Published online Aug 15, 2024. doi: 10.4239/wjd.v15.i8.1704
Revised: June 19, 2024
Accepted: June 27, 2024
Published online: August 15, 2024
Processing time: 115 Days and 17 Hours
Exercise has emerged as one of the important and effective non-drug therapies used for management of type 2 diabetes (T2D) in certain nations. The present report summarizes the latest findings from the research on the beneficial effect of exercise on T2D. The objectives were to provide references for the theoretical study and the clinical practice of exercise-based management of T2D, in addition to identify the limitations of the existing literature, thereby provide direction for future research in this field.
Core Tip: Exercise significantly benefits type 2 diabetes (T2D) management by enhancing insulin sensitivity, regulating glucose and lipid metabolism, and reducing inflammation. This review reveals how different exercise types, including aerobic, resistance, and flexibility exercises, contribute to these effects. It also highlights the need for personalized exercise programs to optimize T2D treatment. The article underscores the importance of incorporating exercise into comprehensive care strategies for T2D, pointing towards future research to refine and personalize exercise recommendations for individuals with T2D.
- Citation: Peng CJ, Chen S, Yan SY, Zhao JN, Luo ZW, Qian Y, Zhao GL. Mechanism underlying the effects of exercise against type 2 diabetes: A review on research progress. World J Diabetes 2024; 15(8): 1704-1711
- URL: https://www.wjgnet.com/1948-9358/full/v15/i8/1704.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i8.1704
Diabetes incidence is increasing every year, and the reason is improved living standards, altered lifestyles, and changes in the methods used for food processing. Diabetes has consequently emerged as a global health crisis, contributing enor
In 1935, Joslin, a famous scholar who worked on diabetes, proposed that “physical activity should be regarded as a treatment tool for diabetes”. However, the research exploring exercise therapy for T2D was scarce initially. In 1969, the term “exercise prescription” was officially adopted by the World Health Organization, following which the topic of the therapeutic effects of exercise on T2D began attracting considerable attention. Exercise is currently recommended as an important non-pharmacological therapeutic strategy for the management of T2D by certain major national and international guidelines, and is, therefore, considered critical to managing the cases of T2D and achieving and maintaining the desired therapeutic goals and improving the quality of life of the affected patients[5-9]. According to the statement of the American College of Sports Medicine, various kinds of physical activity, inclusive of although not limited to planned exercise, could greatly enhance the health and glycemic management of individuals of all ages with T2D, such as flexibility and balance exercise in adults[5]. Research has demonstrated that different types of exercise allow for inter
Among the various strategies adopted to combat T2D, exercise has been recognized as one of the most powerful approaches. However, the mechanism through which exercise contributes to diabetes management and prevention remains to be elucidated. In this context, the present review explored the latest findings of the research exploring the mechanism underlying the effect of exercise against diabetes to offer insights that could revolutionize the therapeutic approach adopted to overcome this chronic metabolic condition.
Studies have demonstrated that exercise effectively prevents the development of T2D and its associated complications by improving insulin resistance, lipid metabolism, and inflammatory reaction[10,11]. In the context of the complications associated with T2D, exercise reportedly improves cardiovascular complications by enhancing the expression of extracellular superoxide dismutase (EcSOD) in skeletal muscles to attenuate oxidative stress, aberrant cell signaling, and inflammation[12]. In addition, progressive resistance training reportedly improved muscle strength in knee extensors and flexors and the motor function of individuals with T2D polyneuropathy[13]. The animal experiments conducted to explore diabetes-induced kidney injury in T2D revealed that treadmill exercise training significantly suppressed the levels of albuminuria, tubulointerstitial fibrosis, inflammation, and oxidative stress in the kidneys of Wistar fatty rats[14].
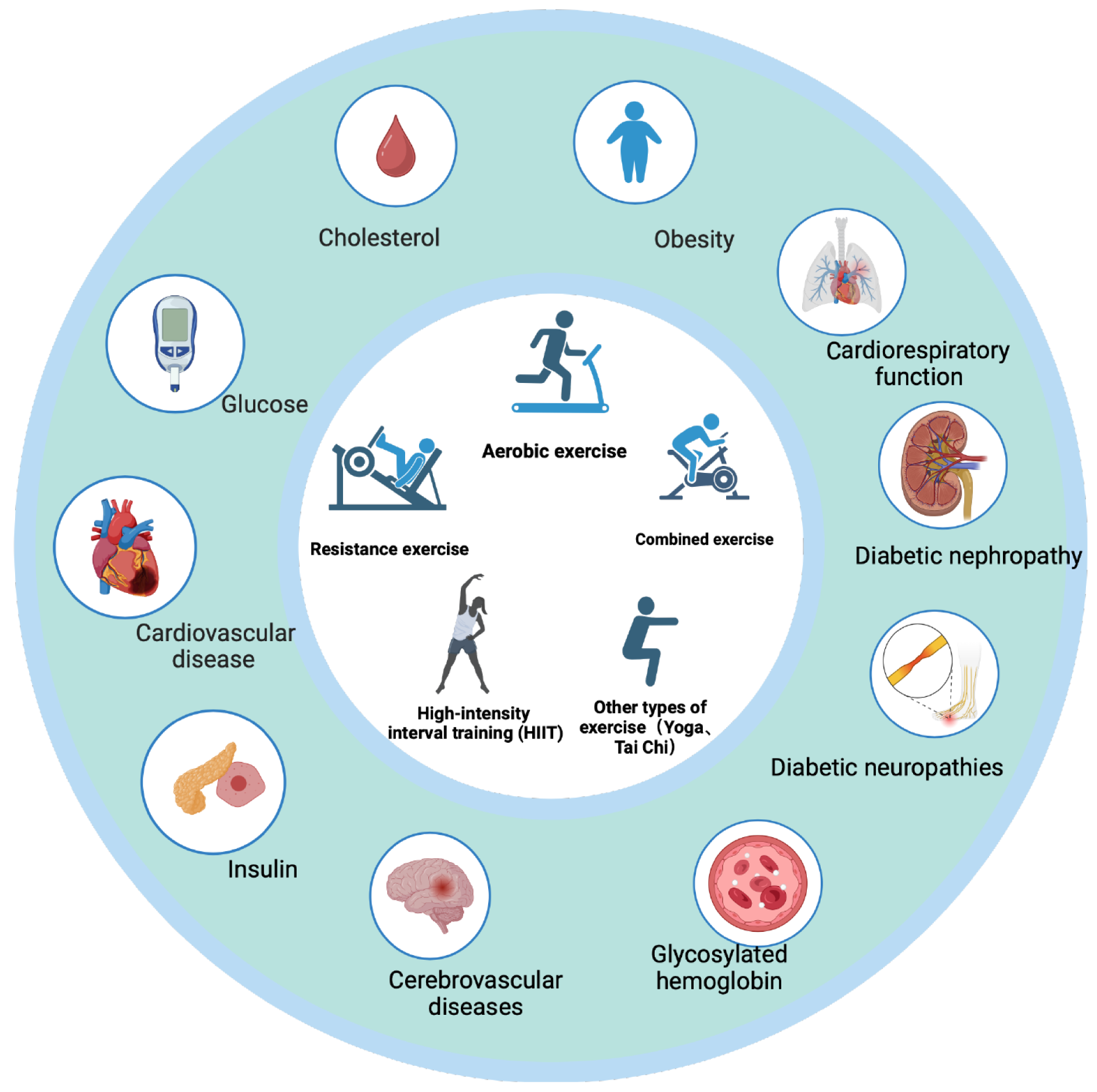
Multiple exercise training modalities, such as aerobic exercise, resistance exercise, combined exercise, and flexibility training, are recommended by the American Diabetes Association, American College of Sports Medicine, European Society of Cardiology, Belgian Physical Therapy Association, and Exercise and Sports Science Australia[15] (Figure 1).
Aerobic exercise training improves insulin sensitivity in adult T2D patients, while paralleling improved mitochondrial function[16]. An even-day training involving vigorous exercise reportedly improved glycemia in T2D, increased peripheral insulin sensitivity and responsiveness, and inhibited the production of hepatic glucose[17]. Aerobic exercise also improved the control of glycated hemoglobin (HbA1c) levels and increased cardiorespiratory fitness[6].
Resistance exercise training reportedly improved strength, bone mineral density, blood pressure, blood lipids, skeletal muscle mass, and insulin sensitivity in adult patients with T2D[5]. According to the guidelines of the American College of Sports Medicine and the American Diabetes Association, resistance exercise training provides optimal benefits for reducing the risk of cardiovascular diseases and minimizing injuries[8]. It has been demonstrated that resistance training exercises improve the control of blood glucose and HbA1c levels[18].
A combined aerobic and resistance exercise intervention might be superior to the implementation of either of these modalities separately and is effective in improving the levels of inflammatory, metabolic, and lipid markers in middle-aged and older adults with T2D[19]. The combined practice of aerobic and resistance exercises reportedly led to a greater reduction in the HbA1c levels than that achieved using any of the training modalities alone in adults with T2D[5].
High-intensity interval training (HIIT) is a regimen comprising aerobic training conducted during the 65%-90% VO2 peak or 75%-95% heart rate peak (HR peak) for a duration of 10 seconds to 4 minutes, followed by 12 seconds to 5 minutes of active or passive recovery.
As a potentially time-efficient modality, HIIT elicits significant physiological and metabolic adaptations. In adults with T2D, one session of HIIT (10 seconds × 60 seconds cycling at approximately 90% HRmax) reduces postprandial hyper
Yoga could lead to significant improvements in several indices significant for the management of T2D, including glycemic control, lipid levels, and BMI. A limited set of data suggests that yoga might also lower oxidative stress and blood pressure, enhance pulmonary and autonomic function, mood, sleep, and quality of life, and reduce the con
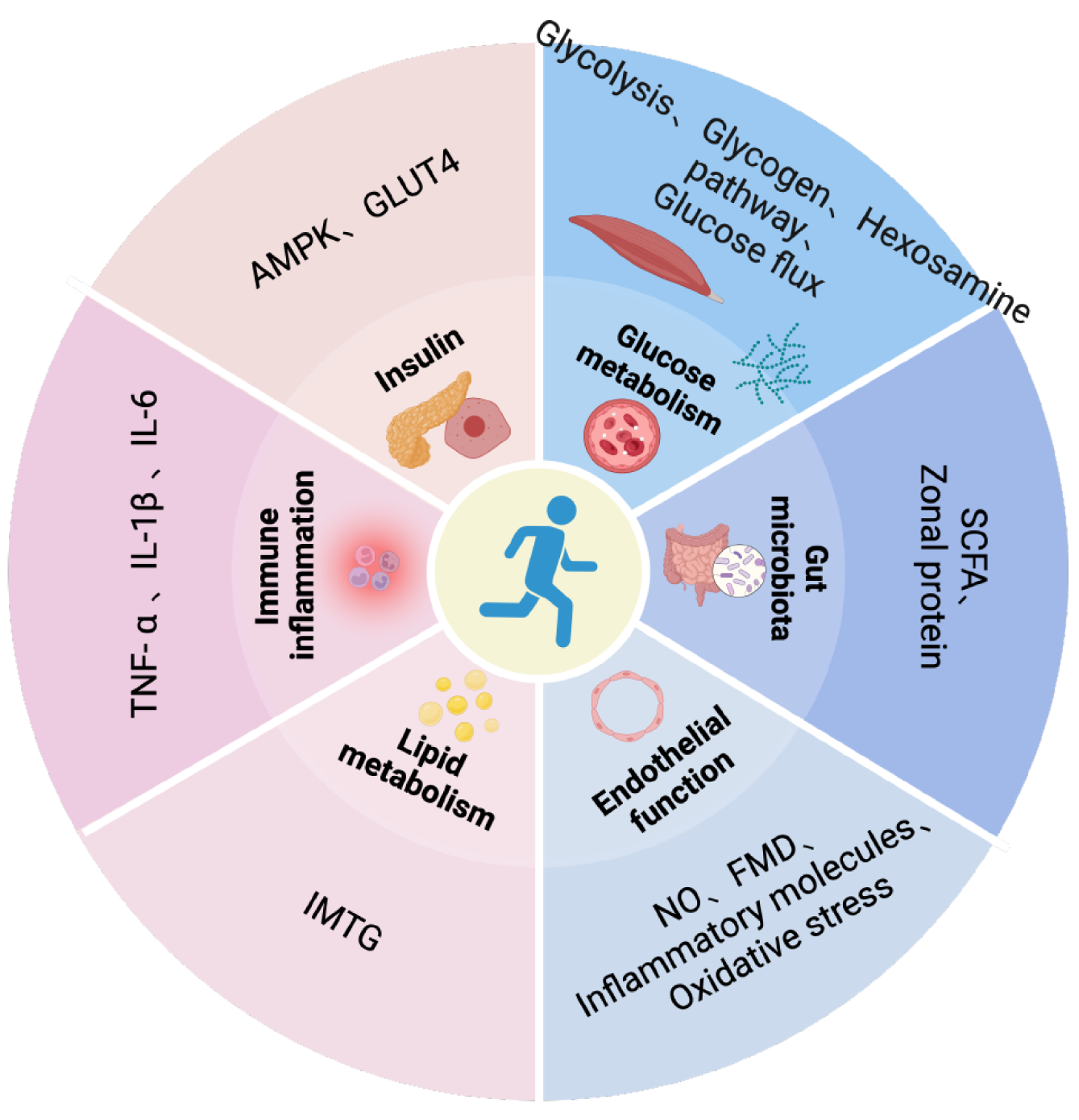
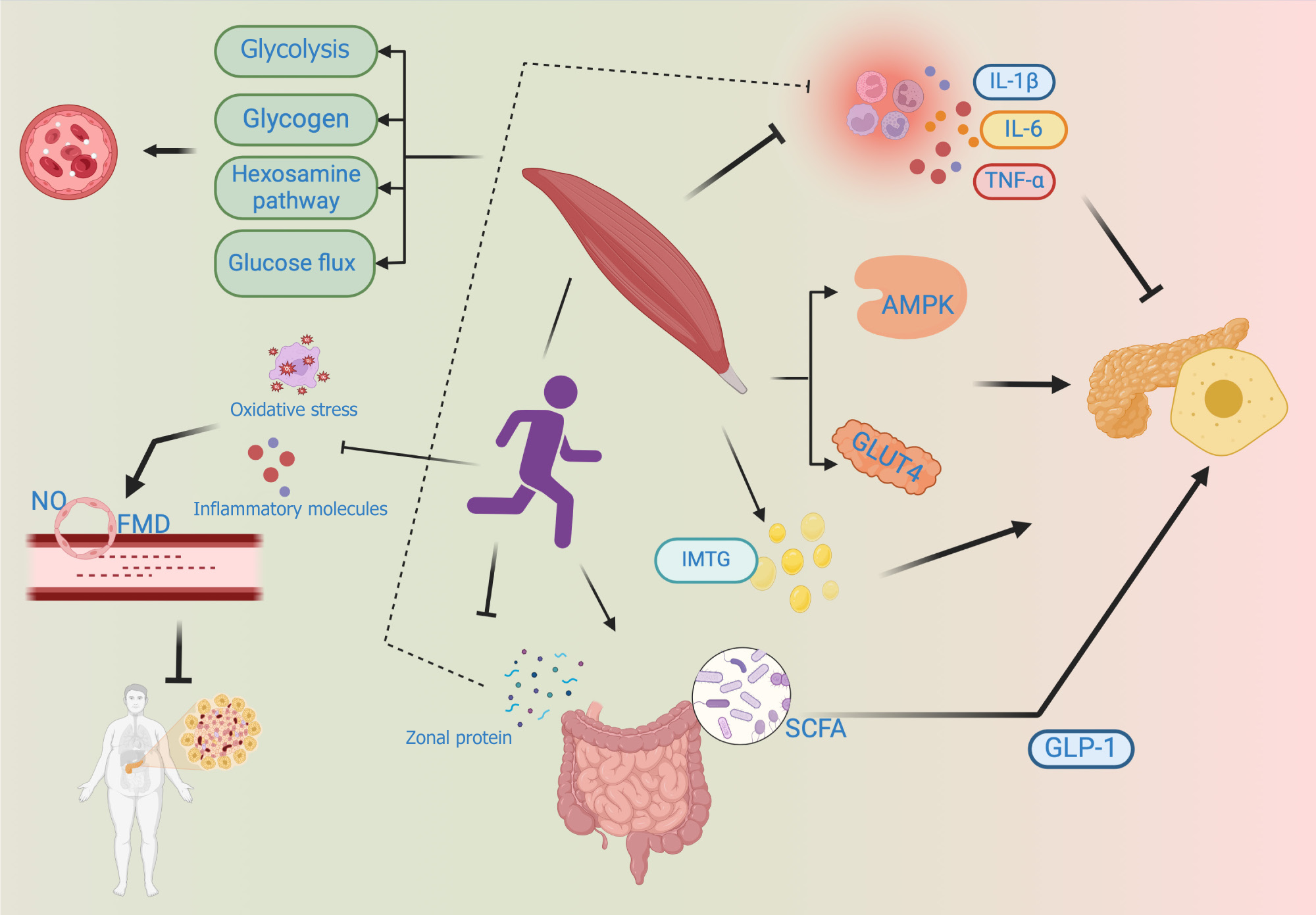
Glucose metabolism: T2D is a chronic metabolic disease characterized by the dysregulation of systemic glucose homeostasis. While the precise etiology of T2D remains to be comprehensively understood, studies have implicated impairments in key glucoregulatory functions in the pathogenesis of this disease. Exercise training, including both aerobic and resistance training, could ameliorate the hyperglycemia associated with T2D by stimulating alterations in skeletal muscle glucose transport and glucose metabolism[23]. Multiple studies have demonstrated that exercise training stimulates alterations in skeletal muscle glucose transport and glucose metabolism by improving the glycolytic capacity of skeletal muscles, decreasing the activity of the hexosamine pathway in skeletal muscles, increasing the glycogen content in skeletal muscles, and stimulating glucose flux via the pentose phosphate pathway in skeletal muscles. Owing to the duration of the exercise program, the exercise intensity, and the number of muscle groups stimulated, aerobic exercise may often lead to a pronounced effect on glucose transport and glucose metabolism compared to resistance exercise[24,25] (Figure 2).
Insulin sensitivity and insulin resistance: Insulin was one of the greatest scientific discoveries of the 20th century. Insulin plays a major role in the regulation of glucose in the body and also in the treatment of diabetes. The dominant control of glucose metabolism by insulin occurs under the regulation of complex and highly regulated hormonal and signaling cascades that may exert different and unique effects on skeletal muscles. As the primary tissue involved in insulin-stimulated glucose metabolism, skeletal muscles are a key driver of systemic glycemic control. Skeletal muscles also respond in a unique manner to muscle contraction or exercise with increased sensitivity to the subsequent insulin stimulation. Exercise training sensitizes the skeletal muscles to exhibit a glucose uptake response after insulin stimulation and might activate 5’ adenosine monophosphate-activated protein kinase (AMPK) in the muscles and promote enhanced translocation of insulin-stimulated glucose transporter 4 (GLUT4)[26]. Exercise training of muscle attenuates the sub
Immune inflammation: Low-grade chronic inflammation in vivo is considered one of the pathogeneses of T2D, and pro-inflammatory cytokines such as tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 reportedly activate intracellular signaling molecules, which then increase the expressions of inflammatory mediators and impair insulin activity through the nuclear translocation of various nuclear factors[28]. A single strenuous exercise stimulates the local muscle tissue to release inflammatory factors such as TNF-α and IL-6 although without releasing these factors into the bloodstream, such that a single strenuous exercise does not exert much effect on the level of systemic pro-inflammatory factors. Long-term regular exercise, however, reduces the basal levels of the corresponding inflammatory factors and causes the body to produce physiological adaptation, such that the levels of inflammatory markers in the entire body are lowered, which then improves the function of pancreatic islets[19].
Lipid metabolism: Cell dysfunction caused by excessive intracellular lipid and ectopic accumulation which is referred to as lipotoxicity, and this phenomenon may subsequently inhibit the insulin signaling pathways, reduce insulin sensitivity, and impact the progression of T2D to a certain degree. It is currently believed that intramuscular lipids do not inherently exhibit lipotoxicity, and rather their metabolic intermediates exhibit lipotoxicity. Intramuscular fat (IMTG) dynamics are disrupted in patients with T2D and its precursor phase, causing the lipophilic intermediates such as diacylglycerol and ceramide to accumulate, and these intermediates then interfere with insulin production. Exercise accelerates the oxidation and conversion of IMTG, and although it increases the levels of intramuscular lipids as well, the reduced levels of lipid intermediates improve insulin function to a certain extent[29,30]. At the same time, patients with T2D have a higher unsaturated intramyocellular fat. Clinical studies have found that patients with T2D can increase contributions of the saturated intramyocellular fatty acid pool through endurance training, adapt to the lipid storage in muscle cells and improve insulin resistance[31].
Endothelial function: Endothelial cells maintain vascular homeostasis, and any imbalance in their physiological function leads to vasoconstriction, possible thrombosis, inflammation, etc., which are the physiological and pathological signs of T2D and are considered important factors for vascular complications in patients with diabetes. Exercise, particularly aerobic exercise and a combination of aerobic and resistance exercises, enhance the vasodilation function[32]. The main mechanism through which exercise produces such changes is as follows: First, exercise increases the intravascular blood flow, enhances the endothelial cell shear force, and increases Nitrogen Oxide (NO) synthesis and bioavailability; next, exercise reduces factors that initiate endothelial dysfunction, such as oxidative stress and reduced expression of pro-inflammatory molecules; finally, exercise restores the function of endothelial progenitor cells, thereby promoting endo
Gut microbiota composition and the intestinal barrier function: Currently, no definite conclusion has been reached on whether alterations in the intestinal microorganisms in patients with T2D are the cause or the consequence of diabetes. However, it is affirmative that changes in the gut microbiota play an important role in the progression of T2D. The diversity of gut bacteria is decreased in patients with T2D, mainly due to a decrease in the abundance of short-chain fatty acid (SCFA)-producing bacteria. In addition, increased permeability facilitates the entry of inflammatory factors present in the intestine into systemic circulation[35]. The interaction between SCFAs and G protein-coupled receptors increases the secretion of GLP-1, thereby regulating blood glucose levels. The mechanism through which exercise training induces changes in gut microbiota and the intestinal barrier function in patients with T2D remains to be understood, although the reasons for improvement in the internal environment of patients with T2D could include the following: On one hand, exercise training increases the abundance of SCFA-producing bacteria in the intestine, which increases the content of SCFA in the intestine and the blood, thereby partially improving insulin resistance; on the other hand, intestinal zonal protein disrupts the intestinal barrier and increases intestinal permeability, while exercise intervention reduces the concentration of zonal proteins[36,37].
Epigenetic mechanisms, including DNA methylation, histone modifications, and RNA-mediated processes, are known to control gene activity and organism development. Disruption of the epigenetic balance could, therefore, lead to various pathologies and contribute to the development of diseases such as T2D (Figure 3). The differential gene expression ana
In addition to methylation, upregulation of the genes involved in insulin function and glucose metabolism, such as fatty acid synthase (FASN), was noted in the skeletal muscles of T2D mice, while the expressions of LPIN1, TBC1D1, HK2, HMOX1, SORBS1, PPARGC1A, and other genes were observed to be downregulated. After exercise intervention, most of the patients with T2D benefited from exercise to a certain extent[39]. The benefits manifested mainly as decreased levels of glycosylated hemoglobin, body fat percentage, and BMI. After exercise, FASN levels decreased in skeletal muscles, while the levels of LPIN1, TBC1D1, HK2, HMOX1, SORBS1, and PPARGC1A increased. However, certain patients presented no significant decrease in glycosylated hemoglobin and body fat percentage after exercise training. In these patients, the mRNA expressions of PPARα and ELOVL1, which are involved in lipid metabolism, and those of CHKB, CISD2, and FOXO1, which are involved in mitochondrial function, were lower compared to the corresponding levels in responsive patients[40].
The understanding of the multifaceted mechanisms through which exercise combats diabetes and its complications is essential for developing effective treatment strategies tailored to individual requirements, emphasizing lifestyle modifications, in addition to medication, as pillars of diabetic care. Numerous studies have demonstrated that exercise could prevent and treat T2D by reducing insulin resistance, improving insulin sensitivity, increasing glucose transport and metabolism, reducing the inflammatory response, and regulating lipid metabolism, among other mechanisms. Exercise might exhibit positive effects on T2D and the associated complications through epigenetic changes as well, although the specific mechanisms underlying such effects are yet to be entirely understood. Significant difficulties remain in issuing personalized exercise prescriptions for different T2D patients in clinical practice. Therefore, future research could include large-scale clinical trials conducted with T2D patients to provide further accurate and efficient guidance for the treatment of these patients.
The clinical evidence of the benefits of physical exercise as a treatment measure for patients with T2D is ample, well-recognized, and widely accepted, and, therefore, deserves to be incorporated into clinical treatment plans. However, several challenges are encountered when the exercise recommendations have to be implemented. The diverse set of exercise modalities available and the inconsistencies in the exercise prescription parameters render it difficult to perform a precise analysis of the dose-response relationship between physical activity and health outcomes. The lack of high-quality evidence regarding the dose-response relationship renders it challenging to recommend measurable and achievable exercise targets in physical activity guidelines. Therefore, exercise prescriptions have to be personalized for individuals according to their habits, preferences, motivations, and tolerance levels, rather than providing generic prescriptions, describing exercise duration, intensity, and frequency based on the clinical testing. Moreover, while exercise has been proven to manage T2D by reducing insulin resistance, improving insulin sensitivity, increasing glucose transport and metabolism, reducing the inflammatory response, and regulating lipid metabolism, it remains unclear whether exercise exerts sustained regulatory effects. These limitations highlight the importance of future research, which could overcome these obstacles by further investigating and elucidating the molecular biology-based mechanisms underlying the effects of exercise against T2D and further comprehensively evaluating the expected type and level-response relationship between exercise and T2D by using more standardized exercise prescription parameters, to realize the full clinical therapeutic effects of exercise. In addition, theoretical demonstration of the effects of exercise therapy against T2D requires further formal evaluation through prospective epidemiological studies.
| 1. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3688] [Article Influence: 461.0] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 2043] [Article Influence: 408.6] [Reference Citation Analysis (1)] |
| 3. | Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30:529-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 594] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 4. | American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S158-S178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 421] [Article Influence: 210.5] [Reference Citation Analysis (1)] |
| 5. | Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, Kirwan JP, Zierath JR. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2022;54:353-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 481] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 6. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2768] [Article Influence: 553.6] [Reference Citation Analysis (0)] |
| 7. | Mendes R, Sousa N, Almeida A, Subtil P, Guedes-Marques F, Reis VM, Themudo-Barata JL. Exercise prescription for patients with type 2 diabetes-a synthesis of international recommendations: narrative review. Br J Sports Med. 2016;50:1379-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ; American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42:2282-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Luo Z, Zhang T, Chen S. Exercise Prescription: Pioneering the "Third Pole" for Clinical Health Management. Research (Wash D C). 2023;6:0284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Luo ZW, Sun YY, Xia W, Xu JY, Xie DJ, Jiao CM, Dong JZ, Chen H, Wan RW, Chen SY, Mei J, Mao WJ. Physical exercise reverses immuno-cold tumor microenvironment via inhibiting SQLE in non-small cell lung cancer. Mil Med Res. 2023;10:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (4)] |
| 11. | Chen Y, Chen X, Luo Z, Kang X, Ge Y, Wan R, Wang Q, Han Z, Li F, Fan Z, Xie Y, Qi B, Zhang X, Yang Z, Zhang JH, Liu D, Xu Y, Wu D, Chen S. Exercise-Induced Reduction of IGF1R Sumoylation Attenuates Neuroinflammation in APP/PS1 Transgenic Mice. J Adv Res. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 12. | Call JA, Chain KH, Martin KS, Lira VA, Okutsu M, Zhang M, Yan Z. Enhanced skeletal muscle expression of extracellular superoxide dismutase mitigates streptozotocin-induced diabetic cardiomyopathy by reducing oxidative stress and aberrant cell signaling. Circ Heart Fail. 2015;8:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Khan KS, Overgaard K, Tankisi H, Karlsson P, Devantier L, Gregersen S, Jensen TS, Finnerup NB, Pop-Busui R, Dalgas U, Andersen H. Effects of progressive resistance training in individuals with type 2 diabetic polyneuropathy: a randomised assessor-blinded controlled trial. Diabetologia. 2022;65:620-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Monno I, Ogura Y, Xu J, Koya D, Kitada M. Exercise Ameliorates Diabetic Kidney Disease in Type 2 Diabetic Fatty Rats. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Han XZ, Sun CZ. The influence of resistance exercise and aerobic exercise on type 2 diabetes: a meta-analysis. J Sports Med Phys Fitness. 2024;64:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714-1721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Pan B, Ge L, Xun YQ, Chen YJ, Gao CY, Han X, Zuo LQ, Shan HQ, Yang KH, Ding GW, Tian JH. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 19. | Xing H, Lu J, Yoong SQ, Tan YQ, Kusuyama J, Wu XV. Effect of Aerobic and Resistant Exercise Intervention on Inflammaging of Type 2 Diabetes Mellitus in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2022;23:823-830.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Ross LM, Porter RR, Durstine JL. High-intensity interval training (HIIT) for patients with chronic diseases. J Sport Health Sci. 2016;5:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Innes KE, Selfe TK. Yoga for Adults with Type 2 Diabetes: A Systematic Review of Controlled Trials. J Diabetes Res. 2016;2016:6979370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Ahn S, Song R. Effects of Tai Chi Exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. J Altern Complement Med. 2012;18:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Gallardo-Gómez D, Salazar-Martínez E, Alfonso-Rosa RM, Ramos-Munell J, Del Pozo-Cruz J, Del Pozo Cruz B, Álvarez-Barbosa F. Optimal Dose and Type of Physical Activity to Improve Glycemic Control in People Diagnosed With Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2024;47:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 24. | Evans PL, McMillin SL, Weyrauch LA, Witczak CA. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 25. | Niyazi A, Yasrebi SMA, Yazdanian M, Mohammad Rahimi GR. High-Intensity Interval Versus Moderate-Intensity Continuous Exercise Training on Glycemic Control, Beta Cell Function, and Aerobic Fitness in Women with Type 2 Diabetes. Biol Res Nurs. 2024;26:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 26. | Knudsen JR, Steenberg DE, Hingst JR, Hodgson LR, Henriquez-Olguin C, Li Z, Kiens B, Richter EA, Wojtaszewski JFP, Verkade P, Jensen TE. Prior exercise in humans redistributes intramuscular GLUT4 and enhances insulin-stimulated sarcolemmal and endosomal GLUT4 translocation. Mol Metab. 2020;39:100998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 27. | Sylow L, Tokarz VL, Richter EA, Klip A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021;33:758-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 28. | Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 1262] [Article Influence: 315.5] [Reference Citation Analysis (0)] |
| 29. | Zacharewicz E, Hesselink MKC, Schrauwen P. Exercise counteracts lipotoxicity by improving lipid turnover and lipid droplet quality. J Intern Med. 2018;284:505-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Badin PM, Langin D, Moro C. Dynamics of skeletal muscle lipid pools. Trends Endocrinol Metab. 2013;24:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Mezincescu AM, Rudd A, Cheyne L, Horgan G, Philip S, Cameron D, van Loon L, Whitfield P, Gribbin R, Hu MK, Delibegovic M, Fielding B, Lobley G, Thies F, Newby DE, Gray S, Henning A, Dawson D. Comparison of intramyocellular lipid metabolism in patients with diabetes and male athletes. Nat Commun. 2024;15:3690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Lobene AJ, Ragland TJ, Lennon SL, Malin SK. Nutrition Interactions With Exercise Training on Endothelial Function. Exerc Sport Sci Rev. 2023;51:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Qiu S, Cai X, Yin H, Sun Z, Zügel M, Steinacker JM, Schumann U. Exercise training and endothelial function in patients with type 2 diabetes: a meta-analysis. Cardiovasc Diabetol. 2018;17:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 34. | Borges Madureira Sabino T, Maria Martins Vancea D, da Cunha Costa M, José Perrier de Melo R, Vilela Dantas I, Nicolas Dos Santos Ribeiro J. Original article - Effect of different resistance training intensities on endothelial function in people with type 2 diabetes mellitus: A systematic review. Diabetes Res Clin Pract. 2023;200:110676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 35. | Wang Z, Peters BA, Yu B, Grove ML, Wang T, Xue X, Thyagarajan B, Daviglus ML, Boerwinkle E, Hu G, Mossavar-Rahmani Y, Isasi CR, Knight R, Burk RD, Kaplan RC, Qi Q. Gut Microbiota and Blood Metabolites Related to Fiber Intake and Type 2 Diabetes. Circ Res. 2024;134:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 36. | Yao T, Wang H, Lin K, Wang R, Guo S, Chen P, Wu H, Liu T, Wang R. Exercise-induced microbial changes in preventing type 2 diabetes. Sci China Life Sci. 2024;67:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Valder S, Brinkmann C. Exercise for the Diabetic Gut-Potential Health Effects and Underlying Mechanisms. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Ling C, Rönn T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019;29:1028-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 621] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 39. | Fu S, Meng Y, Zhang W, Wang J, He Y, Huang L, Chen H, Kuang J, Du H. Transcriptomic Responses of Skeletal Muscle to Acute Exercise in Diabetic Goto-Kakizaki Rats. Front Physiol. 2019;10:872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Stephens NA, Xie H, Johannsen NM, Church TS, Smith SR, Sparks LM. A transcriptional signature of "exercise resistance" in skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism. 2015;64:999-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | BioRender. [cited 2 June 2024]. Available from: https://www.biorender.com/. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: Https://creativecommons.org/Licenses/by-nc/4.0/