Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.942
Peer-review started: December 23, 2022
First decision: February 28, 2023
Revised: April 6, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: July 15, 2023
Processing time: 202 Days and 4.7 Hours
Diabetes-related foot disease (DFD) is a widely feared complication among people who live with diabetes. In Australia and globally, rates of disability, cardio-vascular disease, lower extremity amputation, and mortality are significantly increased in patients with DFD. In order to understand and prevent these outcomes, we analyse the common pathogenetic processes of neuropathy, arterial disease, and infection. The review then summarises important management considerations through the interdisciplinary lens. Using Australian and international guidelines, we offer a stepwise, evidence-based practical approach to the care of patients with DFD.
Core Tip: In Australia, the interdisciplinary service is recognised as a critical component of providing care to people with diabetes-related foot disease (DFD). We give our perspective on the management of DFD based on 6 categories: (1) Assessment and education in high-risk patients; (2) Wound preparation, debridement, and dressing; (3) Offloading and footwear; (4) Diagnosis and management of infection; (5) Interventions including revascularisation, pharmacotherapy, and novel wound therapies; and (6) Integrated interdisciplinary care and patient information.
- Citation: McNeil S, Waller K, Poy Lorenzo YS, Mateevici OC, Telianidis S, Qi S, Churilov I, MacIsaac RJ, Galligan A. Detection, management, and prevention of diabetes-related foot disease in the Australian context. World J Diabetes 2023; 14(7): 942-957
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/942.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.942
Diabetes affects an estimated 463 million people worldwide, a number expected to increase to over 700 million by 2045[1]. The increasing prevalence of diabetes in Australia is driven largely by obesity and an ageing population. People with diabetes have an increased rate of vascular complications even when adjusting for other established risk factors. The combination of obesity and diabetes is associated with higher rates of hospital admission and mortality due to diabetes complications[2]. Foot ulceration and lower extremity amputation (LEA) are catastrophic but preventable complications of diabetes. We review the epidemiology, risk factors, and classification system of diabetes-related foot disease (DFD). Furthermore, we offer a clinical perspective from a National Association of Diabetes Centres accredited High Risk Foot Service Centre of Excellence on the management of DFD based on 6 categories: (1) Assessment and education in high-risk patients; (2) Debridement, wound preparation, and dressings; (3) Offloading; (4) Management of infection; (5) Interventions including revascularisation, pharmacotherapy, and novel wound therapies; and (6) Integrated interdisciplinary care and patient information which summarise and synthesise the major treatment principles in recent guidelines[3,4].
We performed a literature review of PubMed, Medline, and Embase for articles published in English using the following key words: Diabetes-related foot disease; Foot ulceration; Lower extremity amputation; Neuropathy; Peripheral arterial disease; Infection. Recent international guidelines, Australian position statements, systematic reviews, and meta-analyses were preferred to provide an overview of Australian and International prevalence, best practices, and emerging strategies for the management of DFD. We have focused on the International Working Group on the Diabetic Foot (IWGDF) and Diabetes Foot Australia (DFA) guidelines to formulate our narrative review and recommendations.
DFD is defined as ulceration or infection of the foot associated with the key risk factors of peripheral neuropathy and/or peripheral arterial disease (PAD) in people with diabetes[3,5]. There is a huge global disability burden from DFD, which affected 131 million people worldwide in 2016, ranking 11th in the global disease burden[6].
At least 1 in 5 people with diabetes develop DFD in their lifetime, with an annual incidence of around 2%[7,8]. Aboriginal and Torres Strait Islander Australians have a 3-6 fold increased likelihood of experiencing diabetes-related foot complications compared to non-Indigenous Australians[9]. Ulcer recurrence is common, occurring at a rate of 40% within the 1st year after the ulcer has healed, almost 60% within 3 years, and 65% within 5 years[7,8,10].
DFD can be a surrogate marker of systemic disease. Armstrong et al[11] reported the 5-year mortality rates for DFD-related ulceration, and minor and major LEA were 30.5%, 46.2%, and 56.6% respectively, compared with a 5-year pooled mortality for all reported cancer of 31.0%[11]. The 10-year mortality outcomes in a large multi-centre meta-analysis were demonstrated to be 71% in those with DFD, compared to 5% in those without, with a median survival of 7.72 years compared to 12.6 years[12]. Patients with DFD have a 2-fold increased risk of all-cause mortality at 10 years compared to people with diabetes who do not have DFD when controlled for age, sex, education, smoking, and waist circumference[13,14]. Established diabetic nephropathy has been shown to increase mortality in patients with DFD [Odds ratio (OR) 1.47], as has duration of diabetes (OR 1.31) and history of previous amputation (minor OR 1.85, major OR 2.96)[12]. The main causes of death are cardiovascular events (54.7%), respiratory causes (18.9%), and multi-organ failure (12.5%)[15].
Patients with DFD have increased frailty scores and demonstrate significant physical disability in activities of daily living[16]. Frailty is associated with a 5-fold increased risk of re-hospitalisation in patients with non-healing DFD[17]. Sarcopenia, a disorder of muscle mass and function that is highly prevalent in cases of frailty, has increased prevalence in people with diabetes and DFD, particularly in patients with underlying peripheral neuropathy[18]. A recent Australian study found a high likelihood of sarcopenia in 51% of patients with DFD which was associated with significantly lower quality of life based on a validated quality of life questionnaire[19].
DFD is a leading cause of hospitalisation and LEA in people with diabetes. While the association with mortality is clear, there is variability in reported rates of LEA over time[20]. A recent data linkage study in Australia found the overall rates of LEA were as high as 29.8 per 10000 patients (95%CI: 27.7-31.9)[21]. Trends in admission for LEA appear to be decreasing over time in patients with type 2 diabetes with an average annual percentage change of -4.9% between 2004 and 2016[21]. Conversely, no change in rates of LEA over time was observed in patients with type 1 diabetes (age-adjusted annual percentage change 1.4 (95%CI: -0.5-3.3)[21]. In the United States, Geiss et al[22] demonstrated an initial significant reduction in non-traumatic LEA in people with diabetes between 2000 and 2009 of 43%[22]. Subsequently, there was a rebound between 2009 and 2015 to just below the rates seen in 2000, with a particular increase in young and middle-aged adults[22]. Concerningly, increased trends of LEAs in young patients were seen in multiple Australian studies[23,24].
Key risk factors for DFD are loss of protective sensation (LOPS), PAD, foot deformity, ulcer history, and previous amputation[8]. The IWGDF Risk Stratification System describes the risk of foot ulceration in people with diabetes and provides recommendations for screening for frequency for these key risk factors (Table 1).
| Risk category | Ulcer risk | Characteristics | Frequency |
| 0 | Very low | No LOPS and no PAD | Once a year |
| 1 | Low | LOPS or PAD | Once every 6-12 mo |
| 2 | Moderate | LOPS and PAD, or LOPS and foot deformity, or PAD + foot deformity | Once every 3-6 mo |
| 3 | High | LOPS or PAD and one of the following: History of foot ulcer, a previous LEA, end-stage renal disease | Once every 1-3 mo |
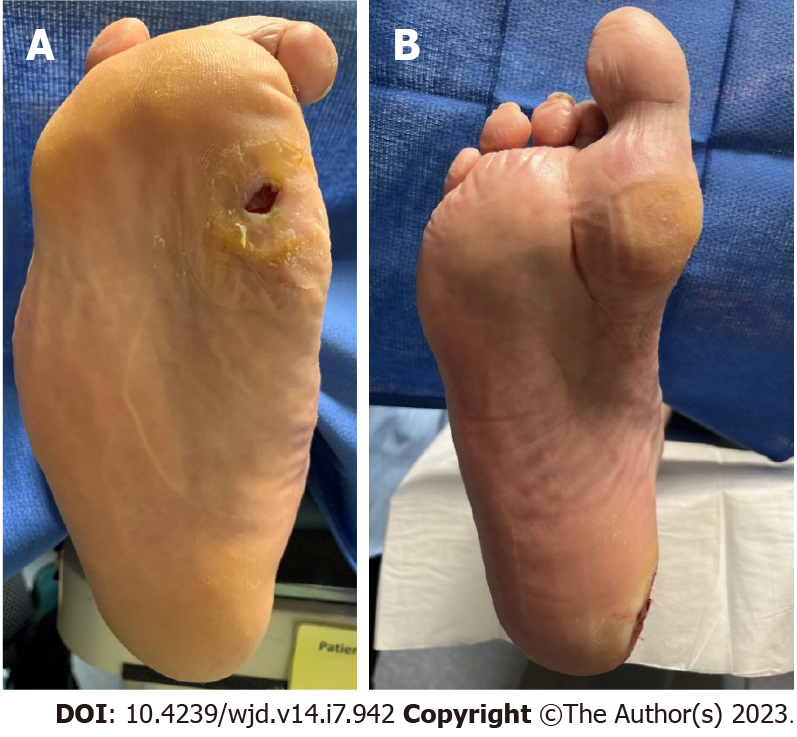
Peripheral neuropathy can lead to changes in gait, foot deformity and soft tissue which can elevate mechanical stress[7,25,26]. The combination of mechanical stress and a LOPS from peripheral neuropathy leads to tissue damage, callus formation and subcutaneous haemorrhage, precipitating ulceration in the neuropathic foot (Figure 1)[7,25].
