Published online Oct 15, 2024. doi: 10.4239/wjd.v15.i10.2081
Revised: August 6, 2024
Accepted: August 30, 2024
Published online: October 15, 2024
Processing time: 143 Days and 2.8 Hours
Dyslipidemia and type 2 diabetes mellitus (T2DM) are chronic conditions with substantial public health implications. Effective management of lipid metabolism in patients with T2DM is critical. However, there has been insufficient attention given to the relationship between thyroid hormone sensitivity and dyslipidemia in the T2DM population, particularly concerning non-high-density lipoprotein cholesterol (non-HDL-C).
To clarify the association between thyroid hormone sensitivity and dyslipidemia in patients with T2DM.
In this cross-sectional study, thyroid hormone sensitivity indices, the thyroid feedback quantile-based index (TFQI), the thyroid-stimulating hormone index (TSHI), the thyrotrophic T4 resistance index (TT4RI), and the free triiodothyronine (FT3)/free thyroxine (FT4) ratio were calculated. Logistic regression analysis was performed to determine the associations between those composite indices and non-HDL-C levels. Random forest variable importance and Shapley Additive Explanations (SHAP) summary plots were used to identify the strength and direction of the association between hyper-non-HDL-C and its major predictor.
Among the 994 participants, 389 (39.13%) had high non-HDL-C levels. Logistic regression analysis revealed that the risk of hyper-non-HDL-C was positively correlated with the TFQI (OR: 1.584; 95%CI: 1.088-2.304; P = 0.016), TSHI (OR: 1.238; 95%CI: 1.034-1.482; P = 0.02), and TT4RI (OR: 1.075; 95%CI: 1.006-1.149; P = 0.032) but was not significantly correlated with the FT3/FT4 ratio. The relationships between composite indices of the thyroid system and non-HDL-C levels differed according to sex. An increased risk of hyper-non-HDL-C was associated with elevated TSHI levels in men (OR: 1.331; 95%CI: 1.003-1.766; P = 0.048) but elevated TFQI levels in women (OR: 2.337; 95%CI: 1.4-3.901; P = 0.001). Among the analyzed variables, the average SHAP values were highest for TSHI, followed by TT4RI.
Impaired sensitivity to thyroid hormones was associated with high non-HDL-C levels in patients with T2DM.
Core Tip: Reduced central thyroid hormone sensitivity was an independent risk factor of high non-high-density lipoprotein cholesterol (non-HDL-C), even after adjusting for multiple confounding factors. The patients with hyper-non-HDL-C were more susceptible to metabolic disorders and impaired sensitivity to thyroid hormones. Meanwhile, the relationships between thyroid hormone sensitivity and non-HDL-C levels were different in male and female, indicating a gender-related regulation of thyroid hormones on serum non-HDL-C levels. This study may provide new evidence for the role of reduced thyroid hormone sensitivity for non-HDL-C levels and lie the groundwork for future therapeutic strategies for diabetes-related cardiovascular disease risk.
- Citation: Duan XY, Fu JL, Sun LN, Mu ZJ, Xiu SL. Association between sensitivity to thyroid hormones and non-high-density lipoprotein cholesterol levels in patients with type 2 diabetes mellitus. World J Diabetes 2024; 15(10): 2081-2092
- URL: https://www.wjgnet.com/1948-9358/full/v15/i10/2081.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i10.2081
Dyslipidemia and type 2 diabetes mellitus (T2DM) are chronic diseases with significant public health implications[1]. Atherogenic dyslipidemia is one of the major risk factors for atherosclerotic cardiovascular disease (ASCVD) in people with T2DM[2]. Patients with diabetes have approximately double the ASCVD risk of those without diabetes[3]. ASCVD, a vascular complication of T2DM, is a leading cause of mortality. Therefore, the management of lipid metabolism in patients with T2DM is crucial.
Thyroid hormones are not only essential determinants of overall energy expenditure but also important regulators of various aspects of lipid metabolism[4,5], including the synthesis, mobilization, and decomposition of fat and other processes through a complex regulatory mechanism[6]. Many studies have revealed a causal association between thyroid dysfunction and dyslipidemia[5,7,8]. However, previous studies have shown that thyroid hormone or thyroid-stimulating hormone (TSH) levels alone may not be sufficient to explain the relationship between the thyroid system and dyslipidemia[6-8], and the calculation of comprehensive indices can systematically reflect the regulation of thyroid hormone homeostasis[9]. The TSH index (TSHI), thyrotrophic T4 resistance index (TT4RI) and thyroid feedback quantile-based index (TFQI) have been well verified for evaluating central sensitivity to thyroid hormones, and the free triiodothyronine (FT3)/free thyroxine (FT4) ratio is an index that reflects the peripheral bioavailability of thyroid hormones[9,10]. An increasing number of studies have shown that higher values of these composite indices are associated with hyperuricemia, homocysteinemia, vitamin D deficiency, obesity, metabolic syndrome, diabetes, hypertension, reduced kidney function, and diabetes-related mortality, even in euthyroid populations[9-16]. These findings have led to new directions in research regarding the relationship between thyroid function and lipid metabolism. Liu et al[17] reported that the risk of dyslipidemia was positively correlated with the TFQI, TSHI, and TT4RI and negatively correlated with FT3/FT4 in patients with coronary heart disease. A recent study indicated that among euthyroid adults, reduced central and peripheral sensitivity to thyroid hormones was associated with high remnant cholesterol (RC) levels[10]. To our knowledge, no study has investigated the association between thyroid hormone sensitivity and dyslipidemia in the T2DM population; in particular, there is a lack of focus on non-high-density lipoprotein cholesterol (non-HDL-C)[17-21]. Non-HDL-C, calculated as total cholesterol (TC) minus high-density lipoprotein (HDL), includes all plasma lipoproteins, such as low-density lipoprotein cholesterol (LDL-C), triglyceride (TG)-rich lipoprotein (TRL), TRL-remnants, and lipoprotein(a)[22]. As non-HDL-C is a measure of all atherogenic cholesterol, it is not surprising that it strongly correlates with ASCVD risk and is also better at predicting ASCVD risk in patients on statin therapy and/or in those with T2DM[23]. Usually, maintaining the optimum level of LDL-C is the primary goal for dyslipidemia management in the general population. However, patients with T2DM who have extremely low LDL-C levels still remain at a very high risk of ASCVD[24]. In line with international guidelines, the 2020 Chinese Guideline on the Primary Prevention of Car-diovascular Diseases recommends non-HDL-C as an alternative treatment target to LDL-C[25]. Previous studies have revealed that TSH levels within the reference range are positively associated with increased non-HDL-C. However, the relationship between thyroid hormone sensitivity and non-HDL-C has rarely been investigated. Thus, the effects of thyroid hormone and thyroid hormone sensitivity on non-HDL-C in individuals with T2DM remain unclear.
Therefore, the purpose of this study was to clarify the association between thyroid hormone sensitivity and non-HDL-C in patients with T2DM and to further explore these associations in different sexes in an attempt to provide new evidence for the role of impaired thyroid hormone sensitivity for serum atherogenic non-HDL-C levels.
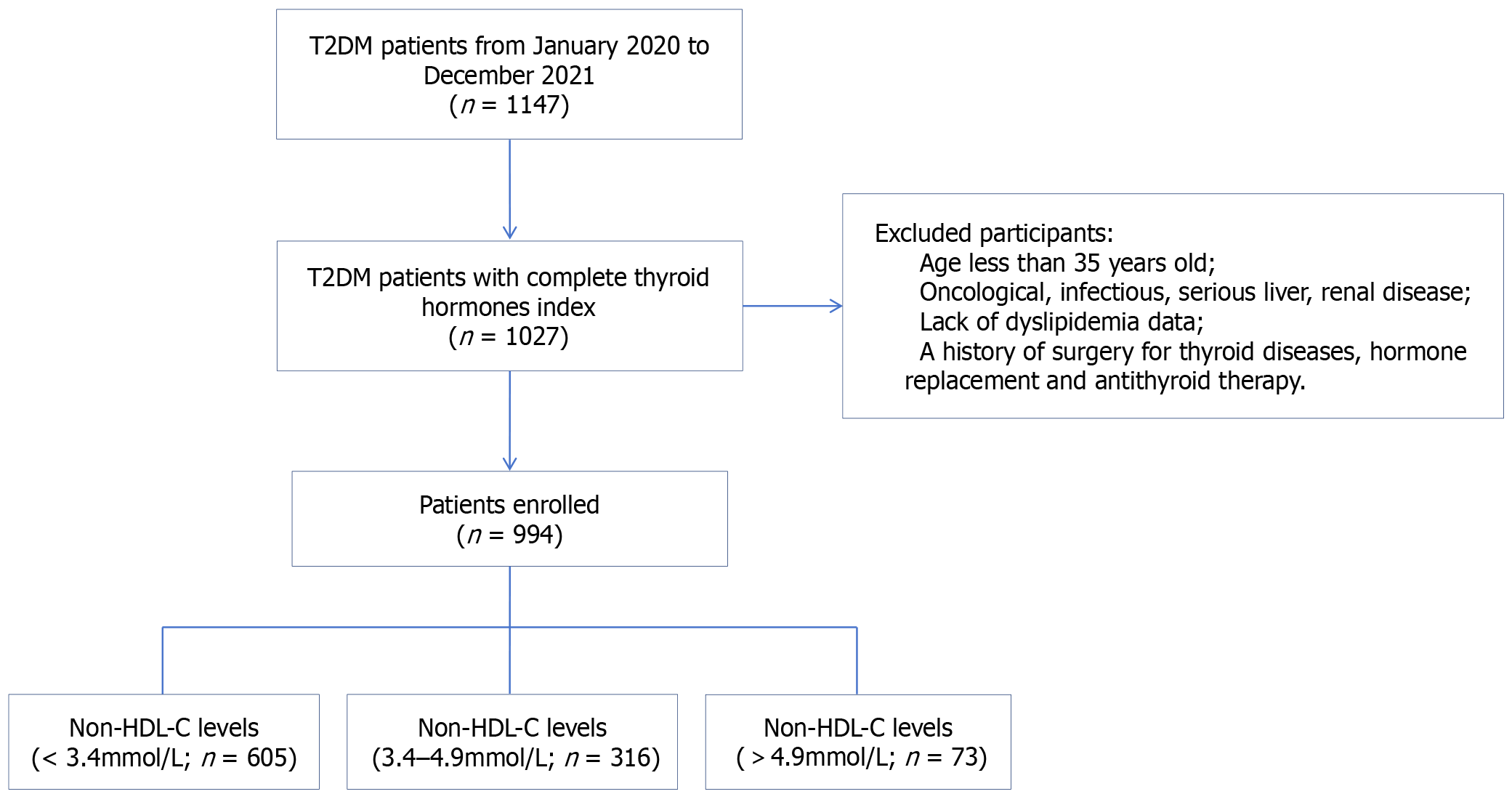
A total of 1147 patients with T2DM were recruited from the Department of Endocrinology, Xuanwu Hospital, Capital Medical University, from January 2020 to December 2021. All participants met the 1999 World Health Organization diagnosis and classification criteria for T2DM. The exclusion criteria were as follows: (1) Age ≤ 35 years; (2) Oncological, infectious, serious liver or renal disease; (3) Lack of data on TSH, FT3, FT4, TC, or HDL cholesterol (HDL-C); and (4) A history of surgery for thyroid diseases, antithyroid therapy and hormone replacement. After exclusion, 994 participants were included in the final analysis (Figure 1).
Blood samples from the participants were obtained after overnight fasting and were measured in the biochemistry laboratory of Xuanwu Hospital of Capital Medical University. Biochemical parameters, including fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), TC, TG, HDL-C, low-density lipoprotein (LDL), uric acid, albumin, prealbumin, 25-hydroxyvitamin D [25(OH)D] and hemoglobin, were measured. Fasting blood insulin and fasting blood C-peptide levels were also measured.
All study subjects fasted for 10 h, and elbow venous blood was collected in the morning to determine FBG, TG, TC, LDL-C, and HDL-C levels. The HbA1c values were determined via liquid chromatography tandem mass spectrometry. Fasting insulin and C-peptide levels were measured via radioimmunoassay. The level of TSH was measured using a third-generation immunoassay. FT3 and FT4 levels were measured via a competitive immunoassay. The reference ranges for FT3, FT4, and TSH were 2.3-4.2 pg/mL, 0.89-1.76 ng/dL, and 0.55-4.78 mLU/L, respectively.
Hyper-non-HDL-C, hypertriglyceridemia, hypercholesterolemia, hypo-HDL cholesterolemia, and hyper-low-density lipoprotein cholesterolemia were defined as non-HDL-C ≥ 3.4 mmol/L, TG ≥ 1.7 mmol/L, TC ≥ 5.2 mmol/L, HDL-C < 1.0 mmol/L, and LDL-C ≥ 3.4 mmol/L[5]. Hypertension was defined as a systolic blood pressure (SBP) ≥ 130 mmHg, a diastolic blood pressure (DBP) ≥ 85 mmHg or specific treatment for previously diagnosed hypertension[13].
Central indices of thyroid hormone sensitivity were calculated with the following formulas: TFQI = cumulative distribution function (CDF) FT4 - (1 - CDF TSH). TSHI = Ln TSH (μIU/mL) + 0.1345 × FT4 (pmol/L). TT4RI = FT4 (pmol/L) × TSH (μIU/mL). Central thyroid hormone sensitivity indicators reflect the response of the hypothalamus-pituitary-thyroid axis to changes in peripheral FT4. Negative values indicate higher central sensitivity, and positive values indicate lower central sensitivity to changes in FT4. For TSHI, TT4RI, and TFQI, the higher the values, the lower is the central sensitivity to thyroid hormones[9].
The peripheral index of thyroid hormone sensitivity was calculated as follows: FT3/FT4 ratio = FT3 (pmol/L)/FT4 (pmol/L). Higher values indicate greater peripheral sensitivity to thyroid hormones.
The fasting RC level was calculated as TC - (HDL-C + LDL-C) (mmol/L)[10].
Non-HDL-C was calculated as TC - HDL-C (mmol/L). Homeostasis Model Assessment of insulin resistance (HOMA-IR) was calculated as the fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5.
For continuous variables, the data are presented as the means ± SDs or medians (upper and lower ranges). Data for categorical variables are expressed as numbers (%). One-way ANOVA, the Kruskal-Wallis H (K) test or the chi-square test was used for comparisons between variables where appropriate. The results of the logistic regression analysis are presented as ORs and 95%CIs. Pearson’s and partial correlation coefficients were used to explore the associations of non-HDL-C with the thyroid-associated variables adjusted for sex, age, body mass index (BMI) and HbA1c. To further evaluate the potential associations of hyper-non-HDL-C with impaired thyroid hormone sensitivity, a logistic regression model was performed, and stratification was performed according to sex. IBM SPSS Statistics software, version 24.0 (IBM Corp., Armonk, NY, United States), and GraphPad 7.0 software were used for analyses of the data. Two-tailed P values < 0.05 were considered statistically significant. The Shapiro-Wilk test was used for the normality test. Random forest variable importance and Shapley Additive Explanations (SHAP) summary plots were used to identify the strength and direction of the association between hyper-non-HDL-C and its major predictor.
A total of 994 adults included in this study were divided into three groups (non-HDL-C < 3.4 mmol/L, 3.4-4.9 mmol/L and > 4.9 mmol/L). The characteristics of the participants according to different non-HDL-C levels are shown in Table 1. The median age was 65.8 years (range 35.0-89.0), and 447 patients (44.9%) were males. Among all individuals with T2DM, 533 (54.1%) had fatty liver, 561 (56.8%) had hypertension, and 155 (27.5%) had cardiovascular disease.
| Characteristics | All (n = 994) | Non-HDL-C levels (mmol/L) | P value | ||
| < 3.4 (n = 605) | 3.4-4.9 (n = 316) | > 4.9 (n = 73) | |||
| Age (years) | 65.8 (35.0-89.0) | 66.0 (36.0-89.0) | 64.0 (35.0-86.0) | 64.0 (36.0-83.6) | 0.010 |
| Sex (male/female) | 447/547 | 278/327 | 140/176 | 29/44 | 0.576 |
| Disease duration of T2DM (years) | 13.0 (0.0-40.0) | 13.0 (0.0-35.0) | 13.0 (0.0-40.0) | 11.0 (0.02-30.0) | 0.336 |
| BMI (kg/m2) | 25.6 (14.38-47.97) | 25.6 (14.38-45.45) | 25.6 (16.0-47.9) | 25.6 (19.5-41.1) | 0.743 |
| SBP (mmHg) | 130 (90-210) | 130 (90-190) | 130 (90-210) | 130 (110-180) | 0.294 |
| DBP (mmHg) | 80 (50-120) | 80 (50-120) | 80 (50-110) | 80 (60-100) | 0.162 |
| FPG (mmol/L) | 8.22 (2.88-24.52) | 7.79 (3.12-24.17) | 8.84 (2.88-23.49) | 9.97 (3.25-24.52) | < 0.001 |
| Fasting C peptide (ng/mL) | 2.30 (0.01-16.34) | 2.21 (0.01-13.35) | 2.37 (0.17-16.34) | 2.78 (0.27-6.93) | 0.008 |
| HOMA-IR | 4.47 (0.04-279.63) | 4.22 (0.05-243.20) | 4.74 (0.47-279.63) | 5.43 (0.04-34.27) | 0.047 |
| HbA1c (%) | 8.1 (4.9-15.1) | 7.8 (4.9-14.8) | 8.7 (5.4-15.1) | 9.2 (6.0-14.7) | < 0.001 |
| Creatinine (μmoI/L) | 62 (28-266) | 62 (30-241) | 60 (28-230) | 65 (30-266) | 0.016 |
| eGFR [mL/min (1.73 m2)-1] | 101.1 (19.1-323.7) | 100.7 (21.1-291.4) | 103.5 (19.1-323.7) | 95.0 (22.4-284.9) | 0.009 |
| UA (mmol/L) | 324 (7-811) | 324 (7-811) | 321 (98-797) | 338 (209-612) | 0.126 |
| UACR (mg/g) | 4.0 (0.0-2404.2) | 4.1 (0.0-1637.8) | 2.6 (0.0-1796.2) | 12.8 (0.1-2402.2) | 0.013 |
| 25(OH)D (ng/mL) | 16.80 (3.00-57.55) | 17.52 (3.00-57.55) | 16.57 (3.00-50.21) | 14.73 (3.31-35.78) | 0.039 |
| Fatty liver, n (%) | 533 (54.1) | 304 (50.6) | 180 (57.7) | 49 (67.1) | 0.002 |
| Hypertension, n (%) | 561 (56.8) | 344 (57.2) | 172 (54.8) | 45 (61.6) | 0.530 |
| Cardiovascular disease, n (%) | 155 (27.5) | 111 (33.5) | 34 (82.9) | 10 (24.4) | 0.002 |
| Diabetic retinopathy, n (%) | 238 (24.1) | 142 (23.6) | 68 (21.8) | 28 (38.4) | 0.011 |
| Diabetic peripheral neuropathy, n (%) | 411 (41.7) | 248 (41.2) | 123 (49.5) | 40 (54.8) | 0.055 |
| Diabetic peripheral vascular disease, n (%) | 157 (38.8) | 89 (38.5) | 55 (38.5) | 13 (41.9) | 0.931 |
| Serum lipid level (mmol/L) | |||||
| TG | 1.35 (0.24-21.80) | 1.37 (0.24-8.59) | 1.69 (0.39-9.99) | 2.51 (0.63-21.80) | < 0.001 |
| TC | 4.25 (2.03-10.35) | 3.74 (2.03-5.74) | 5.07 (4.13-7.20) | 6.53 (5.58-10.35) | < 0.001 |
| LDL-C | 2.51 (0.15-6.88) | 2.08 (0.15-3.80) | 3.28 (1.12-4.66) | 4.32 (1.37-6.88) | < 0.001 |
| HDL-C | 1.10 (0.33-3.42) | 1.10 (0.33-3.42) | 1.10 (0.47-2.66) | 1.09 (0.58-1.90) | 0.896 |
| non-HDL-C | 3.10 (1.02-9.30) | 2.56 (1.02-3.39) | 3.95 (3.40-4.89) | 5.3 (4.90-9.30) | < 0.001 |
| RC | 0.53 (-1.31-7.93) | 0.45 (-1.31-2.40) | 0.67 (-0.34-3.66) | 1.13 (-0.33-7.93) | < 0.001 |
| Thyroid function and indices of thyroid hormone sensitivity | |||||
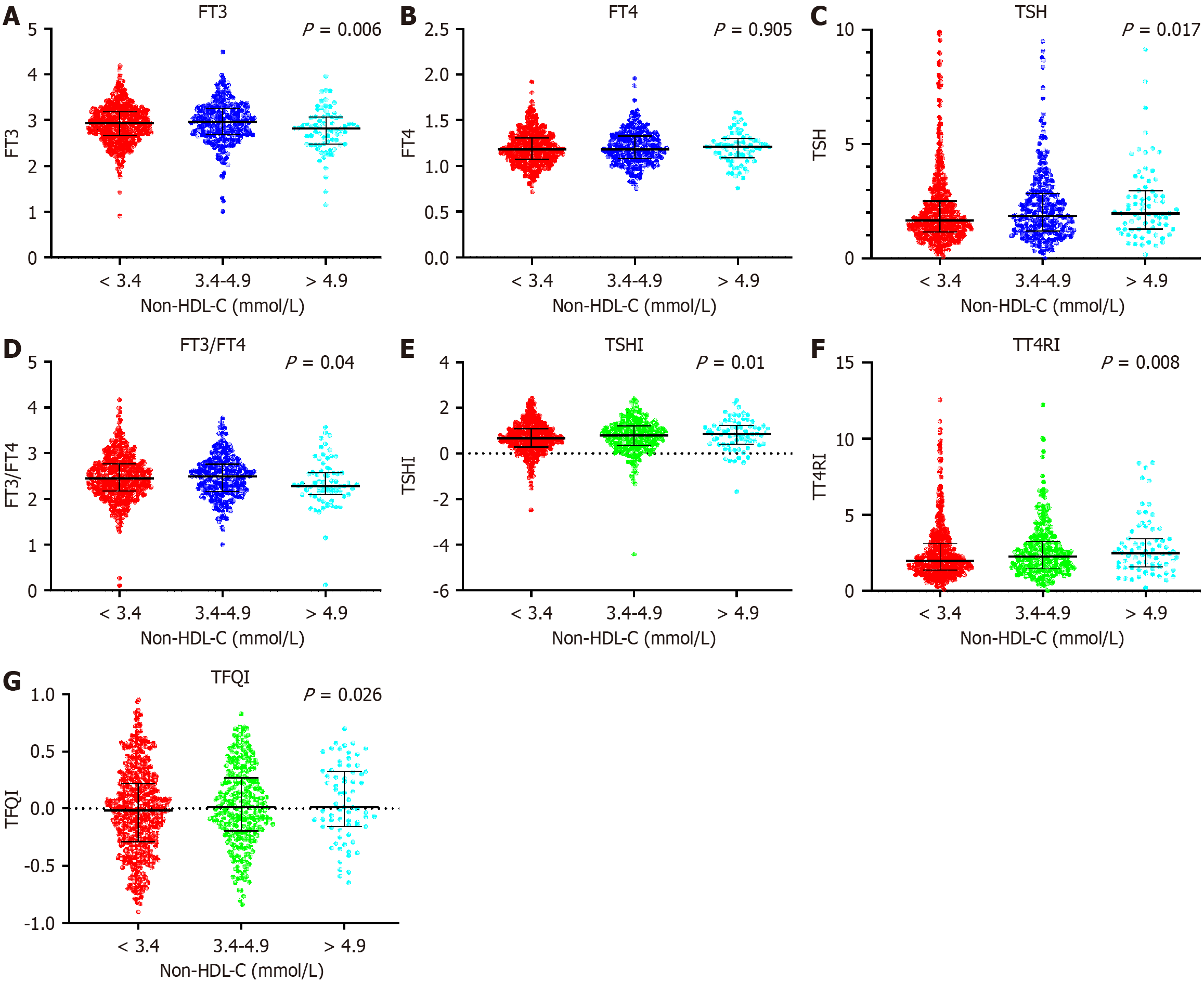
| FT3 (pg/mL) | 2.93 (0.91-4.49) | 2.93 (0.91-4.19) | 2.97 (1.01-4.49) | 2.80 (1.15-3.97) | 0.006 |
| FT4 (ng/dL) | 1.19 (0.72-1.59) | 1.18 (0.72-1.92) | 1.18 (0.75-1.96) | 1.21 (0.76-1.59) | 0.905 |
| TSH (uIU/mL) | 1.74 (0.01-9.89) | 1.65 (0.07-9.89) | 1.86 (0.07-9.48) | 2.03 (0.16-9.12) | 0.017 |
| FT3/FT4 | 2.47 ± 0.47 | 2.47 ± 0.47 | 2.48 ± 0.45 | 2.34 ± 0.52 | 0.040 |
| TT4RI | 2.10 (0.01-12.23) | 1.99 (0.10-9.89) | 2.27 (0.1-12.23) | 2.56 (0.20-8.43) | 0.008 |
| TSHI | 0.72 (-4.43-2.42) | 0.66 (-2.48-2.42) | 0.79 (-4.43-2.42) | 0.91 (-1.66-2.63) | 0.010 |
| TFQI | 0.00 ± 0.35 | -0.02 ± 0.36 | 0.03 ± 0.34 | 0.08 ± 0.34 | 0.026 |
Among them, 389 patients (39.13%) had high non-HDL-C levels (≥ 3.4 mmol/L). Compared with the normal non-HDL-C group (< 3.4 mmol/L), there was no significant difference in BMI, SBP, DBP or the incidence of hypertension. The levels of fasting plasma glucose (FPG), HbA1c, and HOMA-IR were significantly increased in the very high non-HDL-C level group (> 4.9 mmol/L) and decreased in the normal non-HDL-C group. These findings suggested poorer glycemic control in high non-HDL-C group. However, the levels of estimated glomerular filtration rate (eGFR) and 25(OH)D were significantly reduced in the very high non-HDL-C level group and significantly elevated in the normal non-HDL-C group (P < 0.05). TSH, TFQI, TSHI, TT4RI, TG, TC, LDL-C and RC levels tended to increase with increasing HDL level (P < 0.05), whereas the levels of FT3 and FT3/FT4 were significantly lower in the high non-HDL-C group than in the normal non-HDL-C group (P < 0.05; Table 1).
As shown in Figure 2, central thyroid hormone sensitivity indices, including the TFQI, TSHI, and TT4RI, were significantly elevated in patients in the high non-HDL-C group, whereas the peripheral thyroid hormone sensitivity index FT3/FT4 was significantly lower (P < 0.05).
The correlations between non-HDL-C levels and thyroid-associated variables are presented in Table 2. TSH, TT4RI, TSHI, and the TFQI were positively associated with non-HDL-C levels, whereas the FT3/FT4 ratio was negatively associated with non-HDL-C levels in all participants. FT3 and FT4 were negatively correlated with the levels of non-HDL-C in men, whereas TSHI, TT4RI were positively correlated. However, non-HDL-C levels were negatively associated with the FT3/FT4 ratio and positively associated with the TFQI in women (all P < 0.05).
| Variables | All | Male | Female | ||||
| r | P value | r | P value | r | P value | ||
| FT3 (pg/mL) | Model 1 | -0.090a | 0.005 | -0.153a | 0.001 | -0.062 | 0.147 |
| Model 2 | -0.128a | < 0.001 | -0.187a | < 0.001 | -0.095 | 0.027 | |
| Model 3 | -0.113a | < 0.001 | -0.168a | < 0.001 | -0.082 | 0.059 | |
| FT4 (ng/dL) | Model 1 | -0.008 | 0.795 | -0.115b | 0.015 | 0.014 | 0.737 |
| Model 2 | -0.013 | 0.693 | -0.118b | 0.013 | 0.012 | 0.78 | |
| Model 3 | -0.024 | 0.451 | -0.14a | 0.003 | 0 | 0.991 | |
| TSH (uIU/mL) | Model 1 | 0.073b | 0.022 | 0.141a | 0.003 | 0.007 | 0.867 |
| Model 2 | 0.079b | 0.014 | 0.143a | 0.002 | 0.016 | 0.714 | |
| Model 3 | 0.093a | 0.004 | 0.154a | 0.001 | 0.024 | 0.575 | |
| FT3/FT4 | Model 1 | -0.051 | 0.107 | -0.019 | 0.694 | -0.075 | 0.078 |
| Model 2 | -0.076b | 0.017 | -0.037 | 0.441 | -0.101b | 0.019 | |
| Model 3 | -0.033 | 0.301 | -0.003 | 0.942 | -0.052 | 0.228 | |
| TT4RI | Model 1 | 0.076b | 0.018 | 0.121b | 0.01 | 0.037 | 0.394 |
| Model 2 | 0.079b | 0.013 | 0.121b | 0.011 | 0.044 | 0.308 | |
| Model 3 | 0.088a | 0.006 | 0.129a | 0.007 | 0.046 | 0.286 | |
| TSHI | Model 1 | 0.085a | 0.008 | 0.129a | 0.006 | 0.052 | 0.224 |
| Model 2 | 0.087a | 0.006 | 0.129a | 0.007 | 0.058 | 0.175 | |
| Model 3 | 0.103a | 0.001 | 0.14a | 0.003 | 0.069 | 0.111 | |
| TFQI | Model 1 | 0.078b | 0.014 | 0.029 | 0.539 | 0.121a | 0.004 |
| Model 2 | 0.074b | 0.02 | 0.024 | 0.612 | 0.121a | 0.005 | |
| Model 3 | 0.065b | 0.044 | 0.014 | 0.777 | 0.109b | 0.011 | |
We also analyzed the correlations between lipid profiles such as TG, TC, HDL, LDL and RC and thyroid-associated variables (Table 3). Lipid profiles, especially TG, TC and RC, were significantly associated with thyroid hormone sensitivity indices before and after adjusting for age, sex, BMI, and HbA1c. LDL levels were negatively associated with FT3 and the FT3/FT4 ratio and positively associated with TSHI, whereas HDL levels were negatively associated with FT3, TSH, the FT3/TF4 ratio, the TT4RI and the TSHI.
| Variables | TG | TC | LDL-C | HDL-C | RC | ||||||
| r | P value | r | P value | r | P value | r | P value | r | P value | ||
| FT3 (pg/mL) | Model 1 | -0.092a | 0.004 | -0.116a | < 0.001 | -0.086a | 0.007 | -0.093a | 0.004 | -0.037 | 0.246 |
| Model 2 | -0.114a | < 0.001 | -0.149a | < 0.001 | -0.106a | 0.001 | -0.083a | 0.009 | -0.081b | 0.011 | |
| Model 3 | -0.099a | 0.002 | -0.137a | < 0.001 | -0.094a | 0.003 | -0.097a | 0.003 | -0.068b | 0.035 | |
| FT4 (ng/dL) | Model 1 | -0.007 | 0.838 | 0.001 | 0.971 | 0.008 | 0.8 | 0.03 | 0.352 | -0.032 | 0.312 |
| Model 2 | -0.01 | 0.764 | -0.003 | 0.928 | 0.006 | 0.84 | 0.03 | 0.349 | -0.039 | 0.224 | |
| Model 3 | -0.02 | 0.535 | -0.012 | 0.717 | -0.002 | 0.948 | 0.037 | 0.249 | -0.048 | 0.139 | |
| TSH (uIU/mL) | Model 1 | 0.074b | 0.021 | 0.045 | 0.157 | 0.04 | 0.206 | -0.080b | 0.013 | 0.083a | 0.01 |
| Model 2 | 0.077b | 0.016 | 0.05 | 0.12 | 0.043 | 0.177 | -0.082b | 0.01 | 0.09a | 0.005 | |
| Model 3 | 0.082b | 0.01 | 0.063 | 0.05 | 0.056 | 0.084 | -0.08b | 0.012 | 0.095a | 0.003 | |
| FT3/FT4 | Model 1 | -0.082b | 0.01 | -0.093a | 0.003 | -0.065b | 0.041 | -0.141a | < 0.001 | 0.008 | 0.802 |
| Model 2 | -0.095a | 0.003 | -0.114a | < 0.001 | -0.078b | 0.015 | -0.134a | < 0.001 | -0.021 | 0.519 | |
| Model 3 | -0.061 | 0.06 | -0.082b | 0.011 | -0.045 | 0.167 | -0.163a | < 0.001 | 0.01 | 0.749 | |
| TT4RI | Model 1 | 0.079b | 0.013 | 0.052 | 0.102 | 0.046 | 0.147 | -0.066b | 0.039 | 0.078b | 0.014 |
| Model 2 | 0.081b | 0.011 | 0.055 | 0.087 | 0.048 | 0.133 | -0.068b | 0.034 | 0.082b | 0.01 | |
| Model 3 | 0.081b | 0.012 | 0.065b | 0.043 | 0.057 | 0.075 | -0.061 | 0.057 | 0.083b | 0.01 | |
| TSHI | Model 1 | 0.099a | 0.002 | 0.061 | 0.056 | 0.048 | 0.135 | -0.066b | 0.039 | 0.095a | 0.003 |
| Model 2 | 0.1a | 0.002 | 0.063b | 0.049 | 0.049 | 0.126 | -0.067b | 0.036 | 0.098a | 0.002 | |
| Model 3 | 0.103a | 0.001 | 0.079b | 0.014 | 0.064b | 0.047 | -0.059 | 0.065 | 0.102a | 0.002 | |
| TFQI | Model1 | 0.087a | 0.006 | 0.074b | 0.02 | 0.059 | 0.066 | -0.003 | 0.922 | 0.061 | 0.054 |
| Model2 | 0.085a | 0.008 | 0.071b | 0.026 | 0.056 | 0.078 | < 0.001 | 0.989 | 0.057 | 0.076 | |
| Model3 | 0.068b | 0.035 | 0.068b | 0.035 | 0.052 | 0.106 | 0.02 | 0.529 | 0.043 | 0.181 | |
To investigate the relationship between hyper-non-HDL-C and impaired sensitivity to thyroid hormones, we performed logistic regression analyses (Table 4). The risk of hyper-non-HDL-C was positively correlated with the TFQI (OR: 1.584; 95%CI: 1.088-2.304; P = 0.016), TSHI (OR: 1.238; 95%CI: 1.034-1.482; P = 0.02), and TT4RI (OR: 1.075; 95%CI: 1.006-1.149; P = 0.032) but was not significantly correlated with the FT3/FT4 ratio. Even after adjusting for age, sex, BMI, and HbA1c, the associations between hyper-non-HDL-C and impaired central sensitivity to thyroid hormones were significant. However, the relationships between composite indices of the thyroid system and non-HDL-C levels differed according to sex. An increased risk of hyper-non-HDL-C was associated with elevated TSHI levels in men (OR: 1.331; 95%CI: 1.003-1.766; P = 0.048) but elevated TFQI levels in women (OR: 2.337; 95%CI: 1.4-3.901; P = 0.001).
| Variables | All | Male | Female | |||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| FT3 (pg/mL) | 0.91 (0.71-1.166) | 0.456 | 0.698 (0.41-1.191) | 0.187 | 1.002 (0.769-1.306) | 0.987 |
| FT4 (ng/dL) | 0.919 (0.689-1.225) | 0.564 | 0.194 (0.061-0.617) | 0.005 | 1.012 (0.775-1.32) | 0.932 |
| TSH (uIU/mL) | 1.091 (1.015-1.172) | 0.018 | 1.136 (1.02-1.264) | 0.02 | 1.037 (0.935-1.15) | 0.49 |
| FT3/FT4 | 1.01 (0.754-1.352) | 0.949 | 1.369 (0.861-2.177) | 0.184 | 0.846 (0.577-1.24) | 0.392 |
| TT4RI | 1.075 (1.006-1.149) | 0.032 | 1.086 (0.985-1.197) | 0.097 | 1.055 (0.963-1.156) | 0.252 |
| TSHI | 1.238 (1.034-1.482) | 0.02 | 1.331 (1.003-1.766) | 0.048 | 1.155 (0.918-1.452) | 0.218 |
| TFQI | 1.584 (1.088-2.304) | 0.016 | 0.961 (0.545-1.692) | 0.89 | 2.337 (1.4-3.901) | 0.001 |
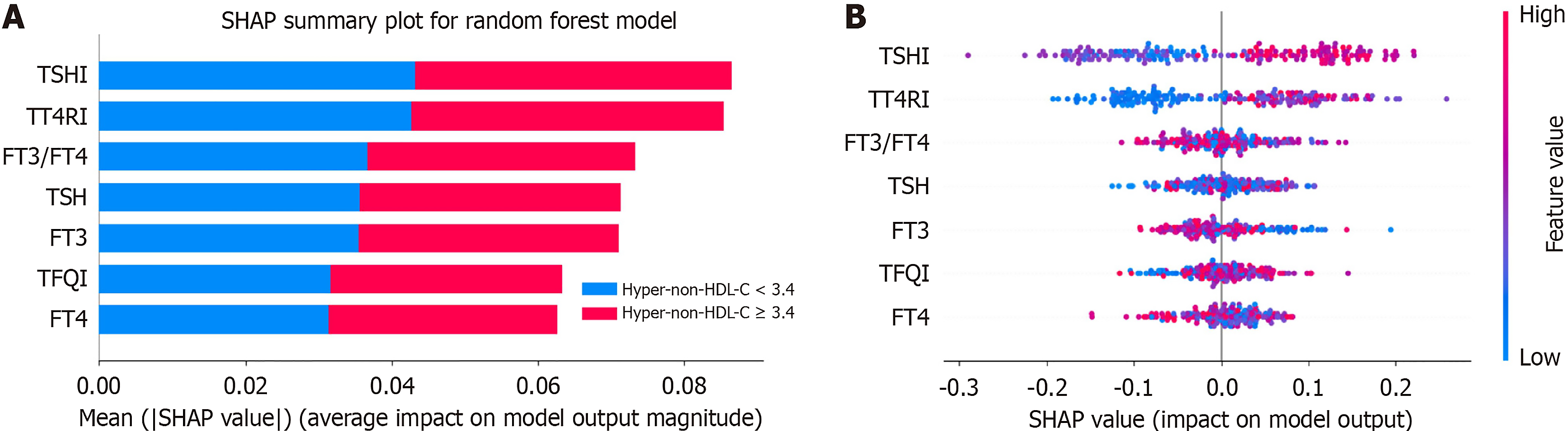
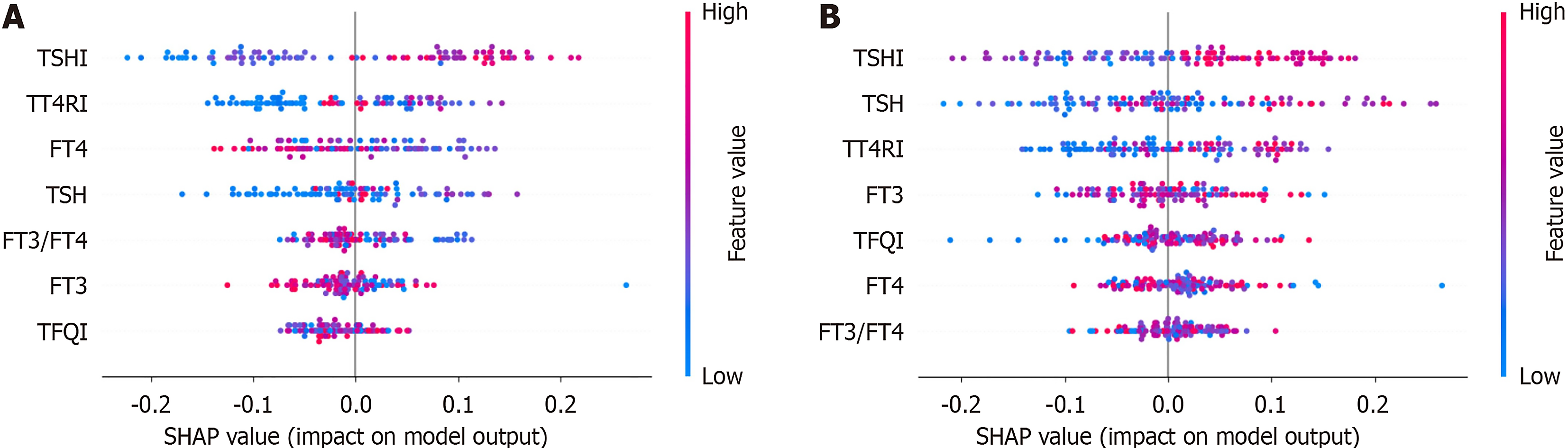
SHAP was employed to assess the importance and contribution of thyroid-related variables within the optimal random forest models for hyper-non-HDL-C, with a prioritized list vividly illustrating their respective impacts (Figure 3). Among the analyzed features, the average SHAP values were highest for TSHI, followed by TT4RI (Figure 3A). The distribution of the SHAP scores was also analyzed for each feature (Figure 3B). As shown in the SHAP summary plot, the red dots indicate high feature values; however, the blue dots represent low feature values. SHAP values above zero suggested higher non-HDL-C values, whereas values below zero indicated lower non-HDL-C values. For example, a higher TSHI, TT4RI and TFQI (depicted in red) correlated with higher SHAP values, suggesting that they were all risk factors for hyper-non-HDL-C. Regarding the TSHI in the different sex models (Figure 4), an increase in the TSHI was strongly associated with an increase in its contribution to the model predictions. In terms of contributors in the sex models, the TT4RI ranked second in the male model and third highest in the female model. Compared with these factors, the other features had smaller contributions. The rankings of these other features also varied between different sex models.
The present study revealed that impaired sensitivity to thyroid hormones was significantly associated with non-HDL-C levels in the T2DM population. Our results indicated that reduced central thyroid hormone sensitivity (increased TSHI, TT4RI, and TFQI) was an independent risk factor for hyper-non-HDL-C, even after adjusting for multiple confounding factors. Moreover, the associations between thyroid hormone sensitivity indices and non-HDL-C levels differed between men and women, suggesting that sex-associated regulation of thyroid hormones impacted serum non-HDL-C levels.
Our study revealed that the levels of TSH, TFQI, TSHI, and TT4RI were significantly greater in individuals with hyper-non-HDL-C than in those with normal non-HDL-C, indicating the presence of central thyroid hormone resistance in participants with high non-HDL-C levels. Moreover, the FT3/FT4 ratio decreased, indicating impaired thyroid hormone sensitivity in peripheral organs. Furthermore, central thyroid hormone sensitivity indices (TFQI, TSHI, and TT4RI) were found to be independent risk factors for hyper-non-HDL-C in patients with T2DM. Moreover, we identified the strength and direction of the association between high non-HDL-C and its major predictor derived from random forest variable importance using SHAP and random forest model analyses. To our knowledge, no study has investigated the association between thyroid hormone sensitivity and non-HDL-C[4,5,17-19]. Non-HDL-C is a better marker of atherogenicity and represents the residual ASCVD risk in patients who have achieved target LDL-C goals[21-23]. Since non-HDL-C is known as ‘bad cholesterol’, it contains all atherogenic lipoproteins, which accumulate in the intima of the arteries, leading to the formation of atherosclerotic plaques[20]. According to international guidelines, the 2023 Chinese Guideline on the Primary Prevention of cardiovascular disease (CVD) recommends non-HDL-C as an alternative treatment target for LDL-C; however, clinicians often do not pay sufficient attention to this point[23]. Asvold et al[24] conducted a large sample cohort study with a follow-up of 11 years and reported that changes in TSH levels were associated with concomitant changes in non-HDL-C and TG levels (all P < 0.001) irrespective of sex. Several studies have implied a close association between higher normal-range TSH and concentrations of total serum non-HDL-C parameters[19,26]. Thyroid hormones within the reference range combined with elevated TSH have been shown to be associated with pronounced lipid disorders and consequently an increased risk of atherosclerotic vascular disease[7,8]. Physiologically, thyroid hormones and TSH are inversely correlated owing to a negative feedback loop. However, high thyroid hormones can coexist with high TSH in individuals with resistance to thyroid hormones[9]. Reduced sensitivity to thyroid hormone in the general population has been shown to be a vital risk factor for various metabolic diseases, such as diabetes and hypertension[5,13,15]. These discoveries have also led to new directions in research regarding the relationship between thyroid function and lipid metabolism. Our findings suggest that the composite thyroid hormone sensitivity indices are better indices than the absolute values of FT3, FT4, and TSH, which could provide more information on thyroid function and directly relate thyroid hormone resistance to lipid metabolism.
Our study demonstrated that lipid profiles, especially TG, TC and RC, were also significantly associated with thyroid hormone sensitivity indices. However, HDL-C levels were negatively associated with peripheral thyroid hormone resistance. In line with previous studies, Liu et al[17] analyzed the associations between thyroid system indices and lipid profiles (TC, TG, HDL-C, LDL-C) and reported that the risk of dyslipidemia was positively correlated with TFQI, TSHI, and TT4RI and negatively correlated with FT3/FT4 in patients with coronary heart disease. A recent large sample study indicated that among euthyroid adults, reduced sensitivity to thyroid hormones was associated with high RC levels[10]. Therefore, sensitivity to thyroid hormones is associated with dyslipidemia, indicating that periodic screening of thyroid hormones in the T2DM population is recommended to aid early prevention of dyslipidemia.
In addition, our findings also revealed that elevated TFQI levels were associated with an increased risk of hyper-non-HDL-C in women. The TFQI is a new index for detecting acquired thyroid hormone resistance that was first proposed in 2019 by Laclaustra et al[9]. The performance of the TFQI was shown to be more stable than that of the TSHI and TT4RI[9]. Lipid abnormalities are especially relevant in women because they escalate rapidly with biological aging and endocrine changes related to menopause[26]. The menopausal transition and loss of estrogen possibly explain this association between TFQI and non-HDL-C in females, which might act as a trigger factor and impede metabolic function[27].
In this study, the patients with high non-HDL-C levels had not only higher TG, TC, LDL-C and RC levels but also significantly higher levels of FPG, HbA1c, and HOMA-IR, suggesting a poorer balance of glucose-lipid metabolism in individuals with high non-HDL-C. Similar findings have also been reported by Vazirian et al[28], indicating that elevated non-HDL-C serves as a significant predictor of glucose-lipid metabolism. In addition, patients with high non-HDL-C levels had worse renal function (lower eGFRs and higher creatinine levels compared with those in the normal group, P < 0.05). Among the previous studies that investigated the association of non-HDL-C and renal function, conclusions have been inconsistent[29,30]. Saland et al[30] reported that longitudinal increases in proteinuria and decreases in eGFR were independently associated with significant concomitant increases in non-HDL-C in children with chronic kidney disease. In another study, the prevalence of hyper-non-HDL-C was not related to chronic kidney disease stage[29]. Moreover, our data revealed that the 25(OH)D level was significantly reduced in the high non-HDL-C group compared with the normal non-HDL-C group. Most previous studies have shown no associations between vitamin D deficiency and elevated non-HDL-C[31,32]. Nwosu et al[33] reported significant inverse correlations between 25(OH)D and non-HDL-C cholesterol. In brief, patients with high non-HDL-C levels could face multiple endocrine and metabolic disorders, which is worth exploring in larger sample studies.
The novelty of this study lies in providing another layer of evidence for resistance to thyroid hormones as an independent risk factor for hyper-non-HDL in the T2DM population, which would be highly important for the T2DM population with an increased risk of ASCVD. The limitations of this study should be acknowledged. First, this was a cross-sectional study that utilized blood sample data from only a single point, which means that the direct causal relationship between non-HDL-C levels and thyroid hormone sensitivity cannot be inferred. However, this study supports the important hypothesis that thyroid hormone sensitivity may be useful for assessing the risk of dyslipidemia. In the future, more studies are needed to demonstrate causality. Second, information on the medication history of the participants was lacking, which might affect the data for thyroid hormone and non-HDL levels. Third, because our analysis was restricted to patients with T2DM selected only from Xuanwu Hospital, it is uncertain whether our findings can be generalized to other populations.
In conclusion, this is the first study to demonstrate an association of high non-HDL-C levels with reduced sensitivity to thyroid hormone in the patients with T2DM, providing new evidence for the role of reduced thyroid hormone sensitivity for non-HDL-C levels. Future investigations are needed to explore the underlying mechanism of this phenomenon and to lay the groundwork for future therapeutic strategies for diabetes mellitus-related CVD risk.
| 1. | Newman CB, Blaha MJ, Boord JB, Cariou B, Chait A, Fein HG, Ginsberg HN, Goldberg IJ, Murad MH, Subramanian S, Tannock LR. Lipid Management in Patients with Endocrine Disorders: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 271] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 3. | Wang C, Xie Z, Huang X, Wang Z, ShangGuan H, Wang S. Prevalence of cardiovascular disease risk factors in Chinese patients with type 2 diabetes mellitus, 2013-2018. Curr Med Res Opin. 2022;38:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Bulum T, Kolarić B, Duvnjak L. Insulin sensitivity modifies the relationship between thyroid function and lipid profile in euthyroid type 1 diabetic patients. Endocrine. 2012;42:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 258] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Mavromati M, Jornayvaz FR. Hypothyroidism-Associated Dyslipidemia: Potential Molecular Mechanisms Leading to NAFLD. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Liu XQ, Rahman A, Bagdade JD, Alaupovic P, Kannan CR. Effect of thyroid hormone on plasma apolipoproteins and apoA- and apoB-containing lipoprotein particles. Eur J Clin Invest. 1998;28:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Beukhof CM, Massolt ET, Visser TJ, Korevaar TIM, Medici M, de Herder WW, Roeters van Lennep JE, Mulder MT, de Rijke YB, Reiners C, Verburg FA, Peeters RP. Effects of Thyrotropin on Peripheral Thyroid Hormone Metabolism and Serum Lipids. Thyroid. 2018;28:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, Mateo-Gallego R, Casasnovas JA, Guallar-Castillon P, Cenarro A, Civeira F. Impaired Sensitivity to Thyroid Hormones Is Associated With Diabetes and Metabolic Syndrome. Diabetes Care. 2019;42:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 10. | Sun H, Zhu W, Liu J, An Y, Wang Y, Wang G. Reduced Sensitivity to Thyroid Hormones Is Associated With High Remnant Cholesterol Levels in Chinese Euthyroid Adults. J Clin Endocrinol Metab. 2022;108:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 11. | Ding X, Wang Y, Liu J, Wang G. Impaired Sensitivity to Thyroid Hormones Is Associated With Elevated Homocysteine Levels in the Euthyroid Population. J Clin Endocrinol Metab. 2022;107:e3731-e3737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Sun Y, Teng D, Zhao L, Shi X, Li Y, Shan Z, Teng W. Impaired Sensitivity to Thyroid Hormones Is Associated with Hyperuricemia, Obesity, and Cardiovascular Disease Risk in Subjects with Subclinical Hypothyroidism. Thyroid. 2022;32:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Mehran L, Delbari N, Amouzegar A, Hasheminia M, Tohidi M, Azizi F. Reduced Sensitivity to Thyroid Hormone Is Associated with Diabetes and Hypertension. J Clin Endocrinol Metab. 2022;107:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 14. | Lai S, Li J, Wang Z, Wang W, Guan H. Sensitivity to Thyroid Hormone Indices Are Closely Associated With NAFLD. Front Endocrinol (Lausanne). 2021;12:766419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Yang S, Lai S, Wang Z, Liu A, Wang W, Guan H. Thyroid Feedback Quantile-based Index correlates strongly to renal function in euthyroid individuals. Ann Med. 2021;53:1945-1955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Zhou L, Wang Y, Su J, An Y, Liu J, Wang G. Vitamin D Deficiency Is Associated with Impaired Sensitivity to Thyroid Hormones in Euthyroid Adults. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Ma M, Li L, Liu F, Li Z, Yu L, Yang T, Wang Y, Gao S, Gao S, Yang R, Yu C. Association between sensitivity to thyroid hormones and dyslipidemia in patients with coronary heart disease. Endocrine. 2023;79:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society; Geriatric Professional Committee of Beijing Medical Award Foundation; National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua Nei Ke Za Zhi. 2022;61:12-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |
| 19. | Asvold BO, Vatten LJ, Nilsen TI, Bjøro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 5938] [Article Influence: 989.7] [Reference Citation Analysis (0)] |
| 21. | Luo Y, Peng D. Residual Atherosclerotic Cardiovascular Disease Risk: Focus on Non-High-Density Lipoprotein Cholesterol. J Cardiovasc Pharmacol Ther. 2023;28:10742484231189597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Brandts J, Tittel SR, Bramlage P, Danne T, Brix JM, Zimny S, Heyer CHJ, Holl RW, Müller-Wieland D. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in type 1 diabetes and type 2 diabetes: Lipid goal attainment in a large German-Austrian diabetes registry. Diabetes Obes Metab. 2023;25:3700-3708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 23. | Joint Committee on the Chinese Guidelines for Lipid Management. [Chinese guidelines for lipid management (2023)]. Zhonghua Xin Xue Guan Bing Za Zhi. 2023;51:221-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 24. | Asvold BO, Bjøro T, Vatten LJ. Associations of TSH levels within the reference range with future blood pressure and lipid concentrations: 11-year follow-up of the HUNT study. Eur J Endocrinol. 2013;169:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Witte T, Ittermann T, Thamm M, Riblet NB, Völzke H. Association between serum thyroid-stimulating hormone levels and serum lipids in children and adolescents: a population-based study of german youth. J Clin Endocrinol Metab. 2015;100:2090-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Duntas LH, Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Med Clin North Am. 2012;96:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Oh HS, Kwon H, Ahn J, Song E, Park S, Kim M, Han M, Jeon MJ, Kim WG, Kim WB, Shong YK, Rhee EJ, Kim TY. Association Between Thyroid Dysfunction and Lipid Profiles Differs According to Age and Sex: Results from the Korean National Health and Nutrition Examination Survey. Thyroid. 2018;28:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 28. | Vazirian F, Darroudi S, Rahimi HR, Latifi M, Shakeri B, Abolbashari S, Mohammadpour AH, Esmaily H, Mouhebati M, Samadi S, Mobarhan MG. Non-HDL cholesterol and long-term follow-up outcomes in patients with metabolic syndrome. Lipids Health Dis. 2023;22:165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Zhang YB, Sheng LT, Wei W, Guo H, Yang H, Min X, Guo K, Yang K, Zhang X, He M, Wu T, Pan A. Association of blood lipid profile with incident chronic kidney disease: A Mendelian randomization study. Atherosclerosis. 2020;300:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Saland JM, Kupferman JC, Pierce CB, Flynn JT, Mitsnefes MM, Warady BA, Furth SL. Change in Dyslipidemia with Declining Glomerular Filtration Rate and Increasing Proteinuria in Children with CKD. Clin J Am Soc Nephrol. 2019;14:1711-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Mai XM, Videm V, Sheehan NA, Chen Y, Langhammer A, Sun YQ. Potential causal associations of serum 25-hydroxyvitamin D with lipids: a Mendelian randomization approach of the HUNT study. Eur J Epidemiol. 2019;34:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Ramiro-Lozano JM, Calvo-Romero JM. Effects on lipid profile of supplementation with vitamin D in type 2 diabetic patients with vitamin D deficiency. Ther Adv Endocrinol Metab. 2015;6:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Nwosu BU, Maranda L, Cullen K, Ciccarelli C, Lee MM. Vitamin D status is associated with early markers of cardiovascular disease in prepubertal children. J Pediatr Endocrinol Metab. 2013;26:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/