Published online Oct 15, 2024. doi: 10.4239/wjd.v15.i10.2147
Revised: August 16, 2024
Accepted: August 26, 2024
Published online: October 15, 2024
Processing time: 185 Days and 3.5 Hours
Interleukin-35 (IL-35) is a novel protein comprising IL-12α and IL-27β chains. The IL12A and EBI3 genes are responsible for its production. The study of IL-35 has experienced a substantial increase in interest in recent years, as demonstrated by many research papers. Recent clinical studies have shown that individuals who do not have a C-peptide have notably reduced amounts of IL-35 in their blood serum. This is accompanied by a drop in the percentage of IL-35+ Treg cells, regulatory B cells, and CD8+ FOXP3+ cells that produce IL-35. This article em-phasizes the potential significance of IL-35 expression in governing the immune response and its involvement in chronic inflammatory autoimmune diabetes in pancreatic inflammation. It demonstrates IL-35's ability to regulate cytokine proportions, modulate B cells, and protect against autoimmune diabetes. However, further investigation is necessary to ascertain the precise mechanism of IL-35, and meticulous planning is essential for clinical studies.
Core Tip: Studies suggest interleukin (IL)-35 protects against prediabetes and autoimmune diabetes by regulating immune system function. Development of type 1 diabetes (T1D) can be influenced by various cytokines produced by immune and pancreatic cells. Some cytokines, such as IL-10, transforming growth factor beta (TGF-β), IL-5, IL-4, IL-2, IL-15, IL-33, and IL-35, can stimulate regulatory cells in the immune system, releasing anti-inflammatory cytokines. Regulatory dendritic cells release IL-7, important for maintaining Tregs. In T1D, Tregs express IL-7Rα. Inhibiting TGF-β and activating IFN-γ can increase TC, Th1, and Th17 cells, while TGF-β can stimulate Runx1 expression to convert Th1 cells into Th17 cells.
- Citation: Chakraborty R, Mukherjee AK, Bala A. Interleukin-35: A key player managing pre-diabetes and chronic inflammatory type 1 autoimmune diabetes. World J Diabetes 2024; 15(10): 2147-2151
- URL: https://www.wjgnet.com/1948-9358/full/v15/i10/2147.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i10.2147
The study by Ping et al[1], published in 2024 in the World Journal of Diabetes, elucidates the etiology of prediabetes and its corresponding treatment medications. Nevertheless, the role of interleukin (IL)-35 in regulating the progression of prediabetes has not been investigated, which deserves due attention.
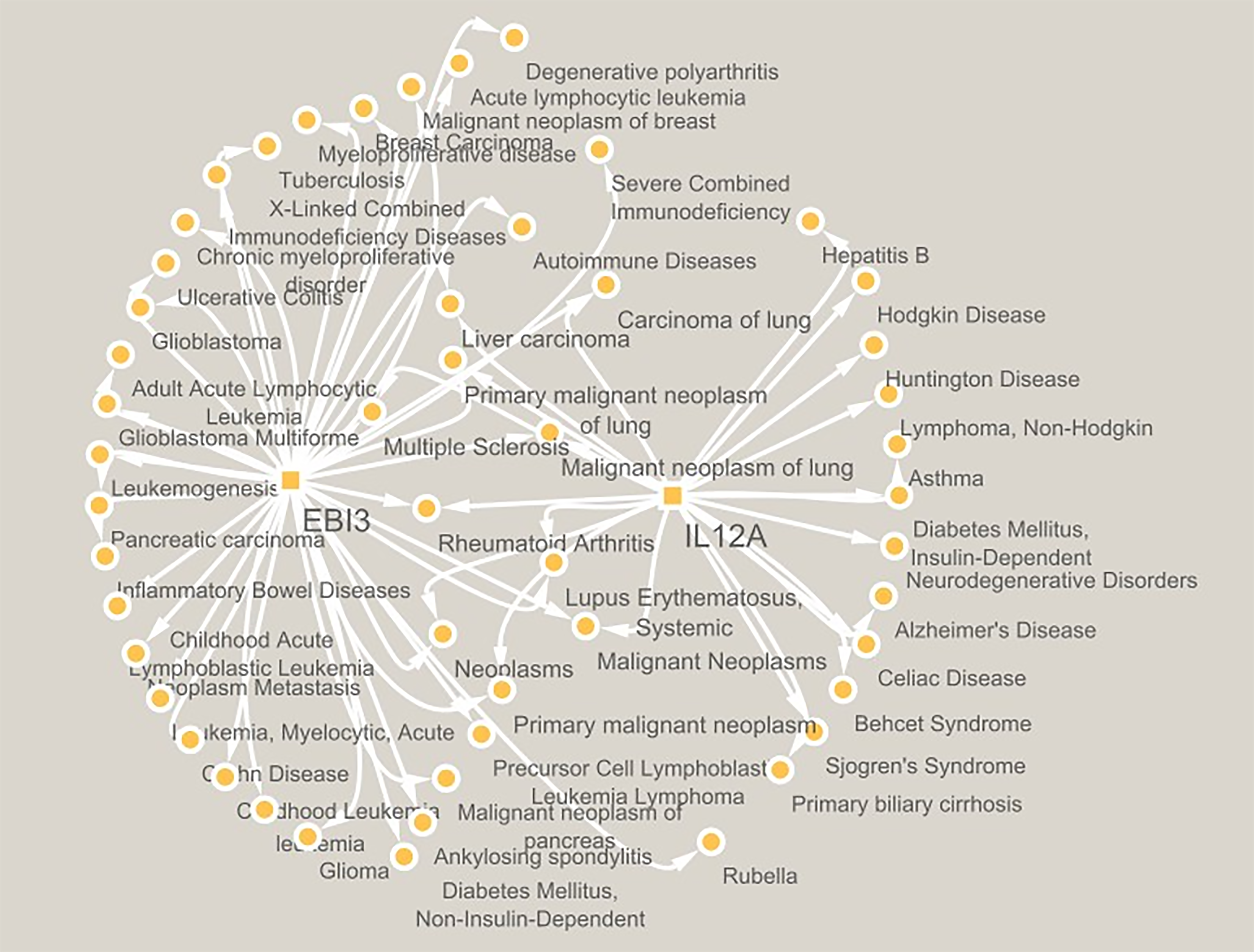
IL-35 has garnered considerable interest in recent years as a potential pivotal controller of diabetes, namely in prediabetes and chronic inflammatory autoimmune diabetes, which are progressively impacting children and adolescents across various locations globally[2,3]. Two separate genes encode IL-35 called IL12A and Epstein–Barr virus-induced 3 (EBI3)[4,5]. Both the genes IL-12A and EBI3 are networked with various diseases, as represented in Figure 1. The data in the PubMed database indicated a direct correlation between IL-35 and EBI3 genes in many immune-inflammatory, autoimmune, cancer, and endocrine diseases[6-8].
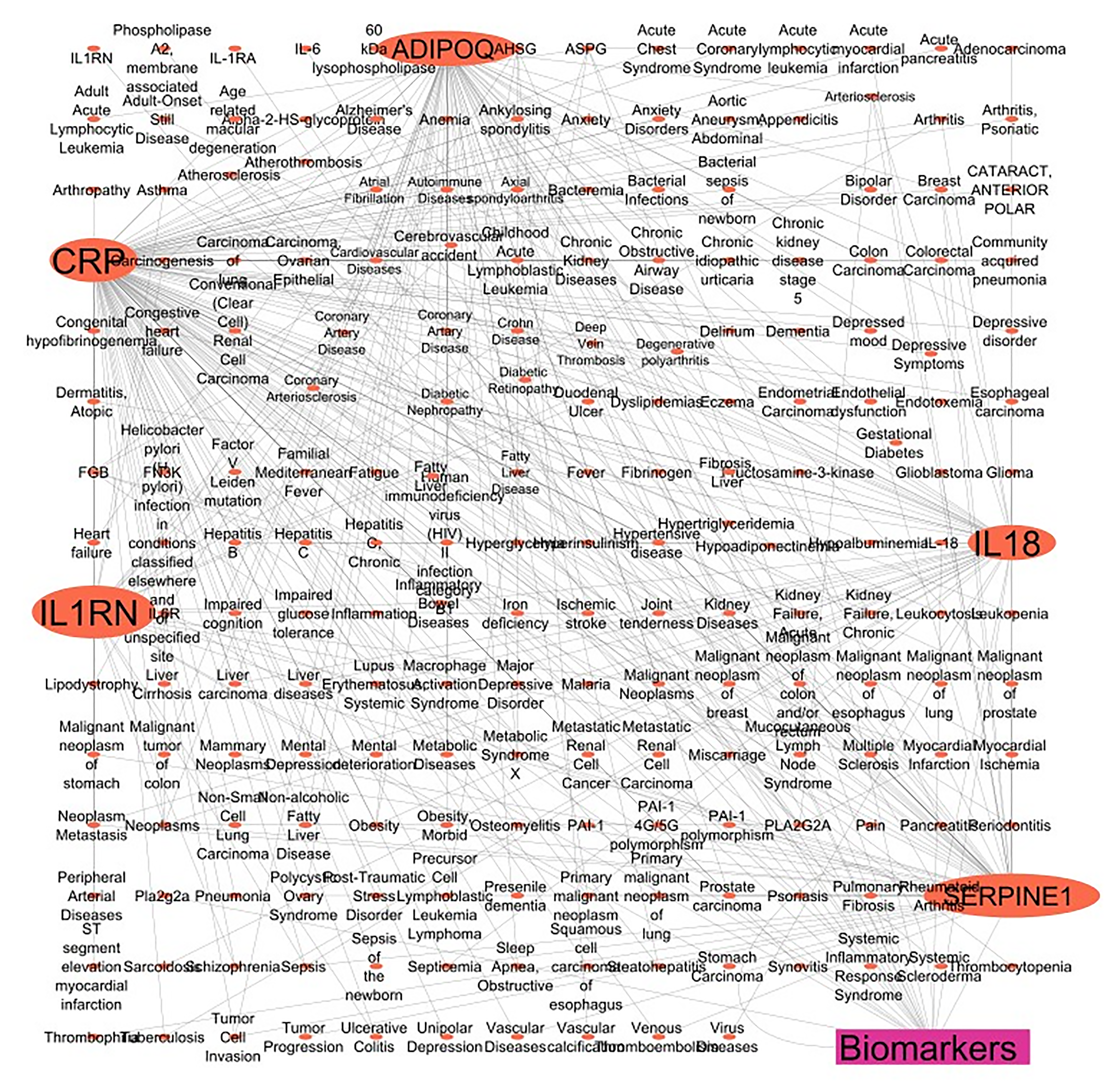
Further, we selected 5 protein/enzyme markers clinically identified with IL12A and EBI3 from the PubMed database. We then searched for their UniProt ID and human gene names in the UniProt databases and looked for their disease associations in the DisGeNET database v7.0. Gene-disease associations with N_PMIDs (citation) greater than or equal to 10 were considered. Finally, we created a gene-disease target network using CYTOSCAPE version 3.10.0[9]. The schematic representation of networking of the total of five genes named ADIPOQ, CRP, IL18, IL1RN, and SERPINE1 encodes the protein Progestin and adipoQ receptor family member 3, C-reactive protein, IL-18, IL-1 receptor antagonist protein, and SERPINE1 mRNA-binding protein 1, respectively are shown in Figure 2.
The network pharmacological analysis revealed that these five genes, highlighted in the figure, exhibited the most significant interaction with the disease. The PubMed database establishes a correlation between 5 genes and various disorders, encompassing immune-inflammatory, autoimmune, cancer, and endocrine diseases.
Based on several PubMed literature searches, there is a direct correlation between proinflammatory mediators such as CRP and IL-6R, which has been re-validated through networking. As a result, IL-35, an anti-inflammatory immune suppressant, may help counteract the proinflammatory signals that occur during prediabetes, diabetes, and its complica
IL-35 is a protective factor against prediabetes and plays a significant role in macrophage polarization[10]. Treg and Th1 cells are crucial for this protection[11]. Studies on non-obese diabetic mice have revealed that IL-35 expression reduces conventional T cells, dendritic cells, and Treg cells against beta cells[12]. The administration of IL-35 also reduces the number of Th1 and Th17 cells and IFN-γ or IL-17A-expressing CD8+ T cells[13]. Thereby, IL-35 plays a critical regulatory role in T1D by decreasing the infiltration of mononuclear cells in the islets[14,15]. Clinical research has provided additional insights, indicating that C-peptide-negative patients exhibit markedly lower serum levels of IL-35[8-13]. This decrease is associated with a simultaneous reduction in the proportion of IL-35+ Treg cells, IL-35+ regulatory B cells, and IL-35-producing CD8+ FOXP3+ cells[15,16].
The results above emphasize the possible importance of IL-35 expression in regulating the immune response and its involvement in the autoimmune mechanisms underlying type 1 diabetes. Immunotherapy with IL-35 has demonstrated encouraging outcomes in combating the consequences of prediabetes and diabetes. Research indicates that IL-35 can alter the balance of cytokines, modulate the activity of B cells, and offer defense against autoimmune diabetes. Nevertheless, additional investigation is necessary to ascertain the precise mechanism of action, accompanied by meticulous design of clinical studies.
The authors sincerely thank the Institute of Advanced Study in Science and Technology (IASST) and the Department of Science and Technology, Government of India for their assistance and contributions.
| 1. | Ping WX, Hu S, Su JQ, Ouyang SY. Metabolic disorders in prediabetes: From mechanisms to therapeutic management. World J Diabetes. 2024;15:361-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (2)] |
| 2. | Ye C, Yano H, Workman CJ, Vignali DAA. Interleukin-35: Structure, Function and Its Impact on Immune-Related Diseases. J Interferon Cytokine Res. 2021;41:391-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 3. | Zysk W, Gleń J, Trzeciak M. Current Insight into the Role of IL-35 and Its Potential Involvement in the Pathogenesis and Therapy of Atopic Dermatitis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Zhang SM, Liang J, Xia JP, Li L, Zheng L, Wang YL, Li YH, Li Y, Lu Y. Interleukin 35: protective role and mechanism in type 1 diabetes. Cent Eur J Immunol. 2023;48:48-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Lu J, Liu J, Li L, Lan Y, Liang Y. Cytokines in type 1 diabetes: mechanisms of action and immunotherapeutic targets. Clin Transl Immunology. 2020;9:e1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 6. | Cano-Cano F, Gómez-Jaramillo L, Ramos-García P, Arroba AI, Aguilar-Diosdado M. IL-1β Implications in Type 1 Diabetes Mellitus Progression: Systematic Review and Meta-Analysis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Zhang J, Zhang Y, Wang Q, Li C, Deng H, Si C, Xiong H. Interleukin-35 in immune-related diseases: protection or destruction. Immunology. 2019;157:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Wetzel A, Scholtka B, Schumacher F, Rawel H, Geisendörfer B, Kleuser B. Epigenetic DNA Methylation of EBI3 Modulates Human Interleukin-35 Formation via NFkB Signaling: A Promising Therapeutic Option in Ulcerative Colitis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Devroy P, Chatterjee SK, Singh R, Mohapatra S, Haldar S, Mukherjee AK, Bala A. Candy leaf - Stevia rebaudiana (Bertoni) Bertoni attenuated LPS-induced protein kinase C phosphorylation in mouse macrophages cells: Target search by network pharmacology and validation using ex vivo and in vivo assays. Food Biosci. 2024;61:104809. [DOI] [Full Text] |
| 10. | Chakraborty R, Mukherjee AK, Bala A. Breakthroughs in road mapping IL-35 mediated immunotherapy for type-1 and autoimmune diabetes mellitus. Cytokine. 2024;181:156692. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Espes D, Singh K, Sandler S, Carlsson PO. Increased Interleukin-35 Levels in Patients With Type 1 Diabetes With Remaining C-Peptide. Diabetes Care. 2017;40:1090-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Singh K, Martinell M, Luo Z, Espes D, Stålhammar J, Sandler S, Carlsson PO. Cellular immunological changes in patients with LADA are a mixture of those seen in patients with type 1 and type 2 diabetes. Clin Exp Immunol. 2019;197:64-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Ouyang H, Wen J, Song K. Decreased interleukin-35 levels and CD4(+)EBI3(+) T cells in patients with type 1 diabetes and the effects of the antibody against CD20 (rituximab). Arch Med Sci. 2021;17:258-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Luo Z, Lundin S, Mejia-Cordova M, Hassani I, Blixt M, Hjelmqvist D, Lau J, Espes D, Carlsson PO, Sandler S, Singh K. Interleukin-35 Prevents Development of Autoimmune Diabetes Possibly by Maintaining the Phenotype of Regulatory B Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Sawant DV, Hamilton K, Vignali DA. Interleukin-35: Expanding Its Job Profile. J Interferon Cytokine Res. 2015;35:499-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Yan A, Zhang Y, Wang X, Cui Y, Tan W. Interleukin 35 regulates interleukin 17 expression and T helper 17 in patients with proliferative diabetic retinopathy. Bioengineered. 2022;13:13293-13299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/