Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.1112
Peer-review started: March 13, 2023
First decision: May 12, 2023
Revised: May 17, 2023
Accepted: May 30, 2023
Article in press: May 30, 2023
Published online: July 15, 2023
Processing time: 122 Days and 6.2 Hours
Commonly used glucocorticoids replacement regimens in patients with hypopituitarism have difficulty mimicking physiological cortisol rhythms and are usually accompanied by risks of over-treatment, with adverse effects on glucose metabolism. Disorders associated with glucose metabolism are established risk factors of cardiovascular events, one of the life-threatening ramifications.
To investigate the glycometabolism profile in patients with hypopituitarism receiving prednisone (Pred) replacement, and to clarify the impacts of different Pred doses on glycometabolism and consequent adverse cardiovascular outcomes.
Twenty patients with hypopituitarism receiving Pred replacement [patient group (PG)] and 20 normal controls (NCs) were recruited. A flash glucose monitoring system was used to record continuous glucose levels during the day, which provided information on glucose-target-rate, glucose variability (GV), period glucose level, and hypoglycemia occurrence at certain periods. Islet β-cell function was also assessed. Based on the administered Pred dose per day, the PG was then regrouped into Pred > 5 mg/d and Pred ≤ 5 mg/d subgroups. Comparative analysis was carried out between the PG and NCs.
Significantly altered glucose metabolism profiles were identified in the PG. This includes significant reductions in glucose-target-rate and nocturnal glucose level, along with elevations in GV, hypoglycemia occurrence and postprandial glucose level, when compared with those in NCs. Subgroup analysis indicated more significant glucose metabolism impairment in the Pred > 5 mg/d group, including significantly decreased glucose-target-rate and nocturnal glucose level, along with increased GV, hypoglycemia occurrence, and postprandial glucose level. With regard to islet β-cell function, PG showed significant difference in homeostasis model assessment (HOMA)-β compared with that of NCs; a notable difference in HOMA-β was identified in Pred > 5 mg/d group when compared with those of NCs; as for Pred ≤ 5 mg/d group, significant differences were found in HOMA-β, and fasting glucose/insulin ratio when compared with NCs.
Our results demonstrated that Pred replacement disrupted glycometabolic homeostasis in patients with hypopituitarism. A Pred dose of > 5 mg/d seemed to cause more adverse effects on glycometabolism than a dose of ≤ 5 mg/d. Comprehensive and accurate evaluation is necessary to consider a suitable Pred replacement regimen, wherein, flash glucose monitoring system is a kind of promising and reliable assessment device. The present data allows us to thoroughly examine our modern treatment standards, especially in difficult cases such as hormonal replacement mimicking delicate natural cycles, in conditions such as diabetes mellitus that are rapidly growing in worldwide prevalence.
Core Tip: Glucocorticoids (GCs) replacement regimens for patients with hypopituitarism are hard to mimic physiological cortisol rhythms and carry risks of over-treatment, which can have adverse effects on glucose metabolism. We assessed the glucose metabolism profile of patients with hypopituitarism receiving prednisone (Pred) replacement, using a flash glucose monitoring system, to clarify impacts of different GCs preparations and prescriptions on glycometabolism, along with the resultant risks of consequent cardiovascular events. The study showed that Pred replacement disturbed glycometabolic homeostasis in patients with hypopituitarism. A dose of > 5 mg/d Pred caused more adverse effects on glycometabolism than ≤ 5 mg/d, contributing to the higher risks of cardiovascular events.
- Citation: Han MM, Zhang JX, Liu ZA, Xu LX, Bai T, Xiang CY, Zhang J, Lv DQ, Liu YF, Wei YH, Wu BF, Zhang Y, Liu YF. Glucose metabolism profile recorded by flash glucose monitoring system in patients with hypopituitarism during prednisone replacement. World J Diabetes 2023; 14(7): 1112-1125
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/1112.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.1112
As the growing amount of information consolidated in the field of glucocorticoids’ (GCs) hyperglycemia effect[1,2], whether GCs replacement therapy disturbs glycometabolism homeostasis in patients with hypopituitarism has garnered considerable interest. Timely and adequate GCs replacement has been commonly recognized as a lifesaving prescription for those patients with hypopituitarism, which aims to restore hormone deficiency and improve well-being. Hydrocortisone (HC) is the default choice for GCs replacement because of its similarity to endogenously-generated cortisol. Prednisone (Pred), cortisone, and dexamethasone represent other viable alternatives[3].
Choosing an optimum GCs replacement regimen for patients with hypopituitarism continues to be a challenging problem as the physiological cortisol rhythm is difficult to replicate. The most commonly used GCs replacement regimen is usually accompanied by a risk of insufficient trough levels or subtly excessive post-dose peaks[4,5]. An inability to mimic physiological cortisol rhythms or over-treatment may make those patients receiving GCs replacement susceptible to metabolic disturbances and subsequent cardiovascular events[6,7]. To date, the majority of evidence collected suggests that the occurrence of cardiovascular events is reportedly higher in patients with hypopituitarism who undergo GCs replacement than that in healthy controls[8,9]. New evidence has also emerged revealing that GCs replacement increases the prevalence of glycometabolism disorders[10], which are established risk factors for cardiovascular disease.
As the prevalence of adverse events increases in patients with hypopituitarism receiving GCs replacement, greater emphasis has been placed on choosing a suitable replacement regimen with as little influence on glycometabolism as possible. Therefore, this study was designed to assess the glucose metabolism profile recorded by a flash glucose monitoring system (FGMS) in patients with hypopituitarism, illuminating the impact of GCs preparation and prescription doses on glucose metabolism. In doing so, we hope to add novel insights into the existing body of evidence and provide references to guide the treatment choices for those patients with hypopituitarism, in order to reduce the incidence of cardiovascular events.
This study was conducted at the Department of Endocrinology, First Hospital of Shanxi Medical University from December, 2018 to August, 2022. The study protocol was approved by Ethic Committee in First Hospital of Shanxi Medical University. Written informed consent was obtained from all the subjects after explanation of study design and purpose.
Patients diagnosed with hypopituitarism and receiving Pred replacement were recruited in this study as patient group (PG). The hypopituitarism was diagnosed by the following criteria[3]: Medical history (including postpartum hemorrhage, hypophysectomy, and pituitary crisis); clinical manifestations (including hyponatremia, hypotension, and hypoglycemia); pituitary magnetic resonance imaging (including empty sella, pituitary hypoplasia, and pituitary stalk interruption); hormone assay (lower levels of hormones relevant to pituitary-adrenal/thyroid/gonad function); corticotropin stimulation test (stimulated cortisol < 500 nmol/L). Those who were under interventions known to influence cortisol metabolism and glucose metabolism were excluded. Age- and sex-matched normal controls (NCs) without known hypopituitary dysfunction or glycometabolic disorders were enrolled. At baseline, all the recruited patients underwent hypopituitary-adrenal/thyroid function assessment, along with electrolyte and glucose metabolism evaluation, including plasma sodium, glycosylated hemoglobin, fasting blood glucose, and fasting insulin. The NCs received laboratory tests similar to those of PG.
Relevant studies have corroborated that > 20 mg/d of HC correlated with unfavorable metabolic profile and car-diovascular events[11-13], however, whether an equivalent dose of Pred (> 5 mg/d) contributes to similar consequences is a relatively unexplored field. Due to the limited availability of HC in China, most patients with hypopituitarism received a Pred replacement regimen. In this study, we enrolled patients with hypopituitarism treated with Pred as PG, and divided PG into Pred > 5 mg/d and Pred ≤ 5 mg/d groups, based on the recommended Pred dose per day.
FGMS (FreeStyle Libre, Abbott Diabetes Care, Witney, United Kingdom) was used to record glucose profiles of those in PG and NCs. Due to unexpected dropping of the sensor or other unpredictable interferences, we failed to obtain a complete two-week monitoring data in each person. The data from the first-day of monitoring were removed due to poor accuracy. In the end, a total 222 d of monitoring data were collected from patients in PG (134 d for the Pred > 5 mg/d group, and 88 d for the Pred ≤ 5 mg/d group), while NCs provided 184 d of glucose data. The FGMS required a real-time scanned value within 8-h in order to ensure complete glucose data during the surveillance. Some glucose values at certain time points were therefore missing, owing either to the subjects’ poor adherence or other reasons, such as sleep time exceeding 8 h. These missing values were filled in with the mean of values before and after the missing values.
β-cell function and insulin resistance (IR) were assessed by calculating the homeostasis model assessment (HOMA)-β along with HOMA-IR, fasting glucose/insulin ratio (G/I), and quantitative insulin sensitivity check index (QUICKI).
The statistical methods used in this study were reviewed by Chunni Zhao from Shanxi Medical University. The data of laboratory tests and FGMS in PG, subgroups, and NCs were allocated in a Microsoft Excel datasheet. Data analysis and graph plotting were performed using SPSS 21.0 and Sigmaplot 12.5. All data are shown as mean ± standard error (SE) unless otherwise stated. Some data are represented as median (first quartile, third quartile) due to their wide distribution. A compared two-group Student’s t-test (for normally distributed data) or Mann-Whitney U test (for skewed data) was conducted between PG, each subgroup, and NCs, respectively. Statistical significance was set as P < 0.05.
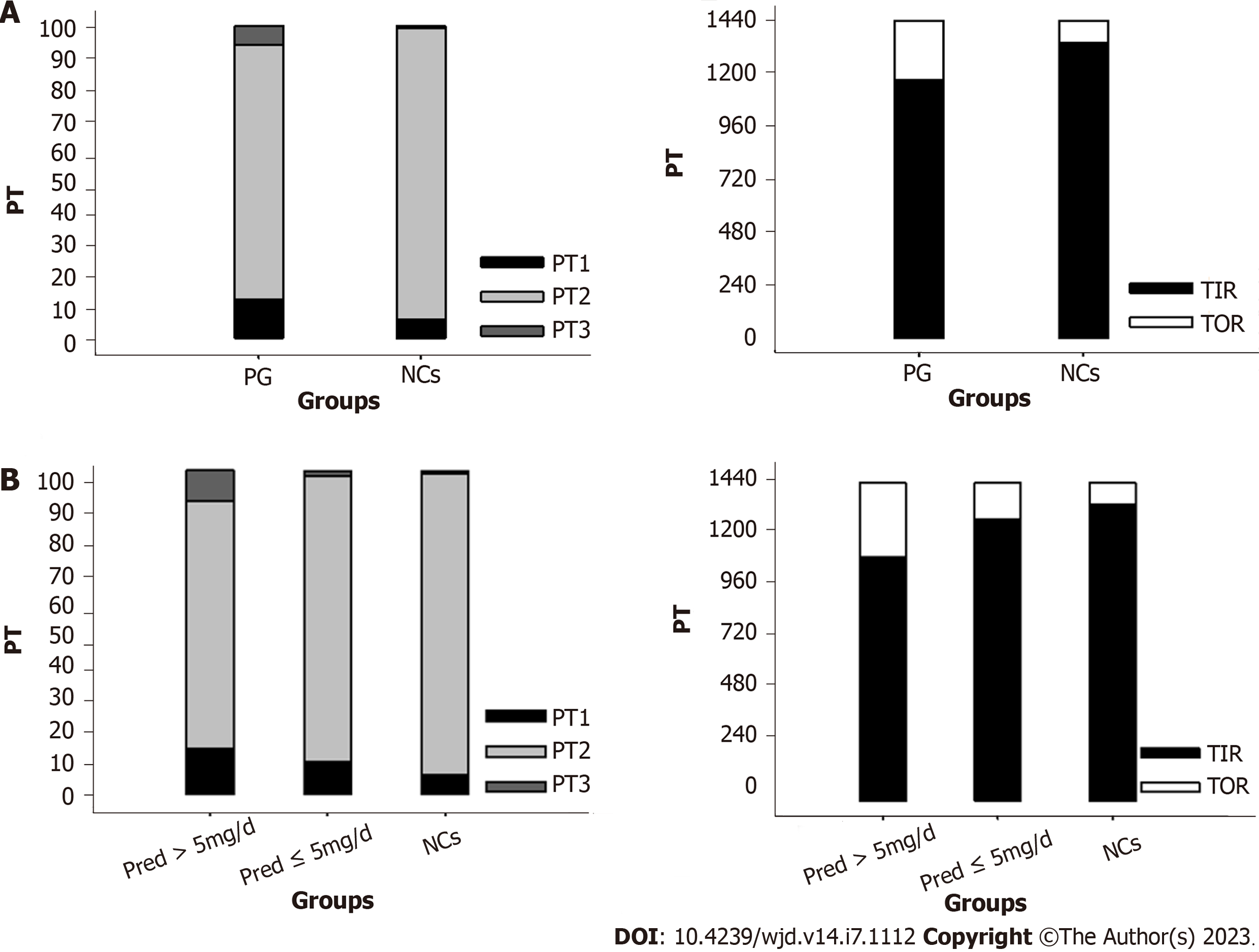
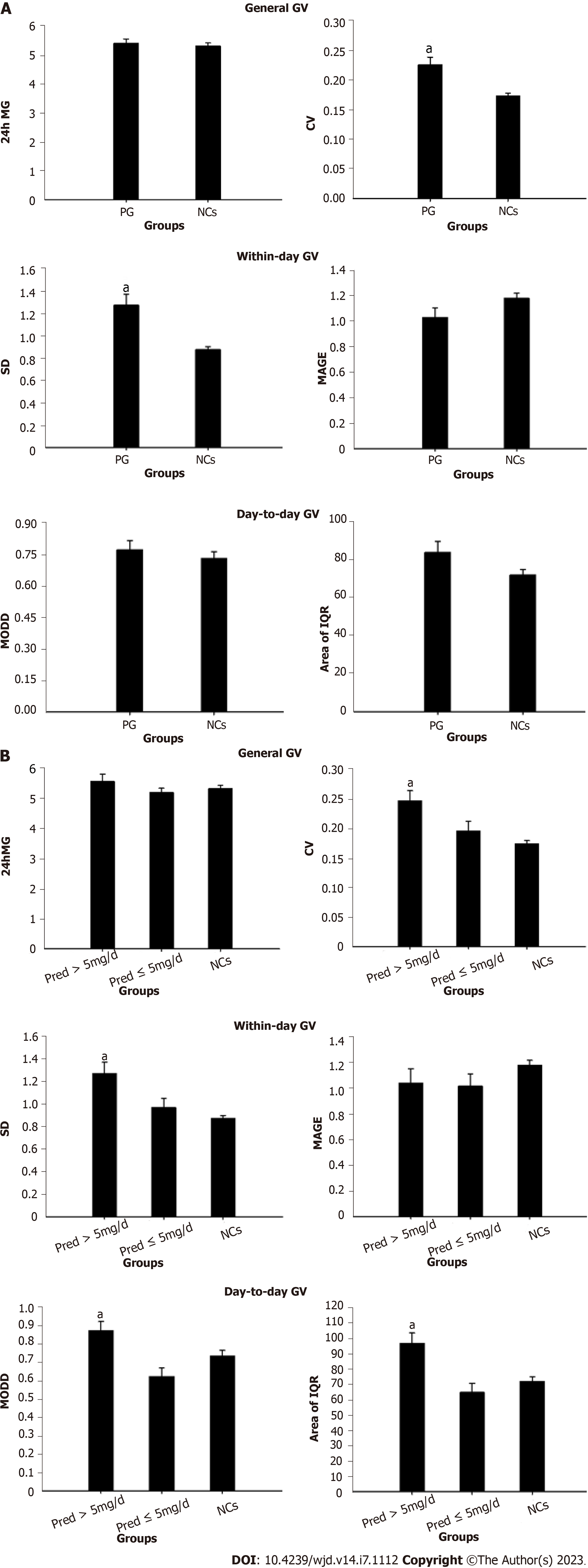
FGMS data were analyzed according to methods used in previous publications[14,15]. Specifically, glucose-target-rate, glucose variability (GV), and period glucose level were analyzed. A sensor glucose value within 3.9-7.8 mmol/L was set as the normal range. Accordingly, parameters representing glucose-target-rate were analyzed, including percentile time 1 (PT1, the percentage of sensor glucose values less than 3.9 mmol/L during the day), PT2 (the percentage of sensor glucose values within the range of 3.9-7.8 mmol/L during the day), PT3 (the percentage of sensor glucose values above 7.8 mmol/L during the day), time in range (TIR, the time of sensor glucose values within 3.9-7.8 mmol/L during the day), and time out of range (the time of sensor glucose values less than 3.9 mmol/L or above 7.8 mmol/L during the day). GV was analyzed from the following perspectives: General GV including 24-h mean glucose and coefficient of variance (CV); within-day GV consisting of SD and mean amplitude of glycemic excursion; mean of daily difference (MODD) and area of interquartile range (IQR) reflecting day-to-day GV.
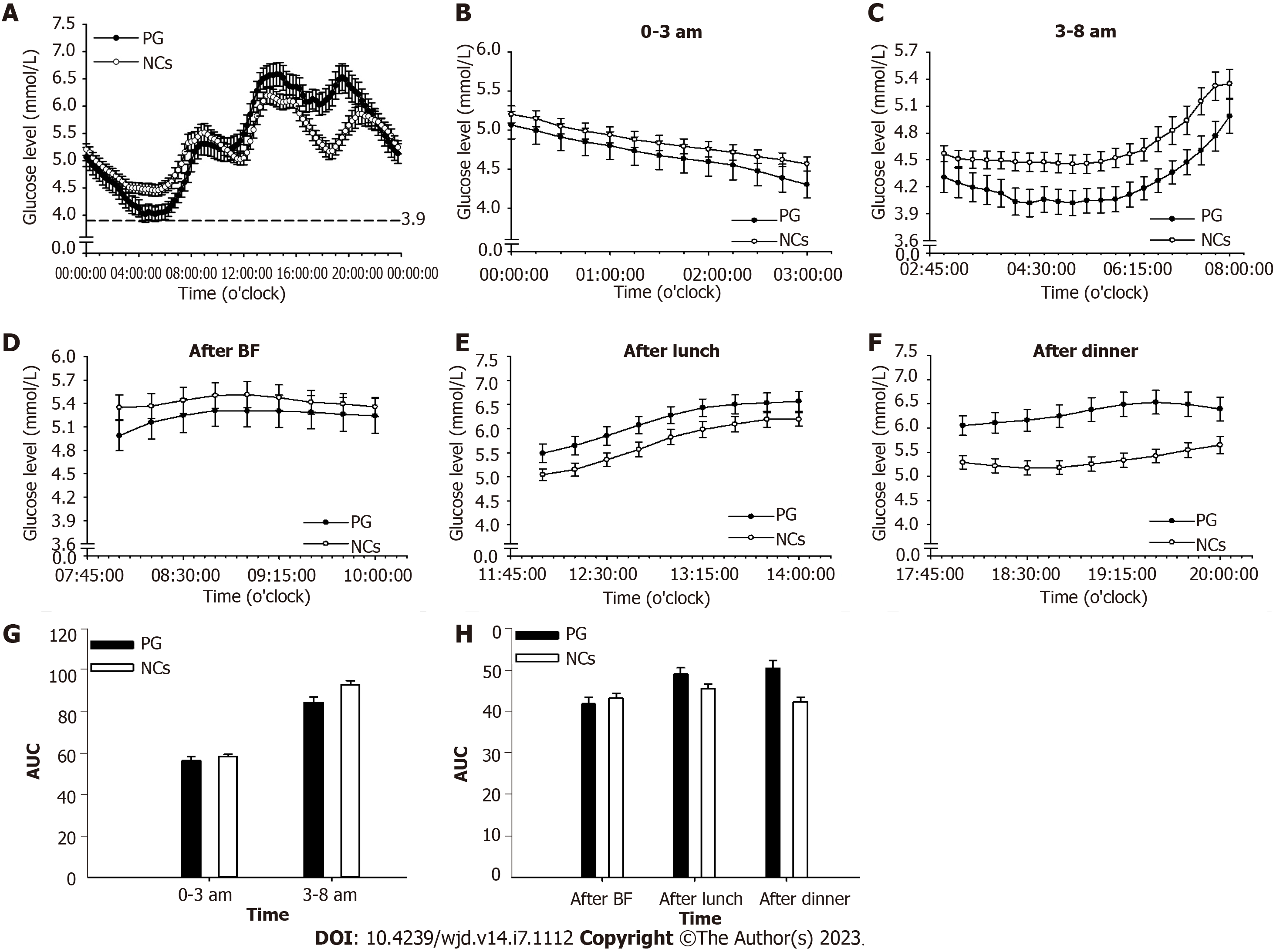
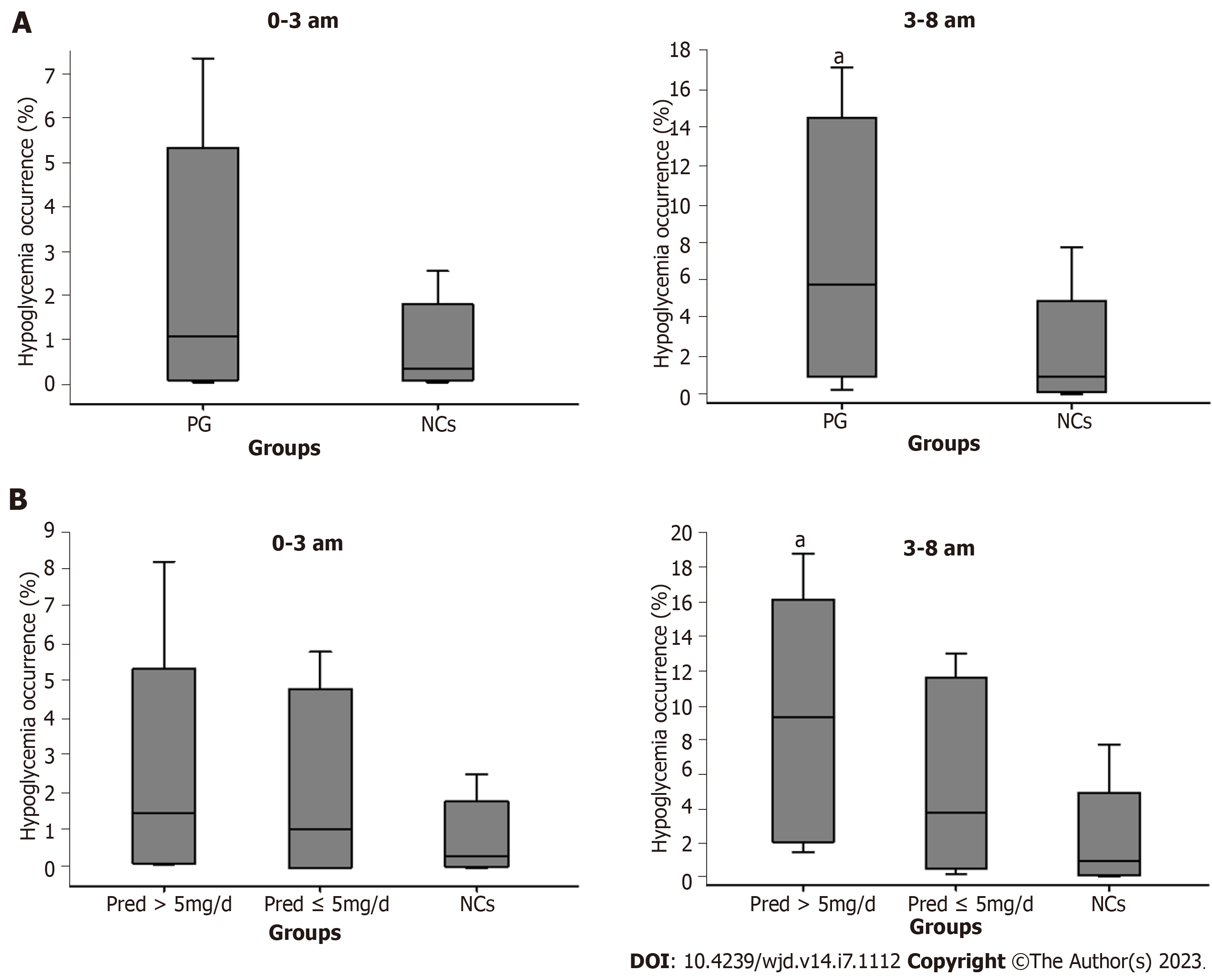
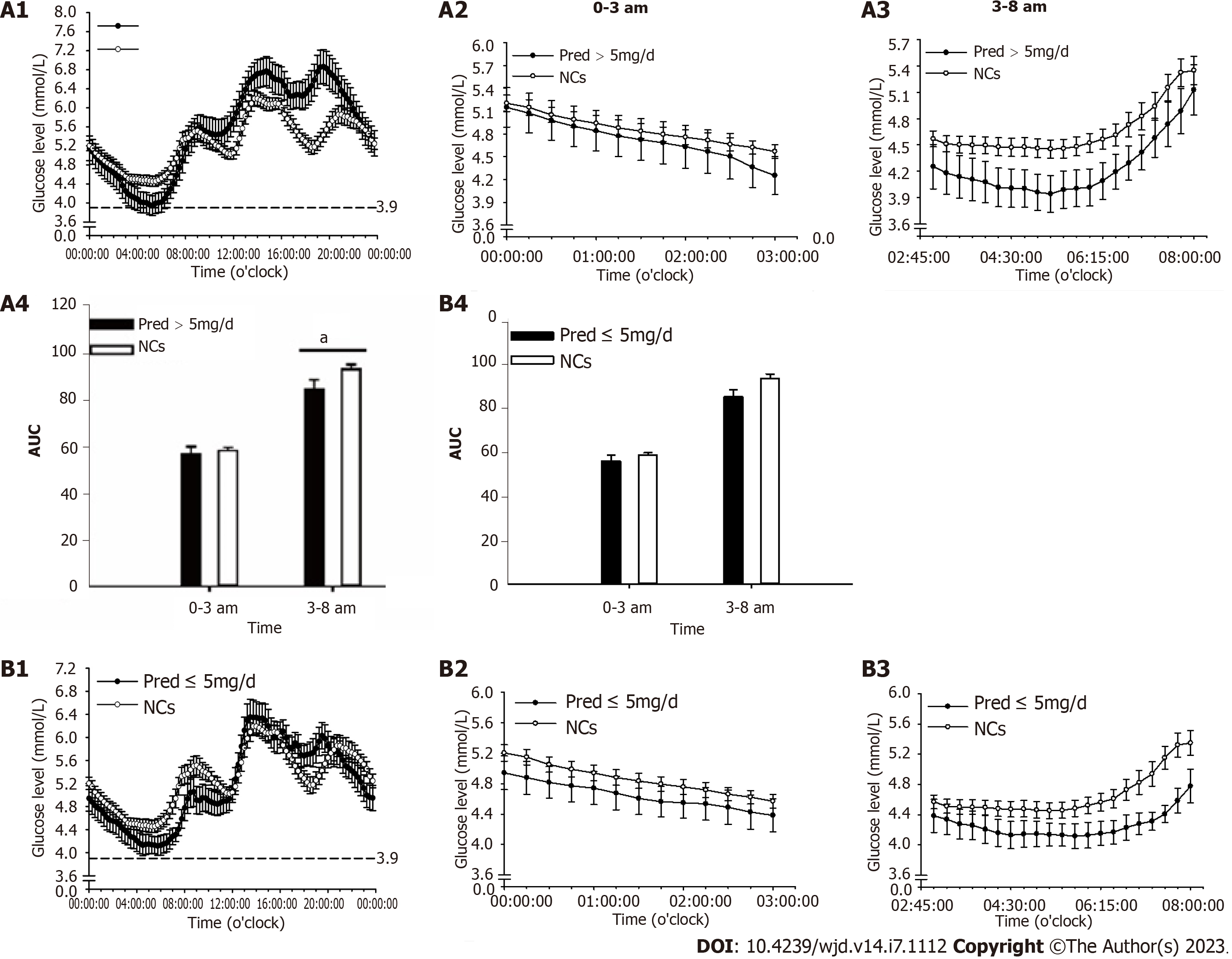
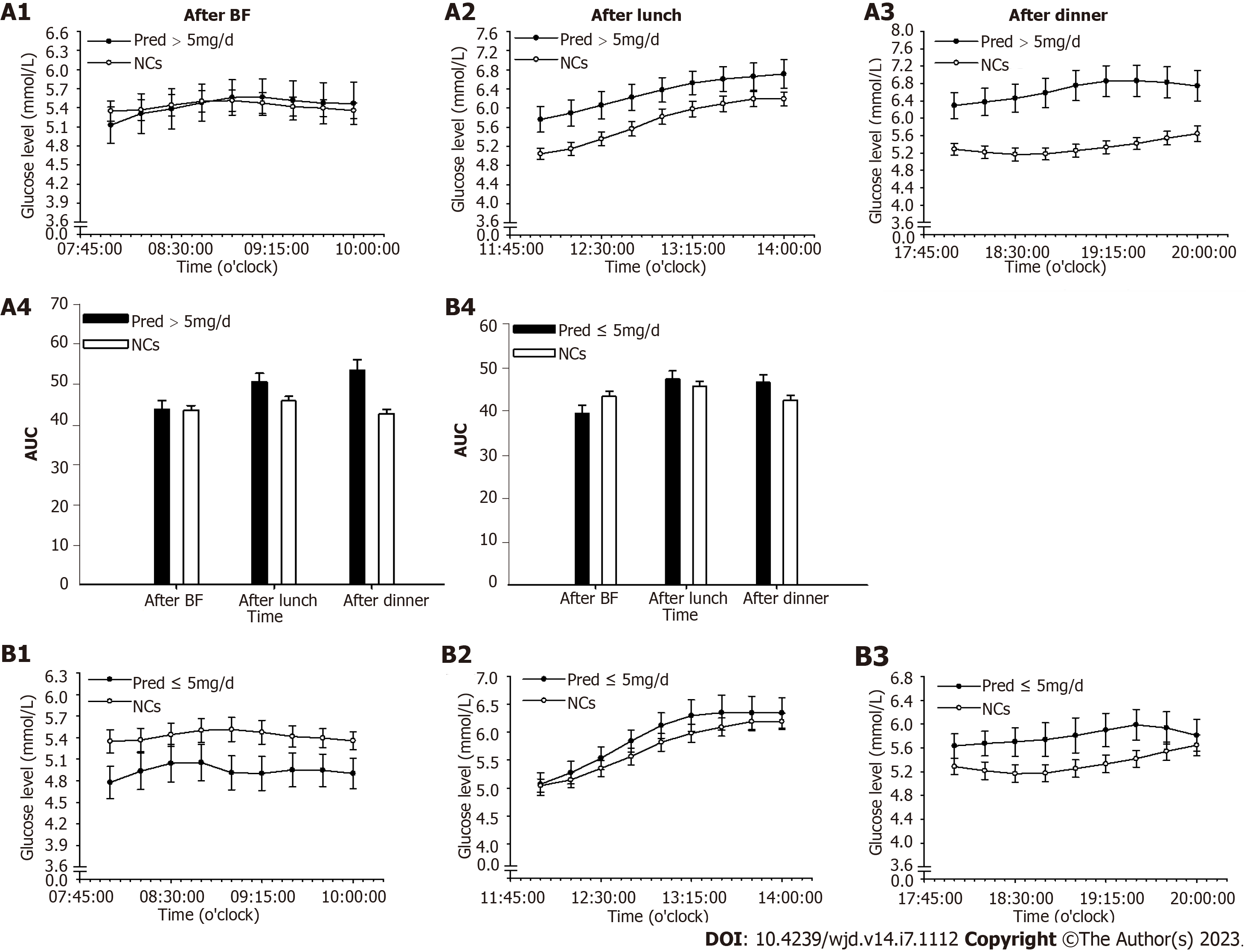
The endogenous cortisol secretion rhythm begins with a rise at around 3 am towards a peak after awaking, and then falls throughout the day until culminates in a nadir around the midnight[16]. Accordingly, nocturnal and fasting periods were merged and readjusted to periods of 0-3 am and 3-8 am. Glucose levels and area under the curve of glucose level at 0-3 am, 3-8 am, and postprandial periods were analyzed. In addition, hypoglycemia occurrence (glucose value less than 3.9 mmol/L) was analyzed during the 0-3 am and 3-8 am periods[17].
Formulas for calculating β-cell function and IR from a previous publication were used[14].
Twenty patients diagnosed with hypopituitarism, including nine with Sheehan’s syndrome, four with empty sella, six with hypophysectomy, and one with pituitary hypoplasia, and receiving Pred replacement were enrolled in this study. Of these patients, 16 had suffered acute hypopituitarism, presenting symptoms of hyponatremia, hypotension, hypoglycemia, etc., and four of them were diagnosed by corticotropin stimulation test (stimulated cortisol < 500 nmol/L). Significantly reduced levels of 24-h urinary free cortisol were detected in all of the patients during the course of the disease. Twelve patients were treated with doses of > 5 mg/d Pred and eight patients were treated with doses of ≤ 5 mg/d. There were also 12 patients undergoing concurrent thyroid hormone replacement therapy. The general characteristics and laboratory results of PG, subgroups, and NCs, including age, sex, disease duration (duration since hypopituitarism had been definitely diagnosed), blood pressure, plasma sodium, and endocrine hormone levels, are listed in the Table 1. Blood pressure, plasma sodium, and hormone levels were within the normal range in PG under the recommended replacement regimen.
| Parameters | PG | Pred (> 5 mg/d) | Pred (≤ 5 mg/d) | NCs | Reference range |
| Age (year-old) | 52.85 ± 3.49 | 51.5 ± 4.78 | 49.88 ± 5.33 | 50.75 ± 3.05 | - |
| Male:famle | 5:15 | 3:9 | 2:6 | 5:15 | - |
| BMI (kg/m2) | 19.46 ± 1.39 | 20.02 ± 1.44 | 18.61 ± 0.81 | 20.5 ± 1.58 | 18.5-23.9 |
| Disease duration (day) | 730 (88.75, 1095) | 730 (50, 1642.5) | 730 (638.75, 1095) | - | - |
| Blood pressure (mmHg) | (108.65 ± 1.15)/(74.85 ± 0.89) | (108.25 ± 1.62)/(75.08 ± 1.36) | (109.25 ± 1.65)/(74.5 ± 0.98) | (111.95 ± 1.17)/(73.5 ± 0.7) | |
| Pred dose (mg/d) | 6.19 ± 1.54 | 7.29 ± 0.48 | 4.53 ± 0.93 | - | - |
| ACTH (pmol/L) | 3.5 ± 0.33 | 3.58 ± 0.51 | 3.37 ± 0.33 | 4.40 ± 0.41 | 1.6-13.9 |
| Cortisol at 8 am (nmol/L) | 228.01 ± 4.42 | 230.24 ± 6.84 | 224.66 ± 4.44 | 372.90 ± 16.69 | 171-536 |
| Cortisol at 4 pm (nmol/L) | 153 ± 11.99 | 165.93 ± 17.48 | 133.6 ± 12.87 | 159.10 ± 14.62 | 64-327 |
| Cortisol at 0 am (nmol/L) | 111.55 ± 8.85 | 113.31 ± 12.01 | 108.91 ± 13.76 | - | - |
| UFC (nmol/24h) | 141.33 ± 11.34 | 131.80 ± 10.41 | 155.61 ± 23.86 | - | 100-279 |
| FT3 (pmol/L) | 4.25 ± 0.13 | 4.24 ± 0.15 | 4.27 ± 0.25 | 5.20 ± 0.14 | 3.1-6.8 |
| FT4 (pmol/L) | 14.11 ± 0.55 | 13.45 ± 0.50 | 15.09 ± 1.10 | 12.98 ± 0.92 | 10-23 |
| TSH (μIU/ml) | 1.076 ± 0.16 | 1.1 ± 0.19 | 1.04 ± 0.28 | 2.30 ± 0.19 | 0.27-4.2 |
| Plasma sodium (mmol/L) | 141.95 ± 0.86 | 140.3 ± 0.65 | 142.63 ± 1.07 | 140.6 ± 0.55 | 137-147 |
Significantly increased PT1 (P = 0.018) and PT3 (P = 0.002) along with decreased TIR (P < 0.001) were identified in PG when compared with that of NCs (Figure 1A).
Remarkable elevations in PT1 (P = 0.02) and PT3 (P < 0.001) along with reduction in TIR (P < 0.001) were identified in Pred > 5 mg/d group when compared with those of NCs (Figure 1B). Comparable PT1, PT3, and TIR were found between Pred ≤ 5 mg/d group and NCs (Figure 1B).
In PG, parameters of general GV were identified significance in CV (P = 0.003) compared with that of NCs. With regard to within-day GV, a notable elevation was found in SD (P = 0.003) when compared to those of NCs. There were no significant differences found in indices of day-to-day GV (Figure 2A).
In Pred > 5 mg/d group (Figure 2B), remarkable elevations were identified in parameters of general GV (CV, P < 0.001), within-day GV (SD, P < 0.001) and day-to-day GV (MODD, P = 0.019; area of IQR, P = 0.002), compared to that of NCs. However, no significant difference was observed in GV parameters between Pred ≤ 5 mg/d group and NCs (Figure 2B).
For PG, period glucose level analysis indicated that glucose level was significantly lower at period of 3-8 am (P = 0.004) than that of NCs (Figure 3). Consistent results were found in the analysis of hypoglycemia occurrence with a remarkable elevation during this period (P = 0.012, Figure 4A). In addition, significantly increased glucose levels were identified at postprandial phase of PG (after lunch, P = 0.028; after dinner, P < 0.001) when compared to that of NCs (Figure 3).
In Pred > 5 mg/d group, notable alterations were found during the 3-8 am period with decreased glucose level (P = 0.025, Figure 5A) and increased hypoglycemia occurrence (P = 0.008, Figure 4B) in comparison with those in NCs. In addition, a remarkable elevation in glucose level was observed at postprandial phase (after lunch, P = 0.015; after dinner, P < 0.001) when compared with that of NCs (Figure 6A).
In Pred ≤ 5 mg/d group, a significant reduction of glucose level at 3-8 am period (P = 0.021) were found in comparison with NCs (Figure 5B), whereas, hypoglycemia occurrence was comparable to that of NCs (Figure 4B). Comparable postprandial glucose levels were also identified at postprandial periods between Pred ≤ 5 mg/d group and NCs (Figure 6B).
In PG, glucose metabolism indicators showed a significant difference in HOMA-β (P = 0.003) compared with that of NCs (Table 2).
| Parameters | PG | Pred (> 5 mg/d) | Pred (≤ 5 mg/d) | NCs | Reference range |
| HbA1c (%) | 5.61 ± 0.101 | 5.71 ± 0.15 | 5.47 ± 0.11 | 5.46 ± 0.06 | 4.8-5.9 |
| FBG (mmol/L) | 4.71 ± 0.12 | 4.81 ± 0.19 | 4.56 ± 0.11 | 5.01 ± 0.13 | 3.9-6.1 |
| FINS (μU/mL) | 7.10 ± 0.64 | 6.82 ± 0.98 | 7.52 ± 0.72 | 5.76 ± 0.62 | 2.6-24.9 |
| HOMA-β | 130.22 ± 13.43a | 113.511 ± 16.18a | 155.28 ± 21.37a | 81.02 ± 6.83 | - |
| HOMA-IR | 1.52 ± 0.17 | 1.52 ± 0.27 | 1.54 ± 0.17 | 1.32 ± 0.16 | - |
| G/I | 0.79 ± 0.09 | 0.898 ± 0.14 | 0.64 ± 0.048a | 1.02 ± 0.082 | |
| QUICKI | 0.69 ± 0.03 | 0.71 ± 0.04 | 0.66 ± 0.02 | 0.73 ± 0.03 |
Glucose metabolism indicators showed a notable difference in HOMA-β (P = 0.021) in Pred > 5 mg/d group when compared with those of NCs (Table 2). As for Pred ≤ 5 mg/d group, significant difference was found in HOMA-β (P < 0.001), and G/I (P = 0.018) in comparison with that in NCs (Table 2).
In this study, we investigated the glucose metabolism profile in patients with hypopituitarism receiving GCs treatment. Significantly decreased glucose-target-rate and glucose level at nocturnal period, along with increased GV, hypoglycemia occurrence, and glucose level at postprandial phase were identified in PG when compared with those of NCs. These results demonstrated that glucose metabolism homeostasis was perturbed in patients with hypopituitarism receiving Pred replacement, despite careful administration. This disturbance may carry a risk of leading to cardiovascular diseases.
A dose of > 5 mg/d Pred was associated with a notable reduction in glucose-target-rate and glucose level at nocturnal period, along with elevation in GV, hypoglycemia occurrence, and glucose level at postprandial phase. However, only glucose level at 3-8 am period was changed significantly in Pred ≤ 5 mg/d group. Accordingly, we concluded that a dose of > 5 mg/d Pred may have a more adverse impact on glucose metabolism.
Given the essential role of GCs in maintaining normal life, authoritative guidelines strongly endorse the paramount importance of exogenously GCs replacement in those patients with endogenous insufficiency[3,18]. In this context, GCs replacement has been recognized as a fundamental therapeutic paradigm for patients with hypopituitarism. The diurnal rhythm of physiological cortisol secretion has been recognized for many years[19]. It is challenging for a GCs replacement regimen to accurately mimic this endogenously rhythmic pattern[20,21], usually leading to nonphysiological and subtly excess cortisol levels.
There is a growing awareness of highly dynamic synchronization in cortisol secretion into the blood circulation and its binding to GCs receptor (GR) in peripheral tissues[22]. Non-physiological GCs replacement fails to achieve a circadian rhythmic pattern, and further disturbs the tissue response mode, ensuing compromised hormone action, such as impaired glycometabolism and water-electrolyte metabolism. Pred is a kind of synthetic steroids, endowed with a great and enduring stimulatory effect on GR[23], and by continuously acting on the target tissues of glycometabolism, it can lead to metabolic disturbance.
In this study, significantly altered PT and TIR were identified in PG when compared with that of NCs. The significantly increased prevalence of hyperglycemia and hypoglycemia led to poor TIR. As an emerging indicator for blood glucose control in diabetic patients, TIR has been demonstrated to be inversely correlated with the risk of cardiovascular events[24,25]. The statistically decreased TIR identified in Pred group may herald a higher risk of cardiovascular events in these patients.
The results of significantly increased GV than that of NCs suggested that Pred replacement brought about an adverse impact on glycometabolism. GV is known to be positively associated with incidence of cardiovascular events in patients with diabetes[26,27]. Accordingly, we hypothesized that this notable elevation of GV found in PG implied that these patients would be prone to developing cardiovascular diseases during the long-term replacement therapy regimen.
The average glucose level throughout the whole day in PG was within the normal range. Nonetheless, a notable reduction was indicated at period of 3-8 am. Increased hypoglycemia occurrence was also identified at this period but not at 0-3 am period. Taken together, one could postulate that there existed relatively insufficient cortisol level at 3-8 am period, which was responsible for the elevated occurrence of hypoglycemia.
It is crucial to reiterate the basic fact that cortisol secretion follows a circadian rhythm in normal subjects, which commences with a rise at approximately 3 am, reaches a peak at around 8-9 am, and then progressively decreases towards a nadir at around midnight[16]. The cortisol trough level seemed sufficient in PG according to the comparable glucose level and hypoglycemia occurrence observed at 0-3 am period, when compared to those of NCs, which allowed us to hypothesize that Pred produced long-term steroids effects due to its delayed disassociation from GR. The applied Pred regimen was enough to maintain sufficient a trough level, although it failed to adequately and synchronously maintain cortisol elevation from 3 am to 8 am compared to the normal cases in NCs. Consequently, there may have existed a pre-dose cortisol insufficiency at period of 3-8 am when the steroids effect of the last administration had been washed out, leading to decreased glucose level and increased hypoglycemia occurrence.
A significantly increased postprandial glucose level was found in PG compared with that of NCs, seemingly indicating a subtly excess cortisol level during the daytime. As an allegedly long-acting GCs preparation, Pred possesses a great affinity for GR, occupying and stimulating the GR over a lengthy period until it is finally degraded[23]. Prolonged exposure to steroids allows continuous accesses to target tissues, eliciting unfavorable side effects such as increased postprandial glucose level, as discovered in this study.
An important consideration when interpreting the influence of GCs replacement on glucose metabolism is whether the daily dose matches with the measured cortisol secretion rate over the course of an entire day in a normal subject. The physiological cortisol production rate is lower than the traditionally recommended GCs replacement dose[28,29]. Clinical evidence suggests that a dose of > 20 mg/d HC correlates with increased incidence of adverse events[11,12,30]. A dose of 5 mg Pred is equivalent to 20 mg HC. Assuming that patients under a replacement regimen of > 5 mg/d Pred were associated with a higher risk of adverse metabolic profile, PG was distributed into two subgroups: Pred > 5 mg/d and Pred ≤ 5 mg/d.
Significantly altered PT and TIR were found in Pred > 5 mg/d group, revealing that poor TIR was attributed to increased hyperglycemia and hypoglycemia occurrence. However, all indicators of glucose-target-rate showed no significant difference between Pred ≤ 5 mg/d group and NCs. According to TIR results, Pred > 5 mg/d group may have a predisposition towards developing cardiovascular diseases.
Significantly increased GV was detected in Pred > 5 mg/d group, while no statistical difference was identified in these parameters when Pred ≤ 5 mg/d group and NCs were compared. It is tempting, therefore, to speculate that patients in Pred > 5 mg/d group experienced more aggressive glycometabolism impairment and a great tendency to experience adverse cardiovascular events.
The average daily glucose level was normal in both Pred > 5 mg/d group and Pred ≤ 5 mg/d group. In Pred > 5 mg/d group, a notable reduction of glucose level was documented at 3-8 am period, highlighting the possibility of insufficient nocturnal hormone level. Moreover, a remarkable elevation of hypoglycemia occurrence was identified at this period, adding credence to the speculation of insufficient nocturnal hormone level. The Pred ≤ 5 mg/d group exhibited a significantly decreased glucose level at 3-8 am period, however, hypoglycemia occurrence at this period was comparable to that in NCs, prompting the assumption that insufficient nocturnal hormone level was relatively mitigated in comparison with the situation in Pred > 5 mg/d group. In addition, the glucose level and hypoglycemia occurrence at 0-3 am period were comparable to those of NCs in Pred > 5 mg/d group and Pred ≤ 5 mg/d group, demonstrating that a sufficient trough level may have been achieved in these two group.
A significantly increased glucose level at postprandial phase was identified in Pred > 5 mg/d group, but not in Pred ≤ 5 mg/d group. The underlying mechanism was assumed to be continuous hormone accessing to the target tissue due to the long-acting property of Pred during the daytime. This continuous stimulation may have disturbed glucose regulation in the relevant organs, leading to reduced glucose disposal and elevated glucose production. The present data allowed us to hypothesize that a Pred dose of > 5 mg/d posed a more profound effect on glucose regulation during the efficacy period than a dose of ≤ 5 mg/d.
Impaired β-cell function was indicated by relevant parameters in PG when compared to that of NCs. Summarizing the yet published literature, there are no consistent data supporting the presence of impaired β-cell function in patients with hypopituitarism undergoing GCs treatment[31-33]. Results generated in this study allowed us to hypothesize that the physiological Pred replacement regimen may exert adverse effects on glucose metabolism, leading to compromised β-dell function.
Of special note is that adrenocorticotropic-hormone (ACTH) levels measured in all the groups were within the normal range, probably indicating partial ACTH deficiency. The normal ACTH levels were suggestive of a reasonable Pred treatment because an excess dose of Pred might suppress the ACTH secretion. However, the usage of FGMS identified impaired glucose metabolism, which is relevant to higher risks of cardiovascular diseases. In this light, FGMS attests its importance in providing reliable information for evaluating a suitable Pred replacement regimen.
Pred replacement in patients with hypopituitarism impaired glucose metabolism, leading to an increased risk of cardiovascular events. A dose of > 5 mg/d Pred had a more significant influence on glucose metabolism than a dose of ≤ 5 mg/d. A suitable Pred replacement regimen necessitates comprehensive and accurate evaluation, for which FGMS is a kind of promising and reliable assessment device. Altogether, the integration of results in this study adds weight to the existing knowledge, and further provides new reference and guidance for future clinical work to effectively avoid the risk of cardiovascular events and improve well-being in patients with hypopituitarism.
As the growing amount of information consolidated in the field of glucocorticoids’ (GCs) hyperglycemia effect, whether GCs replacement therapy disturbs glycometabolism homeostasis in patients with hypopituitarism has garnered considerable interest. Timely and adequate GCs replacement has been commonly recognized as a lifesaving prescription for those patients with hypopituitarism, which aims to restore hormone deficiency and improve well-being. Choosing an optimum GCs replacement regimen for patients with hypopituitarism continues to be challenging problem as the physiological cortisol rhythm is difficult to replicate. An inability to mimic physiological cortisol rhythms or over-treatment may make those patients receiving GCs replacement susceptible to metabolic disturbances and subsequent cardiovascular events.
Commonly used glucocorticoids replacement regimens in hypopituitarism patients have difficulty mimicking physiological cortisol rhythms and are usually accompanied with risks of over-treatment, which will pose adverse effects on glucose metabolism. Disorders associated with glucose metabolism are established risk factors of cardiovascular events, one of the life-threatening ramifications. As the increasing prevalence of adverse events occurs in hypopituitarism patients under GCs replacement, greater emphasis has been placed on choosing a suitable replacement regimen with as little influence on glycometabolism as possible.
This study was designed to assess the glucose metabolism profile recorded by a flash glucose monitoring system in patients with hypopituitarism, illuminating the impact of GCs preparation (Pred) and prescription doses on glucose metabolism. In doing so, we hope to add novel insights into the existing body of evidence and provide references to guide the treatment choices for those patients with hypopituitarism, in order to reduce the incidence of cardiovascular events.
In this study, patients with hypopituitarism treated with Pred were enrolled as patient group (PG), and regrouped into Pred > 5 mg/d group and Pred ≤ 5 mg/d group based on the recommended Pred dose per day. Age- and sex-matched normal controls (NCs) without known hypopituitary dysfunction or glycometabolic disorders were enrolled. At baseline, all the recruited patients underwent hypopituitary-adrenal/thyroid function assessment, along with electrolyte and glucose metabolism evaluation, including plasma sodium, glycosylated hemoglobin, fasting blood glucose, and fasting insulin. The NCs received laboratory tests similar to those of PG. Flash glucose monitoring system (FGMS) was used to record glucose profile of both PG and the NCs. Parameters of glucose-target-rate, glucose variability (GV), and period glucose level were analyzed. β-cell function and insulin resistance (IR) were assessed by calculating the homeostasis model assessment (HOMA)-β along with HOMA-IR, fasting glucose/insulin ratio, and quantitative insulin sensitivity check index.
Twenty patients diagnosed with hypopituitarism receiving Pred replacement were enrolled in this study. Of these, twelve patients were treated with doses of > 5 mg/d Pred and eight patients were treated with doses of ≤ 5 mg/d. Significantly decreased glucose-target-rate and glucose level at nocturnal period, along with increased GV, hypoglycemia occurrence, and glucose level at postprandial phase were identified in PG when compared with those of NCs. These results demonstrated that glucose metabolism homeostasis was perturbed in patients with hypopituitarism receiving Pred replacement, despite careful administration. This disturbance may carry a risk of leading to cardiovascular diseases. A dose of > 5 mg/d Pred was associated with a notable reduction in glucose-target-rate and glucose level at nocturnal period, along with elevation in GV, hypoglycemia occurrence, and glucose level at postprandial phase. However, only glucose level at 3-8 am period was changed significantly in Pred ≤ 5 mg/d group. Accordingly, we concluded that a dose of > 5 mg/d Pred may have a more adverse impact on glucose metabolism. Impaired β-cell function was indicated by relevant parameters in PG when compared to that of NCs.
Pred replacement in patients with hypopituitarism impaired glucose metabolism, leading to an increased risk of cardiovascular events. A dose of > 5 mg/d Pred had a more significant influence on glucose metabolism than a dose of ≤ 5 mg/d. A suitable Pred replacement regimen necessitates comprehensive and accurate evaluation, for which FGMS is a kind of promising and reliable assessment device. Altogether, the integration of results in this study adds weight to the existing knowledge, and further provides new reference and guidance for future clinical work to effectively avoid the risk of cardiovascular events and improve well-being in patients with hypopituitarism.
The integration of results in this study adds weight to the existing knowledge, and further provides new reference and guidance for future clinical work to effectively avoid the risk of cardiovascular events and improve well-being in patients with hypopituitarism.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nishihama K, Japan; Park JH, South Korea S-Editor: Fan JR L-Editor: A P-Editor: Ji MX
| 1. | Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond). 1999;96:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 355] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 2. | van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest. 2009;39:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 312] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, Samuels MH. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:3888-3921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 614] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 4. | Simon N, Castinetti F, Ouliac F, Lesavre N, Brue T, Oliver C. Pharmacokinetic evidence for suboptimal treatment of adrenal insufficiency with currently available hydrocortisone tablets. Clin Pharmacokinet. 2010;49:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Debono M, Ross RJ. What is the best approach to tailoring hydrocortisone dose to meet patient needs in 2012? Clin Endocrinol (Oxf). 2013;78:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, Johannsson G. Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab. 2006;91:4849-4853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 362] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Björntorp P. Visceral obesity: a "civilization syndrome". Obes Res. 1993;1:206-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Rosén T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 918] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 9. | Bülow B, Hagmar L, Eskilsson J, Erfurth EM. Hypopituitary females have a high incidence of cardiovascular morbidity and an increased prevalence of cardiovascular risk factors. J Clin Endocrinol Metab. 2000;85:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | McConnell EM, Bell PM, Hadden DR, McCance DR, Sheridan B, Atkinson AB. Prevalence of diabetes and impaired glucose tolerance in adult hypopituitarism on low dose oral hydrocortisone replacement therapy. Clin Endocrinol (Oxf). 2001;54:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Filipsson H, Monson JP, Koltowska-Häggström M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91:3954-3961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | McConnell EM, Bell PM, Ennis C, Hadden DR, McCance DR, Sheridan B, Atkinson AB. Effects of low-dose oral hydrocortisone replacement versus short-term reproduction of physiological serum cortisol concentrations on insulin action in adult-onset hypopituitarism. Clin Endocrinol (Oxf). 2002;56:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ragnarsson O, Nyström HF, Johannsson G. Glucocorticoid replacement therapy is independently associated with reduced bone mineral density in women with hypopituitarism. Clin Endocrinol (Oxf). 2012;76:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Han M, Cao X, Zhao C, Yang L, Yin N, Shen P, Zhang J, Gao F, Ren Y, Liang D, Yang J, Zhang Y, Liu Y. Assessment of Glycometabolism Impairment and Glucose Variability Using Flash Glucose Monitoring System in Patients With Adrenal Diseases. Front Endocrinol (Lausanne). 2020;11:544752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Li Y, Han MM, He Q, Liu ZA, Liang D, Hou JT, Zhang Y, Liu YF. Exenatide once weekly combined with metformin reduced glycemic variability in type 2 diabetes by using flash glucose monitoring system. World J Diabetes. 2020;11:654-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Henley DE, Lightman SL. Cardio-metabolic consequences of glucocorticoid replacement: relevance of ultradian signalling. Clin Endocrinol (Oxf). 2014;80:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Watanabe T, Ozawa A, Ishii S, Tomaru T, Shibusawa N, Saito T, Yamada E, Horiguchi K, Nakajima Y, Matsumoto S, Yoshino S, Katano-Toki A, Hashimoto K, Mori M, Okada S, Satoh T, Yamada M. Usage of continuous glucose monitoring (CGM) for detecting an unrecognized hypoglycemia and management of glucocorticoid replacement therapy in adult patients with central hypoadrenalism. Endocr J. 2018;65:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Grossman AB. Clinical Review#: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010;95:4855-4863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 807] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Johannsson G, Nilsson AG, Bergthorsdottir R, Burman P, Dahlqvist P, Ekman B, Engström BE, Olsson T, Ragnarsson O, Ryberg M, Wahlberg J, Biller BM, Monson JP, Stewart PM, Lennernäs H, Skrtic S. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab. 2012;97:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 21. | Johannsson G, Bergthorsdottir R, Nilsson AG, Lennernas H, Hedner T, Skrtic S. Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: a pharmacokinetic study. Eur J Endocrinol. 2009;161:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Qian X, Droste SK, Lightman SL, Reul JM, Linthorst AC. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153:4346-4353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 24. | Lu J, Ma X, Shen Y, Wu Q, Wang R, Zhang L, Mo Y, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W, Zhou J. Time in Range Is Associated with Carotid Intima-Media Thickness in Type 2 Diabetes. Diabetes Technol Ther. 2020;22:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 25. | Lu J, Wang C, Shen Y, Chen L, Zhang L, Cai J, Lu W, Zhu W, Hu G, Xia T, Zhou J. Time in Range in Relation to All-Cause and Cardiovascular Mortality in Patients With Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care. 2021;44:549-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 26. | Tang X, Li S, Wang Y, Wang M, Yin Q, Mu P, Lin S, Qian X, Ye X, Chen Y. Glycemic variability evaluated by continuous glucose monitoring system is associated with the 10-y cardiovascular risk of diabetic patients with well-controlled HbA1c. Clin Chim Acta. 2016;461:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, Zhou Y, Ma C. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 28. | Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, Booth JD, Winterer JC, Loriaux DL. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab. 1991;72:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 313] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Crown A, Lightman S. Why is the management of glucocorticoid deficiency still controversial: a review of the literature. Clin Endocrinol (Oxf). 2005;63:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 541] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 31. | Suliman AM, Freaney R, Smith TP, McBrinn Y, Murray B, McKenna TJ. The impact of different glucocorticoid replacement schedules on bone turnover and insulin sensitivity in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2003;59:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Plat L, Byrne MM, Sturis J, Polonsky KS, Mockel J, Féry F, Van Cauter E. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am J Physiol. 1996;270:E36-E42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Bhat MA, Laway BA, Shah ZA, Wani AI, Mubarik I. Insulin resistance, metabolic syndrome and chronic low grade inflammation in Sheehan's syndrome on standard replacement therapy: a case control study. Pituitary. 2015;18:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |