Published online Jan 15, 2025. doi: 10.4239/wjd.v16.i1.97954
Revised: October 12, 2024
Accepted: November 7, 2024
Published online: January 15, 2025
Processing time: 169 Days and 16.2 Hours
The relationship between diabetes mellitus (DM) and asthma is complex and can impact disease trajectories.
To explore the bidirectional influences between the two conditions on clinical outcomes and disease control.
We systematically reviewed the literature on the relationship between DM and asthma, focusing on their impacts, mechanisms, and therapeutic implications. Various studies were assessed, which investigated the effect of glycemic control on asthma outcomes, lung function, and exacerbations. The study highlighted the role of specific diabetes medications in managing asthma.
The results showed that poor glycemic control in diabetes can exacerbate asthma, increase hospitalizations, and reduce lung function. Conversely, severe asthma, especially in obese individuals, can complicate diabetes manage
The complex interrelationship between diabetes and asthma suggests bidirectional influences that affect disease course and outcomes. Inflammation and microvascular complications associated with diabetes may worsen asthma outcomes, while asthma severity, especially in obese individuals, complicates diabetes control. However, the current research has limitations, and more diverse longitudinal studies are required to establish causal rela
Core Tip: This study explores the intricate relationship between diabetes mellitus (DM) and asthma, emphasizing their bidirectional impact. It delves into the influence of glycemic control on lung function and asthma exacerbations, highlighting how poorly controlled DM correlates with reduced lung function. The research underscores various mechanisms linking DM to asthma complications, including inflammation, microangiopathy, and insulin resistance. Medications like metformin and glucagon-like peptide 1 receptor agonists show promise in mitigating asthma exacerbations in diabetic patients. Overall, the study underscores the significance of glycemic management in ameliorating asthma outcomes and identifies potential avenues for targeted therapeutic interventions in this complex interplay between DM and asthma.
- Citation: Al-Beltagi M, Bediwy AS, Saeed NK, Bediwy HA, Elbeltagi R. Diabetes-inducing effects of bronchial asthma. World J Diabetes 2025; 16(1): 97954
- URL: https://www.wjgnet.com/1948-9358/full/v16/i1/97954.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i1.97954
Bronchial asthma is a chronic inflammatory disease affecting the respiratory airways characterized by airway inflammation and hyperresponsiveness. As a heterogeneous disease, it impacts over 300 million people globally, making it a significant health concern across all age groups. The prevalence of bronchial asthma is rising in many countries, particularly among children, leading to frequent school and work absenteeism[1].
Diabetes mellitus (DM) affects approximately 425 million people worldwide aged 20-79, with projections indicating this number could rise to 629 million by 2045. Type 2 DM (T2DM) accounts for 87%-91% of all diabetic cases, while type 1 DM (T1DM) represents 7%-12%, and the remaining 1%-3% includes gestational diabetes mellitus (GDM) and secondary diabetes, which can result from chronic pancreatitis, gene mutations, or medication use. T1DM is the most prevalent form among children and adolescents, though T2DM is increasingly common in this age group due to rising obesity rates. In adults, DM occurs slightly more frequently in men than women, with the incidence rates in 2014 reported at 9% for men and 7.9% for women, expected to increase to 12.8% and 10.4%, respectively, by 2025[2].
T2DM is a common multifactorial disease characterized by decreased insulin sensitivity, reduced insulin reserves, and a defect in insulin secretion associated with chronic inflammation[3]. Obesity is a significant factor linking bronchial asthma and T2DM, as elevated levels of circulating inflammatory cytokines in obese individuals with asthma increase the risk of developing T2DM and insulin resistance[4]. Furthermore, asthma itself can elevate the risk of T2DM indepen
Individuals with asthma are more likely to have comorbidities such as T2DM compared to those without asthma. These comorbidities are often linked to poorer asthma outcomes, increased healthcare utilization, and a diminished quality of life[4-7]. The lungs are one of the target organs affected by diabetic microangiopathy in both type 1 and type 2 diabetes patients[8]. Numerous studies have reported impaired lung function in individuals with diabetes[9]. Bronchial asthma has been associated with an increased risk of developing DM[6]. This review article examines the potential link between bronchial asthma and DM, focusing on T2DM and T1DM. It discusses various epidemiological studies, explores the coexistence of these conditions, and delves into the underlying mechanisms that may explain their association.
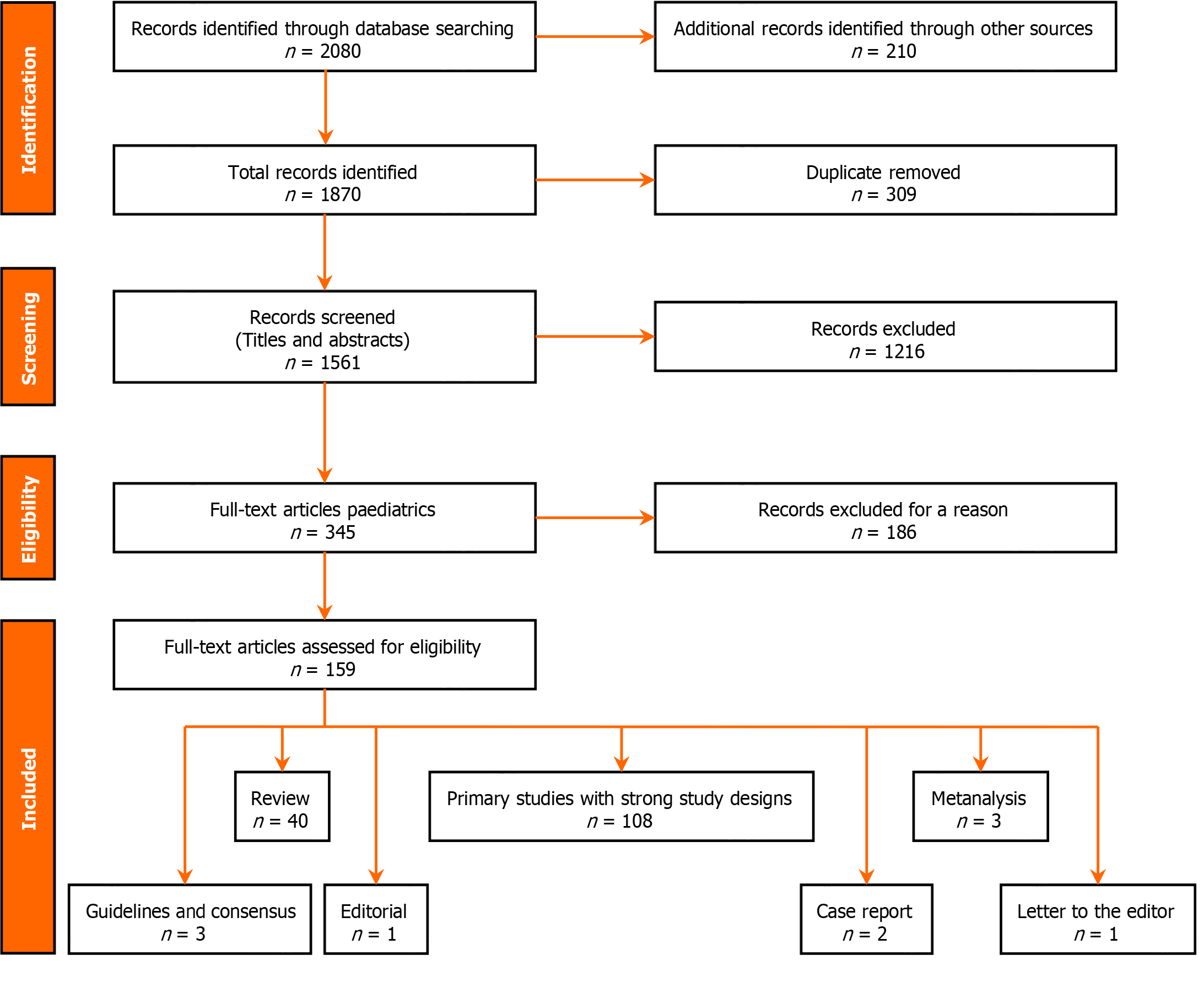
We systematically reviewed electronic data, including PubMed/MEDLINE, Scopus, and Google Scholar, to gather relevant literature on the association between bronchial asthma and DM. We used a combination of keywords and controlled vocabulary terms related to "bronchial asthma" "diabetes mellitus" "association" "epidemiology" and "pathophysiology" to search for articles published from inception to November 28th, 2023. We included articles that met the following criteria: They were written in English, published in peer-reviewed journals, and investigated the epidemiological association between bronchial asthma and DM (Type 1 or Type 2) in any population group. The articles could be meta-analyses, systematic reviews, original research articles, case reports, guidelines, editorial pieces, or letters to the editor discussing the relationship between bronchial asthma and DM. We initially found 1870 articles, but after removing duplicates, we screened 1561 unique articles for relevance. From this, we conducted full-text assessments on 159 articles that met the predefined criteria. The final selection included 108 research articles, 40 review articles, 4 meta-analyses, 3 guidelines, 2 case reports, 1 editorial, and 1 Letter to the editor, as shown in Figure 1. These articles provide information on the relationship between bronchial asthma and DM, including study design, participant characteristics, key findings, and discussions. We synthesized the data from these articles to provide a comprehensive overview and analysis in this review article.
The review showed that poor glycemic control in diabetes correlates with exacerbated asthma, reflected in increased hospitalizations and diminished lung function. Conversely, severe asthma, particularly in obese individuals, affects diabetes management, complicating glycemic control. Mechanisms such as inflammation, microangiopathy, and oxidative stress associated with diabetes may contribute to asthma exacerbations and decreased lung function. Certain diabetes medications exhibit anti-inflammatory effects that show promise in mitigating asthma exacerbations.
Bronchial asthma affects approximately 334 million people worldwide, with incidence rates varying significantly across countries. In 2012, Austria reported the highest incidence at 21%, while China had the lowest at 0.2%[10]. Unlike DM, the global incidence of bronchial asthma has remained relatively stable, although it is rising rapidly in developing countries and declining in developed ones. Boys are about 20% more likely than girls to develop asthma, particularly in childhood. Key risk factors for asthma include cigarette smoking, genetic predisposition, occupational exposure, and obesity[11].
DM is a common comorbidity among adults with asthma. Population-based studies have shown that the prevalence of comorbid diabetes in asthma patients ranges from 5% to 16%[12]. Patients with both asthma and diabetes tend to have higher glucose levels[13], reduced quality-adjusted life expectancy[14], and a greater risk of pneumococcal infections[15] compared to those with either condition alone.
Both DM and bronchial asthma often coexist. A meta-analysis of 11 clinical studies involving over 550000 patients found that those with asthma were more likely to develop DM than those without asthma[6]. In a 12-year study of 38570 women aged 45 and older with asthma or overlap syndrome [asthma and chronic obstructive pulmonary disease (COPD)], 2472 new cases of T2DM were diagnosed. The presence of asthma or COPD was associated with a 1.75-fold increased risk of T2DM, independent of other risk factors like sedentary lifestyle, smoking, hormone replacement therapy, alcohol consumption, family history of DM, systemic hypertension, and familial hypercholesterolemia[5].
In Finland, a study on children found that a prior diagnosis of asthma increased the risk of developing T1DM by 41%. Conversely, having T1DM reduced the risk of subsequent asthma by 18%, independent of age at diagnosis, birth decade, sex, and maternal history of asthma or DM[16]. A Chinese study also identified DM as a major comorbidity in bronchial asthma patients, alongside hypertension, myocardial infarction, and ischemic stroke. This study found a significant relationship between lifestyle factors related to carbohydrate metabolism [e.g., body mass index (BMI), smoking, alcohol consumption, education level, screen time] and the risk of developing asthma[17].
There is a theory that individuals with T1DM, characterized by a prevalence of Th1 cytokines, may be less likely to develop allergic asthma associated with Th2 cytokines. A study by Tosca et al[18] compared the bronchodilator response in children with allergic rhinitis and diabetes to those with only allergic rhinitis. The study found a statistically significant difference, with children having both conditions showing a lower bronchodilator response, potentially suggesting a protective effect against asthma. However, the study had limitations, including a small sample size and lack of follow-up[18]. Another study in Finland by Koskinen et al[19] found higher respiratory disease-related death rates among individuals receiving diabetes medication compared to the general population[19]. Additionally, Kero et al[20] found no significant difference in asthma prevalence among children with T1DM compared to those without, suggesting that Th1 and Th2 diseases can coexist[20].
Yun et al[21] conducted a retrospective study analyzing 2392 patients with reactive airway disease and 4784 matched controls. The study revealed that asthma patients had a significantly higher risk of developing DM. However, the authors could not control for asthma severity, phenotype, or other contributing factors like smoking status, BMI, and physical activity[21]. In Germany and Austria, a study found that 3.4% of patients under 20 with T1DM also had asthma or received asthma-specific treatment. These patients were more likely to be male, older, and have a longer duration of DM[22]. Another study using the Optimum Patient Care Research Database and the British Thoracic Society Difficult Asthma Registry found that T2DM was more prevalent among patients with severe asthma compared to those with mild to moderate asthma[23].
Adults with self-reported asthma have a higher risk of developing T2DM, with an increased risk ranging from 21% to 37% in various longitudinal observational studies[4,5,21]. A Danish twin study by Thomsen et al[24] showed that patients with T2DM were nearly twice as likely to develop asthma compared to those without T2DM[24]. A study using veterans' hospital data revealed that asthma was present in 4.5% of patients with T2DM compared to 2.9% in the control group, regardless of other comorbidities[25]. However, a longitudinal observational study found a negative association between asthma and T2DM[26]. A cross-sectional study of 5045 Korean adults found that the likelihood of developing asthma increased with higher HbA1c levels (1.38-fold) and insulin levels (1.02-fold) but not fasting blood glucose levels. The study also showed that DM rates increased by 1.75-fold with asthma[27].
Individuals with both asthma and T2DM generally have worse asthma control, with a higher likelihood of complications, exacerbations, and hospitalizations[28]. In a retrospective study, Ehrlich et al[29] found that asthma, COPD, pulmonary fibrosis, and pneumonia incidences were higher in patients with DM than in those without. However, no significant increase in asthma risk was observed with higher baseline glycated hemoglobin (HbA1c) levels, suggesting that asthma may be related to factors other than glycemic control in diabetic patients[29]. Hyperglycemia and hyperinsulinemia in lung tissue have been linked to reduced lung function and a higher risk of asthma in diabetic patients[30,31]. The association between asthma and chronic diseases is thought to involve low-grade systemic inflammation, corticosteroid use, genetic pleiotropy, and oxidative stress. However, these mechanisms may vary depending on the asthma phenotype. One limitation of Ehrlich et al's study[29] was the possibility of increased pulmonary disease diagnoses due to more frequent healthcare visits among DM patients[4,23,29,32].
The development of DM results from the interaction of genetic and environmental factors, leading to a gradual loss of pancreatic β-cell mass and/or function. In T1DM, specific variants of the HLA genes contribute to approximately 50%-60% of the genetic risk by influencing the binding of HLA proteins to antigen peptides and their presentation to T cells. Genetic factors and environmental triggers, such as viral infections, dietary components, and autoimmune processes, also affect the disease’s onset. In these cases, autoantibodies in the blood reflect an immune response by T and B cells against
T2DM is characterized by increased insulin resistance and a diminished insulin reserve. Diabetic microangiopathy can complicate DM, leading to conditions like retinopathy, nephropathy, and neuropathy. The lungs are also susceptible to diabetic injury, a condition now recognized as "diabetic pneumopathy" in medical literature[34]. Patients with diabetes often exhibit reduced capillary blood volume, indicating microangiopathy that causes ventilation/perfusion mismatch. Histopathological studies of diabetic patients’ lungs reveal thickening of the alveolar epithelium and the basement membrane of alveolar capillaries. This thickening and ventilation/perfusion mismatch impair diffusion capacity, exacerbated by anemia, a common complication in diabetic patients with kidney impairment[35].
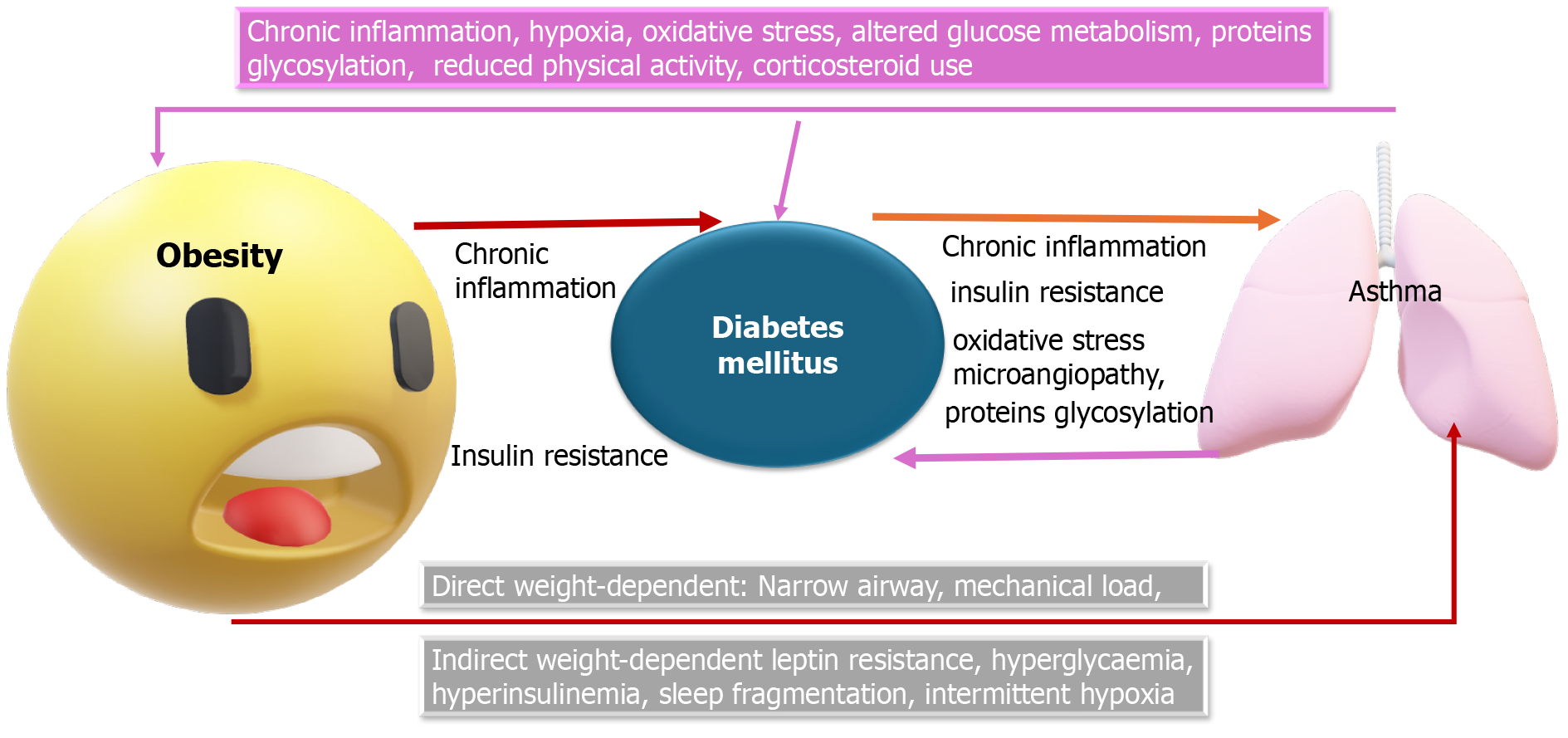
Additional pathological mechanisms linking DM and bronchial asthma include systemic inflammation, obesity, metabolic disturbances, insulin resistance, and the impact of certain medications (Figure 2).
Bronchial asthma is characterized by chronic airway inflammation, leading to structural changes over time. Key players in this inflammation include dendritic cells and Th2 cells, which produce cytokines like interleukin (IL)-4, IL-5, and IL-13. These cytokines promote the maturation and survival of eosinophils, which are recruited to the bronchial mucosa, causing damage. Additionally, these cytokines contribute to the synthesis of IgE, which activates mast cells and further drives inflammation. Genetic factors also play a role, with polymorphisms in genes such as IL-33, IL1RL1/IL18R1, HLA-DQ, SMAD3, IL2RB9, ZPBP2, GSDMB, and ORMDL3 increasing susceptibility to asthma[11,36].
Both bronchial asthma and DM are associated with low-grade systemic inflammation, independent of obesity. Chronic airway inflammation in asthma can contribute to insulin resistance in the liver, skeletal muscle, and vascular endothelium, leading to the clinical manifestations of DM due to elevated inflammatory cytokines[4,37,38]. Patients with T2DM often exhibit irregular immune system function linked to chronic inflammation. For instance, Dandona et al[39] found higher levels of inflammatory markers such as matrix metallopeptidase 9, IL-4, and chemokine receptor type 2 in obese subjects with T2DM compared to healthy non-obese individuals. These markers, excluding nitric oxide metabolites, were also associated with insulin resistance and BMI[39]. Sindhu et al[40] observed elevated monocyte chemoattractant protein-1 levels in patients with both asthma and T2DM, correlating with other inflammation and airway remodeling markers[40].
Though controversies persist, the link between T1DM and bronchial asthma appears stronger than with T2DM. Vaseghi et al s[41] found that T1DM patients had lower expression of T-bet and IFN-γ but higher levels of IL-4 mRNA and plasma IL-4 than non-diabetics[41]. Peters et al[42] noted that IL-6 levels were positively correlated with BMI and diabetes severity, irrespective of asthma severity[42]. Thomsen et al[24] identified an increased risk of asthma among T2DM patients, with positive genetic correlations between asthma and BMI in women[24].
Chronic inflammation, a hallmark of asthma, exacerbates insulin resistance and T2DM risk. High levels of ul
Yun et al[21] conducted a retrospective study revealing an increased risk of T2DM among patients with asthma, attributing it to inflammatory cytokines IL-6 and IL-17, hypoxia, tachyarrhythmia, and corticosteroid use. However, BMI was not evaluated as a potential confounder[21]. Verbovoy et al[45] identified subclinical inflammation in women with T2DM and asthma, characterized by increased leptin and resistin levels, decreased adiponectin, and elevated IL-6 and IL-10[45]. Baek et al[26] found that T2DM patients without retinopathy had a lower risk of asthma than those with reti
The inflammatory pathways of tumor necrosis factor-alpha (TNF-α) and IL-6 link DM, obesity, and bronchial asthma by promoting Th2 cell differentiation and cytokine production involved in eosinophilic asthma[30]. Elevated levels of TNF-α and IL-6 contribute to insulin resistance and T2DM development[46,47]. TLR activation, particularly by elevated free fatty acids in obesity, generates pro-inflammatory signals and activates NF-κB, which inhibits insulin signaling and promotes inflammatory gene expression[32,48-50]. Additionally, advanced glycation end-products and matrix metalloproteinase-9 from hyperglycemia further contribute to airway inflammation and dysfunction[51-53].
The prevalence of asthma, diabetes, and obesity is rising, particularly among young people. Obesity, now recognized as a pro-inflammatory condition, not only increases the risk of asthma but also exacerbates inflammatory processes and insulin resistance, both of which are key contributors to the development of T2DM[13]. Studies indicate that metabolic dysfunction plays a significant role in the severity of asthma[54] and that there is a clear association between obesity, metabolic syndrome (MS), and airway diseases[55]. MS has been identified as an independent risk factor for the incidence of asthma, even when accounting for other metabolic components[56]. Patients with obesity and MS often represent a distinct subtype of bronchial asthma, characterized by more severe symptoms, poorer quality of life, a higher risk of obstructive sleep apnea, and elevated levels of inflammatory markers like leptin and IL-6, compared to non-obese asthmatics and obese asthmatics without MS[47].
A study among Chinese Singaporeans revealed a significant association between bronchial asthma and T2DM, particularly in obese individuals, suggesting that excess body fat and airway inflammation together promote the development of diabetes[4]. Obesity is well-known to be linked with low-grade systemic inflammation, as evidenced by elevated levels of inflammatory markers such as TNF-α, TLR4, IL-6, and IL-1β, which contribute to immune system changes and inflammatory responses[30,32]. Both obesity and insulin resistance are major factors in the pathogenesis of T2DM, with a close correlation between immune system function and excess fat. Dysfunctional adipose cells, caused by caloric overload, produce excessive leptin and reduced adiponectin and are characterized by hypoxia and excessive extracellular matrix deposition, leading to immune cell recruitment, activation, and the formation of proinflammatory cytokines[57].
According to some authors, individuals with obesity and bronchial asthma can be categorized into two phenotypes: Early-onset atopic Th2-high asthma, which is marked by high serum IgE levels and increased bronchial hyperreactivity and is complicated by obesity, and late-onset non-atopic Th2-low asthma, more common in obese women, which develops as a consequence of obesity. This latter phenotype is characterized by lower atopy, reduced bronchial hyperreactivity, less airway obstruction, and fewer exacerbations[58].
Obesity, particularly in women, is associated with treatment-resistant asthma, which tends to have less eosinophilic and more neutrophilic sputum. Obesity also contributes to exertional dyspnea by reducing the functional residual capacity of the respiratory reserve volume[59]. However, the mechanisms underlying the link between obesity, gut microbiota, and bronchial asthma development are not fully understood. It has been suggested that factors such as IgA and calprotectin, exposure to lipopolysaccharides (LPS), bile acids, short-chain fatty acids, and intestinal microflora products may play a role[60].
A study by Cani et al[61] on animal models showed that mice fed a high-fat, carbohydrate-free diet had increased LPS concentrations. This diet altered the gut microbiota, reducing the abundance of Lactobacillus spp., Bifidobacterium spp., and Bacteroides-Prevotella spp., and increased intestinal permeability by decreasing the expression of tight junction proteins such as ZO-1 and Occludin. These changes were associated with elevated mRNA concentrations of PAI-1, IL-1, TNF-α, and F4/80 in visceral adipose tissue. Notably, a 4-week antibiotic treatment reversed these effects, significantly reducing inflammatory markers[60]. Similar effects were observed in groups consuming a carbohydrate-rich diet, which induced insulin secretion, insulin resistance, weight gain, increased energy consumption, and increased visceral and subcutaneous fat[61] (Figure 1).
Prediabetes, a key component of MS, is characterized by elevated blood glucose levels that do not meet the criteria for DM. Though often asymptomatic, prediabetes is frequently associated with obesity, dyslipidemia, and hypertension, all of which contribute to an increased risk of cardiovascular disease. The global prevalence of prediabetes is projected to rise significantly, from 720 million in 2021 to 1 billion by 2045. Over half of those diagnosed with prediabetes eventually develop T2DM[19,26,28,38]. However, lifestyle modifications, such as increased physical activity and a healthy diet, can reduce the risk of progression to T2DM[19,28].
The etiology of prediabetes is multifactorial, involving both genetic and lifestyle factors, ultimately leading to insulin resistance and abnormal blood glucose control. Prediabetes is diagnosed using specific criteria, including fasting blood glucose levels of 110-125 mg/dL (World Health Organization criteria) or 100-125 mg/dL (American Diabetes Association criteria), a glucose tolerance test showing 140-199 mg/dL 2 hours after ingesting a standardized glucose solution, or an HbA1c of 5.7%-6.4%[19,26,28]. Hyperinsulinemia, often preceding both prediabetes and DM, may occur even in individuals with normal glucose levels[19,26,28].
Insulin resistance is known to cause hyperglycemia, leading to hyperinsulinemia as an early compensatory mechanism in DM. Studies in both humans and animals have demonstrated a correlation between asthma and insulin resistance in patients with DM. For example, a cross-sectional study showed a significant association between bronchial asthma and visceral fat accumulation, as indicated by the Homeostasis Model Assessment Index[62]. Another study found that bronchial asthma patients had higher insulin levels than non-asthmatic individuals, with those with elevated insulin levels exhibiting a higher incidence of bronchial asthma[27].
In T2DM patients, hyperglycemia and hyperinsulinemia—hallmarks of insulin resistance—can impair lung function through several mechanisms[63]. These include the stimulation of fibroblast proliferation and differentiation in lung tissue, leading to collagen deposition and airway remodeling, as well as pulmonary microvascular changes, chronic inflammation, autonomic neuropathy, and loss of elastic recoil due to collagen glycosylation in the pulmonary paren
Insulin resistance is an established risk factor for developing bronchial asthma, wheezing, and asthma-like symptoms. However, whether there is a direct causal relationship between insulin resistance and asthma or if inflammatory mediators contribute to asthma pathogenesis remains unclear. This association is more prominent in insulin resistance than in obesity, regardless of gender[50]. Experimental studies have shown that intranasal insulin administration can induce insulin resistance, increase catenin levels, and lead to collagen deposition and bronchial hyperresponsiveness[30,47]. Proposed mechanisms linking insulin resistance with bronchial asthma include hyperinsulinemia potentiating vagally mediated bronchoconstriction by reducing the activity of inhibitory M2 muscarinic receptors on airway parasympathetic neurons, leading to increased acetylcholine release and airway hyperresponsiveness[63,66]. Additionally, insulin resistance can induce fibroblast proliferation and reduce the bioavailability of endogenous nitric oxide, which has bronchodilator effects[47,66].
Inflammation associated with obesity and insulin resistance can lead to asthma in obese children, who are more likely to experience insulin resistance compared to non-asthmatic peers[67-69]. Studies have shown an inverse relationship between insulin resistance and pulmonary function, with higher insulin resistance levels correlating with lower forced expiratory volume in 1 (FEV1)/forced vital capacity (FVC) ratios[70]. Forno et al[71] found that obese adolescents with insulin resistance exhibited a more pronounced inverse association between obesity and spirometric parameters[71]. A prospective study in Korea found a negative linear association between insulin resistance (measured by Homeostasis Model Assessment of Insulin Resistance) and yearly changes in FEV1 and FVC percent predicted, particularly in individuals over 50, even after adjusting for smoking, physical activity, and BMI[72].
Inhaled insulin therapy has also been linked to a reduction in FEV1 in diabetic patients, with effects reversible after discontinuation of treatment[73]. Insulin resistance significantly predicts expiratory reserve volume and may affect the Th1/Th2 immune response ratio, influencing lung function[74]. Even without overt insulin resistance, impaired glucose metabolism is associated with airway hyperresponsiveness, independent of BMI[75]. Insulin receptors in airway smooth muscle cells may contribute to a pro-contractile phenotype in obesity, making these cells more sensitive to neural-mediated bronchoconstriction[47,63]. Experimental studies support this mechanism, with insulin exposure potentiating bronchoconstriction induced by vagal stimulation in mice models, an effect blocked by atropine, indicating the involvement of muscarinic receptors[76,77]. Additionally, insulin exposure can reduce cyclic adenosine monophosphate (cAMP) accumulation, affecting airway β2 adrenergic receptors[78]. Increased insulin resistance is also linked to a higher prevalence of gastro-oesophageal reflux disease, which can worsen bronchial asthma control and contribute to difficult-to-treat asthma[79,80].
Concerns exist regarding the safety of bronchial asthma medications for diabetic patients and the impact of diabetes medications on asthma, particularly in severe cases. Asthma medications are categorized into two main types: Controllers, which suppress inflammation or provide long-acting bronchodilation, and relievers, which offer quick-onset bronchodilation for acute symptoms[81]. These medications include bronchodilators (beta-agonists, anticholinergics), corticosteroids, anti-leukotrienes, and biologics. A 5-year study by Ahmadizar et al[82] found higher usage rates of asthma medication in patients with T1DM. Although diabetic patients more frequently used short-acting muscarinic antagonists, there was no significant difference in the usage of specific asthma medications between patients with and without diabetes. The study also noted a decline in asthma medication use and exacerbations over time, particularly after the first year post-DM diagnosis[82].
Bronchodilators: Bronchodilators are medications used to treat bronchial asthma. They include beta-agonists, anticholinergics, and xanthine derivatives (non-selective phosphodiesterase inhibitors) like theophylline and aminophylline. Jessen et al[83] reported that beta2-agonists at near-therapeutic doses can improve insulin sensitivity in healthy young men due to effects on muscle hypertrophy and glucose disposal[83]. Kalinovich et al[84] demonstrated that clenbuterol, a selective β2-adrenergic agonist, improved glucose tolerance and insulin sensitivity in insulin-resistant mice by stimulating glucose uptake in skeletal muscle and reducing hepatic lipids and glycogen[84]. In humans, nebulized albuterol (2.5 mg) did not significantly increase blood glucose levels in diabetic patients[85]. Anticholinergics, especially inhaled ones, have no reported adverse effects on glucose levels or insulin resistance, though oral forms can attenuate late-phase insulin activity[86]. Aminophylline has been shown to inhibit endogenous glucose production in T2DM by stimulating insulin secretion[87]. Theophylline can improve hypoglycemia unawareness in T1DM patients, though prolonged use leads to tolerance[88].
Corticosteroids: Frequently used in severe asthma and exacerbations, systemic corticosteroids are linked to hyper
ICS: ICS have revolutionized asthma treatment by directly targeting airway inflammation, though systemic absorption (10%-30% of the dose) raises concerns about systemic side effects. These risks are dose-dependent and influenced by patient factors like age, health, and comorbidities. Newer ICS formulations with low oral bioavailability and high serum protein binding are designed to minimize these risks[101,102]. A study on diabetic patients with asthma or COPD found that ICS (e.g., fluticasone propionate) caused a slight, non-clinically relevant increase in HbA1c after 6 weeks[103]. Although some literature suggests ICS is not significantly associated with glycemic changes, careful monitoring is advised[64,94,100].
Montelukast: Montelukast, a leukotriene receptor antagonist, is widely used for asthma management. While no evidence links it to diabetes or insulin resistance, an experimental study by Bapputty et al[104] found that montelukast reduced diabetes-induced retinal capillary leakage, leukocyte adherence, and superoxide generation in a mouse model, indicating potential protective effects[104].
Biologic agents: In severe asthma cases, patients may benefit from biologics, such as anti-IgE (e.g., omalizumab) and anti-IL-5 (e.g., mepolizumab). A case report by Hamada et al[105] highlighted that omalizumab might increase blood sugar levels in patients with T2DM, though switching to mepolizumab improved DM control[105]. Yalcin et al[106] reported transient blood glucose increases after omalizumab injections, possibly due to the sucrose content in the medication, though infrequent use did not seem to affect glycemic control[106]. Despite these effects, omalizumab can reduce the need for corticosteroids, thereby lowering the risk of steroid-induced DM[107] (Table 1).
| Drug | Effect | Mechanism |
| Beta-agonists | Beta2-agonists can improve insulin sensitivity[83]. Improved glucose tolerance and homeostasis[84]. No clinically significant increase in blood glucose in patients with DM[85] | Stimulating glucose uptake in the skeletal muscle, improving whole-body insulin sensitivity, and reducing hepatic lipids and glycogen[84] |
| Anticholinergic | There are no recorded harmful effects of inhaled anticholinergic drugs on the glucose level or insulin resistance. Oral anticholinergics can attenuate the late-phase insulin activity in varying degrees of glycemic status[86] | Insulin secretion is influenced by the cholinergic system[86] |
| Xanthine derivatives (phosphodiesterase inhibitors) | Aminophylline inhibits the endogenous glucose production in T2DM by stimulating insulin secretion[87]. A single dose of theophylline can improve hypoglycemia unawareness in patients with T1DM; however, prolonged theophylline use is associated with tolerance of that effect, causing a sustained effect on different responses to hypoglycemia (cardiovascular, metabolic, and symptom responses)[88] | Paracrine factors, such as adenosine, may be involved in regulating basal insulin secretion[87] |
| Systemic corticosteroids | High risk of developing DM and progression to insulin therapy in persons using long-term systemic steroids[90-93]. Patients with asthma without a diagnosis of DM who used corticosteroids developed hyperglycemia[94]. Risk factors for developing DM in subjects using systemic glucocorticoids include older age, higher fasting blood glucose levels and HbA1c, lower glomerular filtration rate, presence of pregnancy, visceral obesity, insulin resistance, and family history of DM[97] | Increased hepatic gluconeogenesis. The peripheral glucose uptake by the skeletal muscle, adipose tissue, liver, and bone is reduced. Insulin resistance and its effect on glycemic control. Inhibition of insulin secretion by pancreatic β cells. Steroids favor the systemic release of triglycerides and fatty acids that negatively affect β cells' function[96,97] |
| ICS | Systemic bioavailability of ICS increases the risk of systemic adverse effects, especially with higher doses of ICS, increasing the risk of DM and hyperglycemia. Other factors that determine ICS's potential for systemic adverse effects include the dose of the drug, the patient's age, and the presence of other co-morbidities. Newer ICS preparations have essentially zero oral bioavailability and exhibit 98%–99% binding to serum proteins, making them unable to reach the glucocorticoid receptors. Efforts to improve the delivery devices to choose the best form of the ICS are ongoing[101,102] | The same mechanisms as with systemic steroids when the drug reaches the circulation |
| Leukotriene receptor antagonists | No recorded evidence of their effect on DM control or insulin resistance exists. Montelukast has a protective effect against diabetic retinopathy[104] | Leukotriene receptor antagonism by montelukast resulted in a significant decrease in early, diabetes-induced retinal capillary leakage, leukocyte adherence, and superoxide generation[104] |
| Biologic agents | Few case reports show elevation of blood glucose following administration of omalizumab, an anti-IgE antibody. That was not noticed after administering mepolizumab, a monoclonal antibody against IL-5[105-107] | Each vial of omalizumab (150 mg) contains 145.5 mg sucrose. However, because of the infrequent use of omalizumab (every two or four weeks), sucrose included in the omalizumab vial did not seem to influence glycaemic control[105-107] |
Metformin: Metformin is a cornerstone in managing T2DM, recommended by the American Diabetes Association for all patients without contraindications[108]. It has also been shown to reduce airway inflammation[109]. A retrospective cohort study by Chen et al[110] linked metformin use to a lower incidence of bronchial asthma[110]. In vitro and in vivo studies confirm that metformin’s anti-inflammatory effects are mediated by activating 5-AMP-activated protein kinase (AMPK)[111,112], which reduces oxidative stress by regulating cellular proliferation and protein synthesis, particularly affecting nicotinamide adenine dinucleotide phosphate oxidases[113]. Calixto et al[111] observed that metformin reduced allergic eosinophilic inflammation in obese mice on a high-fat diet by inhibiting TNF-α-induced inflammatory signaling and NF-kB-mediated iNOS expression[111]. In a retrospective study by Li et al[109], metformin was associated with fewer asthma exacerbations and hospitalizations despite increased use of short-acting β2-agonists and methylxanthine among treated patients. However, the study did not account for several asthma risk factors like obesity and environmental triggers[109]. Another study found that metformin reduced the risk of asthma-related emergency department visits, though the reduction in asthma exacerbations was not statistically significant[114]. In a cohort of 23920 individuals, metformin use was linked to fewer asthma-related hospitalizations, potentially due to its inhibition of LPS-evoked TLR4 activation, leading to AMPK activation and subsequent reduction in airway inflammation[115-117].
Thiazolidinediones: Thiazolidinediones (TZDs), which act on peroxisome proliferator-activated receptor gamma, are effective in reducing bronchial hyperresponsiveness, airway inflammation, and Th2 cytokine levels[118]. A cohort study of 13528 participants found that TZDs significantly lowered the risk of asthma exacerbations and the need for oral steroids[119]. Pioglitazone, in particular, has shown benefits in controlling asthma in patients with concurrent coronary heart disease, reducing inflammation and improving epithelial function[120]. However, studies by Dixon et al[121] and Anderson et al[122] found no significant changes in asthma control or lung function in patients with asthma treated with pioglitazone over 12 weeks[121,122] (Table 2).
| Drug | Effect | Mechanism |
| Metformin | It reduces the airway inflammation[109,111,112]. It is linked to a lower incidence of asthma[110]. Metformin reduced asthma exacerbation, asthma-related hospitalization, and emergency department visits[109,114,115] | Its anti-inflammatory effect is mediated through the activation of 5 adenosine monophosphate-activated protein kinase[112], which decreases oxidative stress via the regulation of cellular proliferation and protein synthesis with subsequent effects on nicotinamide adenine dinucleotide phosphate-oxidases[113]. Activation of adenosine monophosphate-activated protein kinase also inhibits glycolysis and cytokine production in immune cells with subsequent reduction of airway inflammation, collagen deposition, fibrosis, and airway remodeling[117]. Metformin inhibits tumor necrosis factor-α-induced inflammatory signaling and nuclear factor-kappa B-mediated inducible nitric oxide synthase expression[111]. Activation of lipopolysaccharide evoked Toll-like receptor-4[116] |
| Thiazolidinediones | Thiazolidinediones decrease bronchial hyper-responsiveness[118]. Thiazolidinediones reduce the risk of asthma exacerbation and oral steroid prescription[119]. The use of pioglitazone improves the level of asthma control[120]. Other studies showed no changes in the amount of exhaled nitric oxide, level of asthma control, and lung function parameters using pioglitazone[121,122] | Thiazolidinediones decrease airway inflammation and levels of Th2 cytokines[118]. Thiazolidinediones ameliorate the epithelial function[120] |
| Dipeptidyl peptidase 4 inhibitors | The use of the dipeptidyl peptidase 4 inhibitors did not significantly affect the rate of asthma hospitalization, lower respiratory tract infections, oral glucocorticoid prescriptions, total asthma control, and the number of severe exacerbations[123] | Anti-inflammatory effect |
| GLP1R agonists | GLP1R agonists use results in relaxation of airway smooth muscle cells, lowering of airway eosinophilia, and improvement of airway hyperresponsiveness[126]. GLP1R agonists reduce rates of asthma exacerbations and frequencies of asthma symptoms[128]. GLP1R agonists can improve baseline pulmonary function[126] | GLP1R agonists activate cyclic AMP-dependent protein kinase A in the human airway[126]. GLP1R agonists decrease the expression of IL-5 and IL-13, the lung protein expression of type-2 cytokines and chemokines, the number of perivascular eosinophils, the mucus production, and the airway responsiveness[125]. Liraglutide reduces the number of lung epithelial cells expressing IL-33, the level of IL-33 expression by individual cells, and the level of IL33 in broncho-alveolar lavage fluid |
| Insulin | Increased insulin level increases airway hyperresponsiveness[47,63]. The use of insulin therapy in patients with DM was associated with a higher occurrence of asthma[110]. Other insulin-resistance medications, such as sulfonylureas, improve asthma control[115] | Insulin increases bronchial smooth muscle proliferation[47,63] |
| SGLT2I | SGLT2I use was associated with a reduced risk of incident obstructive airway diseases and a lower rate of obstructive airway diseases exacerbations in comparison with placebo or dipeptidyl peptidase 4[134-136] | The potential anti-inflammatory effect of SGLT2Is was noticed in both animal and human cohort studies[137,138]. SGLT2Is also inhibit NLRP3 inflammasome activation in multiple tissues, including the lung[139]. NLRP3 inflammasome activation has been implicated in asthmatic airway inflammation[140] |
| PDE inhibitors | Nonselective PDE inhibitors such as aminophylline and theophylline increase cAMP and cyclic guanosine monophosphate levels, leading to bronchodilation and reduced airway inflammation. PDE4 Inhibitors such as roflumilast increase cAMP levels in bronchial smooth muscle cells, leading to muscle relaxation and bronchodilation. PDE inhibitors reduce inflammation in the airways. PDE inhibitors can decrease the hyperresponsiveness of airways to allergens and irritants, a key feature of asthma[87,88,141]. PDE inhibitors improve the control of DM and reduce its related complications[142,143] | Modulating cyclic nucleotide signaling pathways that are critical for various cellular functions, including metabolism and smooth muscle relaxation[141,142]. PDE inhibitors have effects on bronchial asthma including bronchodilation, anti-inflammatory effects and reduction of airway hyperresponsiveness. PDE inhibitors reduce inflammation in the airways by inhibiting the breakdown of cAMP, which decreases the release of pro-inflammatory cytokines and reduces the activity of inflammatory cells like eosinophils and neutrophils[87,88,141]. PDE inhibitors have effects on DM, including improved insulin sensitivity and glucose homeostasis, reduction of inflammation, cardiovascular benefits, and potential weight management effects[142,143] |
Dipeptidyl peptidase 4 (DPP-4) inhibitors, a class of oral medications for T2DM, have been studied for their effects on asthma control. A retrospective cohort study (2006-2014) found that one year of DPP-4 inhibitor use did not significantly impact asthma control[123]. Although metformin remains the preferred treatment for T2DM patients with asthma, DPP-4 inhibitors hold promise for future management strategies[124].
Glucagon-like peptide 1 receptor (GLP-1R) agonists, including liraglutide, albiglutide, and semaglutide, have demon
Insulin therapy, essential for T1DM and some T2DM cases, has been associated with increased bronchial smooth muscle proliferation and airway hyperresponsiveness in both animal and in vitro studies[47,63]. A Taiwanese cohort study linked insulin use to a higher incidence of asthma in diabetic patients[110]. Medications that improve insulin resistance, like sulfonylureas, may also enhance asthma control[115].
Sodium/glucose cotransporter 2 inhibitors (SGLT2I) inhibitors, including dapagliflozin and empagliflozin, reduce blood glucose by inhibiting renal glucose reabsorption[133]. A retrospective cohort study in Hong Kong found that SGLT2I use was associated with a reduced risk of obstructive airway disease (OAD) and lower rates of OAD exacerbations than DPP-4 inhibitors[134]. Meta-analyses have supported the association of SGLT2Is with a lower risk of OAD adverse events[135,136]. The anti-inflammatory effects of SGLT2Is, possibly through NLRP3 inflammasome inhibition, may explain their beneficial impact on OAD symptoms[137-140].
Phosphodiesterase (PDE) inhibitors, including aminophylline, theophylline, and roflumilast, treat diabetes and bronchial asthma by modulating cyclic nucleotide signaling pathways. These drugs promote bronchodilation, reduce airway inflammation, and decrease airway hyperresponsiveness[87,88,141,142]. PDE4 inhibitors specifically increase cAMP levels, enhancing insulin signaling and improving glucose uptake[142,143]. Despite their benefits, PDE inhibitors can have undesirable effects on gluconeogenesis regulation.
Research indicates that diabetes significantly increases the risk of asthma attacks[144]. Patients with diabetes exhibit lower lung function parameters than those without the condition, and those with poorly controlled diabetes or higher HbA1c levels experience a more rapid decline in lung function over time[145,146]. This decline is attributed to changes in lung connective tissues, including collagen and elastin, and microangiopathy caused by chronic hyperglycemia-induced nonenzymatic glycosylation of proteins. These changes lead to thickening of the basal lamina of the alveolar epithelium, reducing pulmonary capacity for carbon monoxide diffusion[147].
The Fremantle Diabetes Prospective Study[146] demonstrated that individuals with T2DM have significantly lower lung function, with glycemic exposure being a strong negative predictor of follow-up lung function. This suggests that reduced lung volume and airflow limitations are likely consequences of T2DM. However, the Rancho Bernardo Study[148] found no significant association between pulmonary function and T2DM after adjusting for factors like age, height, and smoking status, though it did note that non-diabetic subjects exhibited significant correlations between fasting plasma glucose levels and spirometric values, suggesting hyperglycemia's harmful effects may begin before diabetes onset. Similarly, Litonjua et al[9] observed impaired lung function in men predisposed to diabetes years before the disease was diagnosed.
Patients with diabetes experience lung function decline due to various mechanisms, including low-grade inflammation, loss of elastic recoil due to lung parenchymal collagen glycosylation, microangiopathy of lung vessels, hypoxia from insulin resistance, and low birth weight. Diabetic polyneuropathy can further impair respiratory muscles, worsening neuromuscular function and impairing diaphragmatic contractility due to phrenic nerve axonal loss[149]. Studies consistently show more significant reductions in FVC than in FEV1 among diabetic patients, regardless of smoking status or obesity, indicating a restrictive rather than obstructive lung function pattern. This deterioration is more pronounced in patients with poor glycemic control and longer disease duration[150].
Diabetes is often associated with obesity and dyslipidemia, conditions that have been linked to more severe and uncontrolled asthma[151]. In a large study from the United Kingdom, Yang et al[152] found that higher HbA1c levels were associated with increased lifetime odds of asthma hospitalization and reduced FEV1 and FVC. Participants with HbA1c in the prediabetic and diabetic range had higher chances of asthma hospitalization and lower lung function[152]. However, limitations included a restricted age range (40 to 69 years) and the study’s cross-sectional design, which did not allow for long-term follow-up[153].
Bronchial asthma in diabetic patients often presents with more severe symptoms, lower quality of life, and reduced responsiveness to asthma medications, which can lead to steroid resistance[154]. While the GEIRD study found no significant difference in bronchial asthma incidence between diabetic and non-diabetic patients, those with diabetes reported more frequent exertional dyspnea and chronic cough[155]. In children and adolescents with T1DM, asthma does not appear to influence the age of diabetes onset. Still, those with both conditions are more likely to use continuous subcutaneous insulin infusion and report hypoglycemic coma, although HbA1c levels remain similar[22].
Several studies have compared lung function parameters between diabetic and non-diabetic patients, consistently finding lower FEV1, FVC, and higher dyspnea scores among diabetic patients, regardless of chronic lung diseases like asthma, chronic bronchitis, or emphysema[156,157]. Smoking cessation has been shown to decrease cardiovascular risk factors, including diabetes, in patients with bronchial asthma[158]. However, hyperglycemia during systemic corticosteroid therapy can delay recovery in acute asthma exacerbations, prolonging hospital stays[89,159]. Additionally, diabetic patients with asthma or COPD are at higher risk of long-term mortality, with diabetes contributing more significantly to this risk than hyperglycemia during hospitalization[28].
Reducing inflammatory markers, BMI, HbA1c, and glucose levels in patients with uncontrolled bronchial asthma and T2DM have improved asthma control[127]. Metformin has also been found to reduce airway inflammation, decreasing the risk of exacerbations and hospitalizations for asthma in diabetic patients[109]. The association between asthma and T2DM can compromise glycemic control and exacerbate asthma, increasing the duration of hospital stays and mortality from respiratory complications[28,156]. Inflammation associated with bronchial asthma significantly contributes to insulin resistance, a key factor in diabetes complications[160-165].
Finally, GDM is more prevalent among patients with asthma and maternal diabetes has been linked to the later need for anti-asthma medications in children born prematurely[166,167]. While ICS do not increase the risk of GDM, there is an increased risk of asthma development in children of mothers with GDM[168,169].
Enhancing glycemic control among diabetes patients is crucial to effectively addressing the interplay between diabetes and asthma. Optimizing blood glucose levels through regular monitoring and medication adjustments can help mitigate the adverse effects on lung function and asthma management. Research indicates that higher HbA1c levels correlate with worsening lung function and increased asthma exacerbations[145,146]. Therefore, maintaining tight control over diabetes can potentially slow the decline in lung function and reduce the frequency of asthma attacks. A multidisciplinary approach is recommended to manage patients with both diabetes and asthma. Coordinating care between endocrinologists, pulmonologists, and primary care providers ensures comprehensive management of these complex conditions. Such collaboration can address the multifaceted needs of patients, including medication management and lifestyle modifications.
Additionally, exploring and utilizing targeted therapies that address diabetes and asthma is beneficial. For example, medications like metformin, known to reduce airway inflammation, may offer dual benefits for patients with comorbid conditions[109]. Understanding the interactions between diabetes and asthma medications can help optimize treatment strategies and improve patient outcomes. Another essential recommendation is to promote lifestyle modifications. Patients should be encouraged to adopt healthy habits such as smoking cessation, weight management, and regular physical activity. Lifestyle factors like smoking and obesity exacerbate both diabetes and asthma, leading to poorer outcomes[151,154]. Addressing these factors can improve overall health and reduce the burden of both conditions.
Patient education and self-management play crucial roles in managing chronic conditions. Comprehensive education on how to manage diabetes and asthma simultaneously can empower patients to adhere to their treatment plans, recognize signs of exacerbations, and maintain regular follow-up appointments. Effective self-management is key to improving health outcomes and quality of life. Further research is needed to explore the long-term effects of diabetes on asthma and lung function, particularly across diverse populations and age groups. Additionally, investigating the impact of early interventions in pre-diabetic individuals on asthma outcomes could provide valuable insights. Current evidence highlights the need for more research to understand the complex interactions between diabetes and asthma and identify effective strategies for prevention and treatment.
Addressing comorbidities such as obesity and dyslipidemia is also essential, as these conditions can worsen both diabetes and asthma control. Targeted interventions to manage these additional risk factors can enhance overall health and improve patient outcomes. Particular attention should be given to pediatric and gestational populations. Monitoring children with diabetes and asthma, as well as those born to mothers with GDM, can help identify and address potential respiratory issues early. Early intervention in these populations can improve long-term outcomes and prevent severe complications associated with both conditions. By implementing these recommendations, healthcare providers can improve the management of patients with both diabetes and asthma, leading to better clinical outcomes and a higher quality of life for these individuals.
It's important to note that the current review has several limitations that may prevent us from drawing a robust conclusion. Some of the studies mentioned in the review are retrospective, which means they rely on pre-existing data and may have potential biases. Additionally, some studies only focused on specific age groups, which may limit their generalizability to broader age ranges. Moreover, some studies utilized cross-sectional designs that make it difficult to establish causality or determine long-term effects. Furthermore, several risk factors that could impact asthma control, such as obesity, allergens, infections, occupational sensitizers, smoking, air pollution, socioeconomic status, and dietary habits, were not included in some studies. This omission could limit our comprehensive understanding of the rela
The relationship between diabetes and asthma is intricate, suggesting bidirectional influences impacting disease course and outcomes. Poor glycemic control in diabetes appears linked to worsened asthma, leading to higher hospitalization rates and reduced lung function. Conversely, asthma severity, particularly in individuals with obesity, seems to affect diabetes control, complicating glycemic outcomes. Inflammation, microangiopathy, and oxidative stress associated with diabetes may contribute to asthma exacerbations and decreased lung function. Certain diabetes medications show promise in mitigating asthma exacerbations through their anti-inflammatory effects. However, limitations in the existing research, primarily retrospective and cross-sectional study designs along with population-specific characteristics, impede the establishment of causal relationships and call for more diverse longitudinal studies to unravel effective treatment strategies for those dealing with both conditions.
We thank the anonymous referees for their valuable suggestions.
| 1. | Holst SS, Sabedin E, Sabedin E, Vermehren C. A Shift in Asthma Treatment According to New Guidelines: An Evaluation of Asthma Patients' Attitudes towards Treatment Change. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2594] [Article Influence: 259.4] [Reference Citation Analysis (0)] |
| 3. | Shu CJ, Benoist C, Mathis D. The immune system's involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012;24:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Mueller NT, Koh WP, Odegaard AO, Gross MD, Yuan JM, Pereira MA. Asthma and the risk of type 2 diabetes in the Singapore Chinese Health Study. Diabetes Res Clin Pract. 2013;99:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Song Y, Klevak A, Manson JE, Buring JE, Liu S. Asthma, chronic obstructive pulmonary disease, and type 2 diabetes in the Women's Health Study. Diabetes Res Clin Pract. 2010;90:365-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Su X, Ren Y, Li M, Zhao X, Kong L, Kang J. Prevalence of Comorbidities in Asthma and Nonasthma Patients: A Meta-analysis. Medicine (Baltimore). 2016;95:e3459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Gershon AS, Guan J, Wang C, Victor JC, To T. Describing and quantifying asthma comorbidity [corrected]: a population study. PLoS One. 2012;7:e34967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Hsia CC, Raskin P. Lung function changes related to diabetes mellitus. Diabetes Technol Ther. 2007;9 Suppl 1:S73-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Litonjua AA, Lazarus R, Sparrow D, Demolles D, Weiss ST. Lung function in type 2 diabetes: the Normative Aging Study. Respir Med. 2005;99:1583-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 1018] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 11. | Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391:783-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 1278] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 12. | Caughey GE, Vitry AI, Gilbert AL, Roughead EE. Prevalence of comorbidity of chronic diseases in Australia. BMC Public Health. 2008;8:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Black MH, Anderson A, Bell RA, Dabelea D, Pihoker C, Saydah S, Seid M, Standiford DA, Waitzfelder B, Marcovina SM, Lawrence JM. Prevalence of asthma and its association with glycemic control among youth with diabetes. Pediatrics. 2011;128:e839-e847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Jia H, Zack MM, Thompson WW. The effects of diabetes, hypertension, asthma, heart disease, and stroke on quality-adjusted life expectancy. Value Health. 2013;16:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70:984-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 16. | Metsälä J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM, Nevalainen J. The association between asthma and type 1 diabetes: a paediatric case-cohort study in Finland, years 1981-2009. Int J Epidemiol. 2018;47:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Ma Q, Yang T. [Prevalence and influential factors for asthma among adults in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Tosca MA, Silòvestri M, D'Annunzio G, Lorini R, Rossi GA, Ciprandi G. May T1 diabetes mellitus protect from asthma? Allergol Immunopathol (Madr). 2013;41:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Koskinen SV, Reunanen AR, Martelin TP, Valkonen T. Mortality in a large population-based cohort of patients with drug-treated diabetes mellitus. Am J Public Health. 1998;88:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001;108:781-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Yun HD, Knoebel E, Fenta Y, Gabriel SE, Leibson CL, Loftus EV Jr, Roger V, Yawn BP, Li B, Juhn YJ. Asthma and proinflammatory conditions: a population-based retrospective matched cohort study. Mayo Clin Proc. 2012;87:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Hörtenhuber T, Kiess W, Fröhlich-Reiterer E, Raile K, Stachow R, Bollow E, Rami-Merhar B, Holl RW; DPV-Wiss Study Group. Asthma in children and adolescents with type 1 diabetes in Germany and Austria: Frequency and metabolic control. Pediatr Diabetes. 2018;19:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, Chaudhuri R, Price D, Brightling CE, Heaney LG; British Thoracic Society Difficult Asthma Network. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 24. | Thomsen SF, Duffy DL, Kyvik KO, Skytthe A, Backer V. Risk of asthma in adult twins with type 2 diabetes and increased body mass index. Allergy. 2011;66:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Hashemzadeh M, Movahed MR. The occurrence of asthma in hospitalized patients with type 2 diabetes mellitus. Intern Med J. 2009;39:699-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Baek JY, Lee SE, Han K, Koh EH; taskforce team of Diabetes Fact Sheet of the Korean Diabetes Association. Association between diabetes and asthma: Evidence from a nationwide Korean study. Ann Allergy Asthma Immunol. 2018;121:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Lee KH, Lee HS. Hypertension and diabetes mellitus as risk factors for asthma in Korean adults: the Sixth Korea National Health and Nutrition Examination Survey. Int Health. 2020;12:246-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Koskela HO, Salonen PH, Romppanen J, Niskanen L. A history of diabetes but not hyperglycaemia during exacerbation of obstructive lung disease has impact on long-term mortality: a prospective, observational cohort study. BMJ Open. 2015;5:e006794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Ehrlich SF, Quesenberry CP Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 30. | Kankaanranta H, Kauppi P, Tuomisto LE, Ilmarinen P. Emerging Comorbidities in Adult Asthma: Risks, Clinical Associations, and Mechanisms. Mediators Inflamm. 2016;2016:3690628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Singh S, Prakash YS, Linneberg A, Agrawal A. Insulin and the lung: connecting asthma and metabolic syndrome. J Allergy (Cairo). 2013;2013:627384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Tesse R, Schieck M, Kabesch M. Asthma and endocrine disorders: shared mechanisms and genetic pleiotropy. Mol Cell Endocrinol. 2011;333:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes. 2017;66:241-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 441] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 34. | Kopf S, Kumar V, Kender Z, Han Z, Fleming T, Herzig S, Nawroth PP. Diabetic Pneumopathy-A New Diabetes-Associated Complication: Mechanisms, Consequences and Treatment Considerations. Front Endocrinol (Lausanne). 2021;12:765201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | van den Borst B, Gosker HR, Zeegers MP, Schols AM. Pulmonary function in diabetes: a metaanalysis. Chest. 2010;138:393-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367:551-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 37. | Wouters EF, Reynaert NL, Dentener MA, Vernooy JH. Systemic and local inflammation in asthma and chronic obstructive pulmonary disease: is there a connection? Proc Am Thorac Soc. 2009;6:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Uppal P, Mohammed SA, Rajashekar S, Giri Ravindran S, Kakarla M, Ausaja Gambo M, Yousri Salama M, Haidar Ismail N, Tavalla P, Hamid P. Type 2 Diabetes Mellitus and Asthma: Pathomechanisms of Their Association and Clinical Implications. Cureus. 2023;15:e36047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 39. | Dandona P, Ghanim H, Monte SV, Caruana JA, Green K, Abuaysheh S, Lohano T, Schentag J, Dhindsa S, Chaudhuri A. Increase in the mediators of asthma in obesity and obesity with type 2 diabetes: reduction with weight loss. Obesity (Silver Spring). 2014;22:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Sindhu S, Koshy M, Al-Roub AA, Akhter N, Al Zanki S, Ali S, Devarajan S, Ahmad R. Differential association of plasma monocyte chemoattractant protein-1 with systemic inflammatory and airway remodeling biomarkers in type-2 diabetic patients with and without asthma. J Diabetes Metab Disord. 2015;15:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Vaseghi H, Sanati MH, Jadali Z. T-helper Cell Type-1 Transcription Factor T-Bet Is Down-regulated in Type 1 Diabetes. Iran J Allergy Asthma Immunol. 2016;15:386-393. [PubMed] |
| 42. | Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SC, Johansson MW, Jarjour NN, Coverstone AM, Castro M, Holguin F, Wenzel SE, Woodruff PG, Bleecker ER, Fahy JV; National Heart, Lung, and Blood Institute Severe Asthma Research Program. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 425] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 43. | Liu Z, Liu Q, Bleich D, Salgame P, Gause WC. Regulation of type 1 diabetes, tuberculosis, and asthma by parasites. J Mol Med (Berl). 2010;88:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Aumeunier A, Grela F, Ramadan A, Pham Van L, Bardel E, Gomez Alcala A, Jeannin P, Akira S, Bach JF, Thieblemont N. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS One. 2010;5:e11484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Verbovoy AF, Kosareva OV, Akhmerova RI. [Leptin, resistin, and hormonal and metabolic parameters in women with type 2 diabetes and in those with its concurrence with asthma]. Ter Arkh. 2015;87:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 292] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 47. | Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, Thompson M, Freeman M, Mabalirajan U, Gosens R, Ghosh B, Pabelick C, Linneberg A, Prakash YS, Agrawal A. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol. 2016;310:L837-L845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 48. | Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4-12. [PubMed] |
| 49. | Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 50. | Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, Hirata K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med. 2011;105:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Bediwy AS, Hassan SM, El-najjar MR. Receptor of advanced glycation end products in childhood asthma exacerbation. Egypt J Chest Dis Tuberc. 2016;65:15-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Yu H, Yang J, Xiao Q, Lü Y, Zhou X, Xia L, Nie D. Regulation of high glucose-mediated mucin expression by matrix metalloproteinase-9 in human airway epithelial cells. Exp Cell Res. 2015;333:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Suratt BT, Ubags NDJ, Rastogi D, Tantisira KG, Marsland BJ, Petrache I, Allen JB, Bates JHT, Holguin F, McCormack MC, Michelakis ED, Black SM, Jain M, Mora AL, Natarajan V, Miller YI, Fessler MB, Birukov KG, Summer RS, Shore SA, Dixon AE; Allergy, Immunology, and Inflammation Assembly. An Official American Thoracic Society Workshop Report: Obesity and Metabolism. An Emerging Frontier in Lung Health and Disease. Ann Am Thorac Soc. 2017;14:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 55. | Agrawal A, Prakash YS. Obesity, metabolic syndrome, and airway disease: a bioenergetic problem? Immunol Allergy Clin North Am. 2014;34:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Brumpton BM, Camargo CA Jr, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 57. | Agrawal M, Kern PA, Nikolajczyk BS. The Immune System in Obesity: Developing Paradigms Amidst Inconvenient Truths. Curr Diab Rep. 2017;17:87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, Moore WC, Peters SP, Yonas M, Teague WG, Wenzel SE. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486-93.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 59. | Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2147] [Cited by in RCA: 2878] [Article Influence: 221.4] [Reference Citation Analysis (0)] |
| 60. | Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O. Gut microbiota in various childhood disorders: Implication and indications. World J Gastroenterol. 2022;28:1875-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (7)] |
| 61. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3224] [Cited by in RCA: 3622] [Article Influence: 201.2] [Reference Citation Analysis (0)] |
| 62. | Murakami D, Anan F, Masaki T, Umeno Y, Shigenaga T, Eshima N, Nakagawa T. Visceral Fat Accumulation Is Associated with Asthma in Patients with Type 2 Diabetes. J Diabetes Res. 2019;2019:3129286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014;51:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Yu D, Simmons D. Association between lung capacity measurements and abnormal glucose metabolism: findings from the Crossroads study. Diabet Med. 2014;31:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Gulcan E, Bulut I, Toker A, Gulcan A. Evaluation of glucose tolerance status in patients with asthma bronchiale. J Asthma. 2009;46:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. Am J Respir Cell Mol Biol. 2011;44:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 67. | Nathan BM, Moran A. Metabolic complications of obesity in childhood and adolescence: more than just diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Al-Beltagi M, Bediwy AS, Saeed NK. Insulin-resistance in paediatric age: Its magnitude and implications. World J Diabetes. 2022;13:282-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (4)] |
| 69. | Arshi M, Cardinal J, Hill RJ, Davies PS, Wainwright C. Asthma and insulin resistance in children. Respirology. 2010;15:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Kim M, Choi S, Choi SH, Shin SH, Kim SK, Shim YS, Jeon YH. Metabolic syndrome and lung function in Korean children and adolescents: A cross-sectional study. Sci Rep. 2019;9:15646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Forno E. A Potential New Treatment Option for Asthma in the Setting of Obesity or Insulin Resistance? Am J Respir Crit Care Med. 2021;203:788-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Kim SH, Kim HS, Min HK, Lee SW. Association between insulin resistance and lung function trajectory over 4 years in South Korea: community-based prospective cohort. BMC Pulm Med. 2021;21:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | McMahon GT, Arky RA. Inhaled insulin for diabetes mellitus. N Engl J Med. 2007;356:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, Khan ZS, Tesfa L, Hall CB, Macian F. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015;191:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 75. | Karampatakis N, Karampatakis T, Galli-Tsinopoulou A, Kotanidou EP, Tsergouli K, Eboriadou-Petikopoulou M, Haidopoulou K. Impaired glucose metabolism and bronchial hyperresponsiveness in obese prepubertal asthmatic children. Pediatr Pulmonol. 2017;52:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Orfanos S, Jude J, Deeney BT, Cao G, Rastogi D, van Zee M, Pushkarsky I, Munoz HE, Damoiseaux R, Di Carlo D, Panettieri RA Jr. Obesity increases airway smooth muscle responses to contractile agonists. Am J Physiol Lung Cell Mol Physiol. 2018;315:L673-L681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 77. | Proskocil BJ, Calco GN, Nie Z. Insulin acutely increases agonist-induced airway smooth muscle contraction in humans and rats. Am J Physiol Lung Cell Mol Physiol. 2021;320:L545-L556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Xu R, Gopireddy RR, Wu Y, Wu L, Tao X, Shao J, Wang W, Li L, Jovanovic A, Xu B, Kenyon NJ, Lu Q, Xiang YK, Fu Q. Hyperinsulinemia promotes heterologous desensitization of β(2) adrenergic receptor in airway smooth muscle in obesity. FASEB J. 2020;34:3996-4008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Hsu CS, Wang PC, Chen JH, Su WC, Tseng TC, Chen HD, Hsiao TH, Wang CC, Lin HH, Shyu RY, Chao YC. Increasing insulin resistance is associated with increased severity and prevalence of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:994-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Bediwy AS, Elkholy MG, Al-Biltagi M, Amer HG, Farid E. Induced Sputum Substance P in Children with Difficult-to-Treat Bronchial Asthma and Gastroesophageal Reflux: Effect of Esomeprazole Therapy. Int J Pediatr. 2011;2011:967460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Elward KS, Pollart SM. Medical Therapy for Asthma: Updates from the NAEPP Guidelines. Am Fam Physician. 2010;82:1242-1251. [PubMed] |
| 82. | Ahmadizar F, Souverein PC, Arets HG, de Boer A, Maitland-van der Zee AH. Asthma related medication use and exacerbations in children and adolescents with type 1 diabetes. Pediatr Pulmonol. 2016;51:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Jessen S, Baasch-Skytte T, Onslev J, Eibye K, Backer V, Bangsbo J, Hostrup M. Muscle hypertrophic effect of inhaled beta(2) -agonist is associated with augmented insulin-stimulated whole-body glucose disposal in young men. J Physiol. 2022;600:2345-2357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Kalinovich A, Dehvari N, Åslund A, van Beek S, Halleskog C, Olsen J, Forsberg E, Zacharewicz E, Schaart G, Rinde M, Sandström A, Berlin R, Östenson CG, Hoeks J, Bengtsson T. Treatment with a β-2-adrenoceptor agonist stimulates glucose uptake in skeletal muscle and improves glucose homeostasis, insulin resistance and hepatic steatosis in mice with diet-induced obesity. Diabetologia. 2020;63:1603-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 85. | König P, Goldstein D, Poehlmann M, Rife D, Ge B, Hewett J. Effect of nebulized albuterol on blood glucose in patients with diabetes mellitus with and without cystic fibrosis. Pediatr Pulmonol. 2005;40:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | Lahiry S, Chatterjee M, Chatterjee S. Impact of oral anticholinergic on insulin response to oral glucose load in patients with impaired glucose tolerance. Indian J Pharmacol. 2021;53:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 87. | Arias AM, Bisschop PH, Ackermans MT, Nijpels G, Endert E, Romijn JA, Sauerwein HP. Aminophylline stimulates insulin secretion in patients with type 2 diabetes mellitus. Metabolism. 2001;50:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | de Galan BE, Tack CJ, Lenders JW, Lutterman JA, Smits P. Effect of 2 weeks of theophylline on glucose counterregulation in patients with type 1 diabetes and unawareness of hypoglycemia. Clin Pharmacol Ther. 2003;74:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 89. | Wytrychowski K, Obojski A, Hans-Wytrychowska A. The Influence of Insulin Therapy on the Course of Acute Exacerbation of Bronchial Asthma. Adv Exp Med Biol. 2016;884:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin Ther. 2017;39:2216-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 377] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 91. | Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141:110-116.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 92. | Daugherty J, Lin X, Baxter R, Suruki R, Bradford E. The impact of long-term systemic glucocorticoid use in severe asthma: A UK retrospective cohort analysis. J Asthma. 2018;55:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 93. | Rodbard HW, Bays HE, Gavin JR 3rd, Green AJ, Bazata DD, Lewis SJ, Fox KM, Reed ML, Grandy S. Rate and risk predictors for development of self-reported type-2 diabetes mellitus over a 5-year period: the SHIELD study. Int J Clin Pract. 2012;66:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Koskela HO, Salonen PH, Niskanen L. Hyperglycaemia during exacerbations of asthma and chronic obstructive pulmonary disease. Clin Respir J. 2013;7:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 96. | Rafacho A, Ortsäter H, Nadal A, Quesada I. Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J Endocrinol. 2014;223:R49-R62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 97. | Katsuyama T, Sada KE, Namba S, Watanabe H, Katsuyama E, Yamanari T, Wada J, Makino H. Risk factors for the development of glucocorticoid-induced diabetes mellitus. Diabetes Res Clin Pract. 2015;108:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 98. | Ernst P, Suissa S. Systemic effects of inhaled corticosteroids. Curr Opin Pulm Med. 2012;18:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Slatore CG, Bryson CL, Au DH. The association of inhaled corticosteroid use with serum glucose concentration in a large cohort. Am J Med. 2009;122:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | O'Byrne PM, Rennard S, Gerstein H, Radner F, Peterson S, Lindberg B, Carlsson LG, Sin DD. Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids. Respir Med. 2012;106:1487-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 101. | Baptist AP, Reddy RC. Inhaled corticosteroids for asthma: are they all the same? J Clin Pharm Ther. 2009;34:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 102. | de Vries TW, de Langen-Wouterse JJ, van Puijenbroek E, Duiverman EJ, de Jong-Van den Berg LT. Reported adverse drug reactions during the use of inhaled steroids in children with asthma in the Netherlands. Eur J Clin Pharmacol. 2006;62:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Faul JL, Wilson SR, Chu JW, Canfield J, Kuschner WG. The effect of an inhaled corticosteroid on glucose control in type 2 diabetes. Clin Med Res. 2009;7:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Bapputty R, Talahalli R, Zarini S, Samuels I, Murphy R, Gubitosi-Klug R. Montelukast Prevents Early Diabetic Retinopathy in Mice. Diabetes. 2019;68:2004-2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 105. | Hamada S, Kuroe A, Tsukino M. Does omalizumab impair glucose homeostasis in a patient with severe persistent asthma and type 2 diabetes mellitus? Rev Port Pneumol (2006). 2017;23:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Yalcin AD, Gorczynski RM, Cilli A, Strauss L. Omalizumab (anti-IgE) therapy increases blood glucose levels in severe persistent allergic asthma patients with diabetes mellitus: 18 month follow-up. Clin Lab. 2014;60:1561-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Macglashan DW Jr, Saini SS. Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol. 2013;132:906-11.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S98-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 698] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 109. | Li CY, Erickson SR, Wu CH. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology. 2016;21:1210-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 110. | Chen CZ, Hsu CH, Li CY, Hsiue TR. Insulin use increases risk of asthma but metformin use reduces the risk in patients with diabetes in a Taiwanese population cohort. J Asthma. 2017;54:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Calixto MC, Lintomen L, André DM, Leiria LO, Ferreira D, Lellis-Santos C, Anhê GF, Bordin S, Landgraf RG, Antunes E. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PLoS One. 2013;8:e76786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 112. | Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, Lee KY, Moon HB, Cho YS. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol. 2012;84:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 113. | Song P, Zou MH. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med. 2012;52:1607-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 114. | Wen L, Zhong W, Chai Y, Zhong Q, Gao J, Guan L, Zhang Mengzhi, Huaiquan L, Haiyang Y, Qingxue W, Changfu Y, Yunzhi C. Association of Metformin Use with Asthma Exacerbation in Patients with Concurrent Asthma and Diabetes: A Systematic Review and Meta-Analysis of Observational Studies. Can Respir J. 2020;2020:9705604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Wu TD, Keet CA, Fawzy A, Segal JB, Brigham EP, McCormack MC. Association of Metformin Initiation and Risk of Asthma Exacerbation. A Claims-based Cohort Study. Ann Am Thorac Soc. 2019;16:1527-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 116. | Vaez H, Najafi M, Toutounchi NS, Barar J, Barzegari A, Garjani A. Metformin Alleviates Lipopolysaccharide-induced Acute Lung Injury through Suppressing Toll-like Receptor 4 Signaling. Iran J Allergy Asthma Immunol. 2016;15:498-507. [PubMed] |
| 117. | Caslin HL, Taruselli MT, Haque T, Pondicherry N, Baldwin EA, Barnstein BO, Ryan JJ. Inhibiting Glycolysis and ATP Production Attenuates IL-33-Mediated Mast Cell Function and Peritonitis. Front Immunol. 2018;9:3026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 118. | Shin NR, Park SH, Ko JW, Cho YK, Lee IC, Kim JC, Shin IS, Kim JS. Lobeglitazone Attenuates Airway Inflammation and Mucus Hypersecretion in a Murine Model of Ovalbumin-Induced Asthma. Front Pharmacol. 2018;9:906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Rinne ST, Feemster LC, Collins BF, Au DH, Perkins M, Bryson CL, O'Riordan TG, Liu CF. Thiazolidinediones and the risk of asthma exacerbation among patients with diabetes: a cohort study. Allergy Asthma Clin Immunol. 2014;10:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Byelan OV, Borzykh OA, Mamontova TV, Kaidashev IP. [Clinical effectiveness of pioglitazone in the combination treatment of patients with asthma concurrent with coronary heart disease]. Ter Arkh. 2015;87:44-51. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Dixon AE, Subramanian M, DeSarno M, Black K, Lane L, Holguin F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir Res. 2015;16:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Anderson JR, Mortimer K, Pang L, Smith KM, Bailey H, Hodgson DB, Shaw DE, Knox AJ, Harrison TW. Evaluation of the PPAR-γ Agonist Pioglitazone in Mild Asthma: A Double-Blind Randomized Controlled Trial. PLoS One. 2016;11:e0160257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 123. | Colice G, Price D, Gerhardsson de Verdier M, Rabon-Stith K, Ambrose C, Cappell K, Irwin DE, Juneau P, Vlahiotis A. The effect of DPP-4 inhibitors on asthma control: an administrative database study to evaluate a potential pathophysiological relationship. Pragmat Obs Res. 2017;8:231-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 124. | Kosmalski M, Różycka-Kosmalska M, Witusik A, Pietras T. The coincidence of diabetes mellitus and asthma, their probable causal relationships and therapeutic opportunities. Adv Respir Med. 2020;88:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Toki S, Goleniewska K, Reiss S, Zhang J, Bloodworth MH, Stier MT, Zhou W, Newcomb DC, Ware LB, Stanwood GD, Galli A, Boyd KL, Niswender KD, Peebles RS Jr. Glucagon-like peptide 1 signaling inhibits allergen-induced lung IL-33 release and reduces group 2 innate lymphoid cell cytokine production in vivo. J Allergy Clin Immunol. 2018;142:1515-1528.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 126. | Rogliani P, Calzetta L, Capuani B, Facciolo F, Cazzola M, Lauro D, Matera MG. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am J Respir Cell Mol Biol. 2016;55:804-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 127. | Yeryomenko GV, Bezditko TV. The treatment of patients with asthma and comorbidity. Med perspektivi (Med perspectives). 2018;23:50-59. [DOI] [Full Text] |
| 128. | Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am J Respir Crit Care Med. 2021;203:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 129. | Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, Maggioni AP, Öhman P, Poulter NR, Ramachandran A, Zinman B, Hernandez AF, Holman RR; EXSCEL Study Group. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 454] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 130. | Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA. 2018;319:1580-1591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 131. | Nguyen DV, Linderholm A, Haczku A, Kenyon N. Glucagon-like peptide 1: A potential anti-inflammatory pathway in obesity-related asthma. Pharmacol Ther. 2017;180:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 132. | Ma W, Jin Q, Guo H, Han X, Xu L, Lu S, Wu C. Metformin Ameliorates Inflammation and Airway Remodeling of Experimental Allergic Asthma in Mice by Restoring AMPKα Activity. Front Pharmacol. 2022;13:780148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 133. | Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S253-S259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 134. | Au PCM, Tan KCB, Lam DCL, Cheung BMY, Wong ICK, Kwok WC, Sing CW, Cheung CL. Association of Sodium-Glucose Cotransporter 2 Inhibitor vs Dipeptidyl Peptidase-4 Inhibitor Use With Risk of Incident Obstructive Airway Disease and Exacerbation Events Among Patients With Type 2 Diabetes in Hong Kong. JAMA Netw Open. 2023;6:e2251177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 135. | Zou HT, Yang GH, Cai YJ, Chen H, Zheng XQ, Hu R. Are High- or Low-dose SGLT2 Inhibitors Associated With Cardiovascular and Respiratory Adverse Events? A Meta-analysis. J Cardiovasc Pharmacol. 2022;79:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 136. | Wang A, Tang H, Zhang N, Feng X. Association between novel Glucose-Lowering drugs and risk of Asthma: A network Meta-Analysis of cardiorenal outcome trials. Diabetes Res Clin Pract. 2022;183:109080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 137. | Leng W, Wu M, Pan H, Lei X, Chen L, Wu Q, Ouyang X, Liang Z. The SGLT2 inhibitor dapagliflozin attenuates the activity of ROS-NLRP3 inflammasome axis in steatohepatitis with diabetes mellitus. Ann Transl Med. 2019;7:429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 138. | Iannantuoni F, M de Marañon A, Diaz-Morales N, Falcon R, Bañuls C, Abad-Jimenez Z, Victor VM, Hernandez-Mijares A, Rovira-Llopis S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 139. | Abd El-Fattah EE, Saber S, Mourad AAE, El-Ahwany E, Amin NA, Cavalu S, Yahya G, Saad AS, Alsharidah M, Shata A, Sami HM, Kaddah MMY, Ghanim AMH. The dynamic interplay between AMPK/NFκB signaling and NLRP3 is a new therapeutic target in inflammation: Emerging role of dapagliflozin in overcoming lipopolysaccharide-mediated lung injury. Biomed Pharmacother. 2022;147:112628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (8)] |
| 140. | Hur J, Kang JY, Kim YK, Lee SY, Lee HY. Glucagon-like peptide 1 receptor (GLP-1R) agonist relieved asthmatic airway inflammation via suppression of NLRP3 inflammasome activation in obese asthma mice model. Pulm Pharmacol Ther. 2021;67:102003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 141. | Goonathilake MR, Waqar S, George S, Jean-Baptiste W, Yusuf Ali A, Inyang B, Koshy FS, George K, Poudel P, Chalasani R, Mohammed L. Can Phosphodiesterase 4 Inhibitor Therapy Be Used in Respiratory Diseases Other Than Chronic Obstructive Pulmonary Disease? Cureus. 2022;14:e27132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 142. | Kilanowska A, Ziółkowska A. Role of Phosphodiesterase in the Biology and Pathology of Diabetes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 143. | Härndahl L, Jing XJ, Ivarsson R, Degerman E, Ahrén B, Manganiello VC, Renström E, Holst LS. Important role of phosphodiesterase 3B for the stimulatory action of cAMP on pancreatic beta-cell exocytosis and release of insulin. J Biol Chem. 2002;277:37446-37455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 144. | Wu TD, Brigham EP, Keet CA, Brown TT, Hansel NN, McCormack MC. Association Between Prediabetes/Diabetes and Asthma Exacerbations in a Claims-Based Obese Asthma Cohort. J Allergy Clin Immunol Pract. 2019;7:1868-1873.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 145. | Walter RE, Beiser A, Givelber RJ, O'Connor GT, Gottlieb DJ. Association between glycemic state and lung function: the Framingham Heart Study. Am J Respir Crit Care Med. 2003;167:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 146. | Davis WA, Knuiman M, Kendall P, Grange V, Davis TM; Fremantle Diabetes Study. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27:752-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 147. | Innocenti F, Fabbri A, Anichini R, Tuci S, Pettinà G, Vannucci F, De Giorgio LA, Seghieri G. Indications of reduced pulmonary function in type 1 (insulin-dependent) diabetes mellitus. Diabetes Res Clin Pract. 1994;25:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Barrett-Connor E, Frette C. NIDDM, impaired glucose tolerance, and pulmonary function in older adults. The Rancho Bernardo Study. Diabetes Care. 1996;19:1441-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 149. | Chance WW, Rhee C, Yilmaz C, Dane DM, Pruneda ML, Raskin P, Hsia CC. Diminished alveolar microvascular reserves in type 2 diabetes reflect systemic microangiopathy. Diabetes Care. 2008;31:1596-1601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 150. | Klein OL, Krishnan JA, Glick S, Smith LJ. Systematic review of the association between lung function and Type 2 diabetes mellitus. Diabet Med. 2010;27:977-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 151. | Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 649] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 152. | Yang G, Han YY, Forno E, Yan Q, Rosser F, Chen W, Celedón JC. Glycated Hemoglobin A(1c), Lung Function, and Hospitalizations Among Adults with Asthma. J Allergy Clin Immunol Pract. 2020;8:3409-3415.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 153. | Hekking PP, Amelink M, Wener RR, Bouvy ML, Bel EH. Comorbidities in Difficult-to-Control Asthma. J Allergy Clin Immunol Pract. 2018;6:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 154. | Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, Stream AR, Fahy JV, Leung DY; Asthma Clinical Research Network. Cluster analysis of obesity and asthma phenotypes. PLoS One. 2012;7:e36631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 155. | De Santi F, Zoppini G, Locatelli F, Finocchio E, Cappa V, Dauriz M, Verlato G. Type 2 diabetes is associated with an increased prevalence of respiratory symptoms as compared to the general population. BMC Pulm Med. 2017;17:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 156. | Klein OL, Aviles-Santa L, Cai J, Collard HR, Kanaya AM, Kaplan RC, Kinney GL, Mendes E, Smith L, Talavera G, Wu D, Daviglus M. Hispanics/Latinos With Type 2 Diabetes Have Functional and Symptomatic Pulmonary Impairment Mirroring Kidney Microangiopathy: Findings From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2016;39:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 157. | Klein OL, Kalhan R, Williams MV, Tipping M, Lee J, Peng J, Smith LJ. Lung spirometry parameters and diffusion capacity are decreased in patients with Type 2 diabetes. Diabet Med. 2012;29:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 158. | Stegberg M, Hasselgren M, Montgomery S, Lisspers K, Ställberg B, Janson C, Sundh J. Changes in smoking prevalence and cessation support, and factors associated with successful smoking cessation in Swedish patients with asthma and COPD. Eur Clin Respir J. 2018;5:1421389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 159. | Ferreira L, Moniz AC, Carneiro AS, Miranda AS, Fangueiro C, Fernandes D, Silva I, Palhinhas I, Lemos J, Antunes J, Leal M, Sampaio N, Faria S. The impact of glycemic variability on length of stay and mortality in diabetic patients admitted with community-acquired pneumonia or chronic obstructive pulmonary disease. Diabetes Metab Syndr. 2019;13:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 160. | Xia C, Rao X, Zhong J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J Diabetes Res. 2017;2017:6494795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 161. | Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 162. | Barros LL, Souza-Machado A, Corrêa LB, Santos JS, Cruz C, Leite M, Castro L, Coelho AC, Almeida P, Cruz AA. Obesity and poor asthma control in patients with severe asthma. J Asthma. 2011;48:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 163. | Adeyeye OO, Ogbera AO, Ogunleye OO, Brodie-Mens AT, Abolarinwa FF, Bamisile RT, Onadeko BO. Understanding asthma and the metabolic syndrome - a Nigerian report. Int Arch Med. 2012;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 164. | Forno E, Zhang P, Nouraie M, Courcoulas A, Mitchell JE, Wolfe BM, Strain G, Khandelwal S, Holguin F. The impact of bariatric surgery on asthma control differs among obese individuals with reported prior or current asthma, with or without metabolic syndrome. PLoS One. 2019;14:e0214730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 165. | Chang YL, Ko HK, Lu MS, Chou CL, Su KC, Hsu CC, Chou KT, Chen TJ, Perng DW, Chou YC. Independent risk factors for death in patients admitted for asthma exacerbation in Taiwan. NPJ Prim Care Respir Med. 2020;30:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 166. | Haataja P, Korhonen P, Ojala R, Hirvonen M, Paassilta M, Gissler M, Luukkaala T, Tammela O. Asthma and atopic dermatitis in children born moderately and late preterm. Eur J Pediatr. 2016;175:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 167. | Baribeau V, Beauchesne MF, Rey É, Forget A, Blais L. The use of asthma controller medications during pregnancy and the risk of gestational diabetes. J Allergy Clin Immunol. 2016;138:1732-1733.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 168. | Zugna D, Galassi C, Annesi-Maesano I, Baïz N, Barros H, Basterrechea M, Correia S, Duijts L, Esplugues A, Fantini MP, Forastiere F, Gascon M, Gori D, Inskip H, Larsen PS, Mommers M, Nybo Andersen AM, Penders J, Petersen MS, Pike K, Porta D, Sonnenschein-van der Voort A, Steuerwald U, Sunyer J, Torrent M, Vrijheid M, Richiardi L, Rusconi F. Maternal complications in pregnancy and wheezing in early childhood: a pooled analysis of 14 birth cohorts. Int J Epidemiol. 2015;44:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 169. | Aspberg S, Dahlquist G, Kahan T, Källén B. Is neonatal phototherapy associated with an increased risk for hospitalized childhood bronchial asthma? Pediatr Allergy Immunol. 2007;18:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/