Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.1013
Peer-review started: January 28, 2023
First decision: March 14, 2023
Revised: March 20, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: July 15, 2023
Processing time: 165 Days and 22.1 Hours
The chronic complications of diabetes mellitus constitute a major public health problem. For example, diabetic eye diseases are the most important cause of blindness, and diabetic nephropathy is the most frequent cause of chronic kidney disease worldwide. The cellular and molecular mechanisms of these chronic complications are still poorly understood, preventing the development of effective treatment strategies. Tight junctions (TJs) are epithelial intercellular junctions located at the most apical region of cell-cell contacts, and their main function is to restrict the passage of molecules through the paracellular space. The TJs consist of over 40 proteins, and the most important are occludin, claudins and the zonula occludens. Accumulating evidence suggests that TJ disruption in different organs, such as the brain, nerves, retina and kidneys, plays a fundamental pathophy
Core Tip: Chronic complications of diabetes mellitus constitute a major public health problem. Tight junctions are epithelial intercellular junctions, and their main function is to restrict the passage of molecules through the paracellular space. TJ disruption plays a fundamental pathophysiological role in the development of diabetic chronic complications. Increased permeability of the blood-brain barrier and the blood-retinal barrier are related to development of diabetic neuropathy and diabetic retinopathy.
- Citation: Robles-Osorio ML, Sabath E. Tight junction disruption and the pathogenesis of the chronic complications of diabetes mellitus: A narrative review. World J Diabetes 2023; 14(7): 1013-1026
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/1013.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.1013
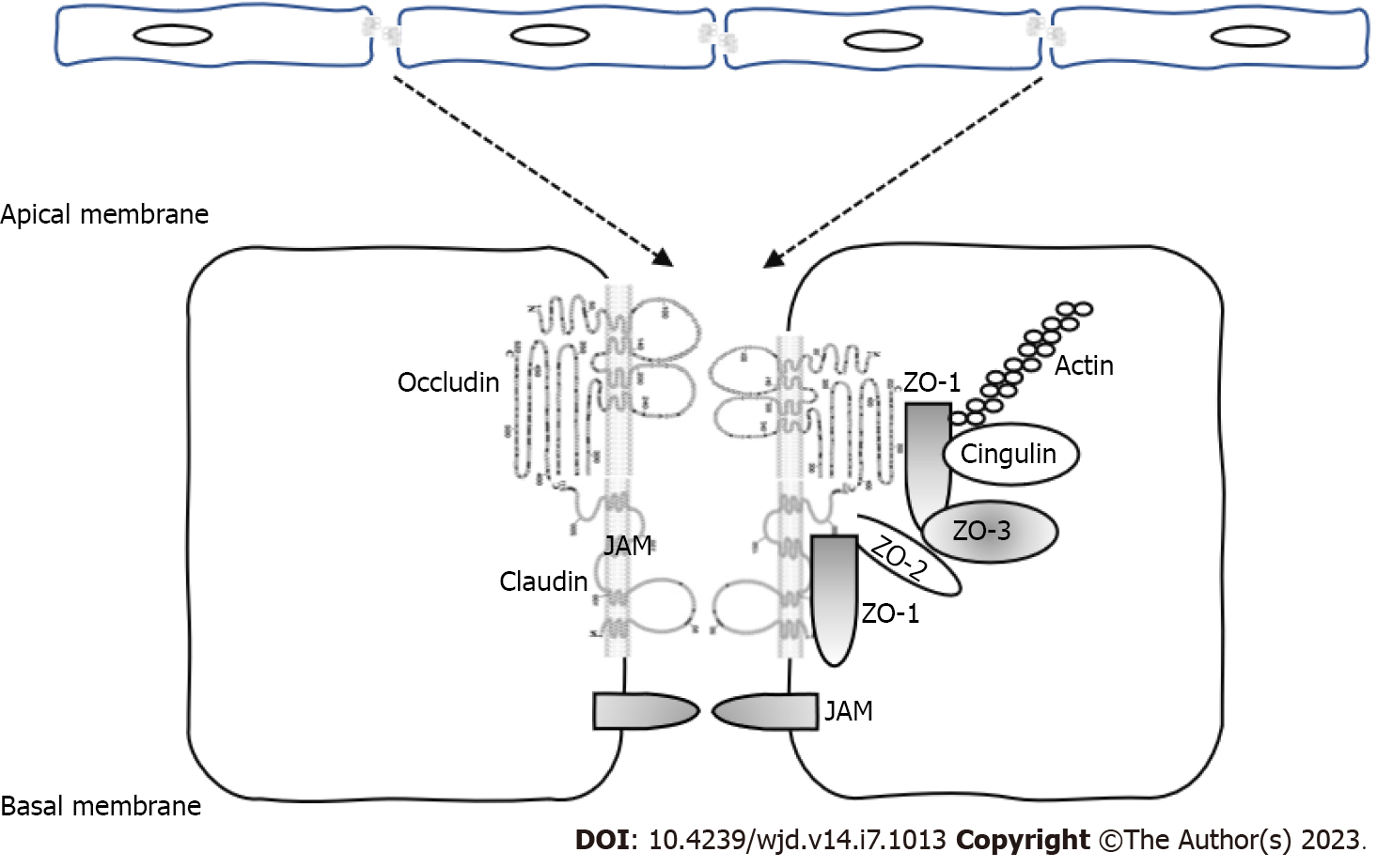
Tight junctions (TJs) are epithelial intercellular junctions located at the most apical region of cell-cell contacts. TJs serve two main functions: (1) Gate function, which restricts the passage of molecules through the paracellular space; and (2) Fence function, which confers cell polarity by preventing the movement of solutes and proteins between the apical and basolateral plasma membrane. Additional functions in cell-signaling processes, cell proliferation and gene expression have been identified[1].
At a molecular level, the TJs consist of over 40 proteins including members of the four-pass trans
Claudins are 21-28 kDa proteins and consist of four transmembrane domains, two extracellular loops, amino- and carboxyl-terminal cytoplasmic domains, and a short cytoplasmic turn. Claudins interact with the ZO-family of scaffolding proteins via their cytoplasmic region and are an essential component of the TJs regulating assembly and permeability[2].
Occludin is a 65 kDa protein that interacts with other TJ proteins such as membrane-associated guanylate kinase-scaffolding proteins. Occludin is expressed in endothelial and epithelial tissues, and its expression is regulated by different tyrosine and threonine kinases such as the non-receptor tyrosine kinase c-Yes and the protein kinase C (PKC). Madin-Darby Canine Kidney (MDCK) cells that express terminally truncated occludin have an increase in the paracellular permeability but preserve the formation of TJ strands[3]. However, occludin null mice did exhibit defects in certain organs, and histological abnormalities were found in several tissues including hyperplasia of the gastric epithelium, brain calcifications and testicular atrophy, suggesting an unknown role of occludin in the homeostasis of these organs[4].
The ZO proteins (members of the membrane-associated guanylate kinase family) are scaffolding proteins that bind and regulate the expression of cytoplasmic (cytoskeleton) and transmembrane components of the TJs. ZO proteins regulate gene transcription, cell proliferation and claudin polymerization. Phosphorylation of these proteins by the PKC and tyrosine kinases regulates TJ permeability and assembly. ZO-1 depletion in MDCK and endothelial cells lead to TJ disruption, delayed formation of TJs and reorganization of the actin and myosin cytoskeleton[5]. Maintenance of the cellular barriers and regulation of the transepithelial permeability to prevent diffusion of small molecules and bacteria to specific organs such as brain and retina is essential to keep the homeostasis at these organs.
Type 2 diabetes mellitus (DM) is a chronic disease that has reached epidemic proportions. Chronic hyperglycemia (CH) combined with defects on insulin secretion and action impair the microvasculature and activate intracellular signaling pathways, eventually leading to diabetic nephropathy (DN), retinopathy and neuropathy with significant negative effects on the quality of life and life expectancy[6].
Many studies have demonstrated that TJ disruption and increased leakage of water, solutes and proteins is associated with development of diabetic chronic complications [diabetic eye disease (DED), diabetic neuropathy and DN][7,8]. Therefore, this review aimed: (1) To summarize the normal structure of the TJs at the different barrier structures (brain, nerves, retina and kidney); (2) To describe the pathophysiological changes caused by DM leading to TJ disruption and increase in paracellular permeability that are associated with the chronic complications; and (3) To summarize these findings with the clinical consequences and pharmacological treatments used in the management of these complications.
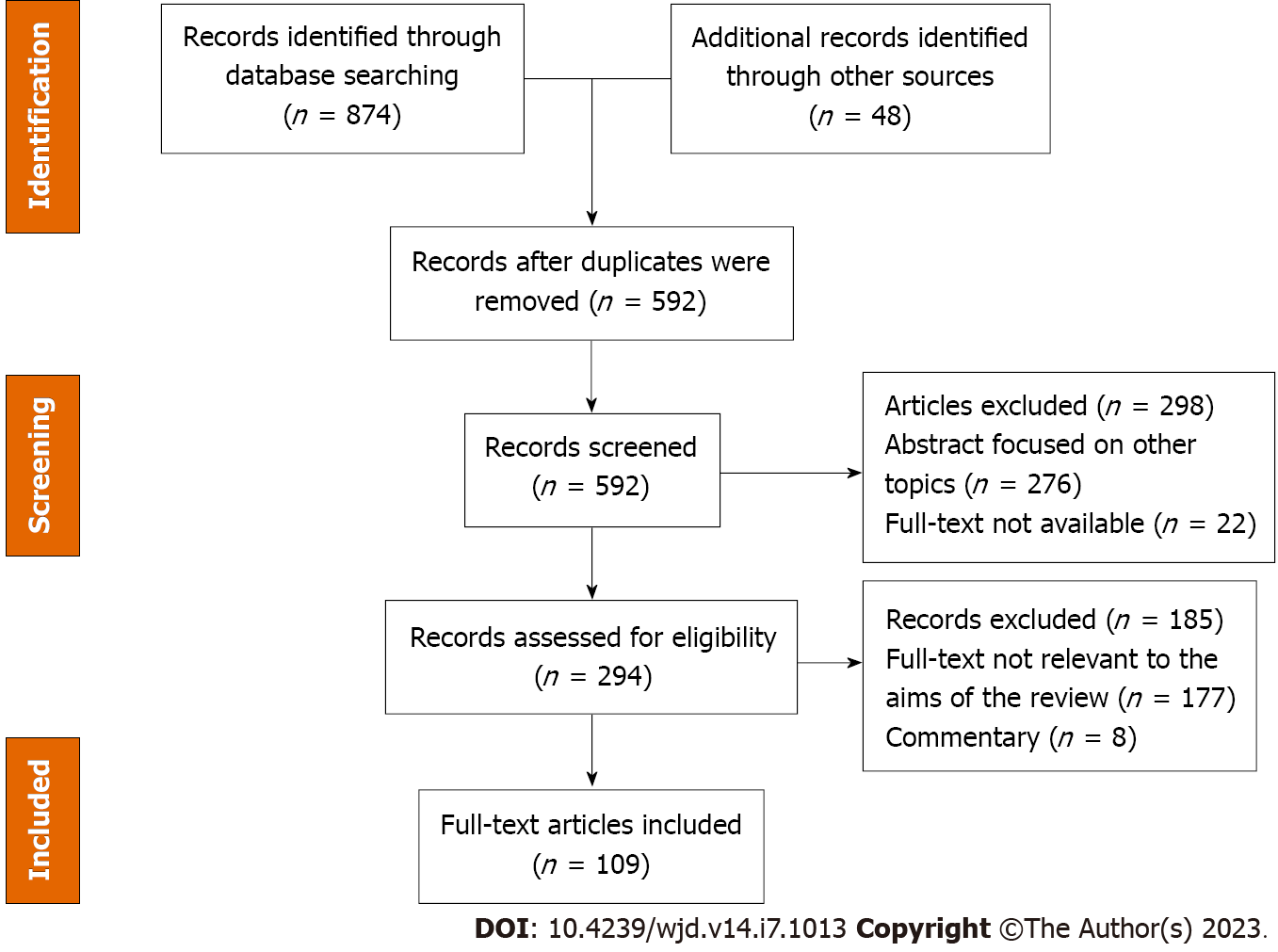
This systematic review was conducted according to the 2021 guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[9]. Both authors (MLRO and ES) systematically searched PubMed, Google Scholar and the Reference Citation Analysis (https://www.referencecitationanalysis.com/) databases to identify published articles from 1978 to December 2022 describing the role of TJs and the chronic complications of DM. Seminal references from selected articles were also searched and included. Both authors independently reviewed the database search results, assessed the titles, evaluated the abstracts and considered the study for full review. The search was performed combining the texts “tight junction” OR “occludin” OR “ZO (zonula occludens) proteins” OR “claudin” OR “blood retinal barrier” OR “brain-blood-barrier” OR “glomeruli” OR “renal tight junctions” with “diabetes mellitus” OR “diabetic retinopathy” OR “diabetic neuropathy” OR “diabetic nephropathy.” Only articles written in English were included (Figure 2). For the final analysis we evaluated 109 research papers.
The blood-brain-barrier (BBB) and the blood-nerve-barrier (BNB) are highly selective semipermeable barriers that regulate the exchange of water and solutes between the blood and the nerve tissue. Both the BBB and the BNB play important roles in maintaining the integrity of the nervous system, and many recent reports suggest that their breakdown drives a cascade of pathogenic events leading to many nervous system diseases[10].
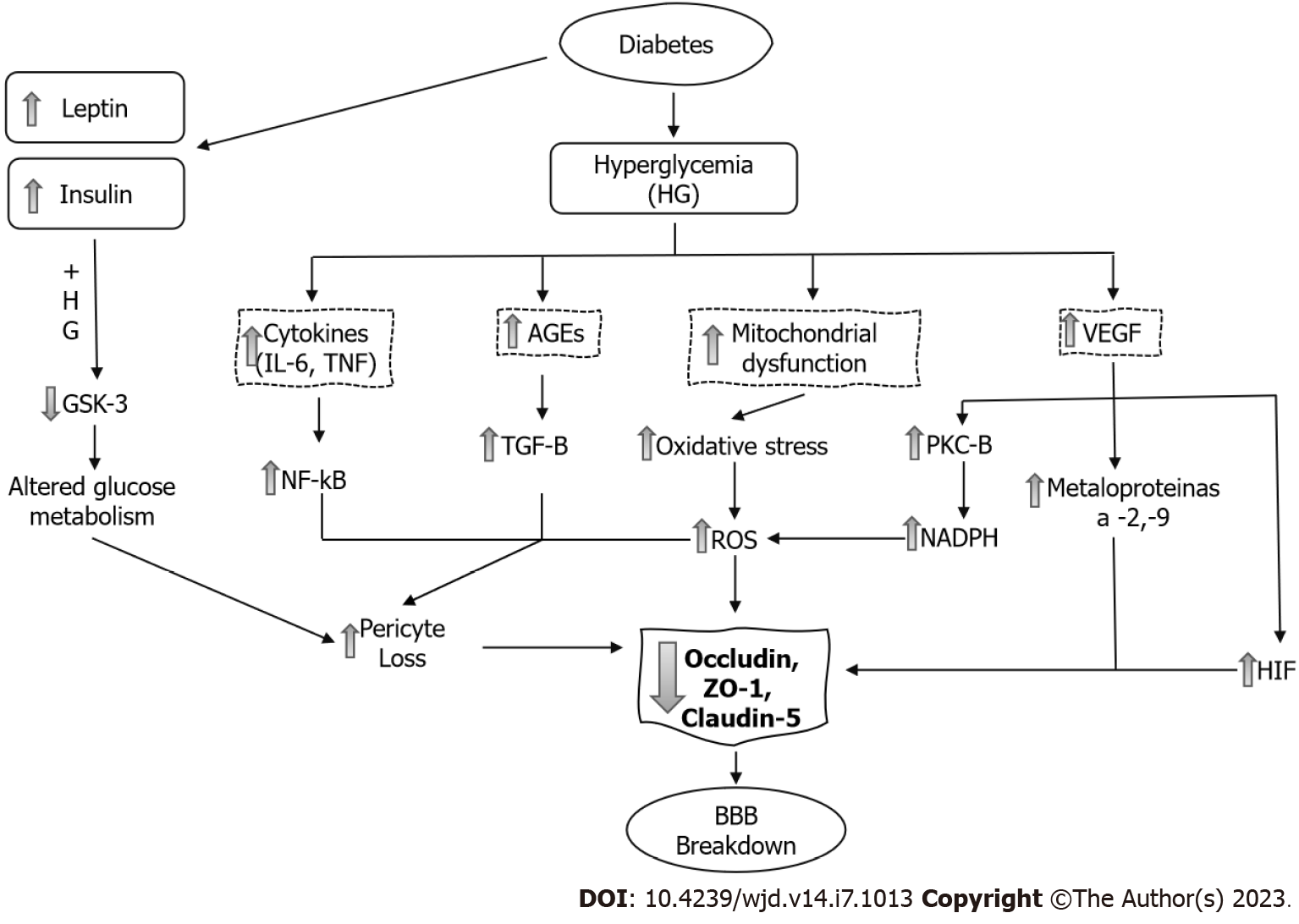
DM and increased permeability of the BBB: Numerous epidemiological studies have shown that DM is an important risk factor for central nervous system (CNS) disorders such as stroke[11], mild cognitive impairment and dementia[12]. The underlying causes related to these complications are multifactorial and are not well understood, although it is now evident that BBB damage adversely affects CNS homeostasis and function[10] (Figure 3).
The BBB consists of a confluent layer of non-fenestrated endothelial cells to tightly regulate the movement of molecules between the blood and the nervous system. Its basic structure is formed by the TJs located between the endothelial cells. The brain capillaries are shielded by pericytes and the foot processes of the astrocytes. These cells are important for the secretion of proteins that forms the basement membrane. The BBB is permeable to small molecules and lipid-soluble proteins, but receptor-mediated transcytosis is required by large molecules to enter the nervous system[13].
The endothelial TJs of the BBB are formed by the transcellular proteins claudins, occludin and junctional adhesion molecules. The loss of claudins increases barrier permeability, suggesting that this family of proteins are particularly important for barrier function. Claudins -1, -3, -5 and -12 take part in the formation of TJs between the endothelial cells[14]; claudin-5 is the most abundant claudin at the BBB and is a critical regulator of brain endothelial cell permeability. In claudin-5 knockout mice the blood vessels of the brain showed normal development and morphology, but the size-selectivity of the BBB was impaired allowing diffusion of small molecules[15].
Occludin is highly expressed at the BBB but does not appear to be essential to barrier function, as occludin-deficient mice have normal BBB permeability. ZO-1, ZO-2 and ZO-3 cross-link the claudins and other TJ proteins to the endothelial cytoskeleton[16]. Increased permeability of the BBB has been demonstrated in both type 1 DM[17] and type 2 DM[18,19], and significant efforts have been made to identify the molecular mechanisms related to BBB breakdown in DM.
Huber et al[20] demonstrated a progressive increase in the BBB permeability to small molecules in mice with streptozotocin-induced DM; the midbrain was particularly susceptible to DM-induced microvascular damage. Insulin administration attenuated BBB disruption during the first few weeks of treatment. However, as DM progressed the microvascular damage occurred even if hyperglycemia was controlled.
There are many proposed mechanisms by which DM leads to pericyte loss and BBB breakdown. Hyperglycemia causes mitochondrial dysfunction and synthesis of reactive oxygen species (ROS) and increases oxidative stress, activation of nuclear factor-kappa B (NF-κB) and the synthesis of inflammatory cytokines[21]. In pericytes and endothelial cells, both hyperglycemia and formation of advanced glycation end-products (AGEs) downregulate the TJ proteins claudin-5, ZO-1 and occludin. There is also a significant increase in the amount of occludin and claudin-5 on the membrane-bound extracellular vesicles[22]. This allows greater influx of blood components into the perivascular space.
Hyperglycemia also stimulates the synthesis of vascular endothelial growth factor (VEGF), increasing both angiogenesis and vascular permeability. Downstream, VEGF activates PKC-β, causing an increase in nicotinamide adenine dinucleotide phosphate-oxidase and an increase in ROS formation. VEGF increases the activation of different matrix metalloproteinases (MMP-2 and MMP-9). These mechanisms increase brain barrier permeability through the decrease in occludin expression and phosphorylation[23].
The hypoxia-inducible factor-1 (HIF-1) is a transcriptional factor that activates cellular adaptation to hypoxia. High glucose upregulates the transcriptional activity and protein level of HIF-1α in brain endothelial cells. In addition, it increased the paracellular permeability and diminished the expression of the TJ proteins occludin and ZO-1[24].
The development of cognitive impairment in diabetic rats was associated with an increase in the BBB permeability. These rats showed an increase in brain levels of interleukin (IL)-6 and a decrease in occludin and claudin-5 expression[25].
Recent studies suggested that many factors other than hyperglycemia, like insulin and leptin, have a pathophysiological role increasing BBB permeability[26]. Insulin crosses the BBB using a saturable transporter, affecting brain functions through mechanisms largely independent of glucose utilization. Insulin transport across the BBB is highly regulated and altered in obesity, starvation and DM[27].
Insulin receptor signaling regulates the integrity of the BBB via inactivation of glycogen synthase kinase 3, a key enzyme in many cellular functions, specifically regulating glycogen synthesis and blood glucose levels. Administration of insulin alone increases BBB resistance, but the combined administration of high glucose/high insulin synergistically impairs TJ integrity[28].
Some drugs have been shown to have effects on BBB structure and function. Statins are known to improve endothelial cell function, and simvastatin treatment improved the barrier function in cerebral tissue of diabetic rats[29]. Administration of valsartan (AT1R antagonist) to db/db mice ameliorated BBB leakage. This finding suggested that neurovascular protection can be obtained blocking the AT1-receptor mediated signaling pathways[30]. Exogenous administration of exendin-4, a glucagon-like peptide 1 agonist that crosses the BBB, reverses the functional changes and restores levels of TJ proteins[31].
In many CNS disorders the BBB integrity is compromised, and treatment with glucocorticoids improves the tightness of the BBB[32]. However, there are no reports about its effects on diabetic animal models.
Diabetic neuropathy and increase permeability of the BNB: Diabetic polyneuropathy (DPN) is the most common chronic complication, with a prevalence of 30%-50%. The duration of DM and HbA1c levels are major predictors of DPN. Other risk factors consistently associated with DPN are hypertriglyceridemia, hypertension, abdominal obesity, low high-density lipoprotein levels, smoking and alcohol ingestion[33].
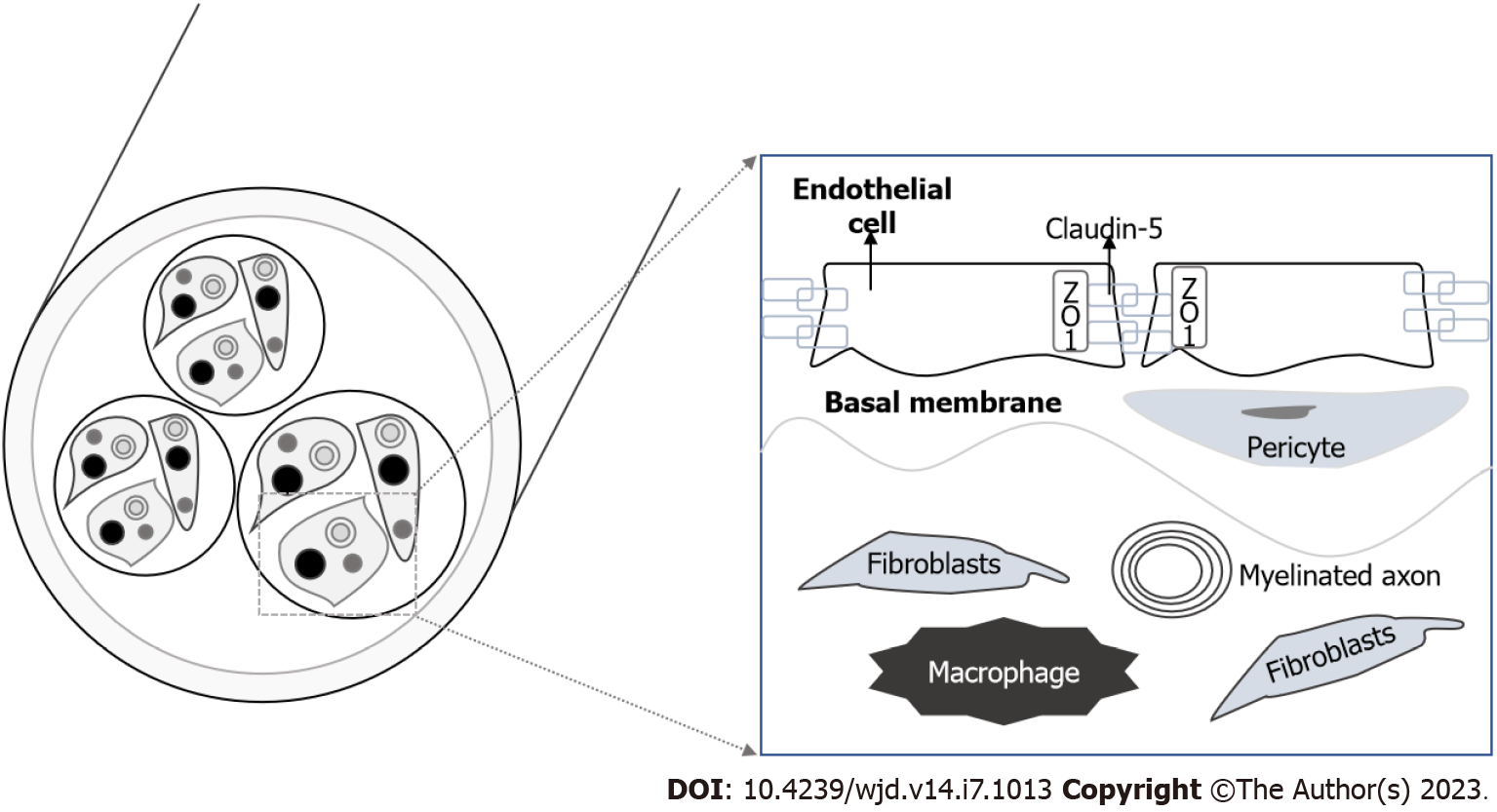
The BNB is localized in the microvessels of the endoneurium or perineurium, and consists of endothelial cells, pericytes and the basement membrane (Figure 4). TJs are an essential component of the BNB cellular architecture to restrict the paracellular flow into the endoneurial milieu and are constituted by occludin, ZO-1 and claudins. Cells of the perineurium express claudin-1, -3 and -19, whereas the endoneurial vessels express claudin-5[34].
There are many mechanisms involved in the axonopathy associated with DPN. Hyperglycemia increases sorbitol pathway activity, reduces myo-inositol nerve content, induces mitochondrial dysfunction with an increase in the synthesis of free radical species and activates metalloproteinases. The formation of AGEs increases protein glycosylation and Schwann cell injury[35].
Initial studies on the effects of hyperglycemia on BNB structure and permeability were controversial as some initial studies conducted in streptozotocin-diabetic rat models did not show increased permeability to large molecules, even in experiments performed with exposition to severe hyperglycemia[36,37]. Other studies showed severe impairment and increased permeability of the BNB[38]. More recent studies showed that the BNB was leaky for small but not for large molecules. Even though no gross changes in TJ proteins were observed, there was a downregulation in the expression of claudin-1[39]. In human subjects with type 1 DM an increase in the extravasation of albumin and immunoglobulin G through the BNB has been demonstrated[40].
Pathological BNB breakdown leads to an increase in the paracellular leakage of potentially harmful molecules into the nerve tissue and the upregulation of adhesion molecules on the vessel walls to permit the transcellular entry of inflammatory cells to the endoneurium initiating a local inflammatory cascade. Inflammation, endoneurial hypoxia and pericyte degeneration are some of the mechanisms associated with BNB disruption[13]. AGE exposition induces basement membrane hypertrophy and disrupts the BNB by increasing autocrine VEGF and transforming growth factor-β signaling. Claudin-5 synthesis was also significantly reduced[41].
