Published online Jan 15, 2025. doi: 10.4239/wjd.v16.i1.98322
Revised: August 26, 2024
Accepted: October 30, 2024
Published online: January 15, 2025
Processing time: 156 Days and 14.1 Hours
Inadequate glycemic control in patients with type 2 diabetes (T2DM) is a major public health problem and a significant risk factor for the progression of diabetic complications.
To evaluate the effects of intensive and supportive glycemic management stra
This prospective observational study investigated glycemic control in patients with poorly controlled T2DM over 12 months. Participants were categorized into four groups based on prior glycemic history: Newly diagnosed, previously well controlled with recent worsening, previously off-target but now worsening, and HbA1c consistently above 10%. HbA1c levels were monitored quarterly, and pa
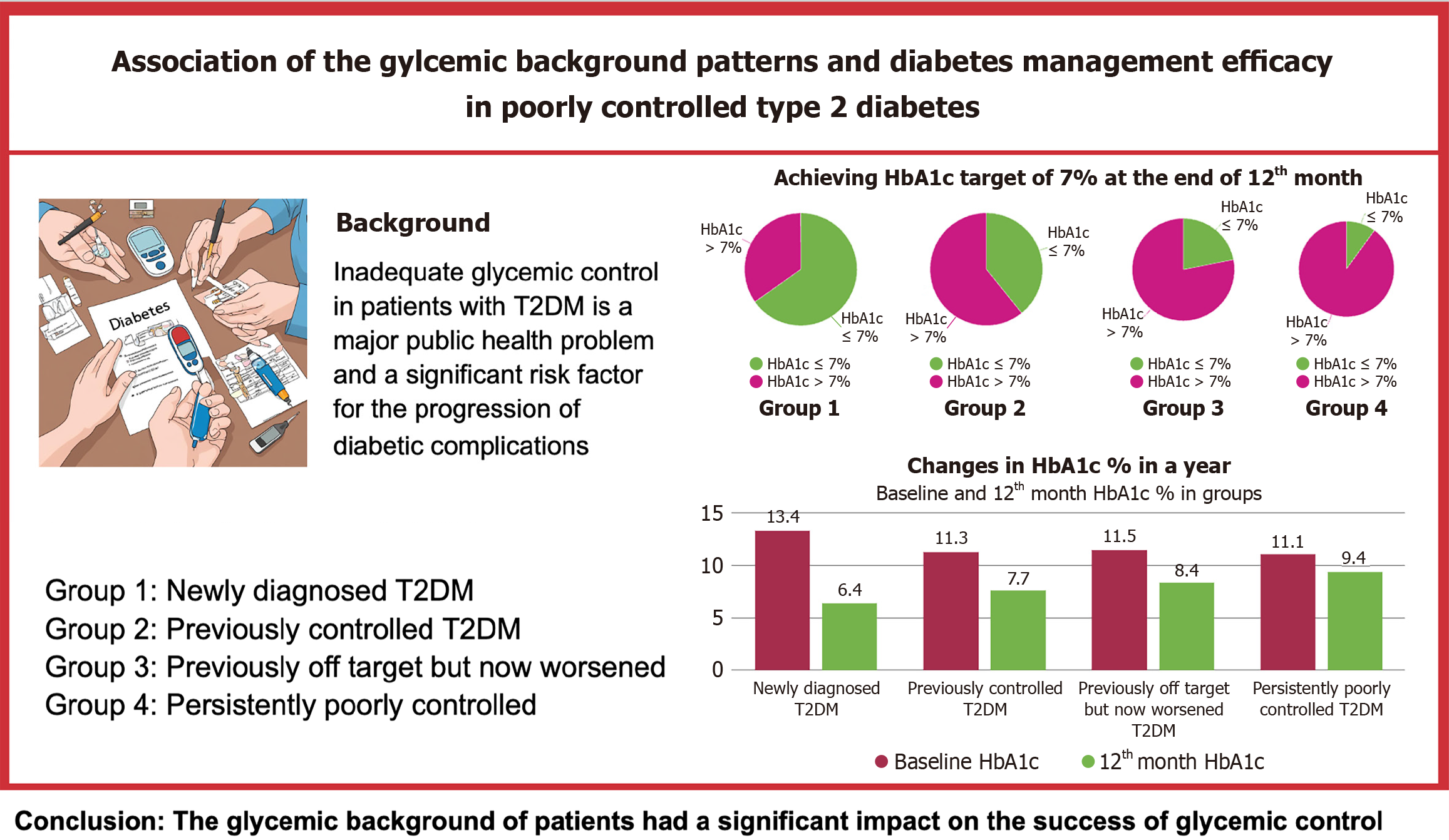
The study showed significant improvements in HbA1c levels in all participants. The most significant improvement was observed in individuals newly diagnosed with diabetes: 65% achieved an HbA1c target of ≤ 7%. The results varied between participants with different glycemic control histories, followed by decreasing success rates: 39% in participants with previously good glycemic control, 21% in participants whose glycemic control had deteriorated compared to before, and only 10% in participants with persistently poor control, with mean HbA1c levels of 6.3%, 7.7%, 8.2%, and 9.7%, respectively. After one year, 65.2% of the “newly diagnosed patients”, 39.3% in the “previously controlled group”, 21.9% in the “previously off-target but now worsened'” group and 10% in the “poorly controlled from the start” group had achieved HbA1c levels of 7 and below.
In poorly controlled diabetes, the rate at which treatment goals are achieved is associated with the glycemic background characteristics, emphasizing the need for tailored strategies. Therefore, different and comprehensive treatment approaches are needed for patients with persistent uncontrolled diabetes.
Core Tip: Twelve months of enhanced monitoring and facilitated access to hospital visits in patients with poorly controlled type 2 diabetes resulted in substantial improvements in glycemic control. However, the glycemic background of patients had a significant impact on the success of glycemic control. The group with newly diagnosed diabetes showed the most favorable results (mean glycated hemoglobin at 12 months, 6.3%), whereas the group with persistently poorly controlled diabetes exhibited the worst results (mean glycated hemoglobin at 12 months, 9.7%), suggesting that new approaches are needed to improve treatment efficacy.
- Citation: Erbakan AN, Arslan Bahadır M, Kaya FN, Güleç B, Vural Keskinler M, Aktemur Çelik Ü, Faydalıel Ö, Mesçi B, Oğuz A. Association of the glycemic background patterns and the diabetes management efficacy in poorly controlled type 2 diabetes. World J Diabetes 2025; 16(1): 98322
- URL: https://www.wjgnet.com/1948-9358/full/v16/i1/98322.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i1.98322
The global incidence of diabetes mellitus is increasing, and the number of people affected by this disease is predicted to increase to over 643 million by 2030. In 2021, diabetes mellitus, particularly type 2 diabetes mellitus (T2DM), and its associated complications led to 6.7 million deaths worldwide[1]. Inadequate glycemic control in patients with T2DM is a major public health problem and a significant risk factor for the progression of diabetic complications[2,3]. Glycemic control remains the most important therapeutic goal for preventing organ dysfunction and other diabetes-related complications[4].
Although there have been significant developments in diabetes treatment in recent decades, such as the development of new medications, various diet and exercise recommendations to control blood glucose levels, and new technologies, less than 50% of people with type 2 diabetes have been reported to achieve a target glycated hemoglobin (HbA1c) level of < 7%[5,6]. Although the success rates reported in clinical trials are likely to be higher, real-life data appear to be different[7-9]. There are a variety of reasons for this, including medication adherence and persistence, physician and patient inertia, prejudice against lifestyle changes (e.g., feeling deprived by the idea of a change in their diet), easy return to unhealthy habits, sleep disturbances, financial conditions that interfere with both healthy eating and medication use, and reaction to the burden of the disease[10].
The definition of “poor glycemic control” is not clearly defined and includes patients with HbA1c levels that exceed the target value without specific upper limits or gradations. Consequently, it is difficult to determine the prevalence of poorly controlled diabetes and its associated factors. This also leads to a heterogeneous group of patients with varying glycemic values and probably different characteristics that influence diabetes management. Even in individuals with “persistently poorly controlled diabetes mellitus”, which characterizes those who have consistently high HbA1c levels despite treatment, the threshold varies between 8.5% and 9.5%. More than 10% of individuals diagnosed with diabetes have an HbA1c value > 10%[11]. This variability indicates the need for personalized treatment plans that consider individual patient factors rather than strictly adhering to uniform HbA1c targets and highlights the limitations of using a single glycemic target for all populations[12]. This discrepancy in achieving glycemic control is a notable and frustrating observation, even in specialized diabetes units. Although some patients achieve glycemic targets effortlessly, others consistently struggle to achieve or maintain these targets despite being treated in the same clinical setting. Early identification of patients who would benefit from additional or different approaches would allow for more effective and timely intervention, potentially leading to improved glycemic control in a greater number of individuals.
The United Kingdom Prospective Diabetes Study showed that every reduction in HbA1c level leads to a 37% reduction in microvascular complications and a 21% reduction in the risk of any endpoint or death associated with diabetes[13]. Patients with persistently poorly controlled diabetes mellitus contribute to the growing burden of diabetes despite receiving clinical-based diabetes care, as they are associated with higher healthcare utilization and higher costs owing to inadequate glycemic control[2]. A study evaluating the impact of lowering HbA1c levels on healthcare costs in patients with uncontrolled diabetes (HbA1c ≥ 9%) found that the group with lower HbA1c levels had an average annual reduction in healthcare costs of 24% in the first year of observation and 17% in the following year[14]. These results highlight the potential financial benefits of lowering HbA1c levels in patients with uncontrolled diabetes.
Therapeutic inertia is defined as the failure to advance or de-intensify therapy when appropriate[15]. It results from a combination of factors involving the patient, physician, and healthcare system[15-17]. Studies have shown that less than 50% of people with type 2 diabetes and treatment failure receive intensification within one year[17-20]. This inertia leads to underutilization of effective therapies and contributes to poor glycemic control and suboptimal management of cardiovascular risk factors in patients with diabetes. The INTEGRA study revealed promising results in terms of the effectiveness of dedicated visits in reducing HbA1c levels, particularly in individuals with initially high HbA1c levels (HbA1c > 9%), reflecting the effect of reducing therapeutic inertia[17]. This is particularly crucial for patients with persistently poorly controlled type 2 diabetes, for whom achieving positive outcomes is of paramount importance. The challenge of managing this particular group of patients and the question of whether their uncontrolled diabetes was truly due to clinical inertia or inherent heterogeneity within the group led us to conduct this study.
In this study, we aimed to investigate the effects of close and intensive monitoring of different glycemic trajectories on lowering HbA1c levels and reaching target levels in individuals with a baseline HbA1c of 10% or more. In addition, we examined the effects of education level, depression, dietary awareness, exercise, and weight change on glycemic control.
In this prospective observational study, patients with an HbA1c level of 10% or higher who underwent initial evaluation in internal medicine diabetes outpatient clinics between August 1st, 2021, and December 31th, 2021, were consecutively invited to participate in the study. Power analysis was performed using the GPower software (version 3.1) to determine the necessary sample size. Based on an assumed medium effect size (Cohen’s d = 0.5), power of 0.80, and alpha level of 0.05, the analysis indicated that at least 128 participants were required to achieve statistical significance. In total, 266 participants were included in this study to account for potential dropouts and missing data. The study was conducted in accordance with the declaration of Helsinki and good clinical practice guidelines. The protocol was approved by the Ethics Committee of the Istanbul Medeniyet University Goztepe Research and Training Hospital (No. 2021/0413). Clinical trial: No. NCT06385899.
Eligible participants were individuals aged ≥ 18 years with a documented diagnosis of type 2 diabetes and an HbA1c level of ≥ 10%. Key exclusion criteria were acute infection or inflammation lasting longer than one-week, acute metabolic decompensation, endocrine disease other than thyroid or active oncologic treatment, and inability to attend follow-up, such as living in another city. All eligible patients were invited to participate in the study and written informed consent was obtained from all patients who agreed. The participants were followed up to the 12th month of follow-up of the last patient.
The subject cohorts were stratified according to their HbA1c levels and glycemic control profiles. Group 1 included subjects who were newly diagnosed with type 2 diabetes within the previous month with no prior documented diabetic pathology or glucose-modulating pharmacotherapy and were hereby categorized as “newly diagnosed patients”.
The remaining participants had an established diagnosis of type 2 diabetes with at least three recorded HbA1c values, which facilitated the assessment of their glycemic background. There had to be a minimum interval of three months between serial HbA1c assessments, with the most recent measurement usually taken six months prior to participation in the study.
Participants in group 2 had previously demonstrated consistently good blood glucose levels, confirmed by an HbA1c threshold of 7% or less at least six months before enrollment. However, as their current HbA1c level exceeded the 10%, these individuals were referred to as the “previously controlled group”.
The participants in group 3 had HbA1c values that varied between 7% and 9.5% on several occasions. The increase in their most recent HbA1c levels beyond the 10% limit indicated a progressive deterioration in their blood glucose management, so they were labeled as the group that had “previously off target but now worsened”.
The final group, group 4, consisted of participants whose previous HbA1c levels were invariably above 10%, and were consequently categorized as “poorly controlled from the start”.
Both group 1 and group 2 were categorized as group A for further analysis, as they had a history of good metabolic control, that is, no diabetes or previous HbA1c levels of 7% or less. Group 3 and group 4, on the other hand, were classified as group B as they had poor metabolic control for more than one year prior to enrollment and were considered “persistently poorly controlled”.
The participants’ characteristics were assessed at baseline. Data were collected through a review of medical records and interviews with patients. Participants’ diabetes type, previous laboratory analyses, medications, dose of medications, and regularity of intake were further investigated as previously described[21]. The participants’ previous HbA1c indices were used to form the patient cohorts. All participants completed three different questionnaires, which are listed below, and then underwent predetermined laboratory tests including C-peptide and HbA1c levels. Participation in the study was initiated after patients completed the questionnaire. All patients were examined by a qualified diabetes nurse educator and dietitian at least once, with follow-up appointments scheduled as necessary according to the guidance of the medical professional or at the request of the patients. All patients received a routine 10-minute diabetes education consultation from both the qualified diabetes nurse educator and dietitian at their first visit. These consultations were not standardized and were conducted independently of the study protocol to replicate real-life clinical conditions. The content of subsequent appointments was determined based on individual needs, with a duration not exceeding the routine practice time of 10 minutes. Patient visits to the diabetes clinic were carried out in collaboration with their respective physicians who specialized in diabetes care. During these visits, patients had the opportunity to discuss their concerns and receive personalized guidance on managing their diabetes. Additionally, participants were encouraged to maintain regular contact with the medical team through phone consultations. This ensured that the patients had easy access to the necessary diagnostic tests and medications, as they were affiliated with a social health institution. This study followed the current diabetes guidelines for diagnostic assessments and treatment interventions. The key focus of this study was to improve patient access to healthcare professionals and enhance their treatment plans.
Participants were evaluated at intervals of at least three months, with the last visit taking place at the end of the twelfth month after enrollment. At the end of the study, the participants were also asked whether they had taken their medi
The primary endpoint was the effect of glycemic background on the change in mean HbA1c from baseline to the end of the 12-month period and the percentage of patients achieving a target HbA1c level of 7% or less in predefined groups with different glycemic backgrounds under close and intensive monitoring.
For the secondary endpoints, this study aimed to investigate how baseline characteristics, such as education, mindful eating, depression, daily physical activity, and changes in weight and BMI influenced changes in HbA1c levels in patients with different glycemic backgrounds over a 12-month period. The analysis predicted the participants’ ability to achieve their HbA1c goals by examining the influence of these factors.
Education, mindful eating, depression, and daily physical activity were selected as secondary measures because of their impact on the adaptation to healthy lifestyle choices. Education is an important social factor that affects health status and facilitates adaptation to changing health challenges. Establishing healthy behaviors, such as improving nutrition, weight management, and integrating regular exercise into lifestyle, is now recognized as a fundamental step in the management of chronic diseases, especially diabetes. Mindful eating entails making conscious food choices to maintain a healthy lifestyle, while monitoring daily physical activity is a means of assessing exercise behavior. We employed the validated Turkish mindful eating questionnaire, which includes 30 items graded on a scale from 1 (never) to 5 (always), assessing seven concepts of eating behavior and mindfulness, with higher scores indicating higher levels of mindful eating[22]. We used the total score for the analysis. Depressive symptoms are associated with challenges in regulating blood glucose levels and reducing adherence to dietary and exercise guidelines. In our study, we used the Turkish version of the beck depression inventory, a 21-question self-assessment tool designed to measure the severity of depression. Depression severity was classified based on the following scores: No depression (0-9 points), mild (10-16 points), moderate (17-24 points), and severe (> 25 points)[23]. The general practice physical activity questionnaire is a brief survey that assesses daily physical activities such as exercise, cycling, walking, housework/childcare, and gardening. It also assesses the level of physical activity in one’s occupation and the walking speed. Based on the results, individuals are categorized as “active”, “inactive”, “moderately inactive” or “moderately active” based on their physical activity index[24].
Normally distributed numerical variables are presented as mean ± SD, while non-normally distributed numerical variables are reported as median and interquartile range (IQR). The median value represents the midpoint of the data and the IQR indicates the range within which the middle 50% of the values fall, highlighting a variable. Categorical variables are presented using frequency and percentage measures. The normality of the numeric variables was assessed using the Kolmogorov-Smirnov test. Group differences in categorical variables were evaluated using the Pearson χ2 test for expected frequencies exceeding 5 in 2 × 2 tables, and Fisher’s exact test for frequencies below 5. When comparing two independent groups, the Student’s t-test was applied to normally distributed variables, and the Mann-Whitney U test was used for non-normally distributed variables. Analysis of variance was used to compare the means of more than two independent groups for normally distributed numerical variables, whereas the Kruskal-Wallis H test was used when the variables did not follow a normal distribution. Pairwise comparisons of numerical variables with normal distributions were conducted using the Tukey’s test. For variables that did not follow a normal distribution, P values were adjusted using the Bonferroni correction. For longitudinal analyses involving measurements from the same patients at different time points, paired t-tests were used for normally distributed numeric variables, Wilcoxon signed-rank tests were used for non-normally distributed data, and McNemar’s test was used for categorical variables. The magnitude of the effect sizes for differences between groups was assessed using Cohen’s d test. Cohen’s d quantifies the size of the difference between two groups in standard deviation units, where values of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effects, respectively. Binary logistic regression was used to explore the effects of the independent variables on binary dependent variables. The significance level was set at P < 0.05. Statistical analyses were performed using the statistical product and service solutions-20 and R programming languages.
The baseline demographic and clinical profiles of participants are shown in Table 1. Group A (n = 51), comprising group 1 and group 2, was characterized by previous normal or good metabolic control, and group B (n = 81), comprising group 3 and group 4, which had poor metabolic control, were compared. There was no statistically significant difference in age between group A (54.4 ± 10.3 years) and group B (56.7 ± 9.6 years). Among 132 patients, 58.3% were male. In group A, 27.5% (n = 14) were female, while 72.5% (n = 37) were male. Group B exhibited a higher proportion of females 50.6% (n = 41) than males 49.4% (n = 40) of males (P = 0.009). At baseline, group B exhibited statistically higher beck depression scores (median: 12, IQR = 12) than group A (7.9 ± 5.9) (P < 0.001). Group A displayed significantly higher glucose levels (283.9 ± 75.9 mg/dL) than group B (238.7 ± 77.4 mg/dL) (P = 0.007).
| Group A (1 + 2) (n = 51) | Group B (2 + 3) (n = 81) | All patients (n = 132) | P value | |
| Age (years) | 54.4 ± 10.3 | 56.7 ± 9.6 | 55.8 ± 9.8 | 0.068 |
| Gender | ||||
| Female | 14 (27.5) | 41 (50.6) | 55 (41.7) | 0.009a |
| Male | 37 (72.5) | 40 (49.4) | 77 (58.3) | |
| Education | 0.141 | |||
| No literacy | 2 (3.9) | 12 (14.8) | 14 (10.6) | |
| Primary school | 27 (52.9) | 43 (53.1) | 70 (53.0) | |
| Middle school | 9 (17.6) | 6 (7.4) | 15 (11.4) | |
| High school | 9 (17.6) | 16 (19.8) | 25 (18.9) | |
| Higher education | 4 (7.8) | 4 (4.9) | 8 (6.1) | |
| Physical activity | 0.088 | |||
| Inactive | 24 (47.1) | 48 (59.3) | 72 (54.5) | |
| Moderately inactive | 18 (35.3) | 19 (23.5) | 37 (28) | |
| Moderately active | 9 (17.6) | 9 (11.1) | 18 (13.6) | |
| Active | 0 (0) | 5 (6.2) | 5 (3.8) | |
| Diabetes course | ||||
| Newly diagnosed T2DM | 23 (45.1) | 0 (0) | 23 (17.4) | |
| Patients with previously controlled T2DM | 28 (54.9) | 0 (0) | 28 (21.2) | |
| T2DM patients whose HBA1c were previously not on target but now worsened | 0 (0) | 32 (39.5) | 32 (24.2) | |
| T2DM patients whose HbA1c were ≥ 10% for a long time | 0 (0) | 49 (60.5) | 49 (37.2) | |
| Weight (kg) | 83 (21) | 81 ± 14 | 81 (19) | 0.350 |
| BMI (kg/m2) | 29.4 ± 3.5 | 29.5 ± 4.8 | 29.5 ± 4.3 | 0.821 |
| Waist circumference (cm) | 102.1 ± 12.8 | 101.8 ± 14.3 | 101.9 ± 13.7 | 0.917 |
| Duration of diabetes (year) | 4 (12) | 10 (8) | 10 (10) | 0.000a |
| CKD | 4 (8) | 4 (5) | 8 (6) | 0.710 |
| Mindful eating | 3.3 ± 0.6 | 3.4 ± 0.6 | 3.4 ± 0.6 | 0.324 |
| BDI | 7.9 ± 5.9 | 12 (12) | 10 (9) | 0.000a |
| Glucose (mg/dL) | 283.9 ± 75.9 | 238.7 ± 77.4 | 254.3 ± 79.4 | 0.007a |
| LDL-C (mg/dL) | 101 ± 41.9 | 106 (53) | 100 (53) | 0.989 |
| HDL-C (mg/dL) | 36 (22) | 43.6 ± 11.3 | 42 (18) | 0.048a |
| Triglyceride (mg/dL) | 203.7 ± 136.7 | 189.1 ± 27.12 | 195.3 ± 96.7 | 0.692 |
| Total cholesterol (mg/dL) | 177 (67) | 188 (49) | 184 (54) | 0.905 |
| C-peptide (ng/mL) | 3.1 ± 1.6 | 2.6 ± 1.2 | 2.5 (1.7) | 0.060 |
| Urea (mg/dL) | 27 (10) | 31.6 ± 7.9 | 31.2 ± 9.7 | 0.137 |
| Creatinine (mg/dL) | 0.8 (0.2) | 0.7 ± 0.2 | 0.8 (0.3) | 0.323 |
| HbA1c (%) | 12.9 ± 2.5 | 11 (2.9) | 11.2 (3.2) | 0.054 |
The baseline clinical outcomes of the four groups are shown in Table 2. Statistically significant differences were observed in glucose (P = 0.016) and HbA1c (P = 0.007) levels among the groups. Pairwise comparisons revealed that the mean glucose level in group 1 (294.1 ± 87.8 mg/dL) was significantly higher than in group 3 (229.1 ± 72.8 mg/dL). Additionally, the HbA1c level in Group 1 (13.6 ± 2%) was significantly higher than that in the other groups.
| Group 1 (n = 23) | Group 2 (n = 28) | Group 3 (n = 32) | Group 4 (n = 49) | P value | |
| Weight (kg) | 84.5 ± 20.1 | 86 ± 16.8 | 80.4 ± 13.7 | 81.6 ± 14.9 | 0.4471 |
| BMI (kg/m2) | 28.8 ± 4.3 | 31.1 ± 4.8 | 29.1 ± 5.1 | 30.3 ± 4.9 | 0.2741 |
| Waist circumference (cm) | 98 (16) | 102.1 ± 10.4 | 100 (11) | 104.3 ± 12.3 | 0.5402 |
| Glucose (mg/dL) | 294.1 ± 87.8 | 276.1 ± 76.3 | 229.1 ± 72.8 | 257.2 ± 76.7 | 0.016a,1 |
| LDL-C (mg/dL) | 110 ± 38.3 | 114.5 (77) | 106.7 ± 33.3 | 115.9 ± 38.3 | 0.8242 |
| HDL-C (mg/dL) | 42.6 (13.4) | 39.2 ± 12.8 | 43.2 ± 10.9 | 45.3 (12.5) | 0.1942 |
| Triglyceride (mg/dL) | 237 ± 122.2 | 244.5 (166.3) | 173 ± 126 | 268.8 ± 259.2 | 0.7222 |
| Total cholesterol (mg/dL) | 200.1 (43.2) | 208.2 ± 72.8 | 195.4 ± 46.6 | 208.7 ± 43 | 0.5932 |
| C-peptide (ng/mL) | 2.8 ± 1.1 | 3.2 ± 1.6 | 2.8 ± 1.4 | 2.4 ± 1 | 0.0681 |
| HbA1c (%) | 13.4 ± 1.9 | 11.3 (2.4) | 11.5 (2.9) | 11.1 (2.3) | 0.007a,2 |
The clinical outcomes at the end of the study were evaluated in group A and group B (Table 3). HbA1c levels were significantly lower in group A (median: 7%, IQR = 1.2%) than in group B (median: 8.5%, IQR = 2%) (P < 0.001). Similarly, group A had a significantly lower glucose level (median: 133.5 mg/dL, IQR = 36.8 mg/dL) than Group B (172.9 ± 57.5 mg/dL) (P < 0.001). Group A also showed a higher C-peptide level (median: 2.7 ng/mL, IQR = 2.0 ng/mL) compared to group B (median: 2.1 ng/mL, IQR = 1.9 ng/mL) (P = 0.012). Conversely, no statistically significant differences were observed in other metabolic parameters such as BMI, waist circumference, or lipid profiles [low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides, and total cholesterol] between the groups after the 12-month period.
| Group A (1 + 2) (n = 51) | Group B (2 + 3) (n = 81) | All patients (n = 132) | P value | |
| Weight (kg) | 84 ± 14 | 80 ± 14 | 82 ± 14 | 0.159 |
| BMI (kg/m2) | 30 ± 4.5 | 30.4 ± 5.2 | 30.2 ± 4.9 | 0.942 |
| Waist circumference (cm) | 102.5 ± 12.4 | 102.7 ± 14 | 102 (16) | 0.741 |
| Glucose (mg/dL) | 133.5 (36.8) | 172.9 ± 57.5 | 145 (77) | 0.000a |
| LDL-C (mg/dL) | 86.1 ± 33.6 | 95.7 ± 36.8 | 91.9 ± 35.7 | 0.335 |
| HDL-C (mg/dL) | 46.1 ± 15.1 | 45 ± 9.5 | 45.4 ± 11.9 | 0.390 |
| Triglyceride (mg/dL) | 153 (123.8) | 155 ± 87 | 155 (100) | 0.566 |
| Total cholesterol (mg/dL) | 166.9 ± 39.7 | 173.2 ± 42.9 | 170.7 ± 41.5 | 0.382 |
| C-peptide (ng/mL) | 2.7 (2) | 2.1 (1.9) | 2.3 (1.8) | 0.012a |
| HbA1c (%) | 7 (1.2) | 8.5 (2) | 7.8 (2.1) | 0.000a |
The clinical outcomes after 12 months in the four groups are summarized in Table 4. Significant differences were noted in glucose (P < 0.001) and HbA1c levels (P < 0.001) among the groups. Pairwise comparisons revealed that the median glucose level in group 4 (199 mg/dL, IQR = 113 mg/dL) was significantly higher than that in the other groups (P < 0.05). Similarly, group 4 exhibited the highest mean HbA1c level (9.4% ± 2%), which was significantly greater than that in the other groups (P < 0.05). Additionally, the mean HbA1c level in group 1 (6.6% ± 0.9%) was significantly lower than that in group 3 (8.4% ± 1.7%) (P = 0.001).
| Group 1 (n = 23) | Group 2 (n = 28) | Group 3 (n = 32) | Group 4 (n = 49) | P value | |
| Weight (kg) | 80.8 ± 14.3 | 86.5 ± 13.8 | 80.9 ± 13.5 | 79.8 ± 14.3 | 0.2531 |
| BMI (kg/m2) | 28.3 ± 3.4 | 31 ± 4.9 | 29.3 ± 5.3 | 30.1 ± 4.7 | 0.2201 |
| Waist circumference (cm) | 101.9 ± 14.4 | 103.8 ± 10.9 | 100.7 ± 15 | 103 ± 14.2 | 0.8441 |
| Glucose (mg/dL) | 125 ± 32.2 | 154.7 ± 45.6 | 137.5 (59) | 199 (113) | 0.000a,2 |
| LDL-C (mg/dL) | 86.3 ± 31.2 | 93.1 ± 39.1 | 97.6 ± 36.7 | 95.8 ± 41.2 | 0.7241 |
| HDL-C (mg/dL) | 51 ± 26 | 45.6 ± 13.4 | 44.7 ± 11 | 45.7 ± 14.6 | 0.5091 |
| Triglyceride (mg/dL) | 158 ± 89.8 | 186.5 (108.5) | 149 (97) | 165 (105) | 0.1202 |
| Total cholesterol (mg/dL) | 164 ± 30.7 | 180 ± 51.7 | 178.1 ± 44.5 | 170 (63) | 0.5282 |
| C-peptide (ng/mL) | 2.4 (2.1) | 3.3 ± 1.4 | 2.5 ± 1.4 | 2.1 (1.1) | 0.0812 |
| HbA1c (%) | 6.6 ± 0.9 | 7.7 ± 1.3 | 8.4 ± 1.7 | 9.4 ± 2 | 0.000a,1 |
Changes in clinical outcomes over time for group A and group B and all patients are detailed in Table 5. In group A, significant and large reductions from baseline were evident in median glucose levels (baseline: 275 mg/dL; 12 months: 131 mg/dL; P < 0.001; Cohen’s d = 2.2) and median HbA1c levels (baseline: 12.1%; 12 months: 7.0%; P < 0.001; Cohen’s d = 3.2). Furthermore, significant and large reductions were observed in median LDL cholesterol (baseline: 111.5 mg/dL; 12 months: 85.5 mg/dL; P = 0.015; Cohen’s d = 0.6) and median total cholesterol (baseline: 198.0 mg/dL; 12 months: 173.0 mg/dL; P = 0.001; Cohen’s d = 0.6). Conversely, a moderate increase in median HDL cholesterol (baseline: 37.0 mg/dL; 12 months: 43.0 mg/dL; P < 0.001; Cohen’s d = 0.4) and a moderate reduction in median triglyceride levels (baseline: 202.0 mg/dL; 12 months: 164.0 mg/dL; P = 0.003; Cohen’s d = 0.4) were also observed. Group B demonstrated significant and substantial reductions in median HbA1c levels (baseline: 11.2%; 12 months: 8.7%; P < 0.001; Cohen’s d = 1.6), alongside modest reductions in median total cholesterol (baseline: 192.0 mg/dL; 12 months: 172.0 mg/dL; P < 0.001; Cohen’s d = 0.5) and median glucose levels (baseline: 242.5 mg/dL; 12 months: 170.0 mg/dL; P < 0.001; Cohen’s d = 0.6). Additionally, significant and moderate reductions were observed in median triglyceride levels (baseline: 181.0 mg/dL; 12 months: 155 mg/dL; P < 0.001; Cohen’s d = 0.3) and mean LDL-cholesterol (baseline: 112.4 mg/dL; 12 months: 96.5 mg/dL; P = 0.040; Cohen’s d = 0.4). No significant changes were detected in any of the other parameters. In all patients, significant and substantial reductions were noted in median glucose levels (baseline: 259.0 mg/dL; 12 months: 146 mg/dL; P < 0.001; Cohen’s d = 1.1) and median HbA1c levels (baseline: 11.7%; 12 months: 7.8%; P < 0.001; Cohen’s d = 2.1). Moreover, significant and moderate reductions were observed in median LDL-cholesterol (baseline: 107.5 mg/dL; 12 months: 91.5 mg/dL; P = 0.001; Cohen’s d = 0.5) and median total cholesterol (baseline: 194.5 mg/dL; 12 months: 172.0 mg/dL; P < 0.001; Cohen’s d = 0.5) levels, indicating notable improvements. Conversely, there was a moderate increase in median HDL-cholesterol (baseline: 41.0 mg/dL; 12 months: 43.0 mg/dL; P = 0.002; Cohen’s d = 0.2) and a moderate reduction in median triglyceride levels (baseline: 189.5 mg/dL; 12 months: 156.5 mg/dL; P < 0.001; Cohen’s d = 0.3).
| Group A differences | P value | Cohen’s d effect size | Group B difference | P value | Cohen’s d effect size | All patients difference | P value | Cohen’s d effect size | |
| Weight (kg) | -1.5 | 0.2822 | 0.1 | -0.9 | 0.3571 | 0.1 | -1.0 | 0.0642 | 0.1 |
| BMI (kg/m2) | -0.7 | 0.3292 | 0.1 | 0.0 | 0.9851 | 0.0 | -0.1 | 0.8281 | 0.0 |
| Waist circumference (cm) | 2.5 | 0.8372 | 0.1 | 0.5 | 0.5072 | 0.0 | 1.5 | 0.5272 | 0.0 |
| Glucose (mg/dL) | -144.0 | 0.000a,2 | 2.2 | -72.5 | 0.000a,2 | 0.6 | -113.0 | 0.000a,2 | 1.1 |
| LDL-C (mg/dL) | -26.0 | 0.015a,2 | 0.6 | -15.8 | 0.040a,1 | 0.4 | -16.0 | 0.001a,2 | 0.5 |
| HDL-C (mg/dL) | 6.0 | 0.000a,2 | 0.4 | 1.0 | 0.7332 | 0.1 | 2.0 | 0.002a,2 | 0.2 |
| Triglyceride (mg/dL) | -38.0 | 0.003a,2 | 0.4 | -26.0 | 0.000a,2 | 0.3 | -33.0 | 0.000a,2 | 0.3 |
| Total cholesterol (mg/dL) | -25.0 | 0.001a,2 | 0.6 | -20.0 | 0.000a,2 | 0.5 | -22.5 | 0.000a,2 | 0.5 |
| C-peptide (ng/mL) | 0.2 | 0.9502 | 0.1 | -0.1 | 0.2992 | 0.1 | -0.1 | 0.417 | 0.0 |
| HbA1c (%) | -5.1 | 0.000a,2 | 3.2 | -2.5 | 0.000a,2 | 1.6 | -3.9 | 0.000a,2 | 2.1 |
Table 6 presents a comprehensive overview of the clinical outcome changes observed over time in each group. In group 1, significant improvements were noted across several clinical outcomes after 12 months compared to baseline. There were marked large reductions in mean glucose levels (baseline: 294.1 mg/dL; 12 months: 125 mg/dL; P < 0.001; Cohen’s d = 2.6), median total cholesterol (baseline: 194 mg/dL; 12 months: 170 mg/dL; P = 0.002; Cohen’s d = 1.0), and mean HbA1c levels (baseline: 13.4%; 12 months: 6.6%; P < 0.001; Cohen’s d = 4.5). Additionally, there were large reductions in mean LDL cholesterol (baseline: 110 mg/dL; 12 months: 86.3 mg/dL; P = 0.029; Cohen’s d = 0.7) and mean triglyceride levels (baseline: 237 mg/dL; 12 months: 158 mg/dL; P < 0.001; Cohen’s d = 0.7), along with a moderate increase in median HDL cholesterol (baseline: 37 mg/dL; 12 months: 43 mg/dL; P = 0.026; Cohen’s d = 0.4). In group 2, significant and substantial decreases were observed in mean glucose levels (baseline: 276.1; 12 months: 154.7 mg/dL; P < 0.001; Cohen’s d = 1.9) and median HbA1c levels (baseline: 11.3%; 12 months: 7.5%; P < 0.001; Cohen’s d = 4.5). Moreover, there were moderate improvements in mean HDL cholesterol (baseline: 39.2 mg/dL; 12 months: 45.6 mg/dL; P < 0.001; Cohen’s d = 0.5) and moderate reduction in mean total cholesterol (baseline: 208.2 mg/dL; 12 months: 180 mg/dL; P = 0.041; Cohen’s d = 0.4). Group 3 demonstrated significant and substantial reductions in median glucose levels (baseline: 218 mg/dL; 12 months: 137.5 mg/dL; P < 0.001; Cohen’s d = 1.1) and median HbA1c levels (baseline: 11.6%; 12 months: 8%; P < 0.001; Cohen’s d = 2.2), as well as moderate reductions in mean total cholesterol (baseline: 195.4 mg/dL; 12 months: 178.1 mg/dL; P = 0.011; Cohen’s d = 0.4) and median triglyceride levels (baseline: 173 mg/dL; 12 months: 149 mg/dL; P = 0.006; Cohen’s d = 0.3). No significant changes were observed in other parameters in this group. Group 4 showed significant and substantial decreases in median HbA1c levels (baseline: 11.1%; 12 months: 9.4%; P < 0.001; Cohen’s d = 1.3) and large reductions in median total cholesterol (baseline: 196 mg/dL; 12 months: 170 mg/dL; P = 0.005; Cohen’s d = 0.6), median glucose levels (baseline: 246.5 mg/dL; 12 months: 199 mg/dL; P = 0.003; Cohen’s d = 0.5), and median triglyceride levels (baseline: 194 mg/dL; 12 months: 165 mg/dL; P = 0.006; Cohen’s d = 0.4). The other parameters remained constant.
| Group 1 differences | P value | Cohen’s d effect size | Group 2 differences | P value | Cohen’s d effect size | Group 3 differences | P value | Cohen’s d effect size | Group 4 differences | P value | Cohen’s d effect size | |
| Weight (kg) | -3.7 | 0.4761 | 0.2 | 0.5 | 0.2732 | 0.0 | 0.5 | 0.5622 | 0.0 | -1.8 | 0.1202 | 0.1 |
| BMI (kg/m2) | -0.5 | 0.4361 | 0.1 | -0.1 | 0.8621 | 0.0 | 0.2 | 0.5441 | 0.0 | -0.2 | 0.4691 | 0.0 |
| Waist circumference (cm) | 2.5 | 0.9842 | 0.0 | 1.7 | 0.9501 | 0.2 | -1.0 | 0.6652 | 0.2 | -1.3 | 0.6621 | 0.1 |
| Glucose (mg/dL) | -169.1 | 0.000a,1 | 2.6 | -121.4 | 0.000a,1 | 1.9 | -80.5 | 0.000 a,2 | 1.1 | -47.5 | 0.003a,2 | 0.5 |
| LDL-C (mg/dL) | -23.7 | 0.029a,1 | 0.7 | -28.5 | 0.0951 | 0.5 | -9.1 | 0.1351 | 0.3 | -20.1 | 0.1471 | 0.5 |
| HDL-C (mg/dL) | 6.0 | 0.026a,2 | 0.4 | 6.4 | 0.000a,1 | 0.5 | 1.5 | 0.3311 | 0.1 | -2.0 | 0.6592 | 0.0 |
| Triglyceride (mg/dL) | -79 | 0.000a,1 | 0.7 | -15.0 | 0.4382 | 0.2 | -24.0 | 0.006a,2 | 0.3 | -29.0 | 0.006a,2 | 0.4 |
| Total cholesterol (mg/dL) | -24. | 0.002a,2 | 1.0 | -28.2 | 0.041a,1 | 0.4 | -17.3 | 0.011a,1 | 0.4 | -26.0 | 0.005a,2 | 0.6 |
| C-peptide (ng/mL) | 0.0 | 0.7842 | 0.3 | 0.1 | 0.9271 | 0.1 | -0.3 | 0.1441 | 0.2 | -0.1 | 0.6922 | 0.0 |
| HbA1c (%) | -6.8 | 0.000a,1 | 4.5 | -3.8 | 0.000 a,2 | 2.6 | -3.6 | 0.000a,2 | 2.2 | -1.7 | 0.000a,2 | 1.3 |
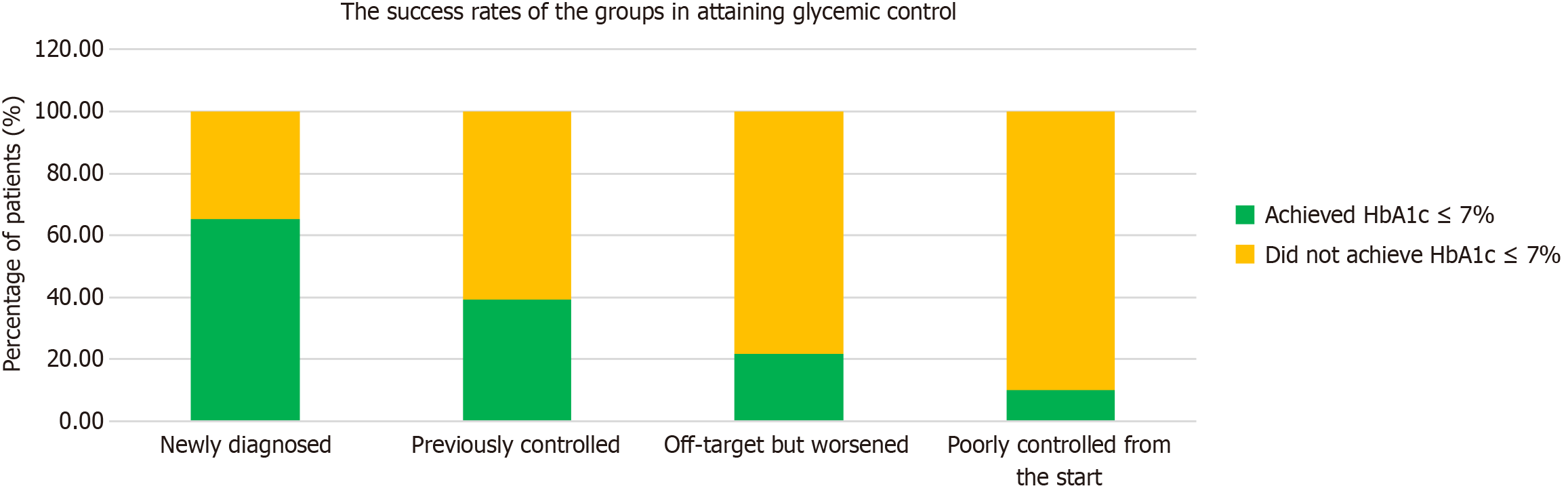
No statistically significant differences were observed in age, sex, education level, physical activity, weight, BMI, waist circumference, total mindful eating score, LDL cholesterol, HDL cholesterol, or triglyceride levels between the patients with HbA1c levels above and below 7% (Table 7). However, a significant difference was found in the distribution of diabetes background between the two groups (P < 0.001). After one year, 65.2% of the “newly diagnosed patients”, 39.3% in the “previously controlled group”, 21.9% in the “previously off-target but now worsened” group and 10% in the “poorly controlled from the start” group had achieved HbA1c levels of 7 and below (Figure 1). Most patients with HbA1c levels > 7% belonged to group 4, who had uncontrolled diabetes with long-standing HbA1c levels ≥ 10%. In contrast, most patients with HbA1c levels ≤ 7% were newly diagnosed with T2DM (39%). Furthermore, patients with HbA1c levels > 7% exhibited significantly higher Beck Depression Index scores and glucose, total cholesterol, C-peptide, and HbA1c levels than those with HbA1c levels < 7%.
| HbA1c ≤ 7% (n = 38) | HbA1c > 7% (n = 94) | P value | |
| Age (years) | 54.2 ± 10.2 | 55.8 ± 10.5 | 0.326 |
| Gender | 0.135 | ||
| Female | 12 (32) | 43 (46) | |
| Male | 26 (68) | 51 (54) | |
| Education | |||
| No literacy | 3 (7.9) | 11 (12) | 0.709 |
| Primary school | 18 (47) | 52 (55) | |
| Middle school | 6 (16) | 9 (9.6) | |
| High school | 8 (21) | 17 (18) | |
| Higher education | 3 (7.9) | 5 (5.3) | |
| Physical activity | |||
| Inactive | 20 (56) | 43 (49) | 0.554 |
| Moderately inactive | 8 (22) | 29 (33) | |
| Moderately active | 7 (19) | 11 (13) | |
| Active | 1 (2.8) | 4 (4.6) | |
| Diabetes course | |||
| Newly diagnosed T2DM | 15 (39) | 8 (8.5) | 0.000a |
| Patients with previously controlled T2DM | 11 (29) | 17 (18) | |
| T2DM patients whose HBA1c were previously not on target but now worsened | 7 (18) | 25 (27) | |
| T2DM patients whose HbA1c were ≥ 10% for a long time | 5 (13) | 44 (47) | |
| Weight (kg) | 83.9 ± 12.8 | 80.7 ± 14.5 | 0.215 |
| BMI (kg/m2) | 29.7 ± 3.8 | 29.8 ± 5.1 | 0.848 |
| Waist circumference (cm) | 102.8 ± 12.6 | 102.3 ± 14.2 | 0.787 |
| Duration of diabetes (year) | 5.4 ± 5.9 | 10.7 ± 6.9 | 0.000a |
| Mindful eating | 3.3 ± 0.6 | 3.4 ± 0.5 | 0.320 |
| BDI | 8.6 ± 5.5 | 12.6 ± 8.5 | 0.022a |
| Glucose (mg/dL) | 116.9 ± 20.8 | 195.5 ± 85.7 | 0.000a |
| LDL-C (mg/dL) | 82.1 ± 33.3 | 98.9 ± 38.6 | 0.074 |
| HDL-C (mg/dL) | 45.3 ± 22.0 | 46.8 ± 13.2 | 0.062 |
| Triglyceride (mg/dL) | 169.5 ± 92.9 | 198.0 ± 144.2 | 0.470 |
| Total cholesterol (mg/dL) | 159.4 ± 33.7 | 184.6 ± 50.6 | 0.029a |
| C-peptide (ng/mL) | 3.3 ± 1.6 | 2.5 ± 1.2 | 0.006a |
| HbA1c (%) | 6.4 ± 0.5 | 9.1 ± 1.7 | 0.000a |
Logistic regression analysis was performed to explore the factors influencing HbA1c levels categorized as ≤ 7% or > 7%. Neither baseline characteristic nor weight change had any relationship with HbA1c levels (categorized as ≤ 7% or > 7%).
The baseline demographic and clinical profiles of participants are shown in Table 1. Depression, mindful eating, and physical activity were the three baseline characteristics examined as secondary outcomes for their impact on glycemic improvement. At baseline, the groups differed only in their depression scores, as measured using the beck depression index. Group B had statistically higher beck depression scores (median: 12; IQR = 12) than Group A (7.9 ± 5.9) (P < 0.001). At the end of the study, none of the three parameters influenced the outcomes.
Of the 132 participants, only 67 had at least a 1 kg change in their weight. The weight changes in both ways resulted in a non-significant change from baseline. Changes in weight had no significant effect on HbA1c levels at the end of the study period.
Although participants were encouraged and recommended to consult a dietitian at each visit, at the end of the study, only 28 of the 132 participants reported that they had contacted a dietitian two or more times a year.
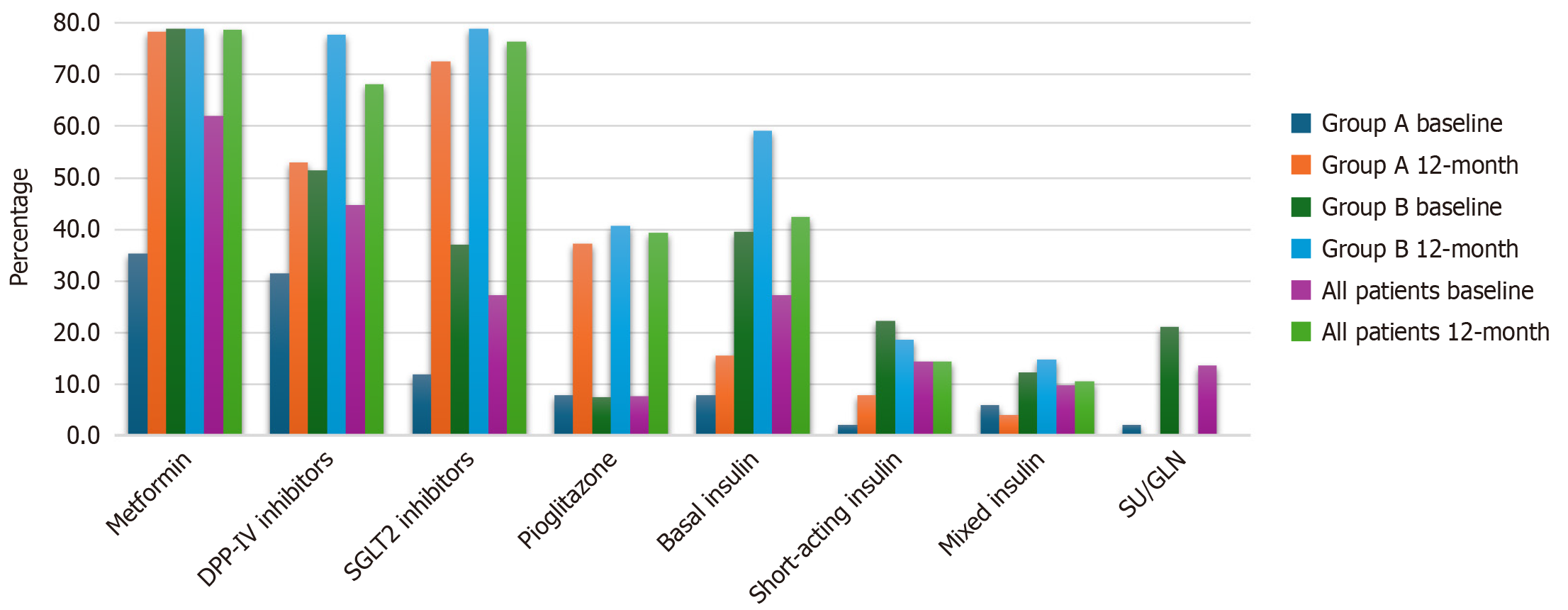
To reflect medication intensification, the use of different medications among the two groups (group A and group B) and the associated P values are presented in Table 8 and Figure 2. Nine participants were not taking any medications even though they had previously been diagnosed with type 2 diabetes. After 12 months, significant differences were found in the use of dipeptidyl peptidase IV (DPP-IV) inhibitors, basal insulin, and mixed insulin, with higher rates in group B than in group A (77.8% vs 52.9%, P = 0.003), 40.7% vs 7.8% (P < 0.001), and 14.8% vs 3.9% (P = 0.048), respectively. No significant differences were observed in the use of sodium-glucose transport protein 2 (SGLT2) inhibitors, pioglitazone, or basal-bolus insulin.
| Medication | Usage | Group A | Group B | All patients | P value |
| MTF | Yes | 40 (78.4) | 64 (79) | 104 (78.8) | 0.937 |
| No | 11 (21.6) | 17 (21) | 28 (21.2) | ||
| DPP-IV | Yes | 27 (52.9) | 63 (77.8) | 90 (68.2) | 0.003a |
| No | 24 (47.1) | 18 (22.2) | 42 (31.8) | ||
| SGLT2 | Yes | 37 (72.5) | 64 (79) | 101 (76.5) | 0.394 |
| No | 14 (27.5) | 17 (21) | 31 (23.5) | ||
| Pioglitazone | Yes | 19 (37.3) | 33 (40.7) | 52 (39.4) | 0.690 |
| No | 32 (62.7) | 48 (59.3) | 80 (60.6) | ||
| Basal insulin | Yes | 4 (7.8) | 33 (40.7) | 37 (28) | 0.000a |
| No | 47 (92.2) | 48 (59.3) | 95 (72) | ||
| Basal-bolus insulin | Yes | 4 (7.8) | 15 (18.5) | 19 (14.4) | 0.089 |
| No | 47 (92.2) | 66 (81.5) | 113 (85.6) | ||
| Mixed insulin | Yes | 2 (3.9) | 12 (14.8) | 14 (10.6) | 0.048a |
| No | 49 (96.1) | 69 (85.2) | 118 (89.4) |
After 12 months, SGLT2 inhibitors, metformin, and DPP-IV inhibitors were the most commonly used medications in group A and group B. The use of metformin was higher among patients with HbA1c levels ≤ 7% than among those with levels > 7% (Table 9). Conversely, the utilization of basal insulin, basal-bolus insulin, and mixed insulin was statistically higher in patients with HbA1c levels > 7% than in those with levels equal to or below 7%.
| Medication | Usage | HbA1c ≤ 7% | HbA1c > 7% | P value |
| MTF | Yes | 34 (89.5) | 70 (74.5) | 0.046a |
| No | 4 (10.5) | 24 (25.5) | ||
| DPP-IV | Yes | 24 (63.2) | 66 (70.2) | 0.431 |
| No | 14 (36.8) | 28 (29.8) | ||
| SGLT2 | Yes | 29 (76.3) | 72 (76.6) | 0.973 |
| No | 9 (23.7) | 22 (23.4) | ||
| Pioglitazone | Yes | 16 (42.1) | 36 (38.3) | 0.685 |
| No | 22 (57.9) | 58 (61.7) | ||
| Basal insulin | Yes | 4 (10.5) | 33 (35.1) | 0.004a |
| No | 34 (89.5) | 61 (64.9) | ||
| Basal-bolus insulin | Yes | 1 (2.6) | 18 (19.1) | 0.014a |
| No | 37 (97.4) | 76 (80.9) | ||
| Mixed insulin | Yes | 0 (0) | 14 (14.9) | 0.012a |
| No | 38 (100) | 80 (85.1) |
The use of SGLT2 inhibitors was similar in both groups. However, metformin and pioglitazone were used more frequently in patients with HbA1c levels ≤ 7%, whereas the use of DPP-IV inhibitors, basal insulin, basal-bolus insulin, and mixed insulin was higher in patients with HbA1c levels > 7%.
Twenty-seven participants were hospitalized at least once during the study.
In this study, 12 months of intensified monitoring and facilitated hospital visits in patients with poorly controlled type 2 diabetes, defined as an HbA1c level ≥ 10%, and with different glycemic backgrounds resulted in a significant improvement in glycemic control, although patients’ glycemic history had a significant impact on HbA1c reduction rates. Patients with newly diagnosed diabetes displayed the most favorable results, with an average HbA1c value of 6.3%. By contrast, participants with varying degrees of previous control had higher average HbA1c levels (7.7%, 8.2%, and 9.7%, respectively) (Figure 1).
The differences were even more pronounced in the percentage of patients who achieved glycemic control, defined as an HbA1c level ≤ 7%. The success rates differed significantly between the groups; while more than half of newly diagnosed patients reached their target, these rates gradually declined, with only 10% of participants with persistently poor control reaching their goal (Figures 1 and 3). This was despite the fact that all participants received similar treatments. Interestingly, similar success rates were also observed in the published data for the first three months, suggesting that the duration of treatment had a minimal impact on success rates[21].
This study differs from other studies in that it included patients with different glycemic backgrounds and poor diabetes control. Patients with newly diagnosed type 2 diabetes are expected to have a much better chance of controlling their blood glucose levels than are patients with established type 2 diabetes. A study conducted in China found that 68.5% of 5770 individuals with newly diagnosed type 2 diabetes achieved an HbA1c level of 7 or less after one year[25]. In another study, 48% of individuals diagnosed at the age of 21-44 and 61.9% at 45-65 years old achieved this value[26]. A multicenter study conducted in Germany and Austria followed 6355 newly diagnosed patients with type 2 diabetes over a 5-year period[27]. Similar to our findings, they reported that approximately 68% of their cohort achieved good glycemic control within the first year. However, even within this newly diagnosed population, they were able to identify four different trajectories of glycemic progression, with 6% having persistently poor control throughout the study[27]. This observation emphasizes that even from the time of diagnosis, diverse trajectories exist, and a subset of patients will face significant challenges in achieving sustained glycemic control. It is worth noting that these studies included individuals with newly diagnosed type 2 diabetes, that is, people with an HbA1c level < 7%. In our study, the probability of having an HbA1c value ≤ 7% after one year was 65% among newly diagnosed patients who had a strikingly high initial HbA1c value.
In a study by Tsai et al[28], mandatory monthly outpatient clinic visits through hospital system notifications improved therapeutic inertia in patients with poorly controlled type 2 diabetes (HbA1c ≥ 9%), particularly those with worsening control, and increased the likelihood of treatment intensification by 21% and 53%, respectively[28]. In the INTEGRA study, a randomized multicomponent intervention to reduce therapeutic inertia led to improved HbA1c levels[17]. These interventions improve not only physicians’ but also patients’ willingness to initiate or intensify diabetes treatment and, most likely, patients’ adherence and persistence. Each visit is also a source of education and empowerment, allowing patients to actively participate in diabetes management and make informed decisions regarding their treatment plans. The problem with these intervention studies is that they may not be generalizable due to the specific patient populations and settings in which they are conducted and the continuity of interventions after the study is completed. Telemedicine, in appropriate settings where possible, is a potentially effective strategy for improving glycemic control, as demonstrated by the positive effects on HbA1c levels observed in various studies[29]. Furthermore, the problem of therapeutic inertia is complex and multifactorial and requires a comprehensive approach that goes beyond interventions for clinicians and patients.
The most important factor influencing glycemic control in our study was baseline glycemic history. Previous studies have investigated the significance of HbA1c trajectories in different ways. For example, An et al[30] identified four different HbA1c patterns over two years in individuals with established type 2 diabetes, emphasizing the dynamic nature of glycemic control. Similarly, Karpati et al[31] used longitudinal HbA1c data to categorize patients into groups with stable, descending, and ascending trajectories, demonstrating the clinical relevance of these patterns in predicting future outcomes and personalizing HbA1c targets. In a separate study with a follow-up period of 3 years, glycemic control was investigated in patients receiving a second antidiabetic agent due to inadequate HbA1c levels[32]. Despite ongoing adjustments of treatment to individual needs, glycemic trajectories varied considerably. While most participants achieved stable, good, or greatly improved long-term glycemic control, 21% had moderate or poor control throughout the follow-up period[32]. This highlights the ongoing challenges in achieving and maintaining optimal glycemic control in this population.
Luo et al[33] shed light on a critical problem in the treatment of type 2 diabetes: While there are recommended glycemic targets, a subset of patients consistently struggle to achieve these targets and have a disproportionately higher risk of complications and mortality. Their study, which categorized patients into different HbA1c trajectory groups (low-, moderate-, moderate-, and high-increased), found that worsening HbA1c patterns, particularly with extremely high baseline HbA1c, correlated significantly with an increased risk of acute myocardial infarction, stroke, end-stage renal disease, and death[33]. Although this study prospectively followed complications, it did not examine the long-term trends in HbA1c control within each trajectory group. Consequently, it remains unclear whether improvements in HbA1c levels, even in those with initially high levels, would result in a lower risk of complications during the follow-up period. This gap in knowledge highlights the inadequacy of a one-size-fits-all approach, as current clinical guidelines often lack specific recommendations tailored to the particular challenges of this high-risk group, who have difficulty meeting existing targets.
Our study aimed to build on these findings by prospectively investigating whether different glycemic trajectories in individuals with type 2 diabetes coupled with closer monitoring to attenuate clinical inertia could improve the likelihood of achieving good glycemic control. To analyze the effects of diabetes management, individuals with a history of effective glycemic control were studied separately from those with a long history of inadequate glycemic control. Participants who had predominantly good glycemic control in the past were more likely to achieve satisfactory control one year later than the other participants. Almost half of the participants with a history of good blood glucose control, including those who were not newly diagnosed, achieved an HbA1c target of 7% or less, compared with only one in six participants with a history of poor blood glucose control. This finding suggests that good glycemic control strongly influences the likelihood of achieving the target HbA1c level in the future.
In this specific cohort, the participants’ educational level, presence of depression, food awareness, and physical activity had no effect on achieving glycemic control. Of these factors, only depression scores were significantly higher in the group with poor glycemic control at the beginning of the study. However, this did not affect the outcomes at the end of the study period. It can be assumed that all groups with poor glycemic control have a high rate of these variables in a negative direction from the beginning, and that they therefore have no influence on the outcome.
During the study, patients had easy access to medical doctors and diabetes education nurses. Dietitian support was provided in the form of recommendations. Participants were referred to dietitians as needed or at the patient’s request as part of their usual care. Health insurance only covered ten minutes of nutritional counselling, and participants were encouraged to take advantage of other alternatives. At the end of the study, only a quarter of the patients, evenly distributed between groups, stated that they had visited a dietician at least twice. The factors that hinder dietary adherence in individuals with uncontrolled type 2 diabetes are still under investigation. The modification of dietary patterns has several intriguing facets[10,34,35]. This emphasizes the importance of addressing these barriers and providing education and support to patients with type 2 diabetes so that they can overcome these challenges and successfully adhere to a healthy diet. Although almost half of the participants had lost at least one kilogram of weight by the end of the year, no significant weight change was observed throughout the study. Weight change also had no effect on HbA1c levels.
Medication prescriptions were also analyzed to assess medication intensification when needed to achieve glycemic targets. There was no difference in the frequency of prescription of DPP-IV inhibitors, pioglitazone, and SGLT2 inhibitors, whereas metformin, basal insulin, basal-bolus insulin, and mixed insulin were used more frequently in patients whose HbA1c levels did not reach the target. When analyzing medication use in the predefined groups, DPP-IV inhibitors, basal insulin, and mixed insulin were more frequently prescribed to participants whose HbA1c levels had been poorly controlled for a long time. Most patients adhered to the treatment recommendations of their physicians and no differences were observed between the groups. Medication adherence is a challenging problem, and even in participants with newly diagnosed diabetes who have a relatively short duration of diabetes, the prevalence of medication adherence can be as high as 65%[36].
This study has some limitations. First, patient adherence and compliance were not assessed in this study. In addition, the participants’ self-reports were used to assess lifestyle changes. Patients received advice and counselling based on their answers to the questionnaires at baseline. However, no follow-up was conducted at the end of treatment to determine the effectiveness of these interventions. Glucagon-like peptide-1 analog reimbursement issues resulted in a low preference rate for the drug. Although patients were actively sought out and referred for visits, the study lost patients to follow-up, even though they were evenly distributed among the groups. This highlights the challenges of diabetes management and the need to improve patient understanding and behavior towards the disease.
One of the strengths of this study was that all participants had health insurance and received frequent follow-up visits and appointments to ensure convenient access to medication and medical advice. In this study, patients with poorly controlled type 2 diabetes were monitored consistently and thoroughly, including regular follow-up visits and close monitoring of glycemic control, to adjust treatment strategies in a timely manner. In addition, patients were categorized based on their previous glycemic control, which helped to identify differences in diabetes progression and response to treatment, ultimately increasing the effectiveness of the intervention. Furthermore, the study utilized a multidisciplinary team approach, including diabetes nurse educators, to provide comprehensive care to patients and help them manage their diseases effectively. Finally, the study explored the difference between effectiveness in clinical trials and effectiveness in practice, focusing on understanding how diabetes treatments are performed under conditions other than trials.
It is important not to simply label diabetes as poorly controlled but to identify the specific type of poor control to predict the effectiveness of treatment and tailor diabetes management accordingly. Therefore, a comprehensive approach beyond medical intervention is required. This includes addressing psychosocial factors and providing support in areas such as lifestyle modifications, self-management, and behavioral changes[37]. A recent study examining persistent poor glycemic control in people with type 2 diabetes in developing countries stated that glycemic control remained inadequate over a 12-year period, and that better organization of care and additional measures to improve glycemic control, personalized treatment strategies, early intervention, and comprehensive management approaches, including self-management and achievement of treatment goals, are needed[38].
Our study underscores the major impact of previous glycemic control on the likelihood that patients with poorly controlled type 2 diabetes will achieve their HbA1c target. This finding has significant implications for clinical practice and urges a paradigm shift from a one-size-fits-all approach to personalized diabetes management. Clinicians should prioritize a thorough assessment of a patient's glycemic history, going beyond a single HbA1c measurement, to understand the patient’s long-term trajectory. This can be achieved by reviewing past medical records, engaging patients in conversations about their experience with diabetes management and using technology to track and visualize glycemic trends. This individualized understanding can lead to tailored interventions. For example, patients with persistently poor control, often characterized by therapeutic inertia and adherence issues, may benefit from more intensive monitoring or behavioral interventions.
Conversely, patients who have had good glycemic control in the past, even if not newly diagnosed, are more likely to reach HbA1c targets[30]. Recognizing this pattern can reinforce positive behaviors and encourage continued adherence to treatment plans. Furthermore, consistent with Karpati et al[31], our results suggest that personalized glycemic targets may be warranted. Patients whose HbA1c levels progressively worsen and who have difficulty achieving the traditional < 7% target may have better treatment success and lower risk of complications with alternative, achievable targets.
Future research should focus on the development and validation of tools to efficiently identify different glycemic trajectories and personalize treatment targets and interventions. This approach has the potential to transform diabetes care, moving away from reactive management towards proactive, individualized strategies that empower patients and improve long-term health outcomes.
In conclusion, in patients with poorly controlled type 2 diabetes, different glycemic backgrounds led to different success rates in achieving glycemic goals, with the most favorable results in individuals with newly diagnosed diabetes. These findings suggest that understanding the challenges of achieving glycemic goals through assessment of the patient’s glycemic background may increase the likelihood of effectively managing poorly controlled diabetes. In addition, studying cohorts of patients categorized by their glycemic history may improve our understanding of the barriers that each group encounters in achieving glycemic control. It should be recognized that the same approach in persistently poorly controlled individuals may not lead to similar successes as in those who have recently deteriorated.
| 1. | International Diabetes Federation. IDF Diabetes Atlas 2021. [Cited 18 October 2024]. Available from: https://diabetesatlas.org/atlas/tenth-edition/. |
| 2. | Mata-Cases M, Rodríguez-Sánchez B, Mauricio D, Real J, Vlacho B, Franch-Nadal J, Oliva J. The Association Between Poor Glycemic Control and Health Care Costs in People With Diabetes: A Population-Based Study. Diabetes Care. 2020;43:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Zheng B, Su B, Price G, Tzoulaki I, Ahmadi-Abhari S, Middleton L. Glycemic Control, Diabetic Complications, and Risk of Dementia in Patients With Diabetes: Results From a Large U.K. Cohort Study. Diabetes Care. 2021;44:1556-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S111-S125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 266] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 5. | Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368:1613-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 741] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 6. | Basto-Abreu AC, López-Olmedo N, Rojas-Martínez R, Aguilar-Salinas CA, De la Cruz-Góngora VV, Rivera-Dommarco J, Shamah-Levy T, Romero-Martínez M, Barquera S, Villalpando S, Barrientos-Gutiérrez T. Prevalence of diabetes and glycemic control in Mexico: national results from 2018 and 2020. Salud Publica Mex. 2021;63:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Carls GS, Tuttle E, Tan RD, Huynh J, Yee J, Edelman SV, Polonsky WH. Understanding the Gap Between Efficacy in Randomized Controlled Trials and Effectiveness in Real-World Use of GLP-1 RA and DPP-4 Therapies in Patients With Type 2 Diabetes. Diabetes Care. 2017;40:1469-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Edelman SV, Polonsky WH. Type 2 Diabetes in the Real World: The Elusive Nature of Glycemic Control. Diabetes Care. 2017;40:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. |
|
| 10. | Cheng L, Leung DY, Sit JW, Li XM, Wu YN, Yang MY, Gao CX, Hui R. Factors associated with diet barriers in patients with poorly controlled type 2 diabetes. Patient Prefer Adherence. 2016;10:37-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Centers for Disease Control and Prevention (CDC). National diabetes statistics report, 2023. [cited 18 October 2024]. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html. |
| 12. | Lee EA, Gibbs NE, Martin J, Ziel F, Polzin JK, Palmer-Toy D. Improving Care in Older Patients with Diabetes: A Focus on Glycemic Control. Perm J. 2016;20:15-080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5819] [Cited by in RCA: 6083] [Article Influence: 234.0] [Reference Citation Analysis (0)] |
| 14. | Bansal M, Shah M, Reilly B, Willman S, Gill M, Kaufman FR. Impact of Reducing Glycated Hemoglobin on Healthcare Costs Among a Population with Uncontrolled Diabetes. Appl Health Econ Health Policy. 2018;16:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Khunti K, Davies MJ. Clinical inertia-Time to reappraise the terminology? Prim Care Diabetes. 2017;11:105-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Zhu NA, Harris SB. Therapeutic Inertia in People With Type 2 Diabetes in Primary Care: A Challenge That Just Won't Go Away. Diabetes Spectr. 2020;33:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Molló À, Vlacho B, Gratacòs M, Mata-Cases M, Rubinat E, Berenguera A, Real J, Puig-Treserra R, Cos X, Franch-Nadal J, Khunti K, Mauricio D; INTEGRA research group. Impact of a multicomponent healthcare intervention on glycaemic control in subjects with poorly controlled type 2 diabetes: The INTEGRA study. Diabetes Obes Metab. 2023;25:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Fu AZ, Sheehan JJ. Treatment intensification for patients with type 2 diabetes and poor glycaemic control. Diabetes Obes Metab. 2016;18:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411-3417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 20. | Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 21. | Erbakan AN, Arslan Bahadir M, Kaya FN, Güleç B, Vural Keskinler M, Faydaliel Ö, Mesçi B, Oğuz A. The effect of close and intensive therapeutic monitoring of patients with poorly controlled type 2 diabetes with different glycemic background. Medicine (Baltimore). 2023;102:e36680. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Kose G, Tayfur M, Birincioglu I, Donmez A. Adaptation Study of the Mindful Eating Questiionnare (MEQ) into Turkish. JCBPR. 2017;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Hisli NH. Beck depresyon envanterinin universite ogrencileri icin gecerliligi, guvenilirligi. (A reliability and validity study of Beck Depression Inventory in a university student sample). J Psychol. 1989;7:3-13. |
| 24. | Kaya Noğay AE, Özen M. [Birinci Basamak İçin Fiziksel Aktivite Anketinin Türkçe Uyarlamasının Geçerlilik ve Güvenilirliği]. Konuralp Tıp Dergisi. 2019;11:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Cai X, Hu D, Pan C, Li G, Lu J, Ji Q, Su B, Tian H, Qu S, Weng J, Zhang D, Xu J, Ji L. The risk factors of glycemic control, blood pressure control, lipid control in Chinese patients with newly diagnosed type 2 diabetes _ A nationwide prospective cohort study. Sci Rep. 2019;9:7709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Gopalan A, Mishra P, Alexeeff SE, Blatchins MA, Kim E, Man A, Karter AJ, Grant RW. Initial Glycemic Control and Care Among Younger Adults Diagnosed With Type 2 Diabetes. Diabetes Care. 2020;43:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Rathmann W, Schwandt A, Hermann JM, Kuss O, Roden M, Laubner K, Best F, Ebner S, Plaumann M, Holl RW; DPV Initiative. Distinct trajectories of HbA(1c) in newly diagnosed Type 2 diabetes from the DPV registry using a longitudinal group-based modelling approach. Diabet Med. 2019;36:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Tsai YY, Kuo TY, Lin MH, Shen FC, Lin YH. Mandatory monthly outpatient visits could improve therapeutic inertia in patients with poorly controlled type 2 diabetes. J Diabetes Investig. 2024;15:227-236. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | De Groot J, Wu D, Flynn D, Robertson D, Grant G, Sun J. Efficacy of telemedicine on glycaemic control in patients with type 2 diabetes: A meta-analysis. World J Diabetes. 2021;12:170-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (7)] |
| 30. | An LW, Li XL, Chen LH, Tang H, Yuan Q, Liu YJ, Ji Y, Lu JM. Clinical Inertia and 2-Year Glycaemic Trajectories in Patients with Non-Newly Diagnosed Type 2 Diabetes Mellitus in Primary Care: A Retrospective Cohort Study. Patient Prefer Adherence. 2021;15:2497-2508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Karpati T, Leventer-Roberts M, Feldman B, Cohen-Stavi C, Raz I, Balicer R. Patient clusters based on HbA1c trajectories: A step toward individualized medicine in type 2 diabetes. PLoS One. 2018;13:e0207096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Bongaerts B, Kuss O, Bonnet F, Chen H, Cooper A, Fenici P, Gomes MB, Hammar N, Ji L, Khunti K, Medina J, Nicolucci A, Shestakova MV, Watada H, Rathmann W. HbA1c trajectories over 3 years in people with type 2 diabetes starting second-line glucose-lowering therapy: The prospective global DISCOVER study. Diabetes Obes Metab. 2023;25:1890-1899. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Luo M, Lim WY, Tan CS, Ning Y, Chia KS, van Dam RM, Tang WE, Tan NC, Chen R, Tai ES, Venkataraman K. Longitudinal trends in HbA1c and associations with comorbidity and all-cause mortality in Asian patients with type 2 diabetes: A cohort study. Diabetes Res Clin Pract. 2017;133:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Bross R, Genter P, Lu Y, Serpas L, Campa D, Ipp E. Barriers to Healthy Eating and Diabetes Diet Education: Divergent Perspectives of Patients and Their Providers. Health Educ Behav. 2022;49:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 35. | Marcy TR, Britton ML, Harrison D. Identification of barriers to appropriate dietary behavior in low-income patients with type 2 diabetes mellitus. Diabetes Ther. 2011;2:9-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Lin LK, Sun Y, Heng BH, Chew DEK, Chong PN. Medication adherence and glycemic control among newly diagnosed diabetes patients. BMJ Open Diabetes Res Care. 2017;5:e000429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Kadirvelu A, Sadasivan S, Ng SH. Social support in type II diabetes care: a case of too little, too late. Diabetes Metab Syndr Obes. 2012;5:407-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Aschner P, Gagliardino JJ, Ilkova H, Lavalle F, Ramachandran A, Mbanya JC, Shestakova M, Chantelot JM, Chan JCN. Persistent poor glycaemic control in individuals with type 2 diabetes in developing countries: 12 years of real-world evidence of the International Diabetes Management Practices Study (IDMPS). Diabetologia. 2020;63:711-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/