Glycemic control was also more favourable in the teplizumab group, evidenced by more optimal serum glucose concentrations, thus having the potential to reduce the longstanding complications of diabetes mellitus. Notably, the control group exhibited a greater susceptibility to experiencing unfavourable outcomes (RR: 0.71, 95%CI: 0.53-0.95)[4].
The authors have mentioned their findings to be in line with that of other meta-analyses, citing a positive correlation between teplizumab use and reduction in insulin requirement, improvement in C-peptide response and HbA1c levels, while exhibiting minimal adverse effects. In addition, teplizumab has been shown to offer improved glycaemic management.
Several meta-analyses have been conducted on the role of teplizumab in T1DM (Supplementary Table 1)[52-54]. While Ma et al[4] has discussed their results in comparison to other prior meta-analyses, they have not included the findings of a more recent meta-analysis by Kamrul-Hasan et al[54]. The authors in this have included seven reports from six RCTs involving 834 subjects, but excluded studies that were sub-analysis and follow-up reports of the included studies. Differences in C-peptide responses were seen as early as six months and changes in insulin dose were seen at 24 months, though the latter were not significantly different. However, contrary to other meta-analyses, they reported teplizumab use to increase the risk for higher risks of grade 3 or higher adverse events most of which were modest and resolved after discontinuation of therapy. Ma et al[4] have acknowledged their limitations including the possibility of selection bias due to selection of just eight studies with heterogeneity in patient selection, confounding factors like gender, age, or ethnicity, limited sample size of the trials used in the current meta-analysis.
There remain some unanswered questions like factors that determine responsiveness to teplizumab, particularly the role of factors that affect escape from the effects of immune therapy, like levels of pro-inflammatory cytokines or of glucose toxicity. Also, the duration of response varies in different individuals. Overall, the response to teplizumab seems to be better in the younger and those with higher levels of insulin production, although the rate of beta cell decline in younger participants is more rapid than in adults. It is yet unknown whether repeated doses of same medication would prolong its therapeutic effects or offer any additional advantages over single dose administration.
Immunotherapy in type 1 diabetes is an emerging area and offers promise for a cure. Teplizumab is a disease modifying therapy for newly diagnosed T1DM and can lead to improvement in C-peptide levels and reduction in insulin requirements with a good safety profile. It has been approved for use in children with stage 3 type 1 diabetes to postpone the onset of overt disease and need for life-long insulin. Several RCTs have confirmed that teplizumab can delay the decline or improve C-peptide response and reduce the need for insulin initiation, lasting up to several years after discontinuation. A recent meta-analysis showed that teplizumab could lead to reduction in insulin use and better C-peptide with better glycemic control[4]. Overall, although a large number of agents targeting specific cytokine and immunologic vaccination have been tried in T1DM, the results have been conflicting with few trials showing promising results with regards to improvement in C-peptide, HbA1c% and/or insulin independency[43-51]. Adverse effects were minimal and mild. However, almost all the studies with teplizumab have been conducted in high-income countries. Future studies should include cost-effectiveness analysis and studies conducted in low-middle income countries, which have a high prevalence of T1DM. Also, evaluation of the role of therapy in more advanced stages of T1DM are the need of the hour[55].
We are thankful to Ms. Jovitta Fernandez Jerrin for her voice clip for the audio core tip.
| 1. | Bluestone JA, Buckner JH, Herold KC. Immunotherapy: Building a bridge to a cure for type 1 diabetes. Science. 2021;373:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 2. | Lin C, Hu S, Cai X, Lv F, Yang W, Liu G, Yang X, Ji L. The opportunities and challenges of the disease-modifying immunotherapy for type 1 diabetes: A systematic review and meta-analysis. Pharmacol Res. 2024;203:107157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | US Food and Drug Administration. Tzield (teplizumab-mzwv) injection. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/761183Orig1s000ltr.pdf. |
| 4. | Ma XL, Ge D, Hu XJ. Evaluation of teplizumab's efficacy and safety in treatment of type 1 diabetes mellitus: A systematic review and meta-analysis. World J Diabetes. 2024;15:1615-1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Williams JM, Poudel B, Shields CA. Chapter 15-Sex Differences in Autoimmune Type-1 Diabetes. In: LaMarca B, Alexander BT, editors. Sex Differences in Cardiovascular Physiology and Pathophysiology. Academic Press, 2019: 239-249. [DOI] [Full Text] |
| 6. | Toren E, Burnette KS, Banerjee RR, Hunter CS, Tse HM. Partners in Crime: Beta-Cells and Autoimmune Responses Complicit in Type 1 Diabetes Pathogenesis. Front Immunol. 2021;12:756548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes. 2017;66:241-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 443] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 8. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 2050] [Article Influence: 410.0] [Reference Citation Analysis (1)] |
| 9. | Bogun MM, Bundy BN, Goland RS, Greenbaum CJ. C-Peptide Levels in Subjects Followed Longitudinally Before and After Type 1 Diabetes Diagnosis in TrialNet. Diabetes Care. 2020;43:1836-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238-3245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Nagy G, Szekely TE, Somogyi A, Herold M, Herold Z. New therapeutic approaches for type 1 diabetes: Disease-modifying therapies. World J Diabetes. 2022;13:835-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Skyler JS, Rabinovitch A. Cyclosporine in recent onset type I diabetes mellitus. Effects on islet beta cell function. Miami Cyclosporine Diabetes Study Group. J Diabetes Complications. 1992;6:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Canakinumab Study Group, Pickersgill L, de Koning E, Ziegler AG, Böehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castaño L, Wägner A, Lervang HH, Perrild H, Mandrup-Poulsen T; AIDA Study Group. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 14. | Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Marwaha AK, Chow S, Pesenacker AM, Cook L, Sun A, Long SA, Yang JHM, Ward-Hartstonge KA, Williams E, Domingo-Vila C, Halani K, Harris KM, Tree TIM, Levings MK, Elliott T, Tan R, Dutz JP. A phase 1b open-label dose-finding study of ustekinumab in young adults with type 1 diabetes. Immunother Adv. 2022;2:ltab022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Greenbaum CJ, Serti E, Lambert K, Weiner LJ, Kanaparthi S, Lord S, Gitelman SE, Wilson DM, Gaglia JL, Griffin KJ, Russell WE, Raskin P, Moran A, Willi SM, Tsalikian E, DiMeglio LA, Herold KC, Moore WV, Goland R, Harris M, Craig ME, Schatz DA, Baidal DA, Rodriguez H, Utzschneider KM, Nel HJ, Soppe CL, Boyle KD, Cerosaletti K, Keyes-Elstein L, Long SA, Thomas R, McNamara JG, Buckner JH, Sanda S; ITN058AI EXTEND Study Team. IL-6 receptor blockade does not slow β cell loss in new-onset type 1 diabetes. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Lernmark A, Larsson HE. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol. 2013;9:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Russell WE, Bundy BN, Anderson MS, Cooney LA, Gitelman SE, Goland RS, Gottlieb PA, Greenbaum CJ, Haller MJ, Krischer JP, Libman IM, Linsley PS, Long SA, Lord SM, Moore DJ, Moore WV, Moran AM, Muir AB, Raskin P, Skyler JS, Wentworth JM, Wherrett DK, Wilson DM, Ziegler AG, Herold KC; Type 1 Diabetes TrialNet Study Group. Abatacept for Delay of Type 1 Diabetes Progression in Stage 1 Relatives at Risk: A Randomized, Double-Masked, Controlled Trial. Diabetes Care. 2023;46:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Patel CM, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Moran A, Russell WE, Pinckney A, Keyes-Elstein L, Howell M, Aggarwal S, Lim N, Phippard D, Nepom GT, McNamara J, Ehlers MR; T1DAL Study Team. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, Zou B, Brusko TM, Hulme MA, Wasserfall CH, Mathews CE, Atkinson MA, Schatz DA. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Haller MJ, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Miller JL, Atkinson MA, Becker DJ, Baidal D, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Herold KC, Marks JB, Moran A, Rodriguez H, Russell W, Wilson DM, Greenbaum CJ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-Dose Anti-Thymocyte Globulin (ATG) Preserves β-Cell Function and Improves HbA(1c) in New-Onset Type 1 Diabetes. Diabetes Care. 2018;41:1917-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 22. | Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, Turka LA, Ehlers MR, Bianchine PJ, Boyle KD, Adah SA, Bluestone JA, Buckner JH, Greenbaum CJ; Diabetes TrialNet and the Immune Tolerance Network. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes. 2012;61:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Bettini M, Bettini ML. Function, Failure, and the Future Potential of Tregs in Type 1 Diabetes. Diabetes. 2021;70:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Herold KC, Pescovitz MD, McGee P, Krause-Steinrauf H, Spain LM, Bourcier K, Asare A, Liu Z, Lachin JM, Dosch HM; Type 1 Diabetes TrialNet Anti-CD20 Study Group. Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol. 2011;187:1998-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Yu L, Herold K, Krause-Steinrauf H, McGee PL, Bundy B, Pugliese A, Krischer J, Eisenbarth GS; Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab selectively suppresses specific islet antibodies. Diabetes. 2011;60:2560-2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Zieliński M, Żalińska M, Iwaszkiewicz-Grześ D, Gliwiński M, Hennig M, Jaźwińska-Curyłło A, Kamińska H, Sakowska J, Wołoszyn-Durkiewicz A, Owczuk R, Młynarski W, Jarosz-Chobot P, Bossowski A, Szadkowska A, Siebert J, Myśliwiec M, Marek-Trzonkowska N, Trzonkowski P. Combined therapy with CD4(+) CD25highCD127(-) T regulatory cells and anti-CD20 antibody in recent-onset type 1 diabetes is superior to monotherapy: Randomized phase I/II trial. Diabetes Obes Metab. 2022;24:1534-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Ostrov DA, Alkanani A, McDaniel KA, Case S, Baschal EE, Pyle L, Ellis S, Pöllinger B, Seidl KJ, Shah VN, Garg SK, Atkinson MA, Gottlieb PA, Michels AW. Methyldopa blocks MHC class II binding to disease-specific antigens in autoimmune diabetes. J Clin Invest. 2018;128:1888-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Ludvigsson J, Krisky D, Casas R, Battelino T, Castaño L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, Veeze HJ, Palmer J, Samuelsson U, Elding Larsson H, Åman J, Kärdell G, Neiderud Helsingborg J, Lundström G, Albinsson E, Carlsson A, Nordvall M, Fors H, Arvidsson CG, Edvardson S, Hanås R, Larsson K, Rathsman B, Forsgren H, Desaix H, Forsander G, Nilsson NÖ, Åkesson CG, Keskinen P, Veijola R, Talvitie T, Raile K, Kapellen T, Burger W, Neu A, Engelsberger I, Heidtmann B, Bechtold S, Leslie D, Chiarelli F, Cicognani A, Chiumello G, Cerutti F, Zuccotti GV, Gomez Gila A, Rica I, Barrio R, Clemente M, López Garcia MJ, Rodriguez M, Gonzalez I, Lopez JP, Oyarzabal M, Reeser HM, Nuboer R, Stouthart P, Bratina N, Bratanic N, de Kerdanet M, Weill J, Ser N, Barat P, Bertrand AM, Carel JC, Reynaud R, Coutant R, Baron S. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 29. | Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, Monzavi R, Moran A, Orban T, Palmer JP, Raskin P, Rodriguez H, Schatz D, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet GAD Study Group. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 30. | Hirst K. Diabetes Prevention Trial of Type 1 Diabetes (DPT-1). NIDDK Cent Repository. 2023. [DOI] [Full Text] |
| 31. | Ludvigsson J, Sumnik Z, Pelikanova T, Nattero Chavez L, Lundberg E, Rica I, Martínez-Brocca MA, Ruiz de Adana M, Wahlberg J, Katsarou A, Hanas R, Hernandez C, Clemente León M, Gómez-Gila A, Lind M, Lozano MF, Sas T, Samuelsson U, Pruhova S, Dietrich F, Puente Marin S, Nordlund A, Hannelius U, Casas R. Intralymphatic Glutamic Acid Decarboxylase With Vitamin D Supplementation in Recent-Onset Type 1 Diabetes: A Double-Blind, Randomized, Placebo-Controlled Phase IIb Trial. Diabetes Care. 2021;44:1604-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Greenbaum C, VanBuecken D, Lord S. Disease-Modifying Therapies in Type 1 Diabetes: A Look into the Future of Diabetes Practice. Drugs. 2019;79:43-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | von Herrath M, Bain SC, Bode B, Clausen JO, Coppieters K, Gaysina L, Gumprecht J, Hansen TK, Mathieu C, Morales C, Mosenzon O, Segel S, Tsoukas G, Pieber TR; Anti-IL-21–liraglutide Study Group investigators and contributors. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9:212-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 34. | Wan XX, Zhang DY, Khan MA, Zheng SY, Hu XM, Zhang Q, Yang RH, Xiong K. Stem Cell Transplantation in the Treatment of Type 1 Diabetes Mellitus: From Insulin Replacement to Beta-Cell Replacement. Front Endocrinol (Lausanne). 2022;13:859638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Herold KC, Gitelman SE, Gottlieb PA, Knecht LA, Raymond R, Ramos EL. Teplizumab: A Disease-Modifying Therapy for Type 1 Diabetes That Preserves β-Cell Function. Diabetes Care. 2023;46:1848-1856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 36. | Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 488] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 37. | Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947-2954. [PubMed] |
| 38. | Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, Gitelman SE, Greenbaum CJ, Gottlieb PA, Hagopian W, Woodwyk A, Dziura J, Herold KC; Immune Tolerance Network. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia. 2019;62:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 39. | Hagopian W, Ferry RJ Jr, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, Ludvigsson J; Protégé Trial Investigators. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901-3908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 40. | Ramos EL, Dayan CM, Chatenoud L, Sumnik Z, Simmons KM, Szypowska A, Gitelman SE, Knecht LA, Niemoeller E, Tian W, Herold KC; PROTECT Study Investigators. Teplizumab and β-Cell Function in Newly Diagnosed Type 1 Diabetes. N Engl J Med. 2023;389:2151-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 154] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 41. | Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG; Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 42. | Hirsch JS. FDA approves teplizumab: a milestone in type 1 diabetes. Lancet Diabetes Endocrinol. 2023;11:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | Ambery P, Donner TW, Biswas N, Donaldson J, Parkin J, Dayan CM. Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet Med. 2014;31:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Aronson R, Gottlieb PA, Christiansen JS, Donner TW, Bosi E, Bode BW, Pozzilli P; DEFEND Investigator Group. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37:2746-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Keyes-Elstein L, Long SA, Kanaparthi S, Lim N, Phippard D, Soppe CL, Fitzgibbon ML, McNamara J, Nepom GT, Ehlers MR. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125:3285-3296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 46. | Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 47. | Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Peakman M, Raskin P, Russell WE, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Abatacept Study Group. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 49. | Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 850] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 50. | Bonifacio E, Ziegler AG, Klingensmith G, Schober E, Bingley PJ, Rottenkolber M, Theil A, Eugster A, Puff R, Peplow C, Buettner F, Lange K, Hasford J, Achenbach P; Pre-POINT Study Group. Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA. 2015;313:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 51. | Salami F, Spiliopoulos L, Maziarz M, Lundgren M, Brundin C, Bennet R, Hillman M, Törn C, Elding Larsson H. Long-Term GAD-alum Treatment Effect on Different T-Cell Subpopulations in Healthy Children Positive for Multiple Beta Cell Autoantibodies. J Immunol Res. 2022;2022:3532685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 52. | Nourelden AZ, Elshanbary AA, El-Sherif L, Benmelouka AY, Rohim HI, Helmy SK, Sayed MK, Ismail A, Ali AS, Ragab KM, Zaazouee MS. Safety and Efficacy of Teplizumab for Treatment of Type One Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocr Metab Immune Disord Drug Targets. 2021;21(10):1895-1904.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Ashraf MT, Ahmed Rizvi SH, Kashif MAB, Shakeel Khan MK, Ahmed SH, Asghar MS. Efficacy of anti-CD3 monoclonal antibodies in delaying the progression of recent-onset type 1 diabetes mellitus: A systematic review, meta-analyses and meta-regression. Diabetes Obes Metab. 2023 Nov;25(11):3377-3389.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 54. | Kamrul-Hasan ABM, Mondal S, Nagendra L, Yadav A, Aalpona FTZ, Dutta D. Role of Teplizumab, a Humanized Anti-CD3 Monoclonal Antibody, in Managing Newly Diagnosed Type 1 Diabetes: An Updated Systematic Review and Meta-Analysis. Endocr Pract. 2024;30:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Nagendra L, Dutta D, Mondal S, Yadav A, Aalpona FTZ, Kamrul-Hasan ABM. Reply to the Letter to the Editor, Endocrine Practice for "Role of Teplizumab, a Humanized Anti-CD3 Monoclonal Antibody, in Managing Newly Diagnosed Type 1 Diabetes: An Updated Systematic Review and Meta-Analysis". Endocr Pract. 2024;30:1012-1013. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/