The consequences of the breakdown of the BNB are the access of hematogenous cells and inflammatory molecules to the endoneurium. These phenomenon take part in the local inflammatory cascade generating neuropathic pain[42]. Unfortunately, there are no effective treatments for this complication. Current analgesics have limited beneficial impact alleviating neuropathic pain, and other than glucose and metabolic control there are no disease-modifying therapies[35].
DED is the most common microvascular complication of DM and manifests as vascular disease with vessel proliferation [diabetic retinopathy (DR)] and vascular leakage (diabetic macular edema). The latest prevalence data from a pooled analysis estimated a prevalence of 35%, and this prevalence increased with DM duration. The most important risk factors associated with DED are CH, age, cholesterol levels and high blood pressure[43].
The retina is the innermost, light-sensitive layer of tissue of the eye that turns light energy from photons into three-dimensional images. The blood–retina barrier (BRB) separates the retina from the systemic circulation to regulate the flow of water, electrolytes, nutrients and metabolic waste products. The BRB is composed of both an inner barrier (iBRB) and an outer barrier (oBRB)[44].
The iBRB is composed of retinal vascular endothelial cells (REC) that line the retinal vasculature, which originates from the central retinal artery and supplies the inner retinal layers. The iBRB has some transport properties because substances from the blood can cross it by transcellular (caveolae-mediated transport) and paracellular transport (dependent on TJs). Intercellular TJs are crucial for the formation of endothelial barriers, as they regulate paracellular diffusion[44].
Claudins are the main determinants to regulate TJ properties. Claudin-5 is the most abundant claudin isoform in the BRB and is essential for the maintenance of the iBRB integrity[45,46]. Claudin-5 interacts with the PDZ domains of ZO-1 to cross-link the transmembrane proteins to the cytoskeleton. ZO-1 has an important role to maintain the iBRB permeability as loss of ZO-1 disrupts TJs and increases the barrier permeability[47]. Claudin-1 is also expressed in TJs on REC and is an important component of these structures to keep the barrier function[48].
The oBRB consists of the choroid, Bruch’s membrane and the retinal pigment epithelial cells. The retinal pigment epithelial cells are a group of epithelial cells divided into apical and basolateral sides. The apical surface is in direct contact with the photoreceptors, and the basolateral side acts as a barrier that interacts with the capillaries of the choroid layer. The TJs of the RPE are located at the apical surface and are mainly responsible for maintaining oBRB integrity. The oBRB is essential for the survival of the photoreceptors by supporting the absorption of out of focus light, the retinal adhesion and the transport of retinoids and other nutrients[49].
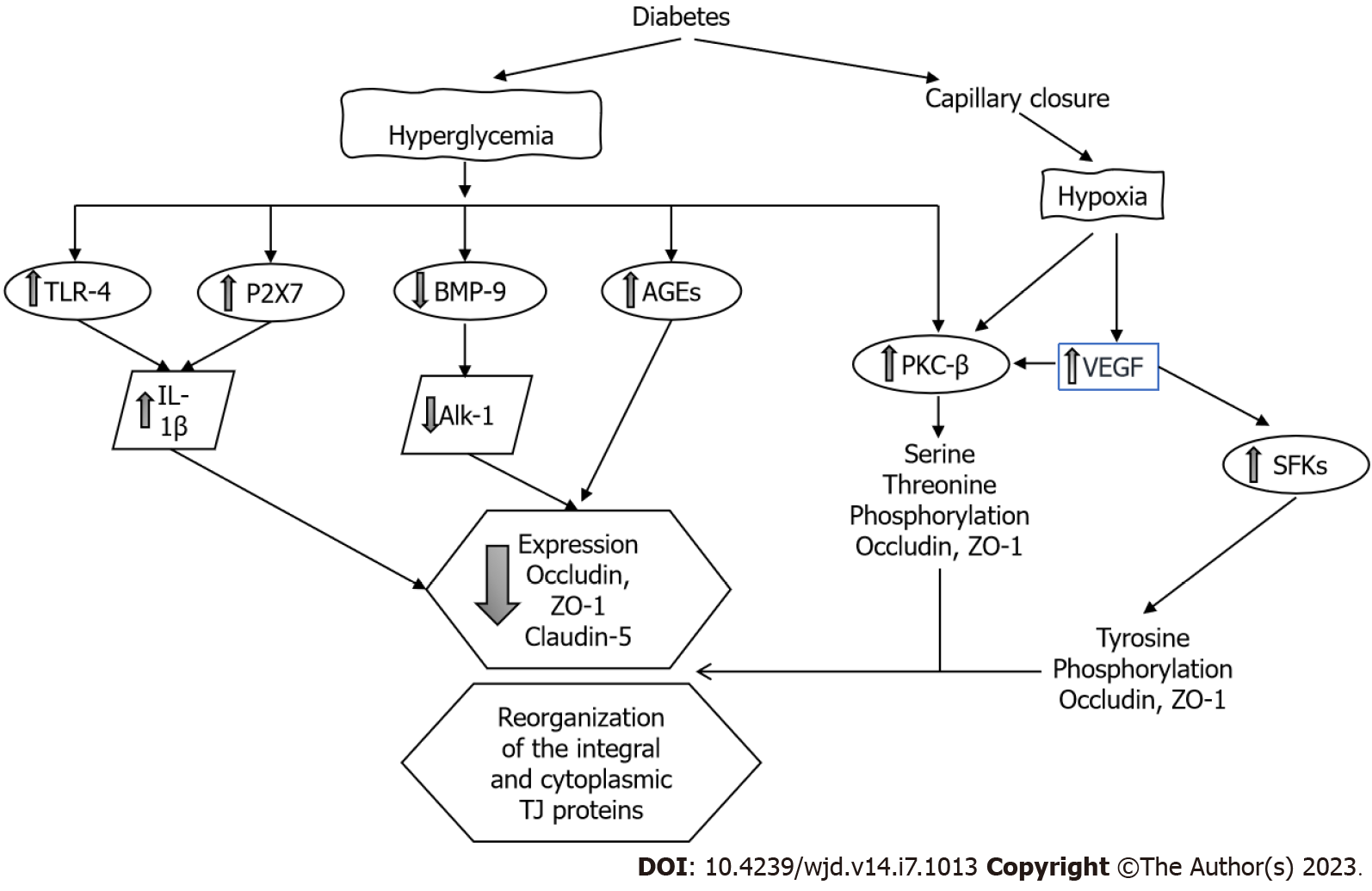
Effects of DM on iBRB: Clinical studies strongly suggest that diabetic macular edema is the result of abnormal fluid accumulation as a consequence of the breakdown and vascular leakage of the iBRB. The predominant molecular mechanisms leading to iBRB breakdown include hypoxia and the direct effects of glucose on the endothelium, activation of VEGF and other intracellular signaling transduction pathways (such as PKC) and the triggering of inflammatory factors like tumor necrosis factor alpha, prostaglandins and toll-like receptor 4 (TLR-4)[50] (Figure 5). Hypoxia activates PKC and directly affects the TJs redistributing occludin and ZO-1[51]; hypoxia is also a key factor to induce the synthesis of VEGF.
VEGF has an important role in the homeostasis of endothelial cells as is an important regulator of vascular permeability, migration and cell proliferation. CH and oxidative stress upregulate VEGF-α and VEGF-β, which induces retinal neovascularization and vascular leakage. In the retina, VEGF is mainly expressed in Müller cells, endothelial cells, astrocytes, RPE cells and ganglion cells. However, recent studies suggest that Müller cell-derived VEGF induces retinal neovascularization, vascular leakage and inflammation playing a major causative role in DR[52].
The process whereby VEGF induces paracellular permeability involves binding to its receptor VEGFR-2 and activation of both the Src family cytoplasmic tyrosine kinases and PKC-β. Tyrosine kinases of the Src family are critically involved in TJ regulation through occludin and ZO-1 tyrosine phosphorylation[53,54]. VEGF also decreases occludin expression[55] and induces occludin serine-threonine phosphorylation through a mechanism mediated by activation of PKC- β. PKC-β is the most crucial PKC isoform that regulates the retinal microvascular permeability[56], and administration of PKC inhibitors prevented this increase in permeability[57]. Endothelial cells with the phosphorylation-resistant Ser490 to Ala form of occludin have preserved TJ organization and reduced VEGF-induced permeability[58].
Hyperglycemia increases the permeability of the REC through decreasing the levels of both ZO-1 and occludin[49]. The expression of claudin-1 and -5 is also decreased[59]. The formation of AGEs also decreases the expression of occludin, ZO-1 and ZO-2 in REC increasing permeability. Interestingly, the administration of silver-nanoparticles inhibited AGE-induced permeability by increasing the expression of the TJ proteins[60].
The increase of intracellular glucose leads to an increase in the synthesis of diacylglycerol, the main endogenous activator of PKC[61]. PKC regulates the function of TJ proteins through the phos
High glucose impairs other signaling cascades in retinal endothelial cells. The bone morphogenetic protein 9/activin receptor-like kinase 1 signaling cascade is necessary to maintain the endothelium integrity; this system is impaired in endothelial cells exposed to hyperglycemic conditions. A decrease in bone morphogenetic protein 9 and alterations in the activin receptor-like kinase 1 cascade contributes to increasing vascular permeability through the disruption of the occludin junctions[63].
