Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.846
Peer-review started: December 16, 2022
First decision: February 20, 2023
Revised: March 21, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: June 15, 2023
Processing time: 181 Days and 7 Hours
Lomatogonium rotatum (LR) is traditionally used in Mongolian folk medicine as a hypoglycemic agent, but its evidence-based pharmacological effects and me-chanisms of action have not been fully elucidated.
To emphasize the hypoglycemic action mechanism of LR in a type 2 diabetic rat model and examine potential biomarkers to obtain mechanistic understanding regarding serum metabolite modifications.
A high-fat, high-sugar diet and streptozotocin injection-induced type 2 diabetic rat model was established. The chemical composition of the LR was identified by high performance liquid chromatography. LR extract administrated as oral gavage at 0.5 g/kg, 2.5 g/kg, and 5 g/kg for 4 wk. Anti-diabetic effects of LR extract were evaluated based on histopathological examination as well as the measurement of blood glucose, insulin, glucagon-like peptide 1 (GLP-1), and lipid levels. Serum metabolites were analyzed using an untargeted metabolomics approach.
According to a chemical analysis, swertiamarin, sweroside, hesperetin, coumarin, 1.7-dihydroxy-3,8-dimethoxyl xanthone, and 1-hydroxy-2,3,5 trimethoxanone are the principal active ingredients in LR. An anti-diabetic experiment revealed that the LR treatment significantly increased plasma insulin and GLP-1 levels while effectively lowering blood glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol, and oral glucose tolerance test compared to the model group. Furthermore, untargeted metabolomic analysis of serum samples detected 236 metabolites, among which 86 were differentially expressed between the model and the LR group. It was also found that LR considerably altered the levels of metabolites such as vitamin B6, mevalonate-5P, D-proline, L-lysine, and taurine, which are involved in the regulation of the vitamin B6 metabolic pathway, selenium amino acid metabolic pathway, pyrimidine metabolic pathway, and arginine and proline metabolic pathways.
These findings indicated that LR may have a hypoglycemic impact and that its role may be related to changes in the serum metabolites and to facilitate the release of insulin and GLP-1, which lower blood glucose and lipid profiles.
Core Tip:Lomatogonium rotatum (LR) is traditionally used in Mongolian folk medicine as a hypoglycemic agent. Its evidence-based pharmacological effects and mechanisms of action have not been elucidated. An anti-diabetic experiment in rats revealed that LR treatment increased insulin and glucagon-like peptide 1 levels and decreased blood sugar, total cholesterol, triglycerides, low-density lipoprotein cholesterol, and oral glucose tolerance test. These findings indicated that LR may have a hypoglycemic impact and that its role may be related to changes in the serum metabolites as well as to facilitating the release of insulin and glucagon-like peptide 1, which lower blood glucose and lipid profiles.
- Citation: Dai LL, Cho SB, Li HF, A LS, Ji XP, Pan S, Bao ML, Bai L, Ba GN, Fu MH. Lomatogonium rotatum extract alleviates diabetes mellitus induced by a high-fat, high-sugar diet and streptozotocin in rats. World J Diabetes 2023; 14(6): 846-861
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/846.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.846
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by chronically elevated blood glucose (BG) (hyperglycemia) and elevated blood insulin (hyperinsulinemia)[1]. T2DM is treated primarily with six classes of anti-diabetic medications, including metformin, glimepiride, repaglinide, pioglitazone, sitagliptin, and acarbose[2]. Traditional medicine has a long history of use as a complementary alternative therapy and has shown promising results in treating T2DM. The demand for complementary and alternative medicine has increased owing to its potential to target a multitude of metabolic pathways for treating T2DM.
Lomatogonium rotatum (LR) is a dried whole herb derived from the Gentianaceae plant Lomatogonium rotatum (L.) Fries ex Nym and is an important medicinal herb utilized in the formulation and practice of Mongolian medicine in China[3]. According to a previous study, LR could decrease the body weight of obese rats induced by a high-fat high-sugar (HFHS) diet[4]. The LR compounds can activate the bitter taste receptors, which have advantageous effects on diabetes[5,6]. The main compounds of LR include flavonoids and xanthones, small amounts of iridoids, alkaloids, steroids, and organic acids[6-9]. Nonetheless, the protective effects of LR against diabetes have not been thoroughly examined.
It is widely known that T2DM comprises several abnormalities in the systemic metabolism of amino acids (AAs), lipids, and glucose[10,11]. The metabolites associated with food metabolism provide a direct functional reading of an organism’s physiological condition. Metabolomics analysis is the untargeted identification and quantification of all low molecular weight metabolic end products (metabolites)[12]. Metabolomics technology is used to investigate the impact of drugs on endogenous metabolite variations and to identify specific biomarkers and their key factors[13]. Moreover, it provides a perspective image of downstream gene expression and vital information regarding drug metabolism[14]. Metabolic profiles of cells, tissues, organs, and biological fluids can be used to infer an individual’s health status and help monitor changes in specific diseases[15]. In recent years, metabolomics has been used to systematically study the metabolites of patients with T2DM and find biomarkers and possible metabolic pathways. The dynamic changes of endogenous metabolites are closely related to the occurrence and development of diabetes. Understanding the hypoglycemic effect of LR on T2DM, identifying its biomarkers, and clarifying its mechanism by metabolomic studies will have considerable clinical significance.
The HFHS diet was provided by Liaoning Changsheng Biotechnology Co., Ltd. (Shenyang, China; Batch No. 20200925). Analytical citric acid and sodium citrate were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). BG, insulin, glucagon-like peptide 1 (GLP-1), total cholesterol (TC), triglycerides (TG), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) kits were provided by Shenzhen Icubio Biotechnology Co., Ltd. (Shenzhen, China). Hematoxylin and eosin (H&E) stain was obtained from Nanjing Jiancheng Technology Co., Ltd. (Nanjing, China).
LR was collected from Xilinhaote grassland, Inner Mongolia, China. Five kilograms of LR was washed, dried, and powdered. Then, LR powder was extracted three times with 95% ethanol for 3 h each time. The extract was combined, concentrated, and freeze-dried at 60 °C under a vacuum. Carboxymethylcellulose sodium salt solvent was employed to suspend the LR extract. Animals received LR at 0.5 g/kg, 2.5 g/kg, and 5 g/kg concentrations by oral gavage according to the previous report[16].
SPF grade male Sprague-Dawley rats (batch no. C-NMG2021012507), aged 6-8 wk (initial body weight of 180-220 g), were obtained from Changsheng Biotechnology Co., Ltd (Shenyang, China). Rats were kept individually in the SPF standard animal room with 30%-40% humidity, 22-25 °C temperature, and a 12-h light/dark cycle. After adaptive feeding for 1 wk, rats were randomly assigned to six groups: control group; model group; LR-0.5 group; LR-2.5 group; LR-5 group; and metformin group (a clinical anti-diabetic drug). The HFHS diet (30% lard oil, 20% sucrose, and 50% standard diet) was fed to the diabetic model for 4 wk along with an injection of streptozotocin (STZ) (30 mg/kg)[17], whereas the control animals received a commercial standard diet. After modeling, rats in the control and dietetic groups received 0.5% carboxymethylcellulose sodium salt (Sigma), whereas rats in the LR and metformin groups received 0.5 g/kg, 2.5 g/kg, and 5 g/kg of LR extract and metformin (150 mg/kg) by oral administration once per day. All animals were given the treatments outlined for 4 wk. At the end of the experimental day, blood was collected from the retro-orbital sinus and centrifuged at 3500 rpm for 10 min at 4 °C. The supernatant was obtained for enzyme-linked immunosorbent assay and meta-bolomic analysis. The liver, kidney, and pancreas tissues were surgically removed from each rat for H&E staining. The Institutional Animal Care and Use Committee, Inner Mongolian University for Nationalities examined and approved all experimental protocols (Approval No. NM-LL-2021-06-15-1).
One gram of LR powder was accurately weighed and placed in a 50 mL conical flask. Then 20 mL of methanol solution was added for 30 min ultrasonic extraction, and the liquid was cooled and weighed. Swertiamarin (2 mg), sweroside (1 mg), hesperetin (1 mg), coumarin (4.9 mg), 1.7-dihydroxy-3,8-dimethoxyl xanthone (1 mg), and 1-hydroxy-2,3,5 trimethoxanthone (1 mg) were carefully weighed and put into a 10 mL flask, dissolved in methanol and diluted to scale, shaken well, and filtered through a 0.45 µm microporous filter membrane (each 1 mL contained 0.2 mg swertiamarin, 0.1 mg sweroside, 0.1 mg hesperidin, 0.49 mg coumarin, 0.1 mg 1,7-dihydroxy-3,8-dimethoxone, and 0.1 mg 1-hydroxy-2,3,5-trimethoxone). High performance liquid chromatography (HPLC) analysis was performed on an Agilent 1260 InfinityII HPLC system. ZORBAX SB-C18 5-Micron column (4.6 mm × 250 mm) with mobile phase water (A) - 0.1% phosphate aqueous solution (B) and gradient elution (0-15 min, 30%-35% B; 15 to 25 min, 35%-50% B; 25 to 35 min, 50%-65% B; 35 to 45 min, 65%-70% B; 45 to 50 min, 70%-80% B; 50 to 55 min, 80%-95%; 55-60 min, 95%-100%). The flow rate was set to 1.0 mL/min, the column temperature was set to 30 °C, and the detection wavelength was set at 234 nm. Standards of six compounds were purchased (Sigma-Aldrich, St. Louis, MO, United States), and calibration curves were performed.
An automated biochemical analyzer (Ichem-340; Icubio, Shenzhen, China) was applied to examine the serum levels of TC, TG, HDL-C, and LDL-C. GLP-1 and insulin were quantified using enzyme-linked immunosorbent assay kits (Bioswamp, Wuhan, China) according to the manufacturer’s instructions. Herein, 450 nm was used to measure the absorbance of 100 μL of serum in this experiment.
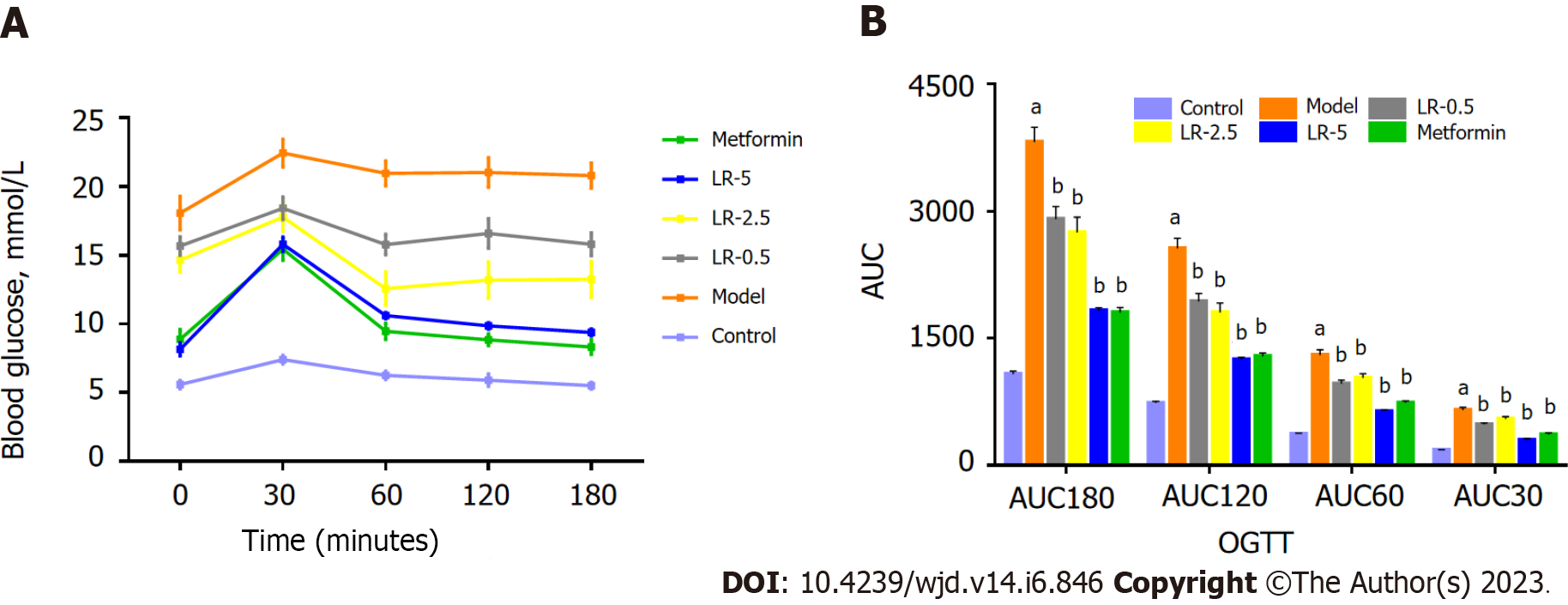
Rats were fasted for 12 h, after which BG levels were determined using glucometer by obtaining a blood sample from the tail vein at 0 min. Subsequently, the rats were orally administered glucose at 2 g/kg body weight, and BG levels were recorded at 30, 60, 120, and 180 min post-administration. The trapezoidal formula was applied to calculate BG levels to compute the area under the curve (AUC). The value of BG at x minutes was denoted by BG (x), and the AUC was determined using the following formula: oral glucose tolerance test (OGTT) calculation formula: AUC = 0.5 × (BG0 + BG30)/2 + 0.5 × (BG30 + BG60)/2 + 1 × (BG60 + BG120)/2 + 1 × (BG0120 + BG180)/2.
The right liver lobe, kidney tissues, and pancreatic biopsy specimens were embedded with formalin at a concentration of 4% and prepared into paraffin slices measuring 3-5 μm thick. Before being examined under an Olympus microscope equipped with a CCD camera (DS-U3; Nikon, Tokyo, Japan), the tissue sections were stained with H&E. A photographic examination software program (Eclipse E100; Nikon) was utilized for microscopic analysis at × 40 magnification.
The serum samples were analyzed for untargeted metabolite profiles using the XploreMET™ (Metabo-Profile Biotechnology, Shanghai, China). A time-of-flight mass spectrometry system (Pegasus HT; LECO Corp., St. Joseph, MI, United States) was used to assay the component with an Agilent gas chromatograph (GC), and a robotic online derivatization station was used to assay the plasma components. The list of chemicals and reagents used in the metabolomic analysis is reported above. The process of analysis is briefly described in the following parts. Prior to processing, plasma samples were stored at -80 °C. After thawing the samples on ice, a metabolite extraction procedure was conducted. Initially, chloroform was removed from the metabolite extracts using a CentriVap vacuum concentrator. Subsequently, a Free Zone freeze dryer (Labconco, Kansas City, MO, United States) was employed to lyophilize the samples into a dry powder. Fifty milligrams of frozen serum samples were deposited in a microcentrifuge container with 25 mg of zirconium oxide beads and 10 µL of internal calibration standards. For automated homogenization, 50 μL of 50% prechilled methanol was added in each aliquot. After 20 min of centrifugation at 14000 g and 4 °C (Microfuge 20R; Beckman Coulter, Indianapolis, IN, United States), the supernatant was transferred carefully to an autosampler vial (Agilent Technologies, Foster City, CA, United States), dissipated to eliminate chloroform in a CentriVap vacuum concentrator, and then lyophilized utilizing a Free Zone freeze dryer (Labconco). The remaining samples were combined for quality control purposes. The desiccated sample was derivatized with 50 μL of methoxyamine (20 mg/mL in pyridine) at 30 °C for 2 h, then 50 μL of MSTFA (1% TMCS) containing FAMEs as retention indices were added at 37.5 °C for 1 h. The sample derivatization and GC-TOF/MS analysis were conducted with a robotic multipurpose sample with dual heads (Gerstel, Mülheim an der Ruhr, Germany).
After obtaining the raw data, the ChromaTOF software was used to automatically export the original GC-TOF/MS data to XploreMET (Metabo-Profile Biotechnology, Shanghai, China). This enabled programmed baseline denoising and smoothing, peak selection and deconvolution, the creation of a database of references from aggregated quality control samples, metabolite spectrum alignment, missing value rectification and imputation, metabolite verification, and data preprocessing (normalization and standardization). Then, all data was converted into comparable data matrices for statistical analysis. The standard deviation of the experimental measures was scaled and applied to each result, which was then mean-centered. The XploreMET software was used to carry out principal component analysis and orthogonal partial least-square discriminant analysis. The sum of squares of the partial least-squares weights was weighted using the value of the variable importance in the projection. The Kyoto Encyclopedia of Genes and Genomes looked at the metabolic process of many metabolites.
The acquired data have been represented as mean ± standard error of the mean. GraphPad prism 7.04 (La Jolla, CA, United States) was used to conduct the statistical analyses. A one-way analysis of variance was applied to the data analysis, and the LSD Multiple Comparison Test was used to evaluate treatment differences. P < 0.05 was considered statistically significant, while P < 0.10 indicated a trend. Data of differentially expressed metabolites were considered to be statistically significant when a variable was variable importance in the projection ≥ 1.2 and a P < 0.05. Univariate statistical analysis (Student’s t-test) was used to analyze differential metabolites.
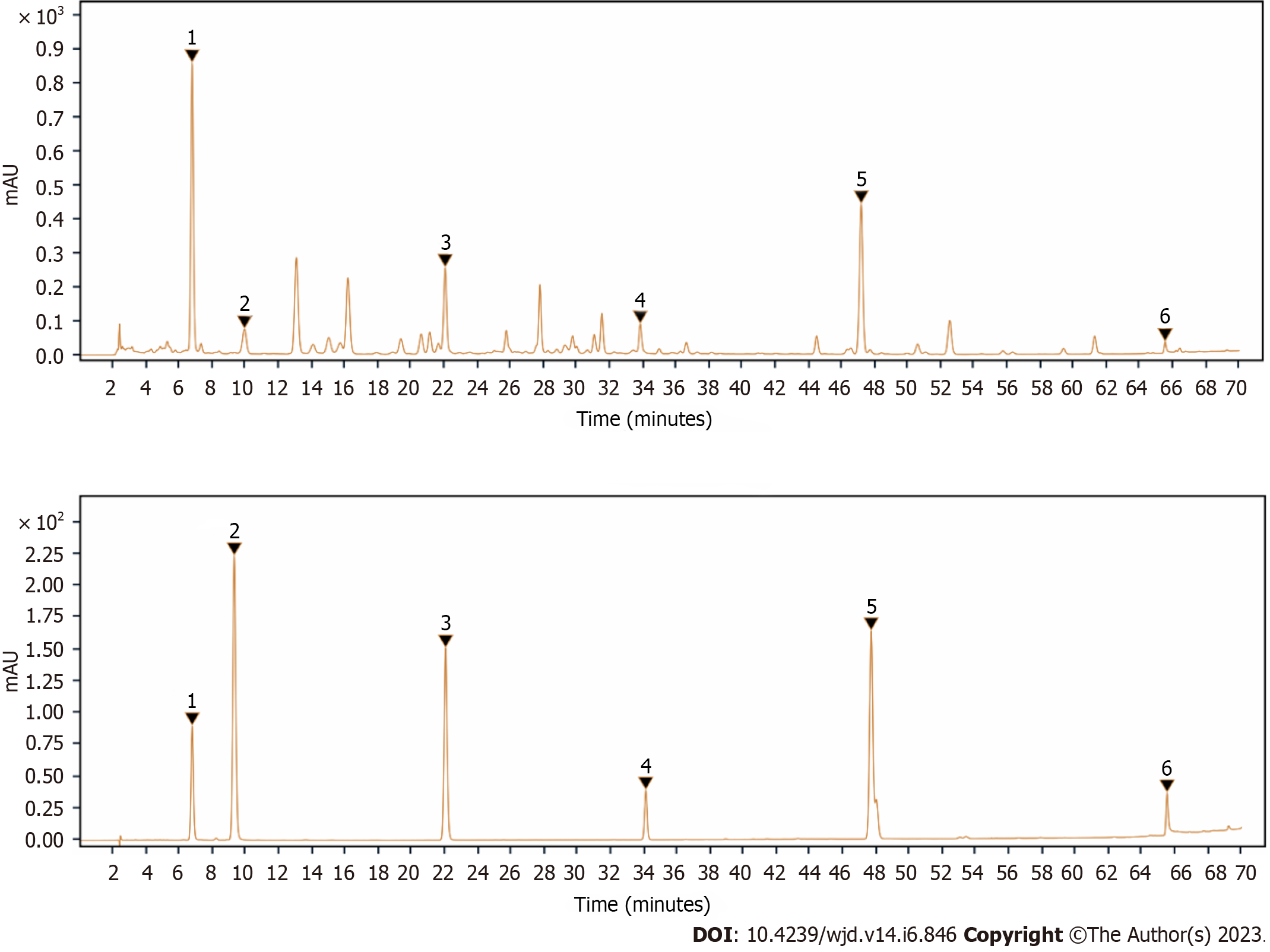
Herein, six bioactive compounds were identified by HPLC analysis as the main bioactive constituents in LR, including swertiamarin, sweroside, hesperetin, coumarin, 1.7-dihydroxy-3,8-dimethoxyl xanthone, and 1-hydroxy-2,3,5 trimethoxanthone with inclusion of 91.10, 6.09, 7.65, 3.04, 29.28, and 3.70 mg/g of dry mater, respectively (Figure 1 and Table 1).
| Active compound | Regression equation | R2 | Linear range, μg | LR extract, mg/g dry mater |
| Swertiamarin | Y = 99.413X + 6.3517 | 1 | 1.953-9.826 | 91.10 |
| Sweroside | Y = 198.81X + 0.8476 | 1 | 0.431-2.154 | 6.09 |
| Hesperetin | Y = 437.89X + 0.0685 | 1 | 0.119-1.002 | 7.65 |
| Coumarin | Y = 365.15X + 0.0103 | 1 | 0.118-0.590 | 3.04 |
| 1.7-dihydroxy-3.8-dimethoxyxanthone | Y = 214.16X + 2.3704 | 0.9999 | 0.098-0.494 | 29.28 |
| 1-hydroxy-2,3,5 trimethoxanthone | Y = 90.498X + 7.1204 | 0.9999 | 2.026-10.305 | 3.70 |
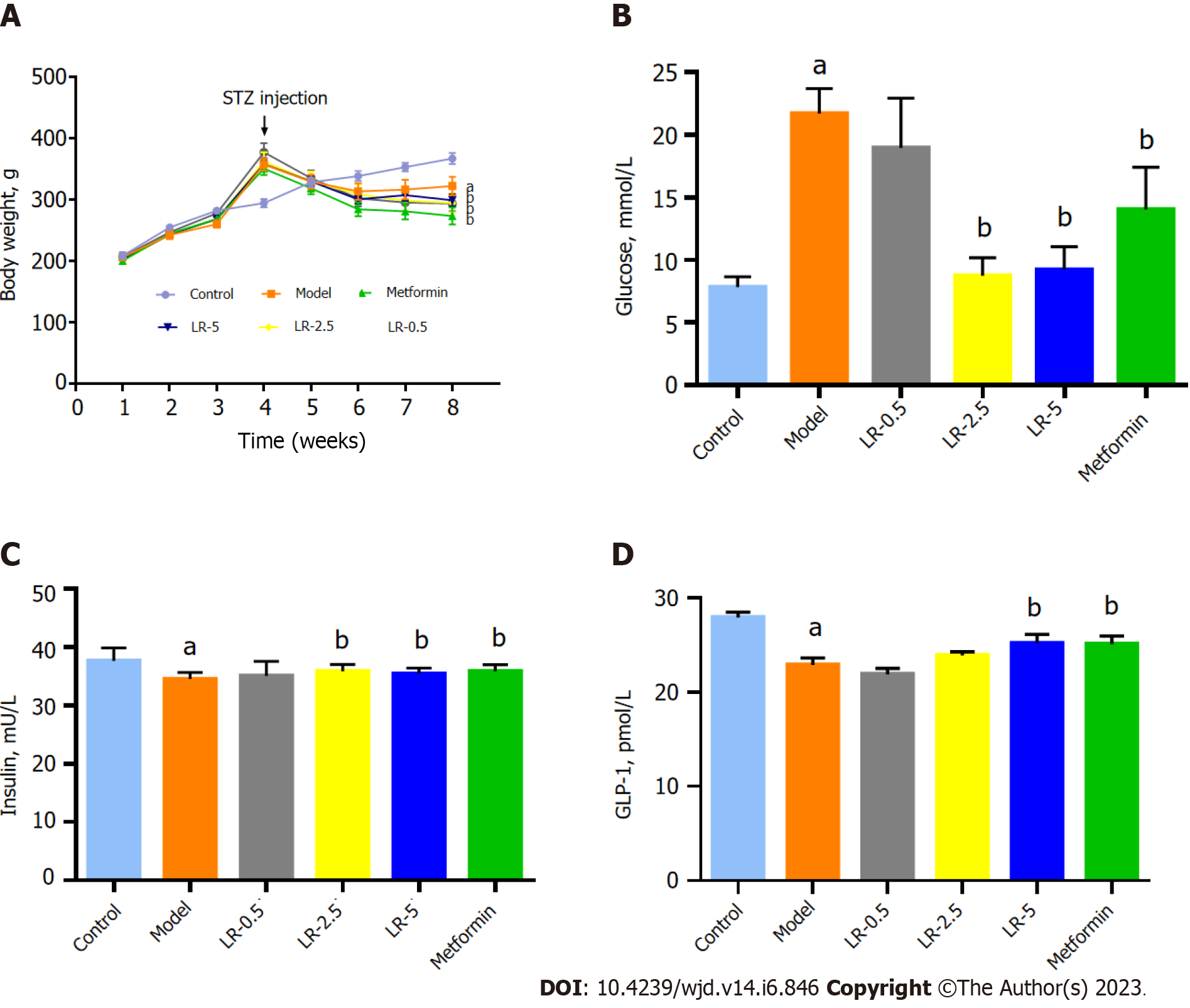
As shown in Figure 2A, the body weight was significantly increased in the HFHS diet-fed mice, while STZ injection sharply decreased the body weight in contrast to those in the control group. LR administration and metformin treatment groups significantly reduced (P < 0.05 and P < 0.01 respectively) the body weight of mice in comparison with those in the model group. In addition, compared with the control group, BG in the model group was significantly increased (P < 0.01), whereas LR at 2.5 g/kg and 5 g/kg doses and metformin treatments significantly decreased the serum BG level (P < 0.05, Figure 2B). The serum insulin level in the model group was significantly higher (P < 0.001) than in the normal group. In comparison to the diabetic model group, LR at 2.5 g/kg and 5 g/kg doses, as well as metformin treatments, significantly (P < 0.05) increased serum insulin concentrations (Figure 2C). In contrast, the level of GLP-1 was significantly reduced in the model group by comparison with the control group (P < 0.001), but metformin and LR at a dose of 5 g/kg enhanced the serum GLP-1 secretion significantly (P < 0.05) as shown in Figure 2D.
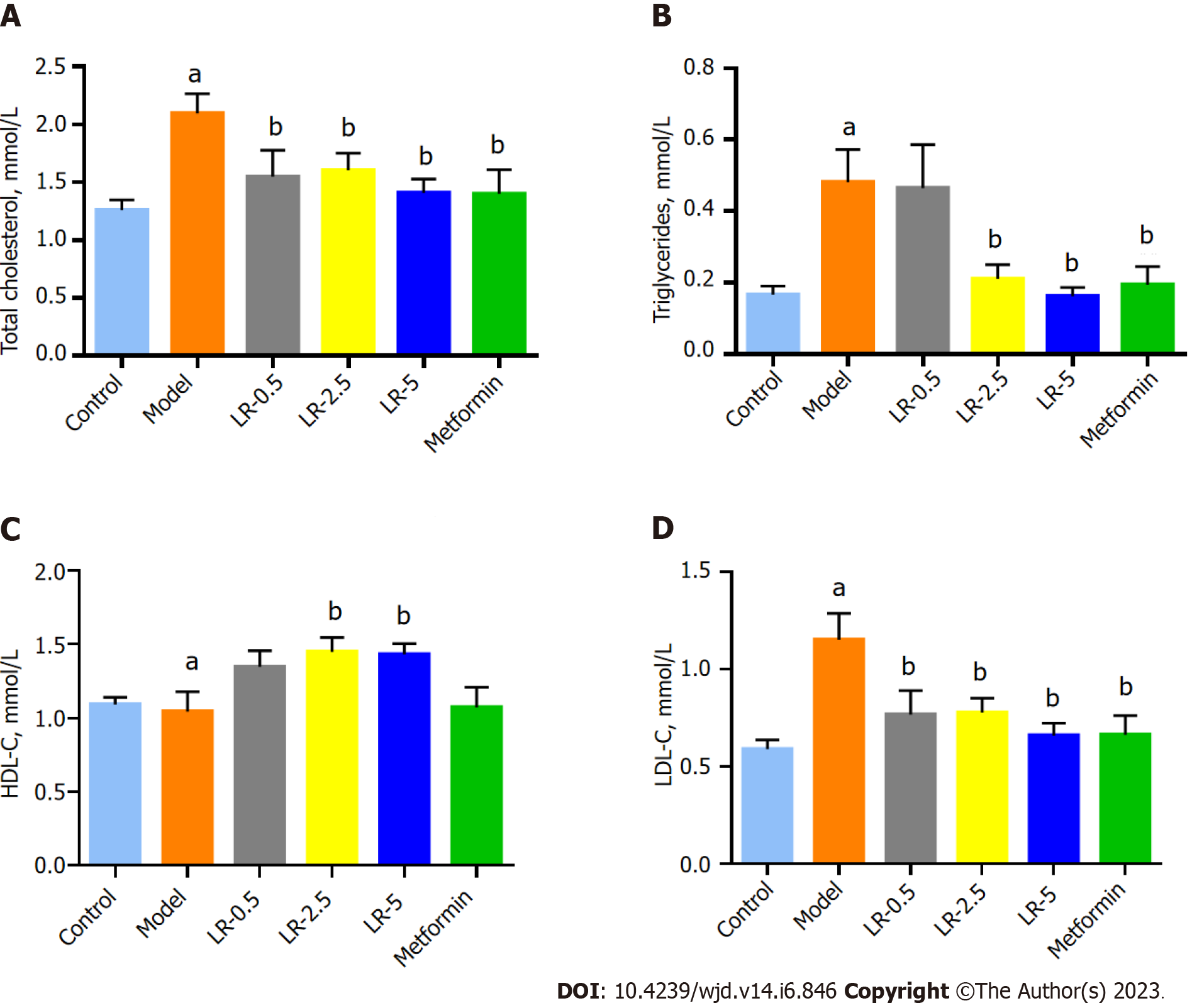
The TC and TG levels of the model group were significantly higher (P < 0.001) than the control group, whereas serum TC content was significantly lower (P < 0.05) in the three LR groups and the metformin group. TG levels were significantly lower in the LR-2.5 and LR-5 groups and the metformin group compared to the diabetic model group (Figure 3A and B). Although the level of HDL-C was not changed in the comparison between the control and model groups, LR treatment at 2.5 g/kg and 5 g/kg doses significantly elevated (P < 0.05) the serum concentration of HDL-C compared to the model group (Figure 3C). In terms of serum LDL-C levels, diabetic model animals had significantly higher (P < 0.05) LDL-C levels than control animals, whereas the three LR treatment groups and the metformin group had significantly lower (P < 0.01) serum LDL-C levels than the model group (Figure 3D).
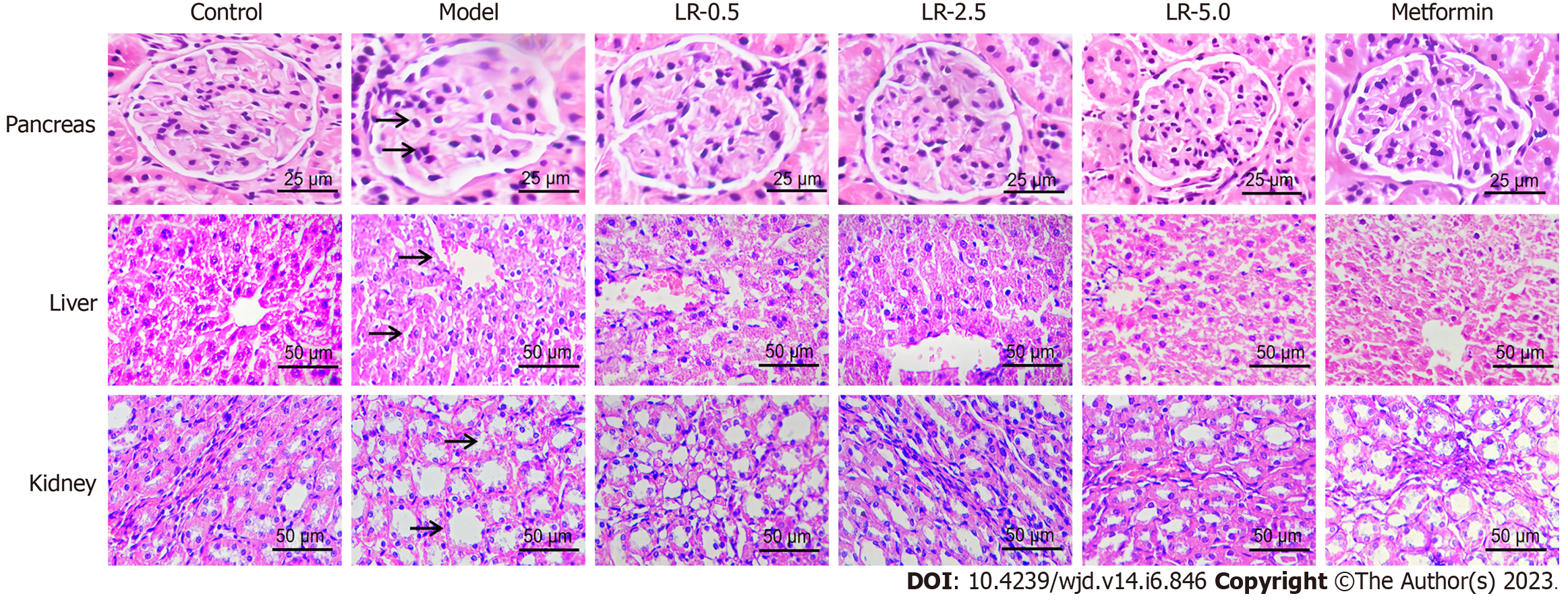
Figure 4 shows that the HFHS diet and STZ-induced diabetic rats had extensive granulation of the β cells and severe vacuolation of the pancreatic islets, while the control rats had normal pancreatic β cells in the islets of Langerhans and the acini. Histological tissue characteristics in the LR and metformin treatment groups showed reduced cell granulation and decreased pancreatic islet vacuolation compared to the diabetic model group. On the other hand, the structure of the liver lobule was complete in the control animals, and cells were organized radially around the central blood vessel. Diabetic model rats had evident macrovesicular steatosis of liver cells. In the LR and metformin groups, the hepatic lobule structure was restored, and the degree of steatosis was significantly lower than in the model group. H&E staining of the kidney sections indicated that the diabetic rats had more visible renal lesions, including glomerular hypertrophy, increased glomerular mesangial cells, and more severe mesangial matrix damage than the control group. The LR and metformin treatments significantly alleviated pathological renal damage in the kidneys of diabetic rats.
In the first 30 min after glucose was given, BG levels were much lower in the LR and metformin groups than in the diabetic group (Figure 5A). Additionally, Figure 5B shows that both LR and metformin treatment significantly improved the AUC values at 30 min, 60 min, 120 min, and 180 min.
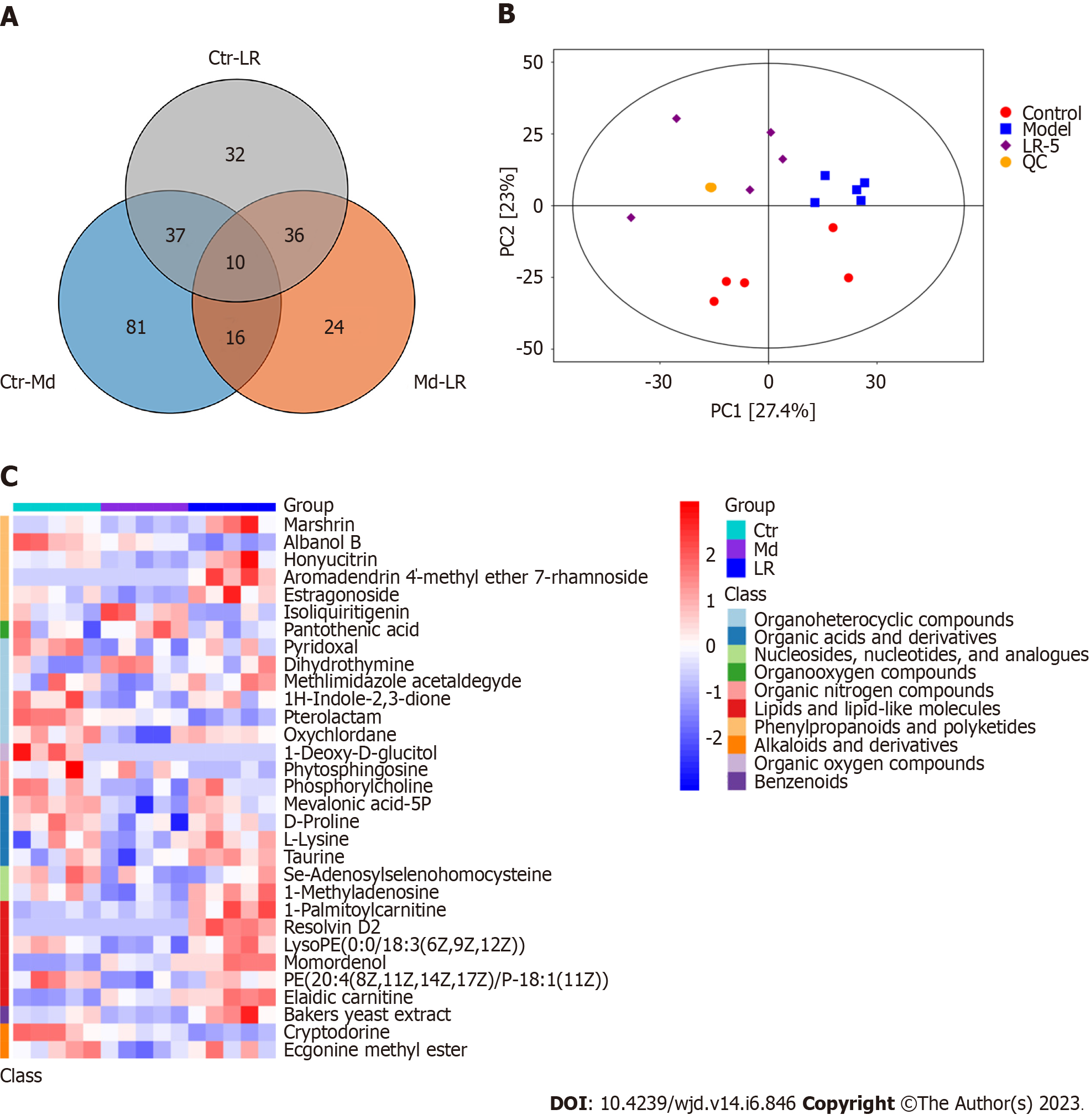
Using untargeted metabolomics, a total of 236 metabolite annotations were determined in the serum samples. Among these, significantly deferentially expressed metabolites mainly include alkaloids and derivatives, lipids and lipid-like molecules, organoheterocyclic compounds, and organic acid and their derivatives. The results of the principal component analysis indicated that the metabolic profiles of the three experimental groups differed significantly, as reflected by the variations between the three sample groups. Furthermore, the quality control sample distances were found to be extremely close, suggesting a high degree of sample data reliability (Figure 6A-C).
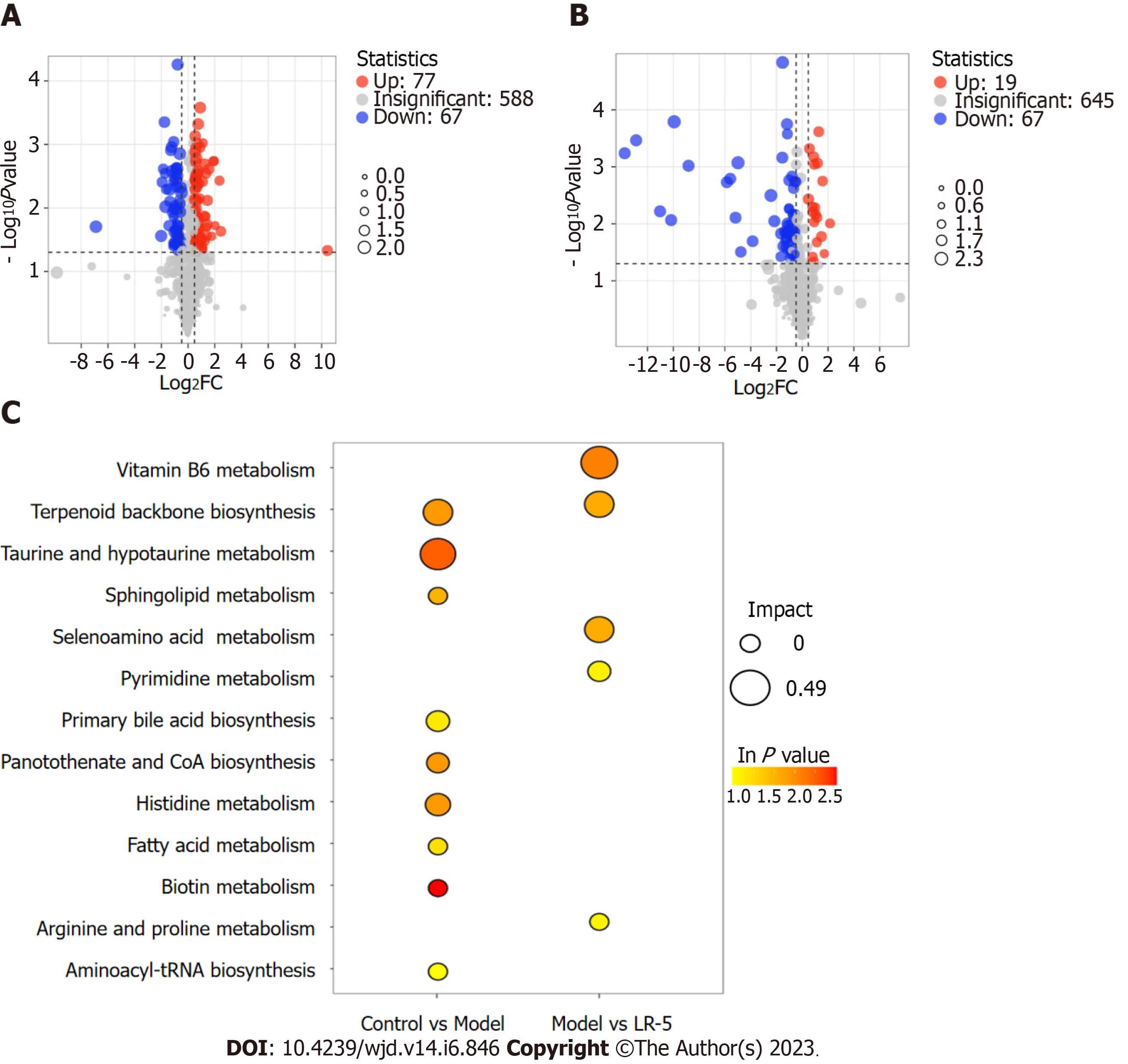
A metabolomic analysis of the differentially expressed metabolites in serum tissues of diabetic rats and the subsequent volcano plot showed that 144 metabolites (67 downregulated and 77 upregulated) were expressed differently between the control and the model group, whereas a comparison of the model and LR-5 groups revealed 86 deferentially expressed metabolites (67 downregulated and 19 upregulated) (Figure 7A and B). According to the order of influencing factors in the LR group comparison, the top 13 metabolic pathways were selected, as shown in Figure 7C, including vitamin B6 metabolism and biosynthesis of terpenoids, taurine and hypotaurine metabolism, lipid metabolism scabbard of taurine, selenium metabolism of AA metabolism, pyrimidine, the original generation of bile acid biosynthesis, pantothenic acid salt and histidine biosynthesis and metabolism of coenzyme A, fatty acid, biotin, arginine and proline, and aminoacyl-tRNA biosynthetic pathway. In the LR group, the metabolic pathway of vitamin B6 was the most influential factor, followed by the metabolic pathway of terpenoid backbone biosynthesis, selenium AA, pyrimidine, arginine, and proline.
The representative differential metabolites obtained from the major altered pathways are shown in Table 2. The levels of mevalonic acid-5P, D-proline, L-lysine, taurine, pyridoxal, marshrin, honyucitrin, isoliquiritigenin, 1H-indole-2,3-dione, oxychlordane, phosphorylcholine, Se-adenosylselenohomocysteine, 1-methyladenosine, LysoPE[0:0/18:3(6Z,9Z,12Z)], PE[20:4(8Z,11Z,14Z,17Z)/P-16:0], Bakers yeast extract, and ecgonine methyl ester showed a significant decrease in the model group in comparison with the control group. By contrast, LR treatment dramatically increased the above metabolites in the serum samples. Moreover, the levels of dihydrothy, pantothenic acid, and aromadendrin 4’-methyl ether 7-rhamnoside were greatly elevated in the model group than in the control group. Nevertheless, LR obviously reduced the levels of these metabolites. The results indicated that most of the metabolites were reversed by LR extract treatment and were regulated to return to levels that were comparable to those of the control group.
| No. | Metabolites | Control group vs model group | Trend | P value | Model group vsLomatogonium rotatum | Trend | P value | ||
| VIP | FC | VIP | FC | ||||||
| 1 | Marshrin | 1.46 | 1.16 | Decreased | 0.016 | 1.59 | 0.68 | Increased | 0.034 |
| 2 | Honyucitrin | 1.67 | 1.23 | Decreased | 0.002 | 1.54 | 0.68 | Increased | 0.047 |
| 3 | Isoliquiritigenin | 1.60 | 1.75 | Decreased | 0.004 | 1.79 | 0.42 | Increased | 0.018 |
| 4 | Pyridoxal | 1.53 | 1.33 | Decreased | 0.029 | 1.73 | 1.47 | Increased | 0.055 |
| 5 | 1H-indole-2,3-dione | 1.21 | 4.08 | Decreased | 0.019 | 1.17 | 0.34 | Increased | 0.039 |
| 6 | Oxychlordane | 1.41 | 1.10 | Decreased | 0.019 | 1.30 | 0.92 | Increased | 0.039 |
| 7 | Phosphorylcholine | 1.55 | 1.25 | Decreased | 0.009 | 1.26 | 0.84 | Increased | 0.045 |
| 8 | Mevalonic acid-5P | 1.32 | 2.16 | Decreased | 0.001 | 1.03 | 0.56 | Increased | 0.010 |
| 9 | D-proline | 1.37 | 1.36 | Decreased | 0.003 | 1.80 | 1.39 | Increased | 0.019 |
| 10 | L-lysine | 1.40 | 1.50 | Decreased | 0.006 | 1.71 | 2.42 | Increased | 0.002 |
| 11 | Taurine | 1.67 | 1.48 | Decreased | 0.017 | 1.61 | 1.59 | Increased | 0.006 |
| 12 | Se-adenosylselenohomocysteine | 1.25 | 1.50 | Decreased | 0.029 | 2.24 | 2.35 | Increased | 0.001 |
| 13 | 1-methyladenosine | 1.47 | 1.46 | Decreased | 0.019 | 1.81 | 0.58 | Increased | 0.001 |
| 14 | LysoPE[0:0/18:3(6Z,9Z,12Z)] | 1.64 | 1.45 | Decreased | 0.003 | 1.80 | 0.63 | Increased | 0.002 |
| 15 | PE[20:4(8Z,11Z,14Z,17Z)/P-16:0] | 1.80 | 1.68 | Decreased | 0.001 | 1.70 | 0.72 | Increased | 0.001 |
| 16 | Bakers yeast extract | 1.51 | 1.17 | Decreased | 0.012 | 1.74 | 0.70 | Increased | 0.016 |
| 17 | Ecgonine methyl ester | 1.48 | 1.10 | Decreased | 0.013 | 1.23 | 0.92 | Increased | 0.033 |
| 18 | Dihydrothy | 1.66 | 0.79 | Increased | 0.013 | 1.59 | 0.79 | Decreased | 0.048 |
| 19 | Pantothenic acid | 1.78 | 0.65 | Increased | 0.028 | 1.97 | 0.61 | Decreased | 0.024 |
| 20 | Aromadendrin 4’-methyl ether 7-rhamnoside | 1.35 | 1.60 | Increased | 0.030 | 1.66 | 2.22 | Decreased | 0.007 |
Obesity-related disorders, specifically T2DM, have become one of the world’s greatest health concerns. According to multiple studies, a disturbance in energy metabolism is the primary risk factor for the development of T2DM. Current clinical applications have recommended single-target medications; however, overcoming the problems with these drugs has been difficult. As a result, traditional medicines with the advantages of multitargets and multimechanisms could be potential treatments for T2DM. LR is a bitter medicinal herb in traditional Mongolian medicine used for bodyweight reduction. However, the pharmacological effects of LR and its specific metabolic changes on T2DM are not entirely understood. Biological cells respond to a disease state by changing the concentration of a large number of metabolites to maintain homeostasis[18]. In this study, serum metabolic profiles were generated using ultra-HPLC, and the potential mechanisms of LR in T2DM were examined.
Herein, the HFHS diets plus STZ is a relatively stable method for modeling T2DM. According to the results, the HFHS-induced rats had increased body weight and considerably elevated TC, TG, and LDL-C plasma levels compared with normal rats. Additionally, increased glucose tolerance significantly impaired the plasma levels of glucose, GLP-1, and insulin. This was confirmed that an HFHS diet causes weight increase, insulin sensitivity, and impaired glucose tolerance[19]. Therefore, the HFHS-induced animal model indicated a typical obesity phenotype.
According to an HPLC analysis, six main compounds were identified from the LR extract. The most abundant components were swertiamarin, hesperetin, and coumarin, which have been previously documented with effects on obesity and hyperglycemia[20-22], making them the likely effectors of the pharmacological activities of the LR extract. The presence of xanthone, another key component in the LR extract, is known to have numerous pharmacological effects, including anti-inflammatory and antimycobacterial properties[23]. Nevertheless, its hypoglycemic action has yet to be investigated. In addition, experimental results showed that high doses and a medium dose of LR administration indicated similar outcomes as metformin. The LR administration significantly reduced the body weight in the model group and showed lower serum glucose and lipid contents. Several studies have reported that abnormalities of lipid contents in serum are highly related to hyperglycemia[24]. In this study, the levels of TC, TG, HDL-C, and LDL-C were significantly reversed after LR administration. TC, TG, LDL-C, and HDL-C are important biomarkers, which indicate hyperlipidemia. Moreover, lipid abnormalities drive the increase in lipid deposition[25]. Our findings indicated that LR may have a potent hypolipidemic effect by decreasing plasma levels of TC, TG, and LDL-C while elevating HDL-C levels. LR could have a positive effect on the control of hyperlipidemia.
Dyslipidemia is caused in part by a correlation between carbohydrate and lipid metabolism and aberrant BG levels[26]. Herein, glucose levels were significantly elevated in the serum, and the OGTT results indicated that diabetic rats developed impaired glucose tolerance. Regarding glucose metabolism, LR treatment lowered BG and greatly improved glucose tolerance. The reduction in BG by LR administration was associated with a significant improvement in glucose intolerance, as revealed by the decreased AUC value in the OGTT response. OGTT was usually used to assess peripheral insulin action and insulin resistance in vivo. The OGTT results were accompanied by insulin levels in serum. Insulin resistance is related to T2DM and is characterized by the decreased response of insulin-sensitive cells or tissues. It can cause impaired peripheral glucose consumption and develop hyperglycemia and compensatory hyperinsulinemia. Moreover, the plasma GLP-1 level was improved by LR treatment. GLP-1 is a hormone primarily produced in the L cells of the distal ileum and colon. It promotes insulin secretion while inhibiting glucagon synthesis. It also plays a significant role in glucose homeostasis and is a key biomarker of abnormalities in glucose metabolism[27]. The exposure of cultured gut endocrine cells to bitter substances stimulates the release of hormones, including GLP-1[28]. Therefore, LR administration significantly improved insulin sensitivity and GLP-1 secretion in diabetic rats. Taken together, the physiological results expressively revealed that LR administration had the effect of reducing obesity and improving lipid and carbohydrate metabolism.
Metabolomics is a high-throughput technology that has been widely used for identifying biomarkers, revealing metabolic pathways, and unraveling the mechanisms of metabolic diseases[29]. In this study, untargeted metabolomics technology was used to analyze serum metabolites and the metabolic pathways of LR administration and to explore its mechanism of lowering BG and anti-diabetic action. Our findings revealed that the metabolic pathway of vitamin B6 was the most influential factor, followed by terpenoid backbone biosynthesis, selenium AA, pyrimidine, arginine, and proline. Metabolites such as pyridoxal, mevalonic acid-5P, proline, lysine, and taurine have been well reported on the regulation of T2DM, dyslipidemia, inflammation, and oxidative stress[30-32]. In addition, LR administration promoted energy metabolism related to AA.
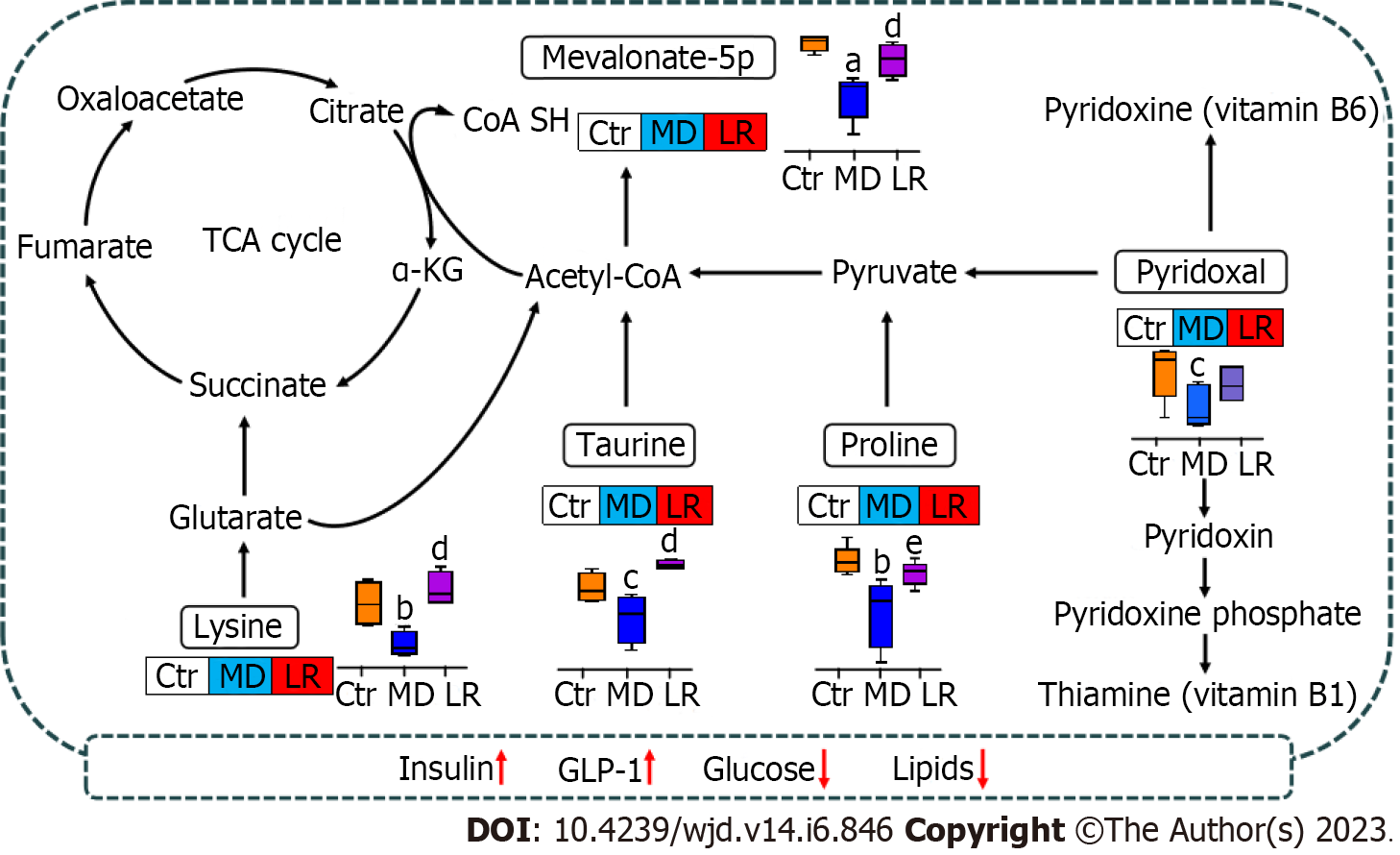
Recent studies reported AAs may be potentially important in the prevention of diabetes and diabetes-associated complications[33]. Protein and glucose metabolism are strongly interconnected and consequently regulated at the metabolic and molecular levels. AAs relate to glucose metabolism via gluconeogenesis, which is a catabolic breakdown of AAs. In metabolomics studies, two important potential biomarkers, i.e. D-proline and L-lysine, were identified.
Lysine supplements decreased diabetic complications linked with T2DM in the diabetic rat models and in vitro[34,35]. Lysine is an essential AA that plays a major role in calcium absorption, building muscle protein, and the body’s production of hormones, enzymes, and antibodies. Animal and human studies have shown that it has also demonstrated various beneficial effects in the treatment/prevention of diabetes and/or its complications. In diabetes-induced animal models, lysine has shown beneficial effects in lowering BG as well as acting as an inhibitor of protein glycation[36]. Lysine is known to react with glucose, with the glycated AA being excreted in the urine, and it has been shown to markedly minimize the glucose response to dietary carbohydrates without influence on insulin response[37]. Lysine could be catabolized to participate in energy metabolism. One mechanism involves the conversion of lysine to glutaryl-CoA, which is then converted to acetyl-CoA[38]. In the tricarboxylic acid cycle, lysine is metabolized to 2-ketoglutaric acid, which then forms succinate. Additionally, proline accelerates insulin secretion in both clonal β cells and isolated mouse islets[39,40].
In the current study, the elevated level of insulin in the LR group could be influenced by the high proline level. Moreover, proline could be converted to glutamate and metabolized to pyruvate, which is a key metabolite joining the tricarboxylic acid cycle[41]. Pyruvate metabolized to acetyl-CoA participates in the regulation of energy metabolism. Subsequently, the inappropriate glucogenic metabolism caused by the HFHS diet could be recovered by LR administration (Figure 8). In this view, LR administration has the potential to elevate lysine, and proline levels may help with diabetes management and blood sugar control.
Vitamin B metabolism was modified after LR administration, and the level of pyridoxal, a key metabolite, was restored in the LR groups. Vitamin B6 is an essential cofactor in various transamination, decarboxylation, glycogen hydrolysis, and synthesis pathways involving carbohydrate, sphingolipid, AA, heme, and neurotransmitter metabolism. The active form of vitamin B6, i.e. 5’-pyridoxine phosphate, is associated with protecting cells from DNA damage. 5’-pyridoxine phosphate acts as a coenzyme in about 160 enzymatic reactions, regulating the metabolism of glucose, lipids, AAs, heme, DNA/RNA, and many neurotransmitters[42]. Furthermore, the effect of vitamin B supplementation in preventing diabetic microvascular complications has long been the subject of study. Studies of vitamin B6 (pyridoxine, pyridoxine 50-phosphate) and high-dose vitamin B1 have shown that proteinuria can be inhibited in diabetic animal models[43]. In patients with T2DM and nephropathy, the combination of vitamin B1 (thiamine) and vitamin B6 (pyridoxine) significantly reduced the glycosylation of leukocyte nuclear DNA[44]. Addressing the vitamin B deficiency associated with diabetes that has been seen in experimental diabetes, particularly in tissues where vascular problems develop, may help to achieve the therapeutic advantage of vitamin B supplementation[45,46].
In this study, an HPLC method was used to identify swertiamarin, sweroside, hesperetin, coumarin, 1.7-dihydroxy-3,8-dimethoxyl xanthone, and 1-hydroxy-2,3,5 trimethoxanthone as the main chemical constituents of LR. Administration of LR extract for 4 wk in T2DM rats resulted in improvement in BG, glucose tolerance, TC, TG, and LDL-C, restoration of insulin and GLP-1 activity, and improvement in the histological properties of tissues and organs. The results suggested that the hypoglycemic effect of LR may be associated with alterations in serum metabolites, which in turn may facilitate insulin and GLP-1 activities, leading to a reduction in BG and lipid profiles.
Although Lomatogonium rotatum (LR) has a long history of usage as a hypoglycemic agent in Mongolian folk medicine, the evidence-based pharmacological properties and mechanisms of action of this medicinal plant have not yet been thoroughly explained.
The current study explored the hypoglycemic effects and mechanism of LR in a high-fat, high-sugar diet and streptozotocin-induced type 2 diabetic rat model.
The current study aimed to emphasize the hypoglycemic action mechanism of LR in a type 2 diabetic rat model and examine potential biomarkers to obtain mechanistic insight into the serum metabolite modifications.
A combination of feeding a high-fat, high-sugar diet and streptozotocin injections were applied to develop type 2 diabetes in rats. The high performance liquid chromatography technique was used to determine the chemical composition of LR. LR extract was given through oral gavage at doses of 0.5 g/kg, 2.5 g/kg, and 5 g/kg on a weekly basis for a period of 4 wk. The histopathological examination, as well as the assessment of blood glucose, insulin, glucagon-like peptide 1 (GLP-1), and lipid levels, were used to evaluate the anti-diabetic effects of LR extract. A method known as untargeted metabolomics was used in order to study the metabolites found in serum.
The primary active components found in LR included swertiamarin, sweroside, hesperetin, coumarin, 1.7-dihydroxy-3,8-dimethoxyl xanthone, and 1-hydroxy-2,3,5 trimethoxanone. When compared to the model group, the LR therapy resulted in a large increase in plasma insulin and GLP-1 levels while simultaneously resulting in a significant reduction in blood glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol, and an oral glucose tolerance test. Analysis of blood samples using an untargeted metabolomic approach found a total of 236 metabolites, of which 86 showed altered levels of expression in the model compared to the LR group. In addition, LR caused significant changes in the levels of metabolites such as vitamin B6, mevalonate-5P, D-proline, L-lysine, and taurine. These metabolites are involved in the regulation of the metabolic pathways for vitamin B6, selenium amino acids, pyrimidine, arginine, and proline.
These findings indicated that the hypoglycemic effect of LR may be associated with alterations in serum metabolites, which in turn may facilitate insulin and GLP-1 activities, leading to a reduction in blood glucose and lipid profiles.
Further research is required to confirm the levels of target gene or protein expression that are linked to the changed metabolic pathways and to demonstrate how LR extract lowers blood glucose at the molecular level.
| 1. | Jaishree V, Narsimha S. Swertiamarin and quercetin combination ameliorates hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced type 2 diabetes mellitus in wistar rats. Biomed Pharmacother. 2020;130:110561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Tsang MW. The management of type 2 diabetic patients with hypoglycaemic agents. ISRN Endocrinol. 2012;2012:478120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Cai MY, Yang Z, Huang XJ, Li J, Bao WY, Hurilebagen, Wulanqiqige, Wuyunsiriguleng, Cui JW, Ma LQ, Tong HY. Mongolian Medicine Areca Thirteen Pill (GY-13) Improved Depressive Syndrome via upregulating cAMP/PKA/CREB/BDNF signaling pathway. J Ethnopharmacol. 2022;293:115310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Bao TRGL. [The study of anti-obesity effect of bitter Mongolian Medicine Digeda on high-fat highenergy diets induced obese rats]. 2020. [DOI] [Full Text] |
| 5. | Yu HZ, Bao TRGL, Ba GN, Fu MH. [Study of dual-directional regulatory effect of Mongolian medicine Lomatogonium rotatum on gastrointestinal motility in mice]. Chin J Clin Pharmacol. 2022;38:2028-2033. [DOI] [Full Text] |
| 6. | Dai LL, Eni RG, Fu MH, Ba GN. Botanical, chemical, and pharmacological characteristics of Lomatogonium rotatum: A review. World J Pharmacol. 2022;11:6-15. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Wang ZW, Long P, Zhang CH, Wu GD, Zhang N. [HPLC Determination of Swertiamain and Swertisin in Herba Lomalogonium rotatum by HPLC]. Chin J of Exp Tradit Med Form. 2013;19:106-108. |
| 8. | Li YL, Suo YR, Liao ZX, Ding LS. The glycosides from Lomatogonium rotatum. Nat Prod Res. 2008;22:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ba GN, Bao ML, Wang XM, Wu YG, Ao WLJ, Wang XL. [Study on Quality Standard of Mongolian Medicine Lomatogonium rotatum]. J Med Pharm Chin Minor. 2018;24:40-42. [DOI] [Full Text] |
| 10. | Liu J, Zhao M, Zhu Y, Wang X, Zheng L, Yin Y. LC-MS-Based Metabolomics and Lipidomics Study of High-Density-Lipoprotein-Modulated Glucose Metabolism with an apoA-I Knockout Mouse Model. J Proteome Res. 2019;18:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Rong G, Weng W, Huang J, Chen Y, Yu X, Yuan R, Gu X, Wu X, Cai Y, Han P, Shao M, Sun H, Ge N. Artemether Alleviates Diabetic Kidney Disease by Modulating Amino Acid Metabolism. Biomed Res Int. 2022;2022:7339611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Nicholson JK, Lindon JC, Holmes E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2918] [Cited by in RCA: 2766] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 13. | Long J, Liu L, Jia Q, Yang Z, Sun Z, Yan C, Yan D. Integrated biomarker for type 2 diabetes mellitus and impaired fasting glucose based on metabolomics analysis using ultra-high performance liquid chromatography quadrupole-Orbitrap high-resolution accurate mass spectrometry. Rapid Commun Mass Spectrom. 2020;34: e8779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Allen J, Davey HM, Broadhurst D, Heald JK, Rowland JJ, Oliver SG, Kell DB. High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat Biotechnol. 2003;21:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, Nicholson JK. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1604] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 16. | Bao S, Wang X, Ma Q, Wei C, Nan J, Ao W. Mongolian medicine in treating type 2 diabetes mellitus combined with nonalcoholic fatty liver disease via FXR/LXR-mediated P2X7R/NLRP3/NF-κB pathway activation. Chin Herb Med. 2022;14:367-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc. 2021;1:e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 515] [Article Influence: 103.0] [Reference Citation Analysis (2)] |
| 18. | Lindon JC, Holmes E, Nicholson JK. So what's the deal with metabonomics? Anal Chem. 2003;75:384A-391A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Ambele MA, Dhanraj P, Giles R, Pepper MS. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 20. | Patel N, Tyagi RK, Tandel N, Garg NK, Soni N. The Molecular Targets of Swertiamarin and its Derivatives Confer Anti- Diabetic and Anti-Hyperlipidemic Effects. Curr Drug Targets. 2018;19:1958-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Yang H, Wang Y, Xu S, Ren J, Tang L, Gong J, Lin Y, Fang H, Su D. Hesperetin, a Promising Treatment Option for Diabetes and Related Complications: A Literature Review. J Agric Food Chem. 2022;70:8582-8592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Pan Y, Liu T, Wang X, Sun J. Research progress of coumarins and their derivatives in the treatment of diabetes. J Enzyme Inhib Med Chem. 2022;37:616-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Elsaman T, Mohamed MS, Eltayib EM, Abdalla AE, Mohamed MA. Xanthone: A Promising Antimycobacterial Scaffold. Med Chem. 2021;17:310-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes. 2008;116 Suppl 1:S4-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Yamabe N, Kim HY, Kang KS, Zhao Q, Matsumoto K, Yokozawa T. Effect of Chinese prescription Kangen-karyu on lipid metabolism in type 2 diabetic db/db mice. J Ethnopharmacol. 2010;129:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Adiels M, Taskinen MR, Borén J. Fatty liver, insulin resistance, and dyslipidemia. Curr Diab Rep. 2008;8:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Kaur N, Fernandez R, Sim J. Effect of Aloe vera on glycemic outcomes in patients with diabetes mellitus: a systematic review protocol. JBI Database System Rev Implement Rep. 2017;15:2300-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Bernard V, Lamothe S, Beau I, Guillou A, Martin A, Le Tissier P, Grattan D, Young J, Binart N. Autocrine actions of prolactin contribute to the regulation of lactotroph function in vivo. FASEB J. 2018;32:4791-4797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Ta N, A L, E E, Qi R, Mu X, Feng L, Ba G, Li Y, Zhang J, Bai L, Fu M. Metabolomics analysis reveals amelioration effects of yellowhorn tea extract on hyperlipidemia, inflammation, and oxidative stress in high-fat diet-fed mice. Front Nutr. 2023;10:1087256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 30. | Stach K, Stach W, Augoff K. Vitamin B6 in Health and Disease. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 31. | Jin Y, Yuan X, Liu J, Wen J, Cui H, Zhao G. Inhibition of cholesterol biosynthesis promotes the production of 1-octen-3-ol through mevalonic acid. Food Res Int. 2022;158:111392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1533] [Cited by in RCA: 1867] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 33. | Anuradha CV. Aminoacid support in the prevention of diabetes and diabetic complications. Curr Protein Pept Sci. 2009;10:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Sulochana KN, Punitham R, Ramakrishnan S. Beneficial effect of lysine and amino acids on cataractogenesis in experimental diabetes through possible antiglycation of lens proteins. Exp Eye Res. 1998;67:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Mirmiranpour H, Bathaie SZ, Khaghani S, Nakhjavani M, Kebriaeezadeh A. L-lysine supplementation improved glycemic control, decreased protein glycation, and insulin resistance in type 2 diabetic patients. Int J Diabetes Dev Ctries. 2021;41:634-643. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ, Banasadegh S. The improvement effect of L-Lys as a chemical chaperone on STZ-induced diabetic rats, protein structure and function. Diabetes Metab Res Rev. 2008;24:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Kalogeropoulou D, LaFave L, Schweim K, Gannon MC, Nuttall FQ. Lysine ingestion markedly attenuates the glucose response to ingested glucose without a change in insulin response. Am J Clin Nutr. 2009;90:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Matthews DE. Review of Lysine Metabolism with a Focus on Humans. J Nutr. 2020;150:2548S-2555S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 39. | Liu Z, Jeppesen PB, Gregersen S, Chen X, Hermansen K. Dose- and Glucose-Dependent Effects of Amino Acids on Insulin Secretion from Isolated Mouse Islets and Clonal INS-1E Beta-Cells. Rev Diabet Stud. 2008;5:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | McClenaghan NH, Barnett CR, Flatt PR. Na+ cotransport by metabolizable and nonmetabolizable amino acids stimulates a glucose-regulated insulin-secretory response. Biochem Biophys Res Commun. 1998;249:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Bakken IJ, White LR, Unsgård G, Aasly J, Sonnewald U. [U-13C]glutamate metabolism in astrocytes during hypoglycemia and hypoxia. J Neurosci Res. 1998;51:636-645. [PubMed] [DOI] [Full Text] |
| 42. | Percudani R, Peracchi A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep. 2003;4:850-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 412] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 43. | di Salvo ML, Contestabile R, Safo MK. Vitamin B(6) salvage enzymes: mechanism, structure and regulation. Biochim Biophys Acta. 2011;1814:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | Percudani R, Peracchi A. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics. 2009;10:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 45. | Polizzi FC, Andican G, Çetin E, Civelek S, Yumuk V, Burçak G. Increased DNA-glycation in type 2 diabetic patients: the effect of thiamine and pyridoxine therapy. Exp Clin Endocrinol Diabetes. 2012;120:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Okada M, Shibuya M, Yamamoto E, Murakami Y. Effect of diabetes on vitamin B6 requirement in experimental animals. Diabetes Obes Metab. 1999;1:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng JT, Taiwan; Yang J, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Xu ZH