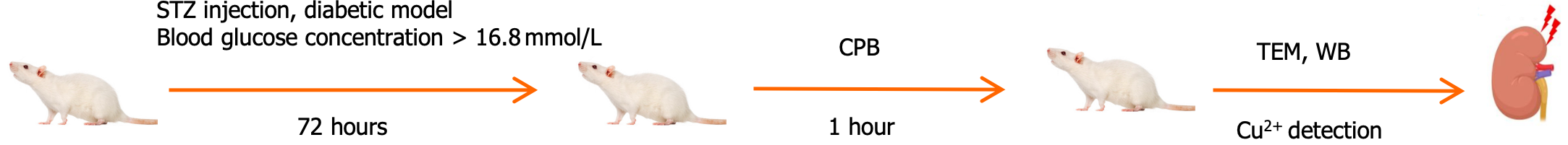Published online Oct 15, 2024. doi: 10.4239/wjd.v15.i10.2123
Revised: August 11, 2024
Accepted: September 5, 2024
Published online: October 15, 2024
Processing time: 77 Days and 21.3 Hours
Cardiopulmonary bypass (CPB) is a common procedure in cardiac surgery. CPB is a high-risk factor for acute kidney injury (AKI), and diabetes is also such a factor. Diabetes can lead to copper overload. It is currently unclear whether AKI after CPB in diabetic patients is related to copper overload.
To explore whether the occurrence of CPB-AKI in diabetic patients is associated with cuproptosis.
Blood and urine were collected from clinical diabetic and non-diabetic patients before and after CPB. Levels of copper ion, lactate, glucose, heat shock protein-70 (HSP-70), and dihydrolipoamide dehydrogenase (DLAT) were determined. A diabetic rat model was established and CPB was performed. The rats were assessed for the development of CPB-AKI, and for the association of AKI with cuproptosis by detecting copper levels, iron-sulfur cluster proteins and observation of mitochondrial structure by electron microscopy.
CPB resulted in elevations of copper, lactate, HSP-70 and DLAT in blood and urine in both diabetic and non-diabetic patients. CPB was associated with pathologic and mitochondrial damage in the kidneys of diabetic rats. Cuproptosis-related proteins also appeared to be significantly reduced.
CPB-AKI is associated with cuproptosis. Diabetes mellitus is an important factor aggravating CPB-AKI and cuproptosis.
Core Tip: This study found that cardiopulmonary bypass (CPB) resulted in elevations of copper, lactate, heat shock protein-70 and dihydrolipoamide dehydrogenase in blood and urine in both diabetic and non-diabetic patients. CPB was associated with pathologic and mitochondrial damage in the kidneys of diabetic rats. Cuproptosis-related proteins also appeared to be significantly reduced. CPB- acute kidney injury (AKI) is associated with cuproptosis. Diabetes mellitus is an important factor aggravating CPB-AKI and cuproptosis. We believe this manuscript is valuable for all the researchers who are interested in.
- Citation: Deng XJ, Wang YN, Lv CB, Qiu ZZ, Zhu LX, Shi JH, Sana SRGL. Effect of cuproptosis on acute kidney injury after cardiopulmonary bypass in diabetic patients. World J Diabetes 2024; 15(10): 2123-2134
- URL: https://www.wjgnet.com/1948-9358/full/v15/i10/2123.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i10.2123
Cardiopulmonary bypass (CPB) has brought new opportunities for valve replacement and coronary artery bypass surgery and is a common procedure in cardiac surgery[1]. However, CPB can lead to a series of complications, the most common of which is acute kidney injury (AKI)[2,3]. One study found that 22.3% of patients with CPB worldwide develop AKI, with severe AKI leading to a mortality rate of more than 35%. Even mild AKI lead to a more than four-fold increase in the risk of death and prolonged hospital stay[4]. Moreover, the incidence of AKI is approximately two times greater with CPB heart bypass surgery compared with non-CPB bypass surgery[5]. Another study reported that 2%-5% of patients with CPB-AKI require renal replacement therapy[6], which seriously affects patient prognosis[6] and increases economic burden. There is also evidence that transient and mild kidney injury is associated with an increased risk of chronic kidney disease[4].
Diabetes mellitus (DM) is an independent risk factor for AKI[7,8]. Approximately 70%-80% of patients with heart disease have comorbid diabetes or abnormal glucose metabolism[9]. Additionally, 30%-40% of diabetic patients will have varying degrees of combined kidney damage[10,11]. CPB-AKI in diabetic patients is currently thought to have two causes. First, the long-term chronic inflammatory response, stress response, and immune imbalance state of the body induced by hyperglycemia, as well as the damage to micro-vessels caused by hyperglycemia can lead to an increased susceptibility to renal injury, causing an increased incidence of AKI. Second, cellular mechanical damage (release of protein hydrolyzing enzymes and metalloproteins) caused by ex vivo dilution of blood and exposure to non-physiologic surfaces during CPB, as well as dysregulation of organ flow and activation of the inflammatory cascade response caused by the non-pulsatile hemodynamics of CPB, can increase the incidence of or exacerbate renal injury. Our previous study found that perioperative hyperglycemia caused an increase in urinary toxic metabolites[12] and also suggested that patients with perioperative hyperglycemia can develop renal injury.
Clinical diagnostic indicators of perioperative AKI include creatinine levels and urine output. These indicators are imprecise and incomplete, which can lead to a delay in the diagnosis of AKI[13]. Therefore, the actual incidence of AKI may be higher than expected. Additionally, the pathogenesis of surgery-related AKI is unclear and may be related to ionic imbalances, immune dysregulation, inflammation, and stress[13]. Currently, there is no effective prevention or treatment for CPB-related AKIs[14,15].
More recently, the copper-dependent cell death driven by mitochondrial stress and damage, termed cuproptosis, has been described[16]. Cuproptosis is a new mode of cell death caused by the accumulation of copper ions and their binding to tricarboxylic acid cycle thioctylated proteins. The binding leads to aberrant oligomerization of the proteins and loss of iron-sulfur (Fe-S) cluster proteins[16]. This type of cell death depends on impaired mitochondrial respiration and subsequent mitochondrial protein stress, rather than mitochondrial oxidative stress[17]. Generally, copper ions have very low intracellular concentrations[18], and mainly bind reversibly with metallothionein to participate in organismal physiological and pathological processes[19], such as antioxidant activity, mitochondrial respiration, and regulation of signaling pathways[20]. There are several isoforms of metallothionein. Metallothionein 2a is mainly found in renal cells. The proximal tubule, Henry’s loop, distal tubule, and collecting ducts of the kidney can reabsorb metal ions, so the kidney is more susceptible to metal ion imbalance. However, it is not known what role(s) copper imbalance and cuproptosis play in AKI.
CPB causes the release of intracellular copper into the bloodstream[21]. Hemodilution involved in CPB in turn leads to a decrease in plasma copper concentration[22]. Additionally, diabetes results in a state of copper overload[23]. To date, it has not been reported domestically or internationally whether the post-CPB copper ion status and cuproptosis in patients with diabetes are involved in the development of diabetic CPB-AKI.
The aim of this study was to explore the effects of diabetes and CPB on copper, metabolism, and cuproptosis by collecting blood and urine from clinically CPB diabetic and non-diabetic patients. Furthermore, a rat model of DM was established using intraperitoneal injection of streptozotocin (STZ), followed by CPB to explore whether the occurrence of CPB-AKI in diabetic rats is associated with cuproptosis.
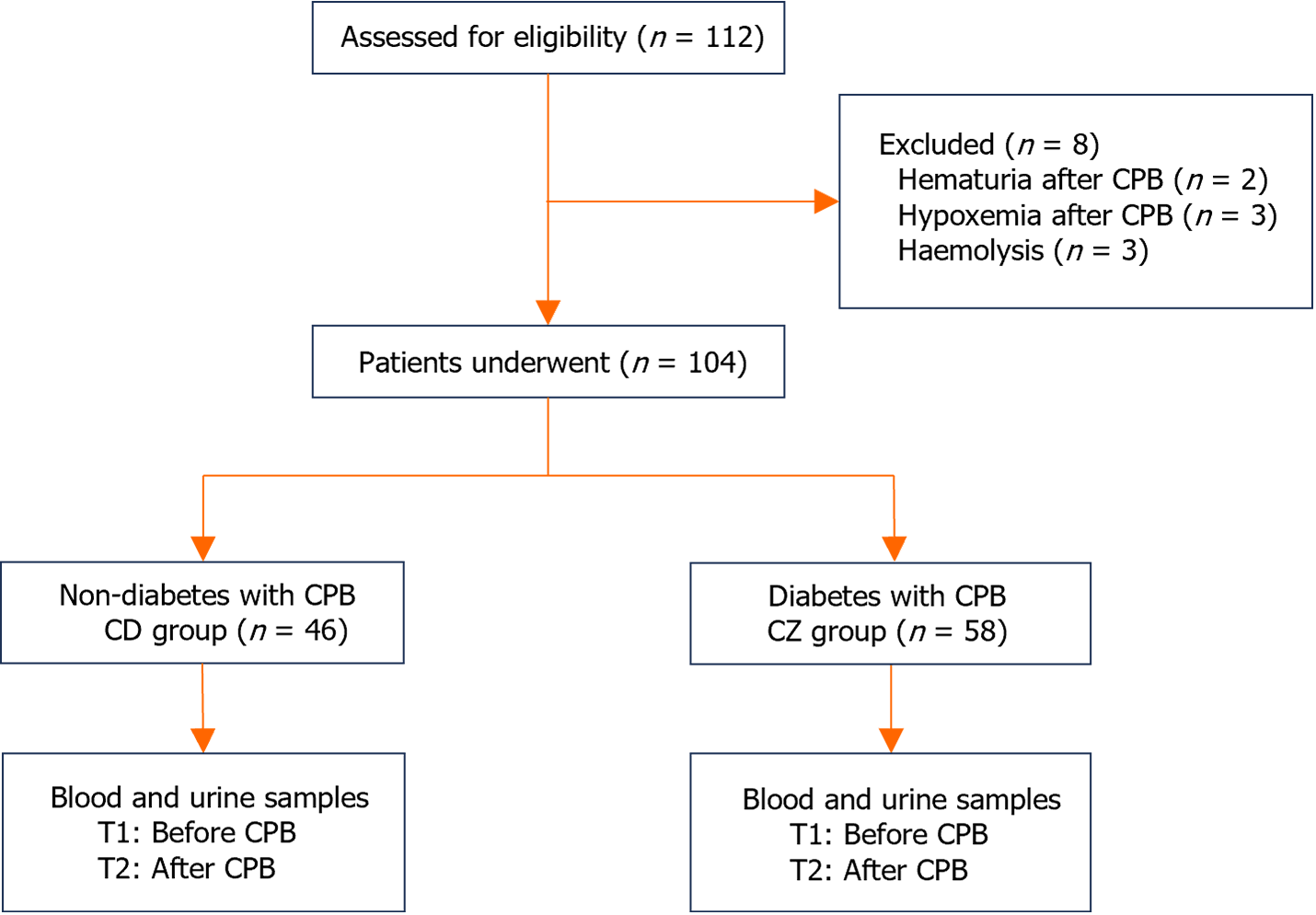
All patients were treated at the First Hospital of Harbin Medical University. Please add IRB number and date in this section. The clinical trial was approved by the Ethics Committee of the Harbin Medical University on May 10, 2022, with the approval No. 202231. A total of 46 non-diabetic patients (CD group) and 58 diabetic patients (CZ group) who underwent CPB were included. Before and after CPB, the basic vital signs of the patients were measured, and blood and urine were collected (Figure 1). Blood samples were collected from patients before and after CPB in this study. Patients were enrolled for subsequent analysis based on the AKI criteria proposed in the kidney disease improving global outcomes guidelines: An increase in serum creatinine of > 0.3 mg/dL (> 26.5 μmol/L) within 48 hours.
Male Sprague-Dawley rats (500-600 g) obtained from Harbin Medical University were randomly divided into three groups: Control group (KB, n = 5), CPB group (CPB, n = 5), and diabetes CPB group (CPB + glucose, n = 5). The rats were housed individually in cages in a room maintained at 22°C-24°C with a 12-hour light/dark cycle. The animal experiments conformed to the guidelines of the National Institutes of Health on the Care and Use of Animals. And the study was approved by the Animal Management Committee of Harbin Medical University on December 25, 2022, with the approval No. 2022IIT037 (Figure 2).
The rats were fasted overnight but had free access to drinking water before induction of diabetes. Diabetes was induced by a single intraperitoneal injection of 60 mg/kg of STZ (S0130, Sigma-Aldrich) freshly dissolved in 0.1 moL/L citrate buffer at potential of hydrogen (PH) 4.5[24]. The presence of hyperglycemia was confirmed 72 hours after STZ administration. Blood samples were collected from the tail veins of rats after a 12-hour fast for blood glucose measurement. The model was considered successful if the blood glucose concentration was equal to or greater than 16.8 mmol/L. Age-matched rats were injected with an equal volume of citrate buffer to serve as non-diabetic normoglycemic controls.
Before the induction of CPB, rats were fasted overnight with free access to drinking water. The rats were anesthetized with 6.0% sevoflurane, intubated using a 16-gauge intravenous catheter, and mechanically ventilated. The ventilator setting included a fraction of inspired oxygen of 0.21 and ventilatory rate of 60 cycles per minute. CPB in the rat was performed using the surgical techniques described previously[24,25]. Heart rate and rectal temperature were con-tinuously monitored, and the rectal temperature was maintained at approximately 37.5 °C. After systemic heparinization using 400 IU/kg, the right femoral artery was cannulated with a 24-gauge angiocatheter. A 14-gauge multi-pore angiocatheter was introduced into the right internal jugular vein and advanced into the right atrium. Mechanical ventilation was terminated upon CPB. The CPB flow rate exceeded 80 mL/kg/minute. Circulation volume was adjusted to ensure the flow rate resulted in a flow time of 60 minutes. During CPB, an oxygen-air mixture in a 1:4 ratio passed through the membrane lung. Mechanical ventilation was restarted before the flow was reduced, and circulation volume was gradually decreased. After the rat resumed spontaneous breathing, tracheal intubation was removed, and the rat was fed in the laboratory for observation. Before and after CPB, 0.5 ml of blood was collected from the right femoral artery into a sterile EP tube. The sample was then centrifuged at 1000g for 15 minutes, and the supernatant was collected, aliquoted, and stored at -80 °C. By detecting the creatinine levels in rats and combining with kidney. Hematoxylin-eosin staining (HE) pathological changes, the establishment of the AKI model was verified. By detecting the serum creatinine levels in rats and combining with HE pathological changes with kidney, the establishment of the AKI model was verified.
Blood and urine were collected before and after CPB according to the actual situation of each surgery. Kidney tissues from diabetic rats were collected at the end of CPB. Kidney tissue was milled in liquid nitrogen and lysed using radio immunoprecipitation assay lysis (RIPA) buffer (P0013B, Beyotime). The supernatant was collected for assay of total protein concentration using a BCA protein assay kit and the manufacturer’s instructions. Copper levels in blood, urine, and kidney tissue supernatants were determined by inductively coupled plasma-mass spectrometry (ICP-MS). Briefly, exactly 0.20 mL of the sample to be tested was pipetted into a 10 mL transparent polytetrafluoroethylene centrifuge tube, followed by exactly 10 mL of concentrated highly pure nitric acid. Tube contents were mixed thoroughly for 1 minute. The sample was heated in a reactor at 120 °C until the mixture evaporated to approximately 1 mL and became clear. This nitrolyzed sample was diluted with ultrapure water to 5 mL, shaken for 1 minute, and filtered through a sterile filter membrane with a 0.22 μm pore size to remove impurities. The sample was diluted according to the concentration and analyzed by ICP-MS. (Shanghai Enzyme-linked Biotechnology Co., Ltd)[26].
The levels of heat shock protein-70 (HSP-70) and dihydrolipoamide dehydrogenase (DLAT) in the plasma of clinical patients and rats before and after CPB were assessed using enzyme-linked immunosorbent assay kits (ab133060, Abcam and NOV-BG-HUM10729-96T, NEWBIOSCIENCE, respectively) following the manufacturers’ instructions.
Renal samples obtained from rats were lysed in complete RIPA buffer (P0013B, Beyotime). Protein concentration of tissue homogenates was measured using the BCA protein assay kit (P0010S, Beyotime). Equal amounts of soluble protein were separated by 10% or 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Each membrane was blocked for 30 minuites using QuickBlock blocking buffer and incubated overnight at 4 °C with antibodies to lipoic acid synthetase [lipoic acid synthase (LIAS); ab246917, Abcam], aconitase 2 (ACO-2; ab110321, Abcam), and succinate dehydrogenase (SDHB; ab175225, Abcam)[16]. This was followed by five washes using tris-buffered saline-tween (TBST) and incubation with secondary antibody for 50 minutes. After five washes with TBST, signals were visualized using an ECL luminescence kit (P0018S, Beyotime) and a gel imaging system.
Renal tissues were fixed in 2% glutaraldehyde and 1% osmium tetroxide. Samples were processed and sectioned. The 70-80 nm thick and sections were placed on copper mesh grids. Sections were stained with uranyl acetate and lead citrate for contrast and viewed by transmission electron microscopy (TEM).
Statistical analysis was performed using statistical product and service solutions 26.0 software, and graphs were created using GraphPad Prism 9.5.1 software. Data are presented as mean ± SD. The Shapiro-Wilk normality test was used to assess normality, and Levene’s test was applied to evaluate homogeneity of variance. For comparisons between two groups, an independent sample t-test was used for data that met the assumptions of normality and homogeneity of variance; a corrected Welch t’ test was employed for data that conformed to normality but not homogeneity of variance. For comparisons among multiple groups, a One-way ANOVA test was conducted for data that satisfied the assumptions of normality and homogeneity of variance; a Kruskal-Wallis test was applied for data that did not meet the assumptions of normality or homogeneity of variance. The level of statistical significance was set at P < 0.05.
The age, height, weight, gender, and CPB time of the two groups of patients did not differ statistically significantly (Table 1). There were also no statistically significant differences in systolic blood pressure (SBP), diastolic blood pressure (DBP), and blood oxygen saturation (SPO2) between the two groups of patients. Intragroup comparison showed that the SBP, DBP, SPO2, hemoglobin (Hb), and heart rate of the two groups of patients before CPB (T1) were statistically significant (P < 0.05) compared to those after CPB (T2) (Table 2).
| Parameter | CD group | CZ group | F value | P value |
| Age (Years) | 64.61 ± 7.43 | 60.07 ± 8.91 | 1.961 | 0.055 |
| Height (cm) | 164.08 ± 10.46 | 164.62 ± 8.99 | -0.198 | 0.844 |
| Weight (kg) | 66.80 ± 12.27 | 64.00 ± 11.18 | 0.860 | 0.394 |
| CPB time (min) | 76.26 ± 12.44 | 80.10 ± 10.54 | -1.205 | 0.234 |
| Sample | Group | T1 | T2 | P value |
| SBP | CD | 148.09 ± 22.14 | 107.17 ± 12.88 | 0.000 |
| CZ | 136.79 ± 22.04 | 103.69 ± 11.90 | 0.000 | |
| P value | 0.073 | 0.317 | ||
| DBP | CD | 71.57 ± 13.85 | 56.56 ± 8.45 | 0.000 |
| CZ | 72.48 ± 10.34 | 54.48 ± 10.27 | 0.000 | |
| P value | 0.786 | 0.437 | ||
| HR | CD | 75.83 ± 10.41 | 99.13 ± 10.49 | 0.000 |
| CZ | 79.34 ± 12.40 | 97.41 ± 12.52 | 0.000 | |
| P value | 0.281 | 0.601 | ||
| SPO2 | CD | 97.30 ± 1.96 | 99.04 ± 1.33 | 0.001 |
| CZ | 97.17 ± 2.47 | 99.07 ± 1.28 | 0.001 | |
| P value | 0.835 | 0.944 | ||
| Hb | CD | 13.91 ± 1.60 | 9.80 ± 0.92 | 0.000 |
| CZ | 13.62 ± 1.62 | 9.94 ± 0.80 | 0.000 | |
| P value | 0.531 | 0.548 |
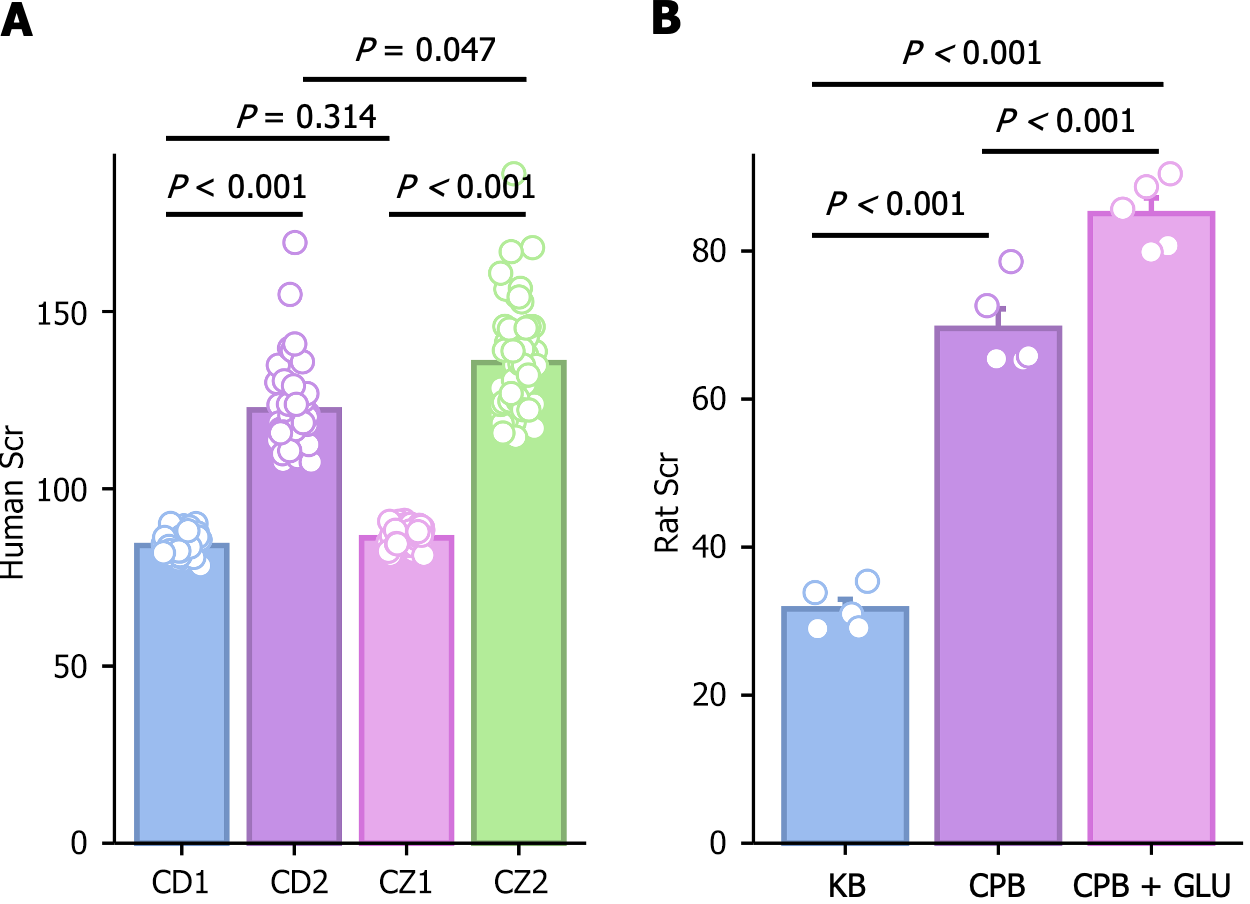
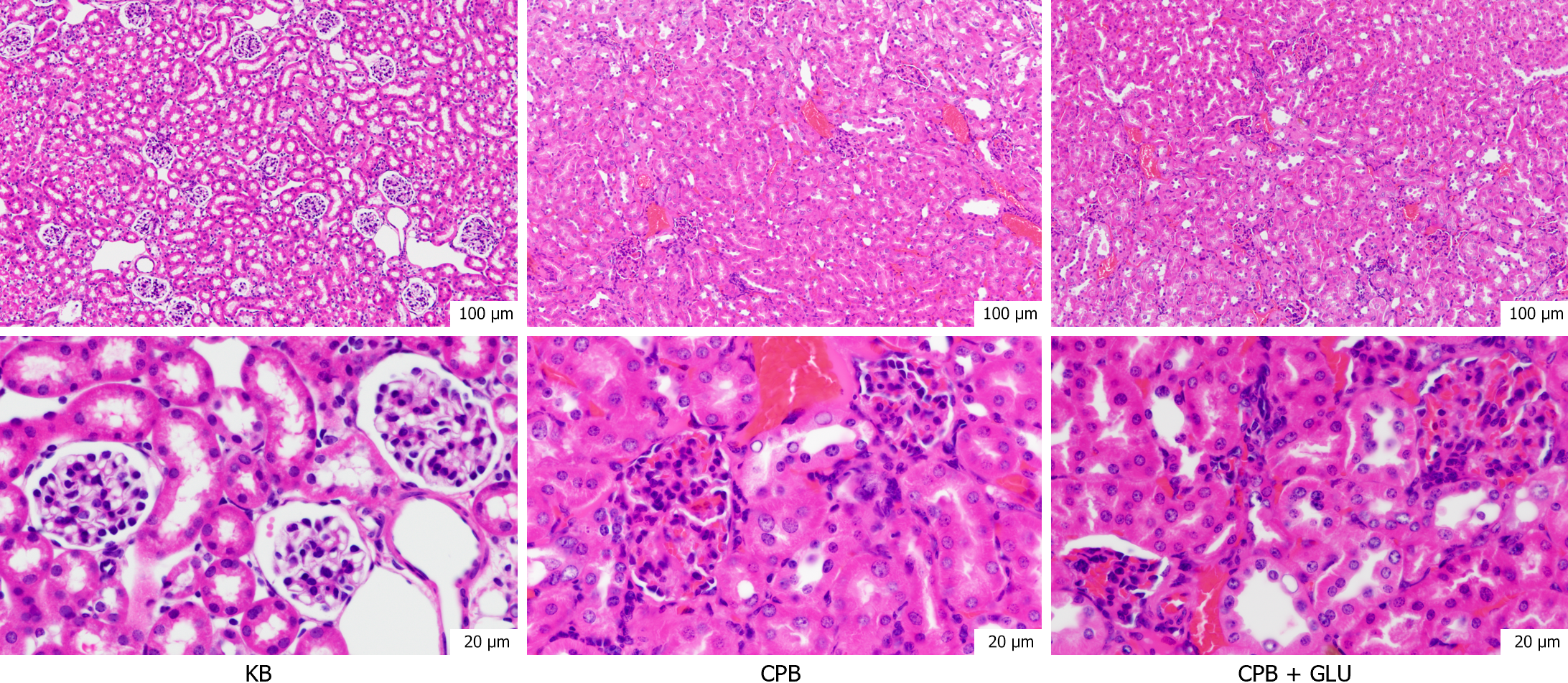
Changes in creatinine concentrations before and after CPB in patients met the corresponding criteria for AKI (Figure 3A). In rats, creatinine levels significantly increased after CPB, and this elevation was further exacerbated in the CPB + glucose group, with statistically significant differences observed (Figure 3B). HE staining of rat kidney tissue revealed damage to renal tubular epithelial cells after CPB, which was more pronounced in the CPB + glucose group (Figure 4).
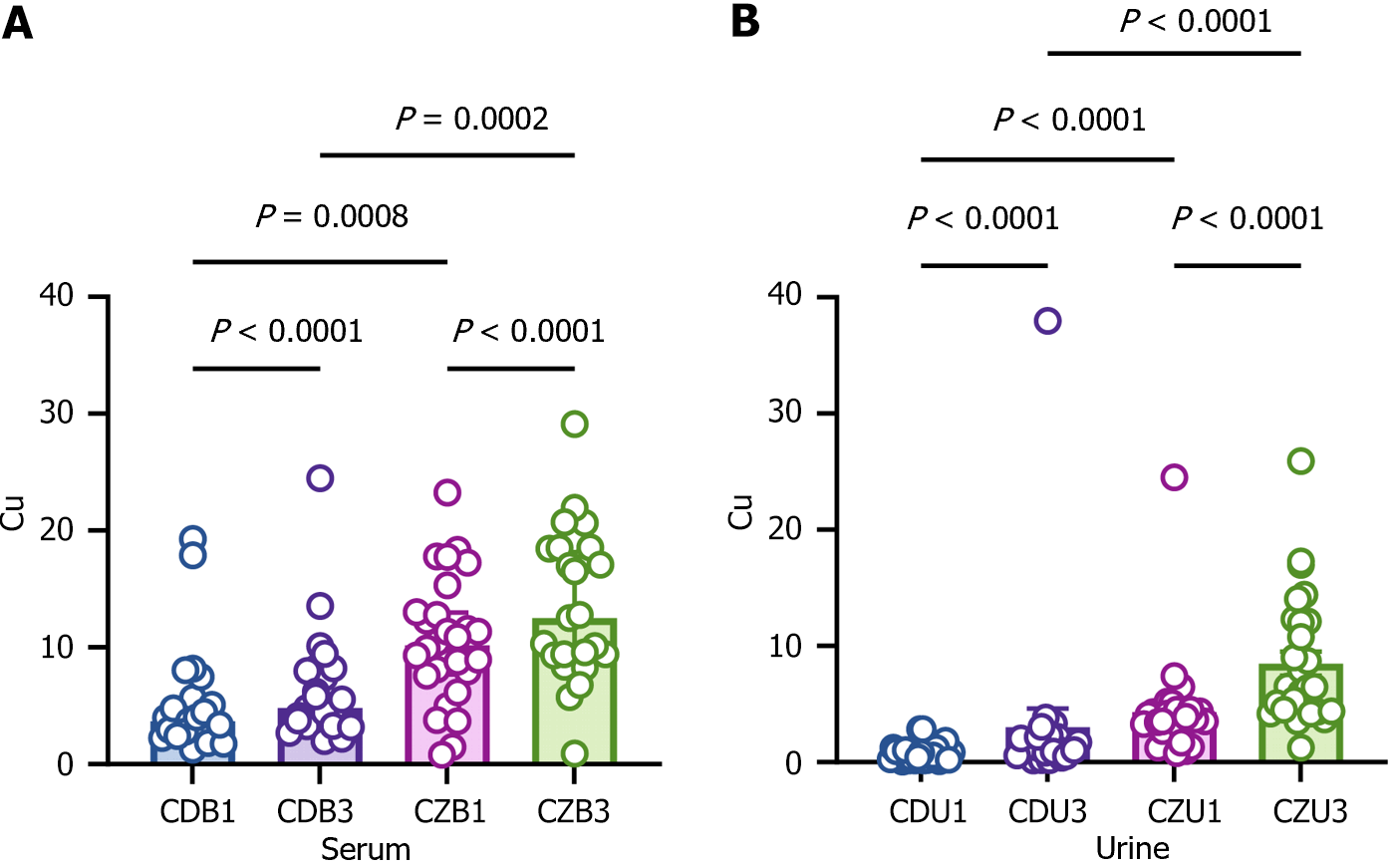
To verify whether CPB and diabetes cause disturbances in body copper, blood and urine were collected for determination of copper levels before and after CPB from diabetic and non-diabetic patients. Copper levels were significantly elevated in the blood of diabetic patients before and after CPB compared to non-diabetic patients (Figure 5). Diabetic patients had significantly higher copper levels in their blood after CPB (Figure 5). Copper levels in urine were determined. The levels reflect kidney function to some extent. The copper levels were significantly higher in diabetic patients before and after CPB (Figure 5). Copper was also significantly elevated in the urine of non-diabetic patients after CPB compared to before CPB (Figure 5). The results suggest that the metabolic disturbances of DM results in elevation of copper in blood and urine, and that these levels increase further following CPB.
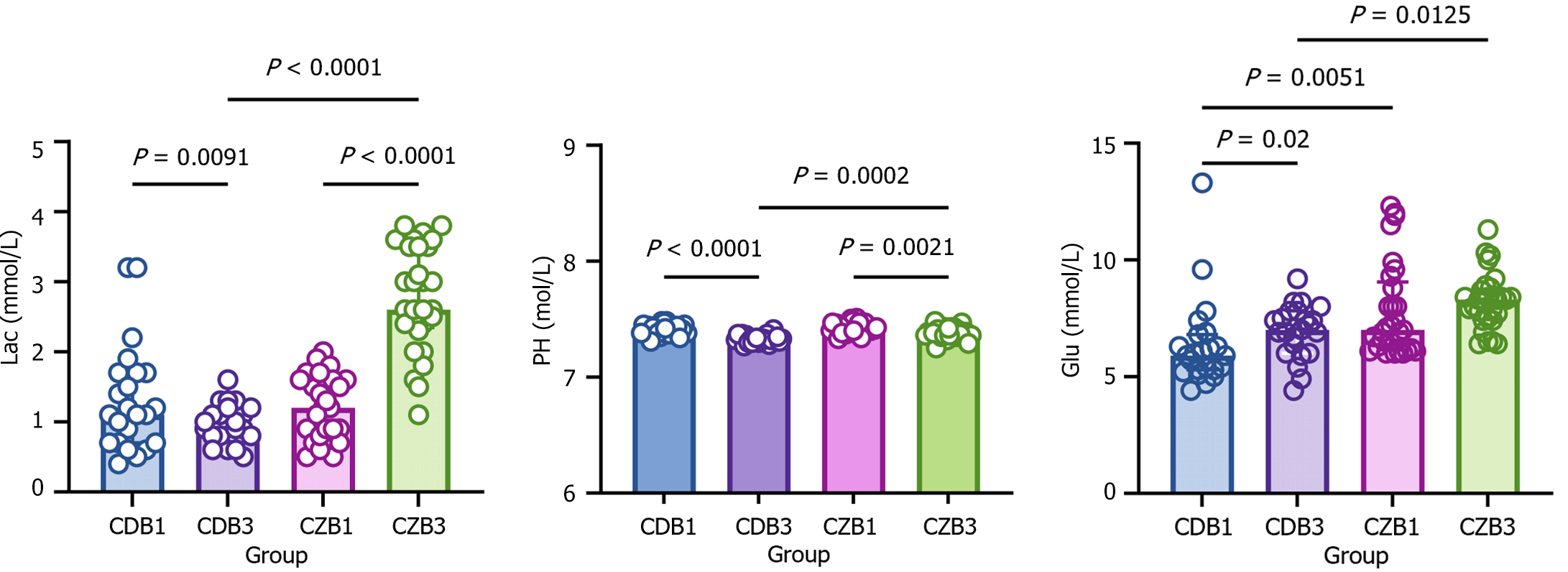
One of the typical features of diabetes is metabolic disorders in the body. To investigate whether CPB exacerbates diabetes-induced metabolic disorders, lactate, glucose, and PH were assayed in diabetic and non-diabetic patients before and after CPB, and the variability in basal vital signs was analyzed. Before CPB, there was no difference in PH and lactate between diabetic and non-diabetic patients, whereas blood glucose was significantly higher in the diabetic group (Figure 6). Following CPB, PH was significantly lower in both diabetic and non-diabetic patients compared to prior to CPB. Following CPB, lactate levels were decreased in non-diabetic patients and increased in diabetic patients (Figure 6). Diabetic patients displayed a significant increase in blood glucose after CPB and non-diabetic patients tended to have higher blood glucose after CPB, with no statistically significant differences (Figure 6). The results suggest that CPB exacerbates metabolic disorders caused by diabetes.
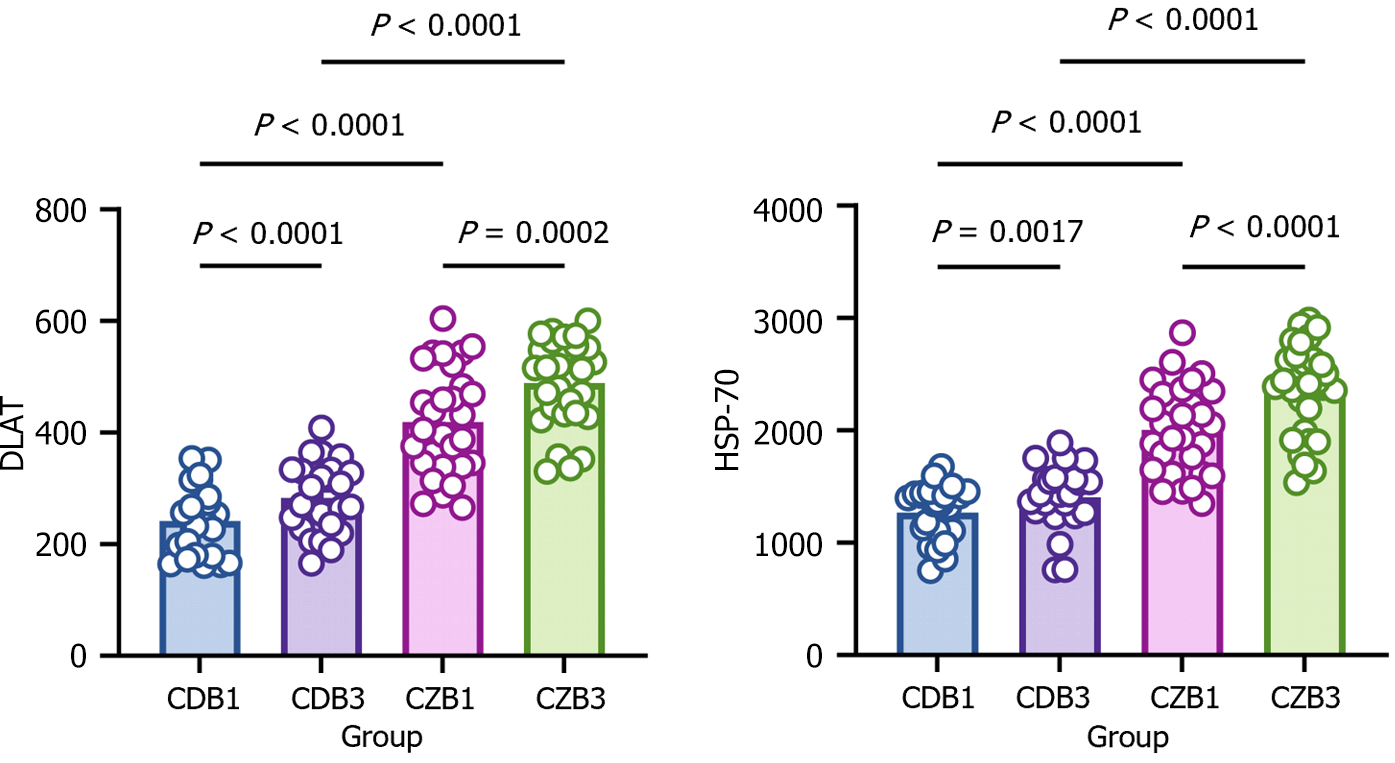
Elevated copper ions cause heterodimerization of DLAT, an important protein in the tricarboxylic acid cycle (TCA) cycle, and increase DLAT deposition[16]. Induction of HSP-70 and reduction of Fe-S cluster proteins are feature of copper-dependent cuproptosis[25]. To verify whether the elevation of copper after CPB in diabetic patients causes an increase in DLAT deposition and HSP-70, which further causes cuproptosis, blood levels of DLAT and HSP-70 were determined. Before CPB, DLAT, and HSP-70 were elevated in the blood of diabetic patients. After CPB, DLAT and HSP-70 were significantly elevated in the blood of diabetic and non-diabetic patients, with more significant elevation in diabetic patients (Figure 7). The results suggest that cuproptosis may occur after CPB, while diabetes may be a risk factor for cuproptosis.
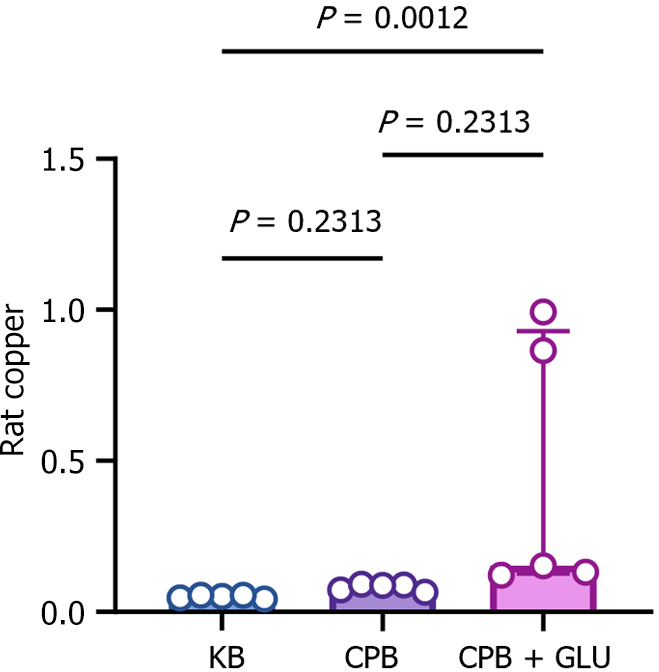
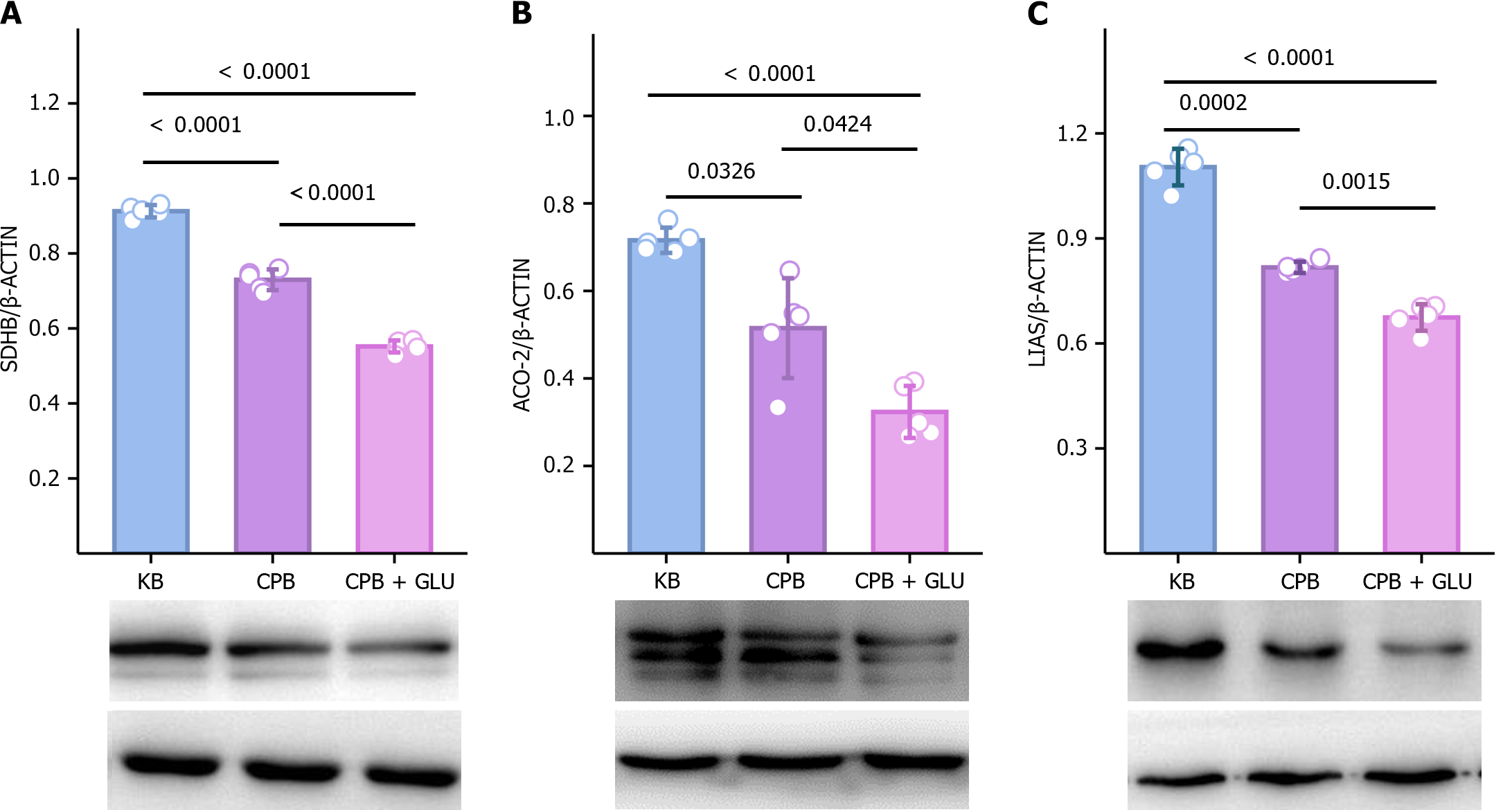
AKI is a common complication after CPB. To investigate whether renal injury caused by CPB is associated with copper deposition and copper-dependent cuproptosis, copper levels and expressions of cuproptosis-related proteins in renal tissues were determined after CPB in diabetic rats. Copper was significantly increased in kidney tissues of diabetic rats after CPB compared with the CPB and KB groups (Figure 8). In addition, cuproptosis-related proteins, including LIAS, ACO-2 and SDHB, were significantly decreased in renal tissues of diabetic rats after CPB (Figure 9).
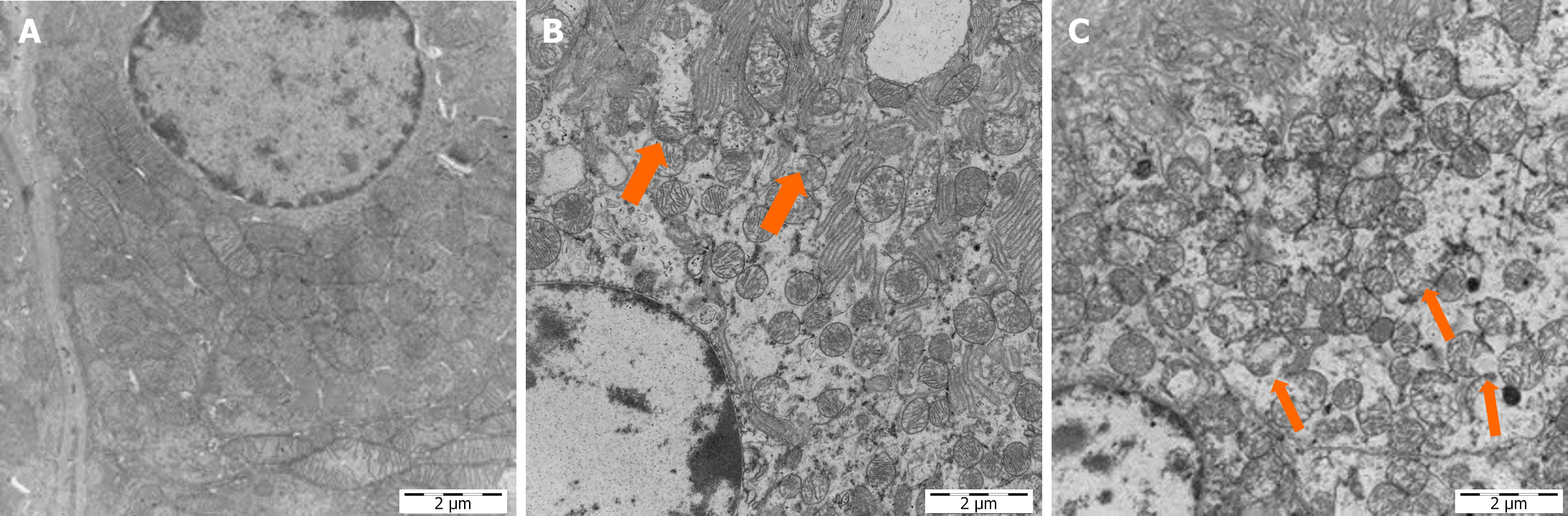
Cuproptosis is associated with proteolipid acylation in the mitochondrial tricarboxylic acid cycle. Mitochondria are a key link in kidney injury. Histologically, mitochondrial swelling and fragmentation are observed after diverse insults to the kidney[26]. Based on these findings, TEM was used to explore the renal mitochondrial damage in diabetic rats after CPB. The mitochondria in renal tissues of diabetic rats in the KB group were oval and rod shaped, with intact mitochondrial membranes. The cristae were perpendicular to the long axis of the mitochondria, and were neatly aligned. In contrast, the mitochondria of kidneys in the CPB + glucose group were swollen, fragmented, and showed vacuolated structures. In addition, the mitochondrial cristae were disarranged (Figure 10).
Cuprotosis was first described in 2022[16]. Since then, copper death has become a hot topic medically. This study is the first clinical evidence of copper ion overload in blood and urine of diabetic patients after CPB. The evidence is consistent with the suggestion that CPB-AKI injury in patients with diabetes might be related to copper overload.
In the present study, marked differential changes in the blood levels of copper and metabolic disorders after CPB were observed in patients with diabetes. Significant mitochondrial damage was observed in renal tissues of diabetic rats after CPB. This damage was associated with high concentrations of copper as well as cuproptosis.
No statistically significant differences were evident in the basic information and vital signs (T1) before CPB between patients with and without diabetes. However, the vital signs of both groups of patients at the T1 were statistically significant different at the T2 time point. This is because during the CPB process, the heart is not beating. When the surgery is over and the heart resumes beating with the assistance of vasoactive drugs. The heart rate is relatively fast and blood pressure is controlled within an appropriate range. The results are statistically significant changes in vital signs before and after CPB in both groups of patients. During the CPB process, to ensure the effective blood volume of the patient, a large amount of blood is diluted. Thus, after CPB, the patient's Hb is relatively reduced. However, organ perfusion during CPB is sufficient, so the differences in vital signs could not have affected the results of this study.
During CPB, the body's circulation is non-physiologic and non-pulsatile. The multitude of inflammatory humoral responses, coagulation, fibrinolysis, and kallikrein cascades occur upon exposure of blood to the artificial surfaces used in CPB[22]. In addition, CPB mechanically damages cells. In this study, plasma and urine copper levels were elevated after CPB in clinical patients, which is also evidence of cellular damage. The increased levels of copper ions in serum and urine in diabetes patients after CPB was even more significant. In animal experiments, compared with the KB group, the copper ions in the CPB group increased, albeit non-significantly, possibly due to the small number of cases. Most of the body’s copper exists in bound form, and copper is one of the metals that is indispensable for mitochondrial function[27]. The most important copper-dependent enzyme in organisms is the cytochrome c multi-subunit enzyme-protein complex, which is found mainly in association with the inner mitochondrial membrane[28]. Mitochondria have a high affinity for copper entry, and thus are important organelles for copper entry and functioning[27]. Another cause of elevated copper in the organism is metabolic disorders, or restricted elimination. Diabetic patients who undergo CPB show significant lactic acid buildup and elevated blood glucose, consistent with elevated copper. This may also be one of the reasons for the disorder of copper ions in diabetes patients after CPB. We also found altered mitochondrial structure in renal tissues after CPB in diabetic rats, a phenomenon that may be due to mitochondrial copper overload and may be attenuated or reversed by copper chelators. These studies are beyond the scope of the present investigation.
The kidney requires many mitochondria to provide sufficient energy to enable removal of waste from the blood, reabsorption of nutrients, regulated balance of electrolytes and fluid, maintenance of acid–base homeostasis, and regulation of blood pressure. Apart from the heart, the kidneys are the most mitochondria-rich organ in the body. Mitochondrial damage occurs early in AKI and diabetic nephropathy, causing an imbalance in renal function. The disruption of mitochondrial homeostasis in the early stages of AKI is an important factor that drives tubular injury and persistent renal dysfunction[26]. A plethora of evidence suggests that mitochondrial dysfunction as an initiator of, and contributor to, AKI and is a therapeutic target[29]. In the present study, pathological and mitochondrial damage in the kidney was observed after CBP in diabetic rats. These results suggest that CPB directly or indirectly caused AKI in the diabetic rats. Adenosine triphosphate (ATP) is required for energy supply during tissue and cellular repair. Interruption of this energy supply due to mitochondrial damage delayed or prevents repair, which further exacerbates the damage. Therefore, the mitochondrial damage found in the kidneys in this study is a hallmark of AKI and also a cause of further aggravation of renal injury.
Copper is an essential component of various enzymes involved in the electron transport chain, cellular metabolism, and antioxidant system[30]. Both copper deficiency and excess can lead to abnormal cellular function and eventually cell death. Copper overload is cytotoxic[16]. In an animal study, CPB led to elevated copper in renal tissues of diabetic rats, indicating that elevated copper is associated with renal injury. In addition, the level of Fe-S cluster proteins were decreased in the kidneys. Another function of mitochondria is the assembly of Fe-S cluster proteins, which is an important physiological process for human survival[31]. Cuproptosis is a type of cell death associated with Fe-S cluster proteins. High levels of copper can block Fe-S cluster formation by inhibiting the activity of relevant mitochondrial assembly proteins[32]. Increased cell death has been associated with the loss of Fe-S cluster proteins. It is also believed that cuproptosis is dependent on mitochondrial respiration; LIAS is a key protein for cuproptosis, and cuproptosis is increased when LIAS production is inhibited[16]. In the present clinical study, after CPB, urinary copper ions in diabetes patients were significantly increased, and DLAT and HSP-70 were both increased. Elevations of DLAT and HSP-70 are associated with cuproptosis[16]. Our previous research confirmed the change in the expression of copper ion related genes in diabetes with renal injury[33]. The prior and present evidence implicate CPB as the direct or indirect cause of cuproptosis in the kidney of diabetic rats.
The main factor in the occurrence of cuproptosis is the accumulation of copper, not the alteration of the associated chaperone proteins. In an organism, the organelle most associated with cuproptosis is the mitochondria. Mitochondria generate and transduce a redox signal that regulates the activity of cellular copper import and export machinery, thereby controlling total copper concentrations. The accumulation of copper in mitochondria impairs mitochondrial membrane integrity and aggravates oxidative stress-related injury[27]. In addition, the occurrence of cuproptosis is associated with mitochondrial respiration and with thioctylated proteins in the mitochondrial tricarboxylic acid cycle[16]. Therefore, we hypothesized that another phenomenon that characterizes the occurrence of copper-dependent cuproptosis is mitochondrial damage, which is mainly manifested as swelling and rupture of mitochondria. Copper ion transport is dependent on two homologous ATPases, ATP7A and ATP7B[30,34]. Whether the impaired ATP synthesis resulting from the damage to mitochondria, which was presently evident in the kidneys after CPB, also attenuates copper clearance and exacerbates copper accumulation, which in turn causes more severe cuproptosis, remains unknown.
There are some limitations in the present study. We did not perform long-term observations after CPB to determine whether this acute-phase cuproptosis and alteration in renal pathology affects long-term renal function or translates to more severe chronic kidney disease.
CPB-AKI is associated with cuproptosis. DM is an important factor aggravating CPB-AKI and cuproptosis.
We thank all participants involved in the original study and all investigators for sharing the data.
| 1. | Song X, Wang H, Kashani KB, Wang C. Extracorporeal Membrane Oxygenation using a Modified Cardiopulmonary Bypass System. J Transl Int Med. 2022;10:175-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 2. | Lagny MG, Jouret F, Koch JN, Blaffart F, Donneau AF, Albert A, Roediger L, Krzesinski JM, Defraigne JO. Incidence and outcomes of acute kidney injury after cardiac surgery using either criteria of the RIFLE classification. BMC Nephrol. 2015;16:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Zhou H, Xiong H, Zheng X, Yang H, Xu S. Coronary Angiography Within 48 Hours Before Cardiac Surgery Increases the Risk of Postoperative Acute Kidney Injury. Heart Surg Forum. 2022;25:E514-E519. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Evans RG, Lankadeva YR, Cochrane AD, Marino B, Iguchi N, Zhu MZL, Hood SG, Smith JA, Bellomo R, Gardiner BS, Lee CJ, Smith DW, May CN. Renal haemodynamics and oxygenation during and after cardiac surgery and cardiopulmonary bypass. Acta Physiol (Oxf). 2018;222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Erlinger S, Arias IM, Dhumeaux D. Inherited disorders of bilirubin transport and conjugation: new insights into molecular mechanisms and consequences. Gastroenterology. 2014;146:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 430] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 7. | Pan S, Li Z, Wang Y, Liang L, Liu F, Qiao Y, Liu D, Liu Z. A Comprehensive Weighted Gene Co-expression Network Analysis Uncovers Potential Targets in Diabetic Kidney Disease. J Transl Int Med. 2022;10:359-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Ai S, Xu L, Zheng K. Acute Kidney Injury Associated with Severe Hypouricemia Caused By a Novel SLC2A9 Mutation: Enlightenment from Rare Disease to Common Disease. J Transl Int Med. 2022;10:369-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, Umpierrez GE. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 478] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | Mendez CE, Der Mesropian PJ, Mathew RO, Slawski B. Hyperglycemia and Acute Kidney Injury During the Perioperative Period. Curr Diab Rep. 2016;16:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Gemalmaz H, Unal C, Gültekin Y. Evaluation of Early Postoperative Period Results of Patients With Type 2 Diabetes Taking Oral Anti-Diabetics Or Insulin Medications, With Microalbuminuria and Normal Creatinine Levels After Coronary Artery Bypass. Heart Surg Forum. 2022;25:E407-E412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Sana SR, Chen GM, Lv Y, Guo L, Li EY. Metabonomics fingerprint of volatile organic compounds in serum and urine of pregnant women with gestational diabetes mellitus. World J Diabetes. 2022;13:888-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Gumbert SD, Kork F, Jackson ML, Vanga N, Ghebremichael SJ, Wang CY, Eltzschig HK. Perioperative Acute Kidney Injury. Anesthesiology. 2020;132:180-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 14. | Bellomo R. Perioperative Statins in Cardiac Surgery and Acute Kidney Injury. JAMA. 2016;315:873-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Ran C, Yu B, Yin H, Yang Y, Wu H, Yin Q. Hugan Buzure Granule Alleviates Acute Kidney Injury in Mice by Inhibiting NLRP3/Caspase-1 Pathway and TLR4/NF-κB Pathway. Front Biosci (Landmark Ed). 2023;28:313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD, Eaton JK, Frenkel E, Kocak M, Corsello SM, Lutsenko S, Kanarek N, Santagata S, Golub TR. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 3032] [Article Influence: 758.0] [Reference Citation Analysis (1)] |
| 17. | Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022;32:417-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 637] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 18. | Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 923] [Article Influence: 230.8] [Reference Citation Analysis (0)] |
| 19. | Sutherland DE, Stillman MJ. The "magic numbers" of metallothionein. Metallomics. 2011;3:444-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Rhodes CJ, Valko M. Essential metals in health and disease. Chem Biol Interact. 2022;367:110173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 498] [Reference Citation Analysis (0)] |
| 21. | Moat NE, Evans TE, Quinlan GJ, Gutteridge JM. Chelatable iron and copper can be released from extracorporeally circulated blood during cardiopulmonary bypass. FEBS Lett. 1993;328:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Hou HT, Xue LG, Zhou JY, Wang SF, Yang Q, He GW. Alteration of plasma trace elements magnesium, copper, zinc, iron and calcium during and after coronary artery bypass grafting surgery. J Trace Elem Med Biol. 2020;62:126612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Dascalu AM, Anghelache A, Stana D, Costea AC, Nicolae VA, Tanasescu D, Costea DO, Tribus LC, Zgura A, Serban D, Duta L, Tudosie M, Balasescu SA, Tanasescu C, Tudosie MS. Serum levels of copper and zinc in diabetic retinopathy: Potential new therapeutic targets (Review). Exp Ther Med. 2022;23:324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc. 2021;1:e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 511] [Article Influence: 102.2] [Reference Citation Analysis (2)] |
| 25. | Zhu Z, Zhao Q, Song W, Weng J, Li S, Guo T, Zhu C, Xu Y. A novel cuproptosis-related molecular pattern and its tumor microenvironment characterization in colorectal cancer. Front Immunol. 2022;13:940774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Harrington CF, Carpenter G, Coverdale JPC, Douglas L, Mills C, Willis K, Schilsky ML. Accurate non-ceruloplasmin bound copper: a new biomarker for the assessment and monitoring of Wilson disease patients using HPLC coupled to ICP-MS/MS. Clin Chem Lab Med. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Zischka H, Lichtmannegger J. Pathological mitochondrial copper overload in livers of Wilson's disease patients and related animal models. Ann N Y Acad Sci. 2014;1315:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Cobine PA, Ojeda LD, Rigby KM, Winge DR. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J Biol Chem. 2004;279:14447-14455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Ishimoto Y, Inagi R. Mitochondria: a therapeutic target in acute kidney injury. Nephrol Dial Transplant. 2016;31:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 1098] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 31. | Roger AJ, Muñoz-Gómez SA, Kamikawa R. The Origin and Diversification of Mitochondria. Curr Biol. 2017;27:R1177-R1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 740] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 32. | Brancaccio D, Gallo A, Piccioli M, Novellino E, Ciofi-Baffoni S, Banci L. [4Fe-4S] Cluster Assembly in Mitochondria and Its Impairment by Copper. J Am Chem Soc. 2017;139:719-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 33. | Ming J, Sana SRGL, Deng X. Identification of copper-related biomarkers and potential molecule mechanism in diabetic nephropathy. Front Endocrinol (Lausanne). 2022;13:978601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Kodama H, Fujisawa C, Bhadhprasit W. Inherited copper transport disorders: biochemical mechanisms, diagnosis, and treatment. Curr Drug Metab. 2012;13:237-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/