Many cell components of the retina including the REC and the RPE express the purinergic receptor (P2X7R). It has been shown that activation of the P2X7R by hyperglycemia has a role in the breakdown of the BRB. Activation of the P2X7R induces the release of IL-1β. IL-1β reduces the transendothelial electrical resistance by decreasing the expression of claudin-5 and ZO-1. These effects were inhibited with the exogenous administration of an P2X7R antagonist[64].
β-adrenergic receptors regulate TLR-4 signaling in the retina, and inhibition of TLR-4 significantly reduces retinal barrier permeability. The exogenous administration of forskolin (a PKA agonist) or compound 49b (β-adrenergic receptor agonist) to retinal endothelial cells restored the high glucose-associated decrease in ZO-1 and occludin through the inhibition of the TLR-4 inflammatory cascade[65]. Histamine increases paracellular permeability and reduces the expression of the TJ protein ZO-1 in cultures of retinal endothelial cells[66].
Angiopoietin 1, derived from pericytes, is known to be an antipermeability factor in the vascular system. Angiopoietin 1 has also been proven to have a protective effect on BRB via inhibiting VEGF-induced retinal vascular leakage[67].
Hydrocortisone increases barrier properties of the retinal endothelial cells. Hydrocortisone increases the occludin content, decreases occludin phosphorylation and promotes the TJ assembly. These changes decrease water and solute endothelial permeability[68].
Effects of DM on oBRB: The role of the oBRB in the pathophysiology of the macular edema has gained importance in recent years. Recent evidence suggests that the TJs of RPE cells are also compromised in DR and may contribute to macular edema. Leaky TJs would dissipate the chloride gradient that RPE uses to pump fluid out of the retina[69]. Treatment of RPE cells with tumor necrosis factor alpha or IL-1 decreased transendothelial electrical resistance, increased permeability and altered the expression or content of TJ molecules[70].
Villarroel et al[71] studied the effects of high glucose concentration in ARPE-19 cells; there was a reduction of permeability with overexpression of claudin-1 and no changes in ZO-1 or occludin. These findings suggested that hyperglycemia per se is not the only factor accounting for the impairment of the oBRB in DR but requires the release of cytokines and ROS to induced damage and increase permeability[72]. At higher glucose exposure, the ARPE-19 cells increased miR-132 expression and decreased the expression of occludin and increased cell permeability[73].
High glucose induces a loss of Na-K-ATPase function impairing the transport of water from the subretinal space contributing to the development of macular edema[74]. Erythropoietin (EPO) is upregulated in DR. EPO overexpression has been found in both the RPE and neuroretina of diabetic eyes. EPO maintains the oBRB integrity through downregulation of HIF-1α and JNK signaling, thus upregulating ZO-1 and occludin expression in RPE cells[75]. Although VEGF has an important role in the pathogenesis of this disease, the RPE has mechanisms for maintaining low concentrations of VEGF in the retinal space. Peng et al[76] showed that VEGF and anti-VEGF drugs (bevacizumab, ranibizumab) have no effects on the TJs of RPE cells.
Diabetic kidney disease or DN is the leading cause of end-stage kidney disease[77]. CH leads to structural, metabolic and hemodynamic changes in the renal glomeruli and tubules, but the pathophysiology of the DN is complex and still poorly understood. CH activates the renin-angiotensin system and increases the activity of PKC, ROS formation and many cytokines and transcription factors that result in structural and functional abnormalities in the kidney[78]. However, the effects of hyperglycemia on the renal TJs have received little interest, except for the important focus on the podocyte slit diaphragms (SD).
TJs are necessary for the proper function of glomeruli and tubules and are the most important structures involved in the paracellular transport of water and solutes. The transepithelial electrical resistance and the complexity of the TJ increases from the proximal to the collecting tubule as does the expression of ZO-1, ZO-2 and occludin[79]. The distribution of claudins through the glomerular endothelium and tubules form selective pores and barriers for water and electrolytes such as sodium, potassium, magnesium, calcium and chloride[80].
The distribution and localization of claudins varies along the nephron. In the glomerular endothelium, claudin-5 forms a barrier for high molecular weight proteins. In the proximal tubule (leaky epithelium), claudin-2 forms a pore for sodium and potassium ions. In the thick ascending limb, claudin-14, -16 and -19 regulate the paracellular reabsorption of calcium and magnesium. In the renal collecting duct (tight epithelium), claudin-4 is expressed (together with claudin-3, -7 and -8) and is the major modulator of the paracellular chloride pathway[81] .
Aldosterone is the main hormonal stimulus of sodium reabsorption in the distal segments of the nephron by increasing the expression and activity of the epithelial sodium channel. Recent evidence has shown that aldosterone also has a role regulating the paracellular flow of sodium. Aldosterone phosphorylates claudin-4 and increases claudin-8 expression. These mechanism in the distal nephron are aimed to prevent the luminal back-flux of reabsorbed sodium as well to reinforce the paracellular chloride reabsorption pathway[81,82].
Effects of DM on TJs of the glomerulus: The glomerulus is a highly specialized structure that functions as an efficient filtration barrier that restricts passage of large molecules but remains highly permeable to water and small molecules. The glomerulus is composed by a network of capillaries, mesangial cells, podocytes and the Bowman’s capsule. The blood is filtered across the fenestra of the glomerular endothelial cells (GEC) and the other components of the glomerular filtration barrier yielding a fluid composed of water plus soluble substances that accumulates at the Bowman’s capsule to enter the renal tubules[83].
The GECs form the first cellular barrier, and the TJs between cells are important for maintaining capillary permeability. Injury to the GECs with disruption of the TJs increases its permeability and induces inflammatory cell infiltration, podocyte damage, albuminuria and progression of kidney disease[84]. High glucose decreases the expression of occludin and translocates ZO-1 to the cytoplasm by activation of RhoA (a member of the family of small GTPases)/ROCK1 system. Simvastatin inhibits the RhoA/ROCK1 signaling, increases occludin expression and restores ZO-1 localization. In db/db mice simvastatin decreases albuminuria by suppressing the RhoA/ROCK1 system[85]. AGEs significantly increase the permeability of GEC monolayers through activation of MMP-2 and MMP-9, which downregulate the expression of occludin and claudin-5[86].
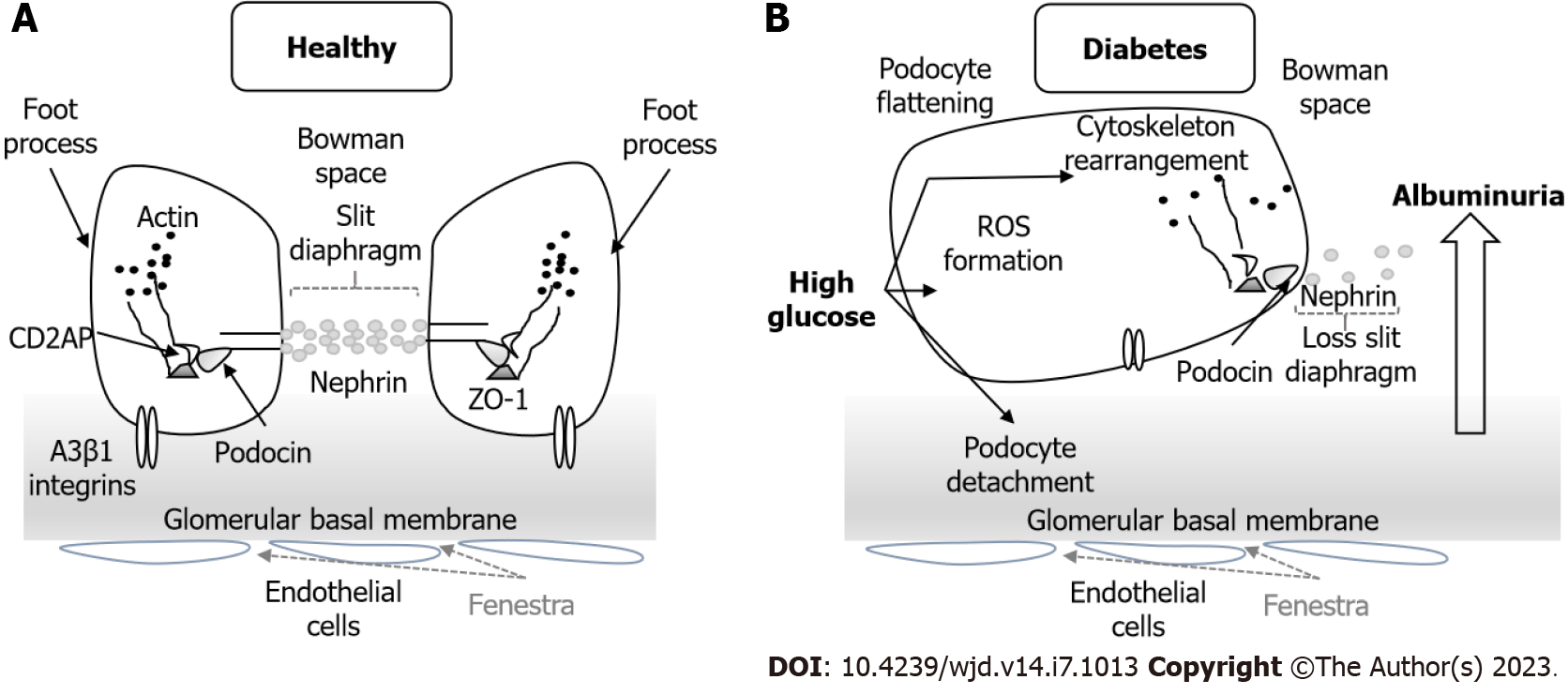
Glomerular podocytes (Figure 6) are highly differentiated cells that cover the glomerular capillaries and have a characteristic morphology with numerous foot processes. The formation of SD between the foot processes serves as a final filtration barrier and is composed by many transmembrane proteins such as nephrin, podocin, Neph1 and Fat1. Podocyte damage causes disruption of the filtration barrier, proteinuria and glomerulosclerosis[87].
During the early stages of embryonic development the TJs connect immature podocytes, but in mature stages they disappear along with the widening of the intercellular spaces and the appearance of SD[88]. TJ proteins such as occludin, claudin-5 and ZO-1, but not claudin-1, have also been found in the SD of the mature podocytes. Their expression and localization are altered in glomerular diseases[89].
DN and other diseases with nephrotic proteinuria are characterized by the loss of the filtration slit, appearance of TJ-like structures and the presence of multiple membrane “fusion” points between the foot processes. This finding has been called the SD to TJ transition and is mediated by the upregulation of claudin-1 in podocytes[90-92].
In normal conditions, claudin-1 is usually absent from podocytes but present in the glomerular parietal cells. In DN, claudin-1 is upregulated in parietal cells and extended ectopically to podocytes[90]. The presence of claudin-1 led to podocyte effacement and albuminuria, presumably through the activation of the β-catenin/Snail signaling system and pathological interactions with nephrin and podocin, which disrupts the SD[92].
Sirtuin-1 (Sirt1) is an NAD(+)-regulated deacetylase with numerous known positive effects on cellular functions, and accumulating evidence shows that Sirt1 plays a crucial role in the pathogenesis of DN[92]. Hasegawa et al[93] found reduced expression of Sirt1 in the proximal tubules and higher expression of claudin-1 in glomeruli in streptozotocin-induced diabetic mice, which led to morphological changes on podocytes and albuminuria. Overexpression of Sirt1 in these mice inhibited the rise of claudin-1 and morphological changes. In kidney biopsy samples from subjects with DN, lower expression of Sirt1 and higher expression of claudin-1 were correlated with higher levels of albuminuria. Altogether, these data indicate a protective role of Sirt1 in glomerular and tubular injury.
Claudin-5 has been classified as a cation barrier and is expressed throughout the plasma membrane of podocytes. Molina-Jijón et al[82] reported that early DN decreases the expression of claudin-5 in glomeruli. This finding was attributed to an increase in oxidative stress and was associated with changes in the localization of ZO-1. Administration of all-trans retinoic acid ameliorated these changes[94]. Spironolactone prevented depletion of claudin-5 in glomeruli, suggesting a role of aldosterone in the regulation of claudin-5 expression and function[82].
Sun et al[95] showed that claudin-5 deletion reduced ZO-1 expression and nuclear translocation of ZO-1-associated nucleic acid-binding protein, followed by activation of the WNT signaling pathway that led to podocyte injury and dysfunction. ZO-1-associated nucleic acid-binding protein is a member of a family of DNA-binding proteins that regulate the expression of genes involved in proliferation and other nuclear signaling processes[96].
As previously stated, the scaffolding protein ZO-1 helps to maintain the permselective properties of the glomerular capillary wall. Experimental proteinuria is associated with cellular redistribution of this protein in the glomeruli, and administration of lisinopril [angiotensin converting enzyme (ACE) inhibitor] prevented these changes[97]. In glomeruli exposed to high glucose ZO-1 expression decreased, was redistributed from the podocyte membrane to the cytoplasm and inhibited serine and tyrosine phosphorylation. Administration of angiotensin II type 1 receptor blockers attenuated these changes[98]. An increase of bradykinin levels associated with the use of ACE-inhibitors also prevented ZO-1 changes[99]. These findings explain some of the beneficial effects of drugs acting on inhibition of the renin-angiotensin system.
Modulation of claudins and other TJ-SD proteins remains a key area of research from a clinical and therapeutic point of view. Many current drugs such as ACE inhibitors and simvastatin have a positive effect on these proteins limiting the glomerular injury and progressive kidney disease. Other potential drugs are shown in Table 1. Further research is necessary to develop specific drugs that target these proteins to evaluate their effect on glomerular cells.
| Drug | Type | Mechanism of action |
| Spironolactone[82] | Mineralocorticoid inhibitor | Decrease oxidative stress |
| Prevent decrease of claudin-5 in glomeruli | ||
| Prevent decrease of claudin-2 and occludin in PT | ||
| Simvastatin[85] | Inhibits HMG-CoA reductase | Inhibit RhoA/ROCK1 signaling |
| Increase occludin expression | ||
| Restore ZO-1 localization | ||
| atRA[94] | Retinoid | Decrease oxidative stress |
| Prevent decrease of claudin-5 in glomeruli | ||
| Prevent decrease of claudin-2 and occludin in PT | ||
| Lisinopril[97] | ACE inhibitor | Preserve glomerular ZO-1 distribution |
| Irbesartan[107] | Antagonist | Avoid nephrin depletion on SD |
| Ang II receptor | ||
| Sitagliptin[108] | Inhibits DPP-4 | Decrease levels of mitochondrial ROS, ameliorate reduction of claudin-5 in GEC |
| Sinomenine[109] | Alkaloid isolated from the root of Sinomenium acutum | Attenuate ROS level, tight junction dysfunction and RhoA/ROCK activation |
Effects of DM on TJs of the renal tubules and tubular transport: The renal tubules and specifically the proximal tubule are uniquely susceptible to a variety of metabolic and hemodynamic factors associated with DM. The development of tubule-interstitial injury is an important risk factor associated with progressive diabetic kidney disease . In early stages of DN, tubular hypertrophy with thickening of the basal membrane is observed, but in advanced stages tubular atrophy with interstitial fibrosis is more prominent[100]. Studies on the effects of DM on tubular TJs are scarce.
The exposition of MDCK II cells to high glucose induced a decrease in the TJ content of claudins-1 and -3, a significant increase in claudin-2 and a decrease in the expression of occludin and ZO-1 junctional content. These changes decreased transendothelial electrical resistance and increased TJ permeability[101]. Claudin-2 expression in the proximal tubule decreased in streptozotocin-induced diabetic rat models[102,103]. The administration of spironolactone and all-trans retinoic acid prevented the decrease in claudin-2 and occludin in proximal tubules by decreasing oxidative stress[82,94].
The consequences of these tubular cell TJ changes on kidney function or progression of kidney disease are currently unknown.
TJs have an important role in maintaining organ homeostasis and are highly selective structures that regulate the paracellular exchange of water and solutes. Altered TJs have an important role in the pathogenesis of the chronic complications of DM. Identification of the mechanisms that lead to TJ disruption will provide better tools for prevention and treatment of these complications in people with DM.
An area of particular interest is the measurement of TJ proteins on plasma and its correlations with clinical outcomes. Halbgebauer et al[104] found significantly increased levels of plasma claudin-5 in trauma patients with hemorrhagic shock that were positively correlated with lactate levels and blood transfusions. These findings indicate that a breakdown of TJ barriers can be related with clinical outcomes in this group of patients. In other diseases, such as bipolar disorders[105] and chronic migraine[106], claudin-5 plasma levels have been found to be significantly higher than in healthy subjects. There are no studies about plasma levels of TJ proteins and clinical outcomes in diabetic patients. This is an area of opportunity for early detection of chronic complications in diabetic subjects.
New findings about the pathophysiology of TJs on the retina, nervous system and kidney may advance the development of delivery systems of insulin and other drugs by targeting these structures.
TJs are essential to the integrity and function of the epithelial and endothelial barriers in the retina, nervous system and kidney. Disruption of these structures contributes to the pathophysiology of the chronic complications in DM. There are many mechanisms of TJ disruption in DM, and hyperglycemia triggers many of the mechanisms that induce TJ disruption. Activation of PKC phosphorylates ZO-1, occludin and claudin increasing the permeability of the TJ; an increase in the oxidative stress, activation of metalloproteinases, synthesis of AGEs and hypoxia induces changes on TJ proteins increasing permeability in these barriers. Claudin-5 is an essential component of the BBB and BRB. A better understanding of the functions of these protein may allow better diagnosis and treatment to prevent injury at these organs.
In the kidney, hyperglycemia induces podocyte detachment and changes in the morphology and function of the SD that leads to albuminuria and progressive kidney disease. More research is required to identify the role of TJ disruption with clinical outcomes in diabetic subjects. Future studies should be directed to develop drugs that target TJ proteins to prevent disruption of these barriers and to improve drug delivery to these organs.
The main limitation of this review was the lack of clinical studies conducted on humans, as most of studies were carried out in animal and cellular models. This increases the difficulty for translating whether the molecular changes and severity of the TJ disruption are associated with worse clinical outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: European Renal Association, No. 11821.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eseadi C, Nigeria; Patel MV, India S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Otani T, Furuse M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020;30:805-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 453] [Article Influence: 75.5] [Reference Citation Analysis (2)] |
| 2. | Heinemann U, Schuetz A. Structural Features of Tight-Junction Proteins. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 3. | Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 382] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131-4142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 909] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 5. | Brunner J, Ragupathy S, Borchard G. Target specific tight junction modulators. Adv Drug Deliv Rev. 2021;171:266-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 334] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 7. | Alves MG, Oliveira PF, Socorro S, Moreira PI. Impact of diabetes in blood-testis and blood-brain barriers: resemblances and differences. Curr Diabetes Rev. 2012;8:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Hanai K, Mori T, Yamamoto Y, Yoshida N, Murata H, Babazono T. Association of Estimated Glomerular Filtration Rate With Progression of Albuminuria in Individuals With Type 2 Diabetes. Diabetes Care. 2023;46:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 50361] [Article Influence: 10072.2] [Reference Citation Analysis (2)] |
| 10. | Li X, Cai Y, Zhang Z, Zhou J. Glial and Vascular Cell Regulation of the Blood-Brain Barrier in Diabetes. Diabetes Metab J. 2022;46:222-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1295] [Cited by in RCA: 1467] [Article Influence: 183.4] [Reference Citation Analysis (2)] |
| 12. | Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW. Diabetes and Cognitive Impairment. Curr Diab Rep. 2016;16:87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 372] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 13. | Richner M, Ferreira N, Dudele A, Jensen TS, Vaegter CB, Gonçalves NP. Functional and Structural Changes of the Blood-Nerve-Barrier in Diabetic Neuropathy. Front Neurosci. 2018;12:1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Gonçalves A, Ambrósio AF, Fernandes R. Regulation of claudins in blood-tissue barriers under physiological and pathological states. Tissue Barriers. 2013;1:e24782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1336] [Cited by in RCA: 1457] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 16. | Bauer HC, Krizbai IA, Bauer H, Traweger A. "You Shall Not Pass"-tight junctions of the blood brain barrier. Front Neurosci. 2014;8:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Mayhan WG, Scott JP, Arrick DM. Influence of type 1 diabetes on basal and agonist-induced permeability of the blood-brain barrier. Physiol Rep. 2015;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 328] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Xu Z, Zeng W, Sun J, Chen W, Zhang R, Yang Z, Yao Z, Wang L, Song L, Chen Y, Zhang Y, Wang C, Gong L, Wu B, Wang T, Zheng J, Gao F. The quantification of blood-brain barrier disruption using dynamic contrast-enhanced magnetic resonance imaging in aging rhesus monkeys with spontaneous type 2 diabetes mellitus. Neuroimage. 2017;158:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Huber JD, VanGilder RL, Houser KA. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol. 2006;291:H2660-H2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Zhang H, Wang S, Guo Y, Fang X, Zheng B, Gao W, Yu H, Chen Z, Roman RJ, Fan F. Reduced pericyte and tight junction coverage in old diabetic rats are associated with hyperglycemia-induced cerebrovascular pericyte dysfunction. Am J Physiol Heart Circ Physiol. 2021;320:H549-H562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Rom S, Heldt NA, Gajghate S, Seliga A, Reichenbach NL, Persidsky Y. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci Rep. 2020;10:7274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Bauer AT, Bürgers HF, Rabie T, Marti HH. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab. 2010;30:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 24. | Yan J, Zhang Z, Shi H. HIF-1 is involved in high glucose-induced paracellular permeability of brain endothelial cells. Cell Mol Life Sci. 2012;69:115-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Geng J, Wang L, Zhang L, Qin C, Song Y, Ma Y, Chen Y, Chen S, Wang Y, Zhang Z, Yang GY. Blood-Brain Barrier Disruption Induced Cognitive Impairment Is Associated With Increase of Inflammatory Cytokine. Front Aging Neurosci. 2018;10:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Corem N, Anzi S, Gelb S, Ben-Zvi A. Leptin receptor deficiency induces early, transient and hyperglycaemia-independent blood-brain barrier dysfunction. Sci Rep. 2019;9:2884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Rhea EM, Rask-Madsen C, Banks WA. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J Physiol. 2018;596:4753-4765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 28. | Ito S, Yanai M, Yamaguchi S, Couraud PO, Ohtsuki S. Regulation of Tight-Junction Integrity by Insulin in an In Vitro Model of Human Blood-Brain Barrier. J Pharm Sci. 2017;106:2599-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Mooradian AD, Haas MJ, Batejko O, Hovsepyan M, Feman SS. Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes. 2005;54:2977-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Cai L, Li W, Zeng R, Cao Z, Guo Q, Huang Q, Liu X. Valsartan alleviates the blood-brain barrier dysfunction in db/db diabetic mice. Bioengineered. 2021;12:9070-9080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Zanotto C, Simão F, Gasparin MS, Biasibetti R, Tortorelli LS, Nardin P, Gonçalves CA. Exendin-4 Reverses Biochemical and Functional Alterations in the Blood-Brain and Blood-CSF Barriers in Diabetic Rats. Mol Neurobiol. 2017;54:2154-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Salvador E, Shityakov S, Förster C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014;355:597-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 34. | Reinhold AK, Schwabe J, Lux TJ, Salvador E, Rittner HL. Quantitative and Microstructural Changes of the Blood-Nerve Barrier in Peripheral Neuropathy. Front Neurosci. 2018;12:936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, Feldman EL. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022;21:922-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 36. | Jakobsen J, Malmgren L, Olsson Y. Permeability of the blood-nerve barrier in the streptozotocin-diabetic rat. Exp Neurol. 1978;60:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Sima AA, Robertson DM. The perineurial and blood-nerve barriers in experimental diabetes. Acta Neuropathol. 1978;44:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Low PA, Nickander KK. Oxygen free radical effects in sciatic nerve in experimental diabetes. Diabetes. 1991;40:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Ben-Kraiem A, Sauer RS, Norwig C, Popp M, Bettenhausen AL, Atalla MS, Brack A, Blum R, Doppler K, Rittner HL. Selective blood-nerve barrier leakiness with claudin-1 and vessel-associated macrophage loss in diabetic polyneuropathy. J Mol Med (Berl). 2021;99:1237-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Poduslo JF, Curran GL, Dyck PJ. Increase in albumin, IgG, and IgM blood-nerve barrier indices in human diabetic neuropathy. Proc Natl Acad Sci U S A. 1988;85:4879-4883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Shimizu F, Sano Y, Haruki H, Kanda T. Advanced glycation end-products induce basement membrane hypertrophy in endoneurial microvessels and disrupt the blood-nerve barrier by stimulating the release of TGF-β and vascular endothelial growth factor (VEGF) by pericytes. Diabetologia. 2011;54:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Lim TKY, Shi XQ, Martin HC, Huang H, Luheshi G, Rivest S, Zhang J. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. Pain. 2014;155:954-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Antonetti DA, Silva PS, Stitt AW. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 2021;17:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 385] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 44. | Díaz-Coránguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017;139:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 45. | Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 531] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 46. | Arima M, Nakao S, Yamaguchi M, Feng H, Fujii Y, Shibata K, Wada I, Kaizu Y, Ahmadieh H, Ishibashi T, Stitt AW, Sonoda KH. Claudin-5 Redistribution Induced by Inflammation Leads to Anti-VEGF-Resistant Diabetic Macular Edema. Diabetes. 2020;69:981-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Hudson N, Campbell M. Tight Junctions of the Neurovascular Unit. Front Mol Neurosci. 2021;14:752781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Morcos Y, Hosie MJ, Bauer HC, Chan-Ling T. Immunolocalization of occludin and claudin-1 to tight junctions in intact CNS vessels of mammalian retina. J Neurocytol. 2001;30:107-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Naylor A, Hopkins A, Hudson N, Campbell M. Tight Junctions of the Outer Blood Retina Barrier. Int J Mol Sci. 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 50. | Zhang J, Zhang J, Zhang C, Gu L, Luo D, Qiu Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 148] [Reference Citation Analysis (0)] |
| 51. | Rudraraju M, Narayanan SP, Somanath PR. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol Res. 2020;161:105115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 52. | Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297-2305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 53. | Werdich XQ, Penn JS. Specific involvement of SRC family kinase activation in the pathogenesis of retinal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:5047-5056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463-23467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 475] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 55. | Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998;47:1953-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 402] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 56. | Murakami T, Frey T, Lin C, Antonetti DA. Protein kinase cβ phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes. 2012;61:1573-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 57. | Kim JH, Kim JH, Jun HO, Yu YS, Kim KW. Inhibition of protein kinase C delta attenuates blood-retinal barrier breakdown in diabetic retinopathy. Am J Pathol. 2010;176:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Goncalves A, Dreffs A, Lin CM, Sheskey S, Hudson N, Keil J, Campbell M, Antonetti DA. Vascular Expression of Permeability-Resistant Occludin Mutant Preserves Visual Function in Diabetes. Diabetes. 2021;70:1549-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Someya H, Ito M, Nishio Y, Sato T, Harimoto K, Takeuchi M. Osteopontin-induced vascular hyperpermeability through tight junction disruption in diabetic retina. Exp Eye Res. 2022;220:109094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 60. | Sheikpranbabu S, Kalishwaralal K, Lee KJ, Vaidyanathan R, Eom SH, Gurunathan S. The inhibition of advanced glycation end-products-induced retinal vascular permeability by silver nanoparticles. Biomaterials. 2010;31:2260-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319-1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 703] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 62. | Cong X, Kong W. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell Signal. 2020;66:109485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (13)] |
| 63. | Akla N, Viallard C, Popovic N, Lora Gil C, Sapieha P, Larrivée B. BMP9 (Bone Morphogenetic Protein-9)/Alk1 (Activin-Like Kinase Receptor Type I) Signaling Prevents Hyperglycemia-Induced Vascular Permeability. Arterioscler Thromb Vasc Biol. 2018;38:1821-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Tassetto M, Scialdone A, Solini A, Di Virgilio F. The P2X7 Receptor: A Promising Pharmacological Target in Diabetic Retinopathy. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Liu L, Jiang Y, Steinle JJ. Forskolin regulates retinal endothelial cell permeability through TLR-4 actions in vitro. Mol Cell Biochem. 2021;476:4487-4492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Gardner TW, Lesher T, Khin S, Vu C, Barber AJ, Brennan WA Jr. Histamine reduces ZO-1 tight-junction protein expression in cultured retinal microvascular endothelial cells. Biochem J. 1996;320 ( Pt 3):717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:3784-3791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 68. | Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC Jr. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Xia T, Rizzolo LJ. Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vision Res. 2017;139:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 70. | Abe T, Sugano E, Saigo Y, Tamai M. Interleukin-1beta and barrier function of retinal pigment epithelial cells (ARPE-19): aberrant expression of junctional complex molecules. Invest Ophthalmol Vis Sci. 2003;44:4097-4104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Villarroel M, García-Ramírez M, Corraliza L, Hernández C, Simó R. Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Exp Eye Res. 2009;89:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 73. | Wang SS, Liao X, Liu F, Zhang Q, Qiu JJ, Fu SH. miR-132 mediates cell permeability and migration by targeting occludin in high-glucose -induced ARPE-19 cells. Endocr J. 2021;68:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Crider JY, Yorio T, Sharif NA, Griffin BW. The effects of elevated glucose on Na+/K(+)-ATPase of cultured bovine retinal pigment epithelial cells measured by a new nonradioactive rubidium uptake assay. J Ocul Pharmacol Ther. 1997;13:337-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Zhang C, Xie H, Yang Q, Yang Y, Li W, Tian H, Lu L, Wang F, Xu JY, Gao F, Wang J, Jin C, Xu G, Xu GT, Zhang J. Erythropoietin protects outer blood-retinal barrier in experimental diabetic retinopathy by up-regulating ZO-1 and occludin. Clin Exp Ophthalmol. 2019;47:1182-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Peng S, Adelman RA, Rizzolo LJ. Minimal effects of VEGF and anti-VEGF drugs on the permeability or selectivity of RPE tight junctions. Invest Ophthalmol Vis Sci. 2010;51:3216-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | McGill JB, Haller H, Roy-Chaudhury P, Cherrington A, Wada T, Wanner C, Ji L, Rossing P. Making an impact on kidney disease in people with type 2 diabetes: the importance of screening for albuminuria. BMJ Open Diabetes Res Care. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Badal SS, Danesh FR. New insights into molecular mechanisms of diabetic kidney disease. Am J Kidney Dis. 2014;63:S63-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 79. | Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000;57:2386-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Muto S. Physiological roles of claudins in kidney tubule paracellular transport. Am J Physiol Renal Physiol. 2017;312:F9-F24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Le Moellic C, Boulkroun S, González-Nunez D, Dublineau I, Cluzeaud F, Fay M, Blot-Chabaud M, Farman N. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol. 2005;289:C1513-C1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Molina-Jijón E, Rodríguez-Muñoz R, González-Ramírez R, Namorado-Tónix C, Pedraza-Chaverri J, Reyes JL. Aldosterone signaling regulates the over-expression of claudin-4 and -8 at the distal nephron from type 1 diabetic rats. PLoS One. 2017;12:e0177362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Swiatecka-Urban A. Membrane trafficking in podocyte health and disease. Pediatr Nephrol. 2013;28:1723-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Fu J, Lee K, Chuang PY, Liu Z, He JC. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol. 2015;308:F287-F297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 85. | Peng H, Luo P, Li Y, Wang C, Liu X, Ye Z, Li C, Lou T. Simvastatin alleviates hyperpermeability of glomerular endothelial cells in early-stage diabetic nephropathy by inhibition of RhoA/ROCK1. PLoS One. 2013;8:e80009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Luo P, Peng H, Li C, Ye Z, Tang H, Tang Y, Chen C, Lou T. Advanced glycation end products induce glomerular endothelial cell hyperpermeability by upregulating matrix metalloproteinase activity. Mol Med Rep. 2015;11:4447-4453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Chen X, Wang J, Lin Y, Liu Y, Zhou T. Signaling Pathways of Podocyte Injury in Diabetic Kidney Disease and the Effect of Sodium-Glucose Cotransporter 2 Inhibitors. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 88. | Menendez-Castro C, Hilgers KF, Amann K, Daniel C, Cordasic N, Wachtveitl R, Fahlbusch F, Plank C, Dötsch J, Rascher W, Hartner A. Intrauterine growth restriction leads to a dysregulation of Wilms' tumour supressor gene 1 (WT1) and to early podocyte alterations. Nephrol Dial Transplant. 2013;28:1407-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG. Slit diaphragms contain tight junction proteins. J Am Soc Nephrol. 2009;20:1491-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 90. | Gong Y, Sunq A, Roth RA, Hou J. Inducible Expression of Claudin-1 in Glomerular Podocytes Generates Aberrant Tight Junctions and Proteinuria through Slit Diaphragm Destabilization. J Am Soc Nephrol. 2017;28:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Wang B, Qian JY, Tang TT, Lin LL, Yu N, Guo HL, Ni WJ, Lv LL, Wen Y, Li ZL, Wu M, Cao JY, Liu BC. VDR/Atg3 Axis Regulates Slit Diaphragm to Tight Junction Transition via p62-Mediated Autophagy Pathway in Diabetic Nephropathy. Diabetes. 2021;70:2639-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Wang W, Sun W, Cheng Y, Xu Z, Cai L. Role of sirtuin-1 in diabetic nephropathy. J Mol Med (Berl). 2019;97:291-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 93. | Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19:1496-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 94. | Molina-Jijón E, Rodríguez-Muñoz R, Namorado Mdel C, Bautista-García P, Medina-Campos ON, Pedraza-Chaverri J, Reyes JL. All-trans retinoic acid prevents oxidative stress-induced loss of renal tight junction proteins in type-1 diabetic model. J Nutr Biochem. 2015;26:441-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Sun H, Li H, Yan J, Wang X, Xu M, Wang M, Fan B, Liu J, Lin N, Li L, Zhao S, Gong Y. Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease. Nat Commun. 2022;13:1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 96. | Lima WR, Parreira KS, Devuyst O, Caplanusi A, N'kuli F, Marien B, Van Der Smissen P, Alves PM, Verroust P, Christensen EI, Terzi F, Matter K, Balda MS, Pierreux CE, Courtoy PJ. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J Am Soc Nephrol. 2010;21:478-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 97. | Macconi D, Ghilardi M, Bonassi ME, Mohamed EI, Abbate M, Colombi F, Remuzzi G, Remuzzi A. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol. 2000;11:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Rincon-Choles H, Vasylyeva TL, Pergola PE, Bhandari B, Bhandari K, Zhang JH, Wang W, Gorin Y, Barnes JL, Abboud HE. ZO-1 expression and phosphorylation in diabetic nephropathy. Diabetes. 2006;55:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Dey M, Baldys A, Sumter DB, Göoz P, Luttrell LM, Raymond JR, Göoz M. Bradykinin decreases podocyte permeability through ADAM17-dependent epidermal growth factor receptor activation and zonula occludens-1 rearrangement. J Pharmacol Exp Ther. 2010;334:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Liu H, Feng J, Tang L. Early renal structural changes and potential biomarkers in diabetic nephropathy. Front Physiol. 2022;13:1020443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 101. | Mongelli-Sabino BM, Canuto LP, Collares-Buzato CB. Acute and chronic exposure to high levels of glucose modulates tight junction-associated epithelial barrier function in a renal tubular cell line. Life Sci. 2017;188:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Molina-Jijón E, Rodríguez-Muñoz R, Namorado Mdel C, Pedraza-Chaverri J, Reyes JL. Oxidative stress induces claudin-2 nitration in experimental type 1 diabetic nephropathy. Free Radic Biol Med. 2014;72:162-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 103. | Rosas-Martínez L, Rodríguez-Muñoz R, Namorado-Tonix MDC, Missirlis F, Del Valle-Mondragón L, Sánchez-Mendoza A, Reyes-Sánchez JL, Cervantes-Pérez LG. Hyperglycemic levels in early stage of diabetic nephropathy affect differentially renal expression of claudins-2 and -5 by oxidative stress. Life Sci. 2021;268:119003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Halbgebauer R, Braun CK, Denk S, Mayer B, Cinelli P, Radermacher P, Wanner GA, Simmen HP, Gebhard F, Rittirsch D, Huber-Lang M. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J Crit Care. 2018;44:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 105. | Kılıç F, Işık Ü, Demirdaş A, Doğuç DK, Bozkurt M. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J Affect Disord. 2020;266:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 106. | Yücel M, Kotan D, Gurol Çiftçi G, Çiftçi IH, Cikriklar HI. Serum levels of endocan, claudin-5 and cytokines in migraine. Eur Rev Med Pharmacol Sci. 2016;20:930-936. [PubMed] |
| 107. | Bonnet F, Cooper ME, Kawachi H, Allen TJ, Boner G, Cao Z. Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia. 2001;44:874-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 187] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 108. | Xu L, Shao F. Sitagliptin protects renal glomerular endothelial cells against high glucose-induced dysfunction and injury. Bioengineered. 2022;13:655-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 109. | Yin Q, Xia Y, Wang G. Sinomenine alleviates high glucose-induced renal glomerular endothelial hyperpermeability by inhibiting the activation of RhoA/ROCK signaling pathway. Biochem Biophys Res Commun. 2016;477:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |