Published online Nov 15, 2024. doi: 10.4239/wjd.v15.i11.2173
Revised: August 21, 2024
Accepted: September 13, 2024
Published online: November 15, 2024
Processing time: 86 Days and 22.7 Hours
This editorial summarizes the latest literature on the roles of neuronal PAS domain protein 2 and KN motif/ankyrin repeat domain 1 in type 2 diabetes (T2D). We highlight their involvement in β-cell dysfunction, explore their potential as therapeutic targets, and discuss the implications for new treatment strategies. We offer valuable insights into relevant gene regulation and cellular mechanisms relevant for the targeted management of T2D.
Core Tip: This editorial explores the pivotal roles of NPAS2 and KANK1 in type 2 diabetes (T2D). It elucidates their contribution to β-cell dysfunction, highlighting their potential for targeted therapies. Insights from this editorial may guide the development of innovative treatment approaches against T2D.
- Citation: Cheng CH, Hao WR, Cheng TH. Targeting neuronal PAS domain protein 2 and KN motif/ankyrin repeat domains 1: Advances in type 2 diabetes therapy. World J Diabetes 2024; 15(11): 2173-2176
- URL: https://www.wjgnet.com/1948-9358/full/v15/i11/2173.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i11.2173
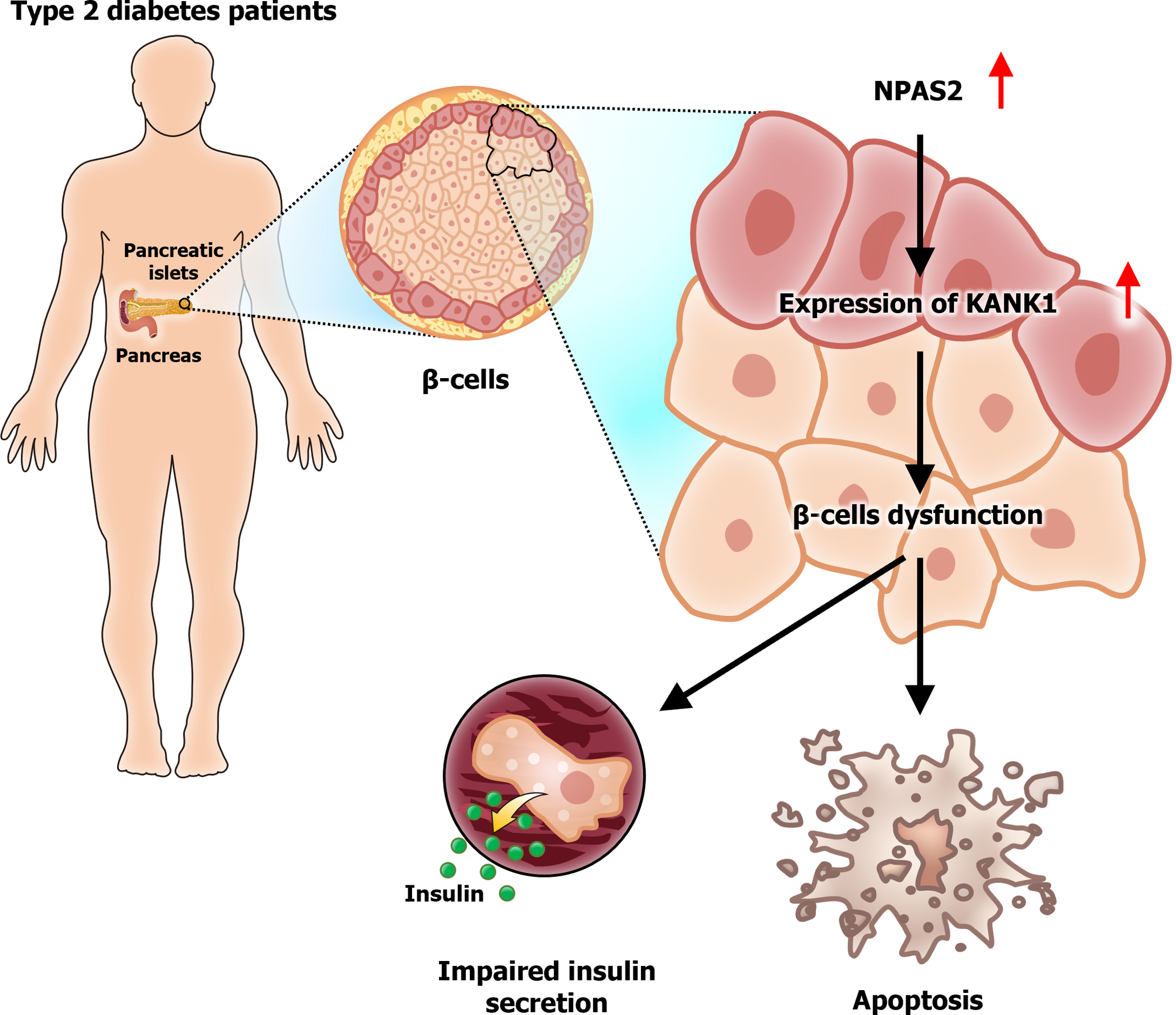
The ongoing fight against type 2 diabetes (T2D) has recently gained momentum with discoveries about the genetic and molecular underpinnings of this widespread disease. A pivotal study by Yin et al[1] in the World Journal of Diabetes has highlighted the significance of the transcription factor NPAS2 (neuronal PAS domain protein 2) and its downstream target KANK1 (KN motif and ankyrin repeat domain 1) in the dysfunction of pancreatic β-cells, a key event in the development of T2D. Since β-cell dysfunction is central to T2D pathogenesis, a deeper understanding of these regulatory mechanisms is crucial for advancing therapeutic approaches. NPAS2 is a component of the circadian clock system, influencing various physiological processes, including glucose metabolism[2]. Its role in β-cell function and insulin secretion has opened new avenues for diabetes research. The study by Yin et al. demonstrates how NPAS2 modulates KANK1 expression, thereby affecting the structural and functional integrity of β-cells. KANK1 is crucial for maintaining cell adhesion and cytoskeletal dynamics, both essential for β-cell function[3]. The interaction between KANK1 and focal adhesion proteins influences the stability and organization of microtubules and actin filaments, thereby affecting insulin secretion[4]. Changes in KANK1 expression can disrupt these processes, causing β-cell malfunction and accelerating T2D development. This editorial contextualizes these findings within the broader scope of T2D research. By exploring the complex pathways involving NPAS2 and KANK1, we can better understand their implications for new T2D therapies. The study by Yin et al[1] underscores the need for probing these molecular pathways to preserve β-cell function and improve T2D outcomes.
Yin et al[1] highlighted the vital role of NPAS2 in the dysfunction of β-cells in T2D. The expression of NPAS2 was upregulated in the islet β-cells of mice with T2D. Upregulation of NPAS2 regulated the expression of KANK1, a gene essential for cellular structure and function. Yin et al[1] demonstrated that NPAS2 overexpression was correlated with elevated KANK1 levels, contributing to β-cell apoptosis and impaired insulin secretion. These findings suggest that NPAS2 is a central regulator of β-cell health, and its dysregulation can drive the progression of T2D[1]. KANK1 is involved in several cellular processes, including protein binding, mechanical force sensing, and phase separation, that are crucial for maintaining cellular integrity and function[3]. Specifically, KANK1 shapes focal adhesions, which are essential for cell signaling and structural support[4]. In addition, these adhesions are integral to β-cell function because they form “secreting adhesions,” which are essential for insulin secretion[5]. The interactions between KANK1 and other cellular components, such as cortical complexes regulating insulin secretion, underscore its importance in maintaining the functionality of β-cells[6]. Dysregulation of these interactions due to NPAS2 overexpression disrupts the delicate balance needed for effective insulin secretion, thus promoting β-cell dysfunction in T2D. The findings of Yin et al[1] are supported by structural studies on KANK1, which revealed its role in coordinating the actin and microtubule cytoskeletons at focal adhesions; this coordination is crucial for maintaining cellular architecture and function[7]. Disruptions in these structures due to NPAS2-induced overexpression of KANK1 lead to cellular dysfunction, facilitating the development of T2D. In summary, NPAS2 serves as a major regulator of β-cell health by modulating KANK1 expression. The upregulation of NPAS2 expression in T2D upregulates the expression of KANK1, disrupting cellular architecture and function and thus causing β-cell apoptosis and impaired insulin secretion. These insights deepen our understanding of the molecular mechanisms underlying β-cell dysfunction in T2D and highlight the potential of NPAS2 and KANK1 as therapeutic targets against T2D progression.
KANK1, a downstream target of NPAS2, contributes to β-cell dysfunction in T2D (Figure 1). Evidence suggests a significant correlation between NPAS2 expression and KANK1 expression in T2D, highlighting a potential pathway for therapeutic intervention[1]. Specifically, KANK1 regulates cellular adhesion and structure, which are essential for maintaining β-cell function and insulin secretion[3]. Knocking down NPAS2 and KANK1 in cell models increased the proliferation of β-cells, implying that downregulating the expression of these genes can help preserve the function of β-cells in patients with T2D[4,5]. Therefore, the NPAS2-KANK1 pathway may be targeted to treat T2D by enhancing β-cell survival and function. KANK1 is essential for structural interactions occurring at focal adhesions; it coordinates the actin and microtubule cytoskeletons, which are vital for β-cell adhesion and insulin secretion[6,8]. These findings highlight the importance of KANK1 in β-cell physiology and underscore its potential as a therapeutic target in T2D management. The ability of KANK1 to influence cellular structures further emphasizes its role in the pathophysiology of T2D and its potential for use in T2D therapies aimed at modulating the expression or function of this protein[7,9].
The discovery of the NPAS2–KANK1 pathway mediating β-cell dysfunction has opened promising new avenues for therapeutic interventions against T2D. Novel T2D therapies can be developed by targeting the molecular mechanisms involving NPAS2 and KANK1 to preserve β-cell function and enhance insulin secretion-factors crucial to managing glycemic control. Yin et al[1] highlighted the feasibility of this approach by demonstrating that knocking down NPAS2 and KANK1 in cell models increased β-cell proliferation-a viable strategy for combating β-cell dysfunction[1]. KANK1 plays a key structural role in coordinating the actin and microtubule cytoskeletons at focal adhesions; this role is essential for maintaining cell integrity and function[3,4]. The aforementioned coordination is crucial for β-cell function because it helps sustain the structural and mechanical properties necessary for insulin secretion. The interplay between cytoskeletal elements and focal adhesions, as described by Guo et al[3], highlights the feasibility of targeting these interactions to improve the resilience and functionality of β-cells. Notably, surgical interventions such as Roux-en-Y gastric bypass surgery have been demonstrated to downregulate the expression of NPAS2 and KANK1; therefore, surgical approaches may be explored to manage T2D by modulating the NPAS2-KANK1 pathway[1]. Pharmacological and surgical interventions may be combined to preserve β-cell health and improve T2D outcomes. The role of the NPAS2-KANK1 pathway in mediating the function of β-cells emphasizes its significance in the pathophysiology of T2D. Targeting this pathway through pharmacological or surgical approaches can optimize therapeutic outcomes by improving β-cell viability and insulin secretion. Future treatments may harness these insights to optimize glycemic control and overall care for patients with T2D.
Yin et al[1] clarified the critical molecular pathways underlying T2D by highlighting the roles of NPAS2 and KANK1 in β-cell dysfunction. The NPAS2-KANK1 pathway may serve as a promising therapeutic target for preserving β-cell function to optimize T2D management. Targeting this pathway can help maintain insulin secretion and glycemic control in patients with T2D. Understanding the structural and functional dynamics of KANK1 at focal adhesions can help researchers devise strategies for protecting β-cells from dysfunction[3,4]. The interaction between talin and KANK1 is essential for coordinating the actin and microtubule cytoskeletons at focal adhesions; this coordination is crucial for β-cell viability and insulin secretion[4,7]. These molecular connections should be leveraged to develop new therapeutic strategies aimed at enhancing the resilience of β-cells. Future studies should explore relevant genetic and molecular interactions, investigating how they can be modified to devise precise and effective therapies for T2D. Knowledge about the NPAS2-KANK1 pathway and its broader implications may guide the development of novel therapies for improving the quality of life of patients with T2D. Targeting this pathway can help preserve β-cell function and thus optimize T2D management[5,6].
| 1. | Yin YB, Ji W, Liu YL, Gao QH, He DD, Xu SL, Fan JX, Zhang LH. cNPAS2 induced β cell dysfunction by regulating KANK1 expression in type 2 diabetes. World Journal of Diabetes. 2024;15:1932-1941. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Karuga FF, Jaromirska J, Sochal M, Białasiewicz P, Gabryelska A. Association between glucose metabolism, the circadian cycle and hypoxia: Evaluation of the NPAS2 and Rev-Erb-α protein serum levels in obstructive sleep apnea patients - a pilot study. Dent Med Probl. 2024;61:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Guo K, Zhang J, Huang P, Xu Y, Pan W, Li K, Chen L, Luo L, Yu W, Chen S, He S, Wei Z, Yu C. KANK1 shapes focal adhesions by orchestrating protein binding, mechanical force sensing, and phase separation. Cell Rep. 2023;42:113321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 4. | Li X, Goult BT, Ballestrem C, Zacharchenko T. The structural basis of the talin-KANK1 interaction that coordinates the actin and microtubule cytoskeletons at focal adhesions. Open Biol. 2023;13:230058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Fye MA, Kaverina I. Insulin secretion hot spots in pancreatic β cells as secreting adhesions. Front Cell Dev Biol. 2023;11:1211482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Noordstra I, van den Berg CM, Boot FWJ, Katrukha EA, Yu KL, Tas RP, Portegies S, Viergever BJ, de Graaff E, Hoogenraad CC, de Koning EJP, Carlotti F, Kapitein LC, Akhmanova A. Organization and dynamics of the cortical complexes controlling insulin secretion in β-cells. J Cell Sci. 2022;135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Bouchet BP, Gough RE, Ammon YC, van de Willige D, Post H, Jacquemet G, Altelaar AM, Heck AJ, Goult BT, Akhmanova A. Talin-KANK1 interaction controls the recruitment of cortical microtubule stabilizing complexes to focal adhesions. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Pan W, Sun K, Tang K, Xiao Q, Ma C, Yu C, Wei Z. Structural insights into ankyrin repeat-mediated recognition of the kinesin motor protein KIF21A by KANK1, a scaffold protein in focal adhesion. J Biol Chem. 2018;293:1944-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Stubb A, Guzmán C, Närvä E, Aaron J, Chew TL, Saari M, Miihkinen M, Jacquemet G, Ivaska J. Superresolution architecture of cornerstone focal adhesions in human pluripotent stem cells. Nat Commun. 2019;10:4756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/