Published online Dec 15, 2024. doi: 10.4239/wjd.v15.i12.2360
Revised: September 22, 2024
Accepted: October 23, 2024
Published online: December 15, 2024
Processing time: 182 Days and 13.2 Hours
Familial partial lipodystrophy disease (FPLD) is a collection of rare genetic diseases featuring partial loss of adipose tissue. However, metabolic difficulties, such as severe insulin resistance, diabetes, hypertriglyceridemia, and hyperte
In 2017, a 31-year-old woman with diabetes, hypertension and hypertriglyceri
We report a rare PPARG mutation, Y151C, which is located in the DBD of PPARG and leads to FPLD, and the preferred agent is PPARG agonists. We then summarized clinical phenotypic characteristics of FPLD3 caused by PPARG gene mutations, and clarified the relationship between different mutations of PPARG gene and the clinical manifestations of this type of FPLD. Additionally, current treatments for FPLD caused by PPARG mutations are reviewed.
Core Tip: This study reports a rare peroxisome proliferator-activated receptor gamma (PPARG) mutation (Y151C) in a 31-year-old woman with familial partial lipodystrophy type 3 (FPLD3), characterized by adipose tissue loss and metabolic complications. The mutation was identified in the PPARG DNA-binding domain. Pioglitazone, a PPARG agonist, effectively improved the patient’s glycemic and blood pressure control. This highlights the importance of genetic testing in FPLD3 diagnosis and the potential of PPARG agonists in managing metabolic complications.
- Citation: Wu CJ, Liu H, Tu LJ, Hu JY. Peroxisome proliferator-activated receptor gamma mutation in familial partial lipodystrophy type three: A case report and review of literature. World J Diabetes 2024; 15(12): 2360-2369
- URL: https://www.wjgnet.com/1948-9358/full/v15/i12/2360.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i12.2360
Lipodystrophy syndrome is a rare congenital or acquired disease that results in partial or systemic loss of adipose tissue[1]. The condition could be a congenital manifestation of genetic mutations or acquired as a result of autoimmune di
FPLD comprises a set of autosomal dominant inherited diseases that feature a reduction of fat in the arms, legs, and glutes[7]. Patients with varying clinical phenotypic features have led to the classification of FPLD into several subtypes, namely FPLD1 through FPLD6[1]. The classification of FPL into different types is primarily attributed to its genetic he
A 31-year-old woman was referred to our hospital in November 2017 due to polydipsia, polyuria, and more food and weight loss for 2 years.
Polydipsia, polyuria, and more food and weight loss for 2 years.
As to her family medical history, her father and grandmother was diagnosed with “Diabetes, hypertension and hypertriglyceridemia”. The family contained 3 generations of diabetics. A total of 5 peoples have been diagnosed with diabetes or impaired glucose tolerance.
She was 162 cm tall, weighed 53 kg, and the body mass index (BMI) was 20.19 kg/m2. Her blood pressure was 160/118 mmHg.
Liver function: Total bilirubin: 25.20 mmol/L; Direct bilirubin: 6.38 mmol/L; Indirect bilirubin: 18.60 μmol/L; Triglyceride (TG): 4.00 mmol/L; Total cholesterol: 3.85 mmol/L; Low-density lipoprotein cholesterol: 2.57 mmol/L; High-density lipoprotein-cholesterol: 0.61 mmol/L; Fasting blood glucose: 9.95 mmol/L; Glycosylated hemoglobin (HbA1c): 10.20%. Insulin release test and C-peptide release test: Pre-meal insulin: 16.47 μIU/mL; 1 hour post-meal insulin: 30.15 μIU/mL; 2 hours post-meal insulin: 37.30 μIU/mL; 3 hours post-meal insulin: 27.24 μIU/mL; pre-meal C-peptide: 1.30 ng/mL; 1 hour post-meal C-peptide: 2.09 ng/mL; 2 hours post-meal: 3.27 ng/mL; 3 hours post-meal: 3.28 ng/mL. Diabetes autoantibodies (glutamate decarboxylase, insulin antibodies, and pancreatic islet cell antibodies) were negative. Ultrasound indicates abnormal cardiac diastolic function and fatty liver. The fundus examination indicates retinal detachment and bleeding, and the fundal vessels were unremarkable. Neuroelectromyography was normal. Renal function normal and 24 hours urinary protein is 65 mg/24 hours.
No imaging examinations were performed.
FPLD3.
