Published online Jan 15, 2025. doi: 10.4239/wjd.v16.i1.99135
Revised: September 17, 2024
Accepted: November 5, 2024
Published online: January 15, 2025
Processing time: 137 Days and 13.9 Hours
Treating diabetes in dialysis patients remains a challenge, with many hypogly
This report describes an 83-year-old female patient with a 30-year history of type 2 diabetes (T2DM) who had struggled to control her blood sugar for more than a year. She had a history of high blood pressure for 30 years, had undergone continuous ambulatory peritoneal dialysis for more than two years, was 163 cm tall, weighed 77 kg, and had a body mass index of 28.98 kg/m2. Despite intensive insulin therapy at a daily dose of 150 units, adding Dorzagliatin at a dosage of
To our knowledge, this report is the first to use Dorzagliatin to treat type 2 dia
Core Tip: Treating diabetes in dialysis patients remains a challenge, with many hypoglycemic drugs requiring dose adjustments or avoidance in these patients. To our knowledge, this report is the first to use Dorzagliatin in the treatment of type 2 diabetes peritoneal dialysis patients with challenging glucose control. Dorzagliatin, a novel glucokinase activator primarily metabolized by the liver, exhibits no pharmacokinetic differences among patients with varying degrees of chronic kidney disease, has a high plasma protein binding rate, and may not be cleared by peritoneal dialysis, potentially offering a new glycemic control option for Type 2 diabetic patients on peritoneal dialysis.
- Citation: Chen F, An B, An WC, Fu G, Huang W, Yan HX. Application of Dorzagliatin in peritoneal dialysis patients with type 2 diabetes mellitus: A case report. World J Diabetes 2025; 16(1): 99135
- URL: https://www.wjgnet.com/1948-9358/full/v16/i1/99135.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i1.99135
Diabetes in dialysis patients is associated with a higher risk of morbidity and mortality due to complications such as cardiovascular diseases, infections, peripheral arterial disease, and diabetic neuropathy, highlighting the complexity of their management requiring a multidisciplinary approach[1]. Physicians are confronted with numerous challenges in the management of type 2 diabetes mellitus (T2DM) in patients undergoing peritoneal dialysis. The alterations in glucose homeostasis, uncertainties in blood glucose indices, and changes in the pharmacokinetics of hypoglycemic drugs make blood glucose management for this group particularly difficult. Treating diabetes in dialysis patients remains a challenge, with many hypoglycemic drugs requiring dose adjustments or avoidance in these patients[1]. In this context, Dor
The patient is an 83-year-old female who presented to the hospital on January 12, 2024, with a 30-year history of diabetes and elevated blood glucose for over a year.
The patient was diagnosed with T2DM thirty years ago during a physical examination, when fasting blood glucose levels were found to be > 7 mmol/L. Following the diagnosis, she received lifestyle guidance, and her blood glucose levels were well controlled within the standard range for several years. However, she later experienced a period of elevated blood glucose levels. Initially, she was prescribed Acarbose 50 mg three times daily, which helped maintain her blood glucose levels between 6-10 mmol/L. Due to good control, this medication was subsequently discontinued. Three years ago, the patient began to experience a progressive rise in blood creatinine levels. By November 2022, her blood creatinine had increased to 400 μmol/L, leading to the initiation of maintenance abdominal dialysis therapy [continuous ambulatory peritoneal dialysis (CAPD)] with daily Icodextrin peritoneal dialysis solution (1500 mL, one bag, from 7:00 to 22:00). Since starting dialysis, her blood glucose levels progressively increased, with fasting blood glucose monitored between 10-15 mmol/L and 2 hours post-meal levels ranging from 10-18 mmol/L. In January 2023, insulin therapy was initiated, and various insulin treatment regimens were adjusted accordingly. By April 2023, the insulin treatment program was modified to an intensive regimen, with a total daily dose of 150 units.
The patient had a 30-year history of primary hypertension and had been taking Amlodipine Besylate 5-10 mg qd for a long time, with blood pressure monitored and controlled at 130-150/80-90 mmHg. She denied a history of infectious diseases such as hepatitis and tuberculosis and had no history of drug allergy.
