Published online Sep 15, 2024. doi: 10.4239/wjd.v15.i9.1874
Revised: June 11, 2024
Accepted: July 18, 2024
Published online: September 15, 2024
Processing time: 130 Days and 21.9 Hours
Type II diabetes mellitus (T2DM) has experienced a dramatic increase globally across countries of various income levels over the past three decades. The persistent prevalence of T2DM is attributed to a complex interplay of genetic and environmental factors. While numerous pharmaceutical therapies have been developed, there remains an urgent need for innovative treatment approaches that offer effectiveness without significant adverse effects. In this context, the exploration of the gut microbiome presents a promising avenue. Research has increasingly shown that the gut microbiome of individuals with T2DM exhibits distinct differences compared to healthy individuals, suggesting its potential role in the disease’s pathogenesis and progression. This emerging field offers diverse applications, particularly in modifying the gut environment through the administration of prebiotics, probiotics, and fecal microbiome transfer. These inter-ventions aim to restore a healthy microbiome balance, which could potentially alleviate or even reverse the metabolic dysfunctions associated with T2DM. Although current results from clinical trials have not yet shown dramatic effects on diabetes management, the groundwork has been laid for deeper investigation. Ongoing and future clinical trials are critical to advancing our understanding of the microbiome’s impact on diabetes. By further elucidating the mechanisms through which microbiome alterations influence insulin resistance and glucose metabolism, researchers can develop more targeted interventions. The potential to harness the gut microbiome in developing new therapeutic strategies offers a compelling prospect to transform the treatment landscape of T2DM, potentially reducing the disease’s burden significantly with approaches that are less reliant on traditional pharmaceuticals and more focused on holistic, systemic health improvements.
Core Tip: Type II diabetes mellitus (T2DM) has surged globally, driven by genetic and environmental factors. Amidst pharmaceutical options, exploring the gut microbiome stands out. Research reveals distinct microbiome differences in T2DM, suggesting its role in pathogenesis. Interventions such as prebiotics, probiotics, and fecal transfers aim to restore balance. While clinical trials have not shown dramatic effects yet, ongoing research holds promise. Understanding microbiome mechanisms could revolutionize T2DM treatment, emphasizing holistic health approaches.
- Citation: Jeyaraman M, Mariappan T, Jeyaraman N, Muthu S, Ramasubramanian S, Santos GS, da Fonseca LF, Lana JF. Gut microbiome: A revolution in type II diabetes mellitus. World J Diabetes 2024; 15(9): 1874-1888
- URL: https://www.wjgnet.com/1948-9358/full/v15/i9/1874.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i9.1874
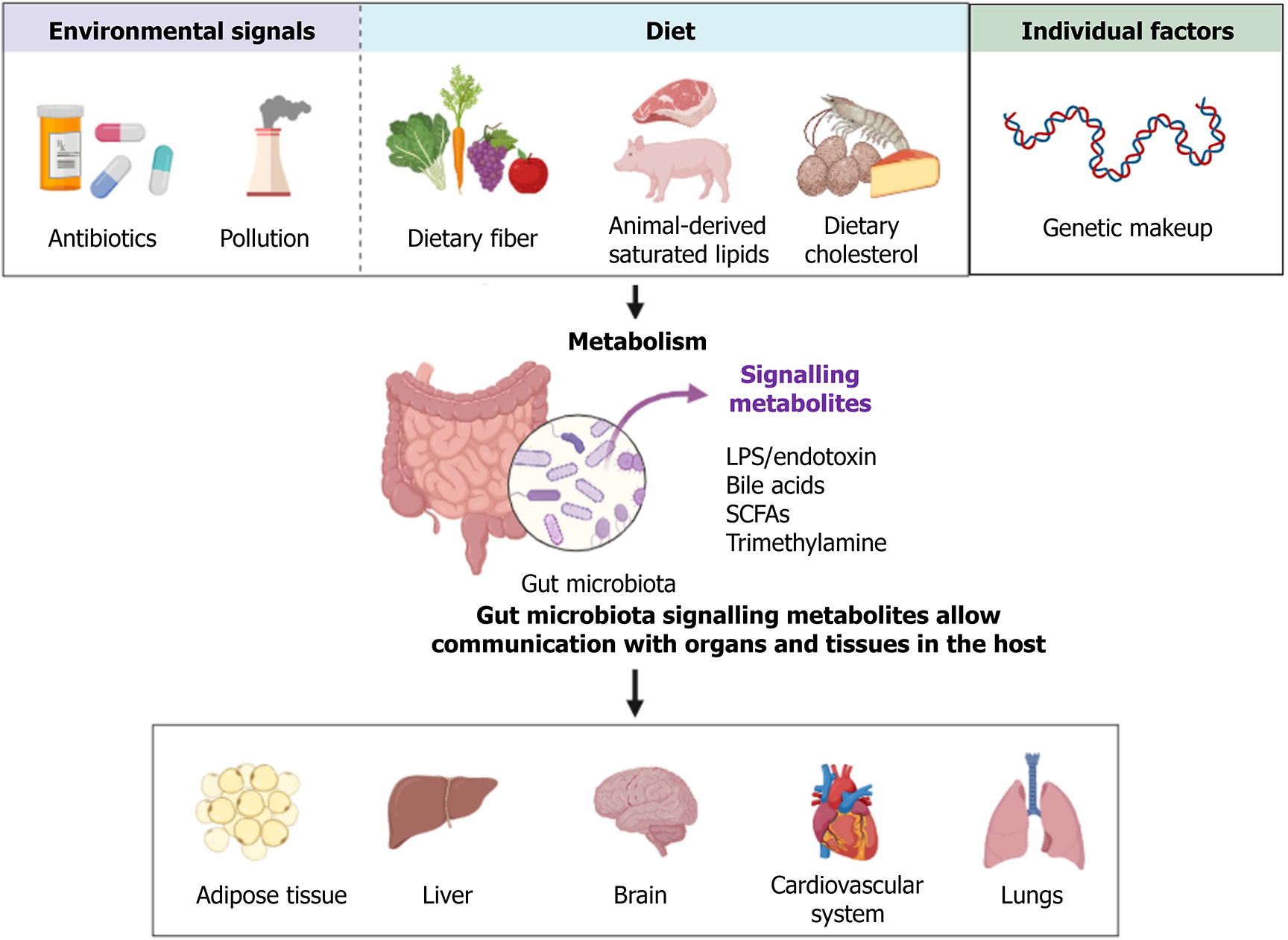
The human gut microbiome is a complex ecosystem, hosting thousands of bacterial species, each exerting a unique influence on host metabolism. This intricate interplay involves a variety of signaling molecules derived from dietary components that are metabolized by these microbiota. The role of the gut microbiome extends beyond digestion, as it actively engages with multiple bodily systems to maintain physiological homeostasis[1]. In the context of type II diabetes mellitus (T2DM), the gut microbiome exhibits notable changes, termed dysbiosis, where there is an increase in bacteria that negatively impact metabolic health and a decrease in beneficial bacteria[2,3]. This shift can lead to a cascade of health issues, including metabolic disorders, cardiovascular complications, neuronal diseases, and various inflammatory conditions, as depicted in Figure 1[4,5].
Despite understanding the broad impacts of gut microbiome dysbiosis, specific pathways, and interactions that lead to T2DM remain underexplored. The mechanisms through which microbial products and toxins contribute to increased intestinal permeability and subsequent inflammation are not fully elucidated. Furthermore, the exact nature of changes in the production of short-chain fatty acids (SCFAs), lipopolysaccharides, and bile acids in diabetics requires detailed investigation[6-8]. These gaps in knowledge hinder the full exploitation of the gut microbiome as a target for therapeutic interventions. Understanding these pathways in greater depth could unveil novel strategies to manage or potentially reverse the effects of dysbiosis in diabetic patients. The primary objective of this review is to synthesize current understandings of the microbiome’s role in T2DM, with a particular focus on the pathophysiological changes associated with dysbiosis. We aimed to identify and discuss novel therapeutic strategies that target the microbiome to ameliorate the symptoms and complications associated with T2DM.
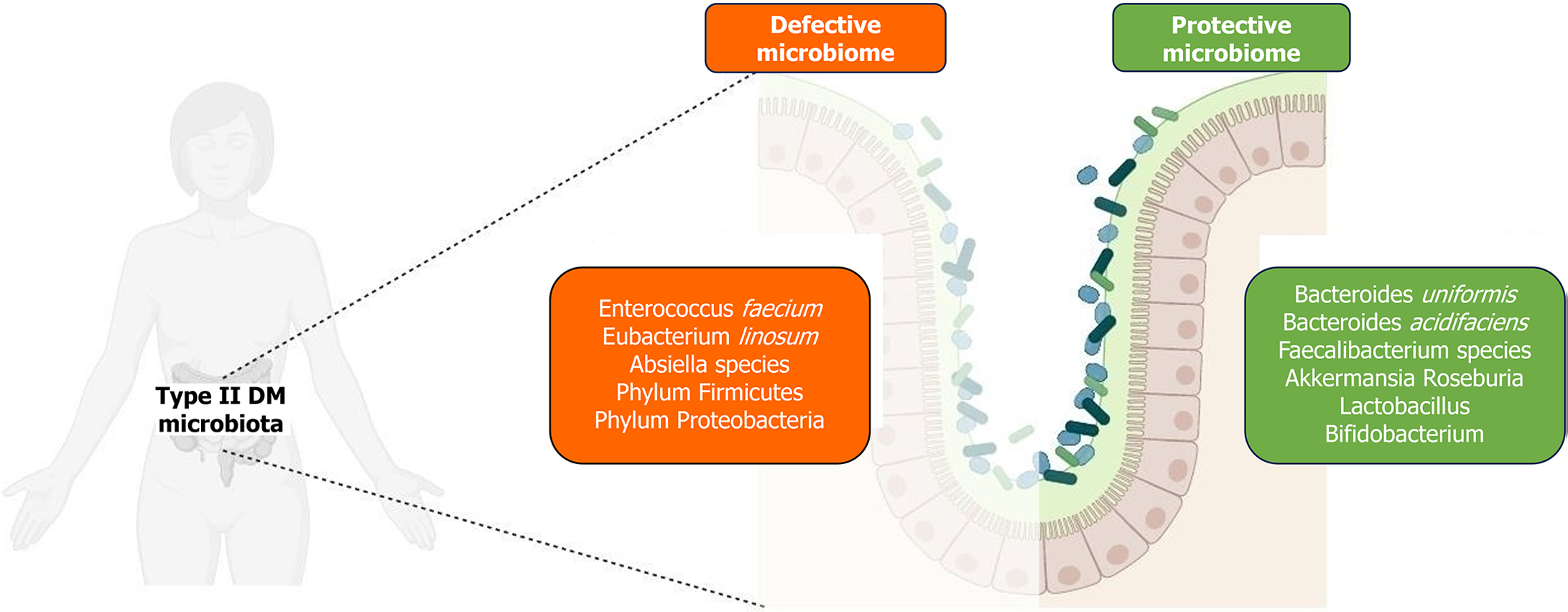
The microbiome composition in individuals with diabetes, particularly T2DM, exhibits significant variations compared to non-diabetic individuals. Notably, the phyla Proteobacteria and Firmicutes[3] are predominantly observed in diabetics. Within Firmicutes, there is a noted increase in the genus Ruminococcus and a decrease in Clostridium species[3], as demonstrated in Figure 2, which outlines the microbiota profile variation in T2DM. However, observations in patients of different ethnicities may vary and needs further investigation.
Research highlights that the Firmicutes-to-Bacteroides ratio, which is typically below 0.8 in healthy individuals, is elevated in those with T2DM and obesity. This altered ratio is indicative of microbial dysbiosis associated with these conditions[9-11]. Using a Predomics approach, three species have been identified as significant biomarkers for T2DM: Enterococcus faecium, Eubacterium linosum, and Absiella spp.[12]. Eubacterium linosum, an anaerobic acetogenic bacterium, is particularly noteworthy for its metabolic functions, including acetate production, sulfate reduction, and the degradation of urea and arginine. These metabolic activities contribute to chronic low-grade inflammation via pro-inflammatory cytokines and metabolites, a hallmark of T2DM[13,14]. Additionally, the production of acetate through the fermentation of galacto-oligosaccharides or inulin is linked to alterations in insulin sensitivity and weight gain[8,15]. Certain species within the Firmicutes phylum are recognized for their enhanced capacity to break down complex sugars and fatty acids, thereby potentially increasing the risk of obesity and T2DM[16]. On the other hand, some species within the Proteobacteria phylum, such as Fusobacterium spp., are implicated in protein fermentation and degradation, leading to dysbiosis. Fusobacterium spp. also contributes to pathogenicity by inducing inflammatory cytokines [interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-17, etc.], which further exacerbate the inflammatory state[17,18].
Several organisms exhibit a negative correlation with T2DM, primarily through mechanisms that involve the production of anti-inflammatory and immunoregulatory metabolites such as butyrate and propionate[14]. Among these, the genus Bifidobacterium is notably protective against T2DM, attributed to its cross-feeding mechanisms that enhance metabolic health[19]. Animal studies further corroborate the benefits of various species within this genus, demonstrating an increase in glucose tolerance[20-24].
Another significant genus negatively correlated with T2DM is Bacteroides. This group, which includes species like Bacteroides uniformis and Bacteroides acidifaciens, is known to improve glucose tolerance and insulin sensitivity, and is instrumental in managing metabolic diseases exacerbated by poor diet[25,26]. Despite their reduced presence in individuals with T2DM, other genera such as Faecalibacterium, Akkermansia, and Roseburia also exhibit similar negative correlations with the disease, though they are not as abundantly reported[27]. Lactobacillus species display variable associations with T2DM; however, species like Lactobacillus plantarum[28], Lactobacillus casei[29], and Lactobacillus reuteri[30] have been shown to improve symptoms when administered as probiotics. In synergy with Bifidobacterium, they confer a collective protective effect against the disease[31-37]. SCFAs such as butyrate play crucial roles beyond their metabolic functions; they regulate pancreatic beta-cell activity, reduce hepatic gluconeogenesis, and modulate immune system functions. The reduction in these critical microbiota directly contributes to the pathogenesis of T2DM.
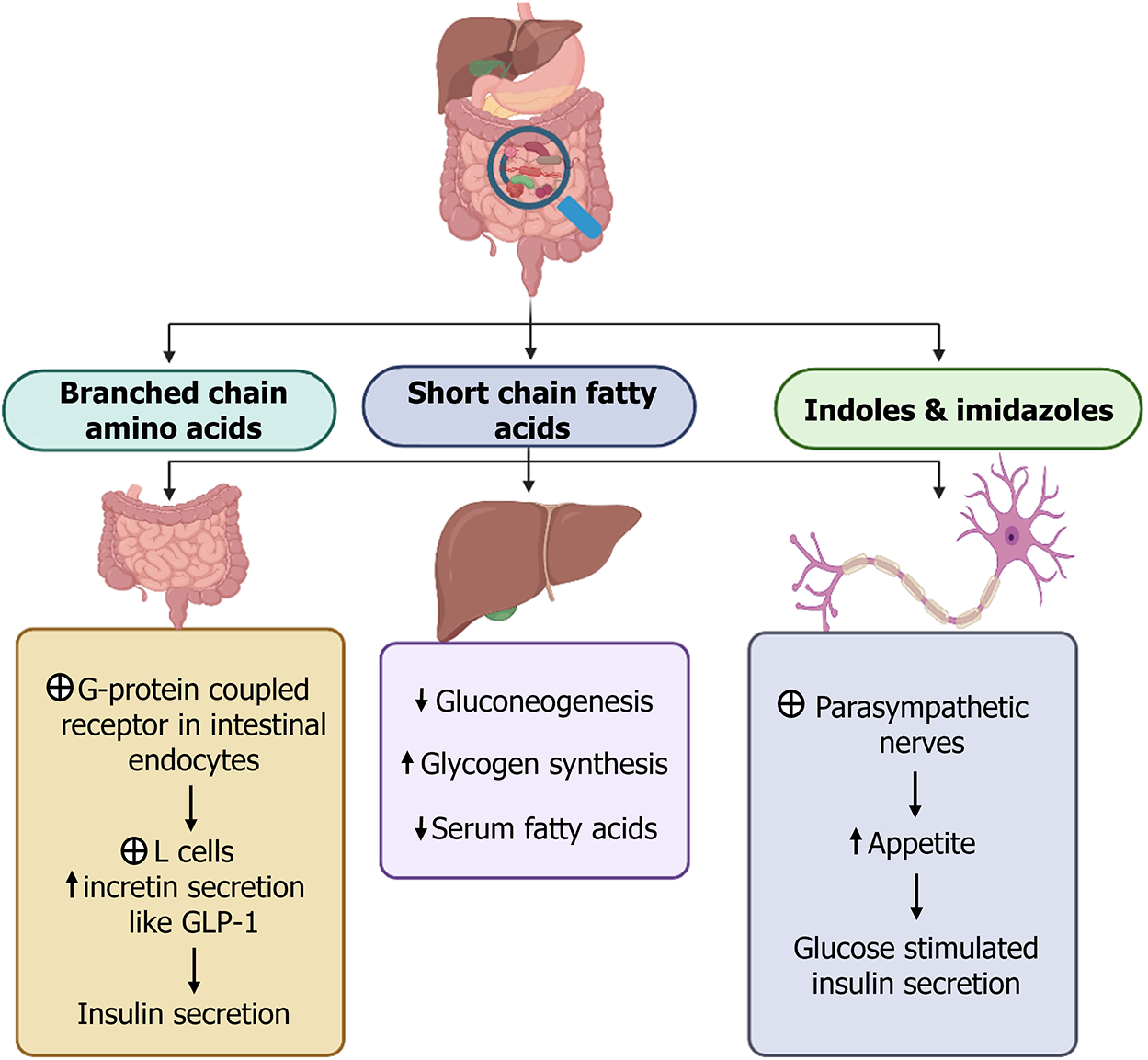
The fermentation of nutrients by gut microbiota leads to the production of metabolites like SCFAs (butyrate, propionate, acetate), branched-chain amino acids, indoles, imidazoles, and succinates. These are predominantly produced by genera including Bacteroides, Akkermansia, Prevotella, Faecalibacterium, Lactobacillus, Clostridium, and Propionibacterium. These metabolites have diverse and significant interactions within the gut environment, as illustrated in Figure 3.
Alteration in permeability and steatosis: Changes in the gut microbiome composition have been associated with T2DM progression, particularly a decrease in species such as Bacteroides and Akkermansia. These microbes are crucial for the regulation of tight junction proteins including occludin and claudin, which maintain intestinal barrier integrity[4,38]. The disruption of these tight junctions leads to increased intestinal permeability. This, in turn, facilitates enhanced nutrient absorption and altered glycemic control, propelling the progression toward steatosis. Concurrently, the reduction in microbial populations that regulate hepatic gluconeogenesis exacerbates liver steatosis.
Inflammation and altered lipopolysaccharides: Increased intestinal permeability allows greater absorption of dietary products and bacterial endotoxins, such as lipopolysaccharides, into the bloodstream. This elevates the production of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-17, and other cytokines linked to T helper (Th)1, Th2, and Th17 responses. This state of endotoxemia drives systemic inflammation and reactive oxygen species production, leading to the destruction of pancreatic beta cells and the onset of insulin resistance[4,38]. The absence of protective microbial effects due to altered microbiota composition, such as those from Roseburia intestinalis, Bacteroides fragilis, Akkermansia spp., and Lactobacillus casei, further compounds the problem. These species induce anti-inflammatory cytokines like IL-10, which may help mitigate low-grade inflammation and potentially improve insulin sensitivity. Roseburia intestinalis, for example, also promotes the production of IL-22, enhancing insulin sensitivity, and transforming growth factor-beta, inhibiting inflammatory processes.
Fatty acid metabolism: The altered composition of gut flora observed in T2DM patients may affect the production of SCFAs. These SCFAs, such as butyrate and propionate, are essential in promoting fatty acid oxidation by inhibiting the expression of peroxisome proliferator-activated receptor-gamma[39]. The absence of these regulatory mechanisms is evident in conditions like increased serum malonaldehyde, a marker for lipid oxidation, which is typically reduced by Lactobacillus casei and Akkermansia muciniphila in experimental models, but is elevated in diabetic subjects[40,41]. This disruption leads to enhanced fat accumulation in adipose tissue and the liver[42-44], triggering a modification in bile acid metabolism. Such alterations further propagate inflammation and microbial imbalance, perpetuating a cyclical exacerbation of T2DM pathogenesis[4]. Having discussed the potential associations between gut metabolites from microbiome dysbiosis and T2DM progression, specific targets to ameliorate their impact remains an area of future research.
The changes in the gut microbiome studied through various human and animal studies, a targeted replenishment of gut microbiota, can lead to delay or prevention in complications of diabetes through supplementation by probiotics, prebiotics, and fecal microbial transplantation, which in turn restores the stability of gut microbiota, as mentioned in Table 1.
| Complication/stage observed | Dysbiosis observed | |
| Decreased | Increased | |
| Diabetic nephropathy | Lactobacillus, Bifidobacterium, Bacteroides, Prevotella, Roseburia, Ruminococcaceae, and Faecalibacterium | Enterococcus, Enterobacteriaceae, Clostridaceae, Klebsiella, and Parabacterides |
| Diabetic neuropathy | Bacteroides and Faecalibacterium | Escherichia, Blautia, Ruminococcus torques, and Lachnoclostridium |
| Diabetic retinopathy | Bacteroidetes and Actinobacteria | Escherihia, Enterobacter, and Acidaminococcus |
| Cerebrovascular disease | Lachnospiraceae, Ruminococcaceae, Bacteroidetes, Prevotella, and Faecalibacterium | Enterobacteriaceae, Veillonellaceae, Bifidobacterium, Lactobacillus, and Oscillobacter |
| Cardiovascular disease | Roseburia, Eubacterium spp, Bacteroides and Faecalibacterium | Collinsella, Escherichia-Shigella, Enterococcus, and the ratio of Firmicutes to Bacteroides |
| Peripheral vascular disease | - | Firmicutes, Actinobacteria, Verrucomicrobia, and Proteobacteria |
Metformin: Metformin is well-documented for its favorable modifications to the gut microbiome. According to Ismail and Evans-Molina[45], metformin treatment leads to changes in the composition of gut microbiota, enhancing the production of SCFAs and altering bile acids. These changes result in increased levels of glucagon-like peptide-1 (GLP-1), which promotes insulin secretion. Additionally, metformin helps normalize the altered microbial community by reversing the Firmicutes/Bacteroides ratio, which is often disrupted in diabetic conditions, thus restoring the microbiome toward a healthier state[4].
Sulfonylureas: The effects of sulfonylureas on the gut microbiome are less clear, with studies presenting conflicting data. One study reports that the use of sulfonylureas is associated with increased levels of phenylalanine and tryptophan, suggesting a potential impact on the microbiome[46]. However, another study found no significant changes in the composition of the gut microbiota with sulfonylurea treatment[47]. This indicates that the influence of sulfonylureas on the gut microbiome may vary depending on other factors such as dosage, duration of treatment, and individual patient microbiome composition.
Alpha-glucosidase inhibitors: Alpha-glucosidase inhibitors prevent the breakdown of oligosaccharides in the small intestine, thereby increasing their availability as nutrients for gut bacteria. This alteration in nutrient availability promotes the growth of beneficial microbes such as Bacteroides, Lactobacillus, and Faecalibacterium, while reducing populations of potentially pathogenic bacteria like Ruminococcus and Butyricicoccus[48]. These microbial shifts are associated with changes in bile acid profiles and improvements in prognostic factors for T2DM.
GLP-1 agonists: GLP-1 agonists, which slow gastric emptying, induce significant shifts in the gut microbial community. Studies indicate a reduction in obesity-promoting organisms within the Firmicutes phylum and an increase in po-pulations of Verrucomicrobia and microbes from the orders Clostridiales and Bacteroidales[49]. These drugs also promote the growth of SCFA-producing bacteria such as Bifidobacterium and Bacteroides, further supporting glycemic control and metabolic health[50].
Sodium-glucose co-transporter type 2 inhibitors: Sodium-glucose co-transporter type 2 inhibitors, such as sotagliflozin, impact the gut microbiome by decreasing the Firmicutes/Bacteroides ratio and enhancing fatty acid production[51]. This alteration not only affects the microbial landscape but also has broader implications for energy metabolism and insulin sensitivity in T2DM patients. A summary of the effects of diabetic medications on the gut microbiome is tabulated in Table 2.
| Medication | Effects on microbiome | Observed outcomes |
| Metformin[45] | Enhances SCFA production, normalizes Firmicutes/Bacteroides ratio | Increased GLP-1 levels, improved insulin secretion |
| Sulfonylureas[46,47] | Conflicting data on impact | Variable influence on microbiome, potential increase in phenylalanine and tryptophan levels |
| Alpha-glucosidase inhibitors[48] | Increases nutrient availability for beneficial bacteria | Growth of beneficial microbes like Bacteroides, improvement in T2DM prognostic factors |
| GLP-1 agonists[49,50] | Changes in gastric emptying rates influence microbiota | Reduction in obesity-promoting organisms, increase in beneficial microbes like Bifidobacterium |
| SGLT-2 inhibitors[51] | Alters microbial ratios favorably | Reduction in Firmicutes/Bacteroides ratio, enhanced fatty acid production |
The influence of diet and exercise on the gut microbiome has significant implications for the management of conditions such as T2DM. Various dietary patterns and physical activities have been shown to differentially affect the composition and functionality of the gut microbiota, with direct consequences on metabolic health (Table 3).
| Factor | Description | Beneficial effects |
| Diet | High fiber plant-based foods[4,52] | Decrease in insulin resistance, stabilization of blood glucose levels, reduction in serum cholesterol |
| High-fat and protein diets[53] | Increase in pro-inflammatory markers; variable effects based on protein source (plant vs animal) | |
| Mediterranean diet[54] | Improvement in SCFA production, enhanced insulin sensitivity, increased beneficial genera like Roseburia | |
| Exercise | Low-intensity physical activity[55-57] | Favorable shifts in microbiota composition, improvement in metabolic health markers |
A diet rich in plant-based fibers such as cellulose, inulin, pectin, and dextrin has been found to confer multiple health benefits, including reduced insulin resistance, lower serum cholesterol levels, and stable blood glucose levels. These effects are largely attributed to the enrichment of beneficial gut bacteria that negatively correlate with metabolic diseases. These bacteria produce SCFAs such as butyrates and propionates, which play a crucial role in reducing the production of pro-inflammatory cytokines, thereby mitigating inflammation associated with T2DM[4,52]. Conversely, diets high in fats and proteins, particularly from animal sources, tend to foster a pro-inflammatory gut environment. This is largely due to an increase in lipopolysaccharides, which are potent inflammatory agents. However, it is important to note that not all protein-rich diets have adverse effects. Diets rich in plant proteins can enhance the proliferation of beneficial microbes such as Lactobacillus spp. and Bifidobacterium spp. In contrast, diets high in animal proteins tend to increase levels of Bacteroides and Bilophila, which have mixed effects on health depending on the overall dietary context[53]. The Mediterranean diet, which is rich in vegetables, fruits, nuts, seeds, and whole grains, has been specifically noted for its positive alterations to the gut microbiota. Adherence to this diet enhances the production of SCFAs, thereby improving insulin sensitivity. Observational studies in obese patients have shown increases in beneficial genera such as Roseburia and Oscillospira, alongside a reduction in Prevotella spp., which are associated with dysbiosis and metabolic disturbances. The beneficial shifts in the gut microbiome are contingent upon strict adherence to the dietary regimen[54].
Physical exercise also plays a critical role in modulating the gut microbiome. Low-intensity physical activities have been associated with favorable changes in the gut microbiota composition, including an increase in the Bacteroides/Firmicutes ratio[55-57]. This shift helps mitigate the adverse effects of an unhealthy diet and contributes to overall metabolic health. Exercise-induced modifications to the microbiome can enhance the resilience of the gut ecosystem, promoting a balance that favors metabolic health and potentially reducing the risk or severity of T2DM.
Lactobacillus and Bifidobacterium are among the most extensively studied probiotics, known for their beneficial effects on glycemic control. Meta-analyses have demonstrated that supplementation with these microbes can significantly decrease hemoglobin A1c, fasting blood glucose, and markers of oxidative stress[58]. Additionally, the introduction of Akkermansia muciniphila, a recent focus in microbiome research, has shown promising results. In murine models, this bacterium has been found to increase GLP-1 levels in colon cells, enhance glucose tolerance, and maintain gut barrier integrity, thereby reducing inflammation and ameliorating liver damage[59]. Research indicates that Akkermansia muciniphila also modulates the Firmicutes/Bacteroides ratio, leading to increased butyrate production. These changes collectively contribute to reduced oxidative stress and improved lipid profiles and glucose tolerance[39].
Beyond probiotics, fecal microbiota transplantation (FMT) represents a more direct method for altering the gut microbiome. Initially popularized through its efficacy in treating Clostridium difficile infections, which often arise from chronic antibiotic use and the resultant suppression of native flora, FMT has since broadened its clinical applications[60]. Data from various human studies suggest that FMT can decrease inflammatory markers and increase the production of secondary bile acids, although its effects on insulin resistance have been relatively mild[61]. This indicates that, while FMT holds potential, its full spectrum of therapeutic benefits in T2DM remains to be fully elucidated through additional clinical trials. Despite its potential, FMT carries risks, particularly the possibility of transferring a dysbiotic microbiome and infectious pathogens. Therefore, while FMT is a promising tool in the arsenal against metabolic diseases, its application must be approached with caution, ensuring rigorous screening and monitoring protocols to mitigate these risks. A summary of evidence of the role of the gut microbiome in T2DM is tabulated in Table 4.
| Ref. | Place of study | Probiotics used | Observed effects |
| Kumari et al[62], 2021 | India | Lactobacillus spp. | Decreased HbA1C, insulin resistance, TNF-α, IL-1β |
| Lactococcus spp. | |||
| Propionibacterium spp. | |||
| Bifidobacterium spp. | |||
| Zhao et al[63], 2020 | China | Selenium enhanced | Decreased FG, HbA1C, and insulin levels and improves glucose tolerance and lipid profile |
| Bifidobacterium spp. | |||
| Palacios et al[64], 2020 | Australia | Lactobacillus plantarum | Decreased FG, HbA1C, and insulin resistance |
| Lactobacillus bulgaricus | |||
| Lactobacillus gasseri | |||
| Bifidobacterium breve | |||
| Bifidobacterium animalis sbsp. lactis | |||
| Bifidobacterium bifidum | |||
| S. thermophiles | |||
| S. boulardii | |||
| Razmpoosh et al[65], 2019 | Iran | Lactobacillus acidophilus | Decreased FG, insulin resistance, and increased HDL cholesterol |
| Lactobacillus casei | |||
| Lactobacillus rhamnosus | |||
| Lactobacillus bulgaricus | |||
| Bifidobacterium breve | |||
| Bifidobacterium longum | |||
| Streptococcus thermophilus | |||
| Madempudi et al[66], 2019 | India | Lactobacillus salivarius | Decreased HbA1C and effects on lipid profile are not significant |
| Lactobacillus casei | |||
| Lactobacillus plantarum | |||
| Lactobacillus acidophilus | |||
| Bifidobacterium breve | |||
| Bifidobacterium coagulans | |||
| Sabico et al[67], 2019 | Saudi Arabia | Bifidobacterium bifidum W23 | Decreased FG, insulin resistance, total cholesterol, and triglycerides |
| Bifidobacterium lactis W52 | |||
| Lactobacillus acidophilus W37 | |||
| Lactobacillus brevis W63 | |||
| Lactobacillus casei W56 | |||
| Lactobacillus salivarius W24 | |||
| Lactobacillus lactis W19 | |||
| Lactobacillus lacis W58 | |||
| Mazruei Arani et al[68], 2019 | Iran | Bacillus coagulans T4 | Decreased FG, insulin resistance, CRP, and improves lipid profile |
| Mohseni et al[37], 2018 | Iran | Lactobacillus acidophilus | Decreased FG, insulin resistance, inflammatory markers, and improves lipid profile |
| Bifidobacterium bifidum | |||
| Lactobacillus casei | |||
| Lactobacillus fementum | |||
| Kobyliak et al[61], 2018 | Ukraine | 14 probiotic strains of Lactobacillus | Decreased HbA1C and insulin resistance |
| Lactococcus | |||
| Bifidobacterium spp. | |||
| Propionibacterium | |||
| Acetobacter | |||
| Kassaian et al[69], 2018 | Iran | Lactobacillus acidophilus | Decreased FG, HbA1C, and insulin resistance |
| Bifidobacterium lactis | |||
| Bifidobacterium bifidum | |||
| Bifidobacterium longum | |||
| Mohseni et al[37], 2018 | Iran | Bifidobacterium bifidum | Decrease FG, insulin resistance, total cholesterol, and increased GSH level |
| Lactobacillus casei | |||
| Lactobacillus acidophilus | |||
| Mofidi et al[35], 2017 | Iran | Lactobacillus casei | Decreased FG and triglycerides |
| Lactobacillus rhamnosus | |||
| Streptococcus thermophilus | |||
| Bifidobacterium breve | |||
| Lactobacillus acidophilus | |||
| Bifidobacterium longum | |||
| Lactobacillus bulgaricus | |||
| Firouzi et al[36], 2017 | Malaysia | Lactobacillus acidophilus | Decreased HbA1C and does not affect lipid profile |
| Lactobacillus casei | |||
| Lactobacillus lactis | |||
| Bifidobacterium bifidum | |||
| Bifidobacterium longum | |||
| Bifidobacterium infantis | |||
| Tajabadi-Ebrahimi et al[33], 2017 | Iran | Lactobacillus acidophilus | Decreased FG, increased insulin sensitivity, and does not affect lipid profile |
| Lactobacillus casei | |||
| Bifidobacterium bifidum | |||
| Ebrahimi et al[70], 2017 | Iran | Lactobacillus spp. | Decreased FG, HbA1C, and no effect on lipid profile |
| Bifidobacterium spp. | |||
| Streptococcus thermophilus and fructo-oligosaccharide | |||
| Asemi et al[71], 2016 | Iran | Probiotic: Lactobacillus sporogenes | Decreased in serum insulin, insulin resistance, triglycerides and increased GSH levels |
| Prebiotic: Inulin, beta-carotene | |||
| Madjd et al[72], 2016 | Iran | Lactobacillus acidophilus LA5 | Decreased HbA1C, 2-h postprandial glucose, insulin resistance, total cholesterol, and LDL levels |
| Bifidobacterium lactis BB12 | |||
| Karamali et al[73], 2016 | Iran | Lactobacillus acidophilus | Decreased fasting glucose, insulin resistance, triglycerides, VLDL, and increased insulin sensitivity |
| Lactobacillus casei | |||
| Bifidobacterium bifidum | |||
| Ostadrahimi et al[74], 2015 | Iran | Lactobacillus acidophilus | Decreased HbA1C, and FG and does not affect lipid profile |
| Lactobacillus casei | |||
| Bifidobacterium lactis | |||
| Eslamparast et al[75], 2014 | Iran | Lactobacillus casei | Decreased FG, insulin resistance and has no effect on lipid profile |
| Lactobacillus rhamnosus | |||
| Streptococcus thermophilus | |||
| Bifidobacterium breve | |||
| Lactobacillus acidophilus | |||
| Bifidobacterium longum | |||
| Lactobacillus bulgaricus | |||
| Rajkumar et al[76], 2014 | India | Bifidobacterium longum | Decreased FG, insulin resistance, total cholesterol, triglycerides, LDL, VLDL, and increased HDL levels |
| Bifidobacterium infantis | |||
| Bifidobacterium breve | |||
| Lactobacillus acidophilus | |||
| Lactobacillus paracasei | |||
| Lactobacillus bulgaricus | |||
| Lactobacillus plantarum | |||
| Streptococcus thermophilus | |||
| Ivey et al[77], 2014 | Australia | Lactobacillus acidophilus La5 | Increased FG and insulin resistance |
| Bifidobacterium lactis Bb12 | |||
| Mohamadshahi et al[78], 2014 | Iran | Bifidobacterium lactis Bb12 | Decreased HbA1C |
| Lactobacillus acidophilus | |||
| Asemi et al[79], 2014 | Iran | Probiotic: Viable & heat-resistant Lactobacillus sporogenes | Decreased FG, HbA1C, insulin resistance, and inflammatory markers |
| Prebiotic: Inulin | |||
| Asemi et al[34], 2013 | Iran | Lactobacillus spp. | Decreased FG and increased insulin levels, total GSH levels, and LDL levels |
| Bifidobacterium spp. | |||
| Streptococcus spp. Fructo-oligosaccharide | |||
| Mazloom et al[80], 2013 | Iran | Lactobacillus acidophilus | Decreased FG, insulin resistance, and improves fasting insulin |
| Lactobacillus bulgaricus | |||
| Lactobacillus bifidum | |||
| Lactobacillus casei | |||
| Shavakhi et al[81], 2013 | Iran | Lactobacillus acidophilus | Decreased FG, triglycerides, and total cholesterol |
| Lactobacillus casei | |||
| Lactobacillus rhamnosus | |||
| Lactobacillus bulgaricus | |||
| Bifidobacterium breve | |||
| Bifidobacterium longum | |||
| Streptococcus thermophilus | |||
| Asemi et al[82], 2013 | Iran | Lactobacillus acidophilus LA5 | Decreased insulin resistance |
| Bifidobacterium animalis BB12 | |||
| Asemi et al[34], 2013 | Iran | Lactobacillus acidophilus | Decreased FG, increased insulin resistance, and LDL levels |
| Lactobacillus casei | |||
| Lactobacillus rhamnosus | |||
| Lactobacillus bulgaricus | |||
| Bifidobacterium breve | |||
| Bifidobacterium longum | |||
| Streptococcus thermophiles | |||
| Moroti et al[31], 2012 | Brazil | Lactobacillus acidophilus | Decreased FG and increased HDL levels |
| Bifidobacterium bifidum | |||
| Ejtahed et al[83], 2012 | Iran | Lactobacillus acidophilus La5 | Decreased FG and HbA1C and does not affect lipid profile |
| Bifidobacterium lactis Bb12 | |||
| Laitinen et al[84], 2009 | Finland | Lactobacillus rhamnosus GG | Decreased FG, insulin resistance, and increased insulin sensitivity |
| Bifidobacterium lactis Bb12 |
One of the principal challenges in leveraging the gut microbiome for diabetes treatment is the inherent variability in microbial composition among individuals. Factors such as genetics, diet, age, and environment significantly influence the gut microbiota, creating a highly personalized microbial ecosystem. This variability can affect the efficacy of probiotic treatments, as the same probiotic strains may not produce identical effects in different individuals. The lack of standardization in probiotic formulations poses another significant challenge. Probiotics are available in various forms, from dietary supplements to fortified foods, with considerable differences in strain specificity, viability, and concentration. These discrepancies can lead to inconsistent study results and confusion about their clinical applicability. Moreover, the regulation of probiotics varies by region, affecting the quality and safety of available products.
While short-term studies have demonstrated the potential benefits of probiotics in managing T2DM, long-term safety and efficacy remain underexplored. Questions about the optimal duration of probiotic therapy, long-term side effects, and the sustainability of beneficial effects need to be addressed through longitudinal studies. A deeper mechanistic understanding of how probiotics interact with both the gut microbiota and the host is crucial. Current knowledge about the pathways through which probiotics influence metabolic health, including their effects on inflammation, insulin sensitivity, and lipid metabolism, is still rudimentary. Enhanced mechanistic insights would facilitate the development of more targeted and effective therapeutic strategies. Interactions between probiotics and other medications commonly used by diabetic patients pose another layer of complexity. The potential for probiotics to affect drug metabolism and efficacy needs thorough investigation to avoid adverse effects and ensure complementary therapeutic outcomes.
Advancing a personalized medicine approach in microbiome research could significantly enhance the efficacy of treatments. By understanding individual microbiome profiles, treatments can be tailored to optimize microbial composition and functionality. This approach would involve integrating detailed genomic, metabolic, and dietary data to predict individual responses to specific probiotic strains. Research into next-generation probiotics, which are specifically engineered or selected based on their beneficial characteristics, is a promising direction. These could include not only bacteria but also other components of the microbiota such as beneficial viruses or fungi that play a role in metabolic regulation.
Combining probiotics with other therapeutic modalities, such as dietary interventions, pharmacotherapy, and lifestyle changes, could enhance overall treatment outcomes. For example, synchronizing probiotic supplementation with a fiber-rich diet might amplify the beneficial effects on the gut microbiome. Conducting more rigorous and comprehensive clinical trials that focus on various demographic groups and different stages of diabetes is crucial. These studies should aim to clarify optimal dosages, treatment durations, and combinations of probiotic strains. Moreover, trials should also assess the impact of probiotics on diabetes complications, offering a broader understanding of their potential benefits. Enhancing healthcare provider and patient education about the potential benefits and limitations of probiotics as part of diabetes management is essential. Increased awareness can lead to more informed decision-making and better clinical outcomes. The limitations and future directions in gut microbiome research for T2DM are summarized in Table 5.
| Limitations | Description | Future directions |
| Variability in microbial composition | Individual differences in microbiome composition complicate standard treatment outcomes | Personalized microbiome interventions: Develop treatments based on individual microbiome assessments to optimize efficacy |
| Lack of standardization | Inconsistencies in probiotic formulations affect study comparability and clinical applicability | Standardization of products: Establish regulations and standards for probiotic formulations to ensure quality and consistency |
| Short-term focus | Most studies have short duration and do not address long-term safety and effectiveness | Longitudinal studies: Conduct long-term studies to assess the sustained effects and safety of microbiome-based interventions |
| Incomplete mechanistic understanding | The pathways through which the microbiome influences diabetes are not fully elucidated | Mechanistic research: Deepen research into the biochemical interactions within the gut microbiome that affect diabetes pathogenesis and treatment |
| Drug-microbiome interactions | Potential interactions between probiotics and anti-diabetic medications are not well understood | Interaction studies: Explore how probiotics interact with common diabetic medications to refine treatment protocols |
| Regulatory hurdles | The global regulatory landscape for probiotics and microbiome therapies varies significantly | Harmonize regulations: Work toward an international consensus on the regulation of microbiome therapies to facilitate global research and application |
T2DM significantly impacts the gut microbiota, leading to dysbiosis, which in turn promotes inflammation and contributes to the development of insulin resistance. The evidence gathered from various trials and studies underscores the pivotal role of nutraceuticals in restoring a balanced gut flora, enhancing insulin sensitivity, and reducing insulin resistance. These beneficial effects have been consistently observed in animal models and, to a lesser extent, in human studies. Although the initial outcomes from human trials have not met all expectations, they lay a solid foundation for future research. Continued exploration and more targeted studies are essential to refine these interventions and fully assess their therapeutic potential. With ongoing advancements and deeper insights into gut microbiome interactions, nutraceuticals hold promise as a novel and effective treatment strategy to manage T2DM. As research progresses, these natural compounds will become an integral part of comprehensive diabetes management, potentially shifting current therapeutic paradigms.
| 1. | Wang B, Yao M, Lv L, Ling Z, Li L. The Human Microbiota in Health and Disease. Engineering. 2017;3:71-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 522] [Article Influence: 58.0] [Reference Citation Analysis (1)] |
| 2. | Barlow GM, Mathur R. Type 2 Diabetes and the Microbiome. J Endocr Soc. 2022;7:bvac184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 3. | Dash NR, Al Bataineh MT, Alili R, Al Safar H, Alkhayyal N, Prifti E, Zucker JD, Belda E, Clément K. Functional alterations and predictive capacity of gut microbiome in type 2 diabetes. Sci Rep. 2023;13:22386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 4. | Sadagopan A, Mahmoud A, Begg M, Tarhuni M, Fotso M, Gonzalez NA, Sanivarapu RR, Osman U, Latha Kumar A, Mohammed L. Understanding the Role of the Gut Microbiome in Diabetes and Therapeutics Targeting Leaky Gut: A Systematic Review. Cureus. 2023;15:e41559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Iatcu CO, Steen A, Covasa M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 6. | Zhang D, Jian YP, Zhang YN, Li Y, Gu LT, Sun HH, Liu MD, Zhou HL, Wang YS, Xu ZX. Short-chain fatty acids in diseases. Cell Commun Signal. 2023;21:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 356] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 7. | Birkeland E, Gharagozlian S, Valeur J, Aas AM. Short-chain fatty acids as a link between diet and cardiometabolic risk: a narrative review. Lipids Health Dis. 2023;22:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 8. | Hernández MAG, Canfora EE, Jocken JWE, Blaak EE. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 401] [Article Influence: 57.3] [Reference Citation Analysis (2)] |
| 9. | Santos VM, Brito AKP, Amorim AT, Souza IR, Santos MB, Campos GB, Dos Santos DC, Júnior ACRB, Santana JM, Santos DB, Mancini MC, Timenetsky J, Marques LM. Evaluation of fecal microbiota and its correlation with inflammatory, hormonal, and nutritional profiles in women. Braz J Microbiol. 2022;53:1001-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 10. | Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 1510] [Article Influence: 251.7] [Reference Citation Analysis (0)] |
| 11. | Stojanov S, Berlec A, Štrukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 1255] [Article Influence: 209.2] [Reference Citation Analysis (0)] |
| 12. | Kolmeder CA, de Vos WM. Roadmap to functional characterization of the human intestinal microbiota in its interaction with the host. J Pharm Biomed Anal. 2021;194:113751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, Gillis CC, Büttner L, Smoot MP, Behrendt CL, Cherry S, Santos RL, Hooper LV, Winter SE. Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe. 2017;21:208-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 14. | Crudele L, Gadaleta RM, Cariello M, Moschetta A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine. 2023;97:104821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 149] [Reference Citation Analysis (1)] |
| 15. | Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1742] [Cited by in RCA: 1902] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 16. | Binda C, Gibiino G, Coluccio C, Sbrancia M, Dajti E, Sinagra E, Capurso G, Sambri V, Cucchetti A, Ercolani G, Fabbri C. Biliary Diseases from the Microbiome Perspective: How Microorganisms Could Change the Approach to Benign and Malignant Diseases. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 17. | Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851-866.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 786] [Article Influence: 87.3] [Reference Citation Analysis (1)] |
| 18. | Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur T, Lagoudas GK, Vatanen T, Fornelos N, Wilson R, Bertha M, Cohen M, Garber J, Khalili H, Gevers D, Ananthakrishnan AN, Kugathasan S, Lander ES, Blainey P, Vlamakis H, Xavier RJ, Huttenhower C. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017;9:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 568] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 19. | Falony G, Vlachou A, Verbrugghe K, De Vuyst L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol. 2006;72:7835-7841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 1745] [Article Influence: 193.9] [Reference Citation Analysis (1)] |
| 21. | Le TK, Hosaka T, Nguyen TT, Kassu A, Dang TO, Tran HB, Pham TP, Tran QB, Le TH, Pham XD. Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed Res. 2015;36:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Moya-Pérez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS One. 2015;10:e0126976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Kikuchi K, Ben Othman M, Sakamoto K. Sterilized bifidobacteria suppressed fat accumulation and blood glucose level. Biochem Biophys Res Commun. 2018;501:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, Hattori M, Koga Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep. 2017;7:43522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 25. | Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, Kim HM, Ahn SH, Kwon BE, Ko HJ, Kweon MN. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 26. | Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van-Hylckama Vlieg JE, Strissel K, Zhao L, Obin M, Shen J. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 545] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 27. | Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 28. | Okubo T, Takemura N, Yoshida A, Sonoyama K. KK/Ta Mice Administered Lactobacillus plantarum Strain No. 14 Have Lower Adiposity and Higher Insulin Sensitivity. Biosci Microbiota Food Health. 2013;32:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, Matsuzaki T, Miyazaki K, Ishikawa F. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Fåk F, Bäckhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe-/- mice. PLoS One. 2012;7:e46837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Moroti C, Souza Magri LF, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 32. | Kijmanawat A, Panburana P, Reutrakul S, Tangshewinsirikul C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J Diabetes Investig. 2019;10:163-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 33. | Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, Raygan F, Karamali F, Razzaghi R, Taheri S, Asemi Z. A Randomized Controlled Clinical Trial Investigating the Effect of Synbiotic Administration on Markers of Insulin Metabolism and Lipid Profiles in Overweight Type 2 Diabetic Patients with Coronary Heart Disease. Exp Clin Endocrinol Diabetes. 2017;125:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 35. | Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 36. | Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. 2017;56:1535-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Mohseni S, Bayani M, Bahmani F, Tajabadi-Ebrahimi M, Bayani MA, Jafari P, Asemi Z. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev. 2018;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1386] [Cited by in RCA: 1184] [Article Influence: 197.3] [Reference Citation Analysis (1)] |
| 39. | den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ, Bakker BM. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes. 2015;64:2398-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 858] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 40. | Zhang L, Qin Q, Liu M, Zhang X, He F, Wang G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog Dis. 2018;76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 41. | Li X, Wang N, Yin B, Fang D, Jiang T, Fang S, Zhao J, Zhang H, Wang G, Chen W. Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J Appl Microbiol. 2016;121:1727-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Ojo O, Feng QQ, Ojo OO, Wang XH. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 43. | Xi Y, Xu PF. Diabetes and gut microbiota. World J Diabetes. 2021;12:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 44. | Gentile JKA, Oliveira KD, Pereira JG, Tanaka DY, Guidini GN, Cadona MZ, Siriani-Ribeiro DW, Perondini MT. THE INTESTINAL MICROBIOME IN PATIENTS UNDERGOING BARIATRIC SURGERY: A SYSTEMATIC REVIEW. Arq Bras Cir Dig. 2022;35:e1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 45. | Ismail HM, Evans-Molina C. Does the Gut Microbiome Play a Role in Obesity in Type 1 Diabetes? Unanswered Questions and Review of the Literature. Front Cell Infect Microbiol. 2022;12:892291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 46. | Đanić M, Pavlović N, Stanimirov B, Lazarević S, Vukmirović S, Al-Salami H, Mikov M. PAMPA model of gliclazide permeability: The impact of probiotic bacteria and bile acids. Eur J Pharm Sci. 2021;158:105668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | van Bommel EJM, Herrema H, Davids M, Kramer MHH, Nieuwdorp M, van Raalte DH. Effects of 12-week treatment with dapagliflozin and gliclazide on faecal microbiome: Results of a double-blind randomized trial in patients with type 2 diabetes. Diabetes Metab. 2020;46:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 48. | Zhang X, Fang Z, Zhang C, Xia H, Jie Z, Han X, Chen Y, Ji L. Effects of Acarbose on the Gut Microbiota of Prediabetic Patients: A Randomized, Double-blind, Controlled Crossover Trial. Diabetes Ther. 2017;8:293-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 49. | Madsen MSA, Holm JB, Pallejà A, Wismann P, Fabricius K, Rigbolt K, Mikkelsen M, Sommer M, Jelsing J, Nielsen HB, Vrang N, Hansen HH. Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Sci Rep. 2019;9:15582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 50. | Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, Wang T, Wang X. Featured article: Structure moderation of gut microbiota in liraglutide-treated diabetic male rats. Exp Biol Med (Maywood). 2018;243:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Cefalo CMA, Cinti F, Moffa S, Impronta F, Sorice GP, Mezza T, Pontecorvi A, Giaccari A. Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives. Cardiovasc Diabetol. 2019;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 52. | Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, Bhutani T, Liao W. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1643] [Cited by in RCA: 1729] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 53. | Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, Moulton L, Glawe A, Wang Y, Leone V, Antonopoulos DA, Smith D, Chang EB, Ciancio MJ. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9:e92193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 54. | Kerry RG, Patra JK, Gouda S, Park Y, Shin HS, Das G. Benefaction of probiotics for human health: A review. J Food Drug Anal. 2018;26:927-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 665] [Cited by in RCA: 504] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 55. | Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, Viggiano A, Cibelli G, Chieffi S, Monda M, Messina G. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid Med Cell Longev. 2017;2017:3831972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 406] [Article Influence: 45.1] [Reference Citation Analysis (2)] |
| 56. | Aragón-Vela J, Solis-Urra P, Ruiz-Ojeda FJ, Álvarez-Mercado AI, Olivares-Arancibia J, Plaza-Diaz J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 57. | Wegierska AE, Charitos IA, Topi S, Potenza MA, Montagnani M, Santacroce L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022;52:2355-2369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (1)] |
| 58. | Singh VP, Sharma J, Babu S, Rizwanulla, Singla A. Role of probiotics in health and disease: a review. J Pak Med Assoc. 2013;63:253-257. [PubMed] |
| 59. | Tao YW, Gu YL, Mao XQ, Zhang L, Pei YF. Effects of probiotics on type II diabetes mellitus: a meta-analysis. J Transl Med. 2020;18:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 60. | Svensson CK, Cold F, Ribberholt I, Zangenberg M, Mirsepasi-Lauridsen HC, Petersen AM, Helms M. The Efficacy of Faecal Microbiota Transplant and Rectal Bacteriotherapy in Patients with Recurrent Clostridioides difficile Infection: A Retrospective Cohort Study. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab Syndr. 2018;12:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 62. | Kumari M, Singh P, Nataraj BH, Kokkiligadda A, Naithani H, Azmal Ali S, Behare PV, Nagpal R. Fostering next-generation probiotics in human gut by targeted dietary modulation: An emerging perspective. Food Res Int. 2021;150:110716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 63. | Zhao D, Zhu H, Gao F, Qian Z, Mao W, Yin Y, Tan J, Chen D. Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice. Food Funct. 2020;11:6528-6541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 64. | Palacios T, Vitetta L, Coulson S, Madigan CD, Lam YY, Manuel R, Briskey D, Hendy C, Kim JN, Ishoey T, Soto-Giron MJ, Schott EM, Toledo G, Caterson ID. Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 65. | Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes Metab Syndr. 2019;13:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 66. | Madempudi RS, Ahire JJ, Neelamraju J, Tripathi A, Nanal S. Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: A double blind, randomized, placebo controlled study. PLoS One. 2019;14:e0225168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | Sabico S, Al-Mashharawi A, Al-Daghri NM, Wani K, Amer OE, Hussain DS, Ahmed Ansari MG, Masoud MS, Alokail MS, McTernan PG. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1561-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 68. | Mazruei Arani N, Emam-Djomeh Z, Tavakolipour H, Sharafati-Chaleshtori R, Soleimani A, Asemi Z. The Effects of Probiotic Honey Consumption on Metabolic Status in Patients with Diabetic Nephropathy: a Randomized, Double-Blind, Controlled Trial. Probiotics Antimicrob Proteins. 2019;11:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 69. | Kassaian N, Feizi A, Aminorroaya A, Jafari P, Ebrahimi MT, Amini M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: a double-blind randomized clinical trial. Acta Diabetol. 2018;55:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 70. | Ebrahimi ZS, Nasli-Esfahani E, Nadjarzade A, Mozaffari-Khosravi H. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: a randomized, double-blind, clinical trial. J Diabetes Metab Disord. 2017;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Asemi Z, Alizadeh SA, Ahmad K, Goli M, Esmaillzadeh A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2016;35:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 72. | Madjd A, Taylor MA, Mousavi N, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: a randomized controlled trial. Am J Clin Nutr. 2016;103:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | Karamali M, Dadkhah F, Sadrkhanlou M, Jamilian M, Ahmadi S, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016;42:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 74. | Ostadrahimi A, Taghizadeh A, Mobasseri M, Farrin N, Payahoo L, Beyramalipoor Gheshlaghi Z, Vahedjabbari M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health. 2015;44:228-237. [PubMed] |
| 75. | Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 294] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 76. | Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm. 2014;2014:348959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 77. | Ivey KL, Hodgson JM, Kerr DA, Lewis JR, Thompson PL, Prince RL. The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. Eur J Clin Nutr. 2014;68:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 78. | Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts. 2014;4:83-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 79. | Asemi Z, Khorrami-Rad A, Alizadeh SA, Shakeri H, Esmaillzadeh A. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2014;33:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 80. | Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. 2013;38:38-43. [PubMed] |
| 81. | Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. Int J Prev Med. 2013;4:531-537. [PubMed] |
| 82. | Asemi Z, Samimi M, Tabassi Z, Naghibi Rad M, Rahimi Foroushani A, Khorammian H, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr. 2013;67:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 83. | Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 84. | Laitinen K, Poussa T, Isolauri E; Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr. 2009;101:1679-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/