Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.1057
Peer-review started: January 20, 2023
First decision: April 11, 2023
Revised: April 14, 2023
Accepted: May 5, 2023
Article in press: May 5, 2023
Published online: July 15, 2023
Processing time: 173 Days and 20.8 Hours
Patients with diabetes mellitus are at higher risk of myocardial ischemia/ reperfusion injury (MI/RI). Shuxin decoction (SXT) is a proven recipe modi-fication from the classic herbal formula "Wu-tou-chi-shi-zhi-wan" according to the traditional Chinese medicine theory. It has been successfully used to alleviate secondary MI/RI in patients with diabetes mellitus in the clinical setting. However, the underlying mechanism is still unclear.
To further determine the mechanism of SXT in attenuating MI/RI associated with diabetes.
This paper presents an ensemble model combining network pharmacology and biology. The Traditional Chinese Medicine System Pharmacology Database was accessed to select key components and potential targets of the SXT. In parallel, therapeutic targets associated with MI/RI in patients with diabetes were screened from various databases including Gene Expression Omnibus, DisGeNet, Genecards, Drugbank, OMIM, and PharmGKB. The potential targets of SXT and the therapeutic targets related to MI/RI in patients with diabetes were intersected and subjected to bioinformatics analysis using the Database for Annotation, Visualization and Integrated Discovery. The major results of bioinformatics analysis were subsequently validated by animal experiments.
According to the hypothesis derived from bioinformatics analysis, SXT could possibly ameliorate lipid metabolism disorders and exert anti-apoptotic effects in MI/RI associated with diabetes by reducing oxidized low density lipoprotein (LDL) and inhibiting the advanced glycation end products (AGE)-receptor for AGE (RAGE) signaling pathway. Subsequent animal experiments confirmed the hypothesis. The treatment with a dose of SXT (2.8 g/kg/d) resulted in a reduction in oxidized LDL, AGEs, and RAGE, and regulated the level of blood lipids. Besides, the expression of apoptosis-related proteins such as Bax and cleaved caspase 3 was down-regulated, whereas Bcl-2 expression was up-regulated. The findings indicated that SXT could inhibit myocardial apoptosis and improve cardiac function in MI/RI in diabetic rats.
This study indicated the active components and underlying molecular therapeutic mechanisms of SXT in MI/RI with diabetes. Moreover, animal experiments verified that SXT could regulate the level of blood lipids, alleviate cardiomyocyte apoptosis, and improve cardiac function through the AGE-RAGE signaling pathway.
Core Tip: Patients with diabetes are susceptible to myocardial ischemia/reperfusion injury (MI/RI). The efficacy of implementing strict glycemic control to reduce cardiovascular mortality in patients with diabetes has not been established to yield significant benefits. Here, we evaluated a recipe [Shuxin decoction (SXT)], which was modified from the classic herbal formula "Wu-tou-chi-shi-zhi-wan" in traditional Chinese medicine. Animal experiments based on findings from network pharmacology indicated that SXT could regulate lipid metabolism, alleviate cardiomyocyte apoptosis, and attenuate MI/RI in diabetes through the advanced glycation end products (AGE)-receptor for AGE signaling pathway. These findings could potentially facilitate developing a novel complementary or alternative form of medicine for effectively managing MI/RI with diabetes.
- Citation: Yang L, Jian Y, Zhang ZY, Qi BW, Li YB, Long P, Yang Y, Wang X, Huang S, Huang J, Zhou LF, Ma J, Jiang CQ, Hu YH, Xiao WJ. Network-pharmacology-based research on protective effects and underlying mechanism of Shuxin decoction against myocardial ischemia/reperfusion injury with diabetes. World J Diabetes 2023; 14(7): 1057-1076
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/1057.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.1057
According to the latest report of the International Diabetes Federation, diabetes is responsible for about 6.7 million deaths globally every year[1]. Most mortality in diabetic patients is associated with cardiovascular disease[2]. Increasing evidence has revealed that larger infarct size and worse cardiac function in diabetes follow with myocardial ischemia/reperfusion injury (MI/RI)[3-6]. Obesity, hyperglycemia, and hyperlipidemia are the most common metabolic diseases in diabetes mellitus, which are recognized as cardiovascular risk factors[7]. However, no significant benefits were obtained from strict glycemic control to decrease cardiovascular mortality in diabetes[8,9]. Thus, regulating lipid metabolism may be a novel strategy for alleviating MI/RI in diabetes.
Shuxin decoction (SXT) is a traditional Chinese medicine (TCM) compound based on modification of “Wu-tou-chi-shi-zhi-wan” recorded in the medical classic “Jin Gui Yao Lue” written by Zhongjing Zhang in the Eastern Han Dynasty. “Wu-tou-chi-shi-zhi-wan” was used to protect the cardiovascular system from various injuries in TCM. SXT was a modification of “Wu-tou-chi-shi-zhi-wan” into seven herbs: Astragalus, Zanthoxylum, Rhizoma zingiberis, Cinnamon, Salvia miltiorrhiza, Panax notoginseng, and Ligusticum wallichii. Recent studies have shown that Astragalus extract can reduce the levels of triglyceride (TG), total cholesterol (TC), and low density lipoprotein (LDL)[10]. Zanthoxylum extract exerts anti-obesity and hypolipidemic effects by reducing liver oxidative stress[11]. Rhizoma zingiberis extract reduces heart structural abnormalities in diabetic rats by improving the levels of apolipoproteins, leptin, cathepsin G, and homocysteine in serum[12]. Cinnamic acid alleviates MI/RI by inhibiting NLRP3/Caspase-1/GSDMD signaling[13]. Salvia miltiorrhiza and Panax notoginseng saponins can reduce oxidative stress and apoptosis to ameliorate myocardial damage[14-17]. Ligusticum wallichii attenuates myocardial injury by activating PI3K/Akt signaling in the myocardium[18]. Our research suggested the effect of SXT in alleviating symptoms of cardiovascular injury in MI/RI in diabetes. However, the details of the SXT mechanism are still unclear due to the complexity of diabetes mellitus with MI/RI.
Network pharmacology is a commonly used tool in identifying multiple components and investigating the mechanisms of herbal medicine. In this study, based on network pharmacology, the main targets and pathways of SXT in the treatment of MI/RI in diabetes were predicted, analyzed, and verified, which will provide evidence for the development of drugs for MI/RI in diabetes.
The Traditional Chinese Medicine System Pharmacology Database (TCMSP) database was used to predict the active compounds and potential targets of SXT with an oral bioavailability ≥ 30% and drug similarity (DL) ≥ 0.18 (http://Lsp.nwu.edu.cn/tcmsp.php)[19]. Then, we constructed the relationship network between the active compounds and potential target genes of SXT via the Cytoscape 3.9.0 software (http://cytoscape.org/)[20].
The therapeutic targets were identified by searching the Gene Expression Omnibus (GEO), DisGeNet, Genecards, Drugbank, OMIM, and PharmGKB with “MI/RI”, “myocardial ischemia/reperfusion injury”, “diabetes mellitus”, and “diabetes” as keywords. We merged the three diabetes related datasets (GSE118139, GSE161355, and GSE193626) and two MI/RI related datasets (GSE36875 and GSE210611) identified in the GEO database separately and then obtained differentially expressed genes (DEGs) via the R package “limma” for batch correction and screening |log 2 (fold change)| > 1 and P < 0.05). Then, we standardized the target names through the UniProt database (https://www.uniprot.org/)[21].
The obtained DEGs from the GEO database were combined with diabetes related targets or MI/RI related targets from the DisGeNet, Genecards, Drugbank, OMIM, and PharmGKB databases separately. Targets that appeared at least twice were regarded as therapeutic targets for diabetes or MI/RI. Then, therapeutic targets for diabetes were intersected with those for MI/RI to obtain potential therapeutic targets for MI/RI in diabetes. Finally, potential targets of SXT obtained from the TCMSP database were intersected with therapeutic targets for MI/RI in diabetes to identify prospective SXT therapeutic targets for MI/RI in diabetes.
We obtained the interactions among potential therapeutic targets of SXT via the STRING (https://string-db.org/)[22] database to construct a protein-protein interaction (PPI) network. Then, we imported the comprehensive data into Cytoscape 3.9.0 software and used its Molecular Complex Detection plugin to select the key subnetworks and therapeutic targets[20]. Default parameters (Degree Cutoff: 2; Node Score Cutoff: 0.2; K-core: 2; maximum depth: 100) were used. The key therapeutic targets were further selected according to the degree value via CytoNCA plugin. To investigate the probable molecular mechanisms of SXT for attenuating MI/RI in diabetes, the Database for Annotation, Visualization and Integrated Discovery (DAVID, v6.8) (https://david.ncifcrf.gov/home.jsp)[23] was used to perform Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses, and the results were visualized using the clusterProfiler package in R[24].
SXT was purchased from Sichuan Hongpu Pharmaceutical Co., Ltd. (Sichuan, China). Triphenyltetrazolium chloride (TTC), Evan’s blue (EB), streptozotocin (STZ), and sodium citrate buffer (SSC, 0.1 mol/L, pH 4.5) were purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). BCA protein analysis reagents were obtained from Shanghai Biyuntian Biotechnology Co., Ltd. (Shanghai, China). Rat insulin (INS), troponin T (cTnT), TG, TC, free fatty acids (FFA), creatine kinase isoenzyme MB (CKMB), lactate dehydrogenase (LDH), oxidized LDL (ox-LDL), LDL cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and advanced glycation endproducts (AGEs) antibodies for ELISA were obtained from Jianglai Company (Shanghai, China). Bax antibody used for Western blot was purchased from Abcam (Shanghai, China), Bcl-2 and receptor for AGE (RAGE) antibodies were purchased from Affinity (Jiangsu, China), and cleaved caspase-3 antibody was purchased from PTGCN (Wuhan, China). Chemical standards (verisoflavone glucoside, tanshinone ⅡA, ginsenosides Rb1, ferulic acid, 6-gingerol, and cinnamaldehyde) with a purity higher than 98 % were purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China).
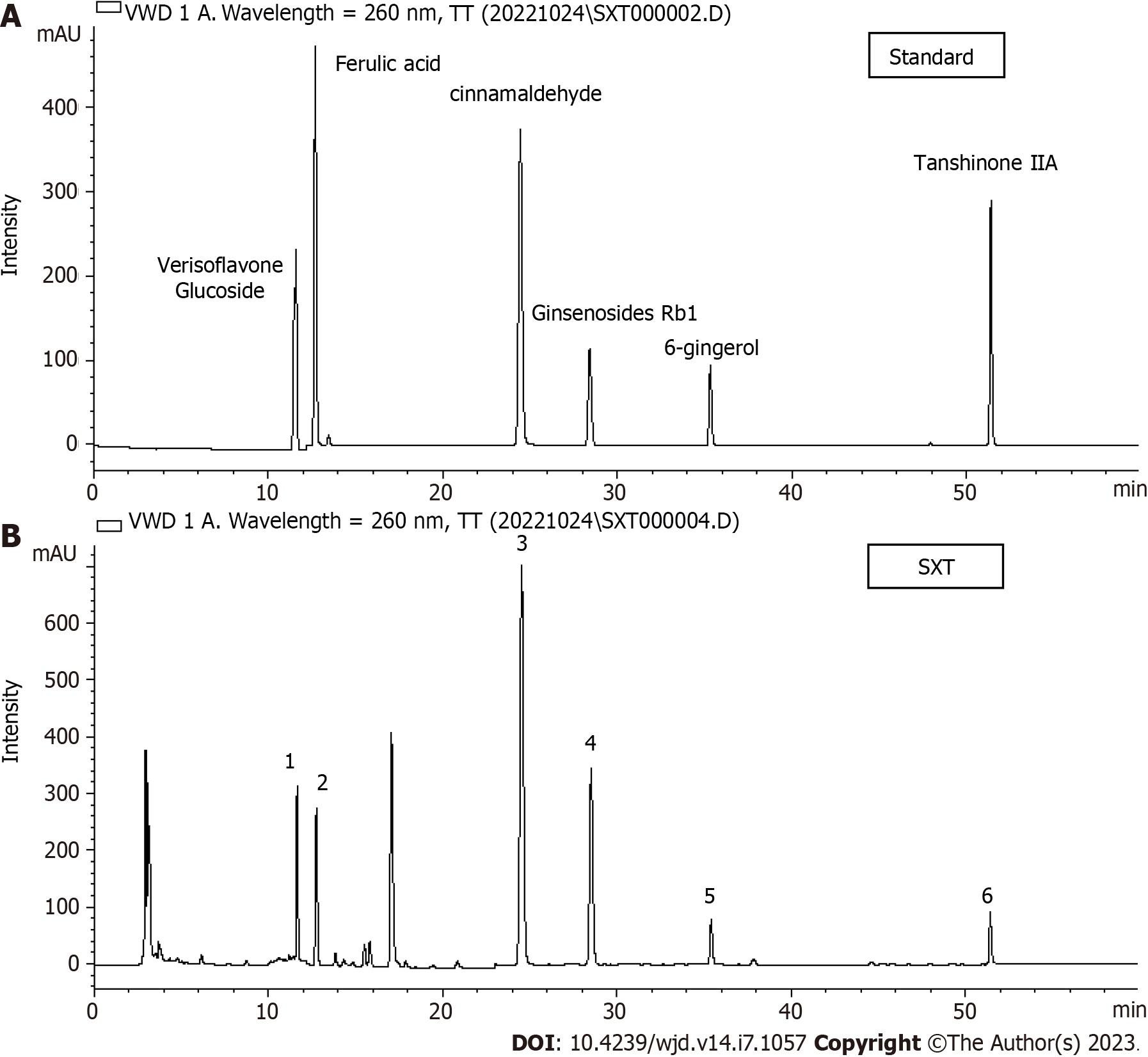
SXT is composed of Astragalus (Huang-Qi, 40 g), Zanthoxylum (Shu-Jiao, 6 g), Rhizoma zingiberis (Gan-Jiang, 12 g), Cinnamon (Rou-Gui, 12 g), Salvia miltiorrhiza (Dan-Shen, 24 g), Panax notoginseng (San-Qi), and Ligusticum wallichii (Chuan-Xiong, 18 g). SXT extract was obtained after sterilization and filtration through a 0.22-μm filter. Mass spectrometry of SXT was performed for quality control by using an HPLC-VWD mass spectrometer (Figure 1A and B).
The animal experimental protocol for this study was approved by the General Hospital of Western Theater Command (No. 2022EC2-ky004). We obtained 60 male Sprague-Dawley rats weighing 120-140 g from Chengdu Dashuo Laboratory Animal Co., Ltd. [Certificate number: SCXK (Chuan) 2020-030]. The animals were housed in an SPF-rated environment. After 1 wk of adaptation, the rats were randomly divided into six groups (n = 10): Normal control group (C), diabetic rats with sham operation group (DS), MI/RI in diabetes group (DMR), MI/RI in diabetic rats receiving SXT 0.7 g/kg/d group (SXTL), MI/RI in diabetic rats receiving SXT 1.4 g/kg/d group (SXTM), and MI/RI in diabetic rats receiving SXT 2.8 g/kg/d group (SXTH). Except group C, other groups were given a high-fat diet (60.65% fat, 18.14% protein, 21.22% carbohydrate; Jiangsu Xietong Pharmaceutical Bioengineering Co., Ltd., China). The intraperitoneal glucose tolerance test (IPGTT) and the intraperitoneal insulin tolerance test (IPITT) were performed on each group of rats. After 4 wk of high-fat diet feeding, rats in all groups except group C were intraperitoneally injected with a single dose of STZ (35 mg/kg, dissolved in 0.1 mol/L citrate buffer, pH 4.5; Solarbio, China). Rats in group C were injected with an equal volume of citrate buffer. After 1 wk, fasting blood glucose (Roche, Germany) level in blood collected from the tail vein was measured, and rats with a blood glucose level ≥ 11.1 mmol/L were considered diabetic[25]. Four weeks after diabetes induction, rats in the SXTL, SXTM, and SXTH groups started to receive SXT gavage treatment. The C, DS, and DMR groups received pure water gavage.
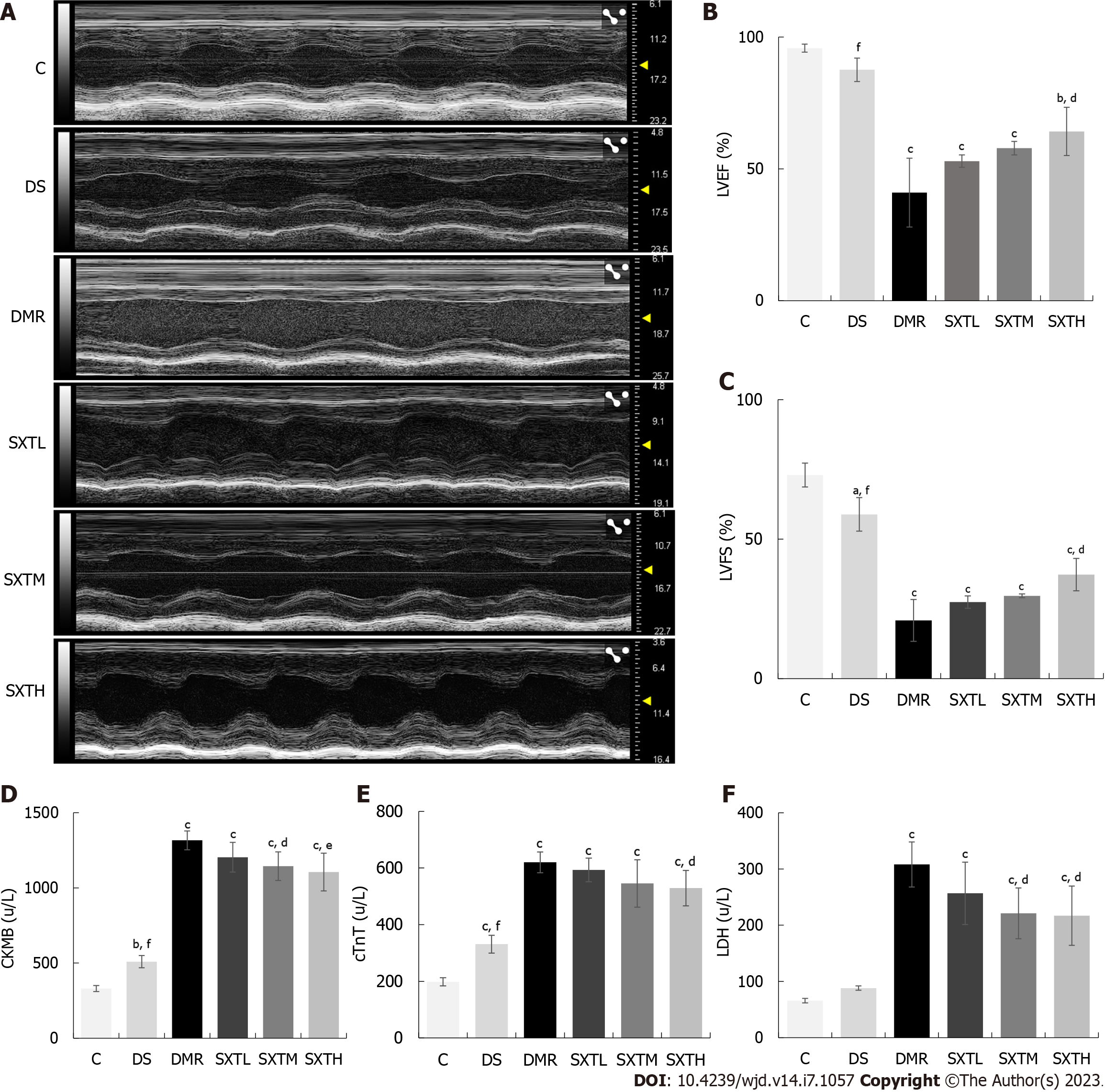
After 8 wk of treatment, the second IPGTT and IPITT experiments were performed. After an overnight fast, an MI/RI model[26] was created by ligation of the left anterior descending artery in the DMR, SXTL, SXTM, and SXTH groups. Briefly, rats were anesthetized with pentobarbital sodium (60 mg/kg) via intraperitoneal injection, and artificial respiration was established using a ventilator (Anhui Zhende Medical Company, Anhui, China) with a respiratory rate of 75 breaths/min, respiratory ratio of 1:1, and tidal volume of 20 mL. After disinfection of the skin, the chest was opened through the left third intercostal space, and a slipknot was made with an 8-0 surgical silk suture to ligate the left anterior descending coronary artery. Coronary artery occlusion was confirmed by ST-segment elevation on electrocardiogram. After 30 min of ligation, the slipknot was released to allow reperfusion for 2 h. The rats in the DS group underwent the same surgical procedure except for ligation of the heart. Cardiac function was assessed by echocardiography 2 h after reperfusion using an M-mode Vevo3100LT high-resolution in vivo imaging system (Visualsonic, Toronto, Canada). The rats were anesthetized with 2.5% isopentyl ether inhalation, and their body temperature was maintained at about 37 °C. We measured the left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS).
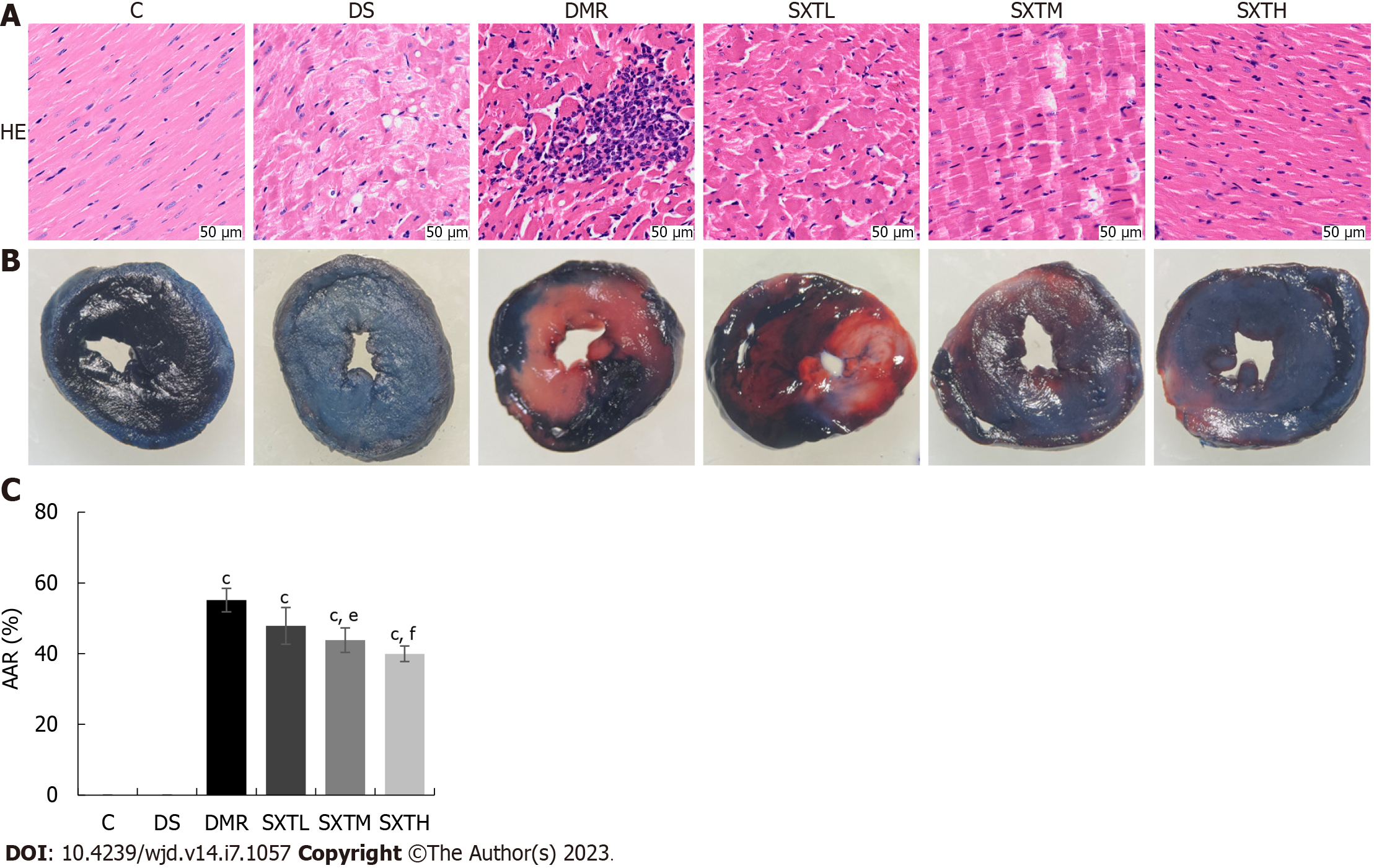
At the end of reperfusion, three rats from each group were randomly selected for TTC and EB staining. The coronary arteries were ligated, and 1% EB was injected into the left ventricular cavity. The heart was rapidly excised. After freezing at -20 °C, sections were stained with 1% TTC at 37 °C for 10 min[27]. The stained area was analyzed with Image J software. Areas at risk (AARs) were indicated by TTC staining in red (infarct border area) and white (infarct area), and normal myocardium was stained dark blue by EB. The AAR was calculated as a percentage of the total area.
Finally, serum and plasma were collected and stored at -20 °C for later experiments. We selected three hearts from each group to fix in 10% formalin for 3 d and then embed in paraffin for hematoxylin and eosin (HE) and immunofluorescence staining. The hearts from the remaining rats were stored at -80 °C.
An ELISA kit was used to detect the levels of INS, ox-LDL, HDL-C, LDL-C, cTnT, CKMB, and LDH in serum and AGEs in plasma. We followed the instructions in the ELISA kit and calculated the concentration of the sample according to the standard concentration and optical density.
Cells in each group were immediately lysed and homogenized with lysis buffer. Total protein samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking, anti-rat RAGE (Affinity, AF5309), cleaved caspase-3 (Affinity, AF7022), Bax (Abcam, ab32503), Bcl-2 (PTGCN, 60178-1-lg), and DAPDH (Affinity, AF7021) antiboides were applied. The membranes were incubated overnight at 4 °C. After three washes, the membranes were incubated with secondary antibody (Bioss, 0295G) for 1 h at room temperature. Proteins were detected by enhanced chemiluminescence (Millipore, WBKLS0100). The integrated optical density of each band was measured with Image J software.
The paraffin sections were dewaxed to water and repaired with proteinase K. After the membranes were ruptured, the buffer was incubated at room temperature for 10 min. According to the number of slices and tissue size, TDT enzyme, dUTP, and buffer from the TUNEL kit at a ratio of 1:5:50 were mixed at a temperature of 37 °C and incubated for 2 h. The nuclei were then counterstained with DAPI and finally mounted with anti-fluorescence quenching mounting medium. Sections were observed under a fluorescence microscope. Nuclei are blue under UV excitation, and positive apoptotic nuclei are green.
The paraffin sections were dewaxed to water and then antigen repair was performed. After blocking, the sections were incubated with anti-rat RAGE (Affinity, AF5309) at 4 °C overnight. Then, we added a secondary antibody and incubated the sections at room temperature for 50 min in the dark. Nuclei were counterstained with DAPI, autofluorescence quencher was added for 5 min, and the sections were washed with running water for 10 min. After drying, the sections were mounted using anti-fluorescence quenching mounting medium. Sections were observed under a fluorescence microscope. Nuclei are blue under UV excitation, and RAGE is stained red.
The data were evaluated by one-way ANOVA, and t-test was used if the variances were not uniform. A P < 0.05 was considered statistically significant. SPSS version 16.0 (SPSS Inc., Chicago, IL) was used to analyze the data.
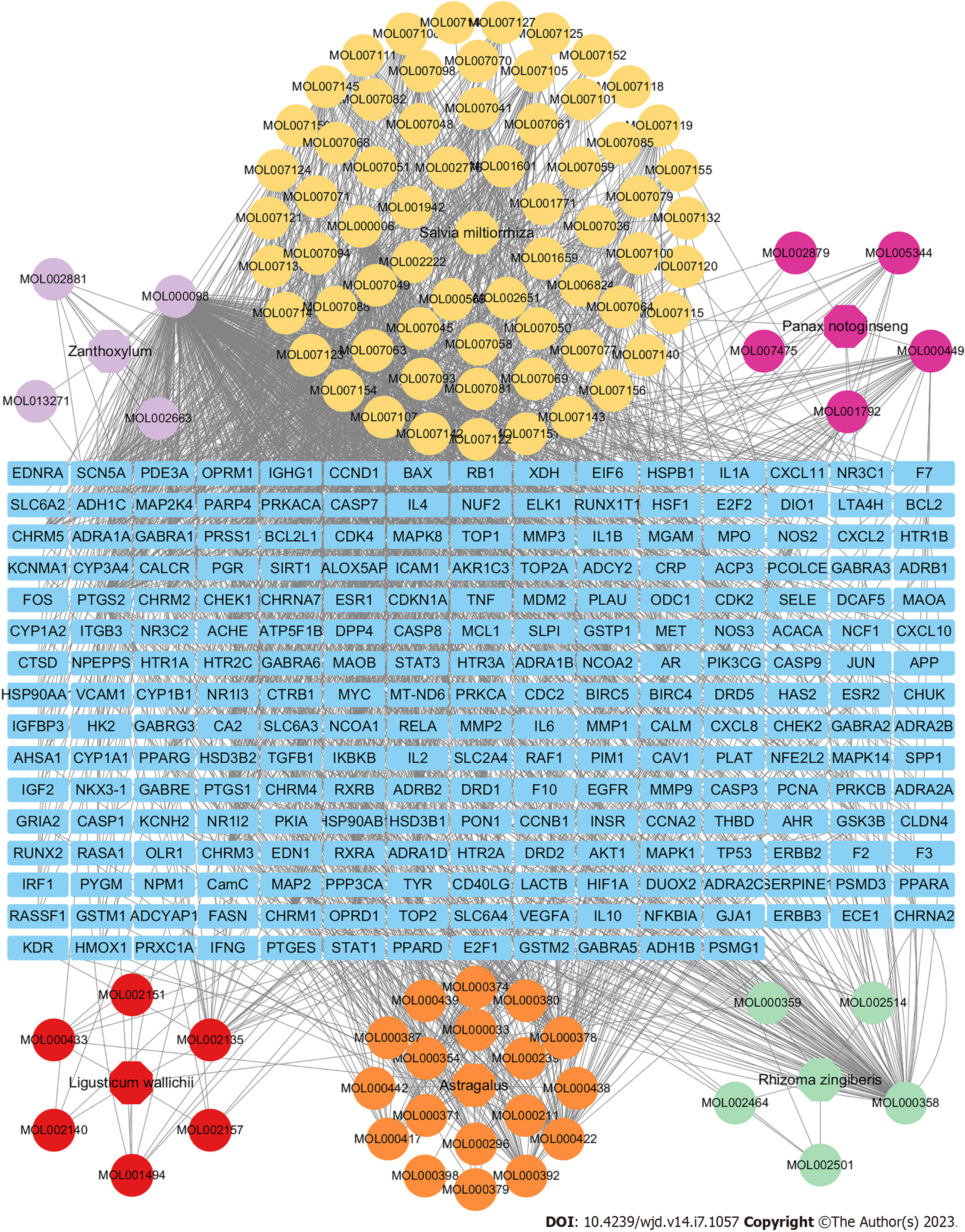
As shown in Figure 2, the pharmacological network of SXT was constructed to indicate the relationships among all the herbs, compounds, and corresponding targets. Finally, 92 active compounds and 237 targets of SXT were identified as the predicted targets for further research (Supplementary Table 1). The top three pharmaceutical compounds of SXT based on degree of value were quercetin, beta-sitosterol, and kaempferol.
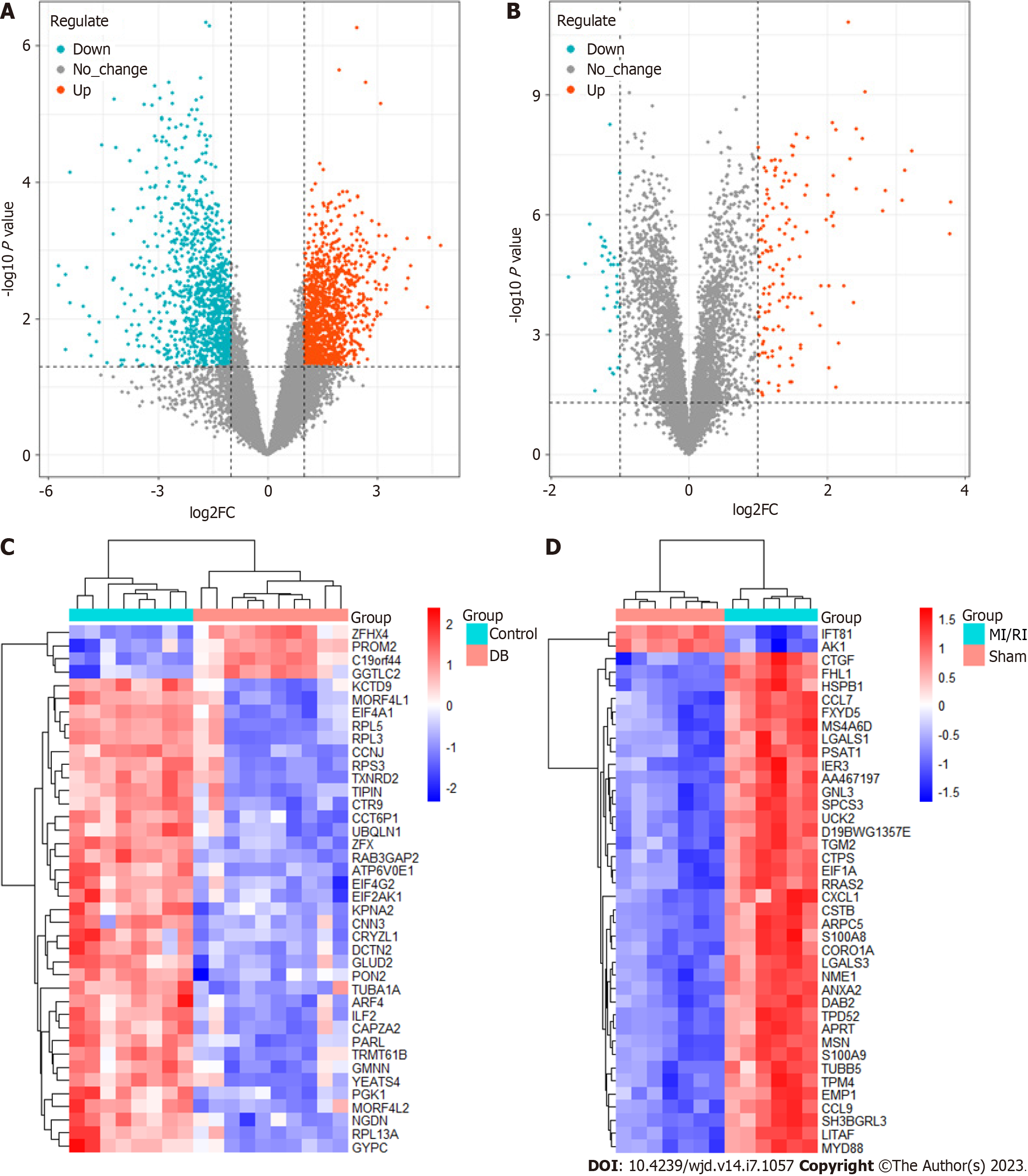
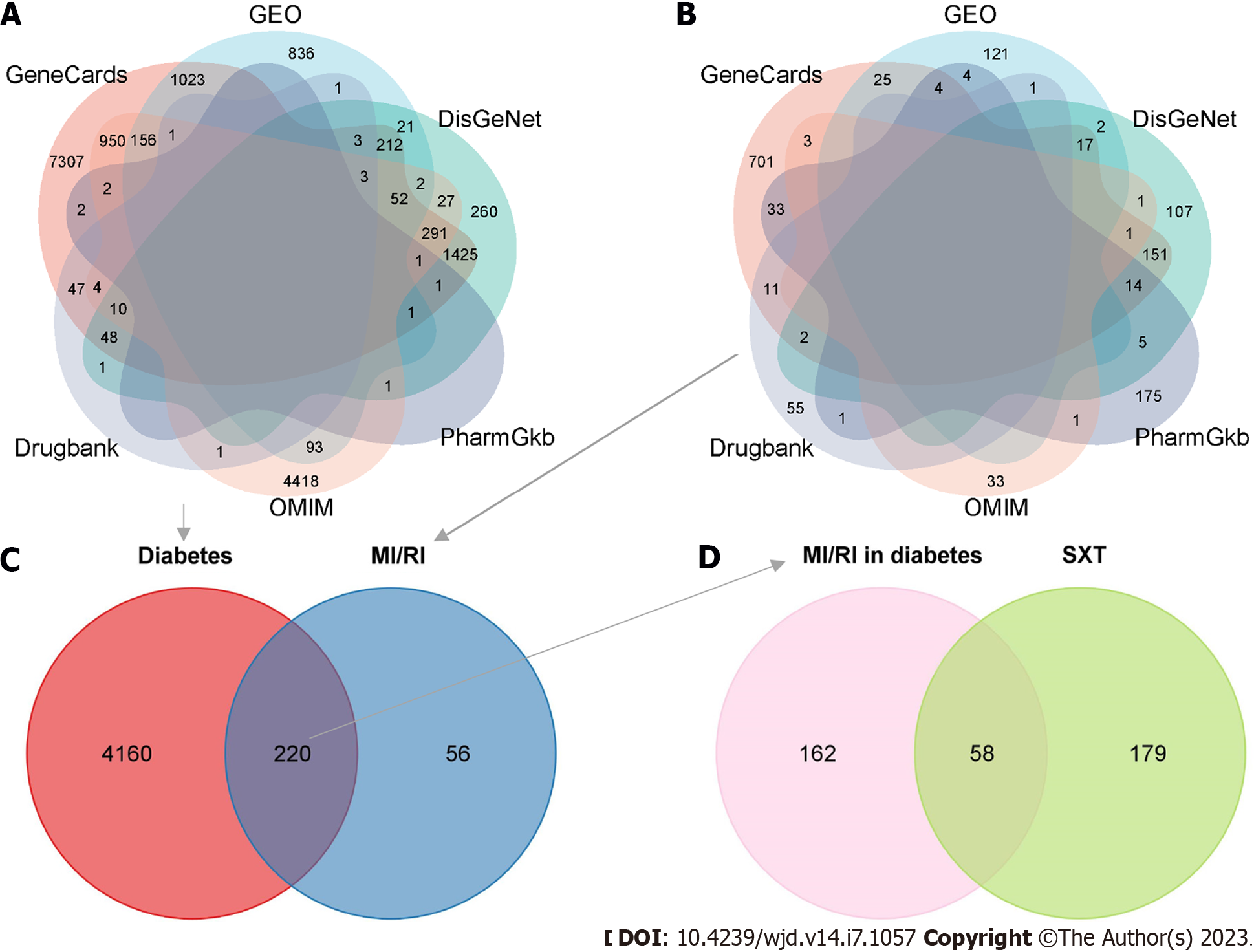
We utilized the R package "limma" to detect 2404 DEGs linked to diabetes and 174 DEGs linked to MI/RI. In Figure 3A and B, the red dots on the right represent up-regulated genes in diabetes or MI/RI patients, while the blue dots on the left represent down-regulated genes in diabetes or MI/RI patients. Figure 3C and D shows the expression of the top 40 DEGs that were ranked high and low in patients vs healthy individuals, respectively. Next, we found 2359, 11539, 119, 6012, and 8 therapeutic targets related to diabetes and 300, 962, 70, 39, and 237 therapeutic targets related to MI/RI in the DisGeNet, Genecards, Drugbank, OMIM, and PharmGKB datasets, respectively. Finally, targets that appeared at least twice were regarded as therapeutic targets for diabetes or MI/RI, and this resulted in 4380 potential therapeutic targets for diabetes and 276 potential therapeutic targets for MI/RI (Figure 4A and B).
After the therapeutic targets for diabetes and MI/RI were intersected, we obtained 220 potential therapeutic targets for MI/RI in diabetes (Figure 4C). Then, 220 potential therapeutic targets were intersected with 237 targets of SXT to identify 58 potential SXT therapeutic targets for MI/RI in diabetes (Figure 4D).
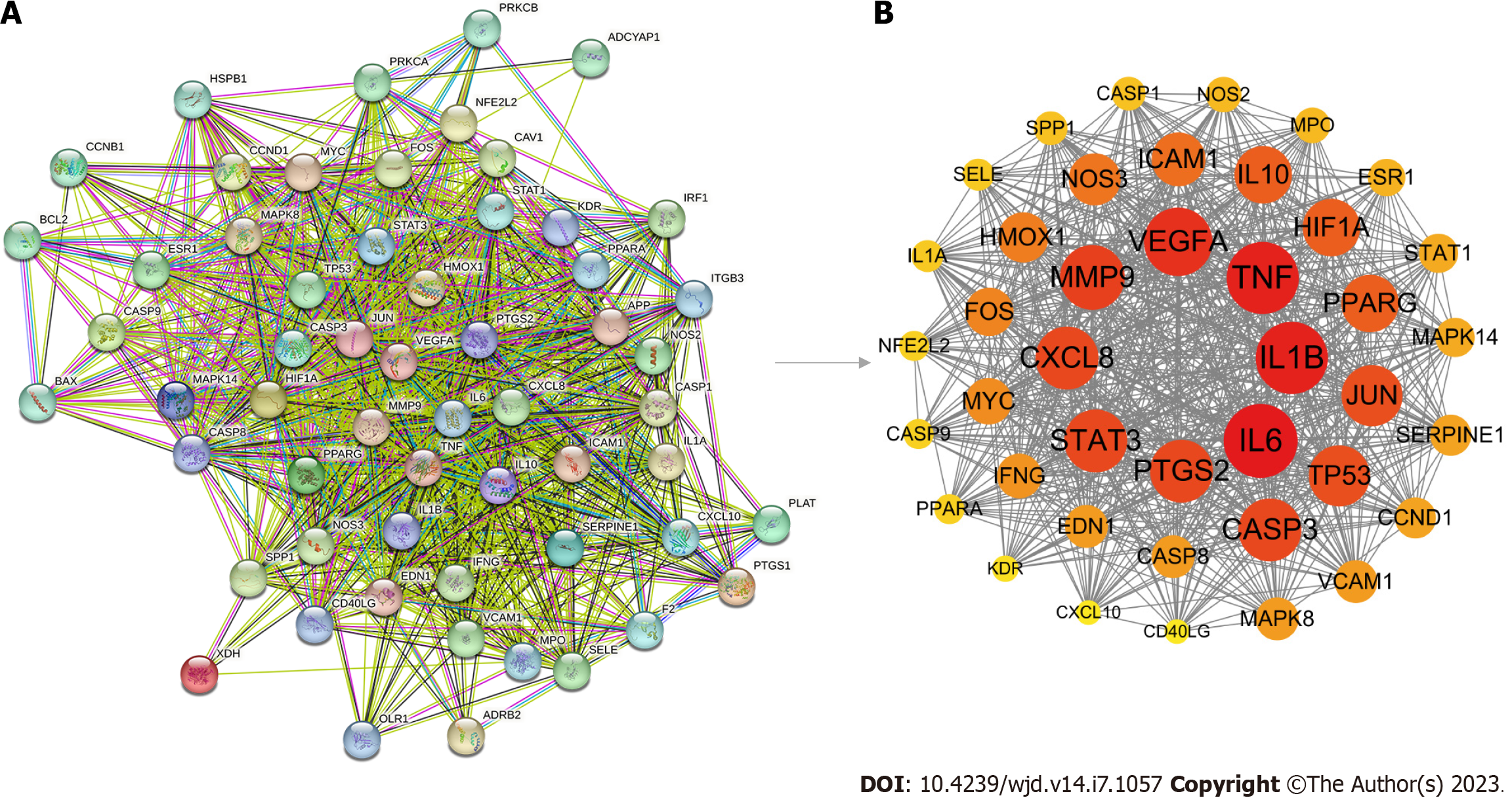
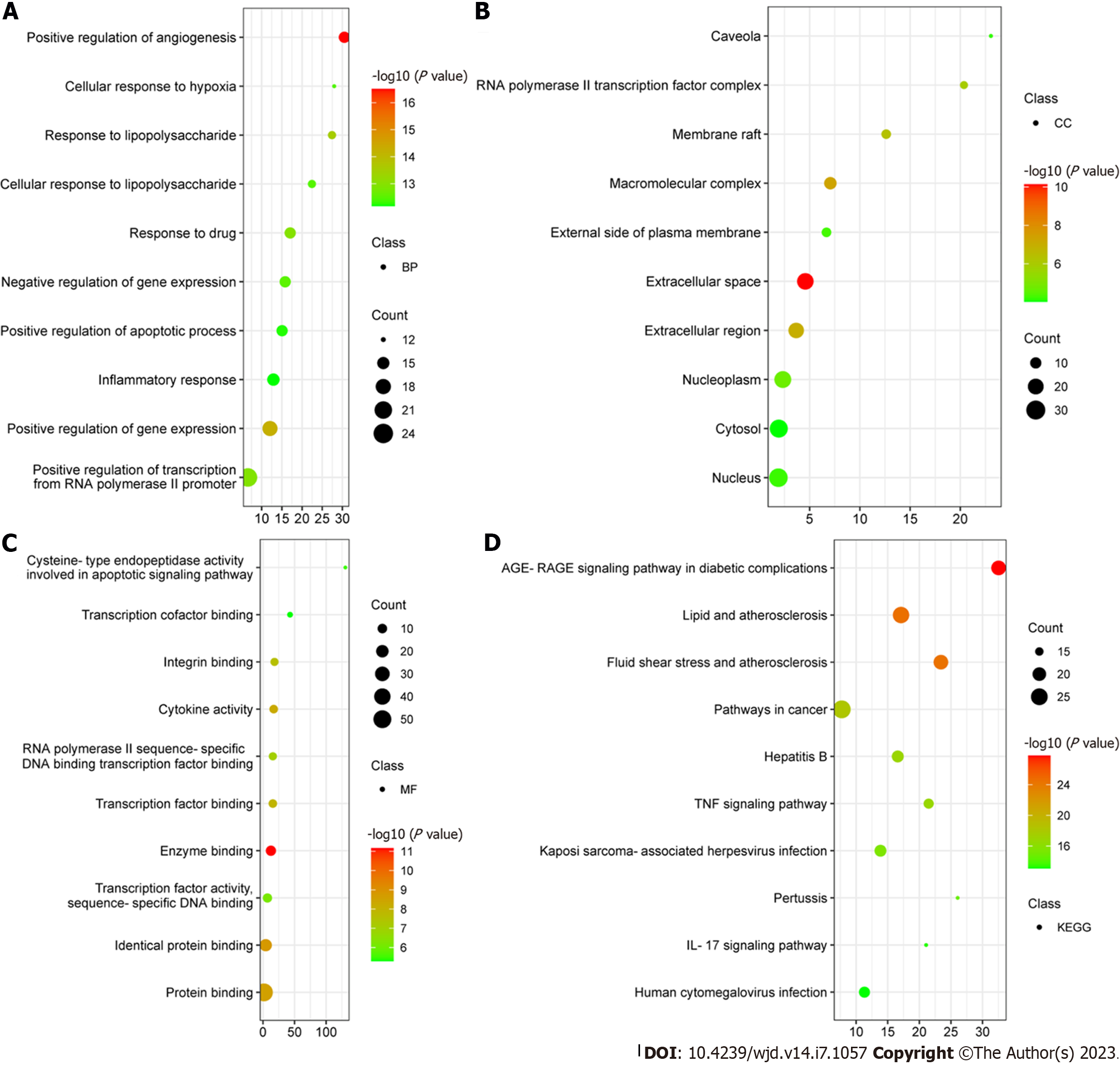
To obtain a PPI network, 58 potential SXT therapeutic targets for MI/RI in diabetes were uploaded to the STRING database (Figure 5A). Then, we imported the comprehensive data into Cytoscape to obtain 41 key therapeutic targets by MCODE plugin (Table 1). A total of 41 key therapeutic target nodes were connected by 680 edges, with an average node degree of 32.8 and clustering coefficient of 0.799 (Figure 5B). According to the DAVID database, a total of 489 GO items were obtained, including 395 biological processes (BPs), 26 cellular components, and 68 molecular functions. The first 10 items were selected in terms of the P value for visual analysis (Figure 6A-C). The results showed that the treatment of MI/RI in diabetes with SXT mainly involves BPs such as angiogenesis, cellular response to hypoxia, apoptotic process, and inflammatory response. These targets have enzyme binding, protein binding, cytokine activity, transcription factor binding, cysteine-type endopeptidase activity, and other functions, and they play a role in the extracellular space, macromolecular complex, membrane raft, nucleoplasm, external side of plasma membrane, and the nucleus. KEGG enrichment analysis showed that these targets were mainly enriched in the AGE-RAGE signaling pathway in diabetic complications and the lipids and atherosclerosis signaling pathway (Figure 6D).
| ID | Target | Protein name | Degree | Betweenness | Closeness |
| 1 | IL-6 | Interleukin-6 | 54 | 0.047667394 | 0.95 |
| 2 | IL-1β | Interleukin-1beta | 53 | 0.029250817 | 0.93442623 |
| 3 | TNF | Tumor Necrosis Factor | 53 | 0.029250817 | 0.93442623 |
| 4 | VEGFA | Vascular Endothelial Growth Factor A | 51 | 0.022294667 | 0.904761905 |
| 5 | MMP9 | Matrix Metallopeptidase 9 | 49 | 0.016493589 | 0.876923077 |
| 6 | CXCL8 | C-X-C Motif Chemokine Ligand 8 | 48 | 0.017645476 | 0.863636364 |
| 7 | STAT3 | Signal Transducer and Activator of Transcription 3 | 48 | 0.014004747 | 0.863636364 |
| 8 | PTGS2 | Prostaglandin-Endoperoxide Synthase 2 | 48 | 0.012914899 | 0.863636364 |
| 9 | CASP3 | Caspase 3 | 48 | 0.017524977 | 0.863636364 |
| 10 | TP53 | Tumor Protein P53 | 47 | 0.013024421 | 0.850746269 |
| 11 | JUN | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit | 47 | 0.010160499 | 0.850746269 |
| 12 | PPARG | Peroxisome Proliferator Activated Receptor Gamma | 45 | 0.017202087 | 0.826086957 |
| 13 | HIF1A | Hypoxia Inducible Factor 1 Subunit Alpha | 45 | 0.01117217 | 0.826086957 |
| 14 | IL-10 | Interleukin-10 | 45 | 0.010195923 | 0.826086957 |
| 15 | ICAM1 | Intercellular Adhesion Molecule 1 | 43 | 0.009172336 | 0.802816901 |
| 16 | NOS3 | Nitric Oxide Synthase 3 | 42 | 0.021291387 | 0.791666667 |
| 17 | HMOX1 | Heme Oxygenase 1 | 41 | 0.005343813 | 0.780821918 |
| 18 | FOS | Fos Proto-Oncogene, AP-1 Transcription Factor Subunit | 40 | 0.017888649 | 0.77027027 |
| 19 | MYC | MYC Proto-Oncogene, BHLH Transcription Factor | 39 | 0.007362402 | 0.76 |
| 20 | IFNγ | Interferon Gamma | 38 | 0.003891145 | 0.75 |
| 21 | EDN1 | Endothelin 1 | 37 | 0.007764542 | 0.74025974 |
| 22 | CASP8 | Caspase 8 | 37 | 0.007408849 | 0.74025974 |
| 23 | MAPK8 | Mitogen-Activated Protein Kinase 8 | 37 | 0.009018794 | 0.74025974 |
| 24 | VCAM1 | Vascular Cell Adhesion Molecule 1 | 37 | 0.00621228 | 0.74025974 |
| 25 | CCND1 | Cyclin D1 | 36 | 0.005854834 | 0.730769231 |
| 26 | SERPINE1 | Serpin Family E Member 1 | 36 | 0.004739025 | 0.730769231 |
| 27 | MAPK14 | Mitogen-Activated Protein Kinase 14 | 35 | 0.0033994 | 0.721518987 |
| 28 | STAT1 | Signal Transducer and Activator of Transcription 1 | 35 | 0.002891632 | 0.721518987 |
| 29 | ESR1 | Estrogen Receptor 1 | 34 | 0.005648692 | 0.7125 |
| 30 | MPO | Myeloperoxidase | 33 | 0.007272867 | 0.703703704 |
| 31 | NOS2 | Nitric Oxide Synthase 2 | 33 | 0.002943975 | 0.703703704 |
| 32 | CASP1 | Caspase 1 | 32 | 0.003275919 | 0.695121951 |
| 33 | SPP1 | Secreted Phosphoprotein 1 | 32 | 0.002473838 | 0.695121951 |
| 34 | IL1A | Interleukin 1 Alpha | 31 | 0.001235526 | 0.686746988 |
| 35 | SELE | Selectin E | 31 | 0.003528291 | 0.686746988 |
| 36 | NFE2L2 | Nuclear Factor, Erythroid 2 Like 2 | 30 | 0.003874065 | 0.678571429 |
| 37 | CASP9 | Caspase 9 | 30 | 0.003883128 | 0.678571429 |
| 38 | PPARA | Peroxisome Proliferator Activated Receptor Alpha | 30 | 0.001620936 | 0.678571429 |
| 39 | KDR | Kinase Insert Domain Receptor | 28 | 0.001361612 | 0.662790698 |
| 40 | CXCL10 | C-X-C Motif Chemokine ligand 8 | 27 | 0.000671 | 0.655172414 |
| 41 | CD40LG | CD40 ligand | 27 | 0.003513977 | 0.655172414 |
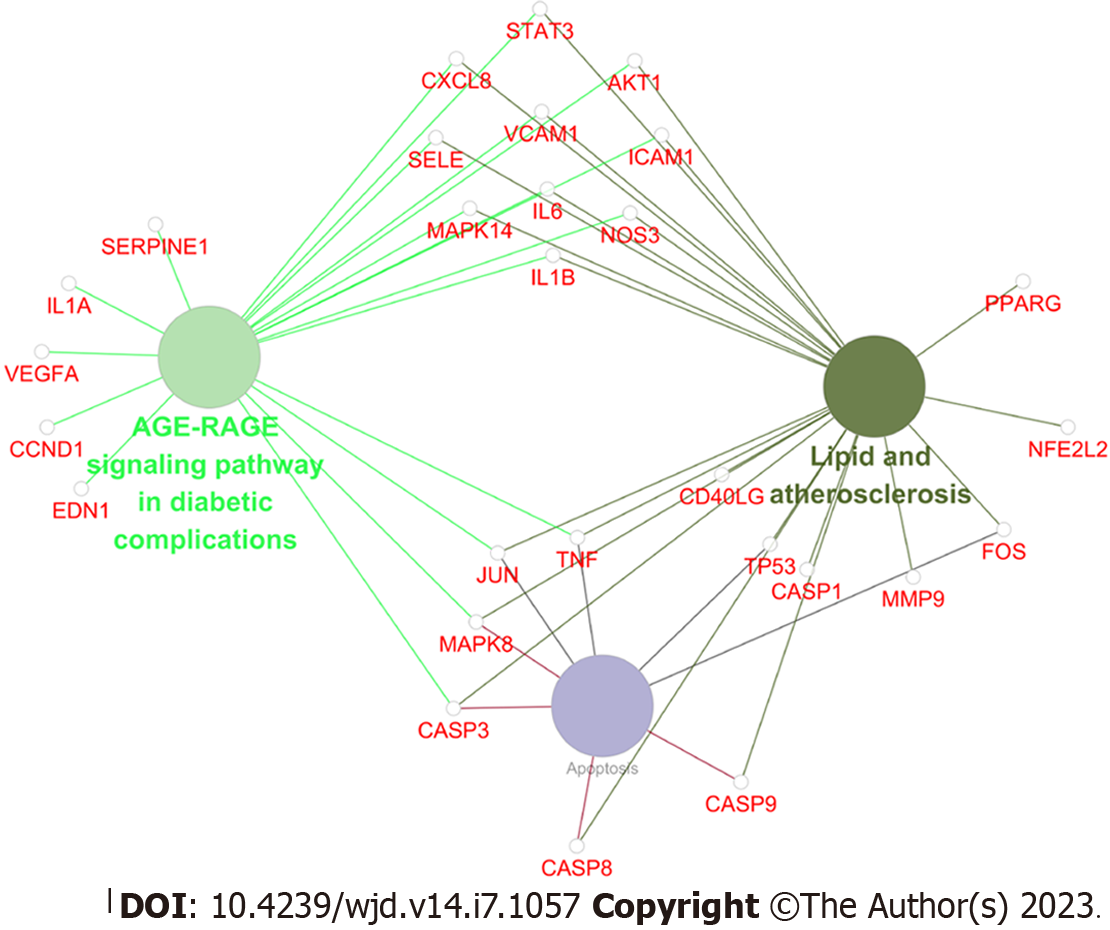
Based on the above results of network pharmacology analysis, we observed that the AGE-RAGE signaling pathway in diabetic complications is a downstream pathway of the lipids and atherosclerosis signaling pathway. As shown in Figure 7, among 41 key therapeutic targets, the AGE-RAGE signaling pathway in diabetic complications, lipids and atherosclerosis signaling pathway, and apoptosis were mainly enriched. Interestingly, these two signaling pathways largely participate in lipid metabolism and apoptotic processes. LDL, AGEs, and RAGE are key proteins of the lipids and atherosclerosis signaling pathway, along with the AGE-RAGE signaling pathway in diabetic complications. LDL is subject to oxidative modifications to become ox-LDL and promotes the binding of AGEs to their receptor RAGE[28]. Studies have shown that AGE level in diabetic patients is much higher than that in non-diabetic patients, and its level is positively correlated with the risk of cardiovascular diseases[29]. AGE-RAGE subsequently activates the expression of nicotinamide adenine dinucleotide phosphate to produce many reactive oxygen species, which further promotes the generation of AGEs and forms a positive cycle, constantly aggravating the occurrence of oxidative stress in the body, and further promoting apoptosis[30-33]. The above results provided great support for clarifying the anti-lipid metabolism disorders and anti-apoptotic mechanisms of SXT on MI/RI in diabetic rats. These indicated that SXT may inhibit the AGE-RAGE signaling pathway via reducing ox-LDL to ameliorate lipid metabolism disorders and anti-apoptotic effects in MI/RI in diabetes. However, further experimental validation is required to confirm the predicted results of network pharmacology.
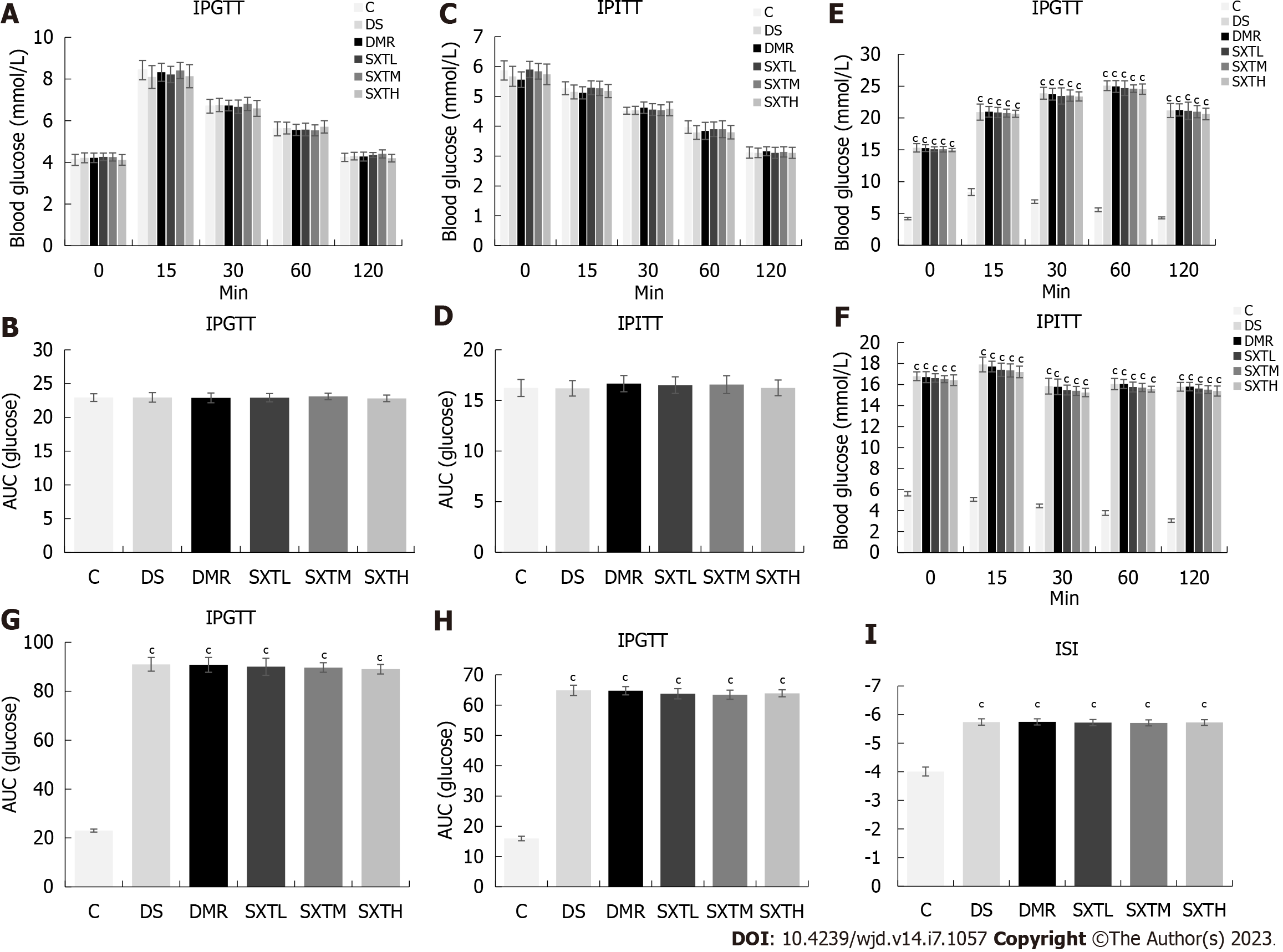
At baseline, there were no significant differences in IPGTT or IPITT between each group of rats, and no insulin resistance or increase in blood glucose was observed (Figure 8A-D). At the end of the experiment, the rats in the DS, DMR, SXTL, SXTM, and SXTH groups exhibited impaired glucose tolerance and significantly increased blood glucose levels at all time points compared with group C (P < 0.001) (Figure 8E and F). The average areas under the curves of the DS, DMR, SXTL, SXTM, and SXTH groups during IPGTT and IPITT were all increased (Figure 8G and H), and the international sensitivity index was significantly decreased compared with group C (P < 0.001) (Figure 8I). However, there were no differences among the DS, DMR, SXTL, SXTM, and SXTH groups. This indicated that SXT could not reduce blood glucose levels in MI/RI in diabetic rats, nor could it relieve the impaired insulin sensitivity and insulin resistance.
The ELISA results given in Table 2 show that compared with group C, TC, TG, FFA, and LDL-C values in each group were significantly increased, and HDL-C was significantly decreased (P < 0.001). Compared with the DMR group, TC, TG, FFA, and LDL-C values were significantly decreased in the SXTH group, and HDL-C was significantly increased (P < 0.05).
| Group | TC (mmol/L) | TG (mmol/L) | FFA (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) |
| C | 1.10 ± 0.20 | 0.60 ± 0.10 | 0.41 ± 0.02 | 0.74 ± 0.08 | 1.66 ± 0.11 |
| DS | 2.50 ± 0.20c | 1.54 ± 0.07c | 0.78 ± 0.02c | 1.53 ± 0.06c | 0.81 ± 0.07c |
| DMR | 2.63 ± 0.15c | 1.54 ± 0.08c | 0.77 ± 0.03c | 1.51 ± 0.03c | 0.81 ± 0.06c |
| SXTL | 2.17 ± 0.21c | 1.46 ± 0.09c | 0.69 ± 0.03c,d | 1.47 ± 0.03c | 0.97 ± 0.06c |
| SXTM | 1.90 ± 0.20b,e | 1.30 ± 0.08c,d | 0.66 ± 0.04c,e | 1.32 ± 0.04c,e | 1.05 ± 0.08c |
| SXTH | 1.85 ± 0.15b,e | 1.30 ± 0.02c,d | 0.61 ± 0.02c,f | 1.34 ± 0.05c,d | 1.08 ± 0.14c,d |
To verify the cardioprotective effects of SXT on MI/RI in diabetic rats, we initially assessed left ventricular function, cardiac damage markers, and histopathologic changes. As shown in Figure 9A-C, echocardiography showed that LVEF and LVFS values were remarkably reduced in the DMR group compared with the C and DS groups (P < 0.001). In the SXTH group, LVEF and LVFS values were significantly increased compared with the DMR group (P < 0.05). Figure 9D-F shows that the cardiac damage markers CKMB, cTnT, and LDH levels in serum were significantly higher in the DMR group than in the C and DS groups (P < 0.001). Conversely, a high dose of SXT markedly attenuated these changes (P < 0.05). Furthermore, HE staining showed that the DMR group showed regional necrosis, interstitial edema, inflammatory cell infiltration, disordered and swollen muscle fibers, rupture of myocardial fibers, and dark staining. However, in the group that received different doses of SXT, these histopathologic changes were replaced by well-arranged myocardial cells (Figure 10A). The percentage of AAR to total area was calculated via the EB-TTC double-staining method. As shown in Figure 10B and C, compared with the C group, the proportion of AAR in the DMR group was significantly higher (P < 0.001). However, in the SXTM and SXTH groups , the proportion of AAR was significantly reduced compared with the DMR group (P < 0.01). These results demonstrate that SXT could improve the cardiac dysfunction of diabetic rats with MI/RI.
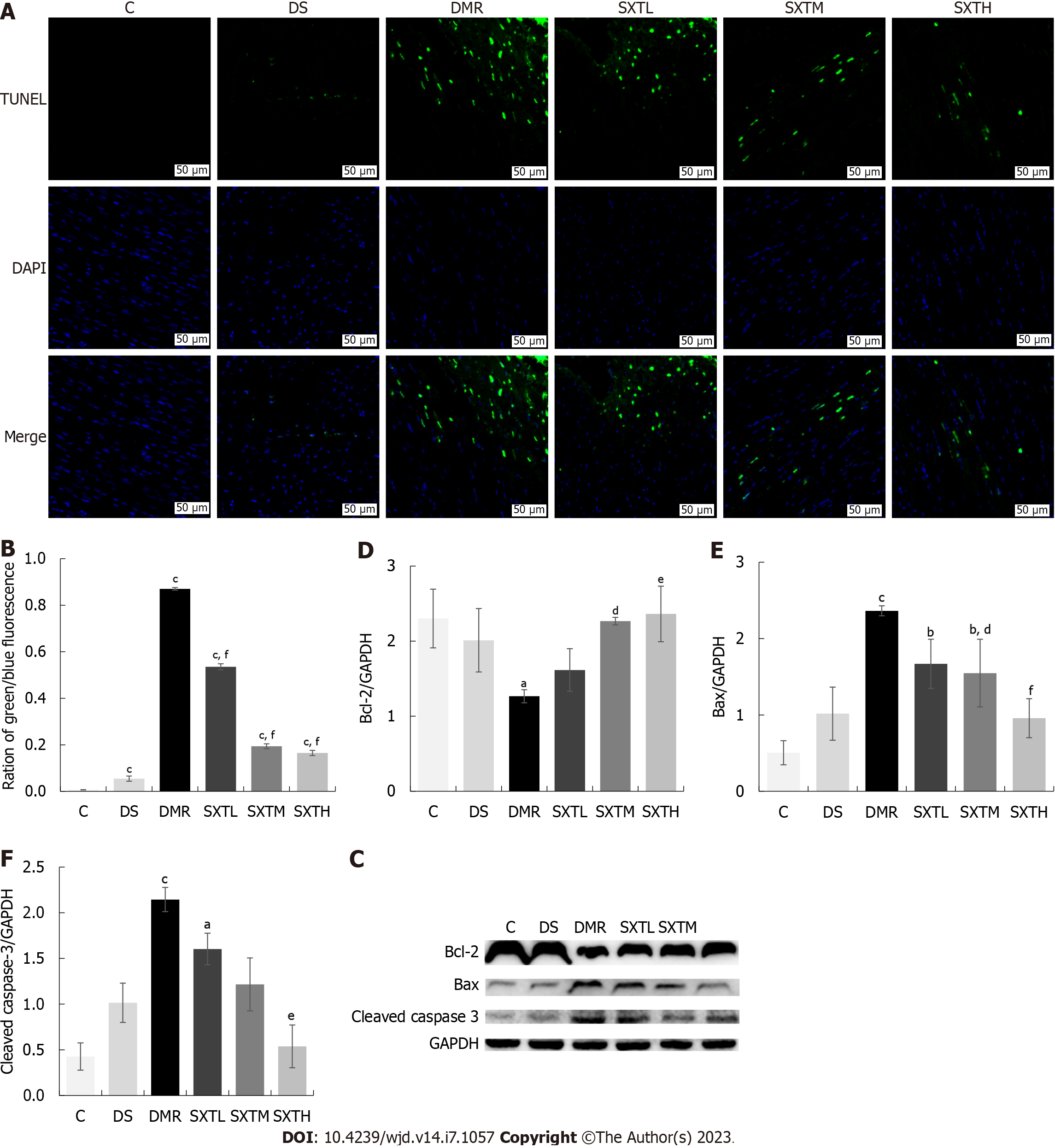
TUNEL assay was used to detect myocardial apoptosis. As shown in Figure 11A and B, the DMR group showed a significant increase in the number of apoptotic myocytes compared with the C and DS groups (P < 0.001). Compared with the DMR group, SXT significantly decreased the number of apoptotic myocytes (P < 0.001). Moreover, the expression of apoptosis-related proteins was evaluated by Western blot analysis. As shown in Figure 11C-F, compared with the C group, the DMR group had significantly decreased anti-apoptotic protein Bcl-2 expression and increased pro-apoptotic proteins Bax and cleaved caspase-3 expression (P < 0.05). The SXTH group had increased Bcl-2 expression and decreased Bax and cleaved caspase-3 expression (P < 0.05). These results indicated that a high dose of SXT attenuated MI/RI in diabetic rats by inhibiting apoptosis.
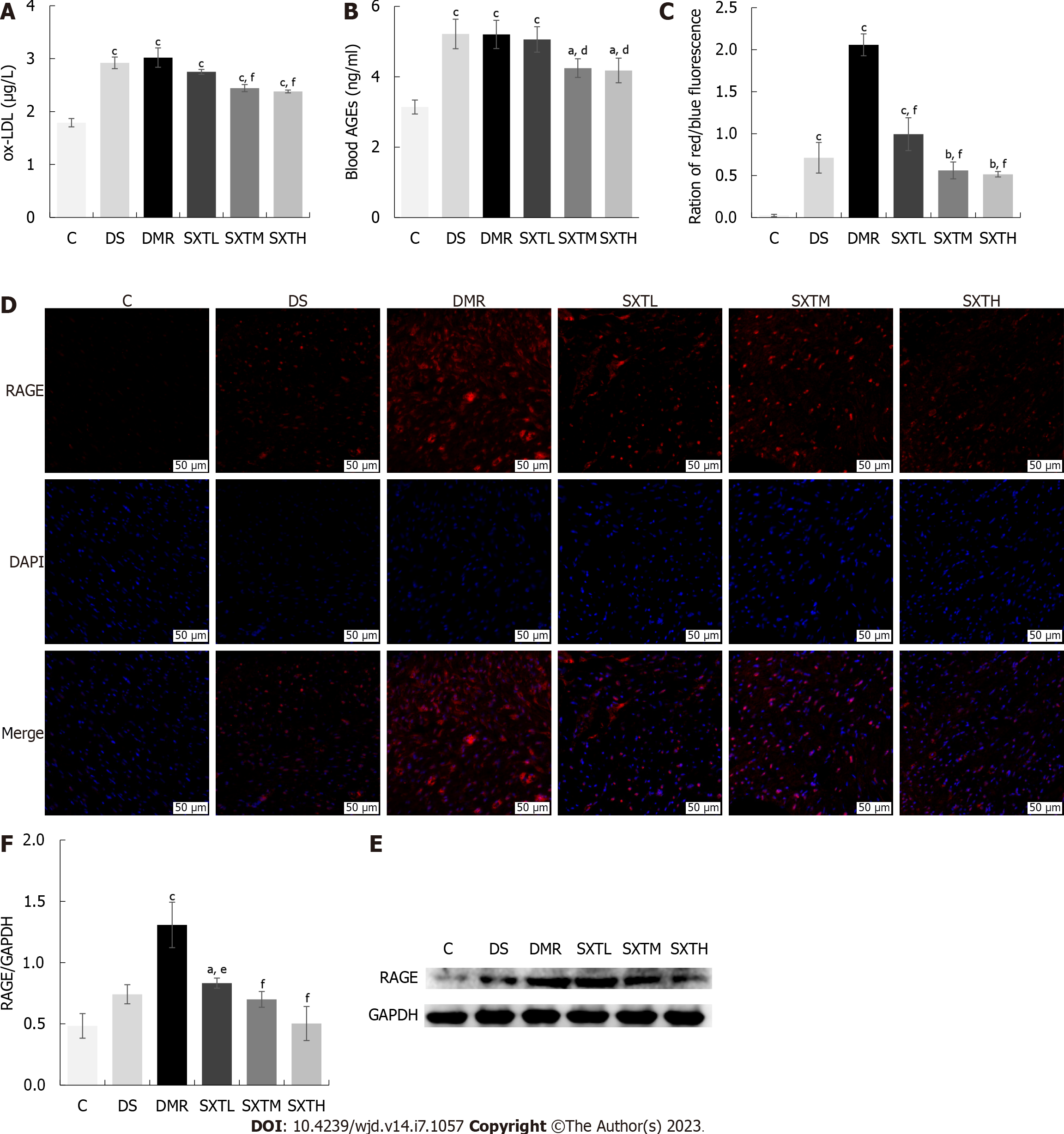
To explore the mechanism of SXT regulating lipid metabolism and attenuating myocardial apoptosis in diabetic rats with MI/RI, we measured the ox-LDL, AGE, and RAGE protein expression based on the results of network predictive analysis. As shown in Figure 12A and B, ELISA revealed that, compared with the C group, the levels of ox-LDL and AGEs in the DMR group were significantly increased (P < 0.001). In the SXTM and SXTH groups, the levels of ox-LDL and AGEs were significantly decreased compared with those in the DMR group(P < 0.05). The results of immunofluorescence (Figure 12C and D) revealed that the average density of RAGE in the DMR group was significantly higher than that of the C group (P < 0.001). The average density of RAGE was significantly lower in the SXTL, SXTM, and SXTH groups compared with the DMR group (P < 0.001). As shown in Figure 12E and F, the expression of RAGE was significantly up-regulated compared with the C group (P < 0.001), while SXTM and SXTH down-regulated the expression of RAGE compared with the DMR group (P < 0.05). These results suggested that the anti-apoptosis mechanism of SXT in MI/RI of diabetic rats might be related to a reduction in ox-LDL and the inhibition of the AGE-RAGE signaling pathway.
In this study, we discovered that SXT could significantly reduce the level of blood lipids, and alleviate cardiomyocyte apoptosis and myocardial injury without glycemic control. SXT targets the pathogenesis of MI/RI in diabetes by reinforcing Qi and promoting blood circulation, regulating the level of blood lipids, alleviating cardiomyocyte apoptosis, and improving cardiac function. It is a problem for TCM formulations to be examined at the molecular level in terms of their multi-component and multi-target features. However, with the rapid development of network pharmacology, systematic research of TCM formulations has been in progress. Therefore, we explored and verified the molecular mechanisms of SXT in the treatment of MI/RI in diabetes via network pharmacology and experimentation.
Based on network pharmacology, quercetin, beta-sitosterol, and kaempferol were found to be the key components of SXT in reducing MI/RI in diabetes according to the degree of value. Quercetin and kaempferol ameliorated lipid metabolism disorders by activating AMPK[34,35], while quercetin could work against mitochondrial apoptosis by regulating ERK1/2/DRP1 signaling[36]. Beta-sitosterol, a plant sterol that has antioxidant activity, has been suggested to increase resistance to oxidative stress and lipid peroxidation[37]. A total of 41 key SXT therapeutic targets for MI/RI in diabetes were identified through network pharmacology analysis, and they were mainly related to the AGE-RAGE signaling pathway in diabetic complications together with the lipids and atherosclerosis signaling pathway. Coincidentally, the AGE-RAGE signaling pathway in diabetic complications is a downstream pathway of the lipids and atherosclerosis signaling pathway, which is closely related to lipid metabolism and apoptosis[38-40]. Therefore, we selected key proteins in these two pathways for validation and predicted that SXT may inhibit the AGE-RAGE signaling pathway via reducing ox-LDL to ameliorate lipid metabolism disorders and exerting anti-apoptotic effects in MI/RI in diabetes. Finally, this study confirmed that a dose of SXT (2.8 g/kg/d) could inhibit the expression of ox-LDL and blood lipids, suppress the expression of AGEs, RAGE, cleaved caspase 3, and BAX proteins, and increase the expression of Bcl-2 protein, thereby reducing MI/RI in diabetes.
Previous studies have found that about half of all patients with type 2 diabetes have complications in the form of dyslipidemia, which is one of the important causes of cardiovascular disease in patients with diabetes[41]. In this study, SXT was not effective in reducing blood sugar and insulin resistance, while it could reduce blood lipids in diabetic rats. This indicates that SXT regulates dyslipidemia, but not due to its hypoglycemic effect. The liver is the main site of lipid metabolism, and ox-LDL plays an important role in lipid metabolism and cardiovascular diseases[42,43]. VLDL is produced in the liver and released into the plasma, where it is metabolized to LDL via intermediate-density lipoproteins[44]. LDL is subjected to oxidization modifications to activate the AGE-RAGE signaling pathway, aggravating oxidative stress and myocardial cell apoptosis[28]. A recent study in the journal of Science suggested a new perspective that liver-heart cross-talk mediated by coagulation factor XI protects attenuated heart failure, which coincides with TCM theory[45]. According to the five elements theory of TCM, the liver pertains to wood, representing the mother organ, while the heart pertains to fire, representing the child organ. Pathologically, disorders of the mother organ involve the child organ, which means that liver disease will lead to heart disease. Our study verified that SXT reduced blood lipids, inhibited the expression of ox-LDL, suppressed the AGE-RAGE signaling pathway, and ultimately alleviated MI/RI in diabetes, which also hinted at the theory of liver-heart crosstalk. However, the specific mechanism of how liver-heart crosstalk mediated by lipid metabolism attenuated MI/RI in diabetes needs further study.
Considering all these results, we uncovered the targets and molecular mechanisms of SXT for attenuating MI/RI in diabetes and confirmed that SXT exerted anti-apoptotic effects in vivo through regulating the AGE-RAGE signaling pathway. Quercetin, beta-sitosterol, and kaempferol are the key components of SXT in reducing MI/RI in diabetes and need further verification.
The occurrence of myocardial ischemia/reperfusion injury (MI/RI) in diabetic individuals is often accompanied by larger infarct sizes and diminished cardiac function, which can have significant implications for patient prognosis. However, the effectiveness of strict glycemic control for the purpose of reducing cardiovascular mortality in diabetes was found to be insignificant. Notablely, Shuxin decoction (SXT) has been successfully used to alleviate secondary MI/RI in patients with diabetes mellitus in the clinical setting.
There is an urgent need to identify and facilitate developing novel complementary or alternative forms of medicine for effectively managing MI/RI with diabetes.
To investigate the protective effects and underlying mechanism of SXT against MI/RI with diabetes.
The Traditional Chinese Medicine System Pharmacology Database was employed to identify critical components and potential targets of SXT. Additionally, various databases such as Gene Expression Omnibus, DisGeNet, Genecards, Drugbank, OMIM, and PharmGKB were searched to identify potential therapeutic targets associated with MI/RI in diabetic patients. The intersection of the potential targets of SXT and the therapeutic targets related to MI/RI in diabetic patients were analyzed through bioinformatics techniques using the Database for Annotation, Visualization and Integrated Discovery. Subsequently, the major results of the bioinformatics analysis were validated through animal experiments.
Through animal experiments, it was demonstrated that the hypothesis generated by network pharmacology pertaining to the potential of the SXT to ameliorate MI/RI in diabetes through the reduction of oxidized low density lipoprotein (ox-LDL) and inhibition of the advanced glycation end products (AGE)-receptor for AGE (RAGE) signaling pathway was valid. The administration of a dose of SXT (2.8 g/kg/day) led to a decline in ox-LDL, AGEs, and RAGE, along with modulation of blood lipid levels. Furthermore, the treatment resulted in a decrease in the expression of apoptosis-related proteins such as Bax and cleaved caspase 3, while increasing the expression of Bcl-2.
SXT could regulate the level of blood lipids, alleviate cardiomyocyte apoptosis, and improve cardiac function through the AGE-RAGE signaling pathway.
The potential utilization of SXT as a complementary or alternative medicinal intervention could represent a valuable strategy for effectively managing MI/RI in diabetes.
The authors would like to acknowledge The General Hospital of Western Theater Command for skillful technical assistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S, Japan; Yildiz K, Turkey S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Diabetes around the world in 2021. 2022 Database: IDF Diabetes Atlas [Internet]. [cited 13 August 2022]. Available from: https://diabetesatlas.org/. |
| 2. | Korkmaz-Icöz S, Lehner A, Li S, Vater A, Radovits T, Hegedűs P, Ruppert M, Brlecic P, Zorn M, Karck M, Szabó G. Mild Type 2 Diabetes Mellitus Reduces the Susceptibility of the Heart to Ischemia/Reperfusion Injury: Identification of Underlying Gene Expression Changes. J Diabetes Res. 2015;2015:396414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Funk F, Kronenbitter A, Isić M, Flocke V, Gorreßen S, Semmler D, Brinkmann M, Beck K, Steinhoff O, Srivastava T, Barbosa DM, Voigt K, Wang L, Bottermann K, Kötter S, Grandoch M, Flögel U, Krüger M, Schmitt JP. Diabetes disturbs functional adaptation of the remote myocardium after ischemia/reperfusion. J Mol Cell Cardiol. 2022;173:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Sun M, Wang R, Xia R, Xia Z, Wu Z, Wang T. Amelioration of myocardial ischemia/reperfusion injury in diabetes: A narrative review of the mechanisms and clinical applications of dexmedetomidine. Front Pharmacol. 2022;13:949754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 5. | Asgari M, Salehi I, Ranjbar K, Khosravi M, Zarrinkalam E. Interval training and Crataegus persica ameliorate diabetic nephropathy via miR-126/Nrf-2 mediated inhibition of stress oxidative in rats with diabetes after myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2022;153:113411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 6. | Ahmad I, Hoda M. Molecular mechanisms of action of resveratrol in modulation of diabetic and non-diabetic cardiomyopathy. Pharmacol Res. 2020;161:105112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019;16:83-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 365] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 8. | Mazzone T. Intensive glucose lowering and cardiovascular disease prevention in diabetes: reconciling the recent clinical trial data. Circulation. 2010;122:2201-2211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3357] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 10. | Choi DJ, Choi BR, Lee H, Kim SC, Yoon D, Lee YS, Han KS, Park SB, Kim GS, Lee DY. Chemical Profiles and Antiobesity Effect of a Mixture of Astragalus membranaceus and Lithospermum erythrorhizon Extract in High Fat Diet Fed Mice. Evid Based Complement Alternat Med. 2022;2022:9642427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Wang L, Fan W, Zhang M, Zhang Q, Li L, Wang J, Zhu L, Wei D, Peng W, Wu C. Antiobesity, Regulation of Lipid Metabolism, and Attenuation of Liver Oxidative Stress Effects of Hydroxy-α-sanshool Isolated from Zanthoxylum bungeanum on High-Fat Diet-Induced Hyperlipidemic Rats. Oxid Med Cell Longev. 2019;2019:5852494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Ilkhanizadeh B, Shirpoor A, Khadem Ansari MH, Nemati S, Rasmi Y. Protective Effects of Ginger (Zingiber officinale) Extract against Diabetes-Induced Heart Abnormality in Rats. Diabetes Metab J. 2016;40:46-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Luan F, Rao Z, Peng L, Lei Z, Zeng J, Peng X, Yang R, Liu R, Zeng N. Cinnamic acid preserves against myocardial ischemia/reperfusion injury via suppression of NLRP3/Caspase-1/GSDMD signaling pathway. Phytomedicine. 2022;100:154047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Shan X, Xiao Y, Hong B, Li L, Chen Y, Wang G, Yu N, Peng D, Zhang C, Wang L, Chen W. Phytochemical profile and protective effects on myocardial ischaemia-reperfusion injury of sweated and non-sweated Salvia miltiorrhiza. Bge alcoholic extracts. J Pharm Pharmacol. 2022;74:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Lian B, Zeng R, Chen Y, Liao P, Guo L, Zhang M. Sodium Tanshinone IIA sulfonate for acute myocardial infarction: a systematic review and Meta-analysis. J Tradit Chin Med. 2021;41:26-35. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Zhang QZ, Fu TT, Dai JN, Zhou ZN, Shen CZ. Sodium Danshensu promotes the healing of stage 2 pressure injury wounds in ischemia/reperfusion injury rat models: possible regulation of apoptosis and inflammatory response. J Tradit Chin Med. 2021;41:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Wang L, Chen X, Wang Y, Zhao L, Zhao X. MiR-30c-5p mediates the effects of panax notoginseng saponins in myocardial ischemia reperfusion injury by inhibiting oxidative stress-induced cell damage. Biomed Pharmacother. 2020;125:109963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Su Q, Lv X, Ye Z. Ligustrazine Attenuates Myocardial Injury Induced by Coronary Microembolization in Rats by Activating the PI3K/Akt Pathway. Oxid Med Cell Longev. 2019;2019:6791457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1623] [Cited by in RCA: 3397] [Article Influence: 283.1] [Reference Citation Analysis (0)] |
| 20. | Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24663] [Cited by in RCA: 35586] [Article Influence: 1617.5] [Reference Citation Analysis (7)] |
| 21. | UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506-D515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4450] [Cited by in RCA: 5451] [Article Influence: 908.5] [Reference Citation Analysis (0)] |
| 22. | Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362-D368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4345] [Cited by in RCA: 5199] [Article Influence: 519.9] [Reference Citation Analysis (0)] |
| 23. | Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24735] [Cited by in RCA: 28540] [Article Influence: 1678.8] [Reference Citation Analysis (0)] |
| 24. | A free online platform for data analysis and visualization 2022. Database: Bioinformatics [Internet]. [cited 30 July 2022]. Available from: https://www.bioinformatics.com.cn. |
| 25. | Liu X, Xu Q, Wang X, Zhao Z, Zhang L, Zhong L, Li L, Kang W, Zhang Y, Ge Z. Irbesartan ameliorates diabetic cardiomyopathy by regulating protein kinase D and ER stress activation in a type 2 diabetes rat model. Pharmacol Res. 2015;93:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Huang L, Ding L, Yu S, Huang X, Ren Q. Propofol postconditioning alleviates diabetic myocardial ischemia-reperfusion injury via the miR200c3p/AdipoR2/STAT3 signaling pathway. Mol Med Rep. 2022;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Cao C, Liu HM, Li W, Wu Y, Leng Y, Xue R, Chen R, Tang LH, Sun Q, Xia Z, Tang QZ, Shen DF, Meng QT. Role of adiponectin in diabetes myocardial ischemia-reperfusion injury and ischemic postconditioning. Acta Cir Bras. 2020;35:e202000107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem. 2014;289:4145-4160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Cai W, He JC, Zhu L, Peppa M, Lu C, Uribarri J, Vlassara H. High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation. 2004;110:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Wang HJ, Kang PF, Wu WJ, Tang Y, Pan QQ, Ye HW, Tang B, Li ZH, Gao Q. Changes in cardiac mitochondrial aldehyde dehydrogenase 2 activity in relation to oxidative stress and inflammatory injury in diabetic rats. Mol Med Rep. 2013;8:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Yildirim SS, Akman D, Catalucci D, Turan B. Relationship between downregulation of miRNAs and increase of oxidative stress in the development of diabetic cardiac dysfunction: junctin as a target protein of miR-1. Cell Biochem Biophys. 2013;67:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol. 2010;88:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Xu Y, Nie L, Yin YG, Tang JL, Zhou JY, Li DD, Zhou SW. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Nasrollahi Z, ShahaniPour K, Monajemi R, Ahadi AM. Effect of quercetin and Abelmoschus esculentus (L.) Moench on lipids metabolism and blood glucose through AMPK-α in diabetic rats (HFD/STZ). J Food Biochem. 2022;46:e14506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 35. | Gao J, Zhang M, Niu R, Gu X, Hao E, Hou X, Deng J, Bai G. The combination of cinnamaldehyde and kaempferol ameliorates glucose and lipid metabolism disorders by enhancing lipid metabolism via AMPK activation. J Funct Foods. 2021;83:104556. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Li F, Li D, Tang S, Liu J, Yan J, Chen H, Yan X. Quercetin Protects H9c2 Cardiomyocytes against Oxygen-Glucose Deprivation/Reoxygenation-Induced Oxidative Stress and Mitochondrial Apoptosis by Regulating the ERK1/2/DRP1 Signaling Pathway. Evid Based Complement Alternat Med. 2021;2021:7522175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Shi C, Wu F, Zhu XC, Xu J. Incorporation of beta-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3beta signaling. Biochim Biophys Acta. 2013;1830:2538-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Wang ZQ, Jing LL, Yan JC, Sun Z, Bao ZY, Shao C, Pang QW, Geng Y, Zhang LL, Li LH. Role of AGEs in the progression and regression of atherosclerotic plaques. Glycoconj J. 2018;35:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Ahotupa M. Oxidized lipoprotein lipids and atherosclerosis. Free Radic Res. 2017;51:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Khodeer DM, Zaitone SA, Farag NE, Moustafa YM. Cardioprotective effect of pioglitazone in diabetic and non-diabetic rats subjected to acute myocardial infarction involves suppression of AGE-RAGE axis and inhibition of apoptosis. Can J Physiol Pharmacol. 2016;94:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Ji L, Hu D, Pan C, Weng J, Huo Y, Ma C, Mu Y, Hao C, Ji Q, Ran X, Su B, Zhuo H, Fox KA, Weber M, Zhang D; CCMR Advisory Board; CCMR-3B STUDY Investigators. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. 2013;126:925.e11-925.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 42. | Ye B, Liang X, Zhao Y, Cai X, Wang Z, Lin S, Wang W, Shan P, Huang W, Huang Z. Hsa_circ_0007478 aggravates NLRP3 inflammasome activation and lipid metabolism imbalance in ox-LDL-stimulated macrophage via miR-765/EFNA3 axis. Chem Biol Interact. 2022;368:110195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 43. | Kumar Singh N, Suri A, Kumari M, Kaushik P. A study on serum homocysteine and oxidized LDL as markers of cardiovascular risk in patients with overt hypothyroidism. Horm Mol Biol Clin Investig. 2022;43:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 44. | Carlier A, Phan F, Szpigel A, Hajduch E, Salem JE, Gautheron J, Le Goff W, Guérin M, Lachkar F, Ratziu V, Hartemann A, Ferré P, Foufelle F, Bourron O. Dihydroceramides in Triglyceride-Enriched VLDL Are Associated with Nonalcoholic Fatty Liver Disease Severity in Type 2 Diabetes. Cell Rep Med. 2020;1:100154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Cao Y, Wang Y, Zhou Z, Pan C, Jiang L, Meng Y, Charugundla S, Li T, Allayee H, Seldin MM, Lusis AJ. Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science. 2022;377:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |