Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1091
Revised: December 22, 2023
Accepted: April 8, 2024
Published online: June 15, 2024
Processing time: 188 Days and 14.6 Hours
Disorders in energy homeostasis can lead to various metabolic diseases, particularly obesity. The obesity epidemic has led to an increased incidence of obesity-related nephropathy (ORN), a distinct entity characterized by proteinuria, glomerulomegaly, progressive glomerulosclerosis, and renal function decline. Obesity and its associated renal damage are common in clinical practice, and their incidence is increasing and attracting great attention. There is a great need to identify safe and effective therapeutic modalities, and therapeutics using chemical compounds and natural products are receiving increasing attention. However, the summary is lacking about the specific effects and mechanisms of action of compounds in the treatment of ORN. In this review, we summarize the important clinical features and compound treatment strategies for obesity and obesity-induced kidney injury. We also summarize the pathologic and clinical features of ORN as well as its pathogenesis and potential therapeutics targeting renal inflammation, oxidative stress, insulin resistance, fibrosis, kidney lipid accumulation, and dysregulated autophagy. In addition, detailed information on natural and synthetic compounds used for the treatment of obesity-related kidney disease is summarized. The synthesis of detailed information aims to contribute to a deeper understanding of the clinical treatment modalities for obesity-related kidney diseases, fostering the anticipation of novel insights in this domain.
Core Tip: There are a few reviews and summaries on obesity-induced renal injuries. We summarize the pathologic and clinical features of obesity-related nephropathy (ORN) as well as its pathogenesis and potential therapeutics targeting renal inflammation, oxidative stress, insulin resistance, fibrosis, kidney lipid accumulation, and dysregulated autophagy, with an aim to provide new insights into the clinical treatment of ORN.
- Citation: Mao TH, Huang HQ, Zhang CH. Clinical characteristics and treatment compounds of obesity-related kidney injury. World J Diabetes 2024; 15(6): 1091-1110
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1091.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1091
Obesity has become one of the epidemics in our era. The prevalence of obesity has significantly surged in the majority of developed nations over the past 20 to 30 years. Based on predictive estimations, it is anticipated that by 2030, more than half of the American population will either be obese or at risk of developing complications associated with obesity[1,2]. The prevalence of obesity in China is increasing, which has been associated with shifts in dietary patterns characterized by a higher intake of animal-derived products, processed grains, energy-dense foods rich in fat and sugar, as well as a sedentary lifestyle[3]. Overweight, obesity, and complications arising from obesity contributed to 11.1% of the deaths associated with noncommunicable diseases in 2019, placing a substantial economic burden on China’s healthcare system[4]. Obesity is an established risk factor for several comorbidities including hypertension, dyslipidemia, atherosclerosis, insulin resistance, diabetes, fatty liver disease, cardiovascular disease, and sleep apnea, all of which can promote chronic kidney disease (CKD). Furthermore, obesity plays a crucial and autonomous role in increasing the risk of CKD[5]. While kidney disease may not be widely acknowledged as a prominent component of metabolic syndrome, it is undeniable that excessive weight gain plays a significant role in the development of hypertension and type 2 diabetes (T2D). These two conditions collectively contribute to approximately 70% of cases that lead to end-stage renal disease (ESRD)[6]. Obesity has been recognized as a distinct risk factor for ESRD in multivariable models, even after considering various epidemiological and clinical factors such as the presence of diabetes mellitus and hypertension[7]. As the prevalence of obesity is rising worldwide, we observed a similar increase in the incidence of obesity-related kidney disease, which markedly impaired human health[8].
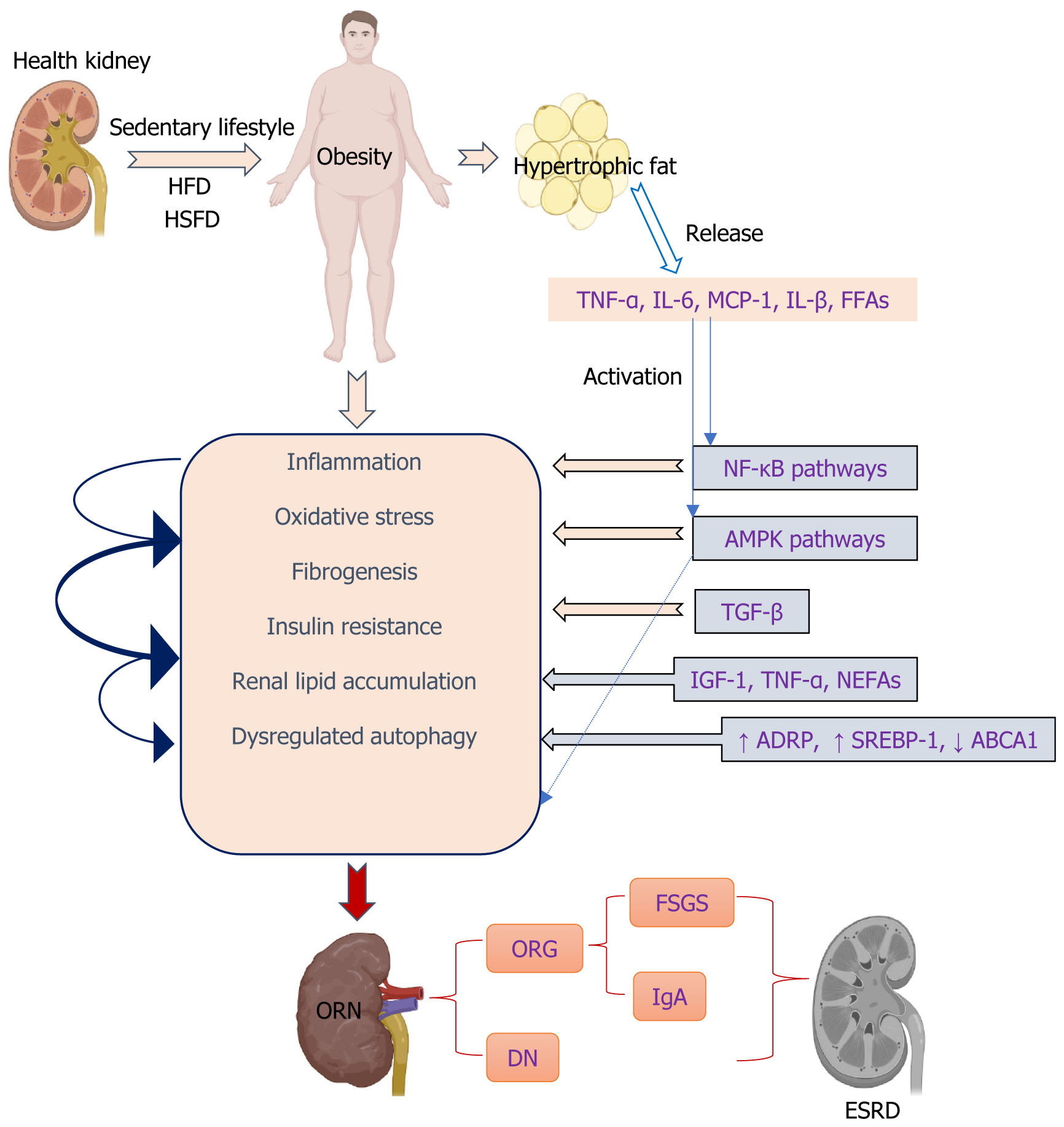
Adults with untreated obesity develop a glomerular injury that can manifest as hyperfiltration, albuminuria, obesity-related glomerulopathy (ORG), focal segmental glomerulosclerosis (FSGS), and ESRD[9-11]. Obesity-related nephropathy (ORN) is a condition characterized by kidney damage, with obesity being identified as a prominent contributing factor[7]. ORG has emerged as a prominent factor contributing to the development of ESRD. A ten year retrospective study demonstrated that ORN includes ORG, which has increased threefold from 2009 to 2018[12]. ORG has the potential to significantly contribute to the development of ORN and can serve as a histopathological indicator for its presence. The pathological state of ORG is characterized by glomerular enlargement and the development of adaptive FSGS. Clinical and experimental research has also revealed that ORG is characterized by enlarged glomeruli accompanied by increasing thickness of the basement membrane in the glomerulus, expansion of the matrix of mesangial cells, and injury to the barrier to filtration in the glomerulus due to hyperfiltration, ultimately leading to the development of enlarged glomeruli and FSGS. These conditions have been associated with severe obesity[13]. Severely obese patients may experience ORG as a secondary form of FSGS, which can lead to the development of renal insufficiency or ESRD in a significant proportion of affected individuals[14]. Podocyte failure occurs in glomerulomegaly and FSGS due to the inability of adaptive podocyte hypertrophy to match the expansion of glomerular tuft, resulting in dysfunction. The presence of perihilar glomerulomegaly and FSGS with relatively mild foot process effacement can be used as distinguishing features between ORG and primary FSGS[8]. However, this unique attribute of ORG proves to be diagnostically valuable, patients with obesity may exhibit a range of glomerular ailments, and the identification of complete nephrotic syndrome helps distinguish ORG from primary FSGS[9,15,16]. Obesity-associated FSGS, clinicopathological features, and its long-term outcomes indicate a poor prognosis, with almost one-half of patients developing advanced renal failure, FSG lesions with glomerulomegaly, and absence of nephrotic syndrome despite nephrotic-range proteinuria[10]. Obesity leads to glomerular enlargement due to increased glomerular filtration rate, renal plasma flow, filtration fraction, and tubular sodium reabsorption, resulting in proteinuria and deterioration of kidney function[6]. While overt clinical renal manifestations may not be evident in all patients with morbid obesity, various glomerular lesions such as increased mesangial matrix, proliferation of mesangial cells, hypertrophy of podocytes, enlargement of glomeruli, and focal and segmental glomerulosclerosis can manifest[17].
CKD is conventionally classified based on the extent of albuminuria, estimated glomerular filtration rate, and various types of kidney-specific injury. The concept of ORN has been introduced to clarify the complex relationship between obesity, CKD, and additional cardiometabolic disorders such as high blood pressure, diabetes, abnormal lipid levels, and systemic inflammation. Subnephrotic proteinuria is frequently observed in patients with ORN, while a minority may exhibit nephrotic-range proteinuria and experience a gradual decline in renal function. However, complete nephrotic syndrome occurs exceptionally rarely[8]. Patients diagnosed with ORG do not exhibit the characteristic manifestations of nephrotic syndrome, likely due to the specific nature of podocyte damage and the gradual onset of proteinuria in adaptive variants of FSGS[9,15,18]. Individuals diagnosed with immunoglobulin A (IgA) nephropathy who had a body mass index (BMI) over 25 at the time of renal biopsy exhibited more severe renal lesions and higher levels of proteinuria. Furthermore, they experienced a significantly accelerated decline in renal function and were more likely to progress to chronic renal failure compared to patients with a BMI below 25[19]. Being overweight has also been found to be a separate risk factor in the occurrence of high blood pressure among individuals diagnosed with IgA nephropathy[20]. Simultaneously, the presence of obesity and renal lipid buildup are crucial factors in the development and onset of diabetic nephropathy (DN) among individuals with T2D. Consequently, DN in T2D can be classified as a form of kidney injury associated with obesity[21,22]. Current treatments focus on addressing obesity, and non-pharmacological approaches are crucial in managing CKD, either as standalone conditions or accompanying comorbidities. These interventions primarily involve making lifestyle adjustments, including dietary modifications and incorporating more physical exercise. Pharmacological measures such as renin-angiotensin system (RAAS) blockers, angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers are advised for the treatment of CKD and albuminuria in all patients. Sodium–glucose cotransporter-2 (SGLT-2) inhibitors and, more recently, non-steroidal mineralocorticoid receptor antagonists (nsMRAs) have emerged as potential pharmacotherapeutic options for patients with CKD, with and without coexisting T2D (SGLT-2 inhibitors)[23,24]. However, although SGLT-2 inhibitors and nsMRAs demonstrate potential in mitigating the likelihood of kidney failure and cardiovascular incidents, their impact on body weight remains minimal or insignificant, particularly among individuals with CKD. Medicines based on glucagon-like peptide-1 (GLP-1) have been developed to assist in weight control for individuals who are overweight or obese, as well as for the treatment of T2D, indicating potential advantages in protecting kidney health[24]. Achieving weight loss through pharmacotherapies may involve both direct effects on the kidneys and indirect effects such as weight reduction, blood pressure reduction, and addressing issues like hyperglycemia, hyperinsulinemia, and excessive adipose tissue. As a result of this, GLP-1-based therapies have potential value in preventing and alleviating ORN.
Identification of the pathogenesis of ORN is very demanding; consequently, the identification of novel therapeutic targets and the determination of options for its management are of vital importance.
While adopting a healthy lifestyle and addressing obesity can serve as preventive measures against ORN, it is imperative to delve into the pathogenesis of this condition in order to discover more efficient and secure treatments or medications. Long-term overnutrition commonly leads to obesity and its associated complications such as kidney injury. This dietary regimen leads to excessive lipid accumulation in the kidney, as evidenced by intensified oil red O staining and signi
Possible mechanisms of obesity induced by glomerular injury and renal lesions are diverse, as discussed below.
Obesity is characterized by a persistent, mild inflammation caused by the excessive production of inflammatory cytokines such as tumor necrosis factor and interleukin-6 (IL-6) in adipose tissue and kidneys[30,31]. Monocyte infiltration and elevated renal expression of systemic proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), IL-6, and monocyte chemoattractant protein-1 (MCP-1), are observed in ORN[32,33]. Furthermore, hyperlipidemia leads to inflammation, resulting in cellular dysfunction and pathological alterations in the renal glomeruli[34,35]. In the presence of elevated sucrose and fat consumption, disturbances in mitochondrial equilibrium can potentially result in glucotoxicity and lipotoxicity, leading to an increase in OS and fibrogenesis[36,37]. The circulation of free fatty acids (FFAs) linked to obesity may result in persistent inflammation, OS, insulin resistance, and cardiovascular diseases. Additionally, they can increase the production of proinflammatory cytokines[38,39]. They have also been associated with the development of glomerulopathy and tubulointerstitial lesions in individuals with T2D[40]. It has also been observed both in vitro and in vivo that FFAs have the ability to activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, leading to an increase in the production of inflammatory cytokines such as TNF-α, interleukin-beta, interleukin-1beta (IL-1β) and interferon-gamma. Additionally, they also enhance the expression of pro-adhesion genes like intercellular adhesion molecule 1 and vascular cell adhesion molecule-1[41-43].
The activation of NF-κB occurs due to an overproduction of ROS, resulting in the subsequent enhancement of expression for pro-inflammatory and pro-fibrotic mediators in renal cells such as nitric oxide synthase, TNF-α, IL-6, IL-1β, and MCP-1[43]. The expression of adhesion molecules in renal cells is controlled by NF-κB, and recent studies have also suggested the involvement of p38 and JNK pathways[44].
The involvement of the MAPK pathway is significant in obesity, as it has been linked to the development of complications associated with obesity, including ORG[45]. Furthermore, emerging findings indicate a potential link between persistently elevated levels of FFAs in individuals with obesity and the presence of systemic inflammation commonly observed in cases of CKD[44]. Therefore, FFAs act as a connection between obesity, inflammation, and CKD, suggesting that targeting inflammation could be a promising approach for ORG treatment. In addition, the infiltration of macr
Disrupted lipid metabolism and buildup in the kidney contribute to OS by promoting excessive oxidation of lipids and generating ROS within mitochondria. This process further exacerbates transforming growth factor-β (TGF-β)-mediated renal fibrosis through lipid peroxidation[52-54]. Renal fibrosis, a process that occurs in the glomerular and tubulointerstitial compartments, is considered the main hallmark of DN and is the final convergent pathway to ESRD. The development of DN is associated with various pathways that are directly or indirectly connected to inflammatory processes, OS, and the formation of fibrous tissue in the kidneys[55-57]. The presence of inflammation in individuals with obesity has been shown to contribute to the progression of kidney injury, resulting in renal glomerular and interstitial fibrosis, as well as the irreversible accumulation of the extracellular matrix (ECM) in the kidneys.
In general, hyperglycemia is the primary and crucial factor responsible for initiating and advancing complications associated with obesity, such as nephropathy. This condition leads to impaired kidney function due to the sudden release of ROS, resulting in OS. The emergence of OS and unregulated ROS production triggers various pathways that contribute to the progression of nephropathy related to obesity.
The etiological factors underlying renal damage caused by obesity suggest that excessive intake of glucose and lipids through dietary means prompts renal cells to excessively uptake these substances, resulting in the abnormal accumulation of lipids within the kidneys[58]. Ectopic lipid deposition in the renal system occurs when there is an imbalance between excessive energy intake and the limited storage capacity of subcutaneous white adipose tissue[59]. Renal lipid buildup in human DN and renal diseases related to obesity primarily occurs within the tubule, although glomerular deposits are also linked to glomerular enlargement and fibrosis of the tubulointerstitium[60]. Distinct impairments have been reported in relevant cellular components, including podocytes, mesangial cells, endothelial cells, and macrophages[61,62]. Lipid buildup in non-adipose tissue is linked to both structural and functional alterations in different kidney cells, thereby playing a role in the progression of renal diseases caused by obesity[63]. In mice with obesity induced by an HFD, the mitochondria of the intrinsic cells of the kidney became small, round, and even broken. Inhibited mitochondrial FFA β-oxidation due to abnormal mitochondrial structure, results in kidney ectopic lipid deposition and renal dys
The expression level of ADRP demonstrates a correlation with the degree of lipid accumulation and adipose-related disorders, such as obesity and diabetes[71]. Emerging evidence indicates that sterol regulatory element-binding protein (SREBP) transcription factors play a crucial role in regulating the synthesis of fatty acids and cholesterol within cells, leading to abnormal lipid deposition. In diabetic mice, excessive expression of SREBP-1 in the kidney results in lipid accumulation, glomerulosclerosis, and fibrosis in the tubulointerstitial region[72]. Increased expression of SREBP has been observed in podocytes that are laden with lipid droplets, both in experimental studies and through a retrospective analysis of renal biopsies from patients with DN or ORG[60,73,74], and this upregulation may lead to the expression of TGF-β and ultimately result in an increase in deposition of the ECM[75]. A diet high in fats leads to increased lipid accumulation and glomerulosclerosis in mice through the SREBP-1c-dependent pathway[76]. The inhibition of SREBP-1 led to a significant improvement in the accumulation of fatty acids and TGs in the renal system[72]. The findings suggest that SREBP-1 may play a crucial role in the development of abnormal lipid accumulation and damage to renal tubules in DKD. Moreover, elevated lipid levels have the potential to stimulate the production of TGF-β1, promote collagen IV accumulation in the kidneys, generate ROS, and facilitate monocyte infiltration into glomeruli[27]. TGF-β1, a crucial regulator of fatty acid metabolism at the upstream level, triggers metabolic reprogramming in renal tubular epithelial cells by promoting reduced utilization of fatty acids and increased lipid accumulation[77]. The overexpression of TGF-β1 is associated with mesangial cell expansion, ECM accumulation, proteinuria, and glomerulosclerosis. Glomerular lipid accumulation occurs in DKD[60], FSGS[74], and Alport syndrome[78] and is associated with the downregulation of ATP-binding cassette transporter (ABCA1)-mediated cholesterol efflux. The storage of lipids is additionally regulated by the interaction between adiponectin and its receptors (AdipoRs), which play a role in modulating insulin sensitivity and facilitating fat oxidation[79]. The adiponectin receptor agonist (AdipoRon) improved diabetes-induced OS, and inhibition of apoptosis in the kidneys ameliorated the relevant intracellular pathways associated with lipid accumulation and endothelial dysfunction. AdipoRon-AdipoR1/AdipoR2 interaction–induced activation of the AMP-activated protein kinase (AMPK)/peroxisome proliferator-activated receptor (PPAR)α pathway ameliorated lipotoxicity, apoptosis, and OS, which in turn alleviated the features of DN[22]. PPARα is another important long-chain fatty acid oxidation gene mediated by AMPK. AdipoRon activates the AMPK/PPARα pathway and protects db/db mice from renal lipid droplet accumulation and lipotoxic damage[80]. In addition, the activation of AMPK/PPARα was found to enhance renal lipo
Autophagy is a lysosomal degradation pathway that has been conserved throughout evolution and is activated in the presence of challenging circumstances[82]. More importantly, enhanced autophagy effectively mitigated OS-induced damage. Autophagic vacuoles have commonly been detected in the renal proximal tubules. As a result, the crucial roles of autophagy in preserving the functionality of proximal tubular epithelial cells (PTECs) have been uncovered through the utilization of autophagy-deficient mice, with a specific focus on PTECs. This has been achieved under both normal and abnormal conditions[83,84]. A novel mechanism of lipotoxicity in the kidneys has been identified by a study, which involves the inhibition of autophagy leading to mitochondrial dysfunction, activation of inflammasomes, and deve
The accumulation of fat in visceral depots, commonly associated with obesity, is linked to an elevated susceptibility to various health conditions, such as insulin resistance[93]. Glucose intolerance, insulin resistance, and compensatory hyperinsulinemia are likely to be influenced by excess weight gain, particularly when accompanied by visceral obesity. These metabolic effects may potentially play a role in the development of renal disease. Insulin resistance serves as the initial stage that leads to the eventual onset of T2D and significantly contributes to kidney disease in certain individuals with obesity. Kidney injury may be influenced by subtle abnormalities in glucose and lipid metabolism, which are associated with insulin resistance. Therefore, the metabolic consequences of insulin resistance may play a more significant role in the development of hypertension and kidney disease, rather than solely attributing them to hyperinsulinemia[6]. Overconsumption of dietary fat can cause an excessive release of insulin, leading to the development of insulin resistance. This, in turn, may contribute to lipotoxicity, changes in kidney morphology, and tissue damage in mice subjected to a HFD challenge[94]. In particular, adipose tissues influence metabolism through the release of non-esterified fatty acids (NEFAs), glycerol, hormones, and proinflammatory cytokines such as IL-6 and insulin growth factor-1. Proinflammatory cytokines like TNF-α secreted by macrophages found in adipose tissue play a significant role in causing insulin resistance associated with obesity and impairing the function of β-cells[44,95,96]
With the rise in obesity rates, it is crucial to gain a deeper understanding of the correlation between obesity and albuminuria, as well as the progression of albuminuria associated with obesity. Albuminuria, which is associated with obesity, can be considered an early indicator of declining kidney function. The potential factors connecting obesity to albuminuria include chronic low-grade inflammation, insulin resistance and T2D, fibrosis development, and dysregulation of adipokines[97]. Recent clinical research has indicated that decreased levels of adiponectin in the bloodstream may be a crucial factor in the development of albuminuria associated with obesity. Adiponectin is believed to have a regulatory effect on podocytes, specialized cells within the kidneys that play a significant role in maintaining the integrity of the glomerular filtration barrier[98]. Renal cells undergo maladaptive responses to the mechanical strain caused by hyperfiltration due to the presence of adipokines and ELD in the kidney, resulting in podocyte depletion, proteinuria, FSGS, and interstitial fibrosis[8]. In the present investigation, scientists aimed to gather evidence regarding the involvement of these factors associated with obesity in the development of certain renal abnormalities, specifically albuminuria and inflammation[97]. Obesity-associated albuminuria is currently being acknowledged as not just a marker of impaired renal function and an initial indicator of DN[99], but it also amplifies the susceptibility to cardiovascular disease[100]. Determining the primary factor contributing to the onset of proteinuria, whether it is diabetes or obesity, can pose a challenge[8]. Renal overexpression of the transcription factor SREBP-1c is implicated in promoting diabetic renal diseases, resulting in increased synthesis and accumulation of TGs. This phenomenon is closely associated with the development of renal sclerosis and proteinuria[101].
Renal tubular sodium reabsorption is enhanced, pressure natriuresis is impaired, and volume expansion occurs due to the activation of the sympathetic nervous system and RAAS. Additionally, visceral obesity can physically compress the kidneys. Renal vasodilation and glomerular hyperfiltration are induced by obesity as initial compensatory mechanisms to preserve sodium balance in response to increased tubular reabsorption. However, over time, these alterations, in con
Lipid accumulation could significantly contribute to the development of tissue injury caused by a condition known as lipotoxicity[75,102]. Lipid deposition occurs in almost all cell types within the kidney, resulting in the condition known as renal lipotoxicity[21]. Lipid toxicity leads to renal impairment through the initiation of inflammatory responses, OS, disruption of mitochondrial function, cellular demise, podocyte harm, tubular injury, mesangial cell proliferation, activation of endothelial cells, and formation of foam cells derived from macrophages[103]. Emerging findings suggest that the accumulation of lipids in the kidneys and their harmful effects, resulting from excessive fat content, are significant factors contributing to renal injury in individuals with metabolic syndrome[104,105]. Lipid accumulation in the renal system can lead to damage in both glomerular and tubulointerstitial regions, thereby exacerbating the progression of DN through its impact on renal tissues[103]. Emerging studies have revealed the significance of ELD in promoting ORN and DN by causing renal lipotoxicity. ELD primarily occurs due to an imbalance in the uptake and oxidation of fatty acids, leading to disruptions in lipid metabolism. These disruptions are mainly associated with the AMPK signaling pathway. AMPK plays a crucial role in regulating several key enzymes involved in both the synthesis and breakdown of lipids, including adipose TG lipase, fatty acid synthase (FAS), hormone-sensitive lipase, and 3-hydroxy-3-methylglutaryl-CoA reductase[22,106]. Activation of AMPK normalizes renal lipid levels, despite prolonged exposure to lipids, and improves the outcomes of obesity-induced renal damage through various mechanisms, such as improving lipid metabolism, reducing inflammation and fibrosis, maintaining mitochondrial balance, promoting autophagy, and alleviating OS[107]. By activating the AMPK/acetyl-CoA carboxylase pathway, AdipoRon, a potent agonist of adiponectin receptors, effectively diminishes renal lipogenesis and lipid accumulation. This intervention plays a crucial role in mitigating the initial susceptibility to obesity-related DN and its subsequent advancement toward ESRD[22].
A comprehensive understanding of the mechanisms contributing to lipotoxicity and DN may facilitate the deve
Despite the significant increase in the prevalence of ORN, therapeutic options remain limited. Like other chronic nephropathies characterized by proteinuria, a substantial reduction in proteinuria is believed to confer renoprotective benefits for ORN. Managing glycemic levels, inhibiting the RAAS blockade, and reducing weight gain are the primary strategies to counteract proteinuria. Nonetheless, the incidence rate of ORN is still prevalent, and cases of ESRD are also increasing because of the restricted effectiveness and numerous undesirable adverse reactions linked to these therapies. Therefore, new therapeutic options for ORG need to be identified.
Numerous studies have highlighted the beneficial impacts of food components derived from organic sources, particularly medicinal flora, in alleviating the detrimental consequences and slowing down the progression or advancement of ORN and DN. Many of these food compounds possess properties that combat oxidation and inflammation, effectively preventing the negative effects caused by high blood sugar levels such as OS and inflammatory reactions.
Numerous research investigations have focused on exploring the potential of natural substances, artificial medications, and compounds as promising alternatives in developing innovative agents for kidney diseases associated with obesity and renoprotection. In this section, we provide a brief overview of observational studies indicating that certain substances have been tested in experimental models of ORN and exhibit renoprotective effects by reducing renal inflammation, OS, insulin resistance, fibrosis, and kidney lipid accumulation, and restoring dysregulated autophagy.
Statins and PPAR agonists: Lipid peroxidation stress biomarkers show a significant elevation in glomeruli and renal microvessels among individuals with T2D. Additionally, statins, known for their lipid-lowering properties, demonstrate the ability to decrease proteinuria and slow down the progression of kidney disease in patients presenting with hyper
In addition to their ability to lower lipid levels, statins possess properties that can reduce inflammation, OS, and cell proliferation. They also have the potential to protect the endothelium and enhance adiponectin levels in renal cells[80].
Given that the interaction between statins and PPARs contributes to their beneficial effects, it is not surprising that researchers have explored the potential of PPAR therapies in addressing obesity and diabetes. This has been de-monstrated in studies involving mesangial cells as well as an experimental model of T2D using leptin receptor-deficient mice (db/db). Hong et al[111] demonstrated the beneficial effects of fenofibrate in reducing albuminuria, suppressing the accumulation of NEFAs and TGs within the kidneys, and protecting against apoptosis and OS[111]. In mice provided with an HFD, fenofibrate demonstrated the ability to decrease OS and lipid buildup within the glomeruli. Additionally, it effectively hindered the progression of albuminuria and glomerular fibrosis[112]. Pharmacological activation of PPAR-γ offers the opportunity to enhance the endogenous plasma levels of adiponectin, thereby further substantiating the protective role of adiponectin in the development of obesity-related kidney disease. We also observed that mice treated with rosiglitazone, a PPAR-γ agonist that belongs to the thiazolidinedione class of drugs, displayed markedly increased plasma levels of adiponectin, a protein predominantly secreted by adipocytes. It is well documented that adiponectin is a cardioprotective adipokine, due to its anti-inflammatory and insulin-sensitizing properties[113]. Furthermore, tesaglitazar, a dual agonist of PPARα/γ, exhibits beneficial effects on lipid metabolism and elevates adip
GLP-1 receptor agonists: Exendin-4 and liraglutide, both as GLP-1 receptor agonists, demonstrate potential in mitigating renal damage caused by obesity through manipulation of the AMPK pathway and promotion of ABCA1-mediated cholesterol efflux[81,115]. The study conducted by Su et al[116] revealed that in rats with T2D, liraglutide, a long-acting derivative of human GLP-1, effectively reduces renal SREBP-1 expression through the activation of AMPK. As a result, it alleviates kidney damage caused by disorders in lipid metabolism[116]. Wang et al[81] demonstrated that the administration of liraglutide effectively restores mitochondrial function in the kidneys of rats fed an HFD. This restoration is achieved through the activation of sirtuin-1/AMPK and PPAR-γ coactivator-1α (PGC-1α) pathways. Additionally, treatment with liraglutide leads to a reduction in renal cholesterol, TG, and fatty acid residue deposition, while simultaneously increasing levels of mitochondrial metabolites[81].
Sodium-glucose cotransporter 2 inhibitors: Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are a recently developed category of medications primarily used in managing individuals with T2D and its associated complications. These drugs have significantly contributed to advancing the field of cardiorenal protective medicine. SGLT2 inhibitors, which inhibit glucose absorption in the kidneys and reduce hyperglycemia, have also shown renoprotective effects. Relevant studies have been conducted to evaluate the effectiveness of SGLT2 inhibitors in preventing kidney damage and progression to ESKD related to T2D, but no significant findings have been reported regarding the efficacy of alternative hypoglycemic medications[87,117]. Remarkably, the beneficial effects of SGLT2 inhibitors on kidney function are not related to blood sugar regulation but instead attributed to their ability to trigger a genetic response that resembles the metabolic state of fasting and oxygen deprivation (an energy-deprived condition)[118]. Hosokawa and colleagues have recently demonstrated that ipragliflozin effectively reduces the accumulation of abnormal lipids in the tubular cells of mice with diabetes[119]. Furthermore, Birnbaum et al[120] have shown that the activation of AMPK is responsible for the im-provement in glomerular hyperfiltration observed in ob/ob mice (a mouse model for obese T2D) when they are treated with dapagliflozin, an inhibitor of SGLT2[120]. Hawley et al[121] demonstrated that lipid synthesis was effectively suppressed by the SGLT2 inhibitor canagliflozin, through a mechanism dependent on AMPK[121]. Therefore, the potential protective effects of SGLT2 inhibitors on obesity-related renal injury and other obesity-related diseases may be attributed to their ability to enhance autophagy, reduce OS, and improve lipid metabolic disorders through AMPK stimulation. Besides, the use of combination therapy may offer a more efficient approach to treating renal injury associated with obesity. While dapagliflozin (an SGLT2 inhibitor) has demonstrated its ability to reduce renal inflammation in mice with T2D by activating AMPK (known for its diverse effects on kidney pathogenesis and dysfunction related to obesity or T2D), the combined administration of saxagliptin (a dipeptidyl peptidase-4 inhibitor) and dapa
While further investigation is necessary, it is conceivable that in addition to the widely recognized impacts of SGLT2i, the potential mitigation of tubular lipid accumulation may emerge as a novel mechanism attributed to these compounds for renoprotection[122-124]. Furthermore, it is crucial to conduct a significant amount of meticulously planned in vivo research to determine the impact of drug interactions and potential adverse effects in renal diseases related to obesity.
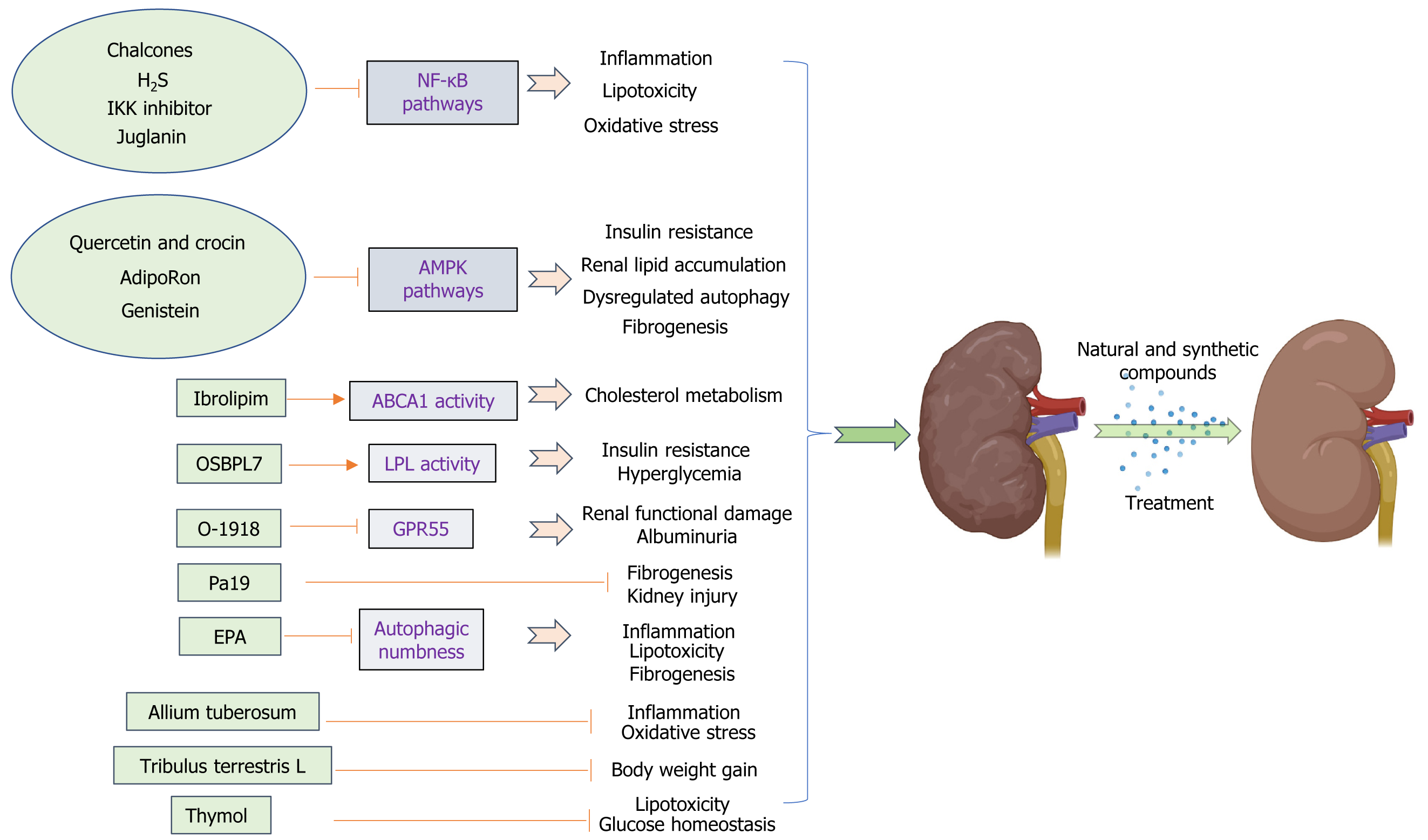
In recent times, numerous research investigations have focused on exploring the potential of natural substances and artificially synthesized compounds as promising alternatives in developing renoprotective agents and new drugs for obesity-related kidney diseases. Numerous studies have highlighted the advantageous impacts of natural food compounds, particularly those derived from medicinal plants, in alleviating the detrimental consequences and slowing down the progression or escalation of chronic diseases such as diabetes, ORN, and DN. Many of these food components possess properties that combat oxidation and inflammation, effectively preventing damage caused by OS, inflammatory reactions, insulin resistance, fibrosis, and lipid buildup in the kidneys associated with obesity, and restoring proper regulation of autophagy (Figure 2).
Synthetic compounds: (1) Oxysterol binding protein like 7 (OSBPL7): The current study presents the discovery of a group of 5-aryl nicotinamide compounds through phenotypic drug exploration. These compounds specifically target OSBPL7 and enhance cholesterol efflux, which is dependent on ABCA1. In preclinical models of kidney disease associated with obesity, these compounds have demonstrated efficacy in reducing lipid accumulation in the kidneys, protecting against podocyte loss, restoring normal levels of proteinuria, and mitigating the decline in renal function. Targeting OSBPL7 with small-molecule drugs offers a potential and secure therapeutic approach for addressing obesity-related renal conditions and other disorders associated with cellular cholesterol balance. This novel mode of action focuses on modulating cellular cholesterol metabolism, providing an alternative means to enhance ABCA1 activity[125]. These agents, in addition to the existing medications that aim to manage blood pressure or glucose levels, have the potential to meet the unmet requirement for ORN.
(2) Lipoprotein lipase (LPL) activator-Ibrolipim: Scientists have successfully demonstrated that the consumption of a diet rich in sucrose and fat leads to lipid accumulation in abnormal locations and a deficiency in LPL, while also reducing the antioxidant capacity within the kidney tissue of Chinese Bama minipigs[126,127]. Therefore, increasing LPL activity could potentially prevent lipid accumulation in the kidney. NO-1886 (also known as Ibrolipim) enhances the expression and functionality of LPL within the kidneys, improving renal lipid accumulation and reducing microalbuminuria in diet-induced diabetic minipigs[25]. This minipig model primarily exhibited abnormal lipid accumulation in unexpected areas, glomerular enlargement, fusion of podocyte processes, thickening of the basement membrane, expansion of mesangial cells, moderate glomerular damage, and fibrosis in the tubulointerstitial region. These changes were accompanied by a slight increase in urinary albumin levels. Furthermore, Ibrolipim effectively reduced the production of ROS induced by an HSFD, while simultaneously enhancing both expression and activity of antioxidant enzymes to promote ROS elimination. As a result, renal ROS levels were significantly suppressed. At the same time, Ibrolipim demonstrated inhibitory effects on the elevated expression of TGF-β and partially restored the reduced levels of matrix metalloproteinase 2, thus hindering the buildup of the ECM. In combination, Ibroipim demonstrates the ability to regulate renal ROS and ECM metabolism, resulting in antioxidative and antifibrotic effects. Consequently, it effectively mitigates the progression of nephropathy in diet-induced diabetic minipigs[58]. Liu et al[58] demonstrated that the administration of Ibrolipim, in combination with an HSFD, for a duration of 5 mo, leads to significant reductions in hyperglycemia, hyper
In summary, the involvement of renal LPL in lipid metabolism and nephropathy is significant, and activation of renal LPL has a protective effect against diet-induced DN. Ibrolipim, an agonist of LPL, shows promising potential for pre
(3) Atypical cannabinoid ligand-O-1918: G protein-coupled receptor 55 (GPR55) plays a crucial role in enhancing insulin action and promoting adipogenesis, and its expression is elevated in renal tissues after high-fat feeding. Research conducted on obesity has revealed a notable disparity in the expression of GPR55 between obese individuals and those who are lean, with significantly higher levels observed in the adipose tissue of the former[128]. Considering the potential antagonistic effect of O-1918 on GPR55 and the known association between chronic low-grade inflammation, obesity, and insulin resistance, it is plausible that this interaction could contribute to an increase in the circulating levels of pro-inflammatory cytokines throughout the body. According to Simcocks et al[129], the administration of O-1918 resulted in a decrease in BAT mass while not affecting overall body fat or lean tissue mass. Interestingly, this compound exhibited a positive effect in reducing albuminuria and renal tubular hypertrophy[129]. The study presents novel findings suggesting that O-1918 has the potential to mitigate both structural and functional damage in the kidneys caused by an HFD. Consequently, O-1918 could be considered a promising therapeutic option for addressing kidney diseases associated with obesity. Future investigations should prioritize exploring how modulating G protein-coupled receptors targeted by O-1602/O-1918 (such as GPR55 and GPR18) impacts specific tissues like adipose tissue or the kidney, aiming to counteract any adverse effects observed with O-1918 on pro-inflammatory signaling pathways implicated in obesity-related kidney disease.
(4) Resveratrol analog-Pa19: Resveratrol, derived from grapes and various plants, has garnered growing interest as a promising therapeutic candidate in the prevention and management of atherosclerosis, cardiovascular diseases, and cerebrovascular diseases due to its anti-inflammatory characteristics and minimal toxicity[130,131]. Various studies have indicated that resveratrol has the potential to reduce obesity and its related complications through multiple mechanisms. PA19 ((1e,4e)-1-{2,4-dimethoxy-6-[(e)-4-methoxystyryl] phenyl}-5-(2,4-dimethoxyphenyl) penta-1,4-dien-3-one), a newly developed anti-inflammatory compound that has been synthesized by the Chemical Biology Research Center at Wenzhou Medical University's School of Pharmaceutical Sciences, is a resveratrol analog; it was investigated in an established mouse model of obesity. Treatment with PA19 for the last 12 wk of the experiment resulted in a significant decrease in both kidney fibrosis and inflammation caused by an HFD. The results suggested that PA19 exhibits renoprotective effects by inhibiting inflammation and inflammatory cell infiltration and effectively inhibits obesity-induced kidney tissue injury and fibrosis[132]. These findings indicate that PA19 is a potential novel agent for treating obesity and obesity-induced renal injury.
(5) Adiponectin receptor agonist-adiporon: Adiponectin, a hormone released by fat cells, plays a crucial role in regu
(6) Hydrogen sulfide (H2S): H2S is a naturally occurring gas that acts as a signaling molecule in mammalian tissues. It is produced by the enzymes cystathionine β-synthase, cystathionine-γ-lyase, and 3-mercaptopyruvate sulfurtransferase[136]. All three enzymes exhibit high levels of expression in renal tissues and play a crucial role in the production of H2S within the kidneys. Growing evidence suggests that H2S plays a significant role in both the normal functioning of the kidneys and kidney diseases[137]. Under normal physiological circumstances, the basal levels of H2S in the kidneys play a role in regulating tubular function and renal hemodynamics, including influencing changes in renal blood flow, sodium and potassium ion excretion, glomerular filtration rate, and urinary excretion[138]. H2S possesses the capability to act as an oxygen sensor in the renal medulla. In situations of low oxygen levels, it can help restore oxygen equilibrium by enhancing blood flow in the medulla, reducing energy demands for tubular transport, and directly inhibiting mitoc
(7) Eicosapentaenoic acid (EPA): EPA reduces TG levels without causing an increase in LDL-C levels[143]. Prior research has suggested that EPA exhibits diverse biological impacts, including anti-inflammatory properties, potential for cancer prevention, and cardiovascular health protection[144]. Furthermore, the presence of EPA has the potential to impact cellular responses mediated by membrane proteins, generation of lipid mediators, signaling pathways within cells, and regulation of gene expression across different cell types[145]. Autophagy has been observed in different cell types upon exposure to EPA, as demonstrated by multiple research studies. Replenishing EPA in cultured PTECs exposed to palmitic acid effectively enhances lysosomal function, leading to notable improvements in autophagic flux. Supplementing EPA in mice fed an HFD effectively mitigates renal lipotoxicity by regulating autophagy, reducing phospholipid accumulation in lysosomes, improving mitochondrial function, and alleviating inflammation and fibrosis associated with lipotoxicity[85]. In summary, EPA effectively counteracts lipotoxicity in the proximal tubule by alleviating autophagic insensitivity, suggesting its potential as an innovative therapeutic approach for kidney diseases associated with obesity.
And (8) IκB kinase (IKK) inhibitor: IKK, a kinase complex, is responsible for phosphorylating IκBα, an inhibitory protein of NF-κB. This process facilitates the ubiquitination and subsequent degradation of IκBα by the proteasome. Nrf2 plays a crucial role in protecting against OS by promoting the production of antioxidant enzymes[146,147]. Cylindromatosis (CYLD) is an enzyme involved in the removal of ubiquitin from specific signaling molecules, thereby regulating their process of ubiquitination[148]. Recent research has revealed that the activation of IKK leads to the phosphorylation of CYLD, resulting in the inhibition of its deubiquitination activity. As a result, this process enhances Nrf2's ubiquitination and exacerbates OS-induced damage. These findings provide a solid foundation for the management of kidney injury caused by ORN. A compound that inhibits IKK was found to decrease the phosphorylation of CYLD and hinder the ubiquitination process of Nrf2, resulting in an improvement in kidney injury induced by OS in ORN[149]. Furthermore, IKK inhibitors may potentially reduce lipid deposition and OS injury, which could be a specific mechanism contributing to kidney damage in ORN.
Natural compounds: (1) Quercetin and crocin: Quercetin is the primary active constituent found in Eucommia species, while crocin is the main active component present in Gardenia species. In the past few years, numerous studies have effectively identified herbs or herbal extracts that exhibit inhibitory effects on renal fibrosis in both live organisms and laboratory settings. Eucommia ulmoides is rich in five primary active components, namely, phenylpropanoids, iridoids, lignans, polysaccharides, and flavonoids. It exhibits various biological activities such as antibacterial properties, anti-inflammatory effects, fever-reducing capabilities, blood pressure-regulating abilities, antioxidant actions, cholesterol-lowering effects, and more[150,151]. Gardenia is recognized for its efficacy in preventing weight gain and enhancing abnormal lipid levels, high insulin levels, impaired glucose tolerance, and lipid peroxidation due to the presence of three primary active components: Iridoids, flavonoids, and crocin[152]. The significant attention garnered by their therapeutic benefits in addressing obesity and diabetes cannot be overlooked. Lai et al[153] extracted quercetin and crocin from Eucommia ulmoides Oliv and Gardenia, respectively, which improved HFD-induced renal lipid accumulation and renal fibrosis, and additionally decreased autophagy-related protein p-AMPK levels in the kidneys, which might exert protective effects against ORN[153];
(2) Juglanin (Jug): Jug is an organic substance derived from the raw material Polygonum aviculare. Overconsumption of energy, such as an HFD, leads to the emergence of metabolic syndrome, which plays a role in the accumulation of lipids in the kidneys and subsequent renal damage. Supplementation with Jug reduced collagen accumulation and lipid deposition in renal tissues, resulting in noticeable histopathological improvements in HFD-challenged mice. The nephroprotective effects of Jug were validated in an in vitro study using hexokinase II cells stimulated with palmitate. The primary mechanism involved inhibiting the nuclear translocation of NF-κB/histone deacetylase 3, leading to the supp
(3) Allium tuberosum: Chinese leek, scientifically referred to as Allium tuberosum, is an onion species that exhibits perennial growth. It originates from the Shanxi province in China; however, it has gained popularity and is now cultivated across various regions globally. Allium tuberosum is extensively utilized as a significant culinary ingredient in various regions globally, and its utilization in traditional medicine has gained substantial popularity due to its pharmacological characteristics. Allium tuberosum has been found to exhibit properties that potentially combat OS, inhibit cancer growth, regulate blood sugar levels, protect cells from damage, preserve kidney function, and reduce inflammation[154,155]. The plant contains abundant amounts of polyphenols, steroidal saponins, nucleosides, nucleotides, and sulfur ethers. The butanol fraction derived from Allium tuberosum (BFAT) exhibited significant reductions in blood glucose and lipid levels (including TG, TC, and LDL-C), as well as serum creatinine, blood urea nitrogen, and urinary albumin levels in rats with diabetes. Additionally, BFAT enhanced the antioxidant enzyme status of the kidneys [such as glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT)] while reducing lipid peroxidation products in diabetic rats. Moreover, it effectively decreased pro-inflammatory cytokine levels in HFD/streptozotocin-induced diabetic rats. Ni et al[156] demonstrated that the renoprotective mechanism of BFAT may primarily be associated with its capacity to suppress elevated blood glucose levels, thereby enhancing renal function through a reduction in OS markers such as malonaldehyde, superoxide dismutase, GSH, CAT and pro-inflammatory mediators including TNF-α, IL-6, and IL-1β. Additionally, BFAT was found to mitigate renal fibrosis by modulating TGF-β1 expression[156]. The potential protective effect of BFAT on the kidneys may be attributed to its ability to alleviate high blood sugar levels, thereby improving renal function through the reduction of OS and inflammatory factors. Additional research is necessary to investigate the direct impact of BFAT on the primary pathways associated with ORN. These findings indicate that Allium tuberosum has the potential to be considered a promising nutraceutical for the prevention or management of ORN;
(4) Tribulus terrestris (TT): TT, which belongs to the Zygophyllaceae family, is widely distributed in subtropical regions across various geographical areas. TT is utilized for its positive effects on cardiovascular function and fluid balance, as well as its potential advantages in enhancing libido, combating the harmful effects of free radicals, facilitating weight management, and supporting herbal recovery after a stroke[157]. According to the bioinformatic annotation, it is suggested that potential factors contributing to ORG may include increased energy metabolism, decreased stress response, and a compromised immune system. After 8 wk of TT administration, there were noticeable reductions in body weight, blood pressure, serum cystatin C levels, and cholesterol levels. Furthermore, TT effectively improved rats' resilience against ORG by reducing energy expenditure and mitigating the likelihood of hemorrhage. Additionally, It enhanced the response to acute-phase reactants and immunity, suggesting that TT may have a protective effect against ORG in rats[158];
(5) Chalcones: Chalcones, which are present in various plant species, belong to a category of compounds that fall within the flavonoid family. They have a variety of pharmacological properties, including antioxidant, anti-hyper
(6) Genistein: Through its interaction with AdipoR, genistein, a prominent isoflavone found in soybeans, has the potential to enhance fatty acid oxidation and consequently decrease lipid accumulation in the kidneys[79]. Genistein inhibits the upregulation and accumulation of ECM proteins induced by ovariectomy. The changes in ECM composition have been associated with metabolic alterations, such as elevated levels of TGF-β, OS, and lipid accumulation. Genistein may contribute to maintaining normal kidney function by mitigating various risk factors for renal damage induced by ovariectomy, including reducing insulin resistance, alleviating renal OS (such as decreasing expression of renal RAGE and H2O2 while increasing expression of renal SOD), preventing lipid accumulation (including decreased expression of renal AdipoR1 and AdipoR2 but increased expression of renal SREBP-1), and inhibiting renal fibronectin production resulting in reduced levels of serum and renal TGF-β[162]. It has been suggested that the removal of ovaries may lead to increased blood sugar levels and insulin levels, as well as fat accumulation in the kidneys and synthesis of fats in the kidneys. However, genistein has shown potential in preserving normal kidney function by mitigating various harmful effects on the kidneys caused by ovariectomy, including obesity-associated kidney damage;
And (7) Thymol: Thymol, a type of dietary monoterpene phenol, can be found in the oils of various plants including Thymus vulgaris, Thymbra spicata, Thymus ciliates, Trachyspermum ammi, Monarda fistulosa, and Nigella sativa seeds. It has been shown to possess several biological properties such as antibacterial effects, anti-inflammatory properties, and antioxidant activity. Additionally, it has also demonstrated potential for preventing myocardial infarction[163,164]. The United States Food and Drug Administration has classified thymol as a safe food additive for consumption, with no toxic effects[165]. The antioxidant properties of thymol, a naturally occurring bioactive compound, resulted in a reduction in the levels of lipid peroxidation products. Additionally, it exhibited positive effects on glucose regulation, decreased kidney weight, and improved various biochemical parameters in both serum and urine. Furthermore, it successfully restored the levels of TGF-β and vascular endothelial growth factor proteins in mice with diet-induced diabetes. Furthermore, thymol exhibits a notable impact on lipid profile reduction through the modulation of SREBP-1c protein expression, preservation of renal architecture, and mitigation of renal fibrosis[27]. Therefore, thymol is a potent nephroprotectant against HFD-induced DN and ORN.
The foremost suggestions are to make lifestyle adjustments, such as adopting a nutritious diet and engaging in consistent physical activity. The primary approach to addressing obesity-related conditions is reducing body weight, as obesity increases the likelihood of developing hypertension, proteinuria, significant histologic abnormalities, and ESRD. Consequently, losing excess weight can lead to a decrease in proteinuria[166]. Furthermore, bariatric surgery may provide renoprotective benefits beyond metabolic control and exercise training in patients with morbid obesity, both of which are recognized as effective therapeutic approaches for managing obesity and its associated conditions. New research has shown that implementing a delayed endurance exercise training (EET) regimen can be an efficient approach to averting complications associated with obesity. One suggestion is to incorporate exercise training into the comprehensive care for individuals with CKD at any stage of its progression[167], employing EET for preventive purposes rather than as an intervention. Juszczak et al[168] conducted a groundbreaking study in which they observed the positive impact of EET when administered for an additional 8 wk on obesity-related CKD. This was evident through notable im-provements in obesity-induced glomerulopathy, interstitial fibrosis, inflammation, OS, and ELD within the kidney. Additionally, there was a reduction in albuminuria and an improvement in overall kidney function. Hence, physical activity training could serve as a promising non-pharmacological approach to activate AMPK in obesity-induced CKD. These findings demonstrate that the administration of EET results in the enhancement of the AMPK pathway in renal tissue. Notably, there is a significant increase in the phosphorylation of downstream signaling molecules mediated by AMPK, leading to elevated fatty acid oxidation and improved autophagy in obese mice[168]. EET is an effective strategy for the prevention of obesity-related complications. EET is expected to be included in renal care at any step in ORN.
Detailed information on natural and synthetic compounds for the treatment of obesity-related kidney diseases is summarized in Table 1.
| Substance | Animal model | The therapeutic effects | Pathway | Ref. |
| Ibrolipim | In diabetic minipigs fed by HSFD | Reduces hyperglycemia, hyperinsulinemia, insulin resistance, hypertriglyceridemia, microalbuminuria, renal fat accumulation, and improves pathological injury | ↑LPL activity | [25] |
| O-1918 | Male Sprague–Dawley rats were fed HFD | Alleviated renal structural and functional damage, reduce albuminuria and reduced renal tubular hypertrophy | O-1918 is a putative antagonist for GPR55 | [129] |
| Pa19 | HFD mice | Inhibiting in flammation and inflammatory cell infiltration and indicate inhibits obesity-induced kidney tissue injury and fibrosis | - | [132] |
| AdipoRon | Male C57BLKS/J db/db mice | Reduce lipotoxicity and to improve insulin resistance | Activation of the AMPK-PPARγ | [22] |
| H2S | HFD-induced obese mice | Reduce kidney lipids, improve kidney function, and reduce the interstitial injury and fibrosis | Downregulating NF-κB expression | [142] |
| EPA | HFD-fed mice | Reduced phospholipid accumulation in the lysosome, mitochondrial dysfunction, inflammation, and fibrosis. Alleviated lipotoxicity | Alleviating autophagic numbness | [85] |
| IKK inhibitor | Male ob/ob mice and homologous C57BL/6 | Reduce lipid deposition and oxidative stress injury | Downregulating NF-κB | [149] |
| Quercetin and crocin | HFD- and streptozotoc induced type 2 diabetes | Prove renal lipid accumulation, renal fibrosis | Decreased autophagy-related protein p-AMPK levels | [153] |
| Juglanin | HFD-challenged mice | Attenuated collagen accumulation, lipid deposition in renal tissues | Blocking the NF-κB/HDAC3 nuclear translocation | [17] |
| Allium tuberosum | HFD/streptozotoc treated diabetic rats | Decreased blood glucose, bad blood lipids, serum creatinine, blood urea nitrogen and urinary albumin, upregulated renal antioxidant enzymes status, decreased lipid peroxidation product, reduced the levels of renal pro-inflammatory cytokines and renal fibrosis | Decreasing oxidative stress and pro-inflammatory mediators | [156] |
| Tribulus terrestris L | Male Wistar rats | Decreased body weight, blood pressure, serum cystatin C and cholesterol were. enhanced the resistance, decreased energy consumption and the hemorrhagic tendency, and improved the response to acute phase reactants and immunity | - | [158] |
| Chalcones | HFD-fed mice | Decreasing the expression of pro-inflammatory cytokines and cell adhesion molecules and improving kidney histology and pathology | Inhibition of the MAPK/NF-κB dependent inflammatory cytokine production | [44] |
| Genistein | Ovariectomy rats | Ameliorated oxidative stress, and lipid accumulation, maintain normal kidney function | Interaction with AdipoR | [162] |
| Thymol | HFD induced diabetic C57BL/6Jmice | Lowered lipid peroxidation products, improved glucose homeostasis, decreased kidney weight, biochemical parameters reduced the lipid profile and also preserved renal architecture and decreased renal fibrosis | - | [27] |
The prevalence of ORN has been increasing with the worldwide obesity epidemic. Therefore, there is an urgent need for new interventions to effectively combat obesity, particularly ORN. In this review, we have explored the mechanisms that establish a connection between obesity and ORN. Additionally, we have provided an overview of potential treatment implications involving both natural and synthetic compounds. These compounds exhibit renoprotective properties by reducing renal inflammation, OS, insulin resistance, fibrosis, and kidney lipid accumulation, as well as by restoring dysregulated autophagy. This comprehensive analysis offers valuable insights for future clinical therapeutic strategies. Furthermore, since kidney damage is typically irreversible, it is crucial to initiate early treatment in order to enhance its potential for recovery. However, due to the complex nature of disease progression in humans compared to animal models, to understand the actual impact of these substances on individuals suffering from ORN, it is imperative to conduct thorough clinical examinations and well-designed research. Furthermore, clinical trials can offer valuable insights into the mechanisms of action and potential adverse reactions associated with various renoprotective medications, thus informing treatment strategies to enhance renoprotection. The potential therapeutic advantages of combining recently discovered medications with conventional natural compounds or botanical extracts could potentially enhance the curative effects for renal injury associated with obesity. Nevertheless, there is currently a lack of adequate clinical trials investigating the utilization of these substances in patients suffering from ORN. Therefore, it is crucial to conduct extensive prospective studies with extended timeframes and meticulously designed in vivo experiments on obese and ORN patients in order to validate the findings obtained from animal models.
| 1. | Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381:2440-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 1449] [Article Influence: 207.0] [Reference Citation Analysis (1)] |
| 2. | Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 3266] [Article Influence: 466.6] [Reference Citation Analysis (0)] |
| 3. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 1035] [Article Influence: 207.0] [Reference Citation Analysis (14)] |
| 4. | Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021;9:446-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 339] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 5. | Lee SJ, Kang JS, Kim HM, Lee ES, Lee JH, Chung CH, Lee EY. CCR2 knockout ameliorates obesity-induced kidney injury through inhibiting oxidative stress and ER stress. PLoS One. 2019;14:e0222352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, Tallam L. Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther. 2004;11:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 1008] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 8. | D'Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, Praga M. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12:453-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 538] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 9. | Praga M, Hernández E, Morales E, Campos AP, Valero MA, Martínez MA, León M. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, Alastrue A, Ariza A. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 483] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 12. | Hu R, Quan S, Wang Y, Zhou Y, Zhang Y, Liu L, Zhou XJ, Xing G. Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34,630 cases. Sci Rep. 2020;10:10994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Verani RR. Obesity-associated focal segmental glomerulosclerosis: pathological features of the lesion and relationship with cardiomegaly and hyperlipidemia. Am J Kidney Dis. 1992;20:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Fowler SM, Kon V, Ma L, Richards WO, Fogo AB, Hunley TE. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol. 2009;24:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 878] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 16. | Sethi S, Glassock RJ, Fervenza FC. Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant. 2015;30:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Li Q, Ge C, Tan J, Sun Y, Kuang Q, Dai X, Zhong S, Yi C, Hu LF, Lou DS, Xu M. Juglanin protects against high fat diet-induced renal injury by suppressing inflammation and dyslipidemia via regulating NF-κB/HDAC3 signaling. Int Immunopharmacol. 2021;95:107340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Praga M, Borstein B, Andres A, Arenas J, Oliet A, Montoyo C, Ruilope LM, Rodicio JL. Nephrotic proteinuria without hypoalbuminemia: clinical characteristics and response to angiotensin-converting enzyme inhibition. Am J Kidney Dis. 1991;17:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Praga M, Hernández E, Herrero JC, Morales E, Revilla Y, Díaz-González R, Rodicio JL. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000;58:2111-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 228] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Bonnet F, Deprele C, Sassolas A, Moulin P, Alamartine E, Berthezène F, Berthoux F. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis. 2001;37:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Yang W, Luo Y, Yang S, Zeng M, Zhang S, Liu J, Han Y, Liu Y, Zhu X, Wu H, Liu F, Sun L, Xiao L. Ectopic lipid accumulation: potential role in tubular injury and inflammation in diabetic kidney disease. Clin Sci (Lond). 2018;132:2407-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ, Choi BS, Kim YS, Chang YS, Park CW. The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes. J Am Soc Nephrol. 2018;29:1108-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 23. | Solis-Herrera C, Triplitt C. Non-steroidal mineralocorticoid receptor antagonists in patients with chronic kidney disease and type 2 diabetes. Diabetes Obes Metab. 2024;26:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Yamada T, Wakabayashi M, Bhalla A, Chopra N, Miyashita H, Mikami T, Ueyama H, Fujisaki T, Saigusa Y, Yamaji T, Azushima K, Urate S, Suzuki T, Abe E, Wakui H, Tamura K. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2021;20:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | Liu Y, Wang ZB, Yin WD, Li QK, Cai MB, Yu J, Li HG, Zhang C, Zu XH. Preventive effect of Ibrolipim on suppressing lipid accumulation and increasing lipoprotein lipase in the kidneys of diet-induced diabetic minipigs. Lipids Health Dis. 2011;10:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Hong SH, Choi KM. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 27. | Saravanan S, Pari L. Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6J mice. Chem Biol Interact. 2016;245:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 555] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 30. | Graziani F, Cialdella P, Liuzzo G, Basile E, Brugaletta S, Pedicino D, Leccesi L, Guidone C, Iaconelli A, Mingrone G, Biasucci LM, Crea F. Cardiovascular risk in obesity: different activation of inflammation and immune system between obese and morbidly obese subjects. Eur J Intern Med. 2011;22:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Cancello R, Clément K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 2006;113:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 32. | Stemmer K, Perez-Tilve D, Ananthakrishnan G, Bort A, Seeley RJ, Tschöp MH, Dietrich DR, Pfluger PT. High-fat-diet-induced obesity causes an inflammatory and tumor-promoting microenvironment in the rat kidney. Dis Model Mech. 2012;5:627-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 586] [Article Influence: 45.1] [Reference Citation Analysis (6)] |
| 34. | Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3400] [Article Influence: 154.5] [Reference Citation Analysis (0)] |
| 35. | Hunley TE, Ma LJ, Kon V. Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens. 2010;19:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hébert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299:F1348-F1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 37. | Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616-39626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 417] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 38. | Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 39. | Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 627] [Cited by in RCA: 627] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 40. | Nosadini R, Tonolo G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Bost F, Aouadi M, Caron L, Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 42. | Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton). 2006;11:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 43. | Zeng C, Zhong P, Zhao Y, Kanchana K, Zhang Y, Khan ZA, Chakrabarti S, Wu L, Wang J, Liang G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J Mol Cell Cardiol. 2015;79:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 44. | Fang Q, Deng L, Wang L, Zhang Y, Weng Q, Yin H, Pan Y, Tong C, Wang J, Liang G. Inhibition of mitogen-activated protein kinases/nuclear factor κB-dependent inflammation by a novel chalcone protects the kidney from high fat diet-induced injuries in mice. J Pharmacol Exp Ther. 2015;355:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Yan Z, Ni Y, Wang P, Chen J, He H, Sun J, Cao T, Zhao Z, Luo Z, Chen L, Liu D, Zhu Z. Peroxisome proliferator-activated receptor delta protects against obesity-related glomerulopathy through the P38 MAPK pathway. Obesity (Silver Spring). 2013;21:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 47. | Xu T, Sheng Z, Yao L. Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics and treatment. Front Med. 2017;11:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Tang J, Yan H, Zhuang S. Inflammation and oxidative stress in obesity-related glomerulopathy. Int J Nephrol. 2012;2012:608397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Habibi J, Hayden MR, Sowers JR, Pulakat L, Tilmon RD, Manrique C, Lastra G, Demarco VG, Whaley-Connell A. Nebivolol attenuates redox-sensitive glomerular and tubular mediated proteinuria in obese rats. Endocrinology. 2011;152:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010;298:F125-F132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Fernandes SM, Cordeiro PM, Watanabe M, Fonseca CD, Vattimo MF. The role of oxidative stress in streptozotocin-induced diabetic nephropathy in rats. Arch Endocrinol Metab. 2016;60:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Lv W, Booz GW, Fan F, Wang Y, Roman RJ. Oxidative Stress and Renal Fibrosis: Recent Insights for the Development of Novel Therapeutic Strategies. Front Physiol. 2018;9:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 53. | Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur J Pharmacol. 2016;791:8-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 54. | Chade AR, Mushin OP, Zhu X, Rodriguez-Porcel M, Grande JP, Textor SC, Lerman A, Lerman LO. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Rane MJ, Song Y, Jin S, Barati MT, Wu R, Kausar H, Tan Y, Wang Y, Zhou G, Klein JB, Li X, Cai L. Interplay between Akt and p38 MAPK pathways in the regulation of renal tubular cell apoptosis associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2010;298:F49-F61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Ha H, Kim KH. Pathogenesis of diabetic nephropathy: the role of oxidative stress and protein kinase C. Diabetes Res Clin Pract. 1999;45:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 848] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 58. | Liu Y, Li H, Wang S, Yin W, Wang Z. Ibrolipim attenuates early-stage nephropathy in diet-induced diabetic minipigs: Focus on oxidative stress and fibrogenesis. Biomed Pharmacother. 2020;129:110321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D, Soulage CO. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie. 2013;95:1971-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 60. | Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 521] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 61. | Song H, Li X, Zhu C, Wei M. Glomerulosclerosis in adriamycin-induced nephrosis is accelerated by a lipid-rich diet. Pediatr Nephrol. 2000;15:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Gheith OA, Sobh MA, Mohamed Kel-S, El-Baz MA, El-Husseini F, Gazarin SS, Ahmed HA, Rasem MW, Amer GM. Impact of treatment of dyslipidemia on renal function, fat deposits and scarring in patients with persistent nephrotic syndrome. Nephron. 2002;91:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, D'Agati VD, Lamb HJ, Pongrac Barlovic D, Hojs R, Abbate M, Rodriquez R, Mogensen CE, Porrini E; ERA-EDTA Working Group Diabesity. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 394] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 64. | Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016;90:997-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 65. | Krahmer N, Farese RV Jr, Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol Med. 2013;5:973-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 365] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 66. | Ferrara D, Montecucco F, Dallegri F, Carbone F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J Cell Physiol. 2019;234:21630-21641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 67. | Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metab. 2012;16:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 68. | Bagby SP. Obesity-initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol. 2004;15:2775-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 69. | Barnes SE, Weinberg PD. Two patterns of lipid deposition in the cholesterol-fed rabbit. Arterioscler Thromb Vasc Biol. 1999;19:2376-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 528] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 71. | Cui J, Chen W, Liu J, Xu T, Zeng Y. Study on quantitative expression of PPARγ and ADRP in muscle and its association with intramuscular fat deposition of pig. Springerplus. 2016;5:1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277:18919-18927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 73. | Merscher S, Pedigo CE, Mendez AJ. Metabolism, energetics, and lipid biology in the podocyte - cellular cholesterol-mediated glomerular injury. Front Endocrinol (Lausanne). 2014;5:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Hara S, Kobayashi N, Sakamoto K, Ueno T, Manabe S, Takashima Y, Hamada J, Pastan I, Fukamizu A, Matsusaka T, Nagata M. Podocyte injury-driven lipid peroxidation accelerates the infiltration of glomerular foam cells in focal segmental glomerulosclerosis. Am J Pathol. 2015;185:2118-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Saito K, Ishizaka N, Hara M, Matsuzaki G, Sata M, Mori I, Ohno M, Nagai R. Lipid accumulation and transforming growth factor-beta upregulation in the kidneys of rats administered angiotensin II. Hypertension. 2005;46:1180-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2786] [Cited by in RCA: 2936] [Article Influence: 101.2] [Reference Citation Analysis (11)] |
| 77. | Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1224] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 78. | Mitrofanova A, Molina J, Varona Santos J, Guzman J, Morales XA, Ducasa GM, Bryn J, Sloan A, Volosenco I, Kim JJ, Ge M, Mallela SK, Kretzler M, Eddy S, Martini S, Wahl P, Pastori S, Mendez AJ, Burke GW, Merscher S, Fornoni A. Hydroxypropyl-β-cyclodextrin protects from kidney disease in experimental Alport syndrome and focal segmental glomerulosclerosis. Kidney Int. 2018;94:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 79. | Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 1841] [Article Influence: 87.7] [Reference Citation Analysis (9)] |
| 80. | Kim Y, Park CW. Mechanisms of Adiponectin Action: Implication of Adiponectin Receptor Agonism in Diabetic Kidney Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 81. | Wang C, Li L, Liu S, Liao G, Chen Y, Cheng J, Lu Y, Liu J. GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis. PLoS One. 2018;13:e0193473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 82. | Packer M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc Diabetol. 2020;19:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 83. | Maejima I, Takahashi A, Omori H, Kimura T, Takabatake Y, Saitoh T, Yamamoto A, Hamasaki M, Noda T, Isaka Y, Yoshimori T. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 2013;32:2336-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 519] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 84. | Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 85. | Yamamoto T, Takabatake Y, Minami S, Sakai S, Fujimura R, Takahashi A, Namba-Hamano T, Matsuda J, Kimura T, Matsui I, Kaimori JY, Takeda H, Takahashi M, Izumi Y, Bamba T, Matsusaka T, Niimura F, Yanagita M, Isaka Y. Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux. Autophagy. 2021;17:1700-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 86. | Yamamoto T, Takabatake Y, Takahashi A, Kimura T, Namba T, Matsuda J, Minami S, Kaimori JY, Matsui I, Matsusaka T, Niimura F, Yanagita M, Isaka Y. High-Fat Diet-Induced Lysosomal Dysfunction and Impaired Autophagic Flux Contribute to Lipotoxicity in the Kidney. J Am Soc Nephrol. 2017;28:1534-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 87. | Packer M. Role of Impaired Nutrient and Oxygen Deprivation Signaling and Deficient Autophagic Flux in Diabetic CKD Development: Implications for Understanding the Effects of Sodium-Glucose Cotransporter 2-Inhibitors. J Am Soc Nephrol. 2020;31:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 88. | Sakai S, Yamamoto T, Takabatake Y, Takahashi A, Namba-Hamano T, Minami S, Fujimura R, Yonishi H, Matsuda J, Hesaka A, Matsui I, Matsusaka T, Niimura F, Yanagita M, Isaka Y. Proximal Tubule Autophagy Differs in Type 1 and 2 Diabetes. J Am Soc Nephrol. 2019;30:929-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 89. | Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224:R15-R30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 90. | Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3239] [Cited by in RCA: 3231] [Article Influence: 190.1] [Reference Citation Analysis (0)] |
| 91. | Zhou D, Zhou M, Wang Z, Fu Y, Jia M, Wang X, Liu M, Zhang Y, Sun Y, Zhou Y, Lu Y, Tang W, Yi F. Progranulin alleviates podocyte injury via regulating CAMKK/AMPK-mediated autophagy under diabetic conditions. J Mol Med (Berl). 2019;97:1507-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 92. | Bao H, Zhang Q, Liu X, Song Y, Li X, Wang Z, Li C, Peng A, Gong R. Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells. FASEB J. 2019;33:14370-14381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 93. | Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1012] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 94. | Shevalye H, Lupachyk S, Watcho P, Stavniichuk R, Khazim K, Abboud HE, Obrosova IG. Prediabetic nephropathy as an early consequence of the high-calorie/high-fat diet: relation to oxidative stress. Endocrinology. 2012;153:1152-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 96. | Henry RR, Chilton R, Garvey WT. New options for the treatment of obesity and type 2 diabetes mellitus (narrative review). J Diabetes Complications. 2013;27:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Morrison MC, Yakala GK, Liang W, Wielinga PY, Salic K, van Koppen A, Tomar T, Kleemann R, Heeringa P, Kooistra T. Protective effect of rosiglitazone on kidney function in high-fat challenged human-CRP transgenic mice: a possible role for adiponectin and miR-21? Sci Rep. 2017;7:2915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Rutkowski JM, Wang ZV, Park AS, Zhang J, Zhang D, Hu MC, Moe OW, Susztak K, Scherer PE. Adiponectin promotes functional recovery after podocyte ablation. J Am Soc Nephrol. 2013;24:268-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 99. | Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 100. | Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2459] [Cited by in RCA: 2384] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 101. | Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 250] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 102. | Gyebi L, Soltani Z, Reisin E. Lipid nephrotoxicity: new concept for an old disease. Curr Hypertens Rep. 2012;14:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 103. | Opazo-Ríos L, Mas S, Marín-Royo G, Mezzano S, Gómez-Guerrero C, Moreno JA, Egido J. Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 104. | Falkevall A, Mehlem A, Palombo I, Heller Sahlgren B, Ebarasi L, He L, Ytterberg AJ, Olauson H, Axelsson J, Sundelin B, Patrakka J, Scotney P, Nash A, Eriksson U. Reducing VEGF-B Signaling Ameliorates Renal Lipotoxicity and Protects against Diabetic Kidney Disease. Cell Metab. 2017;25:713-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 105. | Chowdhury SS, Lecomte V, Erlich JH, Maloney CA, Morris MJ. Paternal High Fat Diet in Rats Leads to Renal Accumulation of Lipid and Tubular Changes in Adult Offspring. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 106. | Lin YC, Wu MS, Lin YF, Chen CR, Chen CY, Chen CJ, Shen CC, Chen KC, Peng CC. Nifedipine Modulates Renal Lipogenesis via the AMPK-SREBP Transcriptional Pathway. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Li Z, Li J, Miao X, Cui W, Miao L, Cai L. A minireview: Role of AMP-activated protein kinase (AMPK) signaling in obesity-related renal injury. Life Sci. 2021;265:118828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 108. | Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, Di Carlo F, Camussi G. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Reisin E, Ebenezer PJ, Liao J, Lee BS, Larroque M, Hu X, Aguilar EA, Morse SA, Francis J. Effect of the HMG-CoA reductase inhibitor rosuvastatin on early chronic kidney injury in obese zucker rats fed with an atherogenic diet. Am J Med Sci. 2009;338:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Blanco S, Vaquero M, Gómez-Guerrero C, López D, Egido J, Romero R. Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens. 2005;18:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Hong YA, Lim JH, Kim MY, Kim TW, Kim Y, Yang KS, Park HS, Choi SR, Chung S, Kim HW, Choi BS, Chang YS, Park CW. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1α in db/db mice. PLoS One. 2014;9:e96147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 112. | Tanaka Y, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Sugimoto T, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int. 2011;79:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 113. | Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 663] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 114. | Yang J, Zhang D, Li J, Zhang X, Fan F, Guan Y. Role of PPARgamma in renoprotection in Type 2 diabetes: molecular mechanisms and therapeutic potential. Clin Sci (Lond). 2009;116:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Yin QH, Zhang R, Li L, Wang YT, Liu JP, Zhang J, Bai L, Cheng JQ, Fu P, Liu F. Exendin-4 Ameliorates Lipotoxicity-induced Glomerular Endothelial Cell Injury by Improving ABC Transporter A1-mediated Cholesterol Efflux in Diabetic apoE Knockout Mice. J Biol Chem. 2016;291:26487-26501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 116. | Su K, Yi B, Yao BQ, Xia T, Yang YF, Zhang ZH, Chen C. Liraglutide attenuates renal tubular ectopic lipid deposition in rats with diabetic nephropathy by inhibiting lipid synthesis and promoting lipolysis. Pharmacol Res. 2020;156:104778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 117. | Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 624] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 118. | Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, Dreyfuss JM, Pan H, Tangcharoenpaisan Y, Morningstar J, Gerszten R, Patti ME. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 119. | Hosokawa K, Takata T, Sugihara T, Matono T, Koda M, Kanda T, Taniguchi S, Ida A, Mae Y, Yamamoto M, Iyama T, Fukuda S, Isomoto H. Ipragliflozin Ameliorates Endoplasmic Reticulum Stress and Apoptosis through Preventing Ectopic Lipid Deposition in Renal Tubules. Int J Mol Sci. 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 120. | Birnbaum Y, Bajaj M, Yang HC, Ye Y. Combined SGLT2 and DPP4 Inhibition Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Nephropathy in Mice with Type 2 Diabetes. Cardiovasc Drugs Ther. 2018;32:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 121. | Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes. 2016;65:2784-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 122. | Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond). 2018;132:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 123. | Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care. 2016;39:S165-S171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 124. | Tanaka S, Sugiura Y, Saito H, Sugahara M, Higashijima Y, Yamaguchi J, Inagi R, Suematsu M, Nangaku M, Tanaka T. Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018;94:912-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 125. | Wright MB, Varona Santos J, Kemmer C, Maugeais C, Carralot JP, Roever S, Molina J, Ducasa GM, Mitrofanova A, Sloan A, Ahmad A, Pedigo C, Ge M, Pressly J, Barisoni L, Mendez A, Sgrignani J, Cavalli A, Merscher S, Prunotto M, Fornoni A. Compounds targeting OSBPL7 increase ABCA1-dependent cholesterol efflux preserving kidney function in two models of kidney disease. Nat Commun. 2021;12:4662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 126. | Li L, Zhao Z, Xia J, Xin L, Chen Y, Yang S, Li K. A Long-Term High-Fat/High-Sucrose Diet Promotes Kidney Lipid Deposition and Causes Apoptosis and Glomerular Hypertrophy in Bama Minipigs. PLoS One. 2015;10:e0142884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 127. | Liu Y, Wang Z, Yin W, Li Q, Cai M, Zhang C, Xiao J, Hou H, Li H, Zu X. Severe insulin resistance and moderate glomerulosclerosis in a minipig model induced by high-fat/ high-sucrose/ high-cholesterol diet. Exp Anim. 2007;56:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Moreno-Navarrete JM, Catalán V, Whyte L, Díaz-Arteaga A, Vázquez-Martínez R, Rotellar F, Guzmán R, Gómez-Ambrosi J, Pulido MR, Russell WR, Imbernón M, Ross RA, Malagón MM, Dieguez C, Fernández-Real JM, Frühbeck G, Nogueiras R. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes. 2012;61:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 129. | Simcocks AC, Jenkin KA, O'Keefe L, Samuel CS, Mathai ML, McAinch AJ, Hryciw DH. Atypical cannabinoid ligands O-1602 and O-1918 administered chronically in diet-induced obesity. Endocr Connect. 2019;8:203-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Della Ragione F. Resveratrol: from basic science to the clinic. Cell Cycle. 2007;6:2495-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 131. | Malhotra A, Bath S, Elbarbry F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxid Med Cell Longev. 2015;2015:803971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 132. | Zhang W, Chen H, Sun C, Wu B, Bai B, Liu H, Shan X, Liang G, Zhang Y. A novel resveratrol analog PA19 attenuates obesityinduced cardiac and renal injury by inhibiting inflammation and inflammatory cell infiltration. Mol Med Rep. 2019;19:4770-4778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 133. | Saxena NK, Anania FA. Adipocytokines and hepatic fibrosis. Trends Endocrinol Metab. 2015;26:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 134. | Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 135. | Choi SR, Lim JH, Kim MY, Kim EN, Kim Y, Choi BS, Kim YS, Kim HW, Lim KM, Kim MJ, Park CW. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism. 2018;85:348-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 136. | Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, Hellmich MR. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A. 2013;110:12474-12479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 137. | Song K, Wang F, Li Q, Shi YB, Zheng HF, Peng H, Shen HY, Liu CF, Hu LF. Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney Int. 2014;85:1318-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 138. | Kasinath BS. Hydrogen sulfide to the rescue in obstructive kidney injury. Kidney Int. 2014;85:1255-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 139. | Aminzadeh MA, Vaziri ND. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol Dial Transplant. 2012;27:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 140. | Peh MT, Anwar AB, Ng DS, Atan MS, Kumar SD, Moore PK. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide. 2014;41:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 141. | Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 142. | Wu D, Gao B, Li M, Yao L, Wang S, Chen M, Li H, Ma C, Ji A, Li Y. Hydrogen Sulfide Mitigates Kidney Injury in High Fat Diet-Induced Obese Mice. Oxid Med Cell Longev. 2016;2016:2715718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 143. | Brinton EA, Mason RP. Prescription omega-3 fatty acid products containing highly purified eicosapentaenoic acid (EPA). Lipids Health Dis. 2017;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 144. | Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 735] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 145. | Mason RP, Jacob RF, Shrivastava S, Sherratt SCR, Chattopadhyay A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta. 2016;1858:3131-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 146. | Xu L, He S, Yin P, Li D, Mei C, Yu X, Shi Y, Jiang L, Liu F. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int J Hyperthermia. 2016;32:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 147. | Park C, Hong SH, Shin SS, Lee DS, Han MH, Cha HJ, Kim S, Kim HS, Kim GY, Park EK, Jeon YJ, Choi YH. Activation of the Nrf2/HO-1 Signaling Pathway Contributes to the Protective Effects of Sargassum serratifolium Extract against Oxidative Stress-Induced DNA Damage and Apoptosis in SW1353 Human Chondrocytes. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 148. | Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 788] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 149. | Chen YY, Hong H, Lei YT, Zou J, Yang YY, He LY. IκB kinase promotes Nrf2 ubiquitination and degradation by phosphorylating cylindromatosis, aggravating oxidative stress injury in obesity-related nephropathy. Mol Med. 2021;27:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 150. | Kojima K, Shimada T, Nagareda Y, Watanabe M, Ishizaki J, Sai Y, Miyamoto K, Aburada M. Preventive effect of geniposide on metabolic disease status in spontaneously obese type 2 diabetic mice and free fatty acid-treated HepG2 cells. Biol Pharm Bull. 2011;34:1613-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 151. | He X, Wang J, Li M, Hao D, Yang Y, Zhang C, He R, Tao R. Eucommia ulmoides Oliv.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 2014;151:78-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 152. | Altinoz E, Oner Z, Elbe H, Cigremis Y, Turkoz Y. Protective effects of saffron (its active constituent, crocin) on nephropathy in streptozotocin-induced diabetic rats. Hum Exp Toxicol. 2015;34:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 153. | Lai LL, Lu HQ, Li WN, Huang HP, Zhou HY, Leng EN, Zhang YY. Protective effects of quercetin and crocin in the kidneys and liver of obese Sprague-Dawley rats with Type 2 diabetes: Effects of quercetin and crocin on T2DM rats. Hum Exp Toxicol. 2021;40:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 154. | Tang X, Olatunji OJ, Zhou Y, Hou X. In vitro and in vivo aphrodisiac properties of the seed extract from Allium tuberosum on corpus cavernosum smooth muscle relaxation and sexual behavior parameters in male Wistar rats. BMC Complement Altern Med. 2017;17:510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 155. | Li QM, Chena HR, Zha XQ, Lu CQ, Pan LH, Luo JP. Renoprotective effect of Chinese chive polysaccharides in adenine-induced chronic renal failure. Int J Biol Macromol. 2018;106:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 156. | Ni Z, Guo L, Liu F, Olatunji OJ, Yin M. Allium tuberosum alleviates diabetic nephropathy by supressing hyperglycemia-induced oxidative stress and inflammation in high fat diet/streptozotocin treated rats. Biomed Pharmacother. 2019;112:108678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 157. | Jiang YH, Guo JH, Wu S, Yang CH. Vascular protective effects of aqueous extracts of Tribulus terrestris on hypertensive endothelial injury. Chin J Nat Med. 2017;15:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 158. | Jiang YH, Jiang LY, Wu S, Jiang WJ, Xie L, Li W, Yang CH. Proteomic Analysis Reveals the Renoprotective Effect of Tribulus terrestris against Obesity-Related Glomerulopathy in Rats. Biol Pharm Bull. 2018;41:1430-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 159. | Shukla P, Singh AB, Srivastava AK, Pratap R. Chalcone based aryloxypropanolamines as potential antihyperglycemic agents. Bioorg Med Chem Lett. 2007;17:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 160. | Wu J, Li J, Cai Y, Pan Y, Ye F, Zhang Y, Zhao Y, Yang S, Li X, Liang G. Evaluation and discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J Med Chem. 2011;54:8110-8123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 161. | Wang HM, Zhang L, Liu J, Yang ZL, Zhao HY, Yang Y, Shen D, Lu K, Fan ZC, Yao QW, Zhang YM, Teng YO, Peng Y. Synthesis and anti-cancer activity evaluation of novel prenylated and geranylated chalcone natural products and their analogs. Eur J Med Chem. 2015;92:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 162. | Choi JS, Song J. Effect of genistein on insulin resistance, renal lipid metabolism, and antioxidative activities in ovariectomized rats. Nutrition. 2009;25:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 163. | Braga PC, Dal Sasso M, Culici M, Bianchi T, Bordoni L, Marabini L. Anti-inflammatory activity of thymol: inhibitory effect on the release of human neutrophil elastase. Pharmacology. 2006;77:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 164. | Wattanasatcha A, Rengpipat S, Wanichwecharungruang S. Thymol nanospheres as an effective anti-bacterial agent. Int J Pharm. 2012;434:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 165. | Dhaneshwar S, Patel V, Patil D, Meena G. Studies on synthesis, stability, release and pharmacodynamic profile of a novel diacerein-thymol prodrug. Bioorg Med Chem Lett. 2013;23:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 166. | Berthoux F, Mariat C, Maillard N. Overweight/obesity revisited as a predictive risk factor in primary IgA nephropathy. Nephrol Dial Transplant. 2013;28:iv160-iv166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 167. | Stump CS. Physical Activity in the Prevention of Chronic Kidney Disease. Cardiorenal Med. 2011;1:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 168. | Juszczak F, Vlassembrouck M, Botton O, Zwakhals T, Decarnoncle M, Tassin A, Caron N, Declèves AE. Delayed Exercise Training Improves Obesity-Induced Chronic Kidney Disease by Activating AMPK Pathway in High-Fat Diet-Fed Mice. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/