Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.705
Peer-review started: December 27, 2022
First decision: February 20, 2023
Revised: March 3, 2023
Accepted: April 13, 2023
Article in press: April 13, 2023
Published online: June 15, 2023
Processing time: 169 Days and 17.9 Hours
The number of people diagnosed with diabetes continues to increase, especially among younger populations. Apart from genetic predisposition and lifestyle, there is increasing scientific and public concern that environmental agents may also contribute to diabetes. Food contamination by chemical substances that originate from packaging materials, or are the result of chemical reactions during food processing, is generally recognized as a worldwide problem with potential health hazards. Phthalates, bisphenol A (BPA) and acrylamide (AA) have been the focus of attention in recent years, due to the numerous adverse health effects associated with their exposure. This paper summarizes the available data about the association between phthalates, BPA and AA exposure and diabetes. Although their mechanism of action has not been fully clarified, in vitro, in vivo and epidemiological studies have made significant progress toward identifying the potential roles of phthalates, BPA and AA in diabetes development and progression. These chemicals interfere with multiple signaling pathways involved in glucose and lipid homeostasis and can aggravate the symptoms of diabetes. Especially concerning are the effects of exposure during early stages and the gestational period. Well-designed prospective studies are needed in order to better establish prevention strategies against the harmful effects of these food contaminants.
Core Tip: One of the most important steps in the prevention and control of diabetes and related disorders is the identification of potential risk factors. Phthalates, bisphenol A (BPA) and acrylamide (AA) are chemicals that are ubiquitously present in the environment and have the ability to act as contributing factors with adverse health effects. Human exposure to phthalates, BPA and AA mainly occurs through ingestion. This paper summarizes the available data about the association between phthalates, BPA and AA exposure and diabetes in order to examine the potential role of these contaminants in the development and progression of this complex disorder.
- Citation: Milanović M, Milošević N, Milić N, Stojanoska MM, Petri E, Filipović JM. Food contaminants and potential risk of diabetes development: A narrative review. World J Diabetes 2023; 14(6): 705-723
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/705.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.705
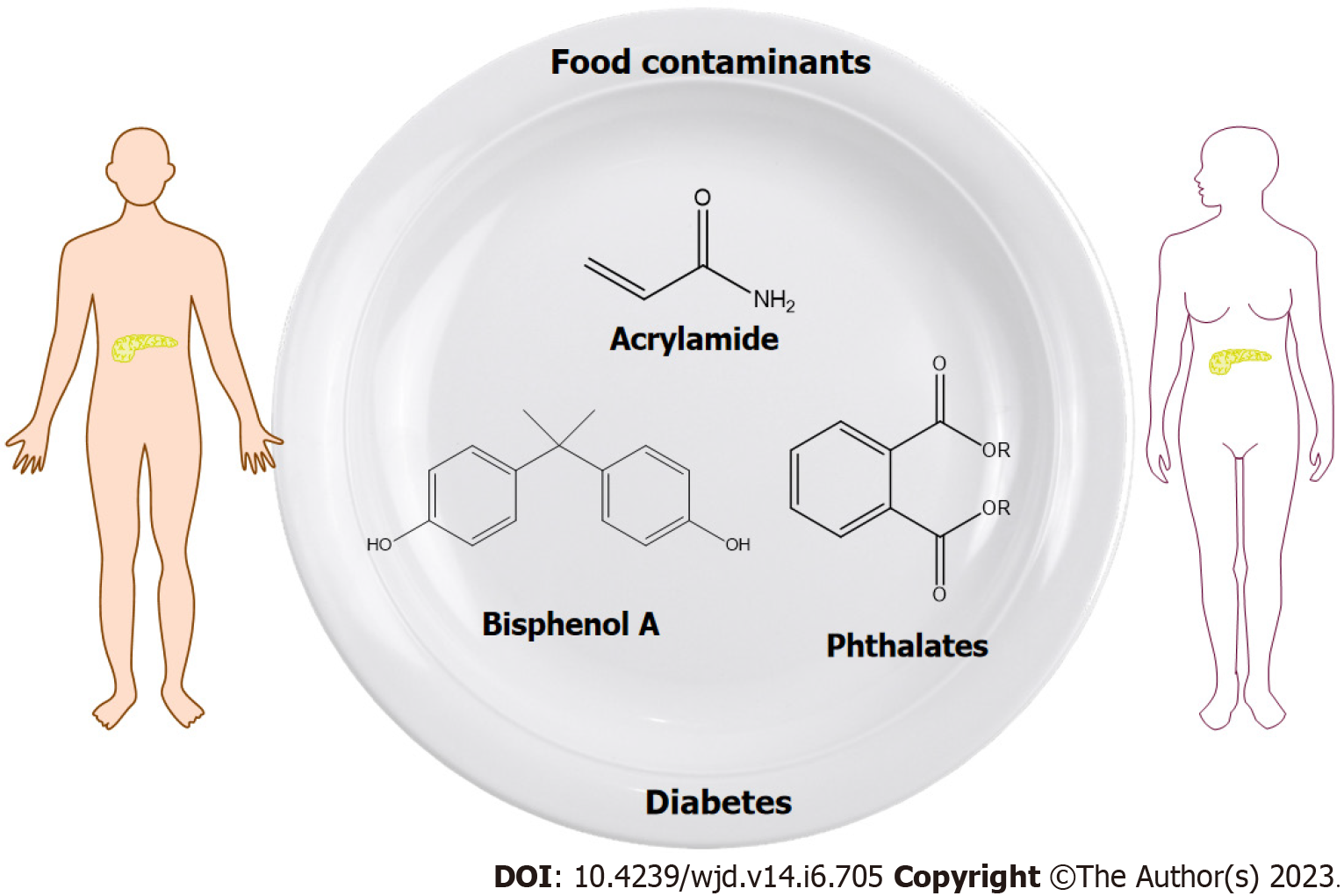
One of our basic human rights is “the right of everyone to have access to safe and nutritious food”[1]. According to the World Health Organization, more than 100 billion dollars is spent each year on medical expenses related to the consummation of unsafe food around the world[2]. Food contamination by chemical substances is generally recognized as an emerging worldwide challenge, with potential health hazards[3,4]. Moreover, diet has been identified as a main source of chemical intake[5]. Chemicals may enter the food chain via several pathways during cultivation, production, handling and processing, packaging, transportation and storage[4]. Numerous studies have confirmed the presence of a wide range of chemicals in drinking water, fruits, vegetables, cereals, meat and poultry, seafood, canned food, dairy products, baked goods, fast foods etc.[6-10]. For instance, humans are exposed daily to multiple chemicals, including environmental and processing contaminants, that may pose a threat to health even at very low concentrations[11]. The continuous ingestion of chemicals that migrate from food packaging, especially plastic packaging materials, or that are the result of chemical reactions during food processing, can lead to adverse health effects such as the development of diabetes.
Among the chemicals that originate from plastic packaging materials, endocrine disrupting chemicals (EDCs) have attracted public attention due to their possible harmful health effects[12]. The Endocrine Society classified EDCs as “a serious public health risk” and since then, data demonstrating their negative effects on human health has been constantly increasing. To date, more than 1400 chemicals have been identified as potential EDCs[13]. EDCs are xenobiotics that interfere with normal endocrine function, which consequently lead to adverse health outcomes[14-17]. Phthalic acid esters (PAEs) and bisphenol A (BPA) are well-known EDCs that are found practically “everywhere” in human societies, and have been the focus of scientific and public attention in recent years.
Among the chemical substances that are inadvertently generated during food preparation, acrylamide (AA) has raised public health concerns since it was first detected in 2002. Over the past twenty years, AA has been recognized as a “potential human carcinogen”, an emerging food contaminant and potential EDC[18,19]. Based on the above, exposure to PAEs, BPA and AA has been associated with a range of adverse health outcomes. Considering that ingestion is the main route of exposure, the objective of this paper is to review the current data concerning the links between PAEs, BPA and AA exposure and diabetes, in order to better understand the potential roles of these compounds in the development and progression of this complex disorder (Figure 1).
A century after the discovery of insulin, diabetes has been transformed from a fatal disorder into a chronic condition[20]. Today, the number of people diagnosed with diabetes continues to increase exponentially and it has been predicted that by 2045 more than 780 million people will have diabetes; with type 2 diabetes (T2D) representing approximately 90% of the total number of cases. It is believed that as many as half of the total number of cases remains undiagnosed, especially in low-income and middle-income countries[21] and that diabetes and its complications have resulted in more than 6.5 million lost lives over the last year alone[21]. In the United States, it is estimated that the non-health costs of diabetes per person per year surpass the costs of heart diseases[22]. Therefore, recognition of potential risk factors is one of the most important steps in the establishment of efficient strategies for the prevention and control of diabetes and related diseases that will consequently reduce the burden on the healthcare system and society.
Diabetes is a chronic disease, associated with a range of metabolic abnormalities. The clinical manifestations of diabetes includes increased serum glucose levels, which are a consequence of insulin deficiency and/or insulin resistance[23]. Type 1 diabetes (T1D) refers to a chronic autoimmune disease characterized by the loss of pancreatic β-cells, which leads to a total lack of insulin secretion and results in elevated blood glucose levels[24,25]. Although the development of T1D is associated with a genetic predisposition, environmental agents (single compounds or mixtures of compounds) can activate autoimmune mechanisms involved in the development of this multi-factorial disorder, through mechanisms that are not completely understood[26]. Insulin resistance is identified as a “key player” in the development and progression of T2D[27]. T2D is known as “adult-onset diabetes”, and develops as a result of increased insulin resistance to a level where overproduction of insulin can no longer cope with insulin insensitivity, leading to β-cell dysfunction[28]. In addition, several other non-communicable disorders are associated with insulin resistance, such as obesity, metabolic syndrome, non-alcoholic fatty liver disease, polycystic ovary syndrome, cardiovascular disease and cancers[20]. However, there is a growing amount of data that also supports a role for food contaminants, such as PAEs, BPA and AA in the onset of diabetes and the development of related conditions.
PAEs are one of the most commonly used plasticizers and additives in a wide-range of products, such as food packaging, detergents, cosmetics, toys, medical tubing, blood-storage containers, and home furnishings. Due to the ability of phthalates to improve the mechanical properties of polymers (e.g., polyethylene, polyethylene terephthalate, polyvinyl acetate and polyvinyl chloride), it is predicted that approximately 500 million tons of PAEs will be produced worldwide by 2050[12,29-31]. Some of the most frequently used PAEs are dimethyl phthalate, diethyl phthalate (DEP), di-n-butyl phthalate (DBP), diisobutyl phthalate (DiBP), di-n-hexyl phthalate, bis (2-ethylhexyl) phthalate (DEHP), diisononyl phthalate, di-n-octyl phthalate and benzylbutyl phthalate[30,32]. Because of their large production volume and widespread applications, these PAEs are omnipresent contaminants[33]. Since PAEs are weakly bound to plastic polymers, they are easily released into the surrounding environment (i.e., in food, water, air, soil) during production, storage, use and disposal of plastic-based products[34]. Because of this, PAEs can be frequently detected in different biological and environmental matrices such as urine, blood, air, soil, sediment, food, surface water and even drinking water[35-40]. The bioaccumulation and biodegradation potential of PAEs is dependent on their physico-chemical properties, which consequently determine their behavior and fate in the environment and their toxicity[41]. Phthalates are associated with negative effects on human health, including obesity, dyslipidaemia, T2D, impaired thyroid function, breast and uterine cancer, endometriosis and low birthweight[42-48]. Upon entering the food chain, the main route of humane exposure to phthalates is by ingestion. In the European Union, it is forbidden to use phthalate-containing materials for infant food and goods which contain high amounts of fats, such as dairy products. Moreover, since January 2022, plastic packaging for fruits and vegetables has been banned in France[49]. DEHP has been estimated as “safe” under 4.8 mg/kg body weight per day (no-observed-adverse-effect level) while the tolerable daily intake (TDI) is 0.05 mg/kg body weight per day[50]. However, data concerning PAE contamination levels in different components of the human diet, especially with respect to vulnerable populations, remains scarce and limited. Hence, PAE-related health risks cannot be neglected even at the “safe dose” exposure levels defined by regulators. Considering that PAEs show additive effects, particular attention must also be given to the potential synergistic effects of mixtures of EDCs[51].
Research status: As EDCs, PAEs have the ability to modulate the activity of multiple nuclear receptors, such as estrogen receptors (ERα and ERβ), androgen receptor (AR), peroxisome proliferator-activated receptors (PPARα and PPARγ), thyroid hormone receptors (TRα and TRβ) and the pregnane X receptor[15,52,53]. In order to understand the connection between PAEs and diabetes, “the dose makes the poison” approach cannot be applied[54]. Although phthalate exposure or mixed exposure with BPA had no influence on T1D development in non-obese mice, a mixture of PAEs and BPA decreased the release of tumor necrosis factor α (TNFα), interleukins (IL-4, IL-6, IL-10) and interferon γ in splenocytes and pancreatic lymphocytes and caused impairment of the immune system[55]. A significant association between PAE exposure and diabetes was probably not observed, due to the use of PAEs in high doses. PAEs as EDCs show non-monotonic effects[56]. Estrogenic compounds in high doses trigger insulin secretion in β-cells, and thus postponed the development of diabetes in non-obese mice[57]. In contrast, administration of DEHP at low levels caused the onset of diabetes symptoms (decrease in serum insulin levels and liver glycogen and an increase in blood glucose levels) followed by thyroid and adrenocortical dysfunction in rats[58]. After oral intake, PAEs undergo two metabolic steps. Short-branched phthalates are hydrolysed into monoester metabolites (mPAEs) and extracted via urine; while after several biotransformation steps in the first phase, long-branched phthalates are conjugated in phase II and eliminated through urine and feces[59]. Therefore, mPAEs should be also considered in order to understand the association between exposure to PAEs and diabetes. Based on in vitro and in vivo studies, mPAEs are more potent at a molecular level compared to their parent diester compounds[60-62]. PAEs and mPAEs have affinity for PPARs receptors, which are involved in complex mechanisms of regulation of glucose homeostasis, insulin sensitivity, differentiation of adipocyte and adipogenesis[63]. However, when the effects of BPA and three phthalate metabolites [monoisobutyl phthalate (MiBP), mono-n-butyl phthalate (MnBP), and mono-(2-ethylhexyl) phthalate (MEHP)] were investigated in pancreatic β-cells at concentrations of 5-500 μM, BPA treatment resulted in a more significant decrease in cellular viability after 72 h of exposure. Although increased insulin secretion was observed for BPA, MEHP, and MnBP after 2 h of simultaneous exposure to chemicals and glucose, no effects on glucose promoted insulin secretion were obtained after exposure for 24-72 h[64]. In contrast, when rats were treated orally with DEHP throughout gestation and lactation, abnormalities in β-cell ultrastructure, together with a decrease in β-cell mass and insulin content in the pancreas were found. Also, in DEHP treated offspring, alterations in pancreas specific gene expression were observed and impairment in β-cell development and function were reported[65]. Particularly, a decrease in the levels of pancreatic and duodenal homeobox-1 (Pdx-1) were observed in DEHP exposed rats of both sexes, as well as an increase in genes involved in endoplasmic reticulum stress, when compared to controls[65]. Considering the fact that Pdx-1 is involved in regulation of insulin gene expression, glucokinase, glucose transporter 2 (GLUT2), islet amyloid polypeptide and somatostatin, Pdx-1 plays crucial roles in the development of β-cells features and functions[66]. Therefore, this decrease in Pdx-1 activity is probably one of the principal mechanisms of DEHP-induced dysregulation of pancreatic β-cells[67]. DEHP exposed offspring had increased blood glucose levels and decreased pancreatic insulin levels and displayed changes in glucose tolerance and glucose stimulated insulin secretion. Despite this observed β-cell dysfunction and wide range of glucometabolic changes, DEHP exposure during the gestational period also induced epigenetic changes and led to inhibition of β-cell development[68]. Particularly, in both sexes a significant decrease in the levels of glucokinase mRNA was observed, which correlated with applied DEHP dose. Moreover, endoplasmic reticulum stress markers were increased, along with the concentrations of plasma membrane bound GLUT2 protein[68]. In addition, DiBP reduced fetal plasma insulin levels in offspring and decreased PPARα mRNA levels in the liver[69]. Additionally, gender and weight differences related to DEHP and diabetes development were seen in adulthood. Namely, DEHP exposed female offspring had lower birth weights, disturbed glucose tolerance, impaired insulin secretion and high blood glucose levels. DEHP exposed male offspring had increased serum insulin levels and lower birth weights at a significant level[65]. When compared to DBP, DEHP induced pancreatic dysfunction and inhibition of insulin secretion was more pronounced in the offspring of rats after in utero and lactational exposure to phthalates[70]. Relative to the effects of DEHP exposure in normal mice and male T2D mice in puberty, female T2D mice in puberty were more sensitive to DEHP. Namely, in DEHP exposed female T2D mice during puberty, higher levels of several parameters were detected such as insulin, C-peptide, fasting blood glucose levels, homeostatic model assessment of insulin resistance (HOMA-IR), low density lipoprotein, C-reactive protein and aspartate aminotransferase (AST). Also, DEHP triggered oxidative stress in terms of higher malondialdehyde (MDA) content and lower superoxide dismutase (SOD) and glutathione (GSH) peroxidase activity in the livers of both normal and T2D mice[71]. DEHP promoted increased body weight in normal adolescent mice. Increases in fasting blood glucose levels and glycated hemoglobin A1c (HbA1c) were more pronounced in adolescent T2D mice in comparison with normal adolescent mice. Additionally, DEHP induced insulin secretion and insulin resistance in normal adolescent mice, inhibited glycogen synthesis in adolescent T2D mice, and caused a decrease in the serum-lecithin cholesterol acyltransferase and hepatic lipase levels. A reduction in insulin levels was found in DEHP-treated adolescent T2D mice[72]. In both DEHP treated groups, a decrease in the expression of insulin receptors (IR-β and IRS-1) and GLUT4 was detected. Hence, DEHP acts as a metabolic toxicant in T2D development through impairment of glucose and lipid metabolism, and disruption of β-cell function and development[72]. Additionally, metabolic toxicity and insulin resistance caused by DEHP were more pronounced in rat liver cells with insulin resistance compared to normal cells[73]. In both cell lines, DEHP promoted cell damage through increased lipid peroxidation, alanine transaminase and AST levels, caspase-3 levels as a marker of cell apoptosis, and downregulated levels of IR-β. DEHP triggered macrophage infiltration in rat adipose tissue and stimulated the production of TNFα and IL-1β, promoting inflammation, while impairing normal lipid metabolism[74].
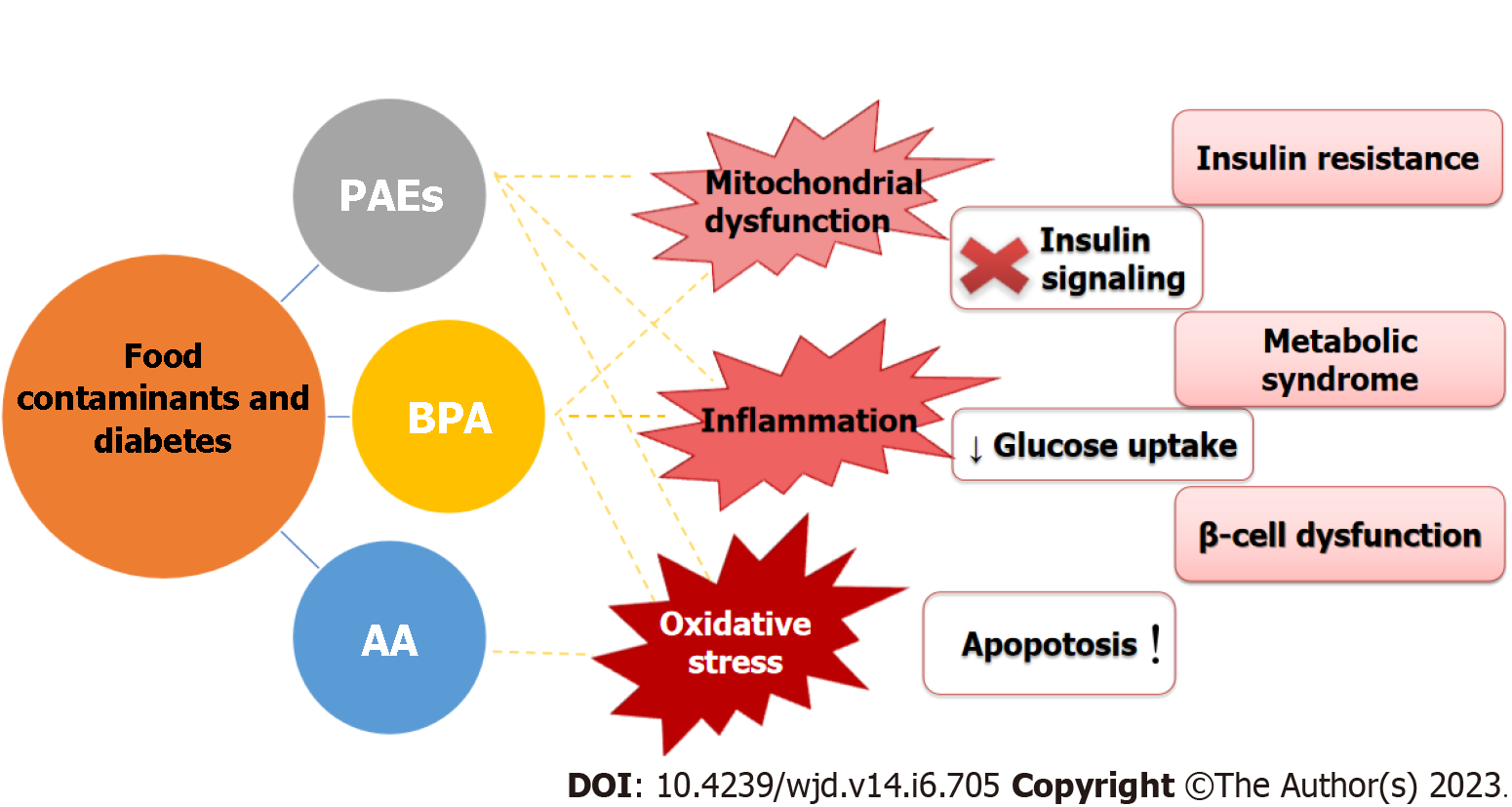
Potential mechanisms associated with diabetes: Although the mechanism of action of PAEs in diabetes has not been fully clarified, in vitro and in vivo studies have made significant progress toward identifying an association between PAE exposure and the development of diabetes. Interactions of PAEs with PPARs receptors impaired molecular signaling pathways (i.e., downregulated Pdx-1, activated JNK and caspase-3 expression, inhibited extracellular signal-regulated kinase (ERK)1/2, activated JAK/STAT pathway, and affected neuropeptide Y expression) that have a significant role in the regulation of glucose and lipid homeostasis[65,68,71,74,75]. Therefore, PAEs induce mitochondrial dysfunction, inflammation and increased oxidative stress, while decreasing the levels of IRs and GLUTs. PAEs also promote β-cell dysfunction, apoptosis, impaired insulin sensitivity and glucose cell uptake, and consequently cause glucometabolic and lipid abnormalities (Figure 2). In addition to their role in the onset of diabetes, PAEs act as obesogenic and diabetogenic chemicals that can aggravate the symptoms of diabetes. Especially concerning is the fact that prenatal PAEs exposure is a potential risk factor for developing diabetes, and pre-clinical studies imply that women are most susceptible to the adverse effects of PAEs.
Epidemiologic evidence: The relationship between PAE exposure and potential risk for development of diabetes has mostly been examined by cross-sectional studies that differ in the race, gender and ages of study participants, sampling size, type of matrix, analytical techniques and kind of phthalates and/or metabolites used as analytes[76-82]. Because PAEs undergo quick metabolism and are excreted via urine as conjugated monoesters, evaluation of mPAEs concentrations in urine is most appropriate for assessment of possible correlations between PAE exposure and diabetes in humans[83]. Different types of PAEs have similar structures and mechanisms of action, and thus their negative effects may be additive[51]. Hence, the sum of phthalate metabolites should be considered as well during assessment of their negative effects[43]. The first evidence for the diabetogenic potential of PAEs in the human population was reported almost 15 years ago in a study where positive correlations were found between mPAE concentrations, abdominal obesity and insulin resistance in males[84]. Although urinary mPAEs concentrations were not associated with T1D at a significant level, in children with new-onset T1D, higher concentrations of MiBP were detected[76]. A high frequency of DEP and DEHP detection in urine was observed in healthy adults, the obese, and people with newly diagnosed T2D[34]. Higher urinary mPAEs levels, especially monomethylphthalate (MMP), MEP and MiBP, were related to a higher prevalence of T2D in both sexes[78,82]. Particularly, MEP and MMP were associated with insulin resistance, while MiBP was correlated with low insulin secretion[82]. Moreover, the association between mPAE concentrations and T2D was more pronounced in young individuals in comparison to older individuals. Interestingly, a positive correlation between specific urinary mPAEs and HbA1c levels was observed in individuals with a lower body mass index, while MEHP concentrations were positively related to fasting glucose levels in men and in the elderly[77]. Additionally, MEHP levels were associated with glucose serum levels in T2D patients and urinary MEP concentrations were positively correlated with HOMA-IR while in healthy participants, positive correlations were found between urinary MEP levels and triglyceride glucose index and triglyceride glucose-body mass index[43]. Both parameters have been proposed as indicators of T2D development in healthy normoglycemic participants[85]. It was found that higher concentrations of specific mPAEs were associated with increased oxidative stress and inflammation in diabetic patients in terms of MDA and TNFα levels, and decreased adiponectin levels[86]. Based on a non-targeted metabolomic study, differences in the serum levels of biomarkers of galactose, amino acids and pyrimidine metabolism were observed between T2D and control groups and mPAEs levels were mostly significantly associated with metabolic biomarkers serum concentrations[87].
In order to examine prospective evidence concerning the association of phthalates with T2D, cohort studies were performed. It was found that, among middle-aged women, T2D may be associated with phthalate exposure[88]. In utero, MEP exposure was associated with poor insulin secretion among pubescent boys, while increased leptin was observed among girls. In utero, and during the peripubertal period, DEHP exposure was associated with higher serum insulin-like growth factor-1, insulin secretion, and insulin resistance[89]. Moreover, in order to better investigate the link between specific phthalates and their metabolites with diabetes, several meta analyses were recently performed. DEHP exposure is mostly related to insulin resistance[90] and a positive correlation was found between phthalate metabolites and increased HOMA-IR[91],while the presence of MMP, MnBP, MiBP, mono-(3-carboxypropyl) phthalate in urine were positively associated with risk of diabetes[92]. Results obtained from epidemiological studies provide additional evidence about the negative effects of phthalates on glucose and lipid metabolism.
BPA is one of the most well-known EDCs because of the numerous adverse health effects associated with its widespread application in different everyday products. BPA is used in the production of polycarbonate plastics and epoxy resins, and can be found in plastic containers and cans for food and beverages, numerous kitchen appliances and utensils, personal care products, toys, paints, electronics, sports equipment, medical devices, dental materials and thermal paper[93,94]. Because of its known reproductive toxicity and endocrine disruption potential, the use of BPA in baby bottles and toys is forbidden in the United States, Canada and the European Union[95]. However, despite continuous debate over more efficient measures to protect especially vulnerable populations from BPA exposure, BPA production and consumption is still increasing. It is expected that BPA commercial sales will exceed 30 billion USD in 2028[96]. Similarly, to PAEs, food can be contaminated with BPA during production, handling, packaging, and transportation[97]. BPA migration from container linings may be increased under high temperature, acid or basic conditions and even due to microwave exposure[95]. Hence, diet is recognized as a main source of BPA exposure, particularly the ingestion of BPA via canned foods[98]. Although the European Food Safety Authority has set a reduced TDI for BPA (0.04 ng of BPA per kg body weight per day), the daily intake of BPA through the diet is several times higher (0.17-0.95 μg of BPA per kg body weight per day)[99]. An extensive number of studies has documented the association between BPA exposure and increased oxidative stress, fertility disorders, obesity in children, adolescents and adults, metabolic disturbances and impaired pancreatic β-cell function, as well as cardiovascular diseases and even increased carcinogenicity[100-106].
Research status: BPA is classified as a “weak estrogen” and “obesogen” due to its endocrine disruptive potential, which is mainly a result of the known ability of BPA to bind to nuclear receptors[15,107]. Acute and long-term effects of low BPA concentrations on the development of diabetes have been documented. Enhanced insulin synthesis was observed through the interaction of ERα with ERK2 in pancreatic β-cells[108]. Similar to 17β-estradiol, picomolar doses of BPA trigger Ca2+ signaling pathways leading to insulin secretion in pancreatic β-cells. In addition, BPA exposure may cause inhibition of the expression of Pdx-1 in pancreatic mice islets, resulting in a decrease in glucose promoted insulin secretion and ATP production. Moreover, microRNA expression and BPA induced insulin secretion dysfunction in pancreatic islets has also been studied. Particularly, BPA suppressed the expression of miR-338, resulting in down-regulation of Pdx-1[109]. The “inverted U-shaped dose-effect curve” corresponds to the impact of BPA on insulin secretion in β-cells and mitochondrial function[110]. It is worth noting that more pronounced effects were exhibited by BPA binding to ERβ receptors. BPA as an insulinotropic pollutant affected β-cell function through inhibition of K(ATP) channel activity, which was observed in ERβ+ mice and human β-cells and islets[111,112]. In comparison with phthalate metabolites (MnBP, MiBP, and MEHP), BPA more strongly affected viability and insulin secretion in pancreatic β-cells[64]. However, in the same study, cytokine-induced cell death, a marker of T1D, was not affected. In spite of this, BPA was found to aggravate T1D in mice by disturbing Ca2+ signaling; indicating that BPA may cause insulin resistance via exacerbation of endoplasmic reticulum stress in pancreatic β-cells[113]. The diabetogenic potential of BPA has also been documented in insulinoma cell lines, where increased insulin secretion was observed together with decreased cell viability at nanomolar BPA levels[114,115]. BPA induced insulin hypersecretion was associated with enhanced β-cell lymphoma 2 family members, caspases and mitochondrial stress, which led to apoptosis[114]. Additionally, apoptosis may be promoted through BPA induced formation of amyloid fibrils. In rat insulinoma cells, BPA at micromolar concentrations induced DNA damage via increased levels of the proteins p53 and p-Chk2, as well as increased production of reactive oxygen species and decreased GSH levels[116]. In pancreatic α-cells, which are responsible for glucagon secretion, BPA reduced the fluctuation of low glucose levels induced by Ca2+[117]. To date, there is no published data concerning the impact of BPA on other Langerhans islets cells (δ, γ, ε). Regarding the data about BPA’s role in autoimmune related disorders, such as T1D, the effects of low and high doses of BPA on T-cell immunity mechanisms have also been examined. Results show that at low doses, BPA acts as a promotor of diabetes, both through modulation of CD4+ T-cells and production of interferon γ, IL-6 and TNFα[118]. BPA effects were not sex-dependent, based on the experiments performed in non-obese diabetic mice models[119]. However, exposure to BPA during the prenatal stage is particularly dangerous, considering that BPA increased the risk for T1D development and metabolic disturbances in juvenile mice models and adult mice offspring, respectively[119,120]. Additionally, changes in gut microbiota and inflammation were recorded in juvenile mice[119,121]. Prenatal BPA exposure during the lactation period led to an increase in body weight in mice[122]. Even at “safe“ levels (below the predicted ‘no adverse effect’ concentration) prenatal BPA exposure led to a significant increase in body and liver weight, abnormalities in adipocytes in terms of mass, number and volume, as well as elevated serum leptin and insulin levels, together with a decrease in adiponectin and glucose tolerance in adult male offspring[120]. Also, BPA exposure during lactation induced body weight gain in mice[122]. In pregnant BPA exposed mice, insulin resistance, together with elevated levels of insulin, triglycerides, and leptin in plasma, as well as glucose intolerance were observed[123]. Prenatal BPA exposure had detrimental effects on β-cells in mice, in terms of cell growth, mass and proliferation[124]. Therefore, exposure during early stages and the gestational period may cause long-term vulnerability to metabolic diseases and the development of glucose intolerance as a collateral effect or through epigenetic modifications[125,126].
Potential mechanisms associated with diabetes: The mechanisms of action of BPA are complex. Besides impairment of β-cell function, pre-clinical studies suggest that BPA is involved in the production of insulin resistance promoters, such as IL-6 and TNFα and inhibition of adiponektine in adipose tissue. In addition, BPA is associated with increased lipid peroxidation and pro-inflammatory cytokines in hepatocytes, as well as alterations in signaling pathways that generate reactive oxygen species, affect T-cell immunity, leading to decreased insulin sensitivity in skeletal muscles and glucose tolerance in the liver (Figure 2)[127-134].
Epidemiologic evidence: Evidence for the diabetogenic effects of BPA could not be completed without biomonitoring studies. Considering that free BPA has higher affinity for nuclear receptors than glucuronide and sulfate conjugates, the adverse effects of BPA are still evaluated mostly by measuring total BPA levels in urine, as a matrix of choice, and are expressed as creatinine-adjusted mean BPA concentrations[135]. Most of these studies are cross-sectional, performed on a limited number of volunteers using spot urine BPA testing. Therefore, the long term effects of BPA could not be estimated. It has been reported that the presence of BPA in urine samples is positively associated with obesity in children, adolescents and adults, as well as with the promotion of obesity, especially the visceral type, increased metabolic risk through hyperinsulinemia, glucose intolerance, insulin resistance, elevated HbA1c and serum leptin levels and dyslipidemia[16,17,103,105,136-142]. Different research groups have reported a positive relationship between BPA levels and T2D[143-147]. It is worth noting that in some studies the obtained outcomes were independent of age, sex, ethnicity, body mass index, and serum cholesterol levels[104,148]. Furthermore, in a meta-analysis that included data from more than 41000 participants, detected BPA concentrations in urine and serum were positively associated with a risk for T2D[149]. In a recently performed cohort study with 1990 participants, the U-shaped curve reflected an association between serum BPA concentrations and risk for T2D[141]. Individuals with increased BPA concentrations and increased diabetes genetic risk score had increased fasting plasma glucose levels and risk for T2D as well[141]. In a longitudinal cohort study performed on more than 2300 adults of both sexes, repeated measurements were conducted in order to investigate the association of urinary BPA levels with glucose homeostasis parameters. The obtained results imply that BPA correlated with compromised glucose homoeostasis in women but not in men before the development of diabetes[150]. Prenatal BPA exposure was connected with an increased risk for lower birth weight, smaller size for gestational age as well as increased leptin and decreased adiponectin levels[151-154]. Significantly higher median urinary BPA levels were observed in children and adolescents with T1D when compared with healthy controls[102].
A limited number of studies have demonstrated BPA detection in adipose tissue, due to the invasive nature of the procedure and the complexity of the matrix. BPA was detected with high frequency (62%) in adipose tissue in children[155]. Moreover, obtained BPA levels in adipose tissue were much higher in children compared with adult women[156]. The levels of BPA in adipose tissue of adults were related to low GSH reductase activity and increased oxidized GSH, confirming that BPA triggers oxidative stress in human adipose tissue[157]. Regarding adipose tissue dysfunction, BPA serum levels were significantly higher in people with T2D in comparison with healthy controls; while a positive correlation with serum leptin levels, and a negative correlation with adiponectin was found in the group with diabetes, strongly suggesting that BPA may worsen diabetes and increase diabetes pathology[147].
AA is an α,β-unsaturated carbonyl compound with electrophilic reactivity that has widespread applications in different industrial and laboratory processes[158]. In particular, AA is applied for the synthesis of polyacrylamide polymers used in water purification, sewage treatment, oil and sugar refinement, the production of soaps and cosmetics, varnishes, plastics, pesticides, adhesives, fibers, pharmaceuticals and textiles, and as a gel medium for electrophoresis methods in research laboratories[159-161]. AA is also found in cigarette smoke[162]. AA is the focus of scientific and public attention since 2002, when it was reported that it can be produced during the processing of certain foods. AA is formed as a result of a Milliard reaction when foods that contain asparagines and sugars are prepared at high temperatures (higher than 120 °C) under low moisture conditions[163-165]. More precisely, AA is formed during the browning of certain foods during frying, baking, grilling and roasting[159]. Hence, the main sources of AA in the diet are fried potatoes, breakfast cereals, cookies, crackers, crisps, bread, toast[166,167] and roasted coffee[168]. It is estimated that chronic average exposure to AA ranges from 0.5-1.9 μg/kg body weight per day in children, to 0.4-0.9 μg/kg body weight per day in adolescents, adults, and the elderly[169].
During detoxification processes, the majority of AA is conjugated to GSH, while less is metabolized to a genotoxic epoxide derivate glycidamide (GA) by the enzyme cytochrome P450 2E1 (CYP2E1)[170]. Genotoxic GA is more reactive than AA, and can produce DNA and Hb adducts[171]. The TDI for AA neurotoxicity is 40 μg/kg/d, while TDIs for cancer are 2.6 and 16 μg/kg/d for AA and GA, respectively[172]. Due to the adverse effects of AA on human health, the European Chemicals Agency ECHA has included AA on a list of candidate substances of very high concern that requires authorization from the European chemical regulation REACH (Registration, Evaluation, Authorization and Restriction of Chemicals)[159,173]. Several regulatory agencies provided different mitigation strategies for the prevention and reduction of AA formation in food[174-179].
Research status: Data about the association between low AA levels from diet and adverse health outcomes are still scarce and limited. To date, there have been only few attempts to investigate the impact of AA exposure on diabetes development. AA exposure disturbed the majority of redox status parameters in vitro in a β-cell line, Rin-5F, a validated β-cell model system[180]. Namely, AA exposure led to increased lipid peroxidation and nitric oxide (NO) production and a decrease in GSH content[180]. In addition, AA treatment affected the activity of antioxidant enzymes SOD and catalase (CAT), and the detoxifiying enzyme GSH S-transferase (GST) in pancreatic β-cells[180]. Formation of AA-GSH conjugates during detoxification could lead to GSH depletion and stimulation of GST activity in AA-exposed β-cells[180-182]. During metabolic processing, most AA is coupled to GSH via GST[158,183]. Elevated lipid peroxidation in pancreatic β-cells could be a result of GSH reduction[182]. AA exposure increased both the expression of inducible NO synthase (iNOS) and NO production in pancreatic β-cells, indicating induction of nitrosative stress[180]. Elevated iNOS and NO levels can cause β-cell dysfunction[184]. Decreased activity, but increased expression of SOD could be a consequence of the inactivation of redundant enzyme that is produced under conditions of high oxidative stress in AA-exposed pancreatic β-cells[185,186]. Upon AA exposure, resulting elevated NO levels reduced CAT activity in pancreatic β-cells[180,187]. In vitro metabolomics analysis revealed AA-induced glycolysis and gluconeogenesis alleviation characterized by diminished levels of glycolitic intermediates and a decreased rate of the tricarboxylic acid cycle[188]. Taken together, in vitro studies suggest that AA induces oxidative stress toxicity in β- cells and alters glucose metabolism.
In rats, AA exposure led to increased blood glucose levels and the development of histopathological changes in the islets[189]. In addition, a decreased β-cell and increased α-cell number was observed in rats upon exposure to AA[190,191]. A similar pattern of islets remodeling characterized by α-cell expansion and β-cell reduction was detected in islets of both diabetic rats and humans[192-197]. These data are in line with the putative prodiabetic properties of AA. AA exposure altered expression of gluconeogenic enzymes in rats and mice, indicating the potential of AA to impair gluconeogenesis[189,198]. Furthermore, AA affected the level of metabolites involved in the pentose phosphate pathway[199]. The pentose phosphate pathway is a significant component of glucose metabolism related to the development of T2D[200]. Taken together, these data demonstrate AA-induced disruption of glucose homeostasis. In addition, AA was shown to affect insulin-regulated IRS/PI3K/Akt/Foxo1 signaling pathways in rats[189]. Furthermore, AA exposure induced the expression of iNOS in rat pancreatic islets[180]. Increased iNOS expression impairs normal β-cell function and insulin secretion, and has been detected in both T1D and T2D[184]. In both in vitro and in vivo model systems, AA treatments reduced the expression of CYP2E1 in pancreatic β-cells[180]. CYP2E1 catalyzes biotransformation of AA to the genotoxic epoxide GA[170]. Reduction of CYP2E1 expression could be a protective mechanism in β-cells, in order to prevent the formation of the more toxic GA[180]. In addition, it has been shown that AA aggravates the diabetic condition in rodents[198,201,202]. Namely, AA worsens the histopathological features of liver and kidney lesions, blood biochemical parameters and redox status in diabetic rodents[198,201,202]. Diabetics are particularly vulnerable individuals, and more susceptible to environmental contaminants than the general population[186,198,203,204]. Collectively, in vivo studies in rodents indicate that AA exposure induces remodeling of pancreatic islets, impairs glucose metabolism and aggravates the overall diabetic state.
Potential mechanisms associated with diabetes: Based on the limited number of performed in vitro and in vivo studies, oxidative stress is the principle mechanism of AA-induced toxicity in pancreatic β-cells[180]. AA related impairment of both pentose phosphate pathway and insulin-regulated signaling is responsible for glucose metabolism disruption and development and aggravation of diabetes (Figure 2)[189,198,199].
Epidemiological evidence: Several epidemiological studies have revealed an association between AA intake and disorders of glucose metabolism[160,205,206]. In a Chinese adult population, a correlation between AA exposure and fasting plasma glucose levels was observed[160]. In line with these findings, data from the United States National Health and Nutrition Examination Survey (NHANES) 2003-2006 showed a significant correlation between high fasting plasma glucose levels and the concentration of HbGA adducts in the general adult population in the United States[205]. This study also reported that AA alters metabolic syndrome biomarkers[205]. Another NHANES study, 2003-2004, reported an association between AA exposure, decreased blood insulin levels and insulin resistance[206]. Subsequent NHANES surveys, 2005-2006 and 2013-2016, further confirmed these data and showed that Hb-AA adducts (HbAA) are linearly and inversely associated with the risk of diabetes development, whereas HbGA/HbAA nonlinearly and positively correlates with the prevalence of diabetes, indicating that HbAA and HbGA/HbAA are significantly associated with diabetes[169]. An association between HBAA adducts and AA intake was also detected in an adult Japanese population[207]. In addition, there is a link between prenatal dietary exposure to AA and the prevalence of obesity[208]. A large prospective study revealed a positive correlation between consumption of french fries and the risk for development of T2D in women[209]. French fries contain a high AA content: a standard portion contains approximately 30 μg of AA[165], indicating a significant contribution of AA to the deve-lopment of T2D. These findings have been further confirmed by two prospective cohort studies, which showed an association between a high intake of ultra-processed foods and the risk of T2D[210,211]. Further epidemiological studies in other populations are required in order to confirm and elucidate the roles of AA exposure in the development of diabetes.
This paper summarizes important data, providing greater understanding of the diabetogenic effects of some PAEs and their metabolites, as well as BPA and AA. Risk assessment of these contaminants in mixtures of EDCs and the exact level of exposure associated with diabetes development over time remained unanswered. The effects of decreased exposure to phthalates, BPA, and AA through avoidance of specific packaging materials, or chemical reactions during food processing on glucose metabolism should also be addressed. Therefore, further prospective, well-designed studies with multiple measurements and longer follow-up, together with experimental studies, are required to completely understand the underlying mechanisms and confirm the causal association between PAEs, BPA, AA and diabetes outcomes. Diabetes is associated with serious complications, such as cardiovascular disease and stroke, chronic kidney disease, liver disease, neuropathy, retinopathy etc. Therefore, more effective prevention and treatment strategies are necessary. New strategies that advocate reduced exposure to food contaminants, while promoting increased physical activity and healthier nutritional choices, may be crucial for the prevention or delay of diabetes progression.
| 1. | Eide A. Human rights requirement to social and economic development: the case of the right to food and nutrition rights. In: Kracht U, Schulz M. Food security and nutrition: the Global Challenge. New York: St. Martins Press Inc. 1999: 329-344. |
| 2. | World Health Organization. World Food Safety Day 2022. [cited 1 November 2022]. Available from: https://www.who.int/campaigns/world-food-safety-day/2022. |
| 3. | Hussain MA. Food Contamination: Major Challenges of the Future. Foods. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Rather IA, Koh WY, Paek WK, Lim J. The Sources of Chemical Contaminants in Food and Their Health Implications. Front Pharmacol. 2017;8:830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Lebelo K, Malebo N, Mochane MJ, Masinde M. Chemical Contamination Pathways and the Food Safety Implications along the Various Stages of Food Production: A Review. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Chakraborty P, Bharat GK, Gaonkar O, Mukhopadhyay M, Chandra S, Steindal EH, Nizzetto L. Endocrine-disrupting chemicals used as common plastic additives: Levels, profiles, and human dietary exposure from the Indian food basket. Sci Total Environ. 2022;810:152200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Thompson LA, Darwish WS. Environmental Chemical Contaminants in Food: Review of a Global Problem. J Toxicol. 2019;2019:2345283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 8. | Wei Q, Liu T, Pu H, Sun DW. Determination of acrylamide in food products based on the fluorescence enhancement induced by distance increase between functionalized carbon quantum dots. Talanta. 2020;218:121152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Mousavi Khaneghah A, Fakhri Y, Nematollahi A, Seilani F, Vasseghian Y. The concentration of acrylamide in different food products: a global systematic review, meta-analysis, and meta-regression. Food Rev Int. 2022;38:1286-1304. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Sharma BM, Bharat GK, Chakraborty P, Martiník J, Audy O, Kukučka P, Přibylová P, Kukreti PK, Sharma A, Kalina J, Steindal EH, Nizzetto L. A comprehensive assessment of endocrine-disrupting chemicals in an Indian food basket: Levels, dietary intakes, and comparison with European data. Environ Pollut. 2021;288:117750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Jeddi MZ, Boon PE, Cubadda F, Hoogenboom R, Mol H, Verhagen H, Sijm DT. A vision on the ‘foodture’role of dietary exposure sciences in the interplay between food safety and nutrition. Trends Food Sci Technol. 2022;120:288-300. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Ong HT, Samsudin H, Soto-Valdez H. Migration of endocrine-disrupting chemicals into food from plastic packaging materials: an overview of chemical risk assessment, techniques to monitor migration, and international regulations. Crit Rev Food Sci Nutr. 2022;62:957-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | The Endocrine Disruption Exchange. TEDX (The Endocrine Disruption eXchange) List of Potential Endocrine Disruptors. [cited 1 November 2022]. Available from: https://endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/search-the-tedx-list#sname=&searchfor=any&sortby=chemname&action=search&searchcats=all&sortby=chemname. |
| 14. | European Commission. Commission staff working document fitness check on endocrine disruptors accompanying the document communication from the commission to the european parliament, the council, the european economic and social committee and the committee of the regions chemicals strategy for sustainability towards a toxic-free environment. [Accessed 1 November 2022]. Available from: https://op.europa.eu/en/publication-detail/-/publication/35f74285-0f02-11eb-bc07-01aa75ed71a1. |
| 15. | Stojanoska MM, Milosevic N, Milic N, Abenavoli L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine. 2017;55:666-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 16. | Milić N, Četojević-Simin D, Milanović M, Sudji J, Milošević N, Ćurić N, Abenavoli L, Medić-Stojanoska M. Estimation of in vivo and in vitro exposure to bisphenol A as food contaminant. Food Chem Toxicol. 2015;83:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Milošević N, Jakšić V, Sudji J, Vuković B, Ičin T, Milić N, Medić Stojanoska M. Possible influence of the environmental pollutant bisphenol A on the cardiometabolic risk factors. Int J Environ Health Res. 2017;27:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Human. Acrylamide. [cited 1 November 2022]. Available from: https://www.iarc.who.int/wp-content/uploads/2018/07/Monographs_Back_QA-1.pdf. |
| 19. | Matoso V, Bargi-Souza P, Ivanski F, Romano MA, Romano RM. Acrylamide: A review about its toxic effects in the light of Developmental Origin of Health and Disease (DOHaD) concept. Food Chem. 2019;283:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 504] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 21. | Evans M, Morgan AR, Patel D, Dhatariya K, Greenwood S, Newland-Jones P, Hicks D, Yousef Z, Moore J, Kelly B, Davies S, Dashora U. Risk Prediction of the Diabetes Missing Million: Identifying Individuals at High Risk of Diabetes and Related Complications. Diabetes Ther. 2021;12:87-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 22. | Rodriguez-Sanchez B, Aranda-Reneo I, Oliva-Moreno J, Lopez-Bastida J. Assessing the Effect of Including Social Costs in Economic Evaluations of Diabetes-Related Interventions: A Systematic Review. Clinicoecon Outcomes Res. 2021;13:307-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1086] [Article Influence: 60.3] [Reference Citation Analysis (1)] |
| 24. | Simmons KM, Michels AW. Type 1 diabetes: A predictable disease. World J Diabetes. 2015;6:380-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (4)] |
| 25. | Lloyd RE, Tamhankar M, Lernmark Å. Enteroviruses and Type 1 Diabetes: Multiple Mechanisms and Factors? Annu Rev Med. 2022;73:483-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Predieri B, Bruzzi P, Bigi E, Ciancia S, Madeo SF, Lucaccioni L, Iughetti L. Endocrine Disrupting Chemicals and Type 1 Diabetes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Mocciaro G, Gastaldelli A. Obesity-Related Insulin Resistance: The Central Role of Adipose Tissue Dysfunction. Handb Exp Pharmacol. 2022;274:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Dendup T, Feng X, Clingan S, Astell-Burt T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 29. | Sardon H, Dove AP. Plastics recycling with a difference. Science. 2018;360:380-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 30. | Baloyi ND, Tekere M, Maphangwa KW, Masindi V. Insights into the prevalence and impacts of phthalate esters in aquatic ecosystems. Front Environ Sci. 2021;9:684190. [DOI] [Full Text] |
| 31. | Huang L, Zhu X, Zhou S, Cheng Z, Shi K, Zhang C, Shao H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 32. | Li X, Zhang W, Lv J, Liu W, Sun S, Guo C, Xu J. Distribution, source apportionment, and health risk assessment of phthalate esters in indoor dust samples across China. Environ Sci Eur. 2021;33:19. [DOI] [Full Text] |
| 33. | Edwards L, McCray NL, VanNoy BN, Yau A, Geller RJ, Adamkiewicz G, Zota AR. Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: a preliminary analysis. J Expo Sci Environ Epidemiol. 2022;32:366-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 34. | Stepanović K, Vuković B, Milanović M, Milošević NP, Bosić-Živanović D, Stojadinović A, Tomić-Naglić D, Lepić S, Milić N, Medić-Stojanoska M. Is there a difference in the phthalate exposure between adults with metabolic disorders and healthy ones? Vojnosanit pregl. 2020;79:93. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Milošević N, Milić N, Živanović Bosić D, Bajkin I, Perčić I, Abenavoli L, Medić Stojanoska M. Potential influence of the phthalates on normal liver function and cardiometabolic risk in males. Environ Monit Assess. 2017;190:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Yao J, Hu M, Yuan F, Ye H, Xu Z, Zhang X, Qiu G, Dong C, Mmereki D, Xu Y, Zheng Y, Bu Z. Exposure to phthalates in the sleeping microenvironment of university dormitories: A preliminary estimate based on skin wipe and dust sampling. Build Environ. 2022;218:109135. [DOI] [Full Text] |
| 37. | Borges Ramirez MM, Dzul Caamal R, Rendón von Osten J. Occurrence and seasonal distribution of microplastics and phthalates in sediments from the urban channel of the Ria and coast of Campeche, Mexico. Sci Total Environ. 2019;672:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 38. | Wang Y, Zhang Z, Bao M, Xu Y, Zhang L, Tan F, Zhao H. Characteristics and risk assessment of organophosphate esters and phthalates in soils and vegetation from Dalian, northeast China. Environ Pollut. 2021;284:117532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Abtahi M, Dobaradaran S, Torabbeigi M, Jorfi S, Gholamnia R, Koolivand A, Darabi H, Kavousi A, Saeedi R. Health risk of phthalates in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in tehran, Iran. Environ Res. 2019;173:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 40. | Milic N, Spanik I, Radonic J, Sekulic MT, Grujic N, Vyviurska O, Milanovic M, Sremački M, Miloradov MV. Screening analyses of wastewater and Danube surface water in Novi Sad locality, Serbia. Fresen Environ Bull. 2014;23:372-377. |
| 41. | Giuliani A, Zuccarini M, Cichelli A, Khan H, Reale M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 42. | Eales J, Bethel A, Galloway T, Hopkinson P, Morrissey K, Short RE, Garside R. Human health impacts of exposure to phthalate plasticizers: An overview of reviews. Environ Int. 2022;158:106903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 43. | Milošević N, Milanović M, Sudji J, Bosić Živanović D, Stojanoski S, Vuković B, Milić N, Medić Stojanoska M. Could phthalates exposure contribute to the development of metabolic syndrome and liver disease in humans? Environ Sci Pollut Res Int. 2020;27:772-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Medic Stojanoska M, Milankov A, Vukovic B, Vukcevic D, Sudji J, Bajkin I, Curic N, Icin T, Kovacev Zavisic B, Milic N. Do diethyl phthalate (DEP) and di-2-ethylhexyl phthalate (DEHP) influence the metabolic syndrome parameters? Pilot study. Environ Monit Assess. 2015;187:526. [PubMed] [DOI] [Full Text] |
| 45. | Villanger GD, Drover SSM, Nethery RC, Thomsen C, Sakhi AK, Øvergaard KR, Zeiner P, Hoppin JA, Reichborn-Kjennerud T, Aase H, Engel SM. Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ Int. 2020;137:105509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Ahern TP, Broe A, Lash TL, Cronin-Fenton DP, Ulrichsen SP, Christiansen PM, Cole BF, Tamimi RM, Sørensen HT, Damkier P. Phthalate Exposure and Breast Cancer Incidence: A Danish Nationwide Cohort Study. J Clin Oncol. 2019;37:1800-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 47. | Cheon YP. Di-(2-ethylhexyl) Phthalate (DEHP) and Uterine Histological Characteristics. Dev Reprod. 2020;24:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Fu Z, Zhao F, Chen K, Xu J, Li P, Xia D, Wu Y. Association between urinary phthalate metabolites and risk of breast cancer and uterine leiomyoma. Reprod Toxicol. 2017;74:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | EUR-Lex. Commission Regulation (EU) 2020/1245 of 2 September 2020 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food (Text with EEA relevance). [cited 1 November 2022]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32020R1245. |
| 50. | EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V, Barat Baviera JM, Bolognesi C, Chesson A, Cocconcelli PS, Crebelli R, Gott DM, Grob K, Lampi E, Mortensen A, Rivière G, Steffensen IL, Tlustos C, Van Loveren H, Vernis L, Zorn H, Cravedi JP, Fortes C, Tavares Poças MF, Waalkens-Berendsen I, Wölfle D, Arcella D, Cascio C, Castoldi AF, Volk K, Castle L. Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J. 2019;17:e05838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Ribeiro E, Ladeira C, Viegas S. EDCs Mixtures: A Stealthy Hazard for Human Health? Toxics. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Hlisníková H, Petrovičová I, Kolena B, Šidlovská M, Sirotkin A. Effects and Mechanisms of Phthalates' Action on Reproductive Processes and Reproductive Health: A Literature Review. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 53. | Engel A, Buhrke T, Imber F, Jessel S, Seidel A, Völkel W, Lampen A. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicol Lett. 2017;277:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 54. | Warner GR, Flaws JA. Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology. Toxicol Sci. 2018;166:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 55. | Bodin J, Kocbach Bølling A, Wendt A, Eliasson L, Becher R, Kuper F, Løvik M, Nygaard UC. Exposure to bisphenol A, but not phthalates, increases spontaneous diabetes type 1 development in NOD mice. Toxicol Rep. 2015;2:99-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 56. | Cha S, Jung K, Lee MY, Hwang YJ, Yang E, Lee SH, Jung HI, Cheon YP. Nonmonotonic Effects of Chronic Low-Dose Di(2-ethylhexyl) Phthalate on Gonadal Weight and Reproductive. Dev Reprod. 2018;22:85-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Guo TL, Germolec DR, Zheng JF, Kooistra L, Auttachoat W, Smith MJ, White KL, Elmore SA. Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol Pathol. 2015;43:435-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Gayathri NS, Dhanya CR, Indu AR, Kurup PA. Changes in some hormones by low doses of di (2-ethyl hexyl) phthalate (DEHP), a commonly used plasticizer in PVC blood storage bags & medical tubing. Indian J Med Res. 2004;119:139-144. [PubMed] |
| 59. | Wang Y, Zhu H, Kannan K. A Review of Biomonitoring of Phthalate Exposures. Toxics. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 512] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 60. | Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 482] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 61. | Gobas FAPC, Otton SV, Tupper-Ring LF, Crawford MA, Clark KE, Ikonomou MG. Chemical activity-based environmental risk analysis of the plasticizer di-ethylhexyl phthalate and its main metabolite mono-ethylhexyl phthalate. Environ Toxicol Chem. 2017;36:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Sree CG, Buddolla V, Lakshmi BA, Kim YJ. Phthalate toxicity mechanisms: An update. Comp Biochem Physiol C Toxicol Pharmacol. 2023;263:109498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 63. | Meling DD, De La Torre KM, Arango AS, Gonsioroski A, Deviney ARK, Neff AM, Laws MJ, Warner GR, Tajkhorshid E, Flaws JA. Phthalate monoesters act through peroxisome proliferator-activated receptors in the mouse ovary. Reprod Toxicol. 2022;110:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Weldingh NM, Jørgensen-Kaur L, Becher R, Holme JA, Bodin J, Nygaard UC, Bølling AK. Bisphenol A Is More Potent than Phthalate Metabolites in Reducing Pancreatic β-Cell Function. Biomed Res Int. 2017;2017:4614379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song L, Wei Z, Lv Z, Chen X, Xia W, Xu S. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am J Physiol Endocrinol Metab. 2011;301:E527-E538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Zhang Y, Fang X, Wei J, Miao R, Wu H, Ma K, Tian J. PDX-1: A Promising Therapeutic Target to Reverse Diabetes. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 67. | Liu J, Lang G, Shi J. Epigenetic Regulation of PDX-1 in Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2021;14:431-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 68. | Rajesh P, Balasubramanian K. Gestational exposure to di(2-ethylhexyl) phthalate (DEHP) impairs pancreatic β-cell function in F1 rat offspring. Toxicol Lett. 2015;232:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Boberg J, Metzdorff S, Wortziger R, Axelstad M, Brokken L, Vinggaard AM, Dalgaard M, Nellemann C. Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology. 2008;250:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 70. | Venturelli AC, Meyer KB, Fischer SV, Kita DH, Philipsen RA, Morais RN, Martino Andrade AJ. Effects of in utero and lactational exposure to phthalates on reproductive development and glycemic homeostasis in rats. Toxicology. 2019;421:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Ding Y, Xu T, Mao G, Chen Y, Qiu X, Yang L, Zhao T, Xu X, Feng W, Wu X. Di-(2-ethylhexyl) phthalate-induced hepatotoxicity exacerbated type 2 diabetes mellitus (T2DM) in female pubertal T2DM mice. Food Chem Toxicol. 2021;149:112003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Ding Y, Gao K, Liu Y, Mao G, Chen K, Qiu X, Zhao T, Yang L, Feng W, Wu X. Transcriptome analysis revealed the mechanism of the metabolic toxicity and susceptibility of di-(2-ethylhexyl)phthalate on adolescent male ICR mice with type 2 diabetes mellitus. Arch Toxicol. 2019;93:3183-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Ding Y, Liu Y, Fei F, Yang L, Mao G, Zhao T, Zhang Z, Yan M, Feng W, Wu X. Study on the metabolism toxicity, susceptibility and mechanism of di-(2-ethylhexyl) phthalate on rat liver BRL cells with insulin resistance in vitro. Toxicology. 2019;422:102-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Zhou L, Chen H, Xu Q, Han X, Zhao Y, Song X, Zhao T, Ye L. The effect of di-2-ethylhexyl phthalate on inflammation and lipid metabolic disorder in rats. Ecotoxicol Environ Saf. 2019;170:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | Liu T, Jia Y, Zhou L, Wang Q, Sun D, Xu J, Wu J, Chen H, Xu F, Ye L. Effects of Di-(2-ethylhexyl) Phthalate on the Hypothalamus-Uterus in Pubertal Female Rats. Int J Environ Res Public Health. 2016;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 76. | Castro-Correia C, Correia-Sá L, Norberto S, Delerue-Matos C, Domingues V, Costa-Santos C, Fontoura M, Calhau C. Phthalates and type 1 diabetes: is there any link? Environ Sci Pollut Res Int. 2018;25:17915-17919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Duan Y, Sun H, Han L, Chen L. Association between phthalate exposure and glycosylated hemoglobin, fasting glucose, and type 2 diabetes mellitus: A case-control study in China. Sci Total Environ. 2019;670:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Nam DJ, Kim Y, Yang EH, Lee HC, Ryoo JH. Relationship between urinary phthalate metabolites and diabetes: Korean National Environmental Health Survey (KoNEHS) cycle 3 (2015-2017). Ann Occup Environ Med. 2020;32:e34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Oktar S, Sungur S, Okur R, Yilmaz N, Ustun I, Gokce C. The relationship between phthalates and obesity: serum and urine concentrations of phthalates. Minerva Endocrinol. 2017;42:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Vafeiadi M, Myridakis A, Roumeliotaki T, Margetaki K, Chalkiadaki G, Dermitzaki E, Venihaki M, Sarri K, Vassilaki M, Leventakou V, Stephanou EG, Kogevinas M, Chatzi L. Association of Early Life Exposure to Phthalates With Obesity and Cardiometabolic Traits in Childhood: Sex Specific Associations. Front Public Health. 2018;6:327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 81. | James-Todd TM, Huang T, Seely EW, Saxena AR. The association between phthalates and metabolic syndrome: the National Health and Nutrition Examination Survey 2001-2010. Environ Health. 2016;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 82. | Lind PM, Zethelius B, Lind L. Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care. 2012;35:1519-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 83. | Metcalfe CD, Bayen S, Desrosiers M, Muñoz G, Sauvé S, Yargeau V. Methods for the analysis of endocrine disrupting chemicals in selected environmental matrixes. Environ Res. 2022;206:112616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 84. | Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115:876-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 474] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 85. | Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev Med. 2016;86:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 86. | Duan Y, Wang L, Han L, Wang B, Sun H, Chen L, Zhu L, Luo Y. Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environ Int. 2017;109:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 87. | Duan Y, Sun H, Yao Y, Han L, Chen L. Perturbation of serum metabolome in relation to type 2 diabetes mellitus and urinary levels of phthalate metabolites and bisphenols. Environ Int. 2021;155:106609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 88. | Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses' Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 89. | Watkins DJ, Peterson KE, Ferguson KK, Mercado-García A, Tamayo y Ortiz M, Cantoral A, Meeker JD, Téllez-Rojo MM. Relating Phthalate and BPA Exposure to Metabolism in Peripubescence: The Role of Exposure Timing, Sex, and Puberty. J Clin Endocrinol Metab. 2016;101:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Radke EG, Braun JM, Meeker JD, Cooper GS. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ Int. 2018;121:764-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 312] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 91. | Shoshtari-Yeganeh B, Zarean M, Mansourian M, Riahi R, Poursafa P, Teiri H, Rafiei N, Dehdashti B, Kelishadi R. Systematic review and meta-analysis on the association between phthalates exposure and insulin resistance. Environ Sci Pollut Res Int. 2019;26:9435-9442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 92. | Zhang H, Ben Y, Han Y, Zhang Y, Li Y, Chen X. Phthalate exposure and risk of diabetes mellitus: Implications from a systematic review and meta-analysis. Environ Res. 2022;204:112109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 93. | Adeyi AA, Babalola BA. Bisphenol-A (BPA) in Foods commonly consumed in Southwest Nigeria and its Human Health Risk. Sci Rep. 2019;9:17458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 94. | Milanovic M, Sudji J, Grujic N, Radonic J, Turk-Sekulić M, Vojinović Miloradov M, Milic N. Seasonal variations of bisphenol A in the Danube River by the municipality of Novi Sad, Serbia. J Serb Chem Soc. 2016;81:95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Almeida S, Raposo A, Almeida-González M, Carrascosa C. Bisphenol A: Food Exposure and Impact on Human Health. Compr Rev Food Sci Food Saf. 2018;17:1503-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 303] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 96. | Emergen Research. Bisphenol A Market, By Application (Epoxy Resins, Polycarbonate Resins, Unsaturated Polyester Resins, Flame Retardants, Polysulfone Resins, Polyacrylate), By Industry Vertical (Automotive, Electronics, Medical, Paints & Coatings, Packaging), By Distribution Channel (Direct, Indirect), and By Region Forecast to 2028. [cited 5 November 2022]. Available from: https://www.emergenresearch.com/industry-report/bisphenol-a-market. |
| 97. | Chen WY, Shen YP, Chen SC. Assessing bisphenol A (BPA) exposure risk from long-term dietary intakes in Taiwan. Sci Total Environ. 2016;543:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 98. | Wang X, Nag R, Brunton NP, Siddique MAB, Harrison SM, Monahan FJ, Cummins E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ Res. 2022;213:113734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 99. | Çiftçi S, Yalçın SS, Samur G. Comparison of daily bisphenol A intake based on dietary and urinary levels in breastfeeding women. Reprod Toxicol. 2021;106:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Chen H, Zhang Y, Zou M, Qi X, Xu S. Bisphenol A aggravates renal apoptosis and necroptosis in selenium-deficient chickens via oxidative stress and PI3K/AKT pathway. J Cell Physiol. 2022;237:3292-3304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 101. | Liu Z, Lu Y, Zhong K, Wang C, Xu X. The associations between endocrine disrupting chemicals and markers of inflammation and immune responses: A systematic review and meta-analysis. Ecotoxicol Environ Saf. 2022;234:113382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 102. | Tosirisuk N, Sakorn N, Jantarat C, Nosoongnoen W, Aroonpakmongkol S, Supornsilchai V. Increased bisphenol A levels in Thai children and adolescents with type 1 diabetes mellitus. Pediatr Int. 2022;64:e14944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Amin MM, Ebrahim K, Hashemi M, Shoshtari-Yeganeh B, Rafiei N, Mansourian M, Kelishadi R. Association of exposure to Bisphenol A with obesity and cardiometabolic risk factors in children and adolescents. Int J Environ Health Res. 2019;29:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 104. | Ahmadkhaniha R, Mansouri M, Yunesian M, Omidfar K, Jeddi MZ, Larijani B, Mesdaghinia A, Rastkari N. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J Environ Health Sci Eng. 2014;12:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 105. | Moon MK, Kim MJ, Lee I, Kim S, Choi S, Park J, Cho YH, Hong S, Yoo J, Park H, Cheon GJ, Park YJ, Choi K. Exposure to Bisphenol A, S, and F and its Association with Obesity and Diabetes Mellitus in General Adults of Korea: Korean National Environmental Health Survey (KoNEHS) 2015-2017. Expos Health. 2023;15:53-67. [DOI] [Full Text] |
| 106. | Yao J, Wang F, Zhang Y, Zhang Z, Bi J, He J, Li P, Han X, Wei Y, Zhang X, Guo H, He M. Association of serum BPA levels with changes in lipid levels and dyslipidemia risk in middle-aged and elderly Chinese. Ecotoxicol Environ Saf. 2022;241:113819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Sonavane M. Chapter 1: Classical and Non-classical Estrogen Receptor Effects of Bisphenol A. In: Gassman NR. Bisphenol A: A Multi-modal Endocrine Disruptor. United Kingdom: Elsevier. [DOI] [Full Text] |
| 108. | Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One. 2008;3:e2069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 305] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 109. | Wei J, Ding D, Wang T, Liu Q, Lin Y. MiR-338 controls BPA-triggered pancreatic islet insulin secretory dysfunction from compensation to decompensation by targeting Pdx-1. FASEB J. 2017;31:5184-5195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | Song L, Xia W, Zhou Z, Li Y, Lin Y, Wei J, Wei Z, Xu B, Shen J, Li W, Xu S. Low-level phenolic estrogen pollutants impair islet morphology and β-cell function in isolated rat islets. J Endocrinol. 2012;215:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Soriano S, Alonso-Magdalena P, García-Arévalo M, Novials A, Muhammed SJ, Salehi A, Gustafsson JA, Quesada I, Nadal A. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One. 2012;7:e31109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 112. | Makaji E, Raha S, Wade MG, Holloway AC. Effect of environmental contaminants on Beta cell function. Int J Toxicol. 2011;30:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 113. | Ahn C, Kang HS, Lee JH, Hong EJ, Jung EM, Yoo YM, Jeung EB. Bisphenol A and octylphenol exacerbate type 1 diabetes mellitus by disrupting calcium homeostasis in mouse pancreas. Toxicol Lett. 2018;295:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, Ye T, Kang M, Shen H, Dong S. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis. 2013;4:e460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 115. | Hectors TL, Vanparys C, Pereira-Fernandes A, Martens GA, Blust R. Evaluation of the INS-1 832/13 cell line as a beta-cell based screening system to assess pollutant effects on beta-cell function. PLoS One. 2013;8:e60030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 116. | Xin F, Jiang L, Liu X, Geng C, Wang W, Zhong L, Yang G, Chen M. Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat Res Genet Toxicol Environ Mutagen. 2014;769:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 117. | Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, Nadal A. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 118. | Cetkovic-Cvrlje M, Thinamany S, Bruner KA. Bisphenol A (BPA) aggravates multiple low-dose streptozotocin-induced Type 1 diabetes in C57BL/6 mice. J Immunotoxicol. 2017;14:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 119. | Xu J, Huang G, Nagy T, Teng Q, Guo TL. Sex-dependent effects of bisphenol A on type 1 diabetes development in non-obese diabetic (NOD) mice. Arch Toxicol. 2019;93:997-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 120. | Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, vom Saal FS, Taylor JA. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013;42:256-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 121. | Xu J, Huang G, Nagy T, Guo TL. Bisphenol A alteration of type 1 diabetes in non-obese diabetic (NOD) female mice is dependent on window of exposure. Arch Toxicol. 2019;93:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151:2603-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 123. | Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 124. | García-Arévalo M, Alonso-Magdalena P, Servitja JM, Boronat-Belda T, Merino B, Villar-Pazos S, Medina-Gómez G, Novials A, Quesada I, Nadal A. Maternal Exposure to Bisphenol-A During Pregnancy Increases Pancreatic β-Cell Growth During Early Life in Male Mice Offspring. Endocrinology. 2016;157:4158-4171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 125. | Tudurí E, Marroqui L, Dos Santos RS, Quesada I, Fuentes E, Alonso-Magdalena P. Timing of Exposure and Bisphenol-A: Implications for Diabetes Development. Front Endocrinol (Lausanne). 2018;9:648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 126. | Provvisiero DP, Pivonello C, Muscogiuri G, Negri M, de Angelis C, Simeoli C, Pivonello R, Colao A. Influence of Bisphenol A on Type 2 Diabetes Mellitus. Int J Environ Res Public Health. 2016;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 127. | Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642-1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 128. | Moon MK, Jeong IK, Jung Oh T, Ahn HY, Kim HH, Park YJ, Jang HC, Park KS. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J Endocrinol. 2015;226:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 129. | Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY, Lee KJ, Kim SH, Yoon YC, Cho BJ, Park KS, Jang HC, Park YJ. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci. 2012;27:644-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 130. | Valentino R, D'Esposito V, Passaretti F, Liotti A, Cabaro S, Longo M, Perruolo G, Oriente F, Beguinot F, Formisano P. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS One. 2013;8:e82099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 131. | Batista TM, Alonso-Magdalena P, Vieira E, Amaral ME, Cederroth CR, Nef S, Quesada I, Carneiro EM, Nadal A. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS One. 2012;7:e33814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 132. | Indumathi D, Jayashree S, Selvaraj J, Sathish S, Mayilvanan C, Akilavalli N, Balasubramanian K. Effect of bisphenol-A on insulin signal transduction and glucose oxidation in skeletal muscle of adult male albino rat. Hum Exp Toxicol. 2013;32:960-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 133. | Boucher JG, Boudreau A, Ahmed S, Atlas E. In Vitro Effects of Bisphenol A β-D-Glucuronide (BPA-G) on Adipogenesis in Human and Murine Preadipocytes. Environ Health Perspect. 2015;123:1287-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 134. | Grasselli E, Cortese K, Voci A, Vergani L, Fabbri R, Barmo C, Gallo G, Canesi L. Direct effects of Bisphenol A on lipid homeostasis in rat hepatoma cells. Chemosphere. 2013;91:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 135. | Ougier E, Zeman F, Antignac JP, Rousselle C, Lange R, Kolossa-Gehring M, Apel P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ Int. 2021;154:106563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 136. | Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res. 2011;111:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 137. | Zhao HY, Bi YF, Ma LY, Zhao L, Wang TG, Zhang LZ, Tao B, Sun LH, Zhao YJ, Wang WQ, Li XY, Xu MY, Chen JL, Ning G, Liu JM. The effects of bisphenol A (BPA) exposure on fat mass and serum leptin concentrations have no impact on bone mineral densities in non-obese premenopausal women. Clin Biochem. 2012;45:1602-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 345] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 139. | Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011;96:3822-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 140. | Silver MK, O'Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One. 2011;6:e26868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 141. | Wang F, Zhang Y, Zhang S, Han X, Wei Y, Guo H, Zhang X, Yang H, Wu T, He M. Combined effects of bisphenol A and diabetes genetic risk score on incident type 2 diabetes: A nested case-control study. Environ Pollut. 2022;307:119581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 142. | Milanović M, Milošević N, Sudji J, Stojanoski S, Atanacković Krstonošić M, Bjelica A, Milić N, Medić Stojanoska M. Can environmental pollutant bisphenol A increase metabolic risk in polycystic ovary syndrome? Clin Chim Acta. 2020;507:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 143. | Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 938] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 144. | Sabanayagam C, Teppala S, Shankar A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol. 2013;50:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 145. | Li AJ, Xue J, Lin S, Al-Malki AL, Al-Ghamdi MA, Kumosani TA, Kannan K. Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia. Environ Res. 2018;166:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 146. | Murphy L, Mérida-Ortega Á, Cebrián ME, Hernández-Garciadiego L, Gómez-Ruiz H, Gamboa-Loira B, López-Carrillo L. Exposure to bisphenol A and diabetes risk in Mexican women. Environ Sci Pollut Res Int. 2019;26:26332-26338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 147. | Jain J, Gupta N, Mathur R, Nimesh S, Mathur SK. A Study on Impact of BPA in the Adipose Tissue Dysfunction (Adiposopathy) in Asian Indian Type 2 Diabetes Mellitus Subjects. Indian J Clin Biochem. 2020;35:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 148. | Rancière F, Botton J, Slama R, Lacroix MZ, Debrauwer L, Charles MA, Roussel R, Balkau B, Magliano DJ; D. E.S.I.R. Study Group. Exposure to Bisphenol A and Bisphenol S and Incident Type 2 Diabetes: A Case-Cohort Study in the French Cohort D.E.S.I.R. Environ Health Perspect. 2019;127:107013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 149. | Hwang S, Lim JE, Choi Y, Jee SH. Bisphenol A exposure and type 2 diabetes mellitus risk: a meta-analysis. BMC Endocr Disord. 2018;18:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 150. | Wang B, Li M, Zhao Z, Lu J, Chen Y, Xu Y, Xu M, Wang W, Wang T, Bi Y, Ning G. Urinary bisphenol A concentration and glucose homeostasis in non-diabetic adults: a repeated-measures, longitudinal study. Diabetologia. 2019;62:1591-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 151. | İnce T, Balcı A, Yalçın SS, Özkemahlı G, Erkekoglu P, Kocer-Gumusel B, Yurdakök K. Urinary bisphenol-A levels in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2018;31:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 152. | Chou WC, Chen JL, Lin CF, Chen YC, Shih FC, Chuang CY. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ Health. 2011;10:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 153. | Volberg V, Harley K, Calafat AM, Davé V, McFadden J, Eskenazi B, Holland N. Maternal bisphenol a exposure during pregnancy and its association with adipokines in Mexican-American children. Environ Mol Mutagen. 2013;54:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 154. | Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, Morisset AS, Taback S, Bouchard MF, Monnier P, Dallaire R, Fraser WD. A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ Health. 2014;13:84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 155. | Olea N, Arrebola JP, Taoufiki J, Fernández-Valades R, Prada R, Navea N, Molina-Molina JM, Fernandez M F. Alkylphenols and bisphenol-A and its chlorinated derivatives in adipose tissue of children. WIT Trans Ecol Environ. 2008;110:129-138. [DOI] [Full Text] |
| 156. | Fernandez MF, Arrebola JP, Taoufiki J, Navalón A, Ballesteros O, Pulgar R, Vilchez JL, Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol. 2007;24:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 157. | Artacho-Cordón F, Ríos-Arrabal S, León J, Frederiksen H, Sáenz JM, Martín-Olmedo P, Fernández MF, Olea N, Arrebola JP. Adipose tissue concentrations of non-persistent environmental phenols and local redox balance in adults from Southern Spain. Environ Int. 2019;133:105118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 158. | Zhang Y, Huang M, Zhuang P, Jiao J, Chen X, Wang J, Wu Y. Exposure to acrylamide and the risk of cardiovascular diseases in the National Health and Nutrition Examination Survey 2003-2006. Environ Int. 2018;117:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 159. | Albiach-Delgado A, Esteve-Turrillas FA, Fernández SF, Garlito B, Pardo O. Review of the state of the art of acrylamide human biomonitoring. Chemosphere. 2022;295:133880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 160. | Wang B, Qiu W, Yang S, Cao L, Zhu C, Ma J, Li W, Zhang Z, Xu T, Wang X, Cheng M, Mu G, Wang D, Zhou Y, Yuan J, Chen W. Acrylamide Exposure and Oxidative DNA Damage, Lipid Peroxidation, and Fasting Plasma Glucose Alteration: Association and Mediation Analyses in Chinese Urban Adults. Diabetes Care. 2020;43:1479-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 161. | Chu PL, Lin LY, Chen PC, Su TC, Lin CY. Negative association between acrylamide exposure and body composition in adults: NHANES, 2003-2004. Nutr Diabetes. 2017;7:e246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 162. | Smith CJ, Perfetti TA, Rumple MA, Rodgman A, Doolittle DJ. "IARC group 2A Carcinogens" reported in cigarette mainstream smoke. Food Chem Toxicol. 2000;38:371-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 163. | Timmermann CAG, Mølck SS, Kadawathagedara M, Bjerregaard AA, Törnqvist M, Brantsæter AL, Pedersen M. A Review of Dietary Intake of Acrylamide in Humans. Toxics. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 164. | World Health Organization. Health Implications of Acrylamide in Food. [cited 4 April 2021]. Available from: https://www.who.int/publications/i/item/health-implications-of-acrylamide-in-food. |
| 165. | Adani G, Filippini T, Wise LA, Halldorsson TI, Blaha L, Vinceti M. Dietary Intake of Acrylamide and Risk of Breast, Endometrial, and Ovarian Cancers: A Systematic Review and Dose-Response Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2020;29:1095-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 166. | Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem. 2002;50:4998-5006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1531] [Cited by in RCA: 1354] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 167. | Törnqvist M. Acrylamide in food: the discovery and its implications: a historical perspective. Adv Exp Med Biol. 2005;561:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 168. | Loaëc G, Jacolot P, Helou C, Niquet-Léridon C, Tessier FJ. Acrylamide, 5-hydroxymethylfurfural and N(ε)-carboxymethyl-lysine in coffee substitutes and instant coffees. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2014;31:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 169. | Yin G, Liao S, Gong D, Qiu H. Association of acrylamide and glycidamide haemoglobin adduct levels with diabetes mellitus in the general population. Environ Pollut. 2021;277:116816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 170. | Parzefall W. Minireview on the toxicity of dietary acrylamide. Food Chem Toxicol. 2008;46:1360-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 171. | Rice JM. The carcinogenicity of acrylamide. Mutat Res. 2005;580:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 172. | Tardiff RG, Gargas ML, Kirman CR, Carson ML, Sweeney LM. Estimation of safe dietary intake levels of acrylamide for humans. Food Chem Toxicol. 2010;48:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 173. | ECHA. Brief Profile on Acrylamide. [cited 13 December 2022]. Available from: https://chemicalsinourlife.echa.europa.eu/web/guest/brief-profile/-/briefprofile/100.001.067. |
| 174. | Marković Filipović J, Miler M, Kojić D, Karan J, Ivelja I, Čukuranović Kokoris J, Matavulj M. Effect of Acrylamide Treatment on Cyp2e1 Expression and Redox Status in Rat Hepatocytes. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 175. | Codex Alimentarius. Code of Practice for the Reduction of Acrylamide in Foods. CAC/RCP 67-2009. [cited 20 December 2019]. Available from: https://www.fao.org/input/download/standards/11258/CXP_067e.pdf. |
| 176. | AECOSAN. Agencia Española de Seguridad Alimentaria y Nutrición. Acrylamide in Food, New Standards and Recommendations for Your Health. [cited 8 December 2022]. Available from: https://www.aesan.gob.es/AECOSAN/web/home/aecosan_inicio.htm. |
| 177. | US Food and Drug Administration. Guidance for Industry Acrylamide in Foods. [cited 18 March 2022]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-acrylamide-foods. |
| 178. | Diario Oficial de la Unión Europea. Commission Regulation (EU) 2017/2158. [cited 20 November 2022]. Available from: https://www.legislation.gov.uk/eur/2017/2158/contents. |
| 179. | Fooddrink Europe. Acrylamide. Toolbox 2019. [cited 25 March 2022]. Available from: https://higieneambiental.com/sites/default/files/images/halimentaria/fooddrinkeurope_acrylamide_toolbox_2019.pdf. |
| 180. | Marković J, Stošić M, Kojić D, Matavulj M. Effects of acrylamide on oxidant/antioxidant parameters and CYP2E1 expression in rat pancreatic endocrine cells. Acta Histochem. 2018;120:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 181. | Awad ME, Abdel-Rahman MS, Hassan SA. Acrylamide toxicity in isolated rat hepatocytes. Toxicol In Vitro. 1998;12:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 182. | Yousef MI, El-Demerdash FM. Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology. 2006;219:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 183. | Doroshyenko O, Fuhr U, Kunz D, Frank D, Kinzig M, Jetter A, Reith Y, Lazar A, Taubert D, Kirchheiner J, Baum M, Eisenbrand G, Berger FI, Bertow D, Berkessel A, Sörgel F, Schömig E, Tomalik-Scharte D. In vivo role of cytochrome P450 2E1 and glutathione-S-transferase activity for acrylamide toxicokinetics in humans. Cancer Epidemiol Biomarkers Prev. 2009;18:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 184. | Keklikoglu N, Akinci S. The role of iNOS in beta cell destruction in diabetes. Oxid Antioxid Med Sci. 2013;2:251. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 185. | Omar RA, Chyan YJ, Andorn AC, Poeggeler B, Robakis NK, Pappolla MA. Increased Expression but Reduced Activity of Antioxidant Enzymes in Alzheimer's Disease. J Alzheimers Dis. 1999;1:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 186. | Marković Filipović J, Karan J, Ivelja I, Matavulj M, Stošić M. Acrylamide and Potential Risk of Diabetes Mellitus: Effects on Human Population, Glucose Metabolism and Beta-Cell Toxicity. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 187. | Sigfrid LA, Cunningham JM, Beeharry N, Lortz S, Tiedge M, Lenzen S, Carlsson C, Green IC. Cytokines and nitric oxide inhibit the enzyme activity of catalase but not its protein or mRNA expression in insulin-producing cells. J Mol Endocrinol. 2003;31:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 188. | Song D, Xu C, Holck AL, Liu R. Acrylamide inhibits autophagy, induces apoptosis and alters cellular metabolic profiles. Ecotoxicol Environ Saf. 2021;208:111543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 189. | Yue Z, Chen Y, Song Y, Zhang J, Yang X, Wang J, Li L, Sun Z. Effect of acrylamide on glucose homeostasis in female rats and its mechanisms. Food Chem Toxicol. 2020;135:110894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 190. | Stošić M, Matavulj M, Marković J. Subchronic exposure to acrylamide leads to pancreatic islet remodeling determined by alpha cell expansion and beta cell mass reduction in adult rats. Acta Histochem. 2018;120:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 191. | Stošić M, Matavulj M, Marković J. Effects of subchronic acrylamide treatment on the endocrine pancreas of juvenile male Wistar rats. Biotech Histochem. 2018;93:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 192. | Li Z, Karlsson FA, Sandler S. Islet loss and alpha cell expansion in type 1 diabetes induced by multiple low-dose streptozotocin administration in mice. J Endocrinol. 2000;165:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 193. | Takeda Y, Fujita Y, Honjo J, Yanagimachi T, Sakagami H, Takiyama Y, Makino Y, Abiko A, Kieffer TJ, Haneda M. Reduction of both beta cell death and alpha cell proliferation by dipeptidyl peptidase-4 inhibition in a streptozotocin-induced model of diabetes in mice. Diabetologia. 2012;55:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 194. | Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151-159. [PubMed] |
| 195. | Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 502] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 196. | Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 493] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 197. | Iki K, Pour PM. Distribution of pancreatic endocrine cells including IAPP-expressing cells in non-diabetic and type 2 diabetic cases. J Histochem Cytochem. 2007;55:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 198. | Zhao T, Guo Y, Ji H, Mao G, Feng W, Chen Y, Wu X, Yang L. Short-term exposure to acrylamide exacerbated metabolic disorders and increased metabolic toxicity susceptibility on adult male mice with diabetes. Toxicol Lett. 2022;356:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 199. | Quan W, Jiao Y, Li Y, Xue C, Liu G, Wang Z, Qin F, He Z, Zeng M, Chen J. Metabolic changes from exposure to harmful Maillard reaction products and high-fat diet on Sprague-Dawley rats. Food Res Int. 2021;141:110129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 200. | Ge T, Jhala G, Fynch S, Akazawa S, Litwak S, Pappas EG, Catterall T, Vakil I, Long AJ, Olson LM, Krishnamurthy B, Kay TW, Thomas HE. The JAK1 Selective Inhibitor ABT 317 Blocks Signaling Through Interferon-γ and Common γ Chain Cytokine Receptors to Reverse Autoimmune Diabetes in NOD Mice. Front Immunol. 2020;11:588543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 201. | Karimani A, Hosseinzadeh H, Mehri S, Jafarian AH, Kamali SA, Hooshang Mohammadpour A, Karimi G. Histopathological and biochemical alterations in non-diabetic and diabetic rats following acrylamide treatment. Toxin Rev. 2021;40:277-284. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 202. | Alanazi IS, Emam M, Elsabagh M, Alkahtani S, Abdel-Daim MM. The protective effects of 18β-glycyrrhetinic acid against acrylamide-induced cellular damage in diabetic rats. Environ Sci Pollut Res Int. 2021;28:58322-58330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 203. | Kim JH, Park HY, Bae S, Lim YH, Hong YC. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PLoS One. 2013;8:e71392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 204. | Deng T, Zhang Y, Wu Y, Ma P, Duan J, Qin W, Yang X, Chen M. Dibutyl phthalate exposure aggravates type 2 diabetes by disrupting the insulin-mediated PI3K/AKT signaling pathway. Toxicol Lett. 2018;290:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 205. | Hung CC, Cheng YW, Chen WL, Fang WH. Negative Association between Acrylamide Exposure and Metabolic Syndrome Markers in Adult Population. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 206. | Lin CY, Lin YC, Kuo HK, Hwang JJ, Lin JL, Chen PC, Lin LY. Association among acrylamide, blood insulin, and insulin resistance in adults. Diabetes Care. 2009;32:2206-2211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 207. | Yamamoto J, Ishihara J, Matsui Y, Matsuda T, Kotemori A, Zheng Y, Nakajima D, Terui M, Shinohara A, Adachi S, Kawahara J, Sobue T. Acrylamide-Hemoglobin Adduct Levels in a Japanese Population and Comparison with Acrylamide Exposure Assessed by the Duplicated Method or a Food Frequency Questionnaire. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 208. | Kadawathagedara M, Botton J, de Lauzon-Guillain B, Meltzer HM, Alexander J, Brantsaeter AL, Haugen M, Papadopoulou E. Dietary acrylamide intake during pregnancy and postnatal growth and obesity: Results from the Norwegian Mother and Child Cohort Study (MoBa). Environ Int. 2018;113:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 209. | Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr. 2006;83:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 210. | Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Debras C, Druesne-Pecollo N, Chazelas E, Deschasaux M, Hercberg S, Galan P, Monteiro CA, Julia C, Touvier M. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern Med. 2020;180:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 211. | Levy RB, Rauber F, Chang K, Louzada MLDC, Monteiro CA, Millett C, Vamos EP. Ultra-processed food consumption and type 2 diabetes incidence: A prospective cohort study. Clin Nutr. 2021;40:3608-3614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YF, China; Moriyama K, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Guo X