Published online Jan 15, 2025. doi: 10.4239/wjd.v16.i1.100376
Revised: October 20, 2024
Accepted: November 8, 2024
Published online: January 15, 2025
Processing time: 107 Days and 12.8 Hours
The onset and progression of type 2 diabetes mellitus (T2DM) are strongly associated with imbalances in gut bacteria, making the gut microbiome a new potential therapeutic focus. This commentary examines the recent publication in World Journal of Diabetes. The article explores the association between T2DM and gut microbiota, with a focus on the pathophysiological changes related to dys
Core Tip: Our editorial uncovers the pivotal role of the gut microbiome in type 2 dia
- Citation: Wen X, Qi LM, Zhao K. Influence of gut bacteria on type 2 diabetes: Mechanisms and therapeutic strategy. World J Diabetes 2025; 16(1): 100376
- URL: https://www.wjgnet.com/1948-9358/full/v16/i1/100376.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i1.100376
Type 2 diabetes mellitus (T2DM) is a prevalent metabolic condition resulting from a mix of genetic, environmental and nutritional influences. Even with growing studies on the causes and treatments of T2DM, the global rates of the disease keep climbing. Increasing research suggests a strong connection between gut microbiota imbalance and the onset and progression of T2DM. This imbalance in gut microbiota can affect the host's immune response and metabolic processes for glucose and lipids, contributing significantly to the development of diabetes. Within this framework, the gut microbiome has been identified as a new therapeutic target for managing T2DM[1].
Jeyaraman et al[2] discovered that the gut microbiome of individuals with T2DM, in contrast to healthy people, exhibited an increase in harmful pathogens and a reduction in helpful bacteria, significantly influencing the body's metabolic functions and disease conditions. The increase in bacterial families like Proteobacteria and Firmicutes, coupled with a decline in advantageous bacteria such as Bifidobacterium and Bacteroides[3-5], paints a multifaceted image of gut microbiota imbalance in individuals with T2DM. These microbial changes not only directly participate in the production of key metabolites but also indirectly regulate physiological processes such as insulin secretion, glycogen synthesis and appetite control. The dysbiosis in the gut microbiota of T2DM patients may exacerbate inflammatory responses and damage to pancreatic β cells by increasing intestinal permeability and promoting endotoxin release[6,7].
Organ-targeted therapy has great advantages in improving curative effect and reducing stress[8]. Fecal microbiota transplantation (FMT) is a groundbreaking approach in recent medical advances for restoring intestinal flora. In 2012, a clinical trial showed that FMT using healthy donor microbiota improved insulin sensitivity and increased butyrate-producing bacteria in metabolic syndrome patients after 6 weeks[9]. Similar results were seen in T2DM patients, with an increase in butyrate producers in their fecal microbiota[10]. Probiotics offer an alternative to FMT for a healthy gut microbiota. A study found that T2DM patients who took Lactobacillus casei daily for 8 weeks had higher sirtuin 1 levels, lower fetuin-A levels, and improved blood glucose compared to the placebo group[11]. Another trial showed that combining multistrain probiotics with metformin for 12 weeks increased beneficial bacteria such as Bifidobacterium and reduced proinflammatory bacteria, leading to lower fasting blood glucose and insulin resistance in T2DM patients[12].
Approaches targeting gut microbiota offer promising avenues for T2DM treatment, such as using probiotics to re-establish microbial equilibrium, using FMT to rebuild a healthy gut environment, making dietary changes to encourage beneficial bacteria, and utilizing traditional Chinese medicine to influence gut flora and metabolic processes. These approaches collectively act on the gut microenvironment to improve the condition of T2DM and its complications. Therefore, the regulation and intervention of gut microbiota have become an emerging focus in the field of T2DM treatment.
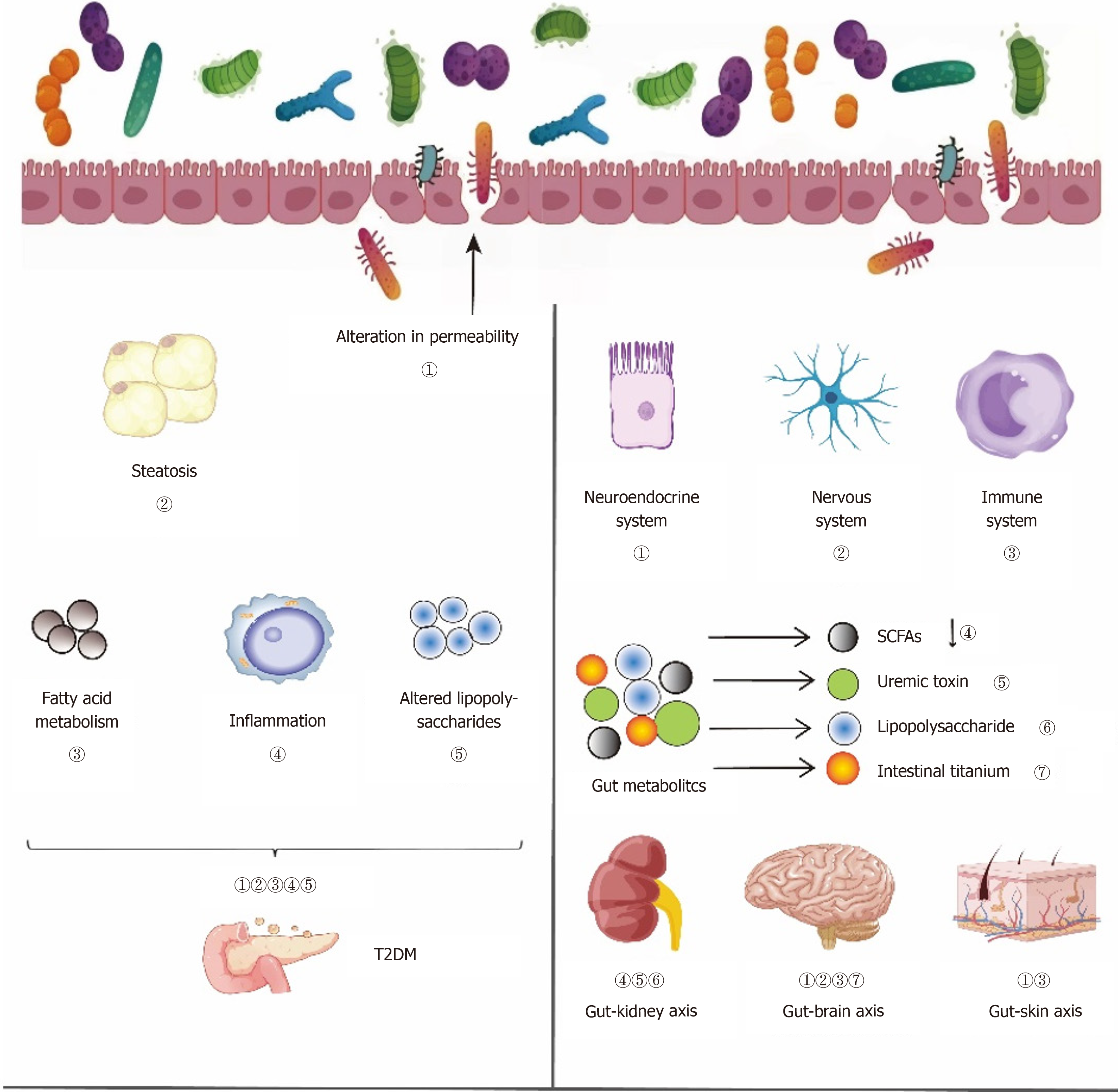
Given the influence of gut microbiota on T2DM complications, we explore how the host–microbiota metabolic axis mediates these effects. In T2DM, the signaling mechanism, involving direct chemical interactions between gut bacteria and the host, influences various organs, including the kidneys, muscles and brain. This aspect is crucial for under
There are similarities and correlations between the physiological and pathological aspects of the skin and gut, with the skin microbiota and skin condition being closely related to the gut microbiota[13]. Probiotic intervention shows pro
Research has found that Bifidobacterium may be related to cognitive function in T2DM patients, and it has also been observed that calcium signaling and the renin–angiotensin system may influence cognitive function in T2DM patients by affecting the metabolism of gut microbiota[15]. Imbalance in gut microbiota can influence the gut–brain connection, affecting glucose regulation and resulting in T2DM. Certain molecules engage directly with enteroendocrine cells and the mucosal immune system, whereas others might traverse the intestinal barrier, enter the bloodstream, and possibly pass through the blood–brain barrier (BBB)[16-18]. Enteroendocrine cells are regarded as crucial elements linking the intestinal microbiota with the nervous system.
In 2011, Meijers and Evenepoel[19] introduced the idea of the gut–kidney axis, demonstrating that changes in gut bacteria might affect the development of diabetic nephropathy by modulating metabolic byproducts. Gut microbiota dysbiosis, coupled with increased intestinal permeability, facilitates the entry of metabolic waste and pathogens into the bloodstream, exacerbating insulin resistance and promoting the progression of diabetes. This is linked to the onset of several long-term illnesses like obesity and metabolic syndrome, which in turn harm nephrons, lower the estimated glomerular filtration rate (eGFR), and impair kidney function[20]. Kidney damage leads to the excessive accumulation of circulating metabolic waste, which, by entering the gut lumen through the impaired gut wall, further aggravates gut dysbiosis, forming a vicious cycle between the gut and kidneys, and worsening diabetic nephropathy. In Figure 1, the left part is the previous author's opinion, and the right part is our opinion.
Studies indicate that Lactobacillus, Bifidobacterium and Faecalibacterium are inversely associated with the onset of T2DM, whereas Ruminococcus and Clostridium show a direct correlation with the condition. Beneficial bacteria associated with T2DM include Bacteroides, Bifidobacterium and Faecalibacterium, whereas harmful bacteria include Clostridium and Ruminococcus[21]. Comprehending how these particular gut microbiota species affect T2DM can facilitate focused research on gut microbiota for T2DM prevention and therapy.
Lactobacillus and Lactococcus can alleviate diabetic symptoms by improving the function of the intestinal mucosal barrier[22], enhancing immune function[23], and improving insulin resistance, thereby promoting glucose utilization in target organs[24]. Given its few side effects and nontoxic nature, Lactobacillus presents a new method for future diabetes prevention and treatment. As one of the gut microbiota that plays a positive role in the intestines, Bifidobacterium supplementation can stabilize the gut microecology and enhance the intestinal barrier function, thereby reducing bacterial translocation[25].
Faecalibacterium prausnitzii (F. prausnitzii), a core strain of Faecalibacterium, has been found to be significantly reduced in abundance in both T1DM and T2DM[26]. F. prausnitzii has been proposed as a marker of a healthy gut. It can convert acetate into butyrate via the butyryl-coenzyme A transferase pathway, thus providing a balanced potential of hydrogen environment in the gut[27].
Fusobacterium triggers the release of proinflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor-α and IL-17, which worsens the inflammation[28,29]. Research indicates that Fusobacterium nucleatum enhances the production of 2-hydroxybutyric acid, a significant contributor to insulin resistance and T2DM. Possible biochemical processes include enhanced fat oxidation and oxidative stress, which could result in higher insulin resistance and reduced glucose tolerance[30,31]. Studies have shown a positive link between Ruminococcus gnavus (R. gnavus) and the occurrence of T2DM[32]. Ruminococcus has been shown to help intestinal epithelial cells absorb sugars, which may lead to weight gain in the host[33]. Studies have found that the R. gnavus group is specific to T2DM, with high abundance in T2DM rats, while short-chain fatty acids (SCFAs) levels are significantly reduced[34]. SCFAs can stimulate G-protein-coupled receptors that play a role in glucose and fat metabolism, thereby demonstrating inherent regulatory functions[35]. Ruminococcus may decrease SCFAs levels in the intestines, disrupting various pathways, which can result in imbalances in lipid and glucose metabolism, ultimately contributing to the onset and advancement of type 2 diabetes.
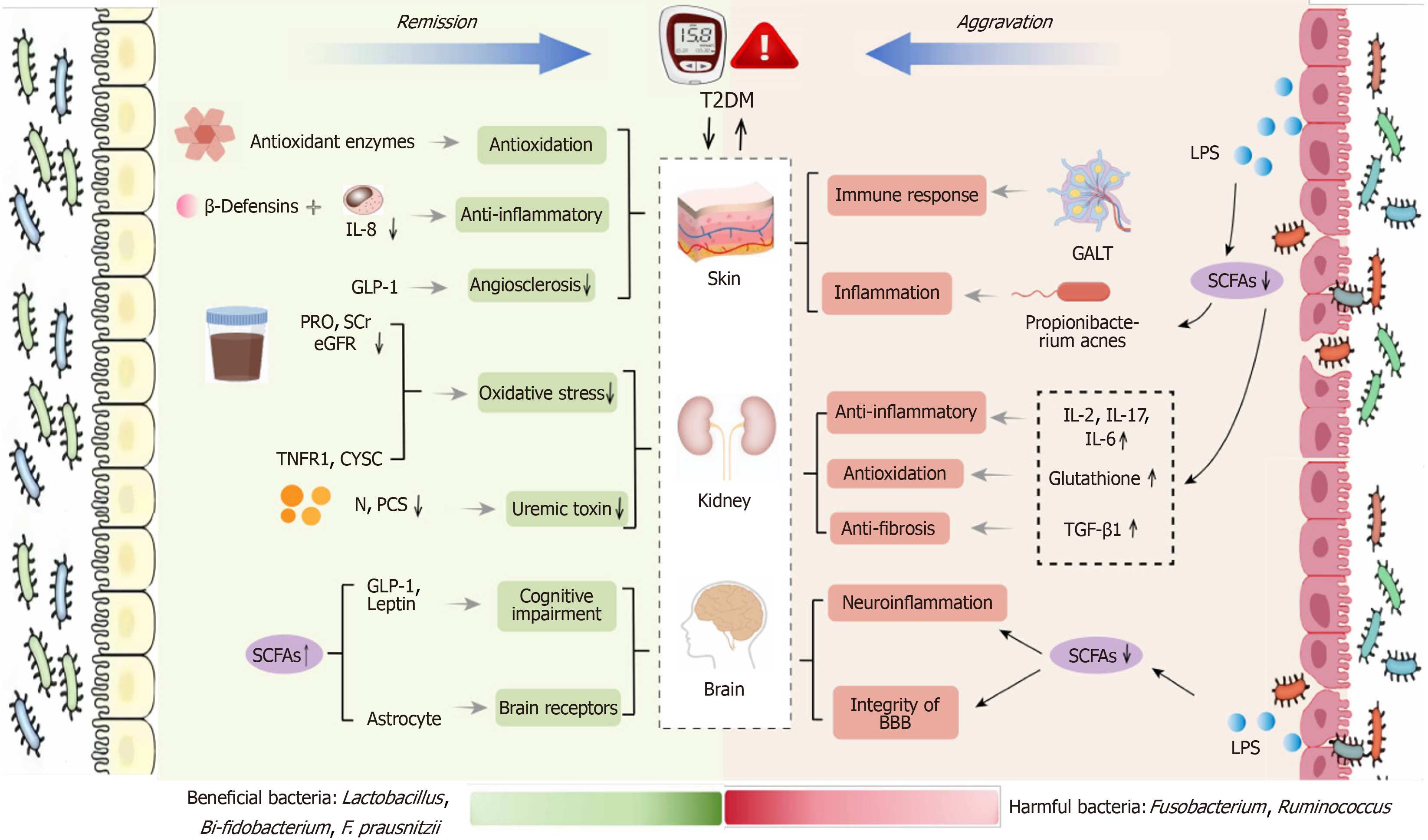
Probiotics such as Lactobacillus, Bifidobacterium and Propionibacterium acnes exert antioxidant effects through the action of antioxidant enzymes like catalase[36], inhibit the cleavage of inhibitory molecules like IkB[37], and reduce the expression of IL-8 to alleviate skin inflammation. Additionally, they can increase the levels of glucagon-like peptide-1 and insulinotropic hormones, enhancing insulin sensitivity[38], thereby mitigating vascular hardening and improving local ischemia. Probiotic soy milk can ameliorate renal oxidative stress, including urinary protein, serum creatinine, and eGFR, and can also reduce the production of serum p-cresol sulfate[39]. Lactobacillus promotes the activation of astrocytes[40], improves the BDNF/TrkB/CREB signaling pathway, and reduces the level of neuronal apoptosis[41,42]. Lactobacillus and others can increase SCFAs, which in turn induce the interaction of intestinal hormones with brain receptors.
In contrast, harmful bacteria such as Fusobacterium and Ruminococcus primarily affect the skin, brain, and kidneys by releasing lipopolysaccharides and reducing SCFAs (Table 1)[22-34]. Ruminococcus may regulate immune responses by affecting gut-associated lymphoid tissue, thereby influencing the skin's reactivity and inflammatory state in response to external stimuli[43]. The reduction of SCFAs can lead to increased glutathione peroxidase activity[44] and promote the production of transforming growth factor β1, exacerbating renal fibrosis[45]. SCFAs can also regulate the integrity of the BBB, thereby alleviating neuroinflammation and the maturation of microglia (Figure 2)[46].
| Specific bacterial species | Influence path | Ref. | |
| Beneficial bacteria | Lactobacillus | Improve the intestinal mucosal barrier function, enhance the immune function of the body, and promote the utilization of glucose by target organs | [22-24] |
| Bifidobacterium | Maintain intestinal barrier function, reduce bacterial translocation, improve metabolic endotoxemia and reduce low level chronic inflammation | [25] | |
| Faecalibacterium prausnitzii | Produce butyrate, provide a balanced potential of hydrogen for the intestine | [26,27] | |
| Harmful bacteria | Fusobacterium | Exacerbating the inflammatory state, produces 2HB | [28-31] |
| Ruminococcus | Reduced short-chain fatty acids, helps the intestinal epithelial cells absorb sugar | [32-34] | |
This research was chosen for editorial comment as it explores the complex connection between the gut microbiota and T2DM, emphasizing its potential for treatment[2]. The global surge in T2DM is affected by genetic predispositions and environmental conditions, with the gut microbiome playing a pivotal role in the development of the disease. The persistent dysbiosis observed in T2DM patients presents opportunities for innovative treatment approaches.
The research outlines changes in gut microbiota in T2DM, highlighting the disrupted Firmicutes/Bacteroidetes ratio and identifying particular bacterial species linked to metabolic issues. These results highlight the possibility of using probiotics, prebiotics, and fecal microbiota transplants to reestablish a balanced gut ecosystem. These measures might provide new ways to enhance insulin responsiveness and lessen the issues linked to T2DM.
Additionally, the review discusses the impact of diabetic medications on gut microbiota, revealing how these drugs can alter microbial composition and influence metabolic outcomes. This insight is crucial for developing more effective, personalized therapeutic strategies that account for individual variations in gut microbiota. The study emphasizes the importance of integrating microbiome research into the broader context of diabetes management to advance the treatment and understanding of T2DM.
While studies on the connection between T2DM and gut bacteria are still in their infancy, gut microbiota shows potential as a novel treatment target for T2DM. Exploring the mechanisms by which the host–microbiota metabolic axis influences T2DM, with a focus on specific gut microbiota species, will require extensive future research. In summary, investigating the more reliable connections and mechanisms between gut microbiota and T2DM could pave the way for safer and more effective approaches to the personalized and precision-based prevention, diagnosis and treatment of T2DM.
| 1. | Qi L, Chen Z, Wang D, Wang L, Soliman MM, El-Bahy SM, Guo Z, El-Bahy ZM, Zhang M, Hu P, Zhao K. Structural characterization of red yeast rice-derived polysaccharide and its promotion of lipid metabolism and gut function in high-fat diet-induced mice. Int J Biol Macromol. 2024;282:136744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Jeyaraman M, Mariappan T, Jeyaraman N, Muthu S, Ramasubramanian S, Santos GS, da Fonseca LF, Lana JF. Gut microbiome: A revolution in type II diabetes mellitus. World J Diabetes. 2024;15:1874-1888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van-Hylckama Vlieg JE, Strissel K, Zhao L, Obin M, Shen J. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 545] [Article Influence: 45.4] [Reference Citation Analysis (1)] |
| 4. | Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, Kim HM, Ahn SH, Kwon BE, Ko HJ, Kweon MN. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 344] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 655] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 6. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1386] [Cited by in RCA: 1187] [Article Influence: 197.8] [Reference Citation Analysis (1)] |
| 7. | Sadagopan A, Mahmoud A, Begg M, Tarhuni M, Fotso M, Gonzalez NA, Sanivarapu RR, Osman U, Latha Kumar A, Mohammed L. Understanding the Role of the Gut Microbiome in Diabetes and Therapeutics Targeting Leaky Gut: A Systematic Review. Cureus. 2023;15:e41559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Hu H, Zhang W, Zhou Y, Zhao K, Kuang J, Liu X, Li G, Xi Y. Engineered mitochondrial ROS scavenger nanocomplex to enhance lung biodistribution and reduce inflammation for the treatment of ARDS. Adv Compos Hybrid Mater. 2024;7:194. [DOI] [Full Text] |
| 9. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2078] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 10. | Ng SC, Xu Z, Mak JWY, Yang K, Liu Q, Zuo T, Tang W, Lau L, Lui RN, Wong SH, Tse YK, Li AYL, Cheung K, Ching JYL, Wong VWS, Kong APS, Ma RCW, Chow EYK, Wong SKH, Ho ICH, Chan PKS, Chan FKL. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. 2022;71:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 11. | Khalili L, Alipour B, Asghari Jafar-Abadi M, Faraji I, Hassanalilou T, Mesgari Abbasi M, Vaghef-Mehrabany E, Alizadeh Sani M. The Effects of Lactobacillus casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran Biomed J. 2019;23:68-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 12. | Palacios T, Vitetta L, Coulson S, Madigan CD, Lam YY, Manuel R, Briskey D, Hendy C, Kim JN, Ishoey T, Soto-Giron MJ, Schott EM, Toledo G, Caterson ID. Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study. Nutrients. 2020;12:2041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Benyacoub J, Bosco N, Blanchard C, Demont A, Philippe D, Castiel-Higounenc I, Guéniche A. Immune modulation property of Lactobacillus paracasei NCC2461 (ST11) strain and impact on skin defences. Benef Microbes. 2014;5:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Mohtashami M, Mohamadi M, Azimi-Nezhad M, Saeidi J, Nia FF, Ghasemi A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol Appl Biochem. 2021;68:1421-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Lu S, Yang Y, Wang Z, Wang B, Zhang B, Yu J, Lu W, Pan M, Zhao J, Guo S, Cheng J, Chen X, Hong K, Li G, Yu Z. The diversity of gut microbiota in type 2 diabetes with or without cognitive impairment. Aging Clin Exp Res. 2021;33:589-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767-16772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1270] [Cited by in RCA: 1169] [Article Influence: 64.9] [Reference Citation Analysis (2)] |
| 17. | Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698-3703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1732] [Cited by in RCA: 2098] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 18. | Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2560] [Article Influence: 232.7] [Reference Citation Analysis (0)] |
| 19. | Meijers BK, Evenepoel P. The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant. 2011;26:759-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. 2017;8:646-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 21. | Das S, Gnanasambandan R. Intestinal microbiome diversity of diabetic and non-diabetic kidney disease: Current status and future perspective. Life Sci. 2023;316:121414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 22. | Michail S, Abernathy F. Lactobacillus plantarum inhibits the intestinal epithelial migration of neutrophils induced by enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 2003;36:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Freitas M, Tavan E, Cayuela C, Diop L, Sapin C, Trugnan G. Host-pathogens cross-talk. Indigenous bacteria and probiotics also play the game. Biol Cell. 2003;95:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Zhao K, Wu X, Han G, Sun L, Zheng C, Hou H, Xu BB, El-Bahy ZM, Qian C, Kallel M, Algadi H, Guo Z, Shi Z. Phyllostachys nigra (Lodd. ex Lindl.) derived polysaccharide with enhanced glycolipid metabolism regulation and mice gut microbiome. Int J Biol Macromol. 2024;257:128588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 25. | Wu J, Wang X, Cai W, Hong L, Tang Q. Bifidobacterium adolescentis supplementation ameliorates parenteral nutrition-induced liver injury in infant rabbits. Dig Dis Sci. 2010;55:2814-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Fassatoui M, Lopez-Siles M, Díaz-Rizzolo DA, Jmel H, Naouali C, Abdessalem G, Chikhaoui A, Nadal B, Jamoussi H, Abid A, Gomis R, Abdelhak S, Martinez-Medina M, Kefi R. Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus. Biosci Rep. 2019;39:BSR20182348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 28. | Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur T, Lagoudas GK, Vatanen T, Fornelos N, Wilson R, Bertha M, Cohen M, Garber J, Khalili H, Gevers D, Ananthakrishnan AN, Kugathasan S, Lander ES, Blainey P, Vlamakis H, Xavier RJ, Huttenhower C. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017;9:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 571] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 29. | Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 789] [Article Influence: 87.7] [Reference Citation Analysis (1)] |
| 30. | Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmüller G, Adamski J, Tuomi T, Lyssenko V, Groop L, Gall WE. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 31. | Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E; RISC Study Group. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5:e10883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 573] [Cited by in RCA: 563] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 32. | Ruuskanen MO, Erawijantari PP, Havulinna AS, Liu Y, Méric G, Tuomilehto J, Inouye M, Jousilahti P, Salomaa V, Jain M, Knight R, Lahti L, Niiranen TJ. Gut Microbiome Composition Is Predictive of Incident Type 2 Diabetes in a Population Cohort of 5,572 Finnish Adults. Diabetes Care. 2022;45:811-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 33. | Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, Chung J, Sohn J, Barber CM, Goldfarb DS, Raju K, Abubucker S, Zhou Y, Ruiz VE, Li H, Mitreva M, Alekseyenko AV, Weinstock GM, Sodergren E, Blaser MJ. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. 2015;6:7486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 34. | An Y, Dai H, Duan Y, Cheng L, Shi L, He C, Wang C, Lv Y, Li H, Zhang H, Huang Y, Fu W, Sun W, Zhao B. The relationship between gut microbiota and susceptibility to type 2 diabetes mellitus in rats. Chin Med. 2023;18:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 35. | De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1674] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 36. | Kiprono S, Wambani J, Langat V, Rono J, Yang G. Microencapsulation of Probiotics and Its Application as Co-Delivery Systems: Review of Literature. ES Food Agrofor. 2024;17. [DOI] [Full Text] |
| 37. | Sharma S, Singh A, Sharma S, Kant A, Sevda S, Taherzadeh MJ, Garlapati VK. Functional foods as a formulation ingredients in beverages: technological advancements and constraints. Bioengineered. 2021;12:11055-11075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Mohseni S, Bayani M, Bahmani F, Tajabadi-Ebrahimi M, Bayani MA, Jafari P, Asemi Z. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev. 2018;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 39. | Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant. 2010;25:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 40. | Li ZH, Jiang YY, Long CY, Peng Q, Yue RS. The gut microbiota-astrocyte axis: Implications for type 2 diabetic cognitive dysfunction. CNS Neurosci Ther. 2023;29 Suppl 1:59-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 42. | Morshedi M, Saghafi-Asl M, Hosseinifard ES. The potential therapeutic effects of the gut microbiome manipulation by synbiotic containing-Lactobacillus plantarum on neuropsychological performance of diabetic rats. J Transl Med. 2020;18:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Mahmud MR, Akter S, Tamanna SK, Mazumder L, Esti IZ, Banerjee S, Akter S, Hasan MR, Acharjee M, Hossain MS, Pirttilä AM. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 256] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 44. | Marzocco S, Fazeli G, Di Micco L, Autore G, Adesso S, Dal Piaz F, Heidland A, Di Iorio B. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance Hemodialysis: Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins, A Pilot Study (PLAN Study). J Clin Med. 2018;7:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Zhang Y, Gao F, Tang Y, Xiao J, Li C, Ouyang Y, Hou Y. Valproic acid regulates Ang II-induced pericyte-myofibroblast trans-differentiation via MAPK/ERK pathway. Am J Transl Res. 2018;10:1976-1989. [PubMed] |
| 46. | Du L, Chen J, Yan J, Xie H, Wang L, Wang R, Han X, Wang Y. Lingguizhugan decoction ameliorates cognitive impairment in AD-like mice by influencing the microbiome-gut-brain axis mediated by SCFAs. Phytomedicine. 2024;133:155942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/