Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.549
Peer-review started: December 3, 2022
First decision: December 26, 2022
Revised: February 2, 2023
Accepted: April 11, 2023
Article in press: April 11, 2023
Published online: May 15, 2023
Processing time: 163 Days and 0.1 Hours
Fatty liver disease is defined as liver condition characterized by hepatic steatosis, closely related to pathological conditions in type 2 diabetes and obesity. The high prevalence of fatty liver disease in obese patients with type 2 diabetes reached 70%, reflecting the importance of these conditions with fatty liver. Although the exact pathological mechanism of fatty liver disease, specifically non-alcoholic fatty liver disease (NAFLD) remains not completely revealed, insulin resistance is suggested as the major mechanism that bridged the development of NAFLD. Indeed, loss of the incretin effect leads to insulin resistance. Since incretin is closely related to insulin resistance and the resistance of insulin associated with the development of fatty liver disease, this pathway suggested a potential me-chanism that explains the association between type 2 diabetes and NAFLD. Furthermore, recent studies indicated that NAFLD is associated with impaired glucagon-like peptide-1, resulting in decreased incretin effect. Nevertheless, improving the incretin effect becomes a reasonable approach to manage fatty liver disease. This review elucidates the involvement of incretin in fatty liver disease and recent studies of incretin as the management for fatty liver disease.
Core Tip: Type 2 diabetes mellitus (T2DM) is correlated with various metabolic disorders, including fatty liver. The influence of T2DM on incretin hormones contributed to fatty liver development. Impairment in lipid and glucose metabolism, fat oxidation, oxidative stress, and other effects lead to liver fat deposition. Therefore, drugs targeting the incretin hormones may provide beneficial effects on patients.
- Citation: Wibawa IDN, Mariadi IK, Somayana G, Krisnawardani Kumbara CIY, Sindhughosa DA. Diabetes and fatty liver: Involvement of incretin and its benefit for fatty liver management. World J Diabetes 2023; 14(5): 549-559
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/549.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.549
Fatty liver disease is a spectrum of inflammatory diseases, ranging from hepatic steatosis to cirrhosis. In a continuous process, it may develop into fibrosis and cirrhosis. The diagnosis of non-alcoholic fatty liver disease (NAFLD) remains challenging since the current definition is a diagnosis of exclusion. Consensus stated that the diagnosis of NAFLD could be made if liver fat accumulates > 5% without any other cause. This makes the diagnosis very challenging due to the influence of other variables. The current definition also suggests that a liver biopsy is required to determine the degree of fat accumulation. The health burden of NAFLD present significant concern, since the prevalence of NAFLD is increased and affects 25% of people globally. The economic impact of NAFLD also become major concern since the financial burden reaching $100 billion per year[1].
The pathophysiology of NAFLD is multifactorial, involving metabolic factors. Among all factors contributing to the development of NAFLD, impairment in hormones become important variables to be considered. Impairment of hormones affected lipid and glucose metabolism, interference with other hormones’ signaling, and oxidative stress[2]. Among hormones associated with the development of NAFLD, incretin hormones become an interest.
Incretin hormones influence glucose homeostasis and are involved with the pathophysiology of type 2 diabetes mellitus (T2DM). Incretin hormone is a gut peptide which secreted after nutritional intake. Incretin hormones consist of GIP (glucose-dependent insulinotropic polypeptide) dan GLP-1 (glucagon-like peptide-1). Both affect lipid metabolism, insulin release, oxidative stress, and other factors associated with glucose metabolism. This important aspect of incretin hormones makes it involved in other metabolic diseases, including NAFLD. Hence, it also served as the target to improve the outcome of metabolic diseases. In this review, we elaborate on the mechanism of incretin hormones and the reported recent studies which evaluate the clinical aspect of incretin hormones in NAFLD[3,4].
The work of incretin hormones, known as the incretin effect, works more effectively when the glucose is administered orally compared to administered intravenously (two to three times more effective). Other substances are also involved in the mechanism of incretin hormone; inhibitors of dipeptidyl peptidase-4 (DPP-4 inhibitors) involved in the therapeutic efficacy of incretin effects. The DPP-4 inhibitors increase the concentration of GLP-1[3,4].
Oral glucose intake leads to an increment of insulin secretion stimulation compared to intravenous glucose infusion. This effect occurred even though the iso glycemic condition was reached[5]. This phenomenon occurred because of incretin hormone release (GIP and GLP-1) after oral glucose intake from the gut entero-endocrine. This condition did not occur after intravenous glucose infusion[5,6]. The secreted incretin hormones acted as endocrine signals to the pancreatic islet of Langerhans. These lead to the increment of insulin secretion and glucagon secretion modulation when glucose concentration is above 66 mg/dL.
Pancreatic β-cells have GIP and GLP-1 receptors in their membrane. In the event of the binding of its receptor with its ligands, the activated receptors will bind with adenylate cyclase. This resulted in increased cyclic adenosine monophosphate (AMP) production, leading to protein kinase A activation[7,8]. However, this signaling pathway did not release pre-formed insulin secretory granules from pancreatic β-cells. In order to release the granules, the closure of the potassium channel, depolarization, and calcium ion influx initiated by the hyperglycemic condition is needed. Therefore, the effects of an increase in insulin release due to incretins always require hyperglycemia in certain limits (66 mg/dL)[9].
Another effect of incretin hormone is glucagon release. GIP molecule stimulates glucagon release, particularly in decreased glucose concentration, while GLP-1 suppresses glucagon secretion in hyperglycemia, resulting in hepatic glucose production[10,11]. The mechanism of incretin hormones in the liver is indirectly mediated since no GLP-1 receptors exist. The mechanism responsible for this phenomenon is the autonomous nervous system.
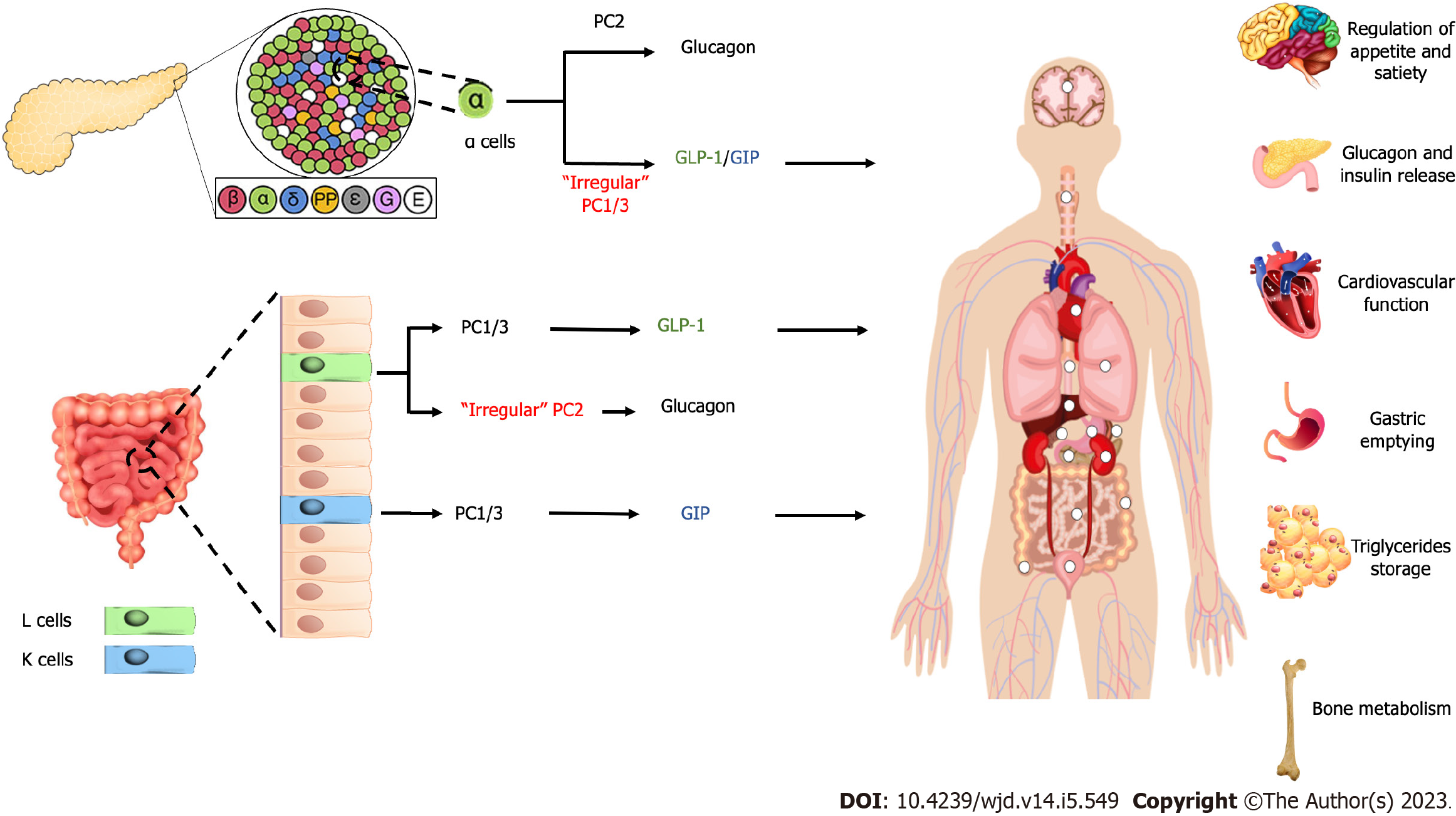
The incretin hormones possess additional biological effects on other organs. GLP-1 hinders appetite and food intake. GLP-1 also increases satiety. The GLP-1 receptors were observed in the hypothalamus[12]. GLP-1, derived from blood flow circulation, enter the brain through the circumventricular organ, characterized by a leaky blood-brain barrier. Therefore, GLP-1, with chronic stimulation of its receptor, is considered a signal to suppress appetite, which acts as a basic mechanism for a decrease in body weight[4,13].
Another additional effect of incretin hormone is the triglycerides storage in adipose tissue. GIP induces lipoprotein lipase, an enzyme that releases fatty acid from triglycerides chylomicrons in adipose tissue; hence it eliminates triglycerides chylomicrons. However, this is still based on animal studies; it is still uncertain whether the same occurred in humans[14,15].
Gastric emptying is also affected by GLP-1 but not by GIP[16,17]. The consequence of this effect is the nutritional delivery to the intestinal lumen is hampered. The decreased absorption of nutrition resulted in the stagnant increase of blood glucose and triglycerides after a meal[18].
Other effects of incretin include bone metabolism and cardiovascular function. Regarding bone metabolism, animal study of GIP found that the signaling pathway through GIP receptors inhibits bone resorption, both from the amount and the function of osteoclast, and supports bone formation (osteoblast function)[19]. The effect of incretins on the cardiovascular system is related to their role in cardiac blood supply, vasodilatation, inflammation response in adipose tissue and blood vessels, substrate intake, cytokine release and atherosclerosis formation, and plaque stabilization[20,21].
It should be noted that the dogma of proglucagon produced in α-cells of the pancreas and GLP produced by intestinal L cells has been challenged. It has been suggested that after total pancreatectomy, glucagon produced by intestinal cells and GLP-1 exist in pancreatic α-cells[22,23]. The animal study suggested that GLP-1 produced by pancreatic α-cells have more potent effect on glucose homeostasis than intestinal cells-produced GLP-1[24]. This showed that the physiological mechanism of the incretin hormones is not as simple as it is known currently. The mechanism of glucagon formation by pancreatic α-cells primarily mediated by prohormone convertase (PC) 2[25], while PC 1/3 acts as the main prohormone for the formation of GLP-1 and GIP[26,27]. It has been suggested that irregular expression of PC 1/3 in the pancreas and PC2 in the intestinal becomes a reason for the existence of GLP-1 in the pancreas[23,28,29] and glucagon in intestinal (Figure 1)[22,30].
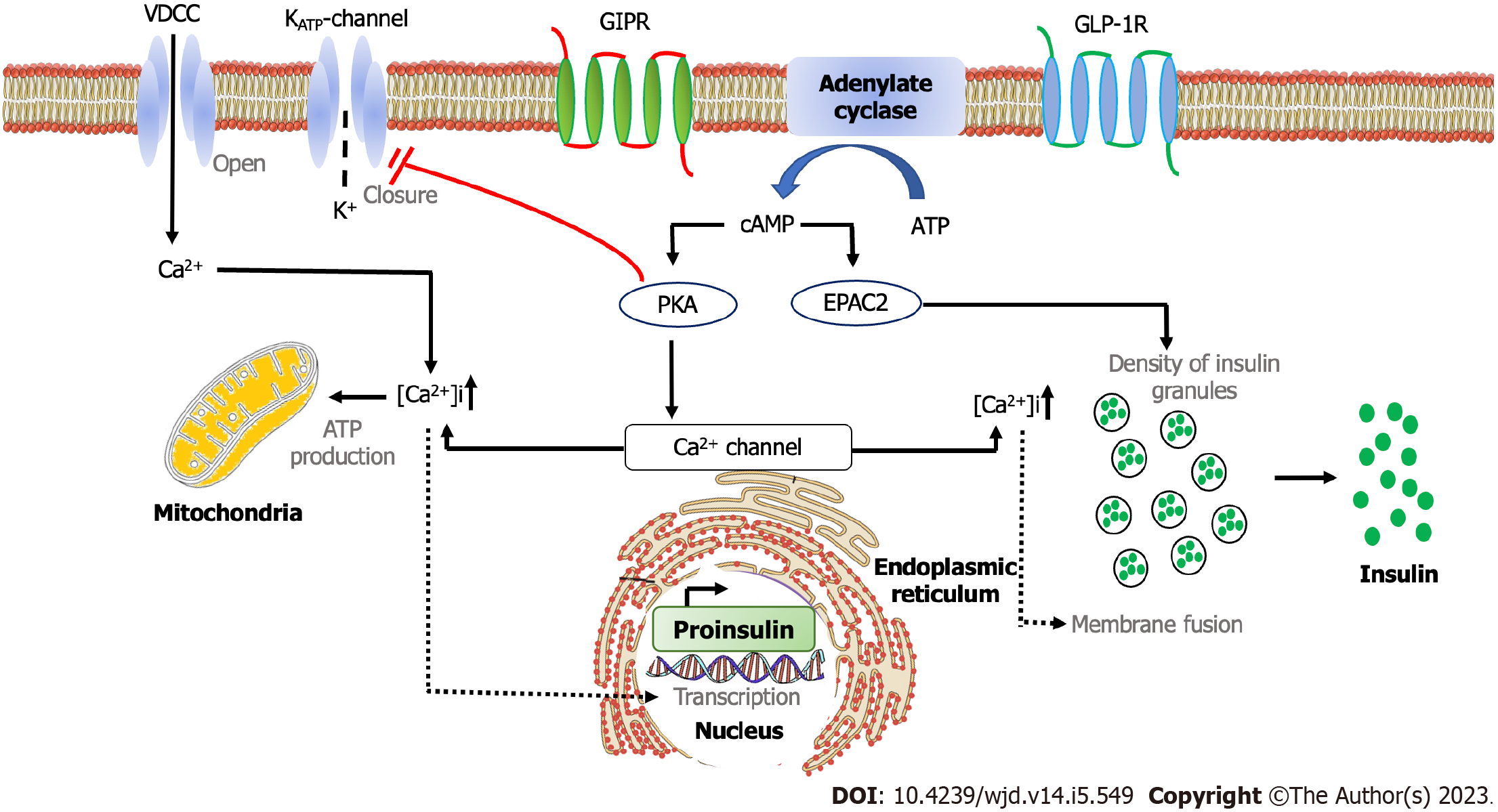
The intracellular mechanism of incretin hormone started with the binding of GIP and GLP-1 with their respective receptors, GIP receptors and GLP-1 receptors. It resulted in the activation of adenylate cyclase and the increase of intracellular cyclic adenosine monophosphate (cAMP), leading to protein kinase A (PKA) activation and protein activated by cAMP2 (EPAC2). The activation of PKA induces the closure of the adenosine triphosphate-sensitive potassium channel and facilitates membrane depolarization and the prolongation of potential action. Depolarization opens the voltage-gated Ca2+ channel, which leads to an increase in intracellular Ca2+. The increased Ca2+ concentration triggers the fusion of insulin-containing granules with the plasma membrane and insulin secretion from pancreatic β cells. The increase of Ca2+ levels also drives the transcription of the proinsulin gene, therefore increasing the insulin content of β cells. Furthermore, the activation of EPAC2 increases the density of insulin-containing granules near the plasma membrane to potentiate the secretion of insulin from β cells (Figure 2)[31].
The pathophysiology of fatty liver disease related to metabolic factors, including NAFLD, is intricate due to its multifactorial nature and related to various comorbidities. The accumulation of liver fat is caused by the imbalance in fatty acid influx (lipolysis of fat tissue), fat disposition (fatty acid disposition), lipogenesis hepatic de novo and very low density lipoprotein secretion by the liver[32]. The progressivity of fatty liver disease involves the interaction of cellular stress response (lipotoxicity and increase of oxidative stress)[33] and liver fat accumulation along with cytotoxicity[33]. The association of gut and hormones released from the pancreas, insulin resistance in muscle, adipose tissue and liver, and gut microbiome are also involved in the pathophysiology of NAFLD. Obesity contributes to fatty liver disease by causing adipocyte hypertrophy and hypoxia, resulting in macrophage influx and pro-inflammatory conditions[34]. The pro-inflammatory condition causing the development of insulin resistance leads to hepatic steatosis. The insulin resistance increases lipolysis and causes the increase of free fatty acids. Hepatic lipotoxicity is caused by the increment of long-chain fatty acids, diacylglycerol, and ceramide, which stored in the liver, causing the release of reactive oxygen species. These contributed to inflammation and liver fibrosis, along with the apoptosis of hepatocytes. Moreover, the increase in hepatic steatosis leads to the resistance of the liver toward insulin, worsening the condition[35].
Type 2 diabetes and metabolic syndromes are closely related to NAFLD[36]. Individuals with T2DM possess a five times greater risk of NAFLD and a greater likelihood to progress toward non-alcoholic steatohepatitis (NASH) when compared to people without T2DM[37]. However, liver steatosis is partly an adaptive and protective response; lipotoxic free fatty acids is stored as a more stable component. However, this protective nature becomes weakened with continuous liver problems along with other contributed factors, e.g., T2DM and genetic predisposition. This, in turn, causes hepatocyte injury and fibrosis[38]. Insulin resistance in the liver is caused by proinflammatory cytokine (tumor necrosis factor α, interleukin-6), proinflammatory pathway, e.g. c-Jun and nuclear factor-kappaB, endoplasmic reticulum stress, and lipid metabolism product.
Incretin hormones are secreted in T2DM patients as well as healthy individuals and obese patients. An early study showed a slight increase of GIP in patients with T2DM and decreased response to GLP-1[39,40], while subjects with impaired glucose tolerance have an intermediate response to GLP-1. Therefore, it has been hypnotized that there is a progressive loss in GLP-1 secretion along with the severity of T2DM. Study has been conducted to compare the secretion of GIP and GLP-1 between healthy and T2DM subjects after oral glucose loads administration and mixed food. There is a slight difference in which lower secretion in T2DM patients. However, another study also found no difference in GIP and GLP-1 between those two populations. A meta-analysis study showed no difference in the secretion of GIP and GLP-1 after nutrition loads between T2DM and healthy subjects[41-43].
Even though the excretion of incretin is approximately normal in T2DM patients, the difference in the characteristic between T2DM and healthy subjects exist in the insulinotropic activity of GIP and GLP-1. GIP is considered a drug candidate for the development of a glucose-lowering agent. In this regard, there is no doubt that physiological and pharmacological concentrations of GLP-1 also exhibit insulinotropic features in T2DM patients[10]. Inappropriate response to GIP may explain the lower effects of incretin hormones in T2DM patients compared to healthy subjects[10,44]. Previously conducted studies have found that the reduced incretin effects occurred after the diagnosis of T2DM was confirmed. Hence it has been suggested that the decrease in incretin effects is secondary to this condition[45]. It is still not fully elucidated which features of T2DM, e.g., inflammatory infiltration of β-cells, hyperglycemia, islet lipid overload, or other mechanisms may trigger this phenomenon[5,45]. The reduced expression of GIP receptors or substances involved in the GIP signaling pathway is also suggested to explain the impairment in insulin secretion[46]. Although animal study with diabetic hyperglycemia has found that GIP receptors are decreased, the same is not found in the human pancreas. In conclusion, type 2 diabetes condition reduces the incretin effect and worsens glycemic control. This situation leads to glucotoxicity. Glucotoxicity resulted in a reduction of beta cell mass in the pancreas and reduced expression of GIP receptors. These will further reduce the incretin effect, creating a vicious cycle. Numerous studies with insulin treatment to control hyperglycemia to reach a near-normal value of glucose concentrations have been done. Insulin treatment may improve the insulinotropic of GIP and GLP-1 in T2DM patients, therefore leads to improvement of the incretin effects[47,48].
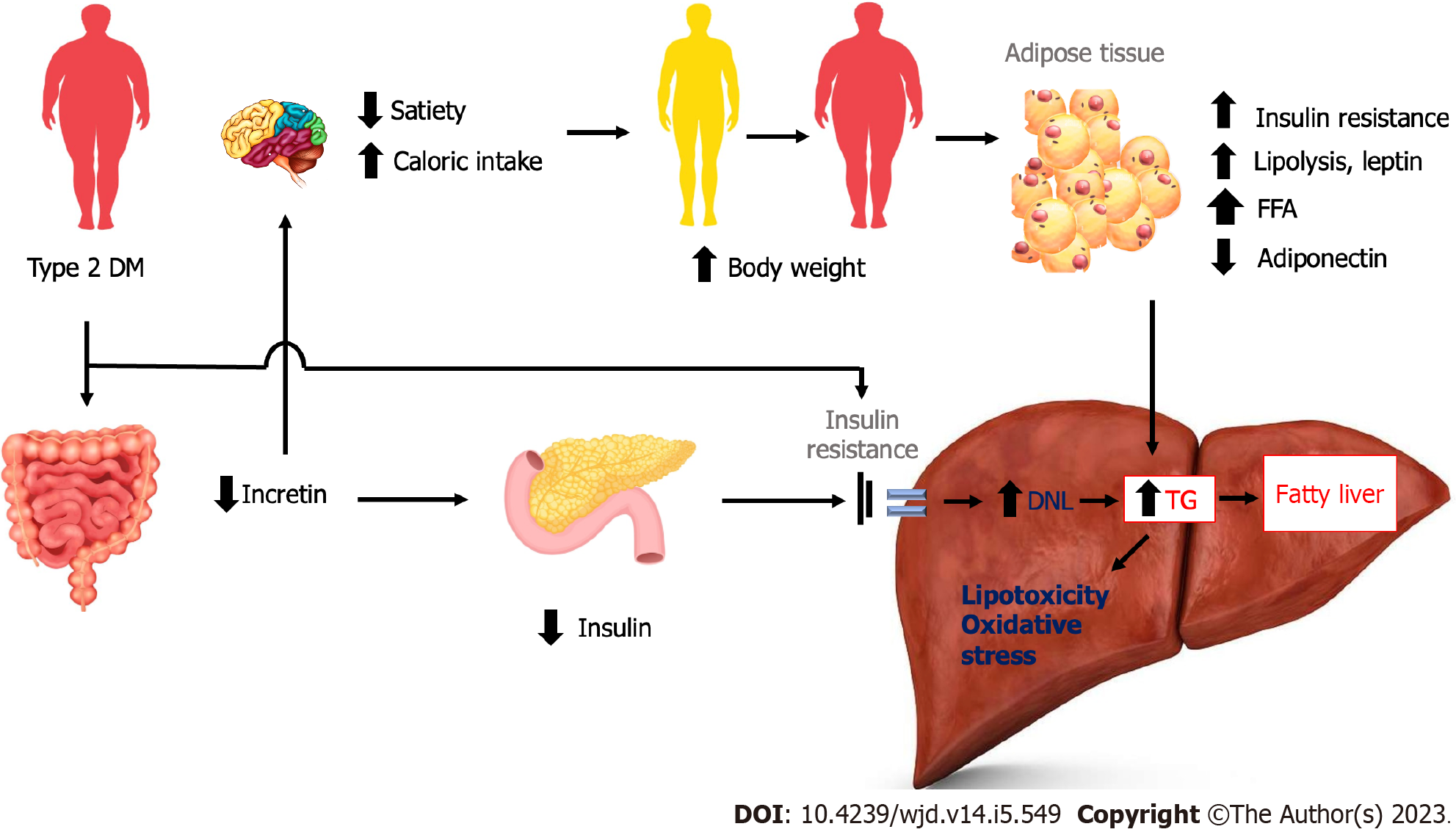
The reduced incretin effects may result in further damage of hepatocytes. Reduced incretin effects may reduce satiety and caloric intake, resulting in increased body weight. The increase in body weight leads to adipose tissue insulin resistance, increased lipolysis and leptin, and decreased adiponectin. The final result leads to increased hepatic insulin resistance, de novo lipogenesis, and hepatic fat deposition. Reduced incretin effects also lead to reduced insulin release, resulting in increased adipose tissue insulin resistance and increased hepatic insulin resistance and hepatic fat deposition. Another mechanism of decreased incretin is increased dietary fats and chylomicrons, resulting in increased hepatic fat deposition (Figure 3)[2].
An approach to modulate the expression and activity of incretin hormones may benefit fatty liver disease. The effect of incretin could improve the satiety, therefore reduced caloric intake. The insulin resistance could be improved, leading to downregulation of lipid in liver, lipotoxicity and oxidative stress, providing beneficial effect in NAFLD patients.
The primary treatment of fatty liver, particularly NAFLD, is decreasing body weight. The decrease of body weight by 10% with regulating diet and physical activity decreases the triglycerides concentration by 60% in overweight people[49]. Another modality is bariatric surgery for patients with severe obesity. This modality may significantly improve lobular inflammation and NASH in 50%-85% of cases[50]. Management with pharmacologic agents remains explored to discover the agent that can give significant efficacy. In short, the pharmacological agents may be classified into agents to improve metabolic impairment, including body weight, inflammation with oxidative stress and dysregulation in the gut-liver axis[51]. In regards to those specific points, the pharmacological agent is ideally able to work in all those mechanisms.
A study showed that GLP-1 had the effect of inducing satiety through the central mechanism in hypothalamus and brain stem. The use of GLP-1 also decreases caloric uptake. These results were obtained from observation of person with obesity and T2DM. A decrease in body weight is also a consistent discovery obtained from clinical trials with GLP-1 receptor agonists (GLP-1RAs). Chronic use of GLP-1 is also expected to improve insulin sensitivity since it is related to its effect on decreasing body weight. Other effects of GLP-1RA administration in NAFLD patients are also related to increased total adiponectin serum concentration and improvement of dysfunctional adipose tissue[52]. Liraglutide also decreases fasting leptin serum levels. Adiponectin is able to repair liver impairment related to fatty liver injury by regulating liver fatty acid oxidation and activity of acetyl-CoA carboxylase as well as fatty acid synthase, which acts as the main enzyme to synthesize fatty acid[53]. The randomized controlled trials of several studies already conducted on the effects of GLP-1RAs toward fatty liver conditions are summarized in Table 1.
| Ref. | Subjects | Intervention (dose) | Comparator | Effects |
| Jendle et al[59], 2009 | T2DM | Liraglutide (1.8-1.2-0.6 mg/day) with additional metformin administration | Glimepiride 4 mg or placebo with metformin | 10% attenuation ratio of liver-spleen |
| Fan et al[60], 2013 | Overweight T2DM | Exenatide (2 x 10µg) | Metformin | Decrease in liver enzyme |
| Shao et al[61], 2014 | Overweight/obese T2DM | Exenatide (2 x 10 µg) | Insulin glargine | Decrease of liver enzymes and degree of fatty liver on ultrasound |
| Tang et al[62], 2015 | Overweight/obese T2DM | Liraglutide 0.6 to 1.8 mg/day | Insulin glargine | No difference in the decrease of liver fat |
| Armstrong et al[63], 2016 | Overweight/obese (17 out of 52 subjects with T2DM) | Liraglutide (1.8 mg/day) | Placebo | Improvement in NASH histology by 39% |
| Smits et al[64], 2016 | Overweight/obese T2DM | Liraglutide (1.8 mg/day) | Sitagliptin, placebo | No difference in liver fat content |
| Dutour et al[65], 2016 | T2DM | Exenatide 5-10 mcg twice a day | Placebo | Significant decrease in body weight and liver fat content in the exenatide group |
| Khoo et al[66], 2017 | Obesity patients without T2DM | Liraglutide (3 mg/day) | Lifestyle intervention | No difference in reducing liver fat |
| Feng et al[67], 2017 | T2DM | Liraglutide (1.8 mg/day) | Metformin or glicazide | Improvement in hepatic/renal index ratio |
| Frøssing et al[68], 2018 | Women with PCOS and NAFLD | Liraglutide 1.8 mg/day | Placebo | Decrease of body weight by 5.2 kg (5.6% from baseline), liver fat content by 44%, decrease the prevalence of NAFLD by about two-thirds and decrease of fasting blood glucose |
| Yan et al[69], 2019 | T2DM and NAFLD | Liraglutide 1.8 mg/day | Insulin glargine and sitagliptin | Decreased liver fat content, reduction of HbA1c levels in all groups, decrease in body weight |
| Khoo et al[70], 2019 | Obese and NAFLD | Liraglutide 3.0 mg/day | Lifestyle changing | The two groups had decrease of liver fat content |
| Liu et al[71], 2020 | T2DM and NAFLD | Exenatide 1.8 mg/day | Insulin glargine | Decrease of liver fat content, greater reduction of visceral adipose tissue |
| Bizino et al[72], 2020 | T2DM and NAFLD | Liraglutide 1.8 mg/day | Placebo | Reduced body weight, but the liver content was not different |
| Kuchay et al[73], 2020 | T2DM and NAFLD | Dulaglutide 1.5 mg/week | Placebo | Control-corrected absolute change in liver fat content of -3.5% and relative change of -26.4% |
| Newsome et al[74], 2020 | NASH and liver fibrosis | Semaglutide 0.1 mg/day, 0.2 mg/day, and 0.4 mg/day | Placebo | A higher percentage of NASH resolution without worsening of fibrosis, dose-dependent decrease of serum ALT and AST, and higher mean percentage weight loss |
Dual incretin receptor agonists are new pharmacological agents that act on GLP-1 and GIP receptors[54]. The new dual incretin receptor agonists have a synergistic effect. The synergistic effects of these pharmacologic agents lead to reduced total liver fat content, risk of cardiovascular disease, body weight and blood glucose levels [determined by glycated hemoglobin, or hemoglobin A1c (HbA1c)][55].
The clinical aspect of dual incretin receptor agonists has been showed in several studies. Tirzepatide, the dual receptor agonist which administered subcutaneously, was approved by the United States US Food and Drug Administration for glycemic control in T2DM patients, In May 2022[56]. Tirzepatide, compared to semaglutide and insulin, showed a greater reduction of HbA1c[56]. A study by Hartman et al[57] in 2020 showed that tirzepatide reduce several biomarkers of steatohepatitis, including N-terminal type III collagen propeptide, keratin-18, aspartate aminotransferase, and alanine aminotransferase. The study also showed the increase of adiponectin levels. A phase 2b, 26-wk trial of tirzepatide in T2DM patients showed superior effect of tirzepatide compared to dulaglutide in terms of glucose control and reduction in body weight[58]. Tirzepatide of 5 mg, 10 mg, and 15 mg decrease HbA1c levels by 1.6%, 2.0%, and 2.4%, respectively. When compared to 1.5 mg of dulaglutide administration, the decrease of HbA1c only 1.1%. A total of 48% of patients achieved normoglycemia (HbA1c 5.7%) compared with 2% of subjects treated with dulaglutide[58].
In conclusion, incretin hormones affect various signaling and mechanisms of lipid and glucose metabolism, insulin release, regulation of glucagon, oxidative stress, the central mechanism of satiety, and various other effects, involved in the development of NAFLD. The importance of the incretin effect on the development and progressivity of NAFLD makes it an ideal target for its management. Clinical research has provide evidence toward beneficial effect on liver content and other metabolic parameters. Further recommendations for drugs targeting the regulation of the incretin effect need to be considered in future studies. Also, future studies on the adverse events of incretin modulation for fatty liver disease should be directed, therefore its safety could be emphasized.
| 1. | Vincent RK, Williams DM, Evans M. A look to the future in non-alcoholic fatty liver disease: Are glucagon-like peptide-1 analogues or sodium-glucose co-transporter-2 inhibitors the answer? Diabetes Obes Metab. 2020;22:2227-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Seghieri M, Christensen AS, Andersen A, Solini A, Knop FK, Vilsbøll T. Future Perspectives on GLP-1 Receptor Agonists and GLP-1/glucagon Receptor Co-agonists in the Treatment of NAFLD. Front Endocrinol (Lausanne). 2018;9:649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2787] [Cited by in RCA: 2894] [Article Influence: 144.7] [Reference Citation Analysis (1)] |
| 4. | Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 6. | Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 495] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1316] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 8. | Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199-E206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 413] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hüfner M, Schmiegel WH. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 393] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1148] [Cited by in RCA: 1234] [Article Influence: 37.4] [Reference Citation Analysis (7)] |
| 11. | Hvidberg A, Nielsen MT, Hilsted J, Orskov C, Holst JJ. Effect of glucagon-like peptide-1 (proglucagon 78-107amide) on hepatic glucose production in healthy man. Metabolism. 1994;43:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473-4488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 729] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 13. | Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 961] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 14. | Eckel RH, Fujimoto WY, Brunzell JD. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes. 1979;28:1141-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Wasada T, McCorkle K, Harris V, Kawai K, Howard B, Unger RH. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. J Clin Invest. 1981;68:1106-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981-E988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 296] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Meier JJ, Goetze O, Anstipp J, Hagemann D, Holst JJ, Schmidt WE, Gallwitz B, Nauck MA. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am J Physiol Endocrinol Metab. 2004;286:E621-E625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Meier JJ, Gethmann A, Götze O, Gallwitz B, Holst JJ, Schmidt WE, Nauck MA. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. 2006;49:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 213] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Tsukiyama K, Yamada Y, Yamada C, Harada N, Kawasaki Y, Ogura M, Bessho K, Li M, Amizuka N, Sato M, Udagawa N, Takahashi N, Tanaka K, Oiso Y, Seino Y. Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol Endocrinol. 2006;20:1644-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology. 1999;140:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Drucker DJ. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016;24:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 505] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 22. | Lund A, Bagger JI, Wewer Albrechtsen NJ, Christensen M, Grøndahl M, Hartmann B, Mathiesen ER, Hansen CP, Storkholm JH, van Hall G, Rehfeld JF, Hornburg D, Meissner F, Mann M, Larsen S, Holst JJ, Vilsbøll T, Knop FK. Evidence of Extrapancreatic Glucagon Secretion in Man. Diabetes. 2016;65:585-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Marchetti P, Lupi R, Bugliani M, Kirkpatrick CL, Sebastiani G, Grieco FA, Del Guerra S, D'Aleo V, Piro S, Marselli L, Boggi U, Filipponi F, Tinti L, Salvini L, Wollheim CB, Purrello F, Dotta F. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55:3262-3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim KS, Gutierrez-Aguilar R, Li B, Drucker DJ, D'Alessio DA, Seeley RJ, Sandoval DA. The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice. Cell Metab. 2017;25:927-934.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 25. | Rouillé Y, Westermark G, Martin SK, Steiner DF. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells. Proc Natl Acad Sci U S A. 1994;91:3242-3246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 159] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Rouillé Y, Martin S, Steiner DF. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. J Biol Chem. 1995;270:26488-26496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 162] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Ugleholdt R, Poulsen ML, Holst PJ, Irminger JC, Orskov C, Pedersen J, Rosenkilde MM, Zhu X, Steiner DF, Holst JJ. Prohormone convertase 1/3 is essential for processing of the glucose-dependent insulinotropic polypeptide precursor. J Biol Chem. 2006;281:11050-11057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Heller RS, Aponte GW. Intra-islet regulation of hormone secretion by glucagon-like peptide-1-(7--36) amide. Am J Physiol. 1995;269:G852-G860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Masur K, Tibaduiza EC, Chen C, Ligon B, Beinborn M. Basal receptor activation by locally produced glucagon-like peptide-1 contributes to maintaining beta-cell function. Mol Endocrinol. 2005;19:1373-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Nauck MA, Quast DR, Wefers J, Pfeiffer AFH. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes Metab. 2021;23 Suppl 3:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 31. | Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010;1:8-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 535] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 32. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2261] [Article Influence: 205.5] [Reference Citation Analysis (1)] |
| 33. | Higarza SG, Arboleya S, Gueimonde M, Gómez-Lázaro E, Arias JL, Arias N. Neurobehavioral dysfunction in non-alcoholic steatohepatitis is associated with hyperammonemia, gut dysbiosis, and metabolic and functional brain regional deficits. PLoS One. 2019;14:e0223019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Parker R. The role of adipose tissue in fatty liver diseases. Liver Res. 2018;2:35-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 35. | Finck BN. Targeting Metabolism, Insulin Resistance, and Diabetes to Treat Nonalcoholic Steatohepatitis. Diabetes. 2018;67:2485-2493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 957] [Article Influence: 95.7] [Reference Citation Analysis (1)] |
| 37. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1631] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 38. | Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016;11:451-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 39. | Jones IR, Owens DR, Luzio S, Williams S, Hayes TM. The glucose dependent insulinotropic polypeptide response to oral glucose and mixed meals is increased in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:668-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 553] [Article Influence: 22.1] [Reference Citation Analysis (8)] |
| 41. | Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 42. | Calanna S, Christensen M, Holst JJ, Laferrère B, Gluud LL, Vilsbøll T, Knop FK. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 43. | Calanna S, Christensen M, Holst JJ, Laferrère B, Gluud LL, Vilsbøll T, Knop FK. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care. 2013;36:3346-3352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 44. | Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002;45:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 392] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 45. | Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes. 2010;59:1117-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 46. | Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, King GL, Weir GC, Bonner-Weir S. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 299] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 47. | Højberg PV, Vilsbøll T, Zander M, Knop FK, Krarup T, Vølund A, Holst JJ, Madsbad S. Four weeks of near-normalization of blood glucose has no effect on postprandial GLP-1 and GIP secretion, but augments pancreatic B-cell responsiveness to a meal in patients with Type 2 diabetes. Diabet Med. 2008;25:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Højberg PV, Vilsbøll T, Rabøl R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 335] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 49. | Johnson NA, George J. Fitness versus fatness: moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology. 2010;52:370-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Aguilar-Olivos NE, Almeda-Valdes P, Aguilar-Salinas CA, Uribe M, Méndez-Sánchez N. The role of bariatric surgery in the management of nonalcoholic fatty liver disease and metabolic syndrome. Metabolism. 2016;65:1196-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 51. | Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 347] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 52. | Cuthbertson DJ, Irwin A, Gardner CJ, Daousi C, Purewal T, Furlong N, Goenka N, Thomas EL, Adams VL, Pushpakom SP, Pirmohamed M, Kemp GJ. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One. 2012;7:e50117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 53. | Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 54. | Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J Clin Endocrinol Metab. 2021;106:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 55. | Ordóñez-Vázquez AL, Beltrán-Gall SM, Pal SC, Méndez-Sánchez N. Editorial: Treatment with Dual Incretin Receptor Agonists to Maintain Normal Glucose Levels May Also Maintain Normal Weight and Control Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Med Sci Monit. 2022;28:e938365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Food and Drug Administration (FDA) News Release. FDA approves novel, dual-targeted treatment for type 2 diabetes in clinical trials, treatment proved more effective than other therapies evaluated. [Internet] [cited 13 May 2022]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes. |
| 57. | Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, Karanikas CA, Duffin KL, Robins DA, Haupt A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care. 2020;43:1352-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 58. | Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, Gimeno RE, Milicevic Z, Robins D, Haupt A. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 610] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 59. | Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, Düring M, Zdravkovic M, Strauss BJ, Garber AJ; LEAD-2 and LEAD-3 Study Groups. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 60. | Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol. 2013;57:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 62. | Tang A, Rabasa-Lhoret R, Castel H, Wartelle-Bladou C, Gilbert G, Massicotte-Tisluck K, Chartrand G, Olivié D, Julien AS, de Guise J, Soulez G, Chiasson JL. Effects of Insulin Glargine and Liraglutide Therapy on Liver Fat as Measured by Magnetic Resonance in Patients With Type 2 Diabetes: A Randomized Trial. Diabetes Care. 2015;38:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 63. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1589] [Article Influence: 158.9] [Reference Citation Analysis (1)] |
| 64. | Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters-van den Bos IC, Hoekstra T, Diamant M, van Raalte DH, Cahen DL. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. 2016;59:2588-2593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 65. | Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, Ronsin O, Pradel V, Lesavre N, Martin JC, Jacquier A, Lefur Y, Bernard M, Gaborit B. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 66. | Khoo J, Hsiang J, Taneja R, Law NM, Ang TL. Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: A pilot randomized trial. Diabetes Obes Metab. 2017;19:1814-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 67. | Feng W, Gao C, Bi Y, Wu M, Li P, Shen S, Chen W, Yin T, Zhu D. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 68. | Frøssing S, Nylander M, Chabanova E, Frystyk J, Holst JJ, Kistorp C, Skouby SO, Faber J. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: A randomized clinical trial. Diabetes Obes Metab. 2018;20:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 69. | Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, Li Y, Dou J, Yang W, Qin G, Yuan H, Xiao X, Luo S, Shan Z, Deng H, Tan Y, Xu F, Xu W, Zeng L, Kang Z, Weng J. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:2414-2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 70. | Khoo J, Hsiang JC, Taneja R, Koo SH, Soon GH, Kam CJ, Law NM, Ang TL. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int. 2019;39:941-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 71. | Liu L, Yan H, Xia M, Zhao L, Lv M, Zhao N, Rao S, Yao X, Wu W, Pan B, Bian H, Gao X. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 72. | Bizino MB, Jazet IM, de Heer P, van Eyk HJ, Dekkers IA, Rensen PCN, Paiman EHM, Lamb HJ, Smit JW. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia. 2020;63:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 73. | Kuchay MS, Krishan S, Mishra SK, Choudhary NS, Singh MK, Wasir JS, Kaur P, Gill HK, Bano T, Farooqui KJ, Mithal A. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial). Diabetologia. 2020;63:2434-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 74. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1399] [Article Influence: 279.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Indonesian Society of Internal Medicine, 111.1986.0015.00692; Indonesian Society of Gastroenterology.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: L Cai, United States; Huang W, China; Liao Z, Singapore; Srinivasan AR, India S-Editor: Liu GL L-Editor: A P-Editor: Zhang XD