Published online Jan 15, 2025. doi: 10.4239/wjd.v16.i1.100675
Revised: October 22, 2024
Accepted: November 13, 2024
Published online: January 15, 2025
Processing time: 99 Days and 11.8 Hours
Ma et al recently reported in the World Journal of Diabetes that ferroptosis occurs in osteoblasts under high glucose conditions, reflecting diabetes pathology. This condition could be protected by the upregulation of the gene encoding poly
Core Tip: We propose using polycytosine RNA-binding protein 1 (PCBP1)-loaded protein nanoparticles to treat type 2 diabetic osteoporosis. While PCBP1 is an active pharmaceutical ingredient due to its druggability and small size, current delivery strategies ineffectively preserve its activity. Intravenous injections may be more suitable because they infuse PCBP1 into circulation without mucosal penetration. Moreover, coating PCBP1 with protein can prevent hydrolysis and oxidation; protein nanoparticles are appropriate vesicles as they are also hydrophilic. Additionally, protein materials react with water and oxygen, thereby protecting PCBP1 function. Furthermore, injection-grade PCBP1 and the large-scale production of protein nanoparticles must be investigated to meet pharmaceutical standards.
- Citation: Zhao ZY, Luo PL, Guo X, Huang ZW. Protein nanoparticles as potent delivery vehicles for polycytosine RNA-binding protein one. World J Diabetes 2025; 16(1): 100675
- URL: https://www.wjgnet.com/1948-9358/full/v16/i1/100675.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i1.100675
A recent article entitled ‘Polycytosine RNA-binding protein 1 regulates osteoblast function via a ferroptosis pathway in type 2 diabetic osteoporosis’ in the World Journal of Diabetes reports that osteoblast viability is reduced under high glucose conditions, reflecting diabetes pathology and likely ascribed to the occurrence of ferroptosis[1]. Interestingly, the expression of polycytosine RNA-binding protein 1 (PCBP1) was upregulated under such conditions. Meanwhile, the expression of ferroptosis-associated factors, such as ferritin and glutathione peroxidase 4, were found to be upregulated upon PCBP1 upregulation, demonstrating the inhibition of ferroptosis. These data suggest that PCBP1 protects osteoblasts from ferroptosis, providing a basis for a PCBP1-based treatment of type 2 diabetic osteoporosis (T2DOP).
Notably, in the aforementioned paper, the administration of PCBP1 was achieved by upregulating its expression using a lentivirus infection assay. The lentivirus infection assay is robust and widely used in biomedicine and pharmacology[2-4]; therefore, the methodology is also acceptable for basic research. In addition, there are lentivirus vectors for cell therapy that exhibit clinical translation potential (NCT02650414, NCT02906371 and NCT04684563)[5]. Nonetheless, the stability of these vectors is usually inadequate, with an in vitro half-life of < 40 hours[6]. Consequently, the cost of cold-chain production and transportation of PCBP1-loaded lentiviruses will be overwhelmingly high, imposing a considerable economic burden on society. The unaffordability of such therapy will substantially limit their application. Thus, it is essential to seek alternative approaches to deliver PCBP1-based therapeutics.
Based on our expertise in protein drug discovery and design, we propose that the direct delivery of PCBP1 via protein nanoparticles may be a viable alternative.
According to the Protein Data Bank (PDB, https://www.rcsb.org/) and UniProt (https://www.uniprot.org/), PCBP1 (PDB accession code: 1ZTG; UniProt accession code: Q15365) has a sequence length of 356 amino acid and a molecular weight of 37.02 kDa and consists of four chains. Compared with major antibody drugs (also denoted as protein drugs) that are used in clinical settings, which typically have molecular weights over 100 kDa, such as adalimumab (148 kDa)[7], dostarlimab (144 kDa)[8], pembrolizumab (149 kDa)[9], nivolumab (146 kDa) and infliximab (160 kDa)[10,11], PCBP1 is much smaller. Thus, its druggability is predicted to be high, and we propose utilizing PCBP1 as an active pharmaceutical ingredient.
A tailored delivery system for PCBP1 should be developed. As protein drugs are intrinsically vulnerable to degradation by gastric acids or digestive enzymes, they cannot penetrate the mucosa easily, making oral delivery unsuitable[12]. PCBP1 exhibits low bioavailability via oral administration. In addition, technologies underlying other non-invasive delivery routes, including topical, pulmonary and nasal, are not yet mature for protein drugs. These delivery routes still face challenges in mucosal penetration[13]. In contrast, intravenous injections are the most suitable delivery system for PCBP1, as PCBP1 can be directly infused into circulation without the need for mucosal penetration[14].
However, most protein drugs are susceptible to hydrolysis and oxidation when administered intravenously[15]. When protein drugs interact freely with water or oxygen, hydrolysis, oxidation and other side reactions may occur[16]. PCBP1 likely faces a similar scenario. Therefore, producing an aqueous solution with PCBP1 is inadvisable. Instead, incorporating PCBP1 into a particulate vehicle can prevent its interaction with water or oxygen.
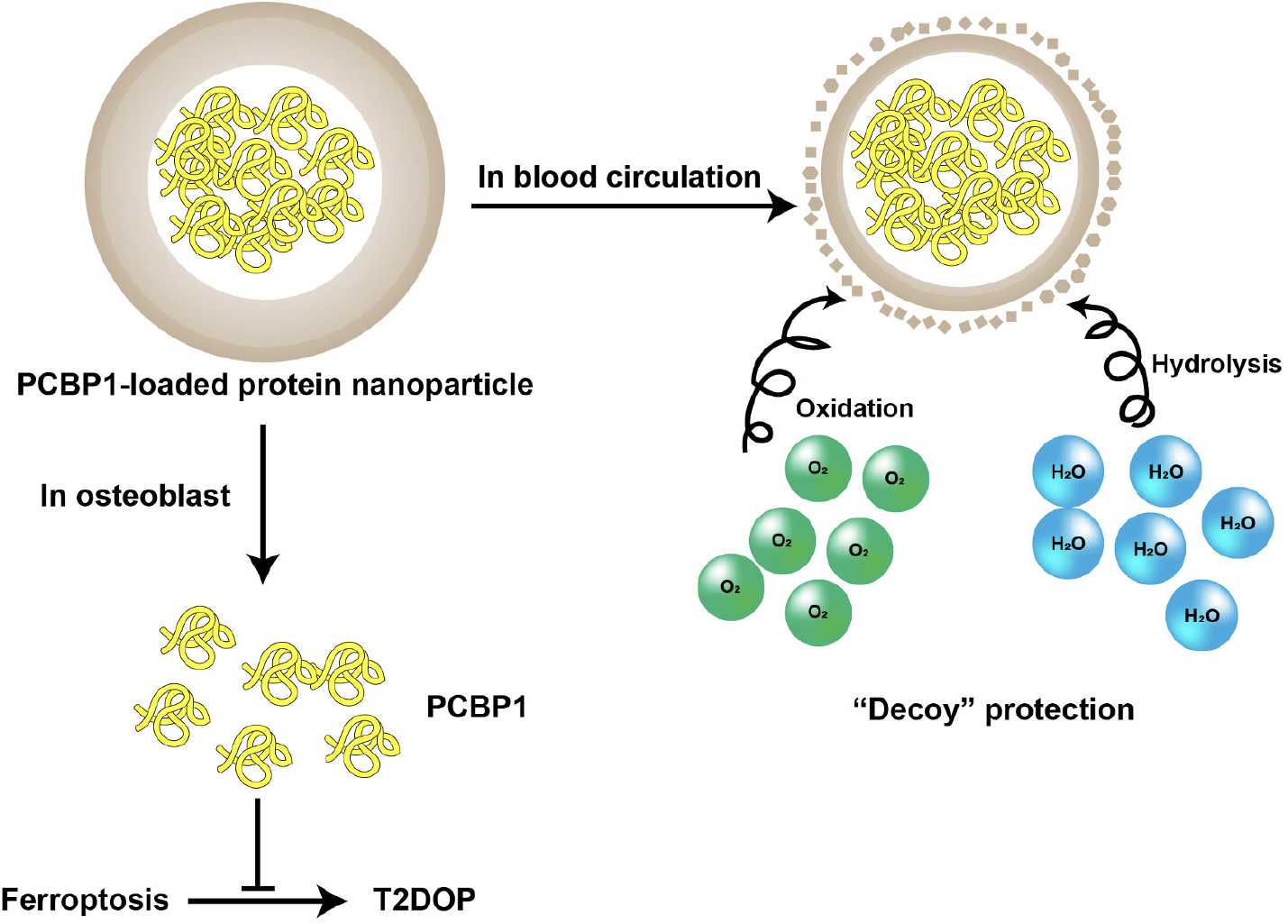
Considering the principle of ‘like dissolves like’[17], protein nanoparticles may be appropriate vesicles to load PCBP1 and protect it from hydrolysis and oxidation. Protein nanoparticles refer to particulate systems composed of protein materials and are at least one-nanosized in dimension[18]. These protein materials mainly involve serum albumin[19], silk fibroin[20], gelatin[21], elastin[22], zein and soybean proteins[23,24]; through coacervation, emulsification, electrospray or other methods[25], they can be assembled into nanoparticles. The preparation methods can vary for different protein materials. For example, serum albumin-based protein nanoparticles (e.g., Abraxane®) have been commercialized in recent years[26]. PCBP1 can be added to and incubated with a protein material, whose amount should far exceed that of PCBP1 to guarantee the formation of the vehicle structure[27]. This way, PCBP1 is embedded as the payload of the prepared protein nanoparticles, and the architecture of the nanoparticles serves as a barrier for PCBP1 and prevents its interaction with water and oxygen. Moreover, the protein within the nanoparticles can act as the decoy for PCBP1; they will react with water and oxygen themselves, consuming them and increasing the protection of PCBP1. It should be noted that injection grade of protein materials and aseptic operation are recommended during the production processes to satisfy the strict pharmaceutical standards for intravenous injections. Thus far, we have proposed the use of PCBP1-loaded protein nanoparticles to treat T2DOP (Figure 1).
Of note, the feasibility of our proposed strategy should be validated, and two major issues must be considered. First, the protein nanoparticle may induce side effects and risks, including gastrointestinal reactions, immune response and liver dysfunction. Thus, the safety of the active pharmaceutical ingredient, PCBP1 and the protein materials must be comprehensively investigated. Second, the industrialized production of protein nanoparticles is limited by some factors, such as introduced organic solvents and the uncontrolled release of ingredients. Thus, the industrialized, large-scale production techniques of protein nanoparticles still require more studies. During the research and development stages, more effort should be paid to settle the above and other unpredicted dilemmas.
We envision that this letter can be a valuable supplement to the original research and help expand its meaning to deepen the research on Peptide drug delivery and diabetic osteoporosis. We also look forward to reading future studies on PCBP1-loaded protein nanoparticles in the World Journal of Diabetes and its sister journals.
| 1. | Ma HD, Shi L, Li HT, Wang XD, Yang MW. Polycytosine RNA-binding protein 1 regulates osteoblast function via a ferroptosis pathway in type 2 diabetic osteoporosis. World J Diabetes. 2024;15:977-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Tandon N, Thakkar KN, LaGory EL, Liu Y, Giaccia AJ. Generation of Stable Expression Mammalian Cell Lines Using Lentivirus. Bio Protoc. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Kandell J, Milian S, Snyder R, Lakshmipathy U. Universal ddPCR-based assay for the determination of lentivirus infectious titer and lenti-modified cell vector copy number. Mol Ther Methods Clin Dev. 2023;31:101120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Kalidasan V, Ng WH, Ishola OA, Ravichantar N, Tan JJ, Das KT. A guide in lentiviral vector production for hard-to-transfect cells, using cardiac-derived c-kit expressing cells as a model system. Sci Rep. 2021;11:19265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Jadlowsky JK, Leskowitz R, McKenna S, Karar J, Ma Y, Dai A, Plesa G, Chen F, Alexander K, Petrella J, Gong N, Hwang WT, Farrelly O, Barber-Rotenberg J, Christensen S, Gonzalez VE, Chew A, Fraietta JA, June CH. Long-term stability of clinical-grade lentiviral vectors for cell therapy. Mol Ther Methods Clin Dev. 2024;32:101186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Dautzenberg IJC, Rabelink MJWE, Hoeben RC. The stability of envelope-pseudotyped lentiviral vectors. Gene Ther. 2021;28:89-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Pelechas E, Voulgari PV, Drosos AA. Preclinical discovery and development of adalimumab for the treatment of rheumatoid arthritis. Expert Opin Drug Discov. 2021;16:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Yadav R, Mathur I, Haokip HR, Pandey AK, Kumar V, Jain N. Dostarlimab: Review on success story and clinical trials. Crit Rev Oncol Hematol. 2024;198:104374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Torrente-López A, Hermosilla J, Salmerón-García A, Cabeza J, Ruiz-Martínez A, Navas N. Comprehensive physicochemical and functional analysis of pembrolizumab based on controlled degradation studies: Impact on antigen-antibody binding. Eur J Pharm Biopharm. 2024;194:131-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Torrente-López A, Hermosilla J, Salmerón-García A, Cabeza J, Navas N. Comprehensive Analysis of Nivolumab, A Therapeutic Anti-Pd-1 Monoclonal Antibody: Impact of Handling and Stress. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 11. | Rodríguez-Prieto T, Hernández-Breijo B, Ortega MA, Gómez R, Sánchez-Nieves J, Guijarro LG. Dendritic Nanotheranostic for the Delivery of Infliximab: A Potential Carrier in Rheumatoid Arthritis Therapy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Hashim LE, Sabri AH, Mohamad MA, Anjani QK, Mustaffa MF, Abdul Hamid K. Circumventing the Gastrointestinal Barrier for Oral Delivery of Therapeutic Proteins and Peptides (PPTS): Current Trends and Future Trajectories. Curr Drug Deliv. 2024;21:211-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Gavali P, Desai J, Shah P, Sawarkar S. Transmucosal Delivery of Peptides and Proteins Through Nanofibers: Current Status and Emerging Developments. AAPS PharmSciTech. 2024;25:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Huang Z, Shu L, Huang Y, Wu C, Pan X. Low Drug Loading Hampers the Clinical Translation of Peptide Drugs-Containing Metered-Dose Inhalers. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 15. | Gupta S, Jiskoot W, Schöneich C, Rathore AS. Oxidation and Deamidation of Monoclonal Antibody Products: Potential Impact on Stability, Biological Activity, and Efficacy. J Pharm Sci. 2022;111:903-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 16. | Houchin ML, Topp EM. Chemical degradation of peptides and proteins in PLGA: a review of reactions and mechanisms. J Pharm Sci. 2008;97:2395-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Zhuang B, Ramanauskaite G, Koa ZY, Wang ZG. Like dissolves like: A first-principles theory for predicting liquid miscibility and mixture dielectric constant. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014;2014:180549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 19. | Hornok V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H. Silk fibroin nanoparticle as a novel drug delivery system. J Control Release. 2015;206:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 21. | Gong H, Zi Y, Kan G, Li L, Shi C, Wang X, Zhong J. Preparation of food-grade EDC/NHS-crosslinked gelatin nanoparticles and their application for Pickering emulsion stabilization. Food Chem. 2024;436:137700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 22. | Phan A, MacKay JA. Steric stabilization of bioactive nanoparticles using elastin-like polypeptides. Adv Drug Deliv Rev. 2024;206:115189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Garavand F, Khodaei D, Mahmud N, Islam J, Khan I, Jafarzadeh S, Tahergorabi R, Cacciotti I. Recent progress in using zein nanoparticles-loaded nanocomposites for food packaging applications. Crit Rev Food Sci Nutr. 2024;64:3639-3659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Wu S, Xia J, Wei Z, Sun W, Zhang X, Xiang N. Preparation, characterization, and foaming properties of soy protein nanoparticles by the cross-linking reaction induced by microbial transglutaminase. Food Hydrocoll. 2023;140:108627. [RCA] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 25. | Tarhini M, Greige-Gerges H, Elaissari A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int J Pharm. 2017;522:172-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 26. | Bhushan B, Khanadeev V, Khlebtsov B, Khlebtsov N, Gopinath P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv Colloid Interface Sci. 2017;246:13-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Pedrozo RC, Antônio E, Khalil NM, Mainardes RM. Bovine serum albumin-based nanoparticles containing the flavonoid rutin produced by nano spray drying. Braz J Pharm Sci. 2020;56:e17692. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/