Published online Aug 15, 2024. doi: 10.4239/wjd.v15.i8.1793
Revised: June 24, 2024
Accepted: July 17, 2024
Published online: August 15, 2024
Processing time: 91 Days and 16.7 Hours
The incidence of diabetes mellitus type 1 (DM1) has been rising worldwide because of improvements in diagnostic techniques and improved access to care in countries with lower socioeconomic status. A new anti-CD4 antibody, Tep-lizumab, has been shown to delay the progression of DM1 and is the only medication approved for this indication. However, more information is needed about the safety profile of this drug.
To identify the odds ratios (OR) of systems-based adverse effects for Teplizumab when compared to Placebo.
An extensive systematic review was conducted from the inception of the medication until December 31, 2023. All clinical trials and studies that evaluated Teplizumab vs placebo were included in the initial review. The study protocol was designed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines guidelines and was registered in PROSPERO (ID: CRD42024496169). Crude OR were generated using RevMan Software version 5.4.
After screening and review, 5 studies were selected to determine the risk of adverse effects of teplizumab compared to placebo. A total of 561 patients were included in the study population. Total adverse effects and system-based adverse effects were studied and reported. We determined that patients receiving Teplizumab had a higher risk of developing gastrointestinal (GI) (OR = 1.60, 95%CI: 1.01-2.52, P = 0.04), dermatological (OR = 6.33, 95%CI: 4.05-9.88, P < 0.00001) and hematological adverse effects (OR = 19.03, 95%CI: 11.09-32.66, P < 0.00001). These patients were also significantly likely to have active Epstein-Barr Virus infection (OR = 3.16, 95%CI: 1.51-6.64, P < 0.002). While our data showed that patients receiving Teplizumab did have a higher incidence of total adverse effects vs placebo, this finding did not reach statistical significance (OR = 2.25, 95%CI: 0.80-6.29, P = 0.12).
Our systematic review suggests that Teplizumab patients are at risk for significant adverse effects, primarily related to GI, dermatological, and hematological systems. The total adverse effect data is limited as study populations are small. More studies should be conducted on this medication to better inform the target population of potential adverse effects.
Core Tip: Teplizumab is an anti-CD4 antibody that has been shown to delay the clinical onset of diabetes mellitus type 1. Our systematic review evaluates the incidence of total adverse effects and a systems-based risk reported in various clinical trials. Our review shows an increased incidence of adverse effects in patients receiving Teplizumab compared to placebo, but this risk is not statistically significant. There is also a statistically significant increased risk of gastrointestinal, dermatological, and hematological adverse effects in the Teplizumab group compared to placebo. More trials need to be conducted to understand better the risk of side effects related to Teplizumab.
- Citation: Buddhavarapu V, Dhillon G, Grewal H, Sharma P, Kashyap R, Surani S. Safety of teplizumab in patients with high-risk for diabetes mellitus type 1: A systematic review. World J Diabetes 2024; 15(8): 1793-1801
- URL: https://www.wjgnet.com/1948-9358/full/v15/i8/1793.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i8.1793
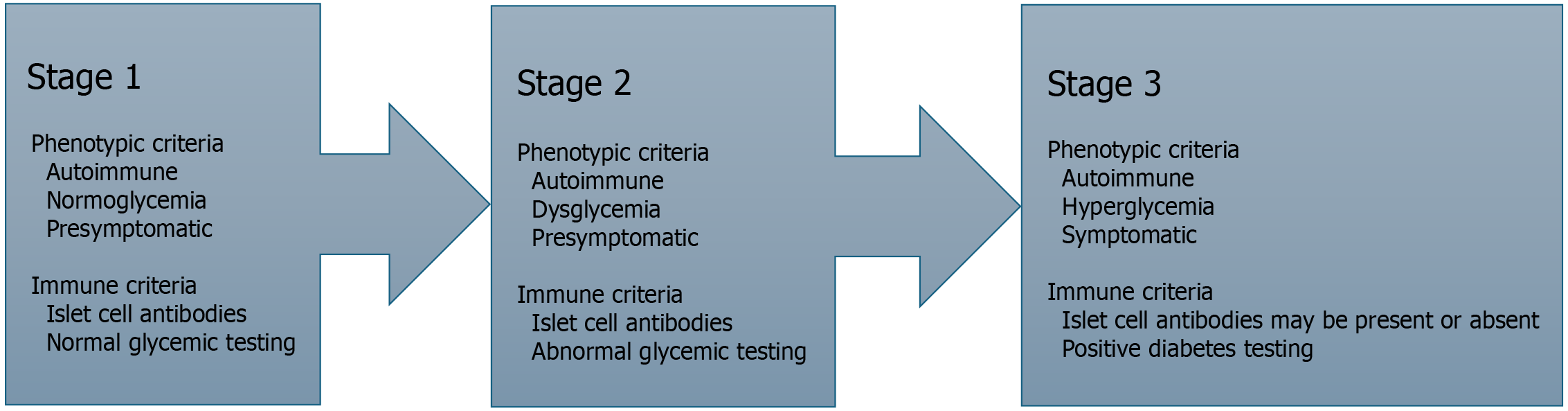
Diabetes mellitus type 1 (DM1) is an autoimmune disease that has been rising in incidence around the world[1]. In 2021 alone, the estimated number of cases was 8.5 million, projected to increase by about 2% to 4% over the next 20 years[1,2]. While the exact cause of this rise is unknown, a reasonable assumption is the improvements in detection in countries of lower socioeconomic status as they gain access to better resources. The condition, characterized by autoimmune destruction of the insulin-producing pancreatic beta cells, often manifests in early childhood or during puberty (10 to 14)[3]. There is strong evidence to support the idea that young patients with DM1 often demonstrate clinical antibodies for many years prior to developing clinical symptoms[4]. This time frame is key for preventative therapies that can ideally halt further immune destruction. As a result, the Endocrine Society and the American Diabetes Association have released a staging system for classifying patients with DM1 based on a combination of immune response and clinical symptoms[5]. This classification is shown in Figure 1.
In the past, significant research has been performed on human leukocyte antigen proteins that conferred a genetic risk for DM1, hoping to identify a potential cure for this illness[6]. Even in high-risk patients, there wasn't a reliable method to predict conversion from asymptomatic to a symptomatic stage. More recently, the focus has shifted towards immunological markers that directly cause islet cell destruction. One of the key drivers of an autoimmune response is CD4+ and CD8+ T-cells that mark beta cells for destruction in patients with DM1. The CD3 surface protein of T-cells has been a popular target in autoimmune conditions, with various monoclonal antibody agents showing excellent efficacy against this protein in clinical trials[7]. The most promising one for patients with a risk of DM1 is Teplizumab, which has been shown to slow progression in patients in stage 2 of DM1[8]. This agent is a modified version of the OKT3 anti-CD3 antibody approved for transplant patients to prevent rejection[9]. The original OKT3 antibody was not extensively used due to its severe adverse effect profile of flu-like symptoms and cytokine storm reaction[10]. Thus, a newer version of the antibody was created by modifying the binding protein to maintain the anti-CD3 properties without causing an adverse reaction[11]. Initial Phase I studies of this new antibody showed that the autoimmune response was sufficiently suppressed, and insulin production was maintained in patients over the course of 2 entire years[12].
As a result, the United States Food and Drug Administration (FDA) has approved Teplizumab for use in this target population. Due to this fast-tracked process, more significant data must be collected on the adverse effects of this medication. In this systematic review, we aim to study the incidence and severity of various side effects of Teplizumab as reported in clinical trials.
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A systematic literature review was conducted from the inception of the medication until December 31, 2023.
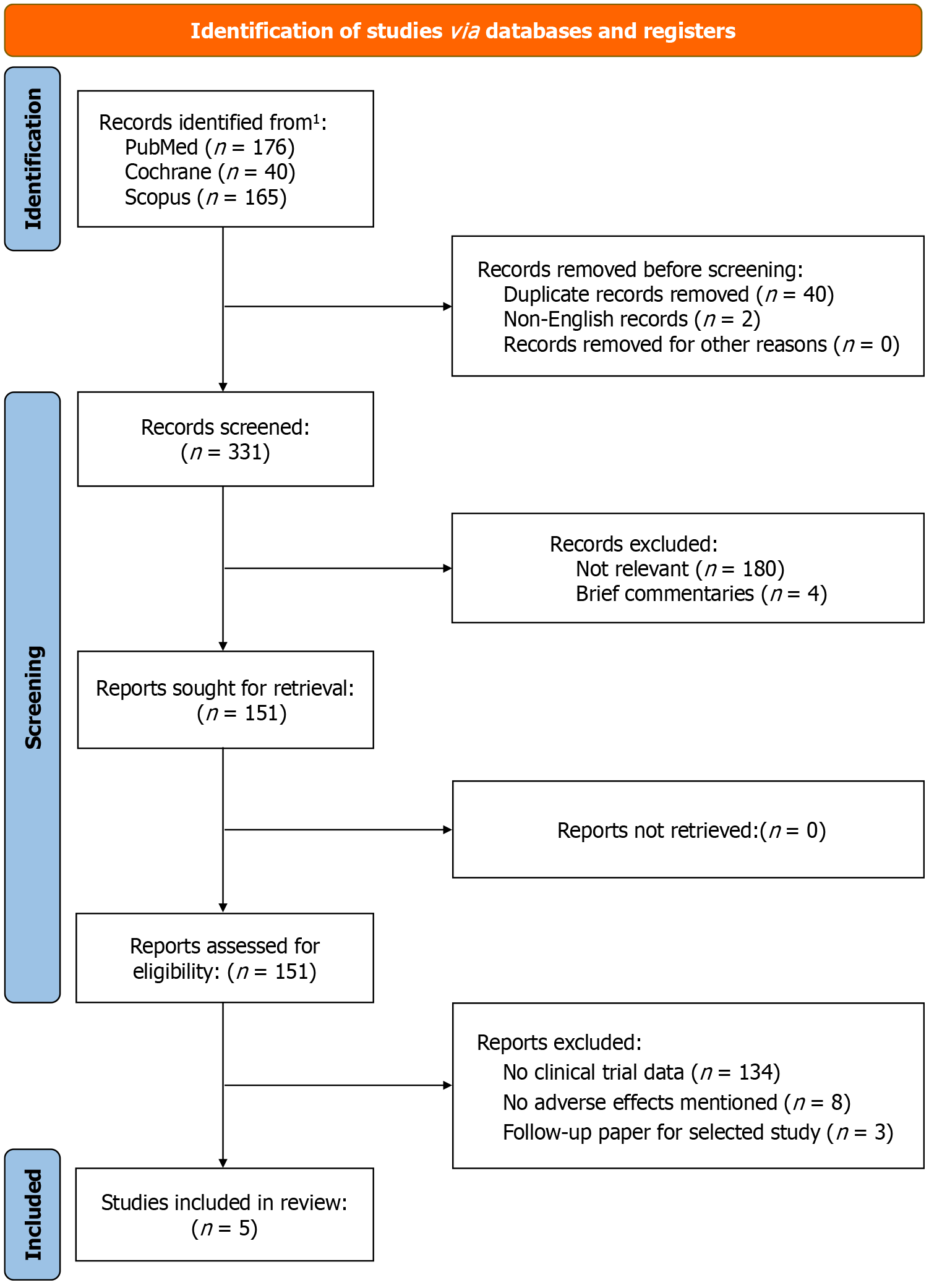
We searched the databases of PubMed, Scopus, and Cochrane Library for titles using the term ‘teplizumab.’ Only articles in the English language were selected for screening. The search dates from the inception of the medication in 2009 until December 31st, 2023. We also manually scanned ClinicalTrials.gov for clinical trials that were in progress or recently completed. The search approach and design are highlighted in Figure 2. The protocol of this systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42024496169).
All clinical trials that met the following criteria were included in this review: (1) Study design: randomized blinded; (2) Reported adverse effects of Teplizumab vs that of Placebo; and (3) Addressed specific adverse effects including but not limited to severe adverse effects, gastrointestinal (GI), and dermatological and hematological adverse effects. All observational studies, cohort studies, review articles, case reports, editorials, conference abstracts, and commentary articles were excluded from our study.
All selected articles were compiled using Rayyan Software[13]. Four authors (Venkata Buddhavarapu, Gagandeep Dhillon, Harpreet Grewal and Pranjal Sharma) independently screened articles after eliminating duplicates to ensure that appropriate manuscripts were selected for final review. Two authors (Venkata Buddhavarapu and Gagandeep Dhillon) reviewed these manuscripts for eligibility in the final evaluation. A third party addressed any discrepancies or conflicts via arbitration.
The primary outcome was the incidence of severe adverse effects in patients receiving Teplizumab when compared to placebo. The secondary outcome was systems-based adverse effects, including GI, dermatological, and hematological adverse effects.
The included articles underwent a thorough evaluation of the full text by two independent reviewers (Venkata Buddhavarapu and Gagandeep Dhillon) to determine eligibility. The articles were assessed using the Population, Intervention, Comparison, Outcomes tool for systematic reviews[14]. The patients selected were those in stage 2 of DM1 who were deemed to be candidates for the intervention, Teplizumab. Studies included matches these patients against placebo-controlled cohorts. The primary outcome was to evaluate the incidence of severe and specific adverse effects related to GI, dermatology, and hematology systems. The studies were evaluated in a blinded manner to control against bias using Rayyan Software. An independent third reviewer (Harpreet Grewal) resolved conflicts following the selection process.
The primary outcomes were analyzed using the RevMan software version 5.4 to generate all results. Crude odds ratios (OR) were calculated using a Random-effects model for each adverse outcome measured with 95%CI. A P value less than 0.05 was considered to be statistically significant. Forest plots were generated to determine the effect of each study. Study heterogeneity was determined using Cochrane Q and I2 statistics, with a low-level heterogeneity being defined at I2 less than 20%.
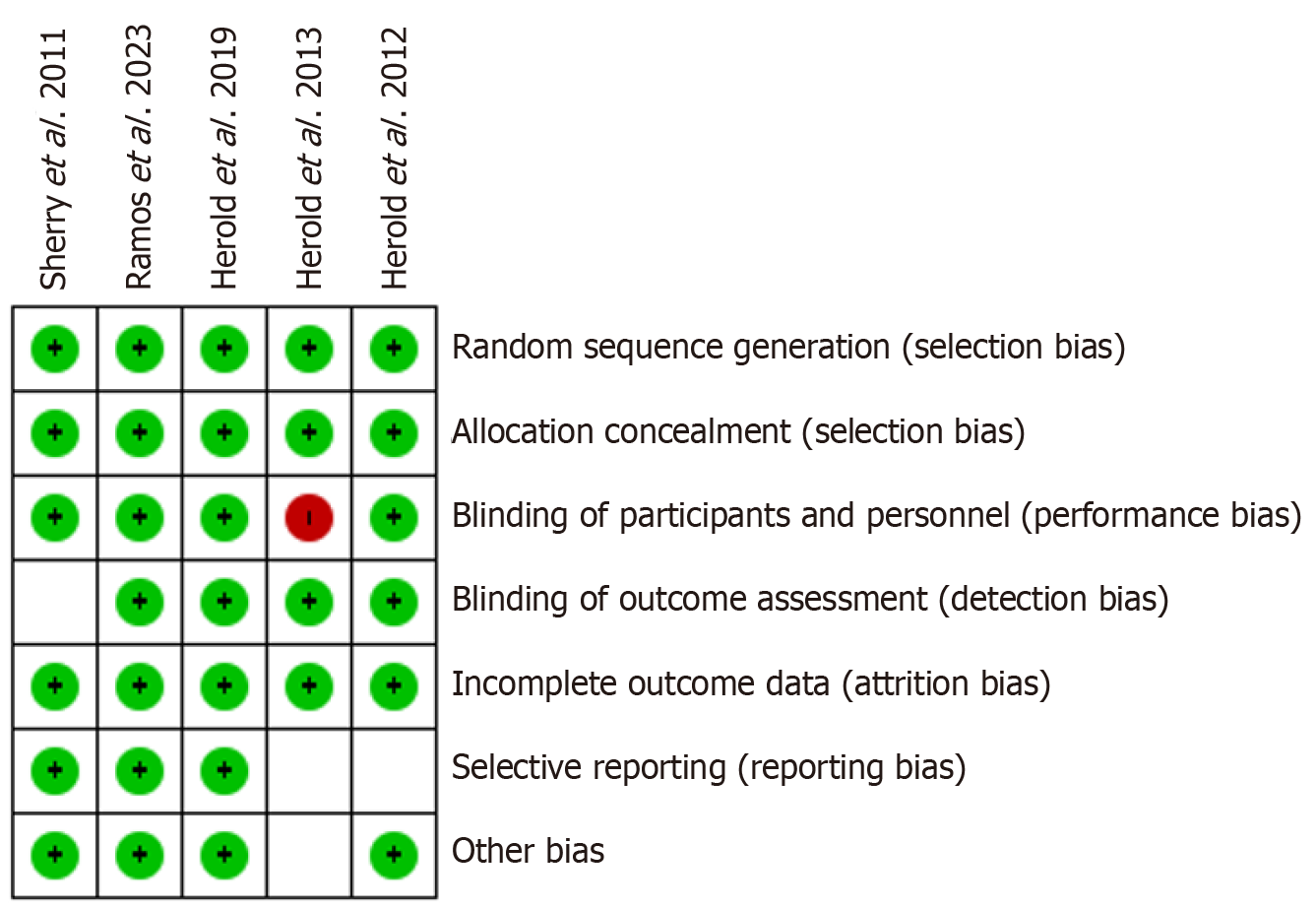
Risk of bias assessment was done by two reviewers (Venkata Buddhavarapu and Harpreet Grewal) using the Cochrane Database Risk of Bias Tool[15]. All studies were evaluated as ‘high’, ‘low’ or ‘unclear’ risk of bias using established domains of risk (Figure 3). All five studies had an overall low risk of bias with only Herold et al[16] in 2013[16], showing an area of concern due to being an open-label study. The authors expressed that the study results were randomized, which mitigated some of the risks.
After screening 331 search results from three databases, 151 articles were selected for secondary review. After reviewing all articles based on the eligibility criteria, a total of. Five studies were selected for final inclusion. These studies included 561 patients in the study group and 298 patients in the control group. The studies were conducted in various countries, including the United States, India, and the United Kingdom. A more detailed outline of the general characteristics of the studies is shown in Table 1[16-20].
| Ref. | Locations | Study design | No. of participants in teplizumab group | No. participants in placebo group | Mean age in teplizumab group | Mean age in placebo group | Male sex% teplizumab | Male sex% placebo |
| Ramos et al[17], 2023 | United States, Canada, Europe | RCT | 217 | 111 | 12 | 12.3 | 54.8 | 62.2 |
| Herold et al[18], 2019 | United States, Canada, Australia, Germany | RCT | 44 | 32 | 14 | 13 | 57 | 53 |
| Herold et al[16], 2013 | United States | RCT | 52 | 25 | 12.7 | 12.3 | 53.8 | 64 |
| Herold et al[19], 2013 | United States | RCT | 33 | 27 | 12.9 | 12 | 52 | 63 |
| Sherry et al[20], 2011 | United States, Canada, Europe | RCT | 209 | 99 | 18.5 | 18.2 | 62.8 | 62.2 |
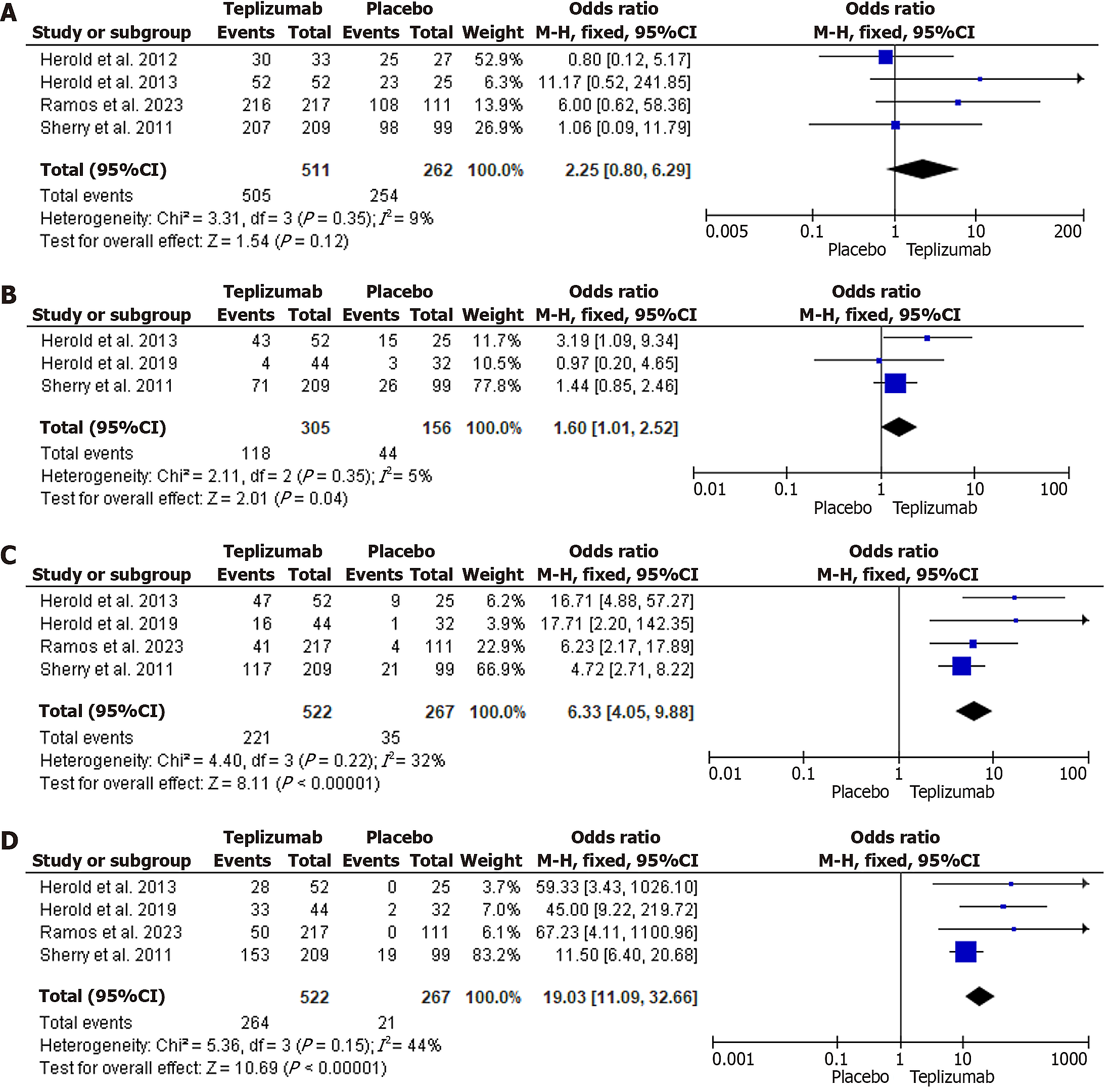
The OR analysis showed that while total adverse effects were higher in the Teplizumab groups when compared to Placebo, this finding was not significant (OR = 2.25, 95%CI: 0.80-6.29, P = 0.12). The heterogeneity was low, with an I2 of 9%. This is shown in Figure 4A.
GI adverse effects, which included nausea, vomiting, diarrhea, and abdominal pain, were more associated with Teplizumab as compared to placebo (OR = 1.60, 95%CI: 1.01-2.52, P = 0.04). Heterogeneity was low at I2 = 5%. Figure 4B demonstrates this association.
There was a strong association with Teplizumab for both dermatological side effects (OR = 6.33, 95%CI: 4.05-9.88, P < 0.00001) and lymphopenia when compared to placebo (OR = 19.03, 95%CI: 11.09-32.66, P < 0.00001). Heterogeneity was also low, with I2 = 32% and I2 = 44%, respectively. Figure 4C and D demonstrate this.
Teplizumab was also shown to activate Epstein-Barr virus (EBV) infections as demonstrated by detectable EBV viral loads in those receiving Teplizumab (OR = 3.16, 95%CI: 1.51-6.64, P < 0.002, I2 = 39%)
Teplizumab has been shown to delay the symptomatic progression of patients with high risk for DM1[21] and is the only medication currently approved by the FDA for this indication[22]. This is achieved by its ability to alter activated T-cells, leading to a decreased immune response[23]. Due to its strong affinity, this response is maintained over an extended period, leading to this delay in progression[23]. Studies have shown that this medication is also associated with a certain side effect profile. While traditional anti-CD3 antibodies led to severe reactions such as fevers, chills, and headaches, the current versions of this antibody, such as Teplizumab, have an altered Fc receptor, which seems to lead to this particular reaction[24]. Due to this change, the side effect profile also appears to be different and has yet to be studied.
Our systematic review reveals that patients are at a much higher risk of developing GI, dermatological, and hematological adverse effects when taking this medication. GI effects included mainly nausea and emesis, the mechanism behind which is not well described. The control group also had many of these effects, an interesting factor related to the placebo. The dermatological adverse effects mainly include rashes and self-limited soft tissue infections. The mechanism behind this is not well described. Still, it could result from a change in the levels of activated CD4 T-cells and a decrease in interferon-gamma, which have been associated with skin conditions[25]. While the shift in ratios from predominantly CD4 T-cells to CD8 memory T-cells is the main factor in lymphopenia, this effect is transient, and the counts are restored to normal shortly after completing the treatment course[26]. During this phase, patients are more susceptible to all infections, with many patients experiencing respiratory and Upper respiratory infection symptoms. Teplizumab has also been shown to block the effect of regulatory and activated CD4 T-cells directly, which are responsible for the immune response against viral infections[21]. This effect explains the dramatic increase in EBV infections in patients receiving this medication.
Our systematic review is the first study to evaluate Teplizumab's pooled risk of system-specific adverse events from the limited clinical data available. Liu et al[27] evaluated both Teplizumab and another CD3 antibody Otelixizumab and also found a higher incidence of total adverse effects but failed to show statistical significance. As their analysis included both drugs, a direct comparison cannot be made with our study. A recent systematic review by Nourelden et al[28] also found lymphopenia and skin disorders to be the most common adverse effects. Still, it did not go into further detail about the incidence and risk of these findings.
The Strengths of our paper mainly involve the significance of the adverse events described with low heterogeneity. The findings are consistent across all clinical trials and should be predictable in any newer studies. The main limitation is that the number of patients enrolled in these studies remains low, which may lead to overestimating adverse effects, especially in systems with lower incidence, such as EBV infections and dermatological events. Additionally, the papers in our review were inconsistent in describing adverse events as systems and often included individual events rather than patients. Some papers described adverse effects of higher incidence, which may make the medication seem worse than predicted. More studies need to be done to describe all potential adverse effects of Teplizumab further. These are currently underway[29].
Teplizumab promises to be an exciting new development in type 1 diabetes and shows great promise in reducing the incidence of this condition in the target group. The type of adverse effects with higher risk may affect compliance with this treatment, especially in the pediatric population, which is more sensitive to these issues and should be weighed against the benefit of the medication. Teplizumab is also being studied in patients with active DM1 who have some preserved pancreatic beta-cell function to assess for reversal[30]. These studies should expand our knowledge of the efficacy and safety of this medication.
Patients on Teplizumab have a much higher risk of developing GI, dermatological, and hematological adverse effects when compared to placebo. These patients are also more likely to have EBV activation when receiving this medication. These adverse effects can lead to poor compliance with treatment protocols, especially in the pediatric population. More studies are needed to identify better the mechanisms behind these effects and other significant adverse effects.
| 1. | Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 836] [Cited by in RCA: 762] [Article Influence: 47.6] [Reference Citation Analysis (5)] |
| 2. | Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, Donaghue KC; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group, Magliano DJ, Maniam J, Orchard TJ, Rai P, Ogle GD. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10:741-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 633] [Article Influence: 158.3] [Reference Citation Analysis (0)] |
| 3. | Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet. 2000;355:873-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 430] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Gianani R, Eisenbarth GS. The stages of type 1A diabetes: 2005. Immunol Rev. 2005;204:232-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 800] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 6. | Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59:1134-1148. [PubMed] |
| 7. | Chitnis T, Kaskow BJ, Case J, Hanus K, Li Z, Varghese JF, Healy BC, Gauthier C, Saraceno TJ, Saxena S, Lokhande H, Moreira TG, Zurawski J, Roditi RE, Bergmark RW, Giovannoni F, Torti MF, Li Z, Quintana F, Clementi WA, Shailubhai K, Weiner HL, Baecher-Allan CM. Nasal administration of anti-CD3 monoclonal antibody modulates effector CD8+ T cell function and induces a regulatory response in T cells in human subjects. Front Immunol. 2022;13:956907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 8. | Grando Alves G, Cunha L, Henkes Machado R, Lins de Menezes V. Safety and efficacy of teplizumab in the treatment of type 1 diabetes mellitus: An updated systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2024;26:2652-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Cosimi AB, Burton RC, Colvin RB, Goldstein G, Delmonico FL, LaQuaglia MP, Tolkoff-Rubin N, Rubin RH, Herrin JT, Russell PS. Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation. 1981;32:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 238] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Norman DJ, Shield CF 3rd, Barry JM, Henell K, Funnell MB, Lemon J. Therapeutic use of OKT3 monoclonal antibody for acute renal allograft rejection. Nephron. 1987;46 Suppl 1:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Alegre ML, Peterson LJ, Xu D, Sattar HA, Jeyarajah DR, Kowalkowski K, Thistlethwaite JR, Zivin RA, Jolliffe L, Bluestone JA. A non-activating "humanized" anti-CD3 monoclonal antibody retains immunosuppressive properties in vivo. Transplantation. 1994;57:1537-1543. [PubMed] [DOI] [Full Text] |
| 12. | Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 902] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 13. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 14342] [Article Influence: 1434.2] [Reference Citation Analysis (1)] |
| 14. | Higgins JP, Deeks JJ. Selecting Studies and Collecting Data. In: Higgins JJ, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration, 2008: 151-185. [DOI] [Full Text] |
| 15. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18678] [Article Influence: 2668.3] [Reference Citation Analysis (0)] |
| 16. | Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA; AbATE Study Team. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766-3774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 327] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 17. | Ramos EL, Dayan CM, Chatenoud L, Sumnik Z, Simmons KM, Szypowska A, Gitelman SE, Knecht LA, Niemoeller E, Tian W, Herold KC; PROTECT Study Investigators. Teplizumab and β-Cell Function in Newly Diagnosed Type 1 Diabetes. N Engl J Med. 2023;389:2151-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 18. | Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ; Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 2019;381:603-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 846] [Cited by in RCA: 820] [Article Influence: 117.1] [Reference Citation Analysis (1)] |
| 19. | Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, Sherr J, Rosenthal SM, Adi S, Jalaludin MY, Michels AW, Dziura J, Bluestone JA. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG; Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 21. | Thakkar S, Chopra A, Nagendra L, Kalra S, Bhattacharya S. Teplizumab in Type 1 Diabetes Mellitus: An Updated Review. touchREV Endocrinol. 2023;19:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 22. | United States Food and Drug Administration. FDA approves first drug that can delay onset of type 1 diabetes. Jan 1, 20223. [cited 3 July 2024]. Available from: https://www.fda.gov/media/164864/download. |
| 23. | Warshauer JT, Bluestone JA, Anderson MS. New Frontiers in the Treatment of Type 1 Diabetes. Cell Metab. 2020;31:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 24. | Masharani UB, Becker J. Teplizumab therapy for type 1 diabetes. Expert Opin Biol Ther. 2010;10:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Skelley JW, Elmore LK, Kyle JA. Teplizumab for treatment of type 1 diabetes mellitus. Ann Pharmacother. 2012;46:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Misra S, Shukla AK. Teplizumab: type 1 diabetes mellitus preventable? Eur J Clin Pharmacol. 2023;79:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Li W, Chen Y, Wang X. Anti-CD3 monoclonal antibodies in treatment of type 1 diabetes: a systematic review and meta-analysis. Endocrine. 2024;83:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 28. | Nourelden AZ, Elshanbary AA, El-Sherif L, Benmelouka AY, Rohim HI, Helmy SK, Sayed MK, Ismail A, Ali AS, Ragab KM, Zaazouee MS. Safety and Efficacy of Teplizumab for Treatment of Type One Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocr Metab Immune Disord Drug Targets. 2021;21:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Provention Bio, Inc. Recent-Onset Type 1 Diabetes Trial Evaluating Efficacy and Safety of Teplizumab (PROTECT). [accessed 2024 Jul 3]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://classic.clinicaltrials.gov/show/NCT03875729 ClinicalTrials.gov Identifier: NCT03875729. |
| 30. | Provention Bio, a Sanofi Company. At-Risk for Type 1 Diabetes Extension Study (TN-10 Extension). [accessed 2024 Jul 3]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://classic.clinicaltrials.gov/show/NCT04270942 ClinicalTrials.gov Identifier: NCT04270942. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/