The patient was given insulin, fenofibrate, irbesartan and levamlodipine besylate (Table 1) with diet and exercise therapy.
| Before pioglitazone treatment | After pioglitazone treatment |
| Insulin glargine injection, 12 μL, QD | Pioglitazone, 30 mg, QD |
| Recombinant lispro insulin injection, 10 μL, TID | Dapagliflozin, 10 mg, QD |
| Fenofibrate, 200 mg, QD | Fenofibrate, 200 mg, QD |
| Irbesartan, 300 mg/day, QD | Irbesartan, 150 mg, QD |
| Levamlodipine besylate, 2.5 mg/day, QD |
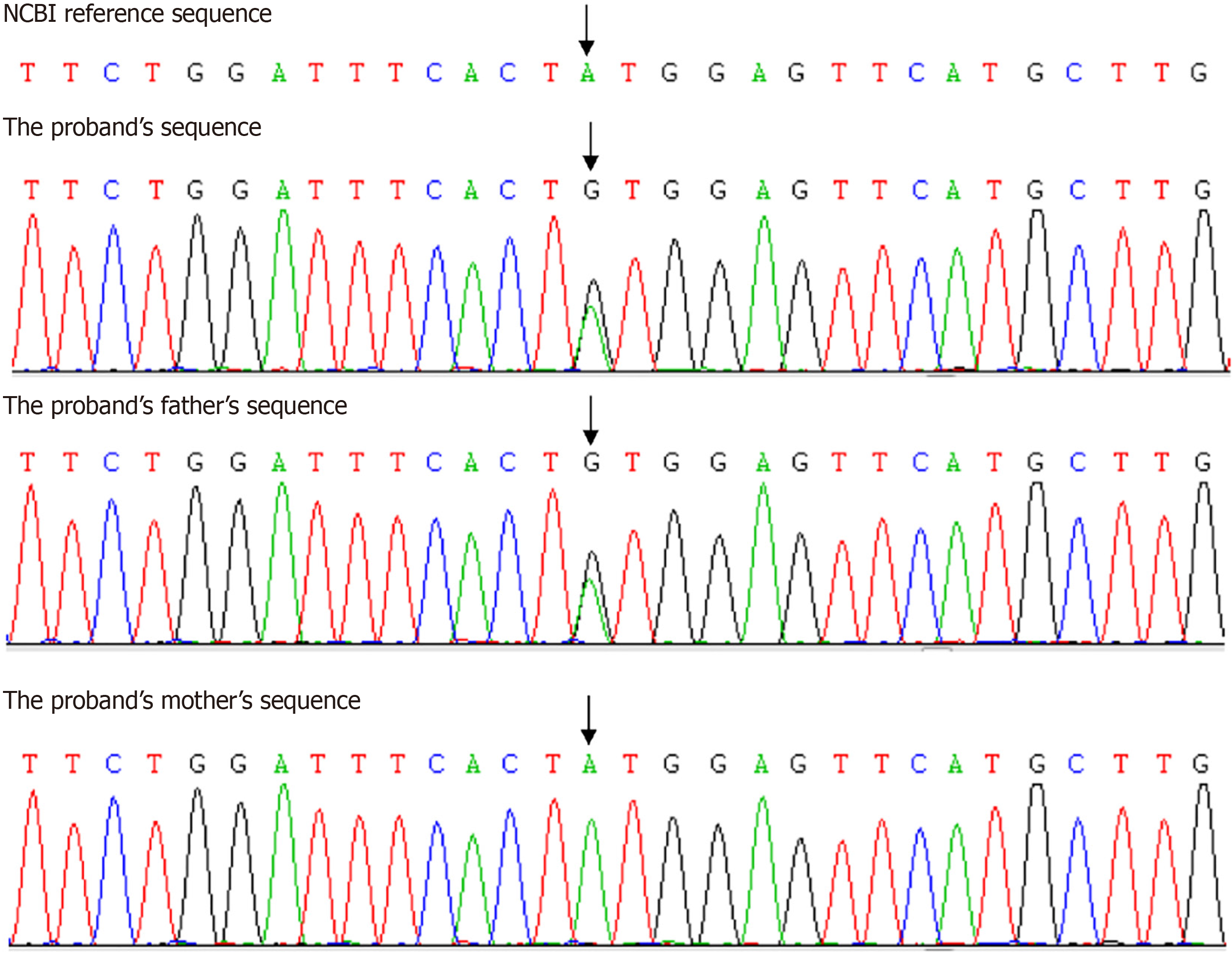
Considering that the patient is relatively young and exhibits multiple symptoms of metabolic disorders, as well as having a family history of three generations with consecutive occurrences, the patient and her parents underwent genetic testing. Whole exome sequencing revealed a heterozygous pathogenic mutation Y151C (c.452A > c.452G) in exon 3 of the PPARG gene in the proband and her father, which led to the diagnosis of FPLD. Sanger sequencing had not found the same mutation in her mother (Figure 1). The patient was given pioglitazone after being diagnosed with FPLD, with significant reductions in the types and dosages of other medications used (Table 1). One year later, she was 73 kg with BMI 26.30 kg/m2. Fasting blood glucose was 7.2 mmol/L and HbA1c was 6.8%, blood pressure was 160/118 mmHg, TG was 4.00 mmol/L.
Referencing the previous text, it is believed that FPL of the third subtype [FPLD3; online mendelian inheritance in man (OMIM) 604367], an autosomal dominant genetic condition, arises from a prevailing non-functional mutation within the PPARG gene[11]. People affected by FPLD3 possess and retain a normal fat distribution pattern during their early childhood; however, as they enter their teen years, they undergo a decrease in the fatty layer beneath the skin in their arms and legs, as well as in specific areas of their torso, creating the appearance of enhanced muscular development. Typically, people exhibit a shortage of subcutaneous fat in their arms and legs as well as their glutes, but they preserve both subcutaneous and visceral fat in the region of their midsection. Furthermore, fat irregularly accumulates in the liver and muscles, giving rise to numerous metabolic disorders including insulin resistance, non-alcoholic fatty liver disease, abnormal blood lipid levels, and type 2 diabetes mellitus, among other conditions[1]. Furthermore, FPLD3 tends to impact female patients with greater frequency and severity compared to male counterparts. Affected females frequently develop features of polycystic ovarian syndrome including polycystic ovaries, hirsutism, and oligomenorrhea[12]. Early onset of cardiovascular disease has also been reported[5].
PPARG, a member of the family of peroxisome proliferator-activated receptors which also includes PPAR-α, PPAR-β, and PPAR-δ among others, is a type of PPAR. PPARG belongs to the vast family of nuclear hormone receptors[13], which includes 48 transcription factors specific to humans. Steroids, thyroid hormones, vitamins, lipid metabolites, and all obiotin exert control by specifically attaching themselves, thereby regulating these elements[14,15]. Activators of peroxisome proliferators attach themselves to compounds that stimulate the multiplication of peroxisomes[16]. The cellular peroxidase organelle facilitates the oxidative breakdown of fatty acids (OMIM 601487), while PPARG, which is produced by the PPARG gene, plays a role in managing fat synthesis and the preservation of adipose tissue[13]. The primary expression of this is observed in fat tissue, particularly within the cells of both white and brown fat[17-20]. The expression of PPARG is more pronounced in monocytes (or macrophages) and colonic regions of adipose tissue, whereas it is less abundant in various additional tissues. The mechanism by which it governs the transcription of specific genes is triggered by a variety of endogenous lipid compounds. Nonetheless, a definitive principal natural ligand remains to be distinctly recognized[15].
PPARG is comprised of four distinct regions with specific roles: These include the activation function 1 domain, DNA binding domain, hinge domain, and ligand binding domain[21]. PPARG1 and PPARG2, which are subtypes of PPARG, exhibit variations at their N-termini; specifically, PPARG2 possesses an additional 30 amino acids compared to PPARG1. These subtypes are derived from different transcription initiation sites; the PPARG gene has nine exons, eight of which encode PPARG1 and seven of which encode PPARG2. G1 and G2 are mainly expressed in adipocytes, while PPAR-G1 is also expressed at lower levels in other tissues, including macrophages, liver, brain, and muscle[22].
PPARG is crucial for the differentiation of adipocytes, metabolic processes, insulin responsiveness, and managing inflammation, due to its wide range of functions[23]. PPARG plays a pivotal role in preadipocyte differentiation into mature adipocytes by binding with retinoid X receptor, a member of the nuclear receptor family, to form a heterodimer, which regulates adipocyte differentiation, lipid storage, and release[15,24,25]. PPARG, an important modulator of adipogenesis and adipose tissue homeostasis, exhibits high expression levels in both white and brown fat tissues, as well as in additional tissue types. It promotes fat production without enlarging fat cells; instead, it leads to an increase in the number of smaller cells. Furthermore, its function includes promoting the transformation of subcutaneous fat into visceral fat[1].
The PPARG gene is essential for the transformation and metabolic processes of fat cells, significantly affecting the clinical manifestations associated with atypical alterations in human fat tissue[26]. In this section, we will provide a brief introduction to the clinical phenotype of FPLD patients with PPARG gene mutations. Additionally, we will summarize the clinical phenotype of FPLD patients with mutations at different sites in the PPARG gene. By doing so, we hope to enhance our understanding of FPLD caused by PPARG mutation and facilitate clinical diagnosis and treatment.
Present-day scholarly articles suggest that divergent mutations at various locations within an identical gene may lead to unique clinical manifestations, a concept referred to as “phenotypic heterogeneity” in the field of genetics. We have compiled clinical phenotype information and PPARG mutation types for FPLD patients diagnosed with PPARG gene mutations, as shown in Table 2. We discovered that patients with FPLD caused by PPARG mutations may experience metabolic syndromes, including diabetes (or insulin resistance), hypertriglyceridemia, hypertension, and other car
| Domain | Gene mutation type | Manifestation | Ref. | |||
| Diabetes/insulin resistance | Hypertrigly-ceridemia | Hyper-tension | Others | |||
| LBD | H449L | Yes | Yes | PCOS, acanthosis nigricans, hepatocyte steatosis, no coronary arteries and other vascular diseases | Demir et al[34] | |
| P467L | Yes | Yes | Yes | Cirrhosis and liver cancer develop in the context of non-alcoholic steatohepatitis | Savage et al[24]; Barroso et al[35] | |
| V290M | Yes | Yes | Yes | Primary amenorrhea, hypertrichosis, acanthosis nigricans | Savage et al[24]; Barroso et al[35] | |
| F310S | Yes | Yes | Fatty liver, liver dysfunction, albuminuria and diabetic peripheral neuropathy | Chen et al[36] | ||
| F162C | Yes | Yes | Hepatomegaly, metabolic steatohepatitis | Melzer et al[38] | ||
| R425C | Yes | Yes | Lipodystrophy of the extremities and face, hirsutism | Agarwal and Garg[39] | ||
| Q157G | Yes | Yes | Yes | Polycystic ovarian syndrome, premature menopause at the age of 38, underwent hysterectomy due to fibroids at the age of 54, osteoporosis at the age of 58 | Lambadiari et al[42] | |
| A261E | Yes | Yes | PCOS, lipodystrophy of the extremities | Agostini et al[16] | ||
| R308P | Yes | Yes | PCOS, Nonalcoholic fatty liver disease | Agostini et al[16]; Majithia et al[50] | ||
| K347T | Yes | Yes | Yes | Lipoatrophy of the limbs, elevated creatine kinase levels, recurrent pancreatitis | Miehle et al[51] | |
| E352Q | Yes | Yes | Low level of plasma leptin, hepatic steatosis, whereas funduscopy was normal | Castell et al[52] | ||
| Y355X | Yes | Yes | Yes | Steatosis hepatis, Family history of similar physical appearance, acute pancreatitis | Francis et al[53] | |
| T356R | Yes | Yes | Non-alcoholic fatty liver, dyslipidemia, and low serum adiponectin levels | Majithia et al[50] | ||
| R385X | Yes | Yes | Yes | Acanthosis nigricans, lipodystrophy affecting gluteal, pancreatitis, cutaneous eruptive xanthomata | Agostini et al[54] | |
| P387S | Yes | Yes | Severe insulin resistance, non-alcoholic fatty liver, dyslipidemia, and low serum adiponectin levels | Majithia et al[50] | ||
| F388L | Yes | Yes | Polycystic ovarian disease, atrophy of gluteal fat | Hegele et al[11] | ||
| A417V | Yes | Yes | Severe insulin resistance, non-alcoholic fatty liver, dyslipidemia, and low serum adiponectin levels | Majithia et al[50] | ||
| D424N | Yes | Yes | Muscular hypertrophy was observed on the legs, slight acanthosis nigricans was present on the neck, axillae and inguinal folds, loss of subcutaneous fat from the arms, accumulation of subcutaneous fat was present in face, chin, trunk and abdomen, ovarian cyst | Lüdtke et al[55] | ||
| L451P | Yes | Yes | Hirsutism, marked loss of subcutaneous fat from the extremities but truncal fat was slightly increased | Broekema et al[56] | ||
| H477L | Yes | Yes | Trunk-sparing lipodystrophy, acanthosis nigricans, multiple acrochordons, an excess of subcutaneous adipose tissue in the abdomen with a decreased amount of subcutaneous adipose tissue in the patient’s lower extremities | Akinci et al[57] | ||
| P495L | Yes | Yes | Severe insulin resistance, non-alcoholic fatty liver, dyslipidemia, and low serum adiponectin levels | Barroso et al[35] | ||
| DBD | Y151C | Yes | Yes | Yes | Pancreatitis, cardiovascular disease, no acanthosis nigricans or hirsutism | Visser et al[37] |
| FS138X | Yes | Bilateral cataracts, bilateral hearing impairment and peripheral neuropathy | Hegele et al[58] | |||
| C114R | Yes | Yes | Yes | PCOS, hepatic steatosis | Agostini et al[54] | |
| E157D | Yes | Yes | Hepatic steatosis, acanthosis nigricans, hirsutism, PCOS | Campeau et al[59] | ||
| C131Y | Yes | Yes | Yes | PCOS, hepatic steatosis | Agostini et al[54] | |
| G161V | Yes | Yes | Yes | Bilateral fallopian tube obstruction, eruptive xanthoma, type V dyslipidemia, pancreatitis, severe hepatic steatosis | Lau et al[26] | |
| R165T | Yes | Yes | Yes | Peripheral lipoatrophy, muscular hypertrophy, liver steatosis | Auclair et al[60] | |
| FS186X | Yes | Yes | Yes | Acanthosis nigricans | Savage et al[61] | |
| C162W | Yes | Yes | Yes | PCOS, hepatic steatosis | Agostini et al[54] | |
| R194W | Yes | Yes | Yes | PCOS, acanthosis nigricans | Monajemi et al[62] | |
Based on current reports, treatments for lipodystrophy are limited to preventing and improving patients’ metabolic complications and cannot achieve a complete cure or radical treatment. Consequently, this part will scrutinize contemporary studies addressing FPLD therapies linked to PPARG mutations, with a focus on three key domains: Overall management strategies, interventions for insulin resistance and diabetes mellitus, and remedies for lipodystrophy.
Dietary control and moderate exercise are the cornerstones of the treatment of all types of lipodystrophy, as this is not only beneficial for lipodystrophy itself, but also helps to ameliorate metabolic disorders such as diabetes mellitus and hypertriglyceridemia, as well as to reduce the risk of coronary atherosclerotic heart disease[6]. Individuals undergoing treatment are advised to adhere to a nutritional regimen comprising 50%-60% carbs, 20%-30% fats, and roughly 20% protein[6,32]. Since some patients with FPLD have hyperphagia due to reduced leptin levels[7], they should be advised to adopt an energy-restricted diet, as overeating is likely to precipitate or exacerbate their metabolic complications and lead to disease progression. In the absence of specific contraindications, most patients should engage in physical activity, but those with cardiac disease should undergo a cardiac evaluation prior to initiating exercise therapy while avoiding strenuous exercise[6].
As with all patients with type 2 diabetes mellitus, improvement of glycemia and insulin resistance will have multiple beneficial metabolic effects, including improved TG levels[33]. Metformin and thiazolidinediones (TZDs) have shown efficacy in the management of diabetes in patients with lipodystrophies[34-39]. An open label study of troglitazone, a TZD, on patients with lipodystrophy showed an improvement in metabolic profile, including HbA1c and fasting TG[39]. Troglitazone is no longer available due to hepatic toxicity[40,41], and evidence is limited for other TZDs. In our case, pioglitazone significantly improved insulin resistance, reduced insulin dosage, and helped with blood pressure and serum lipids. Some case studies using pioglitazone in FPLD patients have shown improvement in dysglycemia and dyslipidemia[36-38]. However, pioglitazone has been associated with an increased incidence of heart failure and fluid retention, so it is generally used with caution[42]. Insulin is also used in the management of diabetes, although increased doses and concentrated preparations of insulin may be required in some lipodystrophy patients with severe insulin resistance[43]. Additional treatments for high blood sugar, such as inhibitors of sodium-glucose cotransporter 2 and agonists for glucagon-like peptide-1 receptors, are utilized for lipodystrophy conditions. However, clinical trials targeting these groups are lacking, even though some animal research and individual case studies suggest they may be effective[44-46].
Metreleptin, a recombinant human leptin analogue, represents leptin replacement therapy and is currently the only effective therapeutic agent for lipodystrophy. The regulatory authorities in the United States, European Union, and Japan have sanctioned the use of metreleptin as a therapeutic option for lipodystrophy[47]. Of note, metreleptin is not currently food and drug administration-approved for use in patients with FPLD and is only available through trials, compassionate use, or special access programs or, to a lesser extent, in Japan[6]. Practice recommendations from various medical societies propose that for patients with partial lipodystrophy who demonstrate leptin deficiency (leptin < 4 ng/mL) and significant metabolic complications (HbA1c > 8% and/or TG > 500mg/dL), Metreleptin could be considered a viable treatment alternative[6]. Based on the lastest reports, the efficacy of metriptyline in patients with lipodystrophy in the pathogenic variant of PPARG, mainly in terms of improvement in serum TG and glycemic parameters (e.g., HbA1c), is controversial, as the improvement in hypertriglyceridemia was not significant in some reported cases[33,48,49].
We reported a rare case of FPLD3 caused by a rare PPARG mutation Y151C. In addition, we reviewed the current un
| 1. | Bagias C, Xiarchou A, Bargiota A, Tigas S. Familial Partial Lipodystrophy (FPLD): Recent Insights. Diabetes Metab Syndr Obes. 2020;13:1531-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 2. | Tsoukas MA, Farr OM, Mantzoros CS. Leptin in congenital and HIV-associated lipodystrophy. Metabolism. 2015;64:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 3. | Non LR, Escota GV, Powderly WG. HIV and its relationship to insulin resistance and lipid abnormalities. Transl Res. 2017;183:41-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Alves MD, Brites C, Sprinz E. HIV-associated lipodystrophy: a review from a Brazilian perspective. Ther Clin Risk Manag. 2014;10:559-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 5. | Knebel B, Müller-Wieland D, Kotzka J. Lipodystrophies-Disorders of the Fatty Tissue. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J Clin Endocrinol Metab. 2016;101:4500-4511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 358] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 7. | Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 615] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Hussain I, Garg A. Lipodystrophy Syndromes. Endocrinol Metab Clin North Am. 2016;45:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Melvin A, Stears A, Savage DB. Recent developments in lipodystrophy. Curr Opin Lipidol. 2019;30:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 10. | Akinci B, Gular MC, Oral EA. Lipodystrophy Syndromes: Presentation and Treatment. 2024 Aug 21. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 11. | Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51:3586-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Broekema MF, Savage DB, Monajemi H, Kalkhoven E. Gene-gene and gene-environment interactions in lipodystrophy: Lessons learned from natural PPARγ mutants. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:715-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Mirza AZ, Althagafi II, Shamshad H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur J Med Chem. 2019;166:502-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 408] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 14. | Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1519] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 15. | Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1167] [Article Influence: 58.4] [Reference Citation Analysis (1)] |
| 16. | Agostini M, Schoenmakers E, Beig J, Fairall L, Szatmari I, Rajanayagam O, Muskett FW, Adams C, Marais AD, O'Rahilly S, Semple RK, Nagy L, Majithia AR, Schwabe JWR, Blom DJ, Murphy R, Chatterjee K, Savage DB. A Pharmacogenetic Approach to the Treatment of Patients With PPARG Mutations. Diabetes. 2018;67:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 752] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 18. | Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1711] [Cited by in RCA: 1738] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 19. | Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 569] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Sears IB, MacGinnitie MA, Kovacs LG, Graves RA. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 1996;16:3410-3419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 223] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Gong S, Han X, Li M, Cai X, Liu W, Luo Y, Zhang SM, Zhou L, Ma Y, Huang X, Li Y, Zhou X, Zhu Y, Wang Q, Chen L, Ren Q, Zhang P, Ji L. Genetics and Clinical Characteristics of PPARγ Variant-Induced Diabetes in a Chinese Han Population. Front Endocrinol (Lausanne). 2021;12:677130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Hu W, Jiang C, Kim M, Xiao Y, Richter HJ, Guan D, Zhu K, Krusen BM, Roberts AN, Miller J, Steger DJ, Lazar MA. Isoform-specific functions of PPARγ in gene regulation and metabolism. Genes Dev. 2022;36:300-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 23. | Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1230] [Article Influence: 55.9] [Reference Citation Analysis (1)] |
| 24. | Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, Williams RL, Umpleby AM, Thomas EL, Bell JD, Dixon AK, Dunne F, Boiani R, Cinti S, Vidal-Puig A, Karpe F, Chatterjee VK, O'Rahilly S. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 314] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731-37734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 906] [Article Influence: 36.2] [Reference Citation Analysis (1)] |
| 26. | Lau E, Carvalho D, Oliveira J, Fernandes S, Freitas P. Familial partial lipodystrophy type 3: a new mutation on the PPARG gene. Hormones (Athens). 2015;14:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2180] [Cited by in RCA: 2068] [Article Influence: 108.8] [Reference Citation Analysis (1)] |
| 28. | Guo WL, Tang Y, Han XY, Ji LN. [Meta-analysis of the association of Pro12Ala polymorphism of peroxisome proliferator activated receptor gamma gene with type 2 diabetes in Chinese Han population]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011;33:593-599. [PubMed] |
| 29. | Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1451] [Cited by in RCA: 1679] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 30. | Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 351] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2717] [Cited by in RCA: 2818] [Article Influence: 88.1] [Reference Citation Analysis (7)] |
| 32. | Araújo-Vilar D, Santini F. Diagnosis and treatment of lipodystrophy: a step-by-step approach. J Endocrinol Invest. 2019;42:61-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 33. | Arioglu E, Duncan-Morin J, Sebring N, Rother KI, Gottlieb N, Lieberman J, Herion D, Kleiner DE, Reynolds J, Premkumar A, Sumner AE, Hoofnagle J, Reitman ML, Taylor SI. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 202] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Demir T, Onay H, Savage DB, Temeloglu E, Uzum AK, Kadioglu P, Altay C, Ozen S, Demir L, Cavdar U, Akinci B. Familial partial lipodystrophy linked to a novel peroxisome proliferator activator receptor -γ (PPARG) mutation, H449L: a comparison of people with this mutation and those with classic codon 482 Lamin A/C (LMNA) mutations. Diabet Med. 2016;33:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 976] [Article Influence: 36.1] [Reference Citation Analysis (1)] |
| 36. | Chen X, Ma Z, Chen P, Song X, Li W, Yu X, Xie J. Case Report: A New Peroxisome Proliferator-Activated Receptor Gamma Mutation Causes Familial Partial Lipodystrophy Type 3 in a Chinese Patient. Front Endocrinol (Lausanne). 2022;13:830708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Visser ME, Kropman E, Kranendonk ME, Koppen A, Hamers N, Stroes ES, Kalkhoven E, Monajemi H. Characterisation of non-obese diabetic patients with marked insulin resistance identifies a novel familial partial lipodystrophy-associated PPARγ mutation (Y151C). Diabetologia. 2011;54:1639-1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Melzer F, Geisler C, Schulte DM, Laudes M. Rapid response to leptin therapy in a FPLD patient with a novel PPARG missense variant. Endocrinol Diabetes Metab Case Rep. 2021;2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 39. | Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am J Gastroenterol. 2000;95:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Chojkier M. Troglitazone and liver injury: in search of answers. Hepatology. 2005;41:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 42. | Lambadiari V, Kountouri A, Maratou E, Liatis S, Dimitriadis GD, Karpe F. Case Report: Metreleptin Treatment in a Patient With a Novel Mutation for Familial Partial Lipodystrophy Type 3, Presenting With Uncontrolled Diabetes and Insulin Resistance. Front Endocrinol (Lausanne). 2021;12:684182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 43. | Jeru I, Vatier C, Vantyghem MC, Lascols O, Vigouroux C. LMNA-associated partial lipodystrophy: anticipation of metabolic complications. J Med Genet. 2017;54:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 44. | Luedtke A, Boschmann M, Colpe C, Engeli S, Adams F, Birkenfeld AL, Haufe S, Rahn G, Luft FC, Schmidt HH, Jordan J. Thiazolidinedione response in familial lipodystrophy patients with LMNA mutations: a case series. Horm Metab Res. 2012;44:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Iwanishi M, Ebihara K, Kusakabe T, Chen W, Ito J, Masuzaki H, Hosoda K, Nakao K. Clinical characteristics and efficacy of pioglitazone in a Japanese diabetic patient with an unusual type of familial partial lipodystrophy. Metabolism. 2009;58:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Sleilati GG, Leff T, Bonnett JW, Hegele RA. Efficacy and safety of pioglitazone in treatment of a patient with an atypical partial lipodystrophy syndrome. Endocr Pract. 2007;13:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Moreau F, Boullu-Sanchis S, Vigouroux C, Lucescu C, Lascols O, Sapin R, Ruimy D, Guerci B, Pinget M, Jeandidier N. Efficacy of pioglitazone in familial partial lipodystrophy of the Dunnigan type: a case report. Diabetes Metab. 2007;33:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Singh S, Loke YK, Furberg CD. Thiazolidinediones and heart failure: a teleo-analysis. Diabetes Care. 2007;30:2148-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 171] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Lane WS, Cochran EK, Jackson JA, Scism-Bacon JL, Corey IB, Hirsch IB, Skyler JS. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract. 2009;15:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Majithia AR, Tsuda B, Agostini M, Gnanapradeepan K, Rice R, Peloso G, Patel KA, Zhang X, Broekema MF, Patterson N, Duby M, Sharpe T, Kalkhoven E, Rosen ED, Barroso I, Ellard S; UK Monogenic Diabetes Consortium, Kathiresan S; Myocardial Infarction Genetics Consortium, O'Rahilly S; UK Congenital Lipodystrophy Consortium, Chatterjee K, Florez JC, Mikkelsen T, Savage DB, Altshuler D. Prospective functional classification of all possible missense variants in PPARG. Nat Genet. 2016;48:1570-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 51. | Miehle K, Porrmann J, Mitter D, Stumvoll M, Glaser C, Fasshauer M, Hoffmann K. Novel peroxisome proliferator-activated receptor gamma mutation in a family with familial partial lipodystrophy type 3. Clin Endocrinol (Oxf). 2016;84:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Castell AL, Hiéronimus S, Lascols O, Fournier T, Fénichel P. Vascular placental abnormalities and newborn death in a pregnant diabetic woman with familial partial lipodystrophy type 3: a possible role for peroxisome proliferator-activated receptor γ. Diabetes Metab. 2012;38:367-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Francis GA, Li G, Casey R, Wang J, Cao H, Leff T, Hegele RA. Peroxisomal proliferator activated receptor-gamma deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3). BMC Med Genet. 2006;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 54. | Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, Rajanayagam O, Semple R, Luan J, Bath L, Zalin A, Labib M, Kumar S, Simpson H, Blom D, Marais D, Schwabe J, Barroso I, Trembath R, Wareham N, Nagy L, Gurnell M, O'Rahilly S, Chatterjee K. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 55. | Lüdtke A, Buettner J, Schmidt HH, Worman HJ. New PPARG mutation leads to lipodystrophy and loss of protein function that is partially restored by a synthetic ligand. J Med Genet. 2007;44:e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Broekema MF, Massink MPG, Donato C, de Ligt J, Schaarschmidt J, Borgman A, Schooneman MG, Melchers D, Gerding MN, Houtman R, Bonvin AMJJ, Majithia AR, Monajemi H, van Haaften GW, Soeters MR, Kalkhoven E. Natural helix 9 mutants of PPARγ differently affect its transcriptional activity. Mol Metab. 2019;20:115-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Akinci B, Onay H, Demir T, Ozen S, Kayserili H, Akinci G, Nur B, Tuysuz B, Nuri Ozbek M, Gungor A, Yildirim Simsir I, Altay C, Demir L, Simsek E, Atmaca M, Topaloglu H, Bilen H, Atmaca H, Atik T, Cavdar U, Altunoglu U, Aslanger A, Mihci E, Secil M, Saygili F, Comlekci A, Garg A. Natural History of Congenital Generalized Lipodystrophy: A Nationwide Study From Turkey. J Clin Endocrinol Metab. 2016;101:2759-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Hegele RA, Ur E, Ransom TP, Cao H. A frameshift mutation in peroxisome-proliferator-activated receptor-gamma in familial partial lipodystrophy subtype 3 (FPLD3; MIM 604367). Clin Genet. 2006;70:360-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Campeau PM, Astapova O, Martins R, Bergeron J, Couture P, Hegele RA, Leff T, Gagné C. Clinical and molecular characterization of a severe form of partial lipodystrophy expanding the phenotype of PPARγ deficiency. J Lipid Res. 2012;53:1968-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Auclair M, Vigouroux C, Boccara F, Capel E, Vigeral C, Guerci B, Lascols O, Capeau J, Caron-Debarle M. Peroxisome proliferator-activated receptor-γ mutations responsible for lipodystrophy with severe hypertension activate the cellular renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2013;33:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Savage DB, Agostini M, Barroso I, Gurnell M, Luan J, Meirhaeghe A, Harding AH, Ihrke G, Rajanayagam O, Soos MA, George S, Berger D, Thomas EL, Bell JD, Meeran K, Ross RJ, Vidal-Puig A, Wareham NJ, O'Rahilly S, Chatterjee VK, Schafer AJ. Digenic inheritance of severe insulin resistance in a human pedigree. Nat Genet. 2002;31:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 62. | Monajemi H, Zhang L, Li G, Jeninga EH, Cao H, Maas M, Brouwer CB, Kalkhoven E, Stroes E, Hegele RA, Leff T. Familial partial lipodystrophy phenotype resulting from a single-base mutation in deoxyribonucleic acid-binding domain of peroxisome proliferator-activated receptor-gamma. J Clin Endocrinol Metab. 2007;92:1606-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/