There was no family history of diabetes mellitus. There was no history of ethanol or drug abuse, herbal use, or depot injections. The patient was vaccinated according to the local vaccination schedule. Her family history was insignificant.
Physical examination revealed blood pressure 135/86 mmHg, height 163 cm, weight 77 kg, body mass index (BMI) 28.98 kg/m2, indicating overweight status, with nephrotic facies. There was no swelling of the lower limbs. Upon further examination, her general appearance was alert and oriented, with no signs of acute distress. The patient demonstrated normal respiratory effort, and her skin was warm and dry, with no rashes or lesions noted. Functional status assessment revealed that the patient was able to perform activities of daily living independently, though she reported some fatigue. Gait was steady, and she did not require assistance while walking.
The main diagnoses were T2DM, chronic kidney disease (CKD), stage 5 continuous peritoneal dialysis, and primary hypertension grade 3.
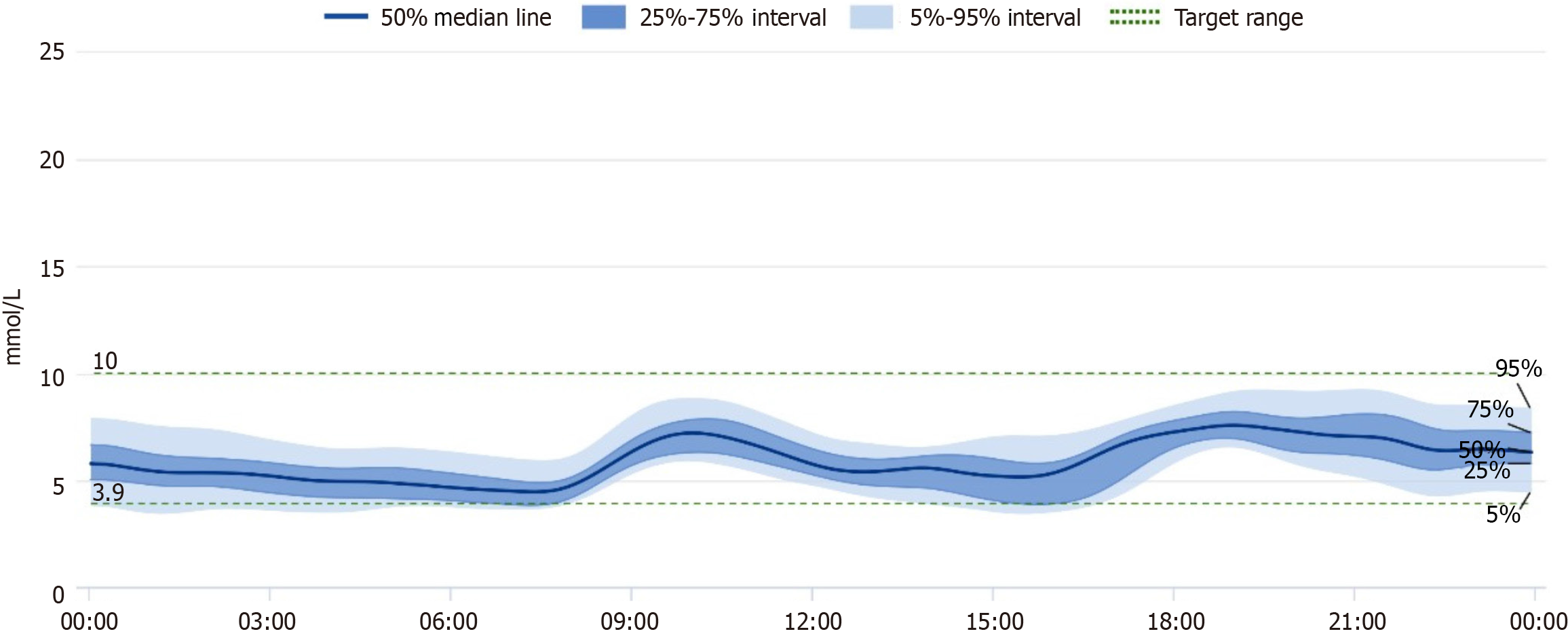
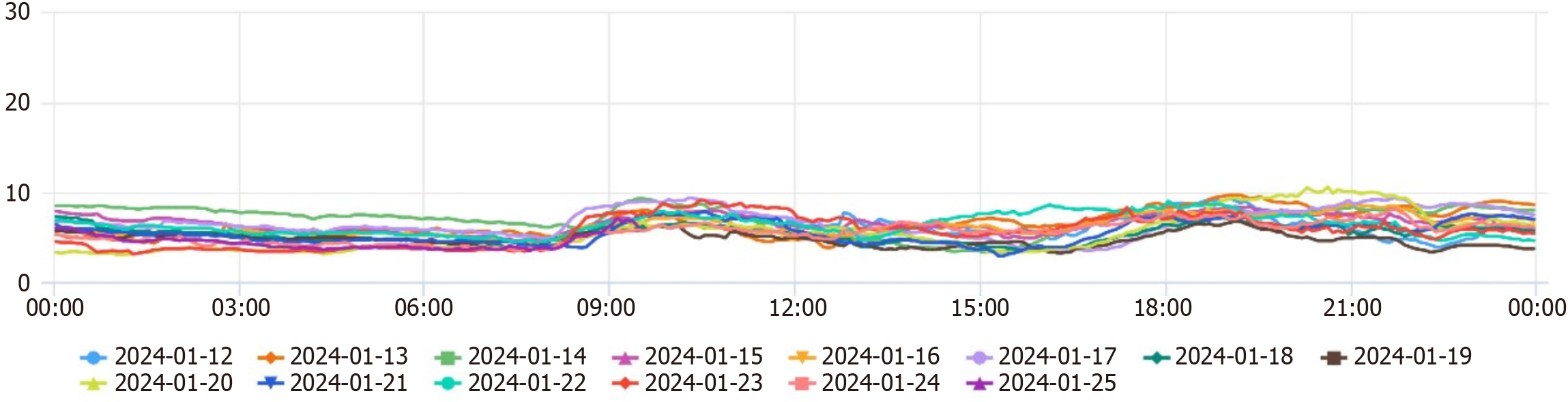
In the outpatient clinic, the glucose-lowering treatment plan was adjusted: Based on intensive insulin therapy, Dorzagliatin 75 mg bid po was added to control blood glucose, other hypoglycemic agents were considered, however, many commonly used medications, such as metformin and sulfonylureas, are contraindicated or require careful dose adjustments in patients with significant renal impairment due to the risk of hypoglycemia and accumulation of metabolites. And a continuous glucose monitoring system (CGMs) was worn, considering that CGM values in patients with abdominal dialysis may be low, the patient's family members were instructed to monitor fasting and postprandial glucose daily to avoid glucose fluctuations and to continue comprehensive treatment such as lowering blood pressure, correcting anemia, regulating lipids, and maintaining water-electrolyte balance. Throughout the adjustment of medication, the patient maintained her established dietary and exercise regimen, which had not changed. She continued to adhere to a balanced diet tailored to her diabetic and renal needs, focusing on portion control and low glycemic index foods. Additionally, she engaged in light physical activity, such as walking for 10-20 minutes daily, which remained consistent and contributed to her overall well-being. After adding Dorzagliatin, CGM data showed a significant decrease in glucose levels on the day of addition (see the CGM report for details). At the same time, the patient monitored terminal blood glucose daily, and blood glucose significantly decreased from January 14th onwards. The insulin dosage was rapidly reduced, with fasting blood glucose monitored at 6-8 mmol/L and 2-hour postprandial blood glucose at 8-12 mmol/L.
After half a month, the patient's daily insulin dosage was reduced to 40U, and blood glucose remained stable. One month later, the patient's treatment regimen was the same as before; blood glucose was still stable (Table 1, Figure 1 and Figure 2). There were no adverse reactions, such as dry mouth and hypoglycemia. Six months later, the patient's treatment regimen was the same as before, daily insulin dosage was 45U; blood glucose was still stable, BMI and blood pressure remains stable as before. Looking ahead, a comprehensive monitoring plan has been established to ensure continued stability in the patient's blood glucose levels. This plan includes regular follow-up appointments every three months, during which blood glucose levels will be assessed, along with HbA1c measurements to evaluate long-term glycemic control. Additionally, the patient will continue using the continuous CGMs to facilitate real-time tracking of glucose fluctuations. The healthcare team will also provide ongoing education regarding diet, exercise, and medication adherence to support optimal management of her condition.
| Date (2024) | Glucose (mmol/L) | Rapid-acting insulin (/U) | Basal insulin (/U) | |||||
| FPG | Breakfast PPG2h | Lunch PPG2h | Supper PPG 2h | Morning | Noon | Evening | 22:00 | |
| Jan 10 | 12.3 | 11.6 | 14.3 | 32 | 32 | 36 | 38 | |
| Jan 11 | 10.5 | 8.9 | 12.5 | 32 | 32 | 36 | 38 | |
| Jan 12 | 9.9 | 7.4 | 8.4 | 16 | 24 | 28 | 30 | |
| Jan 13 | 5.7 | 6.0 | 8.4 | 16 | 16 | 22 | 20 | |
| Jan 14 | 5.8 | 4.4 | 9.1 | 16 | 16 | 20 | 14 | |
| Jan 15 | 6.0 | 6.7 | 10.3 | 0 | 0 | 0 | 14 | |
| Jan 16 | 6.7 | 8.9 | 10.6 | 0 | 0 | 0 | 14 | |
| Jan 17 | 7.8 | 8.7 | 10.9 | 0 | 0 | 0 | 14 | |
| Jan 18 | 6.0 | 7.7 | 9.8 | 0 | 0 | 0 | 16 | |
| Jan 19 | 6.5 | 8.0 | 6.0 | 7.8 | 0 | 20 | 20 | 16 |
| Jan 20 | 5.4 | 6.6 | 6.4 | 12.2 | 0 | 16 | 20 | 16 |
| Jan 21 | 6.3 | 7.5 | 0 | 16 | 20 | 16 | ||
| Jan 22 | 7.6 | 6.1 | 12.5 | 10.9 | 0 | 16 | 20 | 14 |
| Jan 23 | 6.2 | 5.1 | 9.9 | 9.9 | 0 | 16 | 20 | 14 |
| Jan 24 | 5.2 | 7.9 | 10.7 | 9.9 | 0 | 16 | 20 | 14 |
| Jan 25 | 5.8 | 8.1 | 7.6 | 14.2 | 0 | 16 | 20 | 14 |
| Jan 26 | 8.2 | 6.0 | 7.3 | 8.5 | 0 | 16 | 16 | 14 |
| Jan 27 | 6.4 | 5.1 | 13.2 | 0 | 16 | 0 | 14 | |
| Jan 28 | 7.6 | 5.6 | 10.7 | 0 | 16 | 16 | 14 | |
| Jan 29 | 7.4 | 7.6 | 9.0 | 0 | 16 | 16 | 14 | |
| Jan 30 | 7.6 | 9.6 | 12.5 | 0 | 10 | 16 | 16 | |
| Jan 31 | 7.4 | 7.9 | 11.9 | 0 | 10 | 16 | 16 | |
| Feb 1 | 7.1 | 9.5 | 10.9 | 0 | 10 | 16 | 16 | |
| Feb 2 | 7.9 | 9 | 11.7 | 0 | 10 | 16 | 16 | |
| Feb 3 | 7.1 | 12.4 | 11.7 | 0 | 10 | 20 | 16 | |
| Feb 4 | 8.1 | 7.1 | 12.4 | 0 | 10 | 16 | 16 | |
| Feb 5 | 6.8 | 9.1 | 11.9 | 0 | 10 | 16 | 16 | |
According to the international diabetes federation statistics in 2021, China has become the country with the most significant number of diabetic patients in the world, with about 141 million people suffering from the disease[6]. Diabetic nephropathy (DN) is one of the common microvascular complications of T2DM. Statistics indicate that about 40% of diabetic patients will develop DN, and more than half of them will enter the stage of ESRD, requiring renal replacement therapy. Patients with ESRD exhibit increased glucose fragility, and hemodialysis itself interferes with glucose homeostasis, complicating glycemic control. Patients are prone to hypoglycemia during dialysis, and post-dialysis hyperglycemia is also characteristic of hemodialysis patients with DN. Typically, patients with DN have elevated blood glucose levels due to the need for hyperosmolar dialysis fluid for ultrafiltration. In clinical practice, high-dose insulin therapy is often employed, but its glucose-lowering effect is limited and increases the risk of hypoglycemia. Therefore, effectively managing hyperglycemia and avoiding hypoglycemia in patients with diabetic complications during CAPD has become a significant concern for endocrinologists and nephrologists.
Dorzagliatin is the first and only glucokinase activator to have reached the clinical stage. Preclinical studies have confirmed that Dorzagliatin restores the body's correct glucose perception by activating glucokinase in the pancreas and liver, increasing insulin secretion from pancreatic β-cells in a glucose-dependent manner, promoting hepatic glycogen synthesis, and enhancing intestinal secretion of GLP-1, thereby reshaping blood glucose homeostasis[7]. Dorzagliatin has undergone several clinical studies in Chinese patients with T2DM, with results indicating precise glycemic control efficacy. It can lower glycated hemoglobin by more than 1% compared to the baseline, whether used alone or in combination with metformin and has a favorable safety profile and a potential role in protecting β-cell function[2-4]. Additionally, Dorzagliatin has a unique pharmacokinetic profile, has shown dose-proportional pharmacokinetics with a half-life of 4.48 to 7.51 hours and exhibits dose-dependent glucose-lowering effects, primarily metabolized by the liver, with less than 10% excreted by the kidneys[8]. Studies of Dorzagliatin in healthy volunteers and patients with ESRD have shown that its elimination half-life, volume of distribution, and systemic clearance are similar between the two groups, making it suitable for patients with CKD stages 1-5[5]. Dorzagliatin has a plasma protein binding rate greater than 90%[8], and it is hypothesized that it may not be easily cleared by peritoneal dialysis.
This case is the first known report of Dorzagliatin use in a patient with T2DM undergoing CAPD treatment. The patient had a 30-year history of diabetes, with relatively stable blood sugar levels without medication before starting peritoneal dialysis. However, after initiating peritoneal dialysis, blood glucose levels gradually increased, and even a daily insulin dose of 150 units failed to control blood sugar effectively. With limited options for oral hypoglycemic drugs and considering the characteristics of Dorzagliatin, it was analyzed that it might be effective for blood sugar control in this patient. After obtaining consent from the patient and her family, the patient was administered Dorzagliatin 75 mg twice daily, and significant blood sugar improvement was observed on the same day. The application of Dorzagliatin led to a rapid and steady decrease in the patient's hyperglycemia, with a marked reduction in insulin dosage. At the one-month follow-up visit, the patient's daily insulin dosage was 40 units. This suggests that Dorzagliatin may have improved the patient's islet function or slowed insulin resistance, potentially through mechanisms such as increasing the number of pancreatic β-cells. While these proposed mechanisms are insightful, they remain speculative and warrant further investigation. Evidence from existing literature suggests that glucokinase activation may enhance the sensitivity of β-cells to glucose, leading to improved insulin secretion in response to hyperglycemia. Additionally, the modulation of GLP-1 secretion could play a role in appetite regulation and weight management, which are critical factors in diabetes control. Future studies should aim to elucidate these mechanisms through controlled trials and biomarker analysis to provide a more robust understanding of Dorzagliatin's action. Furthermore, the potential for Dorzagliatin to mitigate insulin resistance could be explored through metabolic profiling and insulin sensitivity assays in larger cohorts.
No severe hypoglycemia occurred during treatment, indicating that Dorzagliatin is relatively safe for use in diabetic patients on peritoneal dialysis. Additionally, in previous studies, slight elevations in lipid levels and transaminases were observed as potential adverse reactions. However, in this patient, we did not observe these effects, and we maintained a specific focus on monitoring these parameters throughout the treatment. The absence of severe adverse reactions further supports the safety profile of Dorzagliatin in this patient.
This case report is limited by its single-patient focus and the short follow-up duration. While the initial results are promising, they may not be generalizable to a broader population of dialysis patients with T2DM. Additionally, long-term effects and safety of Dorzagliatin in this specific patient population remain unknown. Further studies are needed to evaluate the efficacy and safety of Dorzagliatin in larger cohorts of dialysis patients with T2DM, including longer follow-up periods to assess sustained glycemic control and potential adverse effects. Investigating the drug's impact on quality of life and overall health outcomes in this population would also be beneficial.
Dorzagliatin may offer a novel therapeutic strategy for T2DM patients on peritoneal dialysis who struggle with glycemic control despite high insulin doses. The case presented here suggests that Dorzagliatin can improve glycemic parameters and reduce insulin requirements, highlighting its potential as a safe and effective treatment option. Further studies are needed to evaluate the efficacy and safety of Dorzagliatin in larger cohorts of dialysis patients with T2DM.
We sincerely thank the endocrinology medical staff for their work.
| 1. | Alalawi F, Bashier A. Management of diabetes mellitus in dialysis patients: Obstacles and challenges. Diabetes Metab Syndr. 2021;15:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Zhu D, Gan S, Liu Y, Ma J, Dong X, Song W, Zeng J, Wang G, Zhao W, Zhang Q, Li Y, Fang H, Lv X, Shi Y, Tian H, Ji L, Gao X, Zhang L, Bao Y, Lei M, Li L, Zeng L, Li X, Hou X, Zhao Y, Hu T, Ge X, Zhao G, Li Y, Zhang Y, Chen L. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: a dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018;6:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Zhu D, Li X, Ma J, Zeng J, Gan S, Dong X, Yang J, Lin X, Cai H, Song W, Li X, Zhang K, Zhang Q, Lu Y, Bu R, Shao H, Wang G, Yuan G, Ran X, Liao L, Zhao W, Li P, Sun L, Shi L, Jiang Z, Xue Y, Jiang H, Li Q, Li Z, Fu M, Liang Z, Guo L, Liu M, Xu C, Li W, Yu X, Qin G, Yang Z, Su B, Zeng L, Geng H, Shi Y, Zhao Y, Zhang Y, Yang W, Chen L. Dorzagliatin in drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2022;28:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Yang W, Zhu D, Gan S, Dong X, Su J, Li W, Jiang H, Zhao W, Yao M, Song W, Lu Y, Zhang X, Li H, Wang G, Qiu W, Yuan G, Ma J, Li W, Li Z, Wang X, Zeng J, Yang Z, Liu J, Liang Y, Lu S, Zhang H, Liu H, Liu P, Fan K, Jiang X, Li Y, Su Q, Ning T, Tan H, An Z, Jiang Z, Liu L, Zhou Z, Zhang Q, Li X, Shan Z, Xue Y, Mao H, Shi L, Ye S, Zhang X, Sun J, Li P, Yang T, Li F, Lin J, Zhang Z, Zhao Y, Li R, Guo X, Yao Q, Lu W, Qu S, Li H, Tan L, Wang W, Yao Y, Chen D, Li Y, Gao J, Hu W, Fei X, Wu T, Dong S, Jin W, Li C, Zhao D, Feng B, Zhao Y, Zhang Y, Li X, Chen L. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2022;28:974-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Miao J, Fu P, Ren S, Hu C, Wang Y, Jiao C, Li P, Zhao Y, Tang C, Qian Y, Yang R, Dong Y, Rong J, Wang Y, Jin X, Sun Y, Chen L. Effect of renal impairment on the pharmacokinetics and safety of dorzagliatin, a novel dual-acting glucokinase activator. Clin Transl Sci. 2022;15:548-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Ling J, Ng JKC, Chan JCN, Chow E. Use of Continuous Glucose Monitoring in the Assessment and Management of Patients With Diabetes and Chronic Kidney Disease. Front Endocrinol (Lausanne). 2022;13:869899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Wang P, Liu H, Chen L, Duan Y, Chen Q, Xi S. Effects of a Novel Glucokinase Activator, HMS5552, on Glucose Metabolism in a Rat Model of Type 2 Diabetes Mellitus. J Diabetes Res. 2017;2017:5812607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Xu H, Sheng L, Chen W, Yuan F, Yang M, Li H, Li X, Choi J, Zhao G, Hu T, Li Y, Zhang Y, Chen L. Safety, tolerability, pharmacokinetics, and pharmacodynamics of novel glucokinase activator HMS5552: results from a first-in-human single ascending dose study. Drug Des Devel Ther. 2016;10:1619-1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/