PAD is associated with an increased risk of non-healing ulcers, infection and LEA[27-29]. The Wound, Ischaemia, and foot Infection (WIfI) classification system was developed to provide risk stratification and predict the risk of amputation and requirement for revascularisation at 1 year[30]. A higher WIfI correlates with an increasing risk of infection, stenosis events and poor wound healing[31].
In patients with diabetic foot infection, other independent risk factors for LEA are osteomyelitis (OR 1.94), retinopathy (OR 1.32), history of amputation (OR 1.47), and history of osteomyelitis (OR 1.94). Male sex and smoking were also associated with increased risk of LEA (OR 1.31 and 1.38, respectively); these risk factors are also associated with the development of PAD caused by atherosclerotic plaque formation[32].
Assessment of risk factors and their management are the basic principles to prevent the development of DFD and the risk of subsequent LEA. Evaluation for LOPS and the presence of PAD, together with specific assessment and management of DFD form the basis of the management recommendations to follow. Optimising glucose control is an established approach to prevent the development and progression of diabetic peripheral neuropathy and LOPS. Attention to strict cardiovascular risk factor modification is the basic management strategy for the prevention of the development and progression of PAD. Furthermore, specific medications may reduce the risk of LEA and/or the development of cardiovascular disease[33,34].
Based on IWGDF and DFA guidelines, DFD treatment can be summarised in 6 main categories[3,4]: (1) Assessment and education in high-risk patients; (2) Debridement, wound bed preparation and dressings; (3) Offloading; (4) Management of infection; (5) Interventions including revascularisation, pharmacotherapy and novel wound therapies; and (6) Integrated interdisciplinary care and patient information.
Cheng and Lazzarini performed Markov modelling to predict the efficacy of the first 4 interventions within a multidisciplinary team against long-term outcomes[35]. Comparing “optimal care” to the current standard of care, the model resulted in an overall cost saving to the health network of Australian dollar $2.7 billion over 5 years and improved the quality of life of participants compared to usual care. This theoretical outcome measure justifies the standardisation of DFD interventions for improved patient care and long-term health economics[35].
The IWGDF and the DFA guidelines recommend an annual foot assessment for signs or symptoms of LOPS and PAD for people with diabetes at very low risk of foot ulceration (IWGDF risk 0)[3,36]. LOPS can be assessed using a 10-g Semmes-Weinstein monofilament, 128-Hz tuning fork or the Ipswich Touch Test[8]. At a minimum, in people with diabetes, taking a relevant history and palpating foot pulses should be done to assess for the presence of PAD. People with DFD will require further non-invasive vascular tests and/or imaging[37]. People with diabetes at higher risk of foot ulceration (IWGDF risk 1-3), including those with a history of foot ulceration or LEA, end-stage renal disease, presence or progression of foot deformity, limited joint mobility, abundant callus, or pre-ulcerative signs should have more frequent screening (i.e. 6-12 mo for IWGDF risk 1, 3-6 mo for IWGDF risk 2, 1-3 mo for IWGDF risk 3)[3,38]. People with diabetes and risk of foot ulceration should be instructed to protect their feet with appropriate footwear, perform daily inspections and be educated on the best foot care[36].
A number of factors should be considered in the approach to wound bed preparation and dressing selection in DFD, including the underlying aetiology/s of the wound and factors impacting healing, the goals of management, patient-centred concerns including pain and access to resources and skilled clinicians[39,40].
Armstrong et al’s[41] mantra “it’s not what you put on, but what you take off” for debriding and offloading techniques for diabetes-related foot ulcer guides the importance of addressing plantar pressure and devitalised tissue that will impact ulcer healing. In a wound with adequate arterial perfusion to heal, active sharp debridement to remove devitalised tissue such as callus, slough and necrosis will promote a moist wound environment to reduce infection risk encourage granulation and conditions conducive to healing[39].
An assessment of the ulcer exudate levels and other local wound conditions guides dressing choice and frequency of dressing change[40]. A dressing may either donate moisture to the wound bed, absorb exudate or maintain the current moisture levels. Moist wound healing is well established to improve outcomes with reduced healing time, pain management and infection rates in wounds with adequate arterial perfusion to heal[42]. In a dry and ischaemic ulcer that is not expected to heal, and where the goal of management is to prevent further deterioration, a dry dressing regime offers the best protection from infection and wound deterioration[39,43].
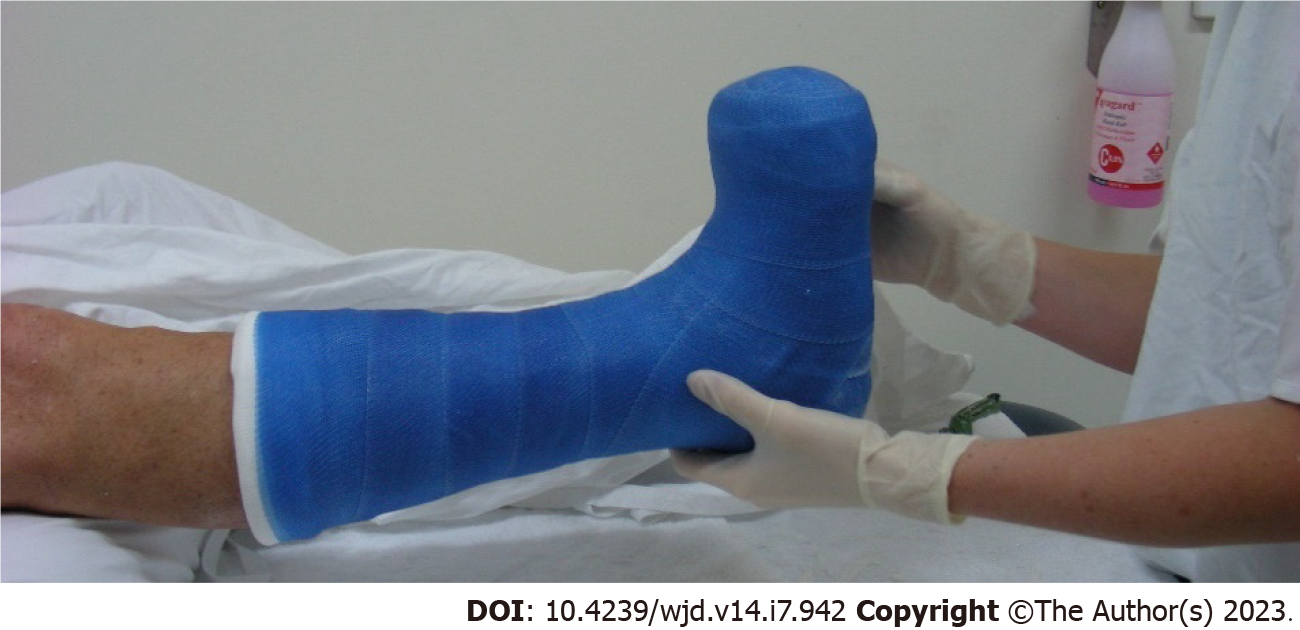
An evidence-based and practical approach to offloading the neuropathic foot ulcer for best healing outcomes is outlined in the DFA and IWGDF guidelines[3,44]. A non-removable knee-high offloading device such as a total contact cast (Figure 2) or a removable cast walker rendered irremovable are the gold standard to promote healing in people with a plantar neuropathic/diabetes-related foot ulcer. Patient factors such as high wound exudate or infection, ischaemia or a risk of falling may preclude the use of an irremovable device. Knee-high removable devices (such as a Controlled Ankle Motion walker) may be a suitable compromise to irremovable devices, and if these are contraindicated or not well tolerated, an ankle-high offloading device should be worn during all weight-bearing activities. Felted foam in combination with an offloading device or custom-made shoe can assist healing when implemented by skilled clinicians. Where other ankle-high and knee-high devices are contraindicated or not tolerated, medical-grade footwear should be used rather than other standard shoes or no footwear.
Most wounds will become colonised with pathogenic organisms from commensal skin flora or the environment. The presence of bacteria in a wound can alter the local pH and prevent wound healing but does not necessarily represent infection[45].
Within a colonised wound there is a perpetual change in the microbiome composition, with certain bacteria (e.g., anaerobes) favouring the development of invasive infection.
The development of infection in diabetic foot wounds is the result of a dynamic interaction between the local host immune response and bacteria colonising the wound. Following colonization, tissue invasion by these microorganisms is facilitated by a combination of diabetes-related factors including hyperglycaemic state, ischaemia-induced tissue injury, neuropathic trophic changes of the skin, impaired local immunity and reduced phagocytosis of bacteria by the macrophages[46,47].
Wound infection in DFD may present with the classical clinical signs of erythema, warmth, pain or tenderness overlying the region[48]. The depth of the wound may be enough to establish the presence of underlying bone infection (osteomyelitis) if clinical signs are present. In a diabetes-related foot ulcer that probes to the bone, a diagnosis of osteomyelitis is very likely[49]. The presence of systemic toxicity or systemic inflammatory response and/or metabolic instability are the clinical hallmarks of a severe infection and can help differentiate between moderate and severe diabetic foot infection[50]. Distinguishing between the two is important as a predictive factor for major amputation and prolonged hospital stay[51].
Once a clinical diagnosis of infection in DFD is made, this may then prompt the use of further radiological investigations depending on the depth and chronicity of the ulcer[47]. The choice of antibiotic, route of administration and duration of treatment relies on a combination of clinical assessment, the severity of presentation, imaging and microbiology. These management issues are discussed in detail below.
Imaging: Imaging can aid with the diagnosis of osteomyelitis when in doubt and inform management decisions and interventions. A plain X-ray can demonstrate cortical erosion to suggest established osteomyelitis, but early changes may not be perceptible. Magnetic resonance imaging (MRI) can detect more acute changes like marrow oedema as well as deep-seated collections. For the diagnosis of osteomyelitis in DFD, MRI has a very high sensitivity (90%) and specificity (79%)[52]. An active Charcot neuroarthropathy is difficult to differentiate from mid-foot septic arthritis with MRI imaging alone and should be further interpreted in a clinical context[53]. A standard three-phase bone scan has long been available for the evaluation of a foot ulcer for osteomyelitis, with a sensitivity of 80%-90% and specificity of 30%-45%. Leucocyte scans with radio-labelled white blood cells can increase specificity to 75%-80% and are now considered the most superior form of nuclear medicine scan for DFD. However, the IWGDF guidelines suggest that MRI is the more useful imaging test for this indication[54]. Fluorodeoxyglucose F18 positron emission tomography or combined 99m technetium white blood cell-labelled single-photon emission and computed tomography (CT) offer additional functional imaging assessment but are costly and are generally not required[54,55].
Microbiology and empirical antibiotics: An acute, superficial wound with clinical signs of spreading cellulitis is most often caused by Gram-positive skin bacteria and can generally be managed without the need for a formal microbiological diagnosis[56]. Similarly, microbiological investigations are often unhelpful for cellulitis without an open wound[57]. The clinician should commence empirical antibiotics with activity against key Gram-positive bacteria such as methicillin-susceptible Staphylococcus aureus (MSSA) and Streptococcus species. The empirical coverage should provide activity against methicillin-resistant Staphylococcus aureus (MRSA) in patients with a known history of colonisation. Narrow-spectrum antibiotics are appropriate unless the patient has recent antibiotic exposure or presents with a water-immersed wound, in which case additional Gram-negative cover may be recommended (Tables 2 and 3). The ulcer should be monitored closely, and antibiotics ceased once infection signs have resolved[58].
| IWGDF classification | Recommended empirical cover | ||||
| Gram-positive (MSSA, Streptococcus spp.) | Gram-negative (enteric, non-pseudomonal) | Obligate anaerobes | MRSA | Pseudomonal | |
| Mild (grade 2) – no recent antibiotics | Yes | No | No | If at risk1 | No |
| Mild (grade 2) – recent antibiotics or water-immersed wound | Yes | Yes | Consider if chronic | If at risk1 | No |
| Moderate (grade 3) | Yes | Yes | Consider | If at risk1 | Tropical climates or recently cultured |
| Severe (grade 4) | Yes | Yes | Yes | If at risk1 | Yes |
| Antibiotic | Antibiotic spectrum | Oral dose frequency | Pill burden (per day) | ||||
| Gram-positive (MSSA, Streptococcus spp.) | Gram-negative (enteric, non-pseudomonal) | Obligate anaerobes | MRSA | Pseudomonal | |||
| Penicillins, anti-staphylococcal1 | Yes | No | No | No | No | 4 | 4-8 |
| Cefalexin | Yes | Some | No | No | No | 4 | 4-8 |
| Amoxicillin-clavulanate | Yes | Yes | Yes | No | No | 2 | 2 |
| Trimethoprim-sulfamethoxazole | Yes | Yes | No | Some2 | No | 2 | 2 |
| Doxycycline | Yes | Some | No | Some2 | No | 2 | 2 |
| Clindamycin | Yes | No | Yes | Some2 | No | 3-4 | 9-16 |
| Metronidazole3 | No | No | Yes | No | No | 2-3 | 2-3 |
| Cefazolin | Yes | Some | No | No | No | ||
| Ceftriaxone | Yes | Yes | No | No | No | ||
| Piperacillin-tazobactam | Yes | Yes | Yes | No | Yes | ||
| Cefepime | Yes | Yes | No | No | Yes | ||
| Meropenem | Yes | Yes | Yes | No | Yes | ||
| Vancomycin | Yes | No | No | Yes | No | ||
| Moxifloxacin | Yes | Yes | Yes | Some2 | No | 1 | 1 |
| Ciprofloxacin4 | No | Yes | No | No | Yes | 2 | 2 |
In a chronic wound, erythema and oedema can suggest the development of a deep-seated infection. In these circumstances, pain often develops even in the presence of peripheral neuropathy. In order to obtain a microbiological diagnosis, a tissue specimen (curettage or biopsy) from the ulcer should be taken after the wound has been cleaned of debris and surface exudate with non-viable tissue debrided. If a tissue sample is not possible, a wound swab can be considered after appropriate cleaning and debridement. Gram-positive and Gram-negative organisms may be present on microbiology specimens, though one pathogen may be predominant. Anaerobic organisms can be difficult to culture but should be suspected if the wound is malodorous or gangrenous in appearance. If the patient with a chronically infected wound is systemically well, delaying antibiotic treatment until microbiology results are available can be considered. If treatment delay poses clinical concern or if microbiology investigations are not available, an empirical treatment with broad-spectrum oral antibiotics is indicated (Tables 2-4). Similar to acute infections, empirical antibiotic regimen should include MRSA coverage if colonisation is established. There is general agreement that empirical coverage for Pseudomonas aeruginosa (P. aeruginosa) is rarely necessary for mild- and most moderate- severity diabetic foot infections, especially in temperate climate regions where prevalence is low[59,60]. This is supported by indirect evidence from randomised controlled trials where outcomes were similar between groups treated with antibiotics with antipseudomonal activity vs those without[61-63]. Furthermore, if P. aeruginosa is identified in microbiological cultures, escalation of treatment may not be necessary in patients improving on antibiotics ineffective against P. aeruginosa[50,64]. Empirical P. aeruginosa coverage in mild- to moderate-severity foot infections may be more important in regions with tropical/sub-tropical climates or in wounds exposed to environmental water.
| IWGDF classification | Example of Empirical Antibiotic | If MRSA Risk1 |
| Mild (grade 2) – no recent antibiotics | Flucloxacillin PO, or cefalexin PO | As a single agent clindamycin PO, or trimethoprim-sulfamethoxazole PO, or doxycycline PO |
| Mild (grade 2) – recent antibiotics or water-immersed wound | Amoxicillin-clavulanate PO | Add one of the agents above, OR as a single agent moxifloxacin PO |
| Moderate (grade 3) | Amoxicillin-clavulanate PO/IV | Add one of the agents above, or if IV required, add vancomycin IV |
| Or cefazolin IV plus metronidazole PO/IV | ||
| Or if Pseudomonas risk2, piperacillin-tazobactam IV | ||
| Severe (grade 4) | Piperacillin-tazobactam IV | Add vancomycin IV |
In the presence of severe deep-seated infection, gangrene or sepsis, the patient requires hospitalisation and empirical treatment with an intravenous broad-spectrum antibiotic regimen with antipseudomonal activity. Additional coverage for MRSA is recommended for those with risk factors (Table 2) but should cease after 48-72 h if microbiological investigations show no evidence of MRSA involvement. In a patient with sepsis, blood cultures should be taken before antibiotics are administered if this does not significantly delay the commencement of treatment. Surgical debridement with or without minor amputation may be required to control the infection. In the case of overwhelming sepsis with ascending infection or very poor distal blood supply, the surgeon may be required to perform a major amputation below or above the knee. The choice of antibiotics used should be reviewed alongside available microbiological investigations within 48-72 h of commencing.
After surgical debridement or distal amputation, taking proximal bone chips from the healthy-appearing bone edge for microbiological culture is useful in determining the choice and duration of antibiotics. When the entirety of the infected bone and soft tissue has been removed (e.g. negative proximal bone chips cultures), antibiotics can be promptly ceased[47,50,65]. If a pathogenic organism is isolated in residual bone, a longer course of 2-4 wk may be required pending clinical progress. Consultation with a medical microbiologist may be required to interpret reported microbiological culture results and/or obtain further antibiotic susceptibilities.
A CT-guided bone biopsy is traditionally considered the gold standard for the diagnosis of chronic osteomyelitis but is only required if an organism has not been isolated in appropriately collected wound swabs or soft tissue biopsy and/or the infection is not responding to empirical treatment[66]. To avoid contamination, the sample must be taken through intact skin, not through the ulcer or sinus[67].
Other investigations: Australian and international guidelines suggest a review of biochemical markers including white cell count, erythrocyte sedimentation rate, C-reactive protein, and/or procalcitonin. These markers are not specific for osteomyelitis in the presence of moderate to severe skin and soft tissue infection or sepsis and need to be considered an adjunct to other investigations[58].
Histopathology from a punch or deep tissue biopsy is indicated if the wound looks atypical in appearance, which may help to diagnose mycobacterial infection, papillomavirus, malignancy, or vasculitis.
Other antibiotic considerations: Beyond wound culture and susceptibilities, the clinician needs to consider several other factors when choosing an antibiotic. Pill burden and dosing interval frequency can be a major factor in patient compliance. For example, broad-spectrum regimens such as amoxicillin with clavulanic acid or trimethoprim with sulfamethoxazole require the patient to take one tablet, twice daily while clindamycin with ciprofloxacin involves up to 12 capsules per day (Table 3). Interactions with other medications, toxicity and tolerance profile, ease of access and out-of-pocket expense are other important considerations which may require consultation with an infectious disease physician and/or pharmacist. Furthermore, many antibiotics used for a long duration require monitoring of a variety of biochemical markers, which may pose an additional burden to the patient and/or health system. If the patient requires intravenous antibiotics beyond the acute presentation, usually for the treatment of P. aeruginosa or multi-drug resistant bacteria, consideration of community-based parenteral antimicrobial therapy for the remaining duration will reduce hospital length of stay and allow the patient to recover in their own home[68].
Revascularisation strategies: PAD is present in up to 50% of people with DFD[38]. In a person with DFD, non –invasive bedside tests including palpation of pedal pulses and evaluation of pedal Doppler arterial waveforms in combination with ankle systolic pressure and systolic ankle brachial index or toe systolic pressure and toe brachial index can be used to assist in the diagnosis of PAD[37]. In people with diabetes, ankle-brachial index can be abnormally high due to non-compressibility of the tibial arteries secondary to calcific changes[69]. This process usually spares the digital arteries, making toe systolic pressures a useful predictor for likelihood of diabetic foot ulcer healing[70]. Vascular imaging in the form of colour duplex ultrasound or CT angiography can be completed for patients with DFD as this will illustrate the level at which the vascular disease is present and assist in surgical planning. Patients with diabetes and PAD typically present with multi-level, long-segment atherosclerotic disease below the knee[71,72]. The underlying pathophysiology is thought to be secondary to the upregulation of vasoconstrictors, abnormal platelet activity, activation of the coagulation cascade and a tendency towards plaque rupture[71]. Optimization of blood supply may be done by revascularisation either by open bypass operation or endovascular intervention (EVI). The revascularisation technique should be decided according to the morphological distribution of PAD, availability of autogenous vein, patient co-morbidities and local expertise[38]. Revascularisation should aim to establish direct blood flow into at least one pedal artery[73]. The preferred vascular target is angiosome-directed, meaning, targeting the vessel that supplies the region of tissue loss directly[72]. Studies have shown that the presence of a complete pedal arch following endovascular intervention is associated with increased wound healing, greater amputation free survival and increased survival at 1 year compared to those without a complete pedal arch[74]. A recent systematic review reported wound healing in patients with diabetes-related foot ulcer at 1 y following EVI was 75% while the healing rates following open revascularisation were reported to be lower, at 52%[72]. Limb salvage rates however, seemed to be greater amongst those undergoing open revascularisation with 85% at 1 year and 87% at 2 years and similar rates of 30 d perioperative mortality[72,75]. Despite these numbers, redo-revascularisation is required in up to 40% of patients undergoing EVI and 31% in those undergoing open surgery[76]. When revascularisation is unsuccessful, amputation may be necessary at a level with adequate perfusion for sufficient wound healing.
Hindfoot ulceration has the highest risk of primary major amputation and secondary major amputation with the risk being reduced in midfoot and forefoot ulcers respectively[77]. Another study has shown that smoking, diabetes duration, hypertension and number of debridements after surgery were significant risk factors for re-amputation[78].
Risk-factor modification and pharmacotherapy: Before the introduction of more recent medications which reduce cardiovascular outcomes independent of glucose lowering effect, early risk factor modification in patients with diabetes has been shown to reduce incident cardiovascular events. The STENO-2 study undertaken in the 1990s, and with over 20 years of mean follow-up time, has continued to demonstrate a reduction in cardiovascular disease and mortality in patients with diabetes who receive early and intensive targeted treatment of blood pressure, cholesterol, microalbuminuria and glycated hemoglobin (HbA1c) as well as smoking cessation[79,80].
All patients with PAD are generally treated with HMG-CoA reductase inhibition using statin therapy[81]. When used as part of optimal medical therapy (renin-angiotensin-aldosterone system inhibitors, beta-blockers and anti-thrombotic agents), they have been shown to reduce major adverse cardiovascular events and all-cause mortality[82]. Treatment with a statin has been shown to reduce LEA by up to 25%[83].
Anticoagulant and antiplatelet medications have long been established therapies for cardiovascular risk reduction in patients with PAD. In patients with stable atherosclerotic disease, rivaroxaban plus aspirin combination has been shown to reduce cardiovascular death, stroke, and myocardial infarction [Hazard ratio (HR) 0.76] compared to either medication alone with no significant increase in intracranial or fatal bleeding[84]. Furthermore, low dose rivaroxaban (2.5 mg) plus aspirin was associated with a significantly reduced incidence of acute limb ischaemia, LEA, myocardial infarction, ischaemic stroke, or death from cardiovascular cause compared to aspirin alone in patients post revascularisation for PAD[85]. In the CAPRIE trial (clopidogrel vs aspirin in patients at risk of ischaemic events), clopidogrel was superior to aspirin in lowering the risk of ischaemic stroke, myocardial infarction, or vascular death[86,87].
Fenofibrate, a lipid-lowering therapy which works through peroxisome proliferator-activated receptor alpha, was shown to prevent microvascular complications of diabetes, particularly diabetic retinopathy through the Fenofibrate Intervention and Event Lowering in Diabetics (FIELD) study[88]. Post-hoc intention to treat analysis of this data demonstrated a reduction in first amputation (HR 0.64) and minor amputation (HR 0.53) in patients on fenofibrate 200 mg per day. This study did not establish statistical significance for a reduction in major amputations (HR 0.93, 95%CI: 0.53–1.62; P = 0.79)[89].
In recent years, two classes of anti-hyperglycaemic agents have shown significant cardiovascular risk reduction independent of their glucose lowering effect. Sodium-glucose cotransporter-2 (SGLT2) inhibitors are an oral medication which inhibits the receptor responsible for re-absorption of 90% of glucose filtered in the nephron. SGLT2 inhibitors induce glucosuria, osmotic diuresis and modest weight loss. In a meta-analysis of 27 cardiovascular outcome studies related to SGLT2 inhibitors, there was an early and sustained reduction in the composite primary outcome of cardiovascular death, non-fatal myocardial infarction or nonfatal stroke primarily driven by a major reduction in heart failure. This effect is independent of HbA1c reduction[90].
Both SGLT2 inhibitors available in Australia have been shown to reduce progression of chronic kidney disease and are generally considered safe to use in patients with renal impairment[91,92]. The main adverse effects of concern include genitourinary infections, euglycaemic diabetic ketoacidosis (in the fasting or unwell patient) and volume depletion[93]. In 2017 the Canagliflozin Cardiovascular Assessment Study, reported an increased risk of LEA in patients treated with canagliflozin (HR 1.97), particularly toe or metatarsal. The overall rates were low (6.3 vs 3.4 participants per 1000 patient years) however the finding caused significant pause in clinicians prescribing this class of medications to patients at risk of DFD[94]. Since then, multiple cardiovascular outcome studies have been published without any signal for increased LEA rates in patients treated with SGLT2 inhibitors. Several high-quality systematic reviews and meta-analyses were undertaken to explore this association. One study demonstrated an increased risk of LEA for canagliflozin (Risk Ratio 1.59), but not for dapagliflozin or empagliflozin, whilst another demonstrated no increased risk of amputation across the entire class including canagliflozin[95,96]. It therefore appears that there is little contemporary data linking SGLT2 inhibitor use with increased LEA rates. Even if there was some risk of LEA associated with SGLT2 inhibitor use, this would need to be balanced against the substantial risk reduction in renal disease progression and protection from cardiovascular disease that this class of medications has shown in major clinical trials. Therefore, in our opinion, the benefits of SGLT2 inhibitor use in patients with DFD who are known to be at high risk for cardio-renal disease far outweighs any possible risk associated with LEA.
Glucagon-like peptide-1 receptor agonist (GLP1-RA) are injectable medications which reduce gastric emptying, stimulate endogenous insulin production and reduce glucagon secretion. Indirect effects of modulation in gut hormone signalling include appetite suppression, weight loss, improved peripheral insulin sensitivity and a more robust reduction in glucose levels. GLP-1RA have demonstrated a statistically significant reduction in major adverse cardiovascular events of 14% (HR 0.86, 95%CI: 0.79–0.95; P = 0.006), with the risk of cardiovascular death reducing by 13% (P = 0.016) and the risk of all-cause mortality reducing by 12% (P = 0.012)[97]. While SGLT2 inhibitor-related cardiovascular risk reduction is largely driven by improved heart failure outcomes, GLP-1 analogues appear to reduce atherosclerotic related cardiovascular events[97].
The GLP-1R analogues available in Australia that have been associated with cardiovascular risk reduction include liraglutide (a daily injection), dulaglutide, and semaglutide (both once weekly injections). Further studies are required to determine whether the reductions in coronary and cerebrovascular events will extrapolate to reduced complications of lower limb arterial disease. Liraglutide has been shown to reduce LEA (HR 0.65) but not other ulcer outcomes[98]. Dulaglutide and semaglutide have been shown to improve metabolic and inflammatory markers commonly associated with peripheral vascular disease but to date have not been shown to reduce DFD or amputation rates[99]. Gastro-intestinal adverse effects are the main limitation to the use of GPL-1 analogues in the Australian setting. Utilisation of these two newer classes of glucose lowering agents which are weight-negative and infer cardiovascular protection is a modern part of the multidisciplinary management of patients with DFD.
Good glycaemic control with a goal HbA1c of less than 53 mmol/mol (7%) has been shown to reduce the incidence of diabetic foot ulcers and the risk of amputation. It has also been associated with improved sensory nerve function compared with less intensive glycaemic control[100]. Whilst many studies have suggested that good glycaemic control improves surrogate markers associated with wound healing, there is currently no randomised trial evidence that clearly shows that good glycaemic control improves the rate of diabetic foot ulcer wound healing[101,102]. However, in clinical practice our opinion is that patients with active ulcers should still aim to maintain good glycaemic control as it is likely that this approach will aid in wound healing and also help to prevent the onset and progression of other diabetes-related microvascular complications[103].
Newer therapies: Future directions in interventions to enhance wound healing include topical oxygen therapy (TOT), sucrose octasulfate-impregnated dressings, topical fibrin and leucocyte platelet patches as well as placenta-derived products[104]. Whilst these therapies rely on small studies, they are demonstrating a positive trend for the future direction of DFD and may be incorporated into future guidelines pending further study.
TOT is based on the idea that oxygen is a necessary factor for wound healing by its action on several oxygen-dependent enzymes, eventually increasing cell metabolism, bacterial defence, angiogenesis and vasodilation, and collagen deposition and crosslinking[105]. The method of delivery for TOT has different levels of effectiveness. Continuous delivery oxygen (CDO) system is a low continuous flow of oxygen (3–15 mL/h) through a sealed, disposable dressing that is changed weekly. Multiple randomised sham control trials have demonstrated a statistically significant improvement in wound healing compared to standard of care, with 32.4%-54% of patients in the CDO group achieving healing, compared to 16.7%-49% in the sham therapy group (P < 0.05)[106,107].
Cyclically pressurised topical wound healing applies high flow oxygen with pressures from 7.5–37.5 mmHg, with the optional addition of humidification. It works by encapsulating the affected limb in an extremity chamber to enable local oxygen and pressure delivery. Cyclically pressurised TOT has been shown in two independent randomised controlled trials to not only improve ulcer healing (41.7% vs 13.5%, P = 0.007), but also to reduce amputations and hospitalisations at 1 year (54.1% vs 41.4%, P < 0.001)[108,109].
Hyperbaric oxygen therapy (HBOT) is a controversial healing modality currently being used at select centres. It is believed to improve wound healing through improving tissue hypoxia, improving perfusion and angiogenesis, as well as downregulating the inflammatory response. Treatment requires the patient to commit to an average of 60 h over many weeks with significant associated costs to the health care system. Although much of the existing evidence lacks the quality to support HBOT, multiple studies have demonstrated a positive trend towards healing rates. Patients most likely to benefit from treatment have indolent lower limb ulcers that have not healed after at least 1 mo of active treatment. Thus, where available, HBOT could be considered as an adjunct to appropriate wound care[110].
Sucrose octasulfate-impregnated dressings have been shown to reduce the action of matrix metalloproteinases and have a statistically significant benefit compared to placebo in wound closure[111]. Newer agents include topical fibrin and leucocyte platelet patches, thought to improve wound closure through the promotion of cytokines and growth factors involved in tissue repair[104]. These platelet-rich patches have been shown to increase wound healing (34% vs 22%; P = 0.0235), however, there are significant cost and organisational issues required to create these products, leading to a cautious recommendation for their use from the IWGDF[112].
A recent multicentre, randomized, double-blind vehicle-controlled study exploring the safety of topical esmolol hydrochloride showed minimal systemic concentration of the drug in plasma and a favourable safety profile in patients who received topical treatment to foot ulceration. Preliminary data suggests a trend to improved ulcer healing. The drugs general availability makes it a potential avenue for treatment of non-healing diabetes-related foot ulcers but larger phase III clinical trials are required to establish a statistically significant improvement in ulcer healing[113].
Finally, placenta-derived products are an area of growing research due to the combination of collagen-rich extracellular matrix and cells, growth factors and various stem cells thought to improve wound healing in human placental membranes. Cryopreserved amniotic membrane allograft has demonstrated increased ulcer closure (62% vs 21.3%; P = 0.001) with reduced median time to healing (42 d vs 69.5 d; P = 0.019), and umbilical cord product has also demonstrated a significant improvement in ulcer healing at 12 wk (70% vs 48%; P = 0.0089)[114,115]. Despite these outcomes, the cost is a major aspect ongoing for placenta-derived products, and these have yet to become utilised in everyday practice[104].
In order to provide the best care, a combination of guideline-based practice and clinical expertise is required. Nuanced decision-making is key, particularly in the presence of infection, vascular compromise and challenging patient factors. In Australia, The National Association of Diabetes Centres has implemented the Foot Forward diabetes education program aiming to detect foot problems early and ultimately prevent amputations[116]. A detailed and integrated Diabetes Foot Care pathway includes risk stratification and triage based on risk factors (Supplementary Materials)[116]. In a nationwide effort to standardise care, the program defines the interdisciplinary approach as the first core service indicator for a high-risk foot centre to achieve accreditation[117]. The standards outline that a High-Risk Foot Service must have access to the necessary core members of the multidisciplinary team, regular ongoing education for staff and patients, required administration and intake criteria, as well as resources to enact on supportive research. Core disciplines represented in the multidisciplinary team may include but are not limited to podiatry, prosthetics and orthotics, nursing (acute and in the home), physiotherapy, endocrinology, infectious diseases, vascular surgery and rehabilitation medicine.
Clinician-to-clinician discussion between disciplines, all within the immediate vicinity of the patient and each other, enables dialogue between specialists to reach the most appropriate outcome for each individual patient. Through case conferences, outpatient clinics or team ward rounds the interdisciplinary team can coordinate the offloading, dressings, infection management, revascularisation strategy, diabetes management and cardiovascular risk modification according to evidence-based guidelines (Supplementary Materials)[116].
Our High-Risk Foot Service is an interdisciplinary service based at a tertiary Australian hospital. Our integrated service includes endocrinologists, podiatrists, rehabilitation physicians, infectious diseases physicians, vascular surgeons, pharmacists, orthotists, diabetes nurse educators and nurses. We are supported by administration assistants and allied health assistants and have on-referral access to other medical, surgical and allied health clinicians. Our services operate multiple interdisciplinary outpatient clinics, an inpatient ward round, case conference and hospital in the home service. We have strong links with regional health services with increasing utilisation of telehealth.
In patients who have received acute hospital care, particularly surgical intervention, timely assessment of rehabilitation goals and options for continuation of hospital-based care in the home reduces readmission and utilization of long-term care facilities with improved patient satisfaction, without an increase in mortality[118].
DFD is a complex condition, acting as a marker of overall systemic disease, increasing morbidity and mortality, and causing a significant burden to health systems. Our review highlights the complexity of DFD and the improved patient outcomes that are associated with an interdisciplinary management approach involving wound bed preparation and offloading, infection management, maintenance of vascular supply, glycaemic control, and cardiovascular risk modification.
We have discussed the above in the framework of our experiences in a multi-disciplinary high-risk foot service located in an Australian tertiary referral centre and university teaching hospital. To our knowledge this is the first review to describe the approach to the management of DFD with an Australian focus in the setting of the recently published national diabetes foot care pathway “Foot Forward for Diabetes” (Supplementary Materials) and to compare this approach to international guidelines. The strength of our recommendations for the care of patients with DFD are in the context of the limitations of a narrative review.
It is our opinion that a guideline-driven strategy with regular and efficient communication between medical, surgical, and allied health specialties within a High-Risk Foot Unit is paramount to the nuanced decision-making required to optimise patient outcomes in the most effective and efficient fashion. We await the further development of novel therapies for DFD. These include better tools and technologies for detecting high-risk feet and improved topical and systemic methods to promote ulcer healing. Unfortunately, apart from promoting good glycaemic control, little progress has been made in preventing the development and progression of diabetic neuropathy. In contrast, promising areas of interest for the management of PAD include the development of novel methods of perfusion assessment and revascularisation. We anticipate that rates of PAD may decrease with trends in improvement of risk factors and wider use of novel pharmacotherapies that infer vascular protection. In the interim, the early detection of high-risk feet and the initiation of strategies to prevent ulcer development within the multidisciplinary team remain essential elements for the care of people with diabetes and in the avoidance of the devastating consequences associated with DFD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Rastogi A, India S-Editor: Li L L-Editor: Filipodia P-Editor: Li L
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 6367] [Article Influence: 909.6] [Reference Citation Analysis (12)] |
| 2. | Galligan A, Greenaway TM. Novel approaches to the treatment of hyperglycaemia in type 2 diabetes mellitus. Intern Med J. 2016;46:540-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA; IWGDF Editorial Board. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 424] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 4. | Lazzarini PA, Raspovic A, Prentice J, Commons RJ, Fitridge RA, Charles J, Cheney J, Purcell N, Twigg SM; Australian Diabetes-related Foot Disease Guidelines & Pathways Project. Guidelines development protocol and findings: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. J Foot Ankle Res. 2022;15:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | van Netten JJ, Bus SA, Apelqvist J, Lipsky BA, Hinchliffe RJ, Game F, Rayman G, Lazzarini PA, Forsythe RO, Peters EJG, Senneville É, Vas P, Monteiro-Soares M, Schaper NC; International Working Group on the Diabetic Foot. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care. 2020;43:964-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 328] [Article Influence: 54.7] [Reference Citation Analysis (2)] |
| 7. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2562] [Article Influence: 284.7] [Reference Citation Analysis (2)] |
| 8. | Bus SA, Lavery LA, Monteiro-Soares M, Rasmussen A, Raspovic A, Sacco ICN, van Netten JJ; International Working Group on the Diabetic Foot. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 9. | West M, Chuter V, Munteanu S, Hawke F. Defining the gap: a systematic review of the difference in rates of diabetes-related foot complications in Aboriginal and Torres Strait Islander Australians and non-Indigenous Australians. J Foot Ankle Res. 2017;10:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Jiang Y, Wang X, Xia L, Fu X, Xu Z, Ran X, Yan L, Li Q, Mo Z, Yan Z, Ji Q. A cohort study of diabetic patients and diabetic foot ulceration patients in China. Wound Repair Regen. 2015;23:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 585] [Article Influence: 97.5] [Reference Citation Analysis (2)] |
| 12. | Rastogi A, Goyal G, Kesavan R, Bal A, Kumar H, Mangalanadanam, Kamath P, Jude EB, Armstrong DG, Bhansali A. Long term outcomes after incident diabetic foot ulcer: Multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study: Epidemiology of diabetic foot complications study. Diabetes Res Clin Pract. 2020;162:108113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 13. | Iversen MM, Tell GS, Riise T, Hanestad BR, Østbye T, Graue M, Midthjell K. History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trøndelag Health Study, Norway. Diabetes Care. 2009;32:2193-2199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 352] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 15. | Rubio JA, Jiménez S, Lázaro-Martínez JL. Mortality in Patients with Diabetic Foot Ulcers: Causes, Risk Factors, and Their Association with Evolution and Severity of Ulcer. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Bôas NCRV, Salomé GM, Ferreira LM. Frailty syndrome and functional disability among older adults with and without diabetes and foot ulcers. J Wound Care. 2018;27:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Maltese G, Basile G, Meehan H, Fuller M, Cesari M, Fountoulakis N, Karalliedde J. Frailty Is Associated with Impaired Diabetic Foot Ulcer Healing and All-Cause Re-Hospitalization. J Nutr Health Aging. 2022;26:169-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Yang Q, Zhang Y, Zeng Q, Yang C, Shi J, Zhang C, Ni X, Du Z, Tang Z, Hu J, Li X, Cai J, Li Q, Cheng Q. Correlation Between Diabetic Peripheral Neuropathy and Sarcopenia in Patients with Type 2 Diabetes Mellitus and Diabetic Foot Disease: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2020;13:377-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Churilov I, Churilov L, Proctor M, Galligan A, Murphy D, Westcott M, MacIsaac RJ, Ekinci EI. The association between SARC-F status and quality of life in High Risk Foot Clinic patients. JCSM Clinical Reports. 4. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Saluja S, Anderson SG, Hambleton I, Shoo H, Livingston M, Jude EB, Lunt M, Dunn G, Heald AH. Foot ulceration and its association with mortality in diabetes mellitus: a meta-analysis. Diabet Med. 2020;37:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 21. | Morton JI, Lazzarini PA, Shaw JE, Magliano DJ. Trends in the Incidence of Hospitalization for Major Diabetes-Related Complications in People With Type 1 and Type 2 Diabetes in Australia, 2010-2019. Diabetes Care. 2022;45:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Geiss LS, Li Y, Hora I, Albright A, Rolka D, Gregg EW. Resurgence of Diabetes-Related Nontraumatic Lower-Extremity Amputation in the Young and Middle-Aged Adult U.S. Population. Diabetes Care. 2019;42:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 23. | Kiburg KV, Galligan A, Sundararajan V, MacIsaac RJ. Temporal trends in non-traumatic lower extremity amputations (LEAs) and their association with 12-month mortality in people with diabetes, 2004-2016. J Diabetes Complications. 2022;36:108221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 24. | Hamilton EJ, Davis WA, Siru R, Baba M, Norman PE, Davis TME. Temporal Trends in Incident Hospitalization for Diabetes-Related Foot Ulcer in Type 2 Diabetes: The Fremantle Diabetes Study. Diabetes Care. 2021;44:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Fernando ME, Crowther RG, Pappas E, Lazzarini PA, Cunningham M, Sangla KS, Buttner P, Golledge J. Plantar pressure in diabetic peripheral neuropathy patients with active foot ulceration, previous ulceration and no history of ulceration: a meta-analysis of observational studies. PLoS One. 2014;9:e99050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Fernando M, Crowther R, Lazzarini P, Sangla K, Cunningham M, Buttner P, Golledge J. Biomechanical characteristics of peripheral diabetic neuropathy: A systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clin Biomech (Bristol, Avon). 2013;28:831-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 27. | Richter L, Freisinger E, Lüders F, Gebauer K, Meyborg M, Malyar NM. Impact of diabetes type on treatment and outcome of patients with peripheral artery disease. Diab Vasc Dis Res. 2018;15:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Elgzyri T, Larsson J, Thörne J, Eriksson KF, Apelqvist J. Outcome of ischemic foot ulcer in diabetic patients who had no invasive vascular intervention. Eur J Vasc Endovasc Surg. 2013;46:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Spreen MI, Gremmels H, Teraa M, Sprengers RW, Verhaar MC, Statius van Eps RG, de Vries JP, Mali WP, van Overhagen H; PADI and JUVENTAS Study Groups. Diabetes Is Associated With Decreased Limb Survival in Patients With Critical Limb Ischemia: Pooled Data From Two Randomized Controlled Trials. Diabetes Care. 2016;39:2058-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G; Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220-34.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 1190] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 31. | Cerqueira LO, Duarte EG, Barros ALS, Cerqueira JR, de Araújo WJB. WIfI classification: the Society for Vascular Surgery lower extremity threatened limb classification system, a literature review. J Vasc Bras. 2020;19:e20190070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Sen P, Demirdal T, Emir B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab Res Rev. 2019;35:e3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 33. | American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S151-S167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (1)] |
| 34. | American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S144-S174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 298] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 35. | Cheng Q, Lazzarini PA, Gibb M, Derhy PH, Kinnear EM, Burn E, Graves N, Norman RE. A cost-effectiveness analysis of optimal care for diabetic foot ulcers in Australia. Int Wound J. 2017;14:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Kaminski MR, Golledge J, Lasschuit JWJ, Schott KH, Charles J, Cheney J, Raspovic A; Australian Diabetes-related Foot Disease Guidelines & Pathways Project. Australian guideline on prevention of foot ulceration: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. J Foot Ankle Res. 2022;15:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, Katsanos K, Mills JL, Nikol S, Reekers J, Venermo M, Zierler RE, Schaper NC, Hinchliffe RJ. Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: A systematic review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Chuter V, Quigley F, Tosenovsky P, Ritter JC, Charles J, Cheney J, Fitridge R; Australian Diabetes-related Foot Disease Guidelines & Pathways Project. Australian guideline on diagnosis and management of peripheral artery disease: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. J Foot Ankle Res. 2022;15:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Sibbald RG, Elliott JA, Persaud-Jaimangal R, Goodman L, Armstrong DG, Harley C, Coelho S, Xi N, Evans R, Mayer DO, Zhao X, Heil J, Kotru B, Delmore B, LeBlanc K, Ayello EA, Smart H, Tariq G, Alavi A, Somayaji R. Wound Bed Preparation 2021. Adv Skin Wound Care. 2021;34:183-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 40. | Wounds Australia. Standard for Wound Prevention and Management. Osborn Park, WA: Cambridge Media; 2016. |
| 41. | Armstrong DG, Lipsky BA. Diabetic foot infections: stepwise medical and surgical management. Int Wound J. 2004;1:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Wodash AJ. Wet-to-Dry Dressings Do Not Provide Moist Wound Healing. J Am Coll Clin Wound Spec. 2012;4:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Expert working group; Satellite expert working group. Wound exudate and the role of dressings. A consensus document. Int Wound J. 2008;5 Suppl 1:iii-i12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Fernando ME, Horsley M, Jones S, Martin B, Nube VL, Charles J, Cheney J, Lazzarini PA; Australian Diabetes-related Foot Disease Guidelines & Pathways Project. Australian guideline on offloading treatment for foot ulcers: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. J Foot Ankle Res. 2022;15:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Schultz G, Bjarnsholt T, James GA, Leaper DJ, McBain AJ, Malone M, Stoodley P, Swanson T, Tachi M, Wolcott RD; Global Wound Biofilm Expert Panel. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25:744-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 46. | Williams H, Campbell L, Crompton RA, Singh G, McHugh BJ, Davidson DJ, McBain AJ, Cruickshank SM, Hardman MJ. Microbial Host Interactions and Impaired Wound Healing in Mice and Humans: Defining a Role for BD14 and NOD2. J Invest Dermatol. 2018;138:2264-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG; International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 48. | Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008;19:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 49. | Lam K, van Asten SA, Nguyen T, La Fontaine J, Lavery LA. Diagnostic Accuracy of Probe to Bone to Detect Osteomyelitis in the Diabetic Foot: A Systematic Review. Clin Infect Dis. 2016;63:944-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1203] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 51. | Wukich DK, Hobizal KB, Raspovic KM, Rosario BL. SIRS is valid in discriminating between severe and moderate diabetic foot infections. Diabetes Care. 2013;36:3706-3711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 53. | Womack J. Charcot Arthropathy Versus Osteomyelitis: Evaluation and Management. Orthop Clin North Am. 2017;48:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Lipsky BA, Aragón-Sánchez J, Diggle M, Embil J, Kono S, Lavery L, Senneville É, Urbančič-Rovan V, Van Asten S; International Working Group on the Diabetic Foot, Peters EJ. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32 Suppl 1:45-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 362] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 55. | Lauri C, Tamminga M, Glaudemans AWJM, Juárez Orozco LE, Erba PA, Jutte PC, Lipsky BA, IJzerman MJ, Signore A, Slart RHJA. Detection of Osteomyelitis in the Diabetic Foot by Imaging Techniques: A Systematic Review and Meta-analysis Comparing MRI, White Blood Cell Scintigraphy, and FDG-PET. Diabetes Care. 2017;40:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 56. | Lipsky BA, Pecoraro RE, Larson SA, Hanley ME, Ahroni JH. Outpatient management of uncomplicated lower-extremity infections in diabetic patients. Arch Intern Med. 1990;150:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Raff AB, Kroshinsky D. Cellulitis: A Review. JAMA. 2016;316:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 58. | Commons RJ, Charles J, Cheney J, Lynar SA, Malone M, Raby E; Australian Diabetes-related Foot Disease Guidelines & Pathways Project. Australian guideline on management of diabetes-related foot infection: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. J Foot Ankle Res. 2022;15:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Noel GJ, Bush K, Bagchi P, Ianus J, Strauss RS. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin Infect Dis. 2008;46:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 60. | Uçkay I, Gariani K, Pataky Z, Lipsky BA. Diabetic foot infections: state-of-the-art. Diabetes Obes Metab. 2014;16:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet. 2005;366:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21:770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 63. | Graham DR, Lucasti C, Malafaia O, Nichols RL, Holtom P, Perez NQ, McAdams A, Woods GL, Ceesay TP, Gesser R. Ertapenem once daily versus piperacillin-tazobactam 4 times per day for treatment of complicated skin and skin-structure infections in adults: results of a prospective, randomized, double-blind multicenter study. Clin Infect Dis. 2002;34:1460-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Uçkay I, Holy D, Schöni M, Waibel FWA, Trache T, Burkhard J, Böni T, Lipsky BA, Berli MC. How good are clinicians in predicting the presence of Pseudomonas spp. in diabetic foot infections? A prospective clinical evaluation. Endocrinol Diabetes Metab. 2021;4:e00225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Rossel A, Lebowitz D, Gariani K, Abbas M, Kressmann B, Assal M, Tscholl P, Stafylakis D, Uçkay I. Stopping antibiotics after surgical amputation in diabetic foot and ankle infections-A daily practice cohort. Endocrinol Diabetes Metab. 2019;2:e00059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Meyr AJ, Singh S, Zhang X, Khilko N, Mukherjee A, Sheridan MJ, Khurana JS. Statistical reliability of bone biopsy for the diagnosis of diabetic foot osteomyelitis. J Foot Ankle Surg. 2011;50:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Tardáguila-García A, Sanz-Corbalán I, García-Morales E, García-Álvarez Y, Molines-Barroso RJ, Lázaro-Martínez JL. Diagnostic Accuracy of Bone Culture Versus Biopsy in Diabetic Foot Osteomyelitis. Adv Skin Wound Care. 2021;34:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Caplan GA, Sulaiman NS, Mangin DA, Aimonino Ricauda N, Wilson AD, Barclay L. A meta-analysis of "hospital in the home". Med J Aust. 2012;197:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 69. | Aerden D, Massaad D, von Kemp K, van Tussenbroek F, Debing E, Keymeulen B, Van den Brande P. The ankle--brachial index and the diabetic foot: a troublesome marriage. Ann Vasc Surg. 2011;25:770-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Brownrigg JR, Hinchliffe RJ, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC; International Working Group on the Diabetic Foot. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32 Suppl 1:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | Lowry D, Saeed M, Narendran P, Tiwari A. A Review of Distribution of Atherosclerosis in the Lower Limb Arteries of Patients With Diabetes Mellitus and Peripheral Vascular Disease. Vasc Endovascular Surg. 2018;52:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 72. | Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, Katsanos K, Mills JL, Nikol S, Reekers J, Venermo M, Zierler RE, Hinchliffe RJ, Schaper NC. Effectiveness of revascularisation of the ulcerated foot in patients with diabetes and peripheral artery disease: A systematic review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Chuter V, West M, Hawke F, Searle A. Where do we stand? The availability and efficacy of diabetes related foot health programs for Aboriginal and Torres Strait Islander Australians: a systematic review. J Foot Ankle Res. 2019;12:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Troisi N, Turini F, Chisci E, Ercolini L, Frosini P, Lombardi R, Falciani F, Baggiore C, Anichini R, Michelagnoli S. Pedal arch patency and not direct-angiosome revascularization predicts outcomes of endovascular interventions in diabetic patients with critical limb ischemia. Int Angiol. 2017;36:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Butt T, Lilja E, Örneholm H, Apelqvist J, Gottsäter A, Eneroth M, Acosta S. Amputation-Free Survival in Patients With Diabetes Mellitus and Peripheral Arterial Disease With Heel Ulcer: Open Versus Endovascular Surgery. Vasc Endovascular Surg. 2019;53:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Noronen K, Saarinen E, Albäck A, Venermo M. Analysis of the Elective Treatment Process for Critical Limb Ischaemia with Tissue Loss: Diabetic Patients Require Rapid Revascularisation. Eur J Vasc Endovasc Surg. 2017;53:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Winkler E, Schöni M, Krähenbühl N, Uçkay I, Waibel FWA. Foot Osteomyelitis Location and Rates of Primary or Secondary Major Amputations in Patients With Diabetes. Foot Ankle Int. 2022;43:957-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Seçkin MF, Özcan Ç, Çamur S, Polat Ö, Batar S. Predictive Factors and Amputation Level for Reamputation in Patients With Diabetic Foot: A Retrospective Case-Control Study. J Foot Ankle Surg. 2022;61:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2459] [Cited by in RCA: 2381] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 80. | Gæde P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, Pedersen O. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016;59:2298-2307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 81. | Jansen-Chaparro S, López-Carmona MD, Cobos-Palacios L, Sanz-Cánovas J, Bernal-López MR, Gómez-Huelgas R. Statins and Peripheral Arterial Disease: A Narrative Review. Front Cardiovasc Med. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Cimaglia P, Bernucci D, Cardelli LS, Carone A, Scavone G, Manfrini M, Censi S, Calvi S, Ferrari R, Campo G, Paola LD. Renin-Angiotensin-Aldosterone System Inhibitors, Statins, and Beta-Blockers in Diabetic Patients With Critical Limb Ischemia and Foot Lesions. J Cardiovasc Pharmacol Ther. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 83. | Kokkinidis DG, Arfaras-Melainis A, Giannopoulos S, Katsaros I, Jawaid O, Jonnalagadda AK, Parikh SA, Secemsky EA, Giri J, Kumbhani DJ, Armstrong EJ. Statin therapy for reduction of cardiovascular and limb-related events in critical limb ischemia: A systematic review and meta-analysis. Vasc Med. 2020;25:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 84. | Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S; COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1748] [Article Influence: 194.2] [Reference Citation Analysis (0)] |
| 85. | Bonaca MP, Bauersachs RM, Anand SS, Debus ES, Nehler MR, Patel MR, Fanelli F, Capell WH, Diao L, Jaeger N, Hess CN, Pap AF, Kittelson JM, Gudz I, Mátyás L, Krievins DK, Diaz R, Brodmann M, Muehlhofer E, Haskell LP, Berkowitz SD, Hiatt WR. Rivaroxaban in Peripheral Artery Disease after Revascularization. N Engl J Med. 2020;382:1994-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 719] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 86. | CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4584] [Cited by in RCA: 4212] [Article Influence: 140.4] [Reference Citation Analysis (0)] |
| 87. | Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ; MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1618] [Cited by in RCA: 1510] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 88. | Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, Crimet DC, O'Connell RL, Colman PG; FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 769] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 89. | Rajamani K, Colman PG, Li LP, Best JD, Voysey M, D'Emden MC, Laakso M, Baker JR, Keech AC; FIELD study investigators. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;373:1780-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 90. | Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, Heerspink HL, Wong MG, Ninomiya T, Wada T, Perkovic V. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:1237-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 242] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 91. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3549] [Article Influence: 591.5] [Reference Citation Analysis (1)] |
| 92. | Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2303] [Cited by in RCA: 2633] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 93. | Martínez-Vizcaíno V, Díez-Fernández A, Álvarez-Bueno C, Martínez-Alfonso J, Cavero-Redondo I. Safety and Efficacy of SGLT2 Inhibitors: A Multiple-Treatment Meta-Analysis of Clinical Decision Indicators. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5636] [Article Influence: 626.2] [Reference Citation Analysis (0)] |
| 95. | Heyward J, Mansour O, Olson L, Singh S, Alexander GC. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: A systematic review and meta-analysis. PLoS One. 2020;15:e0234065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 96. | Miyashita S, Kuno T, Takagi H, Sugiyama T, Ando T, Valentin N, Shimada YJ, Kodaira M, Numasawa Y, Kanei Y, Bangalore S. Risk of amputation associated with sodium-glucose co-transporter 2 inhibitors: A meta-analysis of five randomized controlled trials. Diabetes Res Clin Pract. 2020;163:108136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 97. | Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, Ceriello A, Chiodini P, Esposito K. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 98. | Dhatariya K, Bain SC, Buse JB, Simpson R, Tarnow L, Kaltoft MS, Stellfeld M, Tornøe K, Pratley RE; LEADER Publication Committee on behalf of the LEADER Trial Investigators. The Impact of Liraglutide on Diabetes-Related Foot Ulceration and Associated Complications in Patients With Type 2 Diabetes at High Risk for Cardiovascular Events: Results From the LEADER Trial. Diabetes Care. 2018;41:2229-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 99. | Tuttolomondo A, Cirrincione A, Casuccio A, Del Cuore A, Daidone M, Di Chiara T, Di Raimondo D, Corte VD, Maida C, Simonetta I, Scaglione S, Pinto A. Efficacy of dulaglutide on vascular health indexes in subjects with type 2 diabetes: a randomized trial. Cardiovasc Diabetol. 2021;20:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 100. | Hasan R, Firwana B, Elraiyah T, Domecq JP, Prutsky G, Nabhan M, Prokop LJ, Henke P, Tsapas A, Montori VM, Murad MH. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63:22S-28S.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 101. | Fernando ME, Seneviratne RM, Tan YM, Lazzarini PA, Sangla KS, Cunningham M, Buttner PG, Golledge J. Intensive versus conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2016;2016:CD010764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Dissanayake A, Vandal AC, Boyle V, Park D, Milne B, Grech R, Ng A. Does intensive glycaemic control promote healing in diabetic foot ulcers? - a feasibility study. BMJ Open. 2020;10:e029009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C; Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16426] [Article Influence: 497.8] [Reference Citation Analysis (4)] |
| 104. | Boulton AJM, Armstrong DG, Löndahl M, Frykberg RG, Game FL, Edmonds ME, Orgill DP, Kramer K, Gurtner GC, Januszyk M, Vileikyte L. New Evidence-Based Therapies for Complex Diabetic Foot Wounds. Arlington (VA): American Diabetes Association; 2022 May-. [PubMed] [DOI] [Full Text] |
| 105. | Gordillo GM, Sen CK. Evidence-based recommendations for the use of topical oxygen therapy in the treatment of lower extremity wounds. Int J Low Extrem Wounds. 2009;8:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 106. | Serena TE, Bullock NM, Cole W, Lantis J, Li L, Moore S, Patel K, Sabo M, Wahab N, Price P. Topical oxygen therapy in the treatment of diabetic foot ulcers: a multicentre, open, randomised controlled clinical trial. J Wound Care. 2021;30:S7-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 107. | Niederauer MQ, Michalek JE, Liu Q, Papas KK, Lavery LA, Armstrong DG. Continuous diffusion of oxygen improves diabetic foot ulcer healing when compared with a placebo control: a randomised, double-blind, multicentre study. J Wound Care. 2018;27:S30-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 108. | Frykberg RG, Franks PJ, Edmonds M, Brantley JN, Téot L, Wild T, Garoufalis MG, Lee AM, Thompson JA, Reach G, Dove CR, Lachgar K, Grotemeyer D, Renton SC; TWO2 Study Group. A Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers: The TWO2 Study. Diabetes Care. 2020;43:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 109. | Yellin JI, Gaebler JA, Zhou FF, Niecko T, Novins O, Ockert A, Krzynowek D, Garoufalis MG, Lee AM, Frykberg RG. Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes. Adv Wound Care (New Rochelle). 2022;11:657-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Lipsky BA, Berendt AR. Hyperbaric oxygen therapy for diabetic foot wounds: has hope hurdled hype? Diabetes Care. 2010;33:1143-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018;6:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 112. | Game F, Jeffcoate W, Tarnow L, Jacobsen JL, Whitham DJ, Harrison EF, Ellender SJ, Fitzsimmons D, Löndahl M; LeucoPatch II trial team. LeucoPatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 113. | Rastogi A, Kulkarni SA, Deshpande SK, Driver V, Barman H, Bal A, Deshmukh M, Nair H. Novel Topical Esmolol Hydrochloride (Galnobax) for Diabetic Foot Wound: Phase 1/2, Multicenter, Randomized, Double-Blind, Vehicle-Controlled Parallel-Group Study. Adv Wound Care (New Rochelle). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 114. | Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, Kashefsky H, Owings TM, Nadarajah J; Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11:554-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 115. | Tettelbach W, Cazzell S, Sigal F, Caporusso JM, Agnew PS, Hanft J, Dove C. A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (EpiCord) allograft for the treatment of diabetic foot ulcers. Int Wound J. 2019;16:122-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 116. | National Diabetes Services Scheme. Foot Forward. 2023. [cited 31 March 2023]. Available from: https://www.footforward.org.au/. |
| 117. | National Association of Diabetes Centres. Interdisiplinary HRFS Accreditation. 2019. [cited 31 March 2023]. Avaialble from: https://nadc.net.au/hrfs-accreditation/. |
| 118. | Arsenault-Lapierre G, Henein M, Gaid D, Le Berre M, Gore G, Vedel I. Hospital-at-Home Interventions vs In-Hospital Stay for Patients With Chronic Disease Who Present to the Emergency Department: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e2111568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |