INTRODUCTION
Diabetes mellitus is a chronic metabolic disorder affecting almost 10% of the adult World population. So, around 550 million people are living with diabetes. The incidence is on a steep rise in the middle and lower socioeconomic populations[1]. It affects the kidney, cardiovascular system, brain, peripheral nerves and eye. The prevalence of cataracts is 29% among diabetic individuals compared to 22% who do not have diabetes[2]. As per different studies, the incidence of cataracts is quadrupled in those below 65 years with diabetes and is twofold higher in those above 65 years in comparison to the non-diabetic population[3].
The success of cataract surgery in diabetic patients with implantation of intraocular lenses (IOLs) is variable and dependent on issues related to the challenges during intraocular surgery, choices of IOLs, as well as systemic and ocular conditions. The intraoperative and postoperative considerations are myriad and can have a bearing not only on the patient’s visual outcome but also on the ability to treat subsequently evolving diabetic retinopathy (DR) and its complications.
This review article covers several important aspects related to cataract surgery in diabetic patients, including most importantly, the choice of IOLs best suited for this population.
SEARCH METHODOLOGY
We searched articles from standard databases including Pubmed, Embase, and Scopus, as well as Baishideng Publishing Group, Google Scholar, and ResearchGate. The terms “cataract surgery”, “diabetes”, “complications”, “retinopathy”, “maculopathy”, “macular edema”, “visual acuity”, “vitrectomy”, “capsulorhexis”, “neovascularization”, “intraocular lens”, “monofocal”, “trifocal”, “multifocal”, “extended depth of focus”, “continuous range of vision”, “presbyopia”, “pupil dilation”, “optical coherence tomography”, “posterior capsular opacification”, “retinal laser photocoagulation”, and “prognosis”. We included only those articles that were highly cited and published in the English language from 1983 to 2023. The authors used the Reference Citation Analysis (RCA) Tool to assess the impact and relevance of the referenced articles. Five independent reviewers considered the abstract and full text of 1388 articles and finalized a total of 84 articles to be included in the manuscript.
CHALLENGES IN CATARACT SURGERY IN DIABETES
When IOL implantation was introduced in extra-capsular cataract extraction (ECCE), diabetes was to be considered a contraindication[4,5]. Surgeons in the bygone era feared the possibility of visual loss after any complicated surgery, and considered diabetic subjects to be at high risk of post-operative macular edema and progression of DR[6].
ECCE procedures also carry an additional risk of increased macular edema, neovascularization of the iris (NVI), and neovascular glaucoma (NVG)[7]. This was attributed to the increased degree of ocular inflammation associated with the release of inflammatory cytokines viz. interleukin-1 (IL-1), IL-2, IL-10, IL-12, interferon-alpha, tumor necrosis factor-alpha, vascular endothelial growth factor (VEGF), hepatocyte growth factor, and pigment epithelium-derived factor[8]. The concentrations of these mediators take up to one month to decline to preoperative levels[9]. In this era, it was also recommended that cataract surgery be deferred in patients with DR until visual acuity falls to 20/100 and worse[10].
With the advent of phacoemulsification, it became clear that smaller incisions and shorter surgical duration decreased the inflammation associated with cataract surgery[11]. This was but one aspect of the challenging situations that diabetic cataracts present. The others are discussed as follows.
Visualization
Problems in visualization during cataract surgery in diabetic patients are not limited to the state of the cornea alone. The red reflex may be attenuated depending on the state of the vitreous and retina. Diabetic eyes are also plagued with high chances of poor intra-operative pupillary dilatation, attributed to neuropathic damage to parasympathetic innervation of the pupil and elevated prostaglandin levels[12]. A leathery consistency of the iris is noted in diabetic patients due to vacuolization of the pigment epithelium with glycogen. This increases pre- and intra-operative pupillary miosis, as well as the risk of intraoperative pigment dispersion[3,13]. Pharmacological mydriasis using intracameral adrenaline, preservative-free lignocaine or a combination of adrenaline, tropicamide and phenylephrine can achieve satisfactory pupillary dilation in a significant proportion of cases[14,15]. Intraoperative pupil-expanding manoeuvres such as sphincterotomies or mechanical stretching of the pupillary margin can be planned, or devices such as iris hooks and pupil expander rings can be kept on standby[13]. The relative success of these measures is a matter of debate, and the choice should be individualized by the surgeon based on other considerations.
Capsulorrhexis
The basement membrane of the lens (capsule) is thicker and more friable in diabetic patients, and therefore more prone to rupture during capsulorhexis[13]. Diabetic eyes are associated with an increased incidence of postoperative anterior capsular phimosis[16]. The size of the anterior capsulorrhexis must therefore be continuous, curvilinear, regular, larger than the conventional 5.5 mm diameter, but smaller than the optic diameter of the IOL. This prevents anterior IOL displacement and reduces the rates of posterior capsular opacification (PCO)[17]. Most anterior capsulotomies can be achieved satisfactorily with the use of needle cystitome or capsulorhexis forceps depending on the surgeons’ preference. The Argentinian Flag sign can be further prevented by closed-chamber decompression of intumescent lenses using focal Nd:YAG laser disruption of the anterior capsule immediately before taking in the patient for surgery, or manual or automated vacuum applied after a needle puncture of the anterior capsule, and/or the use of a two-stage or vacuum rhexis technique[18-20]. The use of laser capsulorhexis or precision-pulse capsulotomy devices can help to achieve capsulotomies that are more consistent, however, the benefits of their effects have not been proven in diabetic patients[21].
Previous intravitreal injection may increase the chances of posterior capsule rupture (PCR), or be a cause of direct breach due to trauma with the intravitreal needle[22]. Any previous vitreoretinal intervention may cause zonular instability, or be the cause of cataracts in the first place[23]. Thus, the possibility of vitreous prolapse must be anticipated and the surgery planned accordingly. Lens-bag instability can be challenging to manage in the setting of pre-existing vitreoretinal complications. Therefore, in diabetic eyes, implantation of a capsular tension ring (CTR) during cataract surgery may help to anchor the IOL-bag complex even in subsequent surgeries, should the need arise[23]. There is, however, no guideline regarding the circumstances in which the CTR should be implanted, and the choice is on the surgeon to decide.
Retinal status
The main cause of poor visual outcome (final visual acuity < 20/200) following cataract surgery in diabetic patients is cystoid macular edema (CME), which may be caused by the superimposition of diabetic macular edema (DME) and Irvine-Gass syndrome[6,24]. The cost of the surgery may be doubled due to macular edema[25]. Diabetic patients with preexisting diabetic macular edema are at a heightened danger of aggravation of the macular edema[13]. With the advancement of the cataract, the visibility of the retina is compromised. The status of the retina in such cases is then detectable only by optical coherence tomography (OCT). Historical fluctuation of glycemic control as well as examination of the contralateral eye give clues to the presence of maculopathy in such cases. In addition, tests for macular function such as a potential retinal acuity meter can be done. In the circumstance that the retina cannot be visualized due to the cataract, a preoperative B-scan ultrasonogram is mandatory. Cataract surgery must be performed with explained prognosis, with precise choice and timing of the procedure, appropriate intraocular lens selection and meticulous pre- and post-operative workup, all of which have to be tailored to the patient’s systemic and ocular status.
INTRAOCULAR LENS CHOICE
The choice of IOL in diabetic cataract patients is a challenging scenario. It is recommended to personalize the IOL recommendation based on patient-specific ocular characteristics for optimal surgery outcomes. The following IOL characteristics must be considered when choosing an IOL for a diabetic patient.
IOL design
Several reports emphasize the use of an IOL with a large optic diameter, which, in combination with a larger capsulorhexis, allows for better peripheral retinal visualization post-operatively[13]. A 6.5 mm IOL ensures around 40 per cent more optic area than a 5.5 mm IOL[26]. Most foldable acrylic lenses are available with an optic diameter of 6.0 mm, which is fairly suitable to provide retinal visualization and scope for the treatment of peripheral retinal pathology, for example, by photocoagulation in diabetic patients[13].
A square-edge design of the IOL is preferable as the sharp posterior edge is known to adhere to the posterior capsule and prevent migration of lens epithelial cells (LECs) underneath it, thus reducing the chances of PCO[13,27].
The use of yellow-coloured (blue-light filtering) IOL has been reported to improve colour discrimination in the blue-yellow chromatic axis and appears to be preferable in diabetic patients, however, there is no contraindication for the use of an IOL with clear optic[28]. As there is more incidence of intra- and post-operative reaction in the anterior chamber and uveitis in diabetic patients, heparin-coated hydrophobic IOLs are theoretically suited for these cataract patients to favour in-the-bag implantation of the IOL without uveal trauma (Figure 1).
Figure 1 Heparin Coated Yellow intraocular lens (CT Lucia 621PY, Carl Zeiss, Germany).
The black highlight all around the intraocular lens denotes a surface coating of heparin which facilitates its unfolding within the capsular bag.
IOL material
The preferred choice for IOLs remains the hydrophobic acrylic material, which is now ubiquitous and dependable, and the material of choice in eyes with the possibility of undergoing vitreoretinal surgery in the future[29]. Despite having a risk of increased anterior chamber inflammation, it is associated with low rates of IOL opacification, and posterior capsular opacity (PCO) and has the lowest proclivity for silicon oil adhesion[13,30].
Hydrophilic acrylic IOLs are more prone to IOL opacification and calcification due to higher serum and aqueous humour phosphorus levels observed in patients with proliferative DR, which can then cause visual morbidity and need for IOL exchange[31,32].
The interaction of various factors such as hydrophobic material, smooth optic surface, and sharp posterior optic edge plays a key role in PCO development[33]. Hydrophobic material has a higher contact angle measurement than hydrophilic material, which makes it more difficult for lens epithelial cells to migrate on the IOL surface[34]. Surface modification of the hydrophobic IOL aims to enhance adhesion between the IOL and the capsular bag[35]. This tight adhesion makes it particularly difficult for any residual LECs to proliferate in this area and cause PCO[36].
The silicone material of IOL develops condensations during pars-plana vitrectomy and such IOLs are relatively contraindicated in patients with diabetes[29]. In diabetic patients, who have undergone vitreoretinal surgery with silicone oil insertion, silicone IOLs are absolutely contraindicated.
IOL type and location
In-the-bag implantation of a single-piece IOL is preferred as a general rule in cataract surgery. In case of significant loss of posterior capsular support, a three-piece IOL in the ciliary sulcus can be implanted, preferably with the optic captured through the residual capsular opening(s) to prevent movement and displacement of the IOL[37]. A three-piece IOL can be kept as a backup lens in every case as it can also be scleral-fixated if needed. Most surgeries warrant IOL implantation to preserve visual function, as these patients are poor candidates for long-term aphakic contact lens wear[38]. Aphakia may be preferable if the patient has very low visual potential, with secondary IOL planned subsequently depending upon the corrected visual acuity and anticipated gains following IOL implantation.
An anterior chamber IOL or iris-claw IOL should not be used in diabetic patients due to the risk of NVI. These IOLs are also associated with a theoretical risk of CME, while pupil ovalization and poor mydriasis make post-operative fundus visualization difficult[3,39].
IOL power calculation
Corneal topographic changes during glycemic changes cause errors in IOL power calculation[13]. With stable glycemic control, optical biometry with the inclusion of posterior corneal diameter such as using telecentric keratometry is essential for achieving target refraction in cataract surgery in diabetic patients[40,41]. Tear film instability leads to issues in obtaining consistent keratometry and topography readings[42]. Contact lenses should not be worn for at least one month before the biometry for PMMA lenses, two weeks for gas permeable lenses, and one week for extended-wear and daily-wear soft contact lenses. Findings of automated keratometry can be verified by manual keratometry and observation of the mires in case of ambiguity[13].
Due to sub-basal nerve abnormalities, impaired corneal stem cell and epithelial cell division[3], corneal hypoaesthesia is observed in diabetics, as well as impaired healing of wounds and abrasions[13]. Recurrent corneal erosions can be provoked. Hence, it is best to avoid contact biometry in these cases.
Limbal-relaxing incisions reduce corneal sensation and worsen ocular surface disease, hence are to be avoided in diabetic patients[43]. Toric IOLs should therefore be considered routine in diabetic patients for the correction of astigmatism.
IOL power calculations in diabetic patients with prior laser vision correction or incisional keratorefractive surgeries need caution, as with non-diabetic subjects. Patients need to be counselled that they may not get the intended target, may get visual disturbances, and the lenses have a higher probability of getting exchanged[44,45]. IOL power calculation may also be inaccurate in the presence of vitreoretinal interface abnormalities, or retinal detachment. Aphakia with sequential surgery may be planned if IOL power cannot be satisfactorily calculated.
Correction of presbyopia
Options for the correction of presbyopia include bilateral use of a negative spherical aberration IOL with monovision or micromonovision, implanting an aberration-free monofocal IOL that allows room for the residual normal positive corneal spherical aberration to augment the effect of monovision, and multifocal, trifocal or extended depth of focus (EDOF) lenses, all of which have caveats. An evaluation of pupil diameters, assessment of the patient’s visual needs and the status of retinopathy is essential before even offering these patients the option of presbyopia correction in cataract surgery.
In monovision and micromonovision, the non-dominant eye is made myopic for distance, and the dominant eye is kept emmetropic. They remain viable options for most patients undergoing monofocal IOL implantation, preferably with negative spherical aberration. A preoperative trial with monovision contact lenses whenever possible helps to anticipate post-operative problems[46].
Although the aberration-free Crystalens AO (Bausch & Lomb Incorporated, United States) is regarded as a monofocal accommodative IOL, the final near point is grossly unpredictable. The use of this lens may affect depth perception at a 3 m viewing distance typically observed during walking[47]. Corneal higher-order aberrations also need to be considered. The optic size is 5.0 mm, which is theoretically unfavourable for peripheral retinal visualization. Thus, it should be used with caution.
The use of multifocal (MFIOL), bifocal or trifocal IOLs in diabetic patients remains controversial. These IOLs may make post-operative laser therapy difficult and may interfere with visualization of the fundus during vitrectomy due to their peculiar optics[48]. With multifocal IOLs, reduced contrast sensitivity affecting up to 20% of the quality of vision causes dissatisfaction in patients with pre-existing maculopathy[3]. EDOF lenses have a better contrast profile and provide satisfactory outcomes even if the IOL power calculated is slightly erroneous. Monovision may be enhanced with low-add MFIOL/EDOF in the dominant and high-add MFIOL/EDOF in the non-dominant eye, constituting the so-called blended vision[49]. Continuous range of vision IOLs like Tecnis Synergy (Johnson and Johnson Vision, United States) and hybrid trifocal/EDOF lenses with high modulation transfer function like Adtec Xtnd (Adaptive Ocular Technologies, India) have the advantage of good performance in low ambient illumination[44].
In summary, presbyopia-correcting IOLs can be offered with caution only to patients with stable glycemic control, no ocular surface issues, and minimal retinopathy and maculopathy.
Newer IOLs
The Light-Adjustable Lens (LAL; RxSight, United States), enhanced monofocal lenses like Tecnis Eyhance (Johnson and Johnson Vision, United States) and non-diffractive EDOF lenses like Rayone EMV (Rayner, United States) are known to have low side-effect profile, as they do not split light. It is recommended that for the use of LAL, the patient must have a pupil size of 6mm or more[50].
IC-8 Apthera (Bausch & Lomb Incorporated, United States) has a small aperture with a ring-like blacked-out paracentral area, which may obstruct fundus view. It is, therefore, theoretically not preferable in diabetic patients[51].
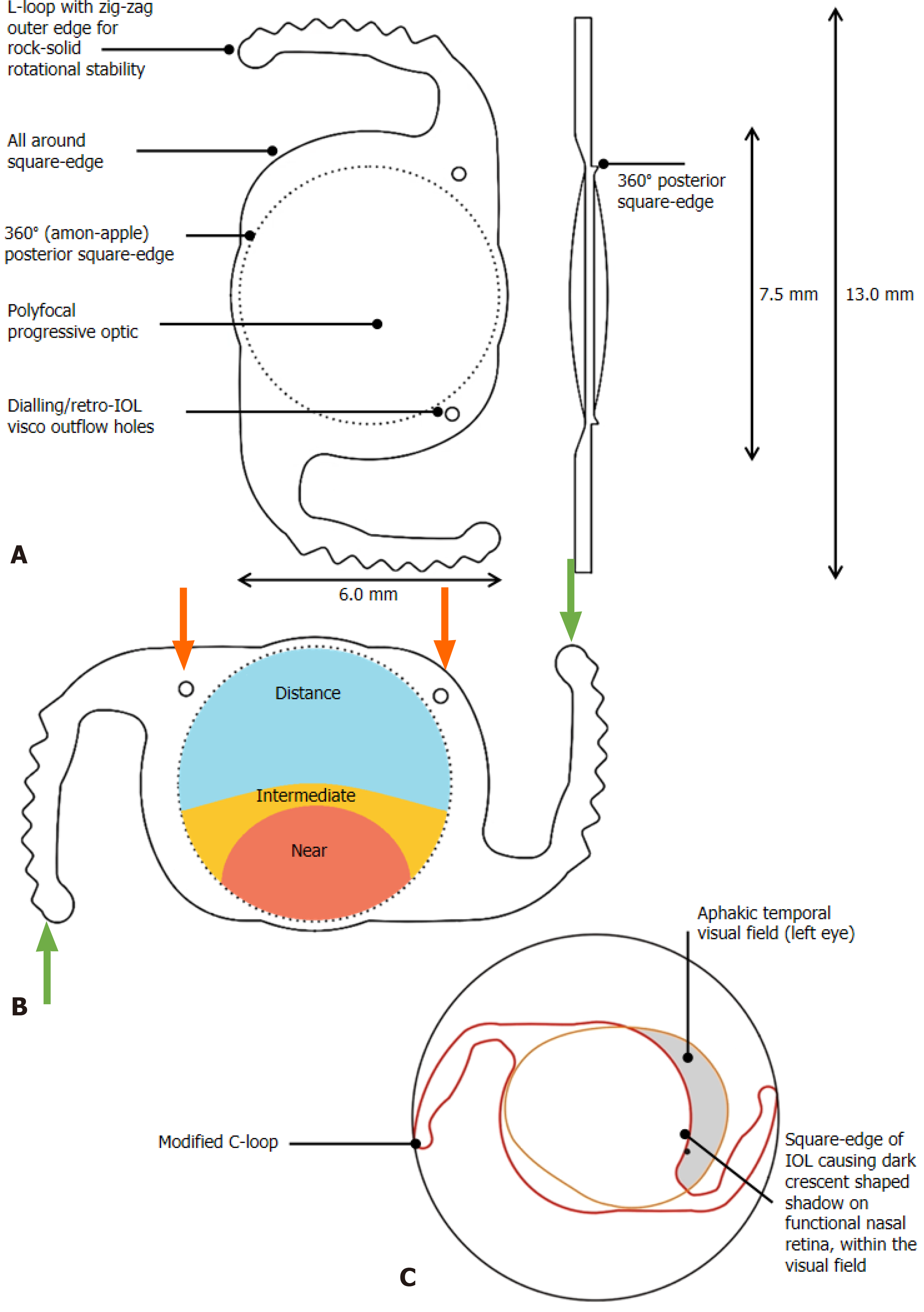
Clearview 3 (Lenstec, United States) is an asymmetric segmented multifocal IOL, where the near addition is sector-shaped and seamlessly blended with an intervening transition zone and is FDA-approved[52]. A similar IOL, Spirant Autofocus Pro (Lifeline Medical Devices Private Limited, India), has a progressive polyfocal optic with GRIN (gradient refractive index) technology akin to a progressive spectacle lens to provide distance, intermediate and near vision with no glare and haloes (Figure 2A)[53]. There is an increased incidence of glare, halos and negative dysphotopsia in diabetic cataract patients. This unique IOL has an optic diameter of 7.2 mm with no refractive or diffractive zones, so these problems are not faced by diabetic patients implanted with Autofocus-Pro (Figure 2B). As it has a larger optic size compared to conventional IOLs, it provides a 45% larger optical area which provides ample visibility and ease in the management of peripheral DR (Figure 2C).
Figure 2 Progressive Polyfocal intraocular lens with GRIN technology (Spirant Autofocus Pro, Lifeline Medical Devices Private Limited, India).
A: Design features of the intraocular lens (IOL); B: Demonstration of polyfocal ringless GRIN Technology and L-loop haptics with zig-zag edges (green arrows). The dialing holes are position indicators to be kept in the upper half of the eye; they also help to drain remaining retro-IOL viscoelastic (red arrows); C: Disadvantages of conventional IOLs that are overcome by the oval optic diameter of 7.2 mm and larger optic size. IOL: Intraocular lens.
However, the real-world evidence regarding the benefit of these newer lenses in diabetic patients is yet to be reported in clinical studies separately.
PRE-OPERATIVE CONSIDERATIONS
The odds for success in a diabetic patient undergoing cataract surgery can be increased when the patient is treated as a whole, and the eye is not viewed in isolation.
Systemic considerations
The need for glycemic control before surgery is critical for the prevention of endophthalmitis, however, rapid glycemic control can irreversibly increase lens opacities and preclude fundus examination. This emphasizes the balance needed to weigh the need for early surgery against any pre-existing retinopathy which is amenable to treatment before surgery and may be compromised with the advancement of the cataract[13].
An internist’s referral should be sought for correction of co-morbidities like hypertension, renal disease, anaemia, and/or dyslipidemia as well as for quitting smoking. Advice regarding diet, exercise and pregnancy is critical for the well-being of such patients. Periocular and hidden infections such as sino-nasal infections or urinary tract infections should be excluded[54,55]. Coexisting nephropathy, neuropathy and periodontitis should be managed simultaneously.
Patients who have suffered adverse systemic events such as myocardial infarction or cerebrovascular accidents ought to be approached with special caution. Surgery in these cases may be undertaken with an anesthesiologist’s monitoring if deemed so.
Ocular considerations
Diabetic corneas suffer from endothelial dysfunction and have a greater predisposition to endothelial cell loss following cataract surgery[56]. Routine specular microscopy is recommended for all people with diabetes. Greater care should be taken about Descemet membrane and endothelial protection when operating on diabetic patients, such as avoiding instrument trauma and using a dispersive viscoelastic with soft-shell techniques[55,57].
Glaucoma is a coexistent problem in the eyes of several patients with diabetes. Glaucomatous changes affect visual prognosis, and control of intraocular pressure might be a challenge pre-operatively, owing to mixed mechanisms of glaucoma, e.g., normotensive or ocular hypertensive states complicated by lens-induced, or neovascular IOP rise. OCT is useful for analyzing optic nerve head morphology, retinal nerve fibre layer and macular ganglion-cell-complex (GCC). The analysis may be compromised due to prior or coexistent DME, and patients may develop significant GCC thinning in diabetes even before retinal vasculopathy[58]. Gonioscopy should be done to exclude neovascularization of the angle (NVA) even in cases that do not have clinically obvious NVI. In case NVA or NVI is detected, retinal laser photocoagulation should be performed[59]. However, the visual outcomes following phacoemulsification in eyes with NVG are usually poor[55].
Pre-existing retinopathy ought to be treated before planning cataract surgery. The ETDRS study entails the utilisation of focal/grid laser photocoagulation and anti-VEGF for the treatment of macular edema, to improve post-operative visual outcomes[60]. Panretinal laser photocoagulation for proliferative DR should be done 6 months to 1 year before cataract surgery, to avoid the risk of an increase in macular edema if cataract surgery is done within 6 months of the procedure[3,13]. Other retinal diseases, if present, compromise the visual prognosis further[61]. For example, an epiretinal membrane can degrade macular image contrast and potentially cause metamorphopsia, increased risk of postoperative macular edema and lower visual gains[45]. Therefore, there is now an increasing preference for earlier cataract surgery in patients with diabetes[55].
The prognosis in cases with pre-existing DR or maculopathy is always guarded and should be explained to the patient. Surgery in these cases requires more chair time incumbent on the surgeon to make sure the patient is properly explained about all aspects of the surgery, and consent taken accordingly[45]. Counselling about different IOLs is also paramount to achieving the best results in diabetic patients undergoing cataract surgery.
INTRAOPERATIVE CONSIDERATIONS
Choice of surgical technique
As has been already established, phacoemulsification is associated with lesser ocular inflammation in comparison to conventional extracapsular cataract surgery, thereby associated with better post-operative visual outcomes[62]. Phacoemulsification is also not known to aggravate pre-existing DR[63]. Progression of DR in patients after uneventful phacoemulsification has been ascribed to poor glycemic control, rather than the procedure itself[55]. It is associated with fewer postoperative complications (barring transient corneal edema), in comparison to conventional techniques, thereby making it an ideal technique for cataract surgery in diabetics[64].
Surgical time
Increased surgical time is associated with an increased risk of progression of retinopathy and poor post-operative visual results. Hence, surgical time should be kept minimal[65].
Cataract extraction procedure and intraoperative adverse events
It is known that the cortex in diabetic patients is sticky, and needs thorough cleanup to reduce post-operative inflammation as well as to enhance short- and long-term visual outcomes[15].
The mere presence of diabetes is not associated with an elevated risk of intra-operative adverse events such as zonular dehiscence, PCR and other such complications[62]. However, these complications are more common in eyes that have previously undergone vitreoretinal interventions, as mentioned previously.
Use of pharmacological adjunctives during surgery
Intraoperative administration of an intravitreal anti-VEGF agent is an effective alternative for patients with DME, in comparison to pre-operative or postoperative injections[56]. Steroids are to be reserved for patients with persistent, or refractory macular edema. Dexamethasone is the better of the steroid injections, owing to its low rates of intraocular pressure elevation, and cataract formation[66]. As an add-on, intravitreal dexamethasone implants require fewer injections and show good improvement in DME[67]. Intravitreal anti-VEGF is also associated with dramatic regression of IOP and reduction of neovascularisation when used in patients with NVG[68].
Combined surgery
Combining trabeculectomy with phacoemulsification may be planned in eyes with coexistent glaucoma to reduce dependence on anti-glaucoma medication, but the theoretical risks associated with trauma to the angle of the anterior chamber have to be weighed in. This is also suggested in cases of coexistent NVG and cataracts when the NVI has regressed post-laser or pharmacologic treatment[3]. The outcomes of minimally invasive glaucoma surgeries in combination with cataract surgeries in the diabetic population are yet unknown.
In patients undergoing isolated vitrectomy, there is an acceleration of development of lenticular opacities post-surgery[69]. The vitreoretinal interface has also been deemed an instigating factor for the development of persistent DME[70]. In consideration of these, phacoemulsification may be combined with pars plana vitrectomy with endolaser for indications such as cataract with refractory vitreous haemorrhage, posterior pole tractional retinal detachment, retinal detachment of combined aetiology, vitreomacular interface abnormalities like significant epiretinal membrane, and macular edema resistant to medical therapy[71]. Combining both procedures reduces the total number of surgeries and results in faster visual recovery to benefit patients[72]. Combined vitrectomy should be avoided in patients with iris neovascularisation, severe traction, and younger patients without significant cataracts[73]. Unilateral surgeries may result in a picture of asymmetric DR which should be considered according to the type of surgery performed[74].
POSTOPERATIVE CONSIDERATIONS
The postoperative period mandatorily entails detailed evaluation of patients with diabetes, more so in patients with an inadequate view of the fundus pre-operatively and in patients with proliferative retinopathy.
Anterior segment complications
Diabetic eyes are prone to corneal epithelial defects and erosions due to neuropathic damage to the cornea[8] and impaired corneal wound healing[75]. Surgical trauma to the anterior surface of the cornea is usually slow to heal. The use of preservative-free postoperative medication may be useful in preventing significant epitheliopathy in diabetic patients.
There is an elevated prospect of developing post-operative iritis, pupillary block due to posterior synechiae, and pigment deposits over the IOL[76]. Inflammation should be aggressively controlled or it may compromise pupillary dilation and fundal view, or degrade the visual quality of the IOL optic.
The compromised blood-aqueous barrier in diabetic patients may also cause increased proliferation of LECs due to more post-operative inflammation[3]. The rates of development of PCO between diabetics and non-diabetics vary, with reports showing an increase in the diabetic group on follow-up after 6, 12, or 18 months[15,77]. Also, PCO rates also are not correlated with the severity of DR[34]. Significant PCO should be cleared at the centre with photodisruptive Nd:YAG laser when thin so that the retinal view is not compromised[78]. Thicker PCO, when present, compromises retinal view and requires more laser energy to disrupt leading to increased chances of intraocular inflammation and macular edema[79].
The most dreaded anterior segment complication remains the development of NVI, whose incidence has remarkably reduced with the development of modern cataract surgeries. Pre-operative pan-retinal laser photocoagulation and allied usage of anti-VEGF drugs have remarkable efficacy in the control of NVI[80].
Posterior segment complications
Pseudophakic CME or Irvine-Gass syndrome: This showed a greater incidence in diabetic patients, even in the absence of retinopathy[81]. Acute CME lasts for less than 4 months and often resolves spontaneously. Chronic CME persists for four months or longer and does not resolve without pharmacotherapy[82].
Pseudophakic CME must be differentiated from DME. The former is more common in diabetics compared to DME, associated with minimal DR changes and a lack of exudates in the posterior pole, while fundus fluorescein angiography reveals optic disc staining and typical flower petal-like pooling of the dye at the macula[60]. CME often resolves spontaneously if it arises post-operatively but not if it was present pre-operatively[25,61,83].
The management of postoperative CME is complex and there is no consensus on the choice of agent to be used[84]. The most commonly used therapy is to suppress postsurgical inflammation using topical non-steroidal anti-inflammatory drugs (NSAIDs) or corticosteroids, either separately or as a combined treatment.
Current evidence on the effects of topical NSAIDs like ketorolac, bromfenac, and nepafenac in patients with CME is very uncertain and further investigation is warranted[82]. Even if NSAIDs reduce CME, this therapeutic effect often does not translate consistently into a measurable benefit on visual function, especially in the case of chronic CME[85].
Steroids as topical drops are routinely prescribed after cataract surgery, but they can also be used in the form of ointments, subconjunctival triamcinolone acetonide (TA) injections, intravitreal dexamethasone and fluocinolone implants, suprachoroidal triamcinolone injection, and posterior subtenon and trans-tenon retrobulbar triamcinolone injections. Steroids may effectively suppress CME but have their own side effects like elevation of intraocular pressure and predisposition to infection due to immunosuppression.
Topical therapy is less effective in vitrectomised eyes wherein the clearance of the drug from the eye is greatly increased. Vitreoretinal pharmacokinetic profiles of intravitreal steroid implants in vitrectomized eyes are similar to those in non-vitrectomized eyes, and they may be the agent of choice in these select groups of patients[86]. Similarly, injections are preferable in other refractory CME cases that do not respond to topical therapy[87].
Surgical intervention may be rarely required, except in case of vitreous loss wherein incompletely lysed vitreous strands may cause persistent traction on the macula. Lasers or vitrectomy may be used to lyse these vitreous strands[87].
Endophthalmitis: Diabetes is also associated with an increased liability of potential post-operative endophthalmitis, which may again hamper visual outcomes[88]. Measures for prevention of endophthalmitis include prior treatment of any infections, perioperative glycemic control, generous use of 5% povidone-iodine preoperatively in the conjunctival cul-de-sac and periocular region, as well as intracameral moxifloxacin injected at the end of the surgery[89]. A subgroup analysis of the Endophthalmitis Vitrectomy Study recommended early vitrectomy for post-operative endophthalmitis in the setting of diabetes[90].
VISUAL PROGNOSIS AFTER CATARACT SURGERY
The advancements in cataract surgery now provide adequate guarantees in terms of lower rates of complications and exceptional visual outcomes, owing to the holistic approach to diabetic and cataract management[91]. The lower the degree of DR, the better the post-operative visual prognosis[92]. Risk factors for poor visual recovery include the presence of DME as well as pre-existing maculopathy, ischemia or traction[92]. CME is the most common cause of poor visual recovery in patients with diabetes undergoing cataract surgery[31]. Visual rehabilitation after uneventful phacoemulsification with in-the-bag IOL implantation is directed by the status of the retinopathy.
Among the factors that hint at a poor visual prognosis, the presence of DME at the time of cataract surgery remains significant[93]. The severity of DR as well as macular ischemic status also plays an important role[94]. Patients with advanced DR may show no functional improvement despite apparent improvement in visual acuity[13]. Untreated PDR pre-operatively, which may bleed and cause vitreous haemorrhage and tractional retinal detachment after phacoemulsification, are also factors that threaten the visual outcome.