INTRODUCTION
The obesity pandemic that started in the latter half of the 20th century was a consequence of adverse lifestyles characterized by physical inactivity, overconsumption of energy-dense, highly processed diets, and various other unfavorable circumstances, including stress, inadequate sleep, adverse childhood experiences, and social determinants of poor health. This relentless and rapidly advancing trend persists into the 21st century. Currently, nearly 2 billion people globally are overweight or obese, and this figure is expected to double by the year 2035, causing a global economic burden of 4 trillion dollars[1]. Childhood obesity figures are also expected to double by 2035 from the figures in 2020 to 208 million boys (100% rise) and 175 million girls (125% increase). Obesity is associated with numerous metabolic and nonmetabolic complications in children and young adults, significantly elevating the risk of morbidity and mortality, not only in the later part of their lives but also throughout the entirety of their life journey.
Development of metabolic syndrome and type 2 diabetes mellitus (T2DM) represent significant consequences of obesity in childhood and adolescence as in adults. A recent global study highlighted that obesity/overweight stands as the primary attributable risk factor for disability-adjusted life years associated with early onset T2DM, regardless of the sociodemographic index of the region studied[2]. Another recent study showed that the incidence of T2DM among children and adolescents in 2021 alone was approximately 41600 new cases[3]. This study from 25 countries/territories showed that the incidence rates varied from 285 to 734 per 100000 population. Obesity and T2DM are associated with a variety of health-related complications affecting every organ system, especially when they occur early in life. It is imperative for the global scientific community to take urgent actions to curtail these pandemics.
THE GLOBAL EPIDEMIOLOGIC SCENARIO
Consumption of a diet rich in highly processed foods, high in fat and carbohydrate contents, with a high glycemic index is likely to lead to both obesity and T2DM. A discerning association emerges when examining the global obesity pandemic, which coincided with the widespread adoption of fast-food culture in many developed countries during the latter half of the 20th century[4,5]. The invention of televisions and computers in the last decades of the 20th century further accelerated the momentum of this obesity pandemic. This technological shift led to a marked reduction in day-to-day physical activities among children and adolescents, thereby perpetuating the obesity prevalence[6]. The widespread introduction of mobile telephones in the early 21st century further aggravated the problem, as the younger generation now spends a significant amount of time browsing the internet and watching videos on their phones, consequently reducing their engagement in regular exercise routines[7,8]. Indiscrete nutritional intake and lack of physical activities in childhood are identified as the major reasons for obesity among adolescents and young adults not only in developed nations but also in developing countries across the globe. Furthermore, decreased sleep, heightened stress levels, inadequate coping mechanisms, structural racism, and adverse social determinants of health all contribute to the obesogenic environment. Many of these children also exhibit genetic and epigenetic predispositions to obesity.
The global prevalence of obesity [based on body mass index (BMI) criteria of ≥ 30 kg/m²) among boys aged 5–19 years was 104 million (10% of all boys), and that among girls was 72 million (8%) in the year 2020[1]. Although most of these children were from the developed world, the prevalence figures are relatively high even in developing countries. Prevalence figures in millions (% of total) from different regions of the world in 2020 were as follows: Americas – boys 24 (20%), girls 18 (16%); Eastern Mediterranean – boys 14 (11%), girls 13 (11%); Europe – boys 11 (13%), girls 7 (8%); South-East Asia – boys 14 (5%), girls 8 (3%); Western Pacific – boys 36 (19%), girls 16 (9%); and Africa – boys 6 (3%), girls 10 (5%)[1]. These figures are expected to exponentially increase in the near future if there are no urgent global actions to curtail the obesity pandemic.
The BMI criteria for assessing the metabolic risk in adults with obesity is not appropriate for non-Caucasian populations due to their higher visceral adiposity[9]. For example, the BMI threshold to define obesity in the Southeast Asian population is ≥ 27.5 kg/m², and that for Indians is ≥ 25 kg/m²[10,11]. Therefore, the age-specific cut-offs for defining obesity in children of non-Caucasian origin should ideally be distinct, given the anticipated higher metabolic risk in these children analogous to the non-White adult population. However, there are no population-based studies specifically examining this possibility. Moreover, waist circumference and fat mass compositions are not widely used in children to determine their obesity risk.
The onset of the COVID-19 pandemic in early 2020 and subsequent social isolation, virtual learning, decrease in physical activity, and alterations in healthy behaviors contributed to worsening obesity and metabolic health and have significantly increased the risk for T2DM among children across the globe[12]. Early adverse childhood experiences and social determinants of poor health may also play a role. Hyperinsulinemia seen in obesity can stimulate the production of an adipose-derived protein that acts as a SARS-CoV-2 chaperone, binding the spike protein and delivering the virus to tissues with ACE-2-expressing cells[13]. The long-term consequences of the COVID-19 pandemic on the metabolic health of children and young adults are yet to unfold in the coming years. Extensive research efforts must be dedicated to this area of uncertainty.
According to a study by the Centers for Disease Control and Prevention, by the year 2060, the number of individuals under the age of 20 with T2DM could potentially reach 220000, representing an alarming increase of 700%[14]. This surge is anticipated to result in significant disparities among various racial and ethnic groups, with Black, Hispanic/Latino, Asian, Pacific Islander, American Indian, and Alaskan Native youths experiencing higher rates of T2DM.
DIFFERENCES IN THE PATHOBIOLOGY OF OBESITY AND T2DM AMONG CHILDREN AND ADULTS
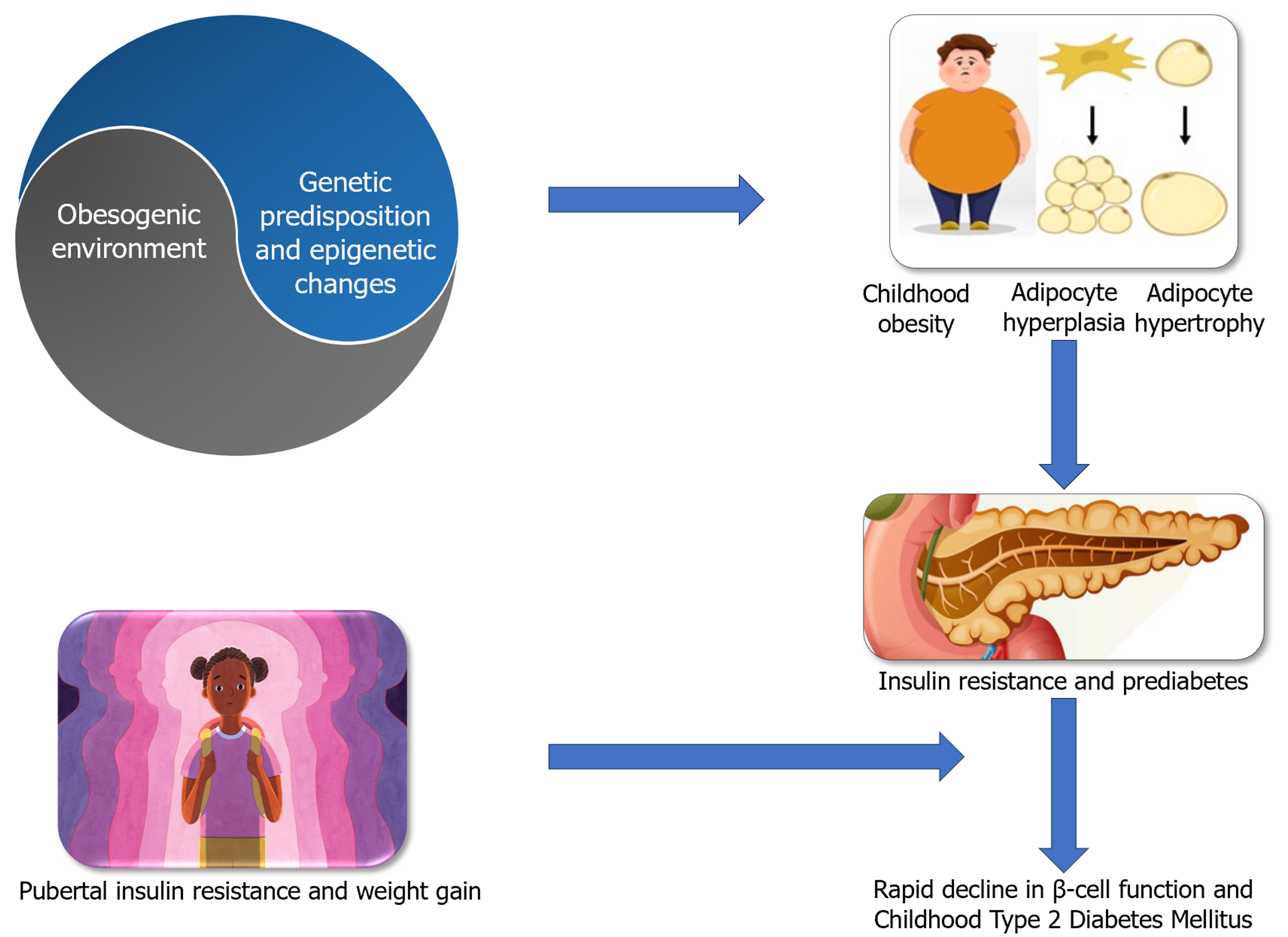
There are fundamental differences in the pathobiology of obesity between children and adults, which need to be explored in detail to have a thorough understanding of the metabolic consequences of obesity, including T2DM. Early onset obesity in children is associated with adipocyte hyperplasia and hypertrophy, whereas the fat cell numbers remain constant in those developing late-onset obesity simply by fat cell hypertrophy, as the adipocyte numbers are set constant in adults[15]. This means the latter type of obesity is predominantly from fat cell hypertrophy. Moreover, adipocyte hyperplasia predominantly occurs in the subcutaneous areas and in a generalized body distribution, whereas fat cell hypertrophy (in adults) occurs mainly in visceral areas and to some extent only in the subcutaneous areas[16]. While subcutaneous adiposity is relatively less harmful [metabolically healthy obesity (MHO)], visceral fat depots are metabolically more active and form the pathogenic substrate for most adiposity-related chronic diseases (metabolically unhealthy obesity), including cardiovascular disease (CVD)[17]. However, we must remember that even MHO can be harmful in the long run. Any form of obesity is associated with elevated inflammatory cytokines and reduced adipokines, leading to adverse long-term health consequences.
Environmental influences play a huge role in childhood obesity, starting from eating behaviors to recreational activities. Various circumstantial influences from the family, community, and socio-political levels are found to create an obesogenic environmental milieu perpetuating the risk of childhood obesity[17,18]. These influences, when coupled with genetic factors, form the fertile soil for the development of childhood and adolescent obesity. Therefore, a gene-environmental interaction initiates obesity onset, while the obesogenic environment perpetuates it. Genome-wide association studies have shown that more than 65 culprit genes can be involved in regulating the metabolic pathways of glucose homeostasis, insulin signaling, and sensitivity, the abnormalities of which can be associated with an elevated risk of T2DM[19]. The complex interactions between these genetic variations in an obesogenic environment potentiate T2DM risk in children and adolescents.
T2DM is the result of beta-cell failure coupled with insulin resistance among children. The disease is usually preceded by a “pre-diabetes” state characterized by impaired glucose tolerance (IGT) and or impaired fasting glucose, which progress to frank T2DM at a rapid pace when compared to disease onset and progression in adults[19,20]. T2DM manifests when beta-cell function fails to compensate for insulin resistance, which is influenced by genetic under-pinnings. In children with obesity, this failure occurs at an accelerated pace. Pubertal insulin resistance and weight gain are attributed to the rapidly declining beta cell function. The prediction of conversion to T2DM in obese children is associated with minority ethnicity, severe obesity, obesity trajectory, IGT, and the disposition index, a composite score that reflects both insulin sensitivity and beta-cell function[21]. In general, T2DM in children and adolescents exhibits an aggressive disease course, and in the presence of multiple comorbidities, runs a disease course characterized by the rapid development of β-cell failure. Progression to insulin dependence can occur within a short period of 3-5 years of initial diagnosis in the absence of appropriate management[20,22]. This rapid decline in β-cell function is a characteristic feature in childhood-onset T2DM when compared to the disease in adults that often remains slowly progressive with total beta-cell failure only after several years.
African Americans and Native Americans tend to have enhanced beta-cell responsiveness, especially enhanced acute-phase insulin response, which theoretically can lead to early impairment of beta-cell function. They also manifest a higher proinsulin-to-C-peptide ratio during fasting and following a glucose challenge. Some African American youth with T2DM exhibit T2DM-related genetic variants, such as those found in the TCF7L2 locus. Additionally, in some Oji-Cree Native Canadian youth, the HNF1A G319S variant is observed[23].
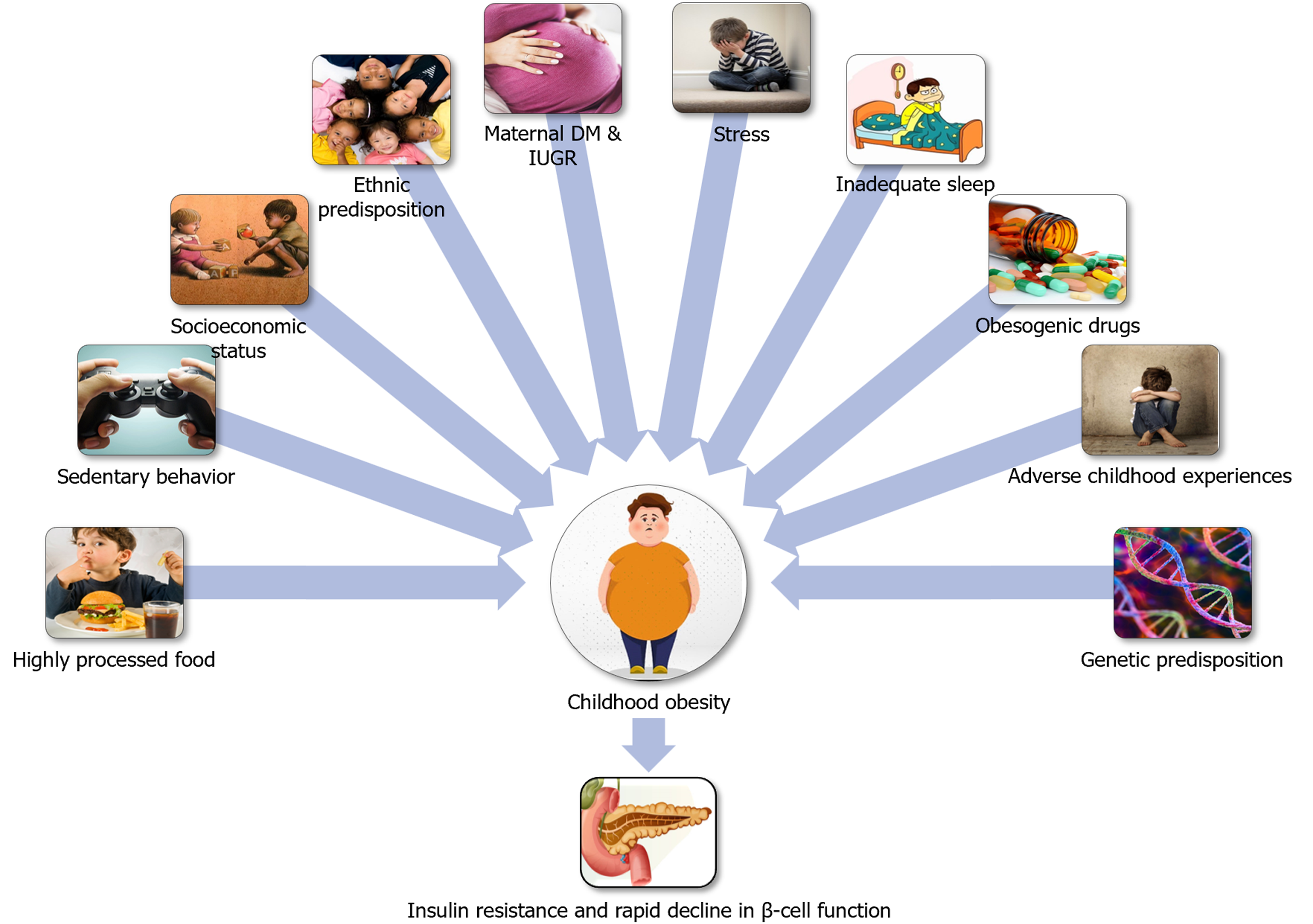
Hispanic Americans exhibit significantly greater genetic risk scores for insulin resistance and display a high prevalence of risk alleles for fatty liver, which is commonly associated with insulin resistance and risk for T2DM. Offspring of parents with T2DM exhibits an impaired acute insulin response to glucose compared to those without diabetes[24], indicating that heritability plays a crucial role in developing T2DM at a younger age when coupled with other environmental influences. Various genetic influences and inter-individual variations determine the response to obesogenic environment. An interaction between genes and a predisposing environment confers susceptibility to diabetes. Figure 1 shows the factors predisposing to diabesity among children and adolescents. Figure 2 shows the etiopathological factors resulting in diabetes among children and adolescents.
Figure 1 Diabesity predisposing environment.
DM: Diabetes mellitus; IUGR: Intrauterine growth retardation.
Figure 2
Determinants of progression to type 2 diabetes.
CLINICAL CONSIDERATION: EVALUATION/SCREENING
Children and adolescents with diabetes should undergo a comprehensive evaluation to exclude type 1 diabetes mellitus (T1DM) and other heritable/syndromic causes of diabetes. T1DM in children often presents with diabetic ketoacidosis (DKA) at or soon after initial diagnosis of the disease, and they often present with symptoms of severe hyperglycemia and failure to thrive (in very young individuals) or weight loss. Insulin dependence is a characteristic feature. The diagnosis is confirmed by the presence of diabetic autoantibodies (DAAs). When all five DAAs are tested, the sensitivity for diagnosis of T1DM can be as high as 97.8%[25]. Measurement of serum C-peptide levels may be useful in proving severe insulin deficiency characteristic of T1DM, though some residual β-cell function may be preserved (c-peptide level ≥ 0.2 nmol/L) for a variable time period between a few months to 3-5 years after initial diagnosis of DM in up to 7% individuals[26]. A recent meta-analysis revealed that a urine C-peptide creatinine ratio of < 0.2 mmol/mol after 3 years of diabetes diagnosis demonstrated a sensitivity and specificity of 84.4% and 91.6%, respectively, making it a reliable indicator for correctly identifying T1DM[27].
In individuals lacking positive DAA, especially in those with a family history of early onset diabetes or atypical presentation such as dysmorphic features, multi-organ involvement, and absence of obesity, various inherited causes of diabetes and diabetes associated with genetic syndromes should be considered[28-30]. A comprehensive history, including current drug intake and past exposure, is also necessary to exclude drug-induced diabetes in children and adolescents[31]. Familial clustering is often a clue to the diagnosis of several genetic and syndromic types of diabetes such as various forms of monogenic diabetes, Bardet-Beidl syndrome, Wolfram syndrome, Alstrom syndrome, and cystic fibrosis.
Up to 50% of children and young people with T2DM are asymptomatic, and the presentation can be indolent. Therefore, the diagnosis is often overlooked, leading to underestimation of the disease burden[22,32]. Moreover, individuals at times revert from having T2DM to prediabetes and vice versa. A high index of clinical suspicion is crucial for clinicians to consider early screening, especially in the presence of risk factors such as obesity, non-Caucasian ethnic background, features of insulin resistance like acanthosis nigricans, metabolic-associated fatty liver disease (MAFLD), hyperandrogenism in females and/or polycystic ovary syndrome (PCOS). Around 6% of pediatric patients with T2DM may present with DKA at the outset. Patients may also present with a hyperosmolar hyperglycemic state without ketoacidosis. Using HbA1c for screening may be misleading for early diagnosis of prediabetes and T2DM in children and adolescents because of the relatively low sensitivity and specificity of the test[22,33]. Although a standard oral glucose tolerance test (OGTT) is more accurate for initial screening, its application is hindered by its cumbersome nature and limited availability in remote, primary care, and outpatient settings. These challenges make OGTT a difficult-to-use clinical screening tool for at-risk children.
COMPLICATION PROFILE
Complications in children and young adults can be directly linked to T2DM or the associated diseases due to obesity. These complications can manifest earlier than that in their adult counterparts due to the more aggressive course of the disease.
Microvascular disease
Diabetic retinopathy: Retinal microvasculature seems to be an early target of diabetic vasculopathy in children and young adults with T2DM. A recent meta-analysis of 27 studies with 5924 patients showed a global prevalence of diabetic retinopathy (DR) in 6.99% of children[34]. The study also observed a steep rise in the prevalence figures over time after initial diagnosis of T2DM, with 1.11% only affected within the first 2.5 years, 9.04% between 2.5-5 years, and 28.14% after 5 years. This underscores the need for more aggressive screening and management strategies for DR in children.
Diabetic neuropathy: Like DR, neuropathy settles early in children with T2DM compared to the onset of diabetic neuropathy (DN) in T1DM. A longitudinal Australian study revealed that at a median duration of 1.3 years, 23% of children with T2DM developed peripheral and 57% autonomic neuropathy[35,36]. Data from more recent studies revealed that disease-free survival without DN was much lower in children and adolescents with T2DM[37], and autonomic involvement[38] and cardiac autonomic neuropathy[39] were quite common within a few years of disease onset in these children. However, a very recent study showed that the prevalence of DN in children with T1DM was only 1.33% at a median follow up period of 8.3 years[40]. The risk factors for DN in this study among T1DM children were female sex, higher age, low body weight, smoking, retinopathy, high cholesterol, longer duration of DM, postprandial hyperglycemia, and high HbA1c in a multivariate analysis of 84390 patients. In the absence of much longer-term study data on prevalence and risk factors for DN among children with T2DM, those with the above predictive risks for DN (as in T1DM) should be more aggressively managed to reduce the development of this dreaded complication.
Diabetic kidney disease: A recent systematic review of 15 studies from seven countries clearly showed evidence for an exaggerated risk for the development of chronic diabetic kidney disease (DKD) in children and adolescents with T2DM than those age-matched T1DM patients[41]. The study shows that incidence rates for various forms of DKD were: Albuminuria – 12.4 to 114.8, macroalbuminuria/ proteinuria – 10 to 35.0, end-stage renal disease – 0.4 to 25.0, CVD – 3.7 to 19.5, and mortality – 1.0 and 18.6 per 1000 person-years at a follow-up duration from 1 year to 12.6 years.
Macrovascular disease
One of the largest follow-up studies investigating the management and long-term outcomes of T2DM among children is the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) clinical trial, which reported a com-plication profile of participants at a mean age of 26.4 ± 2.8 years with a mean diabetes duration of 13.3 ± 1.8 years[42]. The study revealed microvascular complications such as DKD in 54.8%, neuropathy in 32.4%, and retinopathy including advanced diabetic eye disease in up to 51% at this follow-up period. However, the macrovascular disease prevalence of this cohort is not yet reported. The 14-year cumulative incidence of CVD risk factors such as hypertension, high low-density lipoprotein cholesterol, and triglycerides were 59%, 33%, and 37%, respectively, indicating that these children were at a very high risk of macrovascular complications[43].
CVD: A recent meta-analysis examining children with T2DM showed a prevalence of hypertension in 25.3% (95% CI: 19.6%-31.5%) among 3463 cases and microalbuminuria in 22.2% (17.3%-27.4%) among 2250 cases[44]. These estimates indicate that these children are at huge risk of cardiovascular morbidity and mortality as hypertension and albuminuria markedly increase CVD risk in T2DM. The CVD risk among these children is probably much higher than that in their adult counterparts owing to worse metabolic risks in the pediatric populations. Small cohort study-based data suggests that the ischemic heart disease risk in T1DM vs T2DM is 1.2 vs 5.4 per thousand person-years in youth with T2DM[45].
Cerebrovascular disease: Although the risk of cerebrovascular disease in pediatric populations with T2DM is not well estimated based on large long-term population-based studies, it is very likely that this risk is probably higher than that in adults with T2DM. Carotid intima-media thickness and global cerebral blood flow have been shown to be lower in youth-onset T2DM compared to their obese counterparts in small studies[46].
Peripheral vascular disease: The data on the exact prevalence of peripheral vascular disease is not available from good-quality studies reporting T2DM complications in children. However, considering the high risk in these populations, regular follow-up monitoring for complications of T2DM, including foot disease, is mandatory as per the current recommendations[47].
Comorbidities associated with diabesity
Obesity is frequently linked with complications such as MAFLD, obstructive sleep apnea (OSA), PCOS, and dyslipidemia. The presence of diabesity exacerbates the comorbidity profile and consequences. Therefore, early implementation of intense lifestyle and therapeutic strategies is imperative[47,48].
MAFLD: MAFLD has recently become a great public health threat, with about one-third of the global population affected[49]. It is the most common cause of liver dysfunction, affecting 7% to 9% of all and 38% to 41% of obese children[50]. A recent study suggests that 16.8% of children with MAFLD also had T2DM, with an incidence rate of 3000 new cases per 100000 person years at risk[51]. Recent studies also suggest that the presence of T2DM is associated with a 3.5 times higher risk of hepatic decompensation and an 8.4 times higher risk of hepatocellular carcinoma at 5 years of diagnosis among adults with MAFLD compared to those without diabetes[49]. The extrapolated risks of liver-related/general morbidity and mortality among children with coexistent MAFLD and T2DM would be higher than those in their adult counterparts as the complication risks in general are higher in the pediatric populations with diabetes.
OSA: In a recent study among children aged 7-18 years, the prevalence of OSA was found to be 9.1% when BMI was normal, whereas it was 44.6% in those with overweight/obesity[52]. Although OSA was found to be associated with worsening insulin resistance[53] and higher severity of OSA was associated with greater HbA1c levels in children[54], the exact prevalence of OSA among pediatric T2DM patients is not clear in the absence of data from large-scale studies. When we consider the risks among adults, we can assume that OSA is very likely to significantly worsen the CVD-related and general morbidity and mortality in children with T2DM.
PCOS: A recent meta-analysis involving six studies (albeit with a high degree of heterogeneity) and 470 girls with T2DM revealed that the prevalence of PCOS was notably elevated in the range of 19.6% to 24%[55]. Because of the commonality of occurrence, obese children are more likely to develop both PCOS and T2DM than their peers with normal BMI. More aggressive management strategies must be adopted to improve fertility and mitigate the complications of T2DM in these patients when they transition to adulthood.
Dyslipidemia: Recent studies showed high prevalence of diabetic dyslipidemia in pediatric T2DM patients from Canada and Israel with high triglycerides and low high-density lipoprotein cholesterol[56,57]. Hypertriglyceridemia was present in 35% of the Canadian cohort while 45.6% of the Israeli cohort had the disease. Given that dyslipidemia is a significant risk factor for both micro- and macro-vascular disease in T2DM, intense management strategies must be considered with an individualized therapeutic approach in children and adolescents.
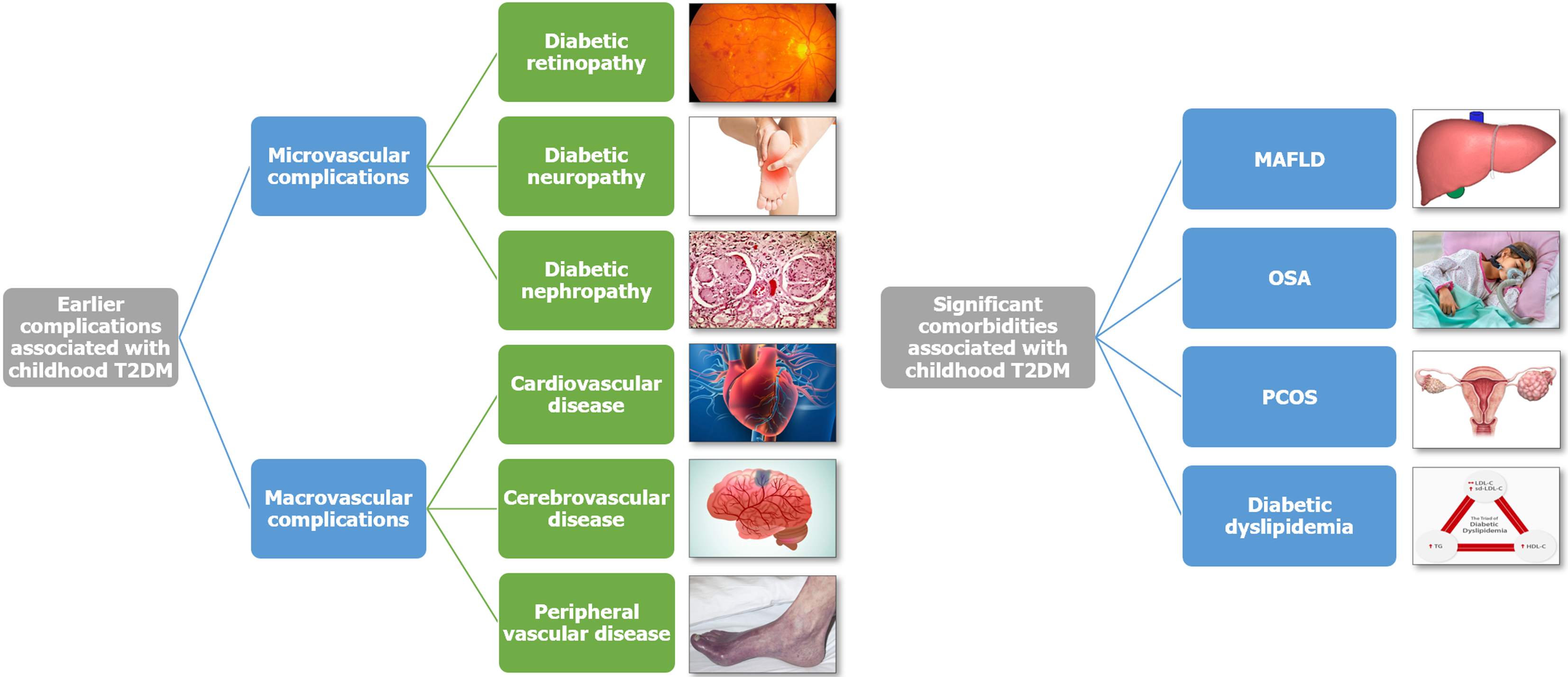
Hypertension: The prevalence of hypertension is significantly higher in youth with T2DM. A comprehensive systematic review and meta-analysis of 60 studies revealed that 25% of children and adolescents with T2DM exhibited hypertension and 22% had albuminuria[44]. Notably, Pacific Islander and indigenous youth faced a heightened risk of these conditions compared to their counterparts from other racial groups in the United States. Figure 3 shows the complication and comorbidity profile of diabesity in children and adolescents.
Figure 3 Complications and comorbidities associated with diabesity in children and young adults.
HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; MAFLD: Metabolic-associated fatty liver disease; OSA: Obstructive sleep apnea; PCOS: Polycystic ovary syndrome; sd-LDL-C: Small dense low-density lipoprotein cholesterol; T2DM: Type 2 diabetes mellitus; TG: Triglyceride.
PREVENTION STRATEGIES
As T2DM is a lifelong disease with myriad complications in every organ system, several of which are quite crippling and potentially lethal, every effort should be taken to prevent it by appropriate interventions well before disease onset. Thorough and intensive lifestyle modifications are the cornerstones in disease prevention, especially in vulnerable populations such as obese, ethnically prone, and those with strong family histories. A recent meta-analysis of 48 studies involving 6630296 participants showed a prediabetes prevalence of 8.84% (6.74-10.95) in children, with an alarming rise in the prevalence figures from 0.93% to 10.66% in the past decades, highlighting the explosive nature of the clinical threat[58]. Unless coordinated and urgent collective global efforts are made by governmental and nongovernmental agencies, professional bodies, and healthcare providers to curtail this healthcare challenge, the global budget to fight diabetes will face significant financial constraints in the near future.
Several studies on nutritional and lifestyle interventions have shown modest benefits in preventing or modifying the risk for incident T2DM in children, though most of these were with high degrees of heterogeneity, low number of participants, and short duration[59,60]. Timely screening of disease-vulnerable children and lifestyle interventions coupled with pharmacotherapy or bariatric interventions when appropriate would be reasonable approaches to the childhood population with T2DM risk. Initial screening of at-risk populations for prediabetes/ T2DM should be considered at puberty or ≥ 10 years (whichever is earlier), and if negative, subsequent screening should be performed at 3-year intervals as per the current American Diabetes Association (ADA) clinical practice recommendations[47].
MANAGEMENT APPROACH
Lifestyle interventions
The new American Association of Pediatric (AAP) Obesity guidelines recommend intensive health behavior lifestyle treatment (IHBLT) as the cornerstone of obesity management, which is family-centered, multimodal, and multi-disciplinary. This suggests more than 26 h of face-to-face contact hours for IHBLT over the course of 1 year[61]. The 2023 ADA guideline recommends providing culturally appropriate comprehensive education and support for children with T2DM and their families[47]. In obese/overweight patients, the goal should be for a weight loss of 7%-10%, accompanied by comprehensive diabetes management. This approach includes 60 minutes of moderate to vigorous physical activities and a nutrient-rich, calorie-reduced diet. A successful weight loss often allows for diabetes remission/reversal along with improvement of major cardiometabolic risks[62]. However, sustainability of such interventions is a big challenge, and continued effort from the healthcare provider and support from family are of immense value.
Pharmacotherapy
Pharmacotherapeutic interventions should be considered early in the course of the disease, given the distinct pathobiological behavior of T2DM in children. Appropriate drug therapy should be planned in alignment with the current ADA recommendations for glycemic control and according to AAP guidelines for obesity treatment considering the pathobiological factors of T2DM[47]. The glycemic targets should be individualized, with an HbA1c of < 7% (53 mmol/mol) as an acceptable goal if not intense reduction < 6.5% (48 mmol/mol). Glucose monitoring is mandatory to ensure optimal management. A desired weight loss goal should be individualized based on previous weight trajectory, comorbidities, severity of obesity, and patient or family preferences. Comorbidities such as dyslipidemia, hypertension, and PCOS should be considered while choosing pharmacotherapy.
Insulin: Insulin should be considered when the initial HbA1c is > 8.5% (69 mmol/mol), when there is persistent hyperglycemia, or there is possibility of T1DM prior to full initial diagnostic evaluation[47]. Insulin is not weight-neutral, so it cannot be the sole medication to treat T2DM in children. Disadvantages of hypoglycemia, weight gain, need for parenteral administration, and compliance issues can influence insulin use for T2DM in pediatric practice. The Restoring Insulin Secretion (RISE) study sought to investigate the potential of early pharmacotherapy in preserving beta cell function[63]. The study revealed that among youths with prediabetes and recently diagnosed T2DM, beta cell function and glycemia experienced deterioration even during treatment with either 12 mo of metformin or a regimen comprising 3 mo of insulin glargine followed by 9 mo of metformin.
Metformin: Metformin can inhibit hepatic glucose production, but it is not a potent insulin sensitizer at the muscle or adipocyte level. Metformin is ideally initiated soon after diagnosis without delay[47]. Obese children treated with metformin have shown a modest reduction of BMI compared to controls in some randomized control trials (RCTs)[64]. The TODAY study results showed that metformin monotherapy was associated with a 5-year treatment failure of 51.7% in children with T2DM compared to 21% in adults[65]. These rates of treatment failure in the TODAY trial occurred in a mean baseline diabetes duration of only 7.8 mo. The main side effect related to metformin use is gastrointestinal intolerance. A recent meta-analysis showed that the addition of probiotics to metformin improved these side effects and HbA1c compared to metformin alone[66].
Glucagon-like peptide-1 receptor agonists: These drugs should be used as second-line agents or even at the beginning of therapy because of the weight loss potential in obese/ overweight patients with T2DM[47]. The disease modifying properties of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in the management of diabesity should be considered in every patient with the disease[67]. GLP-1RA enhances glucose-stimulated insulin secretion, increases satiety due to its actions at the hypothalamic level, and reduces gastric emptying. Liraglutide, exenatide, and dulaglutide are now approved for use in adolescents with T2DM. Newer agents such as semaglutide and tirzepatide are eagerly anticipated for approval following major RCTs showing remarkable benefits as disease modifying agents.
Other antidiabetic agents: Drugs belonging to the gliptin class are generally weight neutral and, therefore, usually used as second-line oral agents or in metformin intolerance in adults with T2DM. However, sitagliptin used as first-line therapy[68] or added as a second-line agent[69] did not show meaningful and durable control of diabetes in recent RCTs. The United States FDA has granted approval for the SGLT2 inhibitor empagliflozin for lowering glucose levels in children aged 10 years and older with T2DM. The majority of the other antidiabetic agents used in adult clinical practice are not endorsed by the latest ADA guidelines. However, some of these medications with reasonable safety profiles may be used on an individual basis through shared decision-making with patients and their caregivers when deemed appropriate with careful monitoring of efficacy.
Bariatric procedures
Surgical management of diabesity should be considered early in the course of the disease when lifestyle and pharmacotherapy fail in patients, especially in those with a BMI of > 35 kg/m2 as per the ADA recommendations, those with class II obesity (BMI > 120th percentile of 95th percentile with comorbidities), and those with class III obesity ((BMI > 140th percentile of 95th percentile)[47]. Multiple RCTs and systematic reviews have established the high efficacy, good safety, and economic advantage of bariatric procedures in addressing diabesity and its comorbidities in adolescents[70,71]. However, longer term data of more than 5 years is still unavailable in this new area of intervention for diabesity in the youth. The choice of surgery depends on the shared decision-making between the healthcare provider and the patient/caretaker.
AREAS OF UNCERTAINTY
As T2DM and diabesity are recent issues in pediatric clinical practice, there are several areas of uncertainty in the current evidence regarding diseases pathobiology and therapeutic aspects. Substantial research efforts must be dedicated to these areas by the global scientific community to fill in knowledge gaps. BMI-based criteria for the approval of GLP-1RAs may disadvantage Asian and other populations with T2DM. Newer pharmacotherapeutic agents such as GLP-1RA/GIP co-agonists and GLP-RA/ GIP/glucagon triple agonists may show promising utility in the management of severe obesity and diabesity[72-74]. These agents also confer remarkable cardiovascular and other metabolic/comorbidity reduction benefits, including MAFLD, dyslipidemia, OSA, and hypertension improvements. However, large long-term multi-center RCTs involving multi-ethnic pediatric populations with these molecules are necessary before the efficacy and safety of such drugs can be proven prior to their use in clinical practice.
Sodium-glucose co-transporter-2 inhibitors are another group of antidiabetic drugs widely used for the management of diabesity with remarkable cardiovascular, renal, and metabolic/comorbidity reduction benefits when used in adult patients[75-78]. However, there are only limited research data on the use of these medications in pediatric practice for the wide use of these drugs in our day-to-day clinical practice[79-81]. More research must also be done in this area. Fur-thermore, in accordance with the latest obesity guidelines from the AAP, anti-obesity medications such as phenteramine, topiramate, naltrexone, or bupropion may be considered as adjuvant therapy for children with T2DM, as deemed appropriate for managing their obesity.
Glucose monitoring is another area that requires attention. While continuous glucose monitoring is recommended for the management of T1DM, its use is not widely recommended in children with T2DM.
CONCLUSION
T2DM and diabesity have emerged as major health challenges in recent years due to the global obesity pandemic, which affects children even more severely than their adult counterparts. The pathobiology of T2DM in children is different from that in adults in various aspects, such as a rapid progression to insulinopenia, an association with multiple comorbidities, and a likelihood of developing complications much earlier in their disease journey. Individualized care incorporating intense lifestyle modifications coupled with early pharmacotherapy and, when necessary, bariatric interventions are crucial elements in the management of diabesity and T2DM in pediatric populations. Substantial efforts from governmental agencies, voluntary organizations, and professional bodies to raise public awareness should contribute to combating the global obesity pandemic, especially in children and adolescents. Additional global research input in the coming years is crucial to shed clearer light on the areas of uncertainty regarding patient care, including the judicious use of newer antidiabetic medications with proven benefits in adults with diabesity. These collaborative multinational efforts are expected to improve our understanding and contribute to addressing the significant healthcare challenge posed by this devastating global pandemic.
ACKNOWLEDGEMENTS
We thank Dr. Marina George Kudiyirickal for providing the voice clip for the audio Core Tip.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Surani S, United States S-Editor: Lin C L-Editor: Filipodia P-Editor: Cai YX