Published online Dec 15, 2023. doi: 10.4239/wjd.v14.i12.1862
Peer-review started: September 21, 2023
First decision: October 10, 2023
Revised: October 20, 2023
Accepted: November 17, 2023
Article in press: November 17, 2023
Published online: December 15, 2023
Processing time: 83 Days and 20.1 Hours
Diabetic cardiomyopathy (DCM) increases the risk of hospitalization for heart failure (HF) and mortality in patients with diabetes mellitus. However, no specific therapy to delay the progression of DCM has been identified. Mitochondrial dysfunction, oxidative stress, inflammation, and calcium handling imbalance play a crucial role in the pathological processes of DCM, ultimately leading to cardio
To investigate the effects of empagliflozin on high glucose (HG)-induced oxidative stress and cardiomyocyte apoptosis and the underlying molecular mechanism.
Twelve-week-old db/db mice and primary cardiomyocytes from neonatal rats stimulated with HG (30 mmol/L) were separately employed as in vivo and in vitro models. Echocardiography was used to evaluate cardiac function. Flow cytometry and TdT-mediated dUTP-biotin nick end labeling staining were used to assess apoptosis in myocardial cells. Mitochondrial function was assessed by cellular ATP levels and changes in mitochondrial membrane potential. Furthermore, intracellular reactive oxygen species production and superoxide dismutase activity were analyzed. Real-time quantitative PCR was used to analyze Bax and Bcl-2 mRNA expression. Western blot analysis was used to measure the phos
In the in vivo experiment, db/db mice developed DCM. However, the treatment of db/db mice with empagliflozin (10 mg/kg/d) for 8 wk substantially enhanced cardiac function and significantly reduced myocardial apoptosis, accompanied by an increase in the phosphorylation of AMPK and PGC-1α protein levels, as well as a decrease in the phosphorylation of MYPT1 in the heart. In the in vitro experiment, the findings indicate that treatment of cardiomyocytes with empagliflozin (10 μM) or fasudil (FA) (a ROCK inhibitor, 100 μM) or overexpression of PGC-1α significantly attenuated HG-induced mitochondrial injury, oxidative stress, and cardiomyocyte apoptosis. However, the above effects were partly reversed by the addition of compound C (CC). In cells exposed to HG, empagliflozin treatment increased the protein levels of p-AMPK and PGC-1α protein while decreasing phos
Empagliflozin partially achieves anti-oxidative stress and anti-apoptotic effects on cardiomyocytes under HG conditions by activating AMPK/PGC-1α and suppressing of the RhoA/ROCK pathway independent of SGLT2.
Core Tip: We established a diabetic cardiomyopathy model in db/db mice and treated the mice with empagliflozin for 8 wk, and found that empagliflozin observably improved cardiac function in diabetic mice, which was maybe related to activation of AMP-activated protein kinase (AMPK)/peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and inhibition of the RhoA/ROCK pathway. In order to exclude the effects of metabolic improvement on the heart in vivo, in vitro experiment in high glucose conditions was performed. The results confirmed that the anti-oxidative stress and anti-apoptotic effects of empagliflozin on cardiomyocytes were achieved by activating AMPK/PGC-1α and inhibiting ROCK. Furthermore, the effects were independent of sodium-glucose cotransporter (SGLT)2 inhibition as no SGLT2 expression was detected on cardiomyocytes.
- Citation: Li N, Zhu QX, Li GZ, Wang T, Zhou H. Empagliflozin ameliorates diabetic cardiomyopathy probably via activating AMPK/PGC-1α and inhibiting the RhoA/ROCK pathway. World J Diabetes 2023; 14(12): 1862-1876
- URL: https://www.wjgnet.com/1948-9358/full/v14/i12/1862.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i12.1862
Diabetic cardiomyopathy (DCM) is characterized by systolic and diastolic dysfunction, eventually resulting in heart failure (HF) in patients with diabetes mellitus (DM) in the absence of hypertension, coronary artery disease, and valvular heart disease[1,2]. To date, no specific treatments have been identified to delay the progression of DCM. The pathological processes of DCM include insulin resistance, mitochondrial dysfunction, oxidative stress, calcium handling imbalance, and inflammation[3]. In hyperglycemia environments, reduced antioxidant enzymes and increased production of reactive oxygen species (ROS), defined as oxidative stress[4], affect various signaling pathways involved in the occurrence of DCM, ultimately leading to cardiomyocyte apoptosis and cardiac dysfunction[5]. Myocardial oxidative stress and apoptosis are key components of its pathogenesis, and their occurrence has been confirmed in patients with DM and diabetic animal models[6,7].
Sodium-glucose cotransporter (SGLT)2 inhibitors are a novel class of glucose-lowering agents that enhance urinary glucose excretion combined with excessive natriuresis independently of insulin. Clinical trials have demonstrated that SGLT2 inhibitors substantially reduced the risk of hospitalization for HF in patients with DM[8-10]. In addition, the cardiac benefits of empagliflozin have been demonstrated in non-diabetic patients with HF and reduced ejection fraction[11]. However, the mechanism by which these observed benefits are mediated remains unclear. Two systematic reviews and meta-analyses demonstrated that the reversal of cardiac remodeling might be a mechanism responsible for the favorable clinical effects of these agents on HF regardless of glycemic status, particularly in the case of empagliflozin[12,13]. Adverse cardiac remodeling is closely associated with increased myocardial apoptosis, decreased autophagy, and altered energy metabolism in the heart[14]. Packer speculated that the cardioprotective effects of SGLT2 inhibitors might be due to the direct effects of these drugs on cardiomyocytes, involving the activation of AMP-activated protein kinase (AMPK), reduction of cellular stress, and restoration of cellular survival[15]. However, further experiments are required to validate the molecular mechanisms underlying the benefits of SGLT2 inhibitors on the heart.
AMPK, which is activated by an increased AMP/ATP ratio, plays a crucial role in regulating the energy metabolism of the heart[16]. Recent investigations have demonstrated that empagliflozin protects the heart from inflammation and energy depletion, and it improves myocardial vascular injury in diabetic mice through the activation of AMPK-mediated inhibition of mitochondrial fission and oxidative stress[17,18]. In addition, a recent study indicated that AMPK activation reduced the myocardial apoptotic effects in diabetic mice[19]. However, there is a scarcity of studies on SGLT2 inhibitors and cardiomyocyte apoptosis in DCM. Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) serves as a pivotal factor in maintaining mitochondrial biogenesis and energy metabolism and plays a critical role in the progression of HF. Deacetylation of silent mating type information regulation 2 homolog 1 (SIRT1) or phosphorylation of AMPK can modulate PGC-1α activity[20]. RhoA is a member of the GTP-binding proteins within the Ras superfamily, and Rho kinase (ROCK) is a serine/threonine protein kinase that acts as a principal effector of RhoA. Many important functions in cells, such as proliferation, apoptosis, and differentiation, and gene transcription are regulated by the RhoA/ROCK pathway. Our previous in vitro and in vivo studies have revealed that the RhoA/ROCK pathway regulates myocardial apoptosis and fibrosis in diabetic rats. Fasudil (FA), a ROCK inhibitor, has been shown to alleviate oxidative stress and improve cardiac function[21,22]. Therefore, the RhoA/ROCK pathway is associated with several cardiovascular conditions, such as hypertension, atherosclerosis, and HF[23].
In this study, we established a DCM model in db/db mice and then treated the mice with empagliflozin for 8 wk. Significant improvements in cardiac function were observed in diabetic mice, along with concurrent activation of AMPK/PGC-1α and the inhibition of the RhoA/ROCK pathway (Figures 1 and 2). An in vitro experiment under high glucose (HG) conditions was performed to exclude the effects of metabolic improvement on the heart in vivo. The aim of this study was to elucidate the molecular mechanisms underlying the protective effects of empagliflozin on cardiomyocytes.
The experiments were conducted using 8-week-old male db/db mice weighing 40–42 g (Nanjing, China). The random blood glucose levels were ≥ 16.7 mmol/L in all db/db mice. Male C57BL/6J mice were used as a control group. The mice were given standard care according to a protocol approved by the Ethics Committee of Hebei Medical University. The animals were acclimatized to laboratory conditions (24 °C, 10 h/14 h light/dark, 55% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. Intragastric gavage administration was carried out with conscious animals, using straight gavage needles appropriate for the animal size. All animals were euthanized by barbiturate overdose (intravenous injection, 200 mg/kg pentobarbital sodium) for tissue collection. All appropriate measures were taken to minimize the pain or discomfort of animals. Approval was granted by the Research Ethics Committee of the Second Hospital of Hebei Medical University (Date 2022.3.5/No. 2022-AE136). The mice were divided into three groups: (1) Normal control group (NG, n = 11); (2) Db/db mice group (DB, n = 7); and (3) empagliflozin (EM)-treated db/db mice group (EM/DB, n = 7). Empagliflozin (Biberach, Germany) was administrated via oral gavage at a dose of 10 mg/kg/d for 8 wk from the age of 12 wk.
At the age of 20 wk, systolic arterial blood pressure (SABP) was measured by tail-cuff micro-photoelectric plethysmography. Fasting blood samples were collected for blood glucose (FBG), glycosylated hemoglobin (HbA1c), and total cholesterol (TC) determination using an automatic biochemical analyzer in the Second Hospital of Hebei Medical University (Shijiazhuang, China), and serum insulin (FINS) was measured using an ELISA kit (Senberga, Nanjing, China) according to the manufacturer’s instructions. The insulin resistance index (HOMA-IR) was calculated as FBG × FINS/22.5.
At the age of 20 wk, the mice were anesthetized with an intraperitoneal injection of pentobarbital sodium at a dose of 200 mg/kg. All measurements were performed with an 11-MHz linear transducer coupled to a high-resolution ultrasound system (Vivid E95, GE Healthcare, United States). Serial M-mode echocardiographic images were taken in the short axis view at the level of the papillary muscles. The left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), and peak velocity of early filling (E) and atrial contraction (A) were obtained to assess cardiac diastolic and systolic functions.
The myocardial tissues were fixed with 2.5% glutaraldehyde overnight at 4 °C, followed by postfixation with 1% acetic acid. After dehydration, the specimens were conventionally processed and examined by transmission electron microscopy (TEM) (JEM-1200EX, JEOL, Japan) for analyses of the myocardium ultrastructure.
The heart tissues were fixed with 4% paraformaldehyde. The paraffin sections of heart tissues were dewaxed, stained with hematoxylin and eosin (HE), dehydrated, and mounted. A microscopic examination was performed to evaluate pathological changes in the heart tissues.
A TdT-mediated dUTP-biotin nick end labeling (TUNEL) assay kit (Beyotime, Shanghai, China) was used to examine cell apoptosis in the myocardial tissues according to the manufacturer’s instructions. Apoptotic cells were observed and photographed using a light microscope (CX2, OLYMPUS, Japan). The nuclei of apoptosis-positive cells were brown. The apoptotic index was calculated as the percentage of TUNEL-positive cells.
Cardiomyocytes were isolated from 1-3-day-old newborn Sprague-Dawley rats in the Animal Experimental Center of Hebei Medical University. Briefly, freshly isolated hearts were minced and washed thrice with D-Hanks (Gibco, Carlsbad, CA, United States), digested with a mixture of enzymes containing 0.04% type II collagenase (Biofroxx, Einhausen, Germany) and 0.1% trypsin (Gibco, Billings, MT, United States) for 8 min for 6 cycles, and then the digestion was stopped with fetal bovine serum (FBS; Gemini, CA, United States). Next, the tissues were disrupted in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, CA, United States) containing 10% FBS and 5.5 mmol/L D-glucose. Fibroblasts were eliminated by attaching to the culture plates for 90 min. Bromodeoxyuridine (BrdU, Solarbio, Beijing, China) was added to the medium to suppress the growth of fibroblasts, resulting in high-purity cardiomyocytes. These cells started spontaneously pulsating in about 3 d at 80-100 beats/min, and 95% of them, which were positive for anti-α-actin, were identified as cardiomyocytes by immunocytochemistry. This experiment was approved by the Experimental Animal Administration Committee of Hebei Medical University.
The isolated cardiomyocytes were maintained for 24 h in DMEM supplemented with streptomycin (100 μg/mL), penicillin (100 U/mL), and 5% FBS. Next, the cells were cultured in different conditions: 5.5 mmol/L D-glucose as normal control (NG) group; 5.5 mmol/L D-glucose plus 24.5 mmol/L D-mannitol as hyperosmotic group (OSM); 30 mmol/L D-glucose as HG group (HG); cells in the HG + EM group were pretreated with 10 μM empagliflozin for 2 h and then incubated with HG for an additional 48 h; cells in the HG + EM + compound C (CC) group were pretreated with AMPK inhibitor and 1 µM CC (MCE, CA, United States) for 2 h, and then the cells were cultured as in the HG + EM group; cells in the HG + FA group were pretreated with 100 μM FA (Hongri, Tianjin, China)) as our previous study[17] and incubated with HG for an additional 48 h; cells in the PGC-1α-GFP-Ad and GFP-Ad groups were transfected with PGC-1α-GFP-Ad and GFP-Ad (Shanghai Genechem Co., Ltd., China), respectively, using liposomes (Invitrogen, United States) and then incubated with HG for an additional 48 h.
Cell counting kit-8 (CCK-8) (Sharebio, Shanghai, China, SB-CCK8S) was used to measure cell viability. The cardio-myocytes were inoculated in the 96-well plate (100 μl/well), followed by intervention with empagliflozin for 48 h at different concentrations (0, 0.05, 0.1, 1, 10, and 20 μM) in HG conditions. The medium was then supplemented with CCK-8 solution for 4 h. Lastly, a microplate reader (Thermo, United States) was used to measure the absorbance value at 450 nm.
After digestion with 0.25% trypsin, the cardiomyocytes were collected and supplemented with Annexin V and propidium iodide (PI) binding buffer in the dark at 4 °C for 30 min. Annexin V and PI are used to distinguish between apoptotic and necrotic cells, and positive Annexin V can be labeled with fluorescent dye to identify early apoptosis. Flow cytometry (Beckman FC500, California, United States) was used to collect and analyze the cells. The early apoptotic cells were located in the upper right quadrant (Annexin V+/PI-).
A TUNEL assay kit (Beyotime, C1086-20T) was used to measure the apoptosis rates of cardiomyocytes in line with the manufacturer’s instructions. The cell slides containing the cells were supplemented with 50 µl TUNEL reaction mixture for 2 h at 37 °C to identify apoptotic cells. The DAPI staining solution was then placed on slides in the dark at 37 °C for 20 min to mark the total cells. An inverted fluorescence microscope (Olympus, Japan) was used to observe the cell slides and take photos. The apoptotic index was calculated as the percentage of TUNEL-positive cells. Ten representative fields were evaluated for each group and the average value was calculated.
The fluorescent probe dichlorodihydrofluorescein (DCFH) diacetate (DCFH-DA, Beyotime, S0033) was used to detect the levels of ROS generation. Intracellular esterases convert DCFH-DA to DCFH, which is oxidized to fluorescent dichlorofluorescein (DCF) by an oxidant. The cells were cultured with 3 μM DCFH-DA in serum-free DMEM for 20 min at room temperature. A fluorescence microscope was used to observe cellular ROS and take photos. The changes in fluorescence were analyzed by the ImageJ program (Bio-Rad, California, United States).
The activity of superoxide dismutase (SOD) in cardiomyocytes was determined with a kit according to the manu
JC-1, which produces red fluorescence in normal mitochondria, changes to green fluorescence with loss of mitochondrial membrane potential (MMP). The change in MMP was determined by the decrease in the red to green fluorescence ratio. The myocardial cells were cultured with JC-1 solution (Beyotime, C2006) at 25 °C for 20 min. The samples were detected using a flow cytometer (Beckman FC500, California, United States) within 30 min.
An ATP bioluminescence kit (Beyotime, S0026) was used to assay the ATP levels in isolated myocardial cells. Briefly, after drug intervention, the cells were collected, lysed, and centrifuged (14000 rpm for 8 min). Next, the firefly luciferase reagent, which emits light in the presence of ATP, was added to the supernatant. The bioluminescence signals were detected using a luminometer (Promega, United States). The concentration of ATP in the sample was calculated from the standard curve.
The myocardial gene expression of Bcl-2 and Bax was determined using real-time quantitative PCR. Trizol reagent (ThermoScientific, United States) was used to separate total RNA in cardiomyocytes. The experimental steps were carried out according to the manufacturer’s procedure. The primers were provided by Sangon Biotechnology (Shanghai, China). Their sequences are as follows:
Bax: Forward, 5′-AGACACCTGAGCTGACCTTGGAG-3′ and reverse, 5′-TTCATCGCCAATTCGCCTGAGAC-3′; Bcl-2: Forward, 5′-TGGAGAGCGTCAACAGGGAGATG-3′ and reverse, 5′-GTGCAGATGCCGGTTCAGGTAC-3′; 18S rRNA: Forward, 5′-TGCGGAAGGATCATTAACGGA-3′ and reverse, 5′-AGTAGGAGAGGAGCGAGCGACC-3.
The Ct value of the target mRNA was calculated as follows: ∆Ct = Ct purpose − Ct internal reference, and the relative expression levels of the target mRNA were decided by 2-∆∆Ct.
The total proteins of myocardial tissues and cardiomyocytes were lysed using RIPA lysate (Solarbio, R0010). The bicinchoninic acid protein assay (Solarbio, PC0020) was used to measure the concentration of proteins in the supernatant. The protein samples (50 µg, 15 μL) were then run on a 10% SDS-PAGE gel and subsequently blotted to a PVDF membrane (Millipore, Billerica, MA, United States, IPVH 0010). After being blocked with 5% nonfat dried milk, the membrane was incubated at 4 °C overnight with primary antibodies, followed by incubation with the goat anti-rabbit IgG as secondary antibody at 37 °C for 1.5 h. The antibodies used were as follows: Anti-phosphorylated myosin phosphatase target subunit 1 (p-MYPT1) and anti-total myosin phosphatase target subunit 1 (t-MYPT1), anti-cleaved caspase 3, anti-AMPK, anti-p-AMPK, anti-PGC-1α (these antibodies were all from Cell Signaling Technology, USA), anti-SGLT1 (Abcam, United States), anti-SGLT2 (Abcam), and anti-β-Tubulin (Abways). Finally, the membrane was detected using chemiluminescent reagents (Solarbio, SB-WB012S) and Image J (Bio-Rad).
Data are expressed as the mean ± SD. All data were verified to be normally distributed. One-way analysis of variance was used to detect the differences among multiple groups followed by the Tukey test if F was significant. The differences between the two groups (SGLT1 and SGLT2 protein) were determined using the Student's t-test. P < 0.05 was considered statistically significant. Data were analyzed using GraphPad Prism.9.0 software (GraphPad, CA, United States).
Diabetic mice exhibited higher levels of FBG, HbA1c, FINS, HOMA-IR, and TC and the high ratio of heart weight to body weight (HW/BW) than control mice (Table 1) (P < 0.05). Among the three groups, no significant differences were observed in SABP. Empagliflozin treatment resulted in a significant reduction in FBG, HbA1C, FINS, and HOMA-IR levels in diabetic mice (P < 0.05). However, HW/BW and TC in diabetic mice were not altered by empagliflozin treatment.
| NC (n = 11) | DB (n = 7) | EM/DB (n = 7) | |
| FBG (mmol/L) | 4.98 ± 0.82 | 33.51 ± 2.961 | 19.26 ± 3.122 |
| HbA1c (%) | 4.47 ± 0.14 | 9.15 ± 1.161 | 6.86 ± 0.812 |
| INS (mU/L) | 82.88 ± 17.85 | 325.80 ± 47.301 | 176.30 ± 30.892 |
| HOMO-IR | 19.22 ± 5.76 | 472.60 ± 83.221 | 190.50 ± 113.502 |
| TC (mmol/L) | 2.57 ± 0.30 | 4.27 ± 0.181 | 4.27 ± 0.271 |
| HW/BW ( 10-3) | 5.21 ± 0.17 | 5.77 ± 0.121 | 5.78 ± 0.091 |
| SABP (mmHg) | 122.70 ± 2.37 | 122.90 ± 3.71 | 123.60 ± 5.03 |
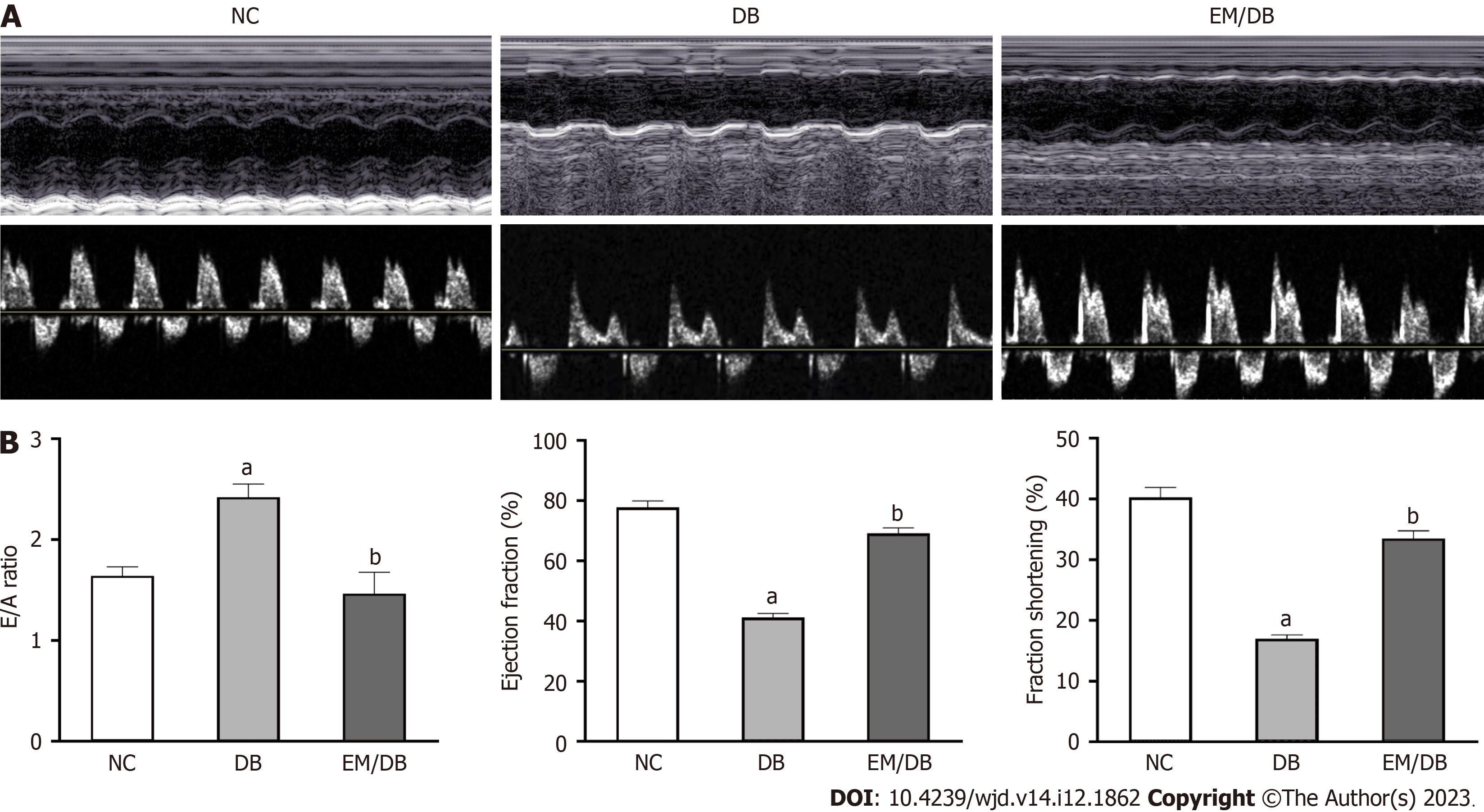
Compared with control mice, LVEF and LVFS were significantly decreased whereas E/A was enhanced in diabetic mice (Figure 1) (P < 0.05). Empagliflozin treatment substantially enhanced cardiac diastolic and systolic function by reducing E/A and increasing LVEF and LVFS in diabetic mice (P < 0.05).
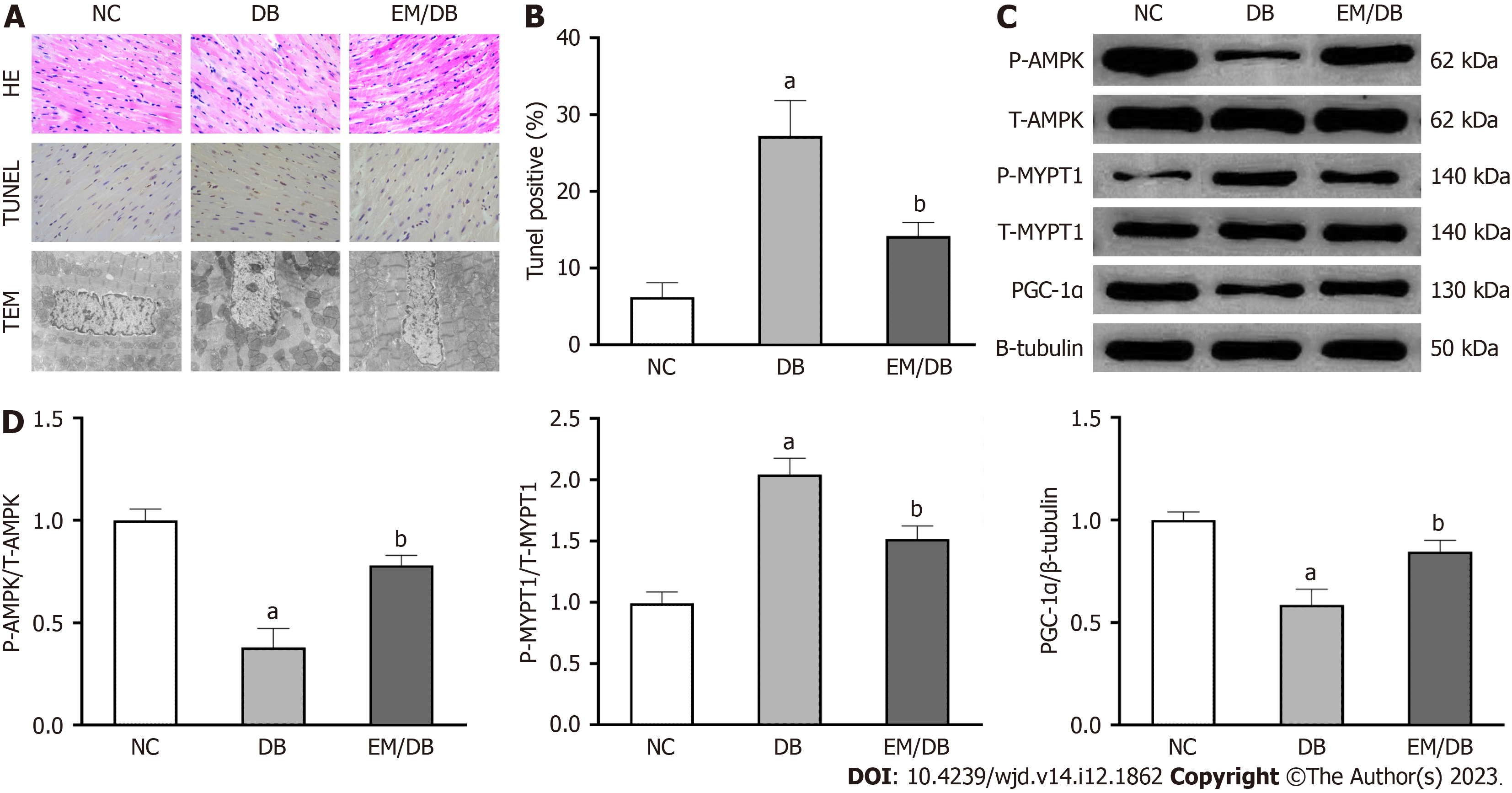
HE histological staining revealed a well-organized arrangement of myocardial fibers in control mice (Figure 2A). In diabetic mice, the myocardial fibers were disorganized. Nevertheless, empagliflozin treatment improved myocardial tissue fiber arrangement in diabetic mice. Similarly, TEM examination of the myocardium ultrastructure indicated that mitochondria in control mice were longitudinally arranged between the muscle bundles, and their membrane structures remained intact. However, in diabetic mice, mitochondria exhibited partial disappearance, swelling, fragmentation, and pyknosis. The morphology of mitochondria in diabetic mice was partially restored by empagliflozin treatment. TUNEL staining revealed that cellular apoptosis in diabetic mice was increased compared with that in the NC group, which was reduced by empagliflozin treatment.
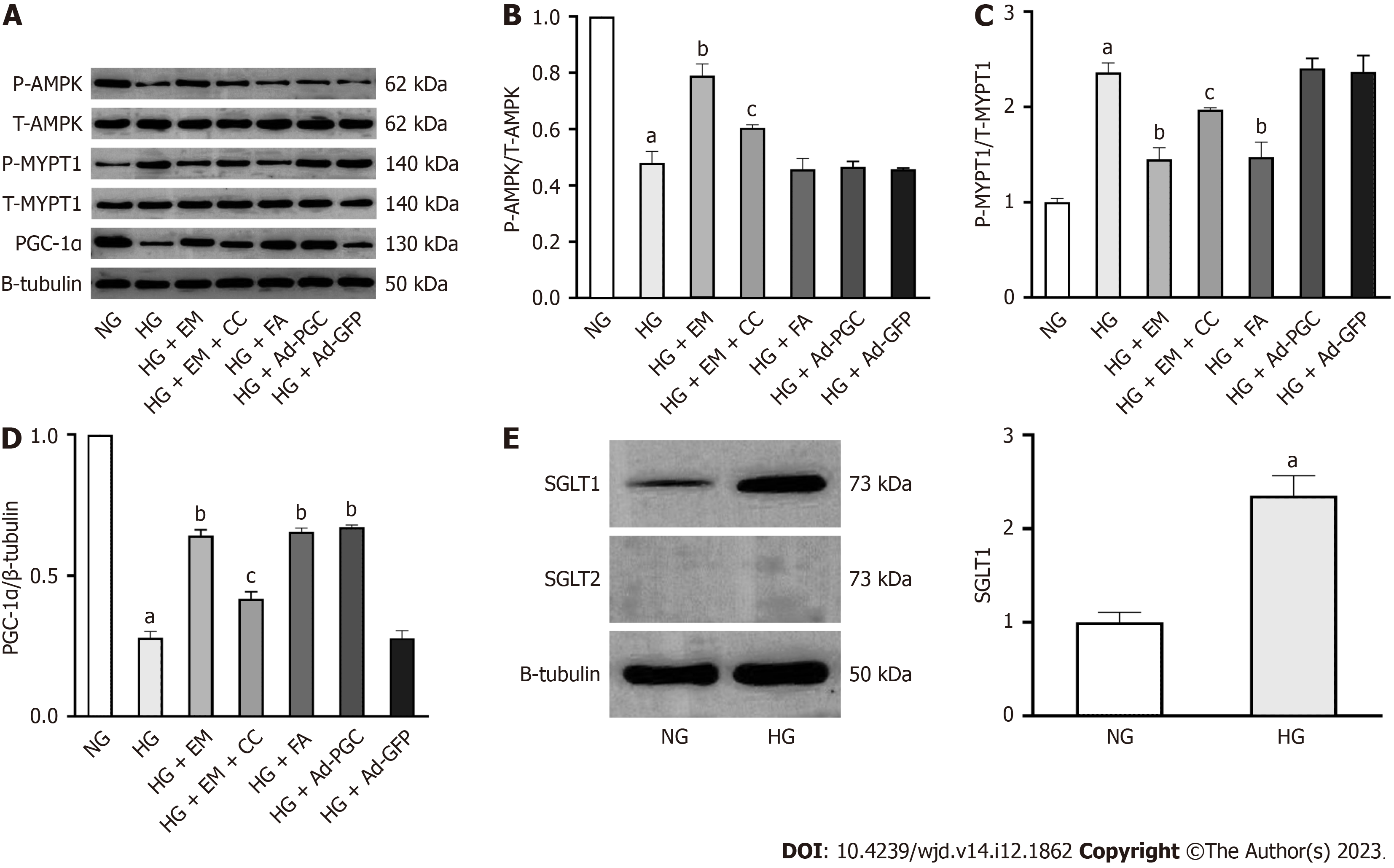
Phosphorylation of AMPK and MYPT1 represents the activation of AMPK and the RhoA/ROCK pathway, respectively. Herein, the protein levels of p-AMPK and PGC-1α in diabetic mice were significantly decreased compared with those in control mice (P < 0.05). The treatment of empagliflozin substantially increased the levels of p-AMPK and PGC-1α (P < 0.05). Meanwhile, the level of p-MYPT1 was significantly increased in diabetic mice compared with control mice (P < 0.05), which was notably inhibited by empagliflozin treatment (P < 0.05). These findings indicate that empagliflozin is associated with AMPK/PGC-1α and the RhoA/ROCK pathway in the myocardium of diabetic mice (Figure 2B and C).
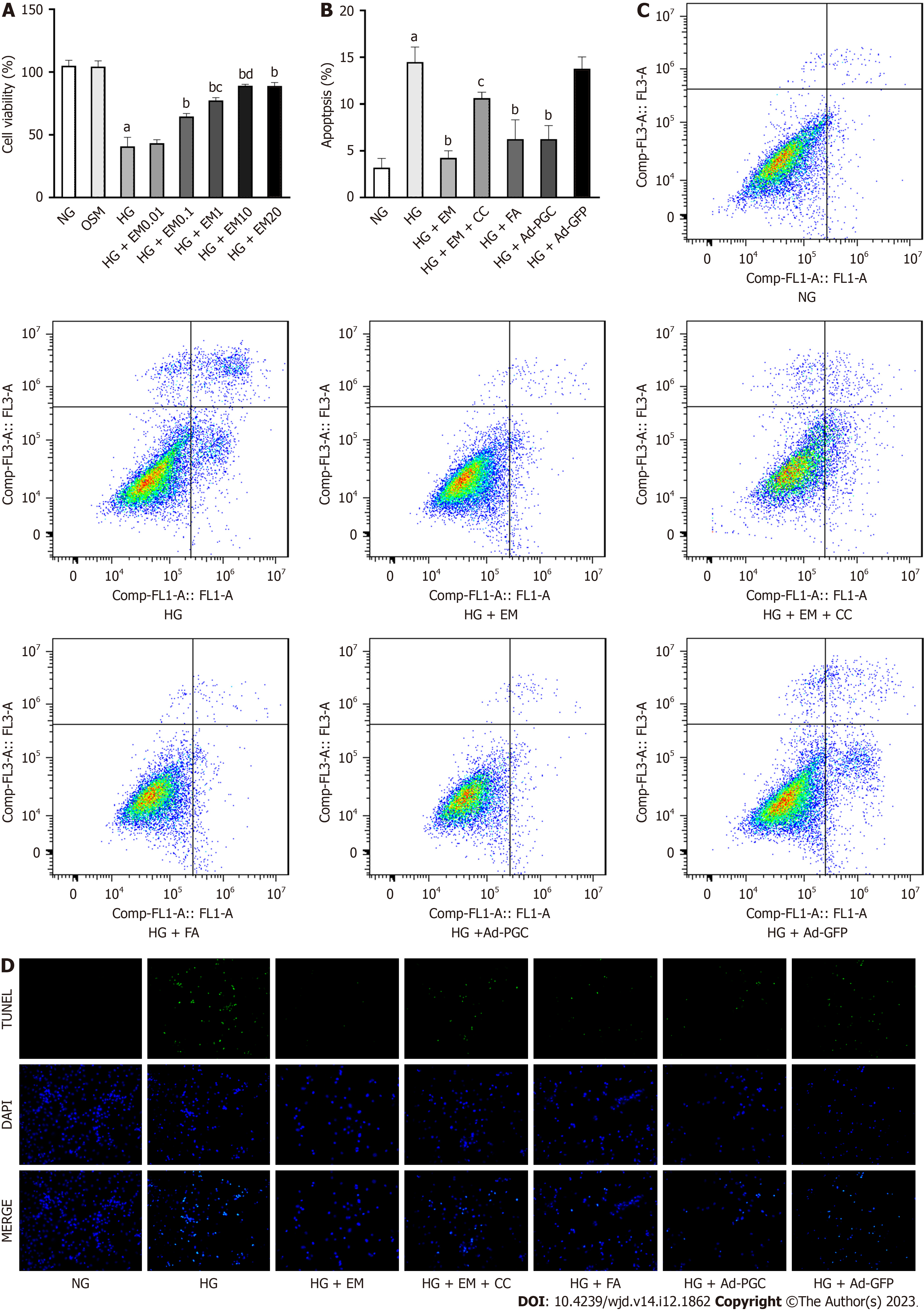
HG significantly decreased cardiomyocyte viability (Figure 3A). Under HG conditions, empagliflozin concentrations ranging from 0.1 to 10 μM increased cell viability in a dose-dependent manner (P < 0.05), whereas 0.01 μM empagliflozin did not induce any changes in the viability of cells exposed to HG. The viability of cardiomyocytes treated with empagliflozin at concentrations between 10 and 20 μM did not show a significant difference. In addition, hyperosmosis did not affect the viability of these cells. Consequently, a 10 μM concentration of empagliflozin was chosen for subsequent experiments.
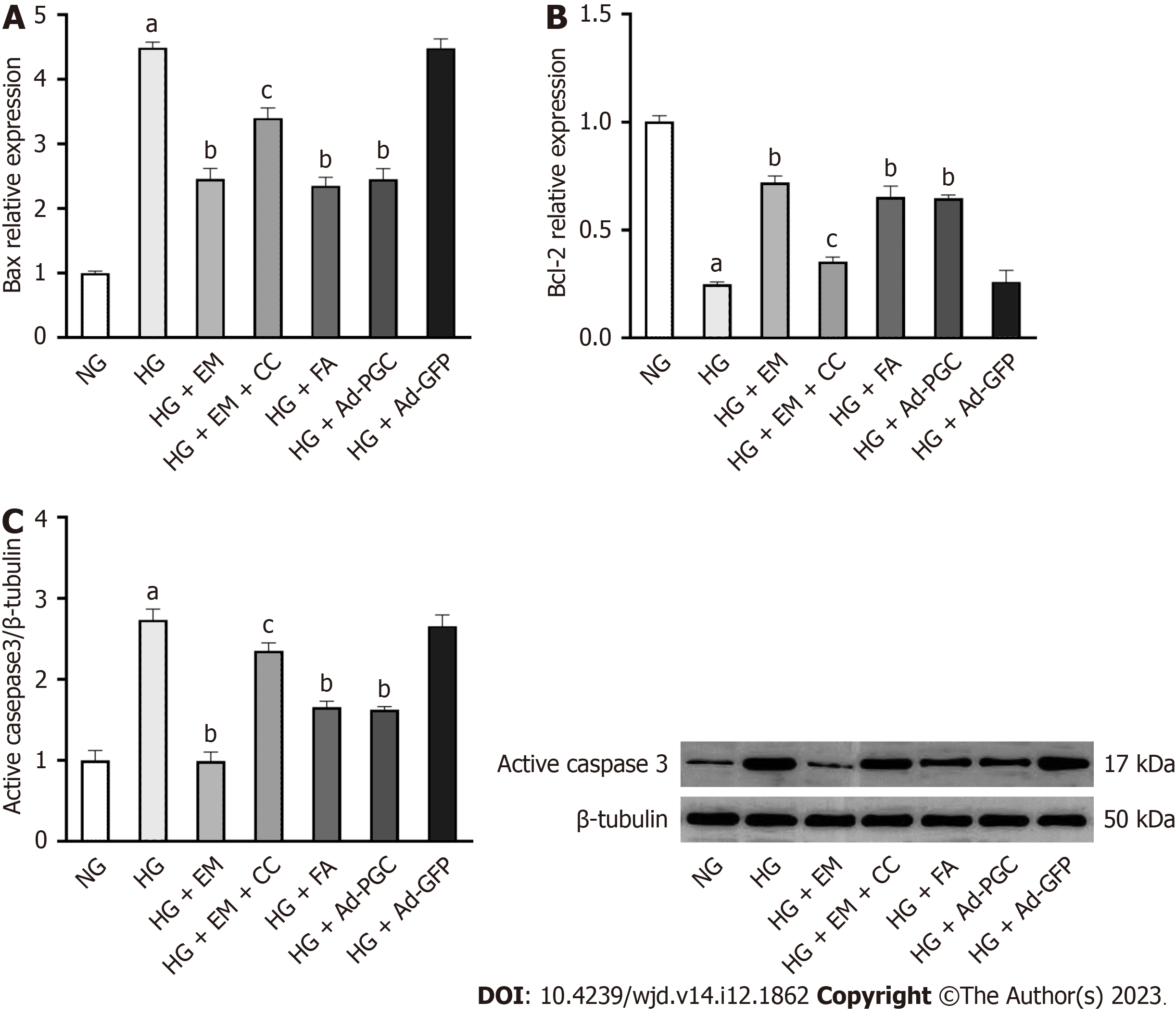
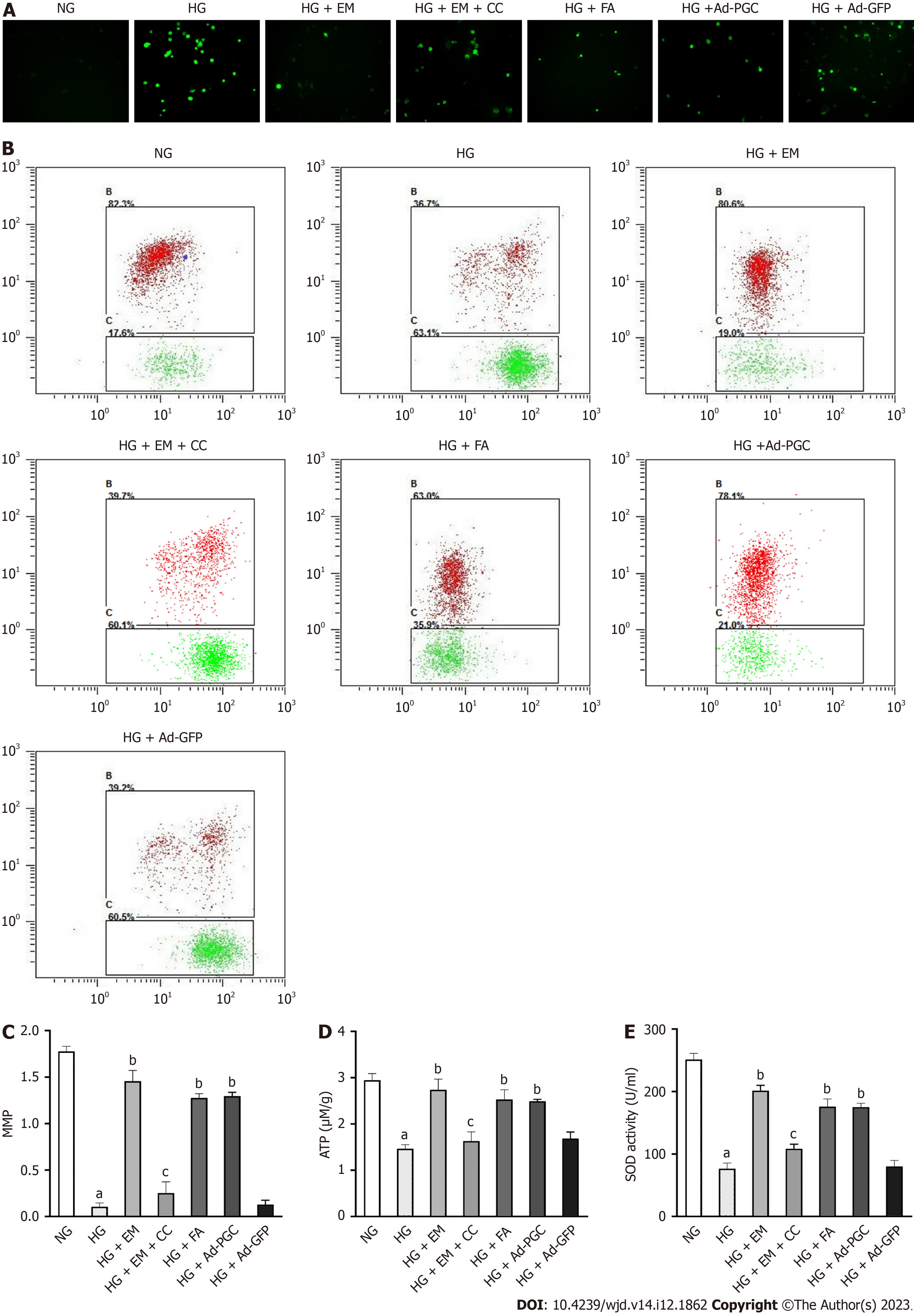
Cell apoptosis was enhanced in HG conditions and was mitigated by empagliflozin, FA, and overexpression of PGC-1α protein (P < 0.05) (Figure 3B-D). Under the HG condition, the mRNA expression of the apoptosis-related gene Bax was upregulated (P < 0.05), whereas that of the anti-apoptosis indicator Bcl-2 was downregulated (P < 0.05). Furthermore, the level of active caspase-3 protein was upregulated in cells exposed to HG (P < 0.05) (Figure 4). However, treatment with empagliflozin, FA, and the overexpression of PGC-1α protein reversed all these changes (P < 0.05). The addition of CC, an AMPK inhibitor, demonstrated the opposite effects of empagliflozin on cardiomyocytes.
Cellular ATP production and MMP reflect mitochondrial function. In this study, MMP and ATP levels in cardiomyocytes exposed to HG were substantially decreased. Furthermore, compared with the NG group, HG reduced the antioxidant enzyme SOD and enhanced cellular ROS levels (P < 0.05). Treatment with empagliflozin or FA or the overexpression of PGC-1α substantially increased MMP, ATP, and SOD levels, and reduced ROS production in cells exposed to HG (Figure 5). The addition of CC to the EM group weakened these effects (P < 0.05). These findings indicate that the activation of AMPK, upregulation of PGC-1α, or inhibition of the RhoA/ROCK pathway can enhance mitochondrial function and mitigate oxidative stress in HG-induced cardiomyocytes.
As illustrated in Figure 6A-D, the protein levels of p-AMPK and PGC-1α were significantly reduced in cells exposed to HG compared with the NG group (P < 0.05), whereas the level of p-MYPT1 was increased (P < 0.05). Compared with the HG group, empagliflozin treatment upregulated the protein levels of p-AMPK and PGC-1α in cells exposed to HG (P < 0.05), accompanied by the downregulation of p-MYPT1 (P < 0.05), which was weakened by the addition of CC. In cells exposed to HG, the addition of FA and the overexpression of PGC-1α significantly upregulated PGC-1α protein level (P < 0.05). These findings indicate that empagliflozin can activate AMPK, inhibit the RhoA/ROCK pathway, and induce PGC-1α expression.
SGLT1 protein was expressed in cardiomyocytes. In cardiomyocytes under HG conditions, the protein expression of SGLT1 was significantly increased compared with the NG group. However, the SGLT2 protein was not expressed in cardiomyocytes under either the NG or HG conditions. (Figure 6E).
Db/db mice are typically employed as an animal model of type 2 DM (T2DM). In this study, db/db mice exhibited hyperglycemia, hyperlipidemia, and insulin resistance, which are consistent with the characteristics of T2DM. The effects of empagliflozin on cardiac function in db/db mice and HG-treated cardiomyocytes were evaluated and four main findings were demonstrated: (1) Db/db mice developed DCM, and hyperglycemia led to mitochondrial injury and increased cardiomyocyte apoptosis in vivo and in vitro; (2) Empagliflozin enhanced cardiac function in db/db mice and prevented mitochondrial injury, oxidative stress, and cardiomyocyte apoptosis in vivo and in vitro; (3) These protective effects of empagliflozin on myocardial cells were achieved through the activation of AMPK, inhibition of the RhoA/ROCK pathway, and upregulation of PGC-1α. AMPK could regulate the RhoA/ROCK pathway and PGC-1α expression, with PGC-1α being a downstream target of the RhoA/ROCK pathway; and (4) Cardiomyocytes expressed SGLT1 protein, but did not express SGLT2 protein in either NG or HG conditions.
DCM can occur in the early stage of DM, manifest as diastolic and systolic dysfunction, and eventually progress to decompensated HF, which results in increased mortality in patients with DM[2]. Our in vivo study demonstrated that at the age of 20 wk, db/db mice developed DCM, which was consistent with the findings of Lew et al[24]. Empagliflozin prevented DCM by enhancing impaired heart diastolic and systolic functions in db/db mice. Hammoudi et al[25] de
Packer concluded that the cardiovascular advantages of SGLT2 inhibitors could not be attributed to the control of hyperglycemia, ketogenesis, and natriuretic action, but might be associated with the stimulation of autophagy and reduction of intracellular sodium in the myocardium[27]. Remarkably, several studies have demonstrated that SGLT2 inhibitors have numerous mechanisms of action on DCM, including the regulation of cardiac iron homeostasis, anti-inflammation, anti-fibrosis, and anti-oxidative stress[28]. Cardiomyocytes were cultured in vitro to clarify the direct effects of empagliflozin on the myocardium, independent of metabolic improvements. First, the protein expression of SGLT1 and SGLT2 in isolated myocardial cells was investigated, indicating that SGLT2 protein was not expressed in primary cardiomyocytes under either NG or HG conditions. This finding is consistent with the results of Di Franco et al[29], who reported that SGLT2 was not expressed in the human myocardium under normal or pathological conditions. Similarly, Mustroph et al[30] reported that SGLT2 expression was not identified in healthy or failing myocardium in humans and mice. Therefore, there may be a direct effect that elucidates the protection offered by empagliflozin to the myocardium independent of the SGLT-2 protein.
Accumulating evidence demonstrated that hyperglycemia and increased cardiac lipid deposition increase cell ROS generation[31]. This increased ROS generation can trigger oxidative stress, disrupt mitochondrial structure, and result in mitochondrial dysfunction in the heart of diabetic patients, which contributes to myocardial cell apoptosis and necrosis[32]. In this study, alterations in mitochondrial morphology were observed in db/db mice, which were mitigated by empagliflozin treatment. HG-induced injury to mitochondria results in the generation of less ATP, decreased MMP, excess ROS, and decreased SOD. In other words, HG triggers oxidative stress, where empagliflozin treatment prevented mitochondrial injury and oxidative stress in myocardial cells. The role of mitochondrial function in maintaining cardiac function is crucial. Empagliflozin was linked to the enhancement of mitochondrial biogenesis and energetics, ultimately resulting in the suppression of cardiac remodeling and dysfunction[33-35]. DCM mice exhibited enhanced myocardial apoptosis, which was closely associated with mitochondrial injury and oxidative stress[36]. This study also demonstrated obvious apoptotic cells in the myocardium exposed to HG in vivo and in vitro, and empagliflozin mitigated HG-induced cardiac cell apoptosis by regulating mitochondria-dependent apoptosis pathways, which was consistent with the findings of a previous study[37].
Some related molecular pathways have been discussed to elaborate the protection mechanism of empagliflozin against cardiomyocytes. AMPK plays a crucial role in cardioprotection[38]. HG suppressed AMPK activity and increased mitochondrial dysfunction and apoptosis of cardiomyocytes, and AMPK activation ablated hyperglycemia-induced cardiac oxidative stress, mitochondrial injury, and myocardial apoptosis, ultimately resulting in enhanced cardiac function in DCM mice[39-41]. Consistent with these findings, our study indicated that empagliflozin increased AMPK activity, and the activation of AMPK resulted in anti-oxidative stress and anti-apoptosis effects on cardiomyocytes. Furthermore, our findings indicated that AMPK could regulate the RhoA/ROCK pathway and PGC-1α expression. It is well-established that AMPK activation results in the suppression of the RhoA/ROCK pathway in AngII-induced human vascular smooth muscle cells[42]. The RhoA/ROCK pathway plays a crucial role in the development of diabetic complications[43]. The inhibition of ROCK reduced HG/lipopolysaccharide-induced myocardial apoptosis and mitochondrial damage through the activation of autophagy[44,45]. The findings of this study demonstrated that inhibiting the RhoA/ROCK pathway prevented oxidative stress and apoptosis of cardiomyocytes under HG conditions, and enhanced mitochondrial dysfunction, which was consistent with the findings of our previous study[21]. In addition, the activation of AMPK could directly phosphorylate PGC-1α in skeletal muscle, or indirectly regulated PGC-1α expression through SIRT1 activation[46,47]. It has been confirmed that ROCK suppression can induce PGC-1α expression in rat striatal neurodegeneration[48]. In a rat model of T2DM, PGC-1α is closely associated with mitochondrial biogenesis and atrial remodeling, and empagliflozin can enhance impaired mitochondrial biogenesis and mitochondrial dysfunction, while also improving atrial remodeling through upregulation of PGC-1α[49]. Similarly, in DCM rats, the restoration of PGC-1α can enhance mitochondrial damage and cardiac dysfunction[50]. Our study demonstrated that empagliflozin up-regulated PGC-1α expression under HG conditions, and inhibited cardiomyocyte oxidative stress, mitochondrial injury, and apoptosis. PGC-1α expression was modulated in part by AMPK and the RhoA/ROCK pathway.
To date, there has been few studies on the effects of empagliflozin on HG-induced cardiomyocyte apoptosis and the underlying mechanism. Cardiomyocyte apoptosis is believed to be the initial factor contributing to HF in DCM[6]. This study offers a novel molecular foundation for the anti-HF effects of empagliflozin. However, this study has some limitations: (1) Db/db mice exhibited hyperglycemia with hyperlipidemia, but the in vitro experiments only employed HG without the addition of palmitate. Consequently, the cellular models did not precisely match the animal models; and (2) No positive drug control was established in in vivo experiments. Further appropriate study should be conducted to solve these limitations.
In conclusion, this study demonstrated that empagliflozin not only controlled glycemic changes but also improved mitochondrial injury and cardiac dysfunction in db/db mice. HG-induced oxidative stress and cardiomyocyte apoptosis were mitigated by empagliflozin through the activation of AMPK/PGC-1α and inhibition of the RhoA/ROCK pathway. These findings demonstrated that empagliflozin exerted direct beneficial effects on cardiomyocytes independent of SGLT2 inhibition.
Diabetic cardiomyopathy (DCM) increases the risk of hospitalization for heart failure in diabetic patients. However, there is no specific therapy to delay the progression of DCM. Empagliflozin has been confirmed to reduce the risk of hospitalization for heart failure in diabetic patients. However, the molecular mechanisms by which these agents exert cardioprotection remain unclear.
To explore the effects of empagliflozin on the development of DCM.
To investigate whether empagliflozin can improve mitochondrial injury and cardiac dysfunction, and prevent high glucose (HG)-induced oxidative stress and cardiomyocyte apoptosis, along with the underlying molecular mechanism.
We used db/db mice and primary cardiomyocytes from neonatal rats stimulated with HG (30 mmol/L) separately as in vivo and in vitro models. Cardiac function was evaluated by echocardiography. We used transmission electron microscopy to observe mitochondrial injury. RT-qPCR, Western blot, flow cytometry, TdT-mediated dUTP-biotin nick end labeling staining, and immunofluorescence were used to investigate the effects of empagliflozin treatment on cellular processes in cardiomyocytes of neonatal rats stimulated with HG.
Empagliflozin significantly improved cardiac dysfunction and dramatically reduced myocardial apoptosis, accompanied by upregulation of AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), as well as downregulation of myosin phosphatase target subunit 1 (MYPT1) in the heart of mice. At the cellular level, treatment of cardiomyocytes with empagliflozin or FA (a ROCK inhibitor) or overexpression of PGC-1α all markedly attenuated HG-induced mitochondrial injury, oxidative stress, and cardiomyocyte apoptosis. However, AMPK inhibitor reversed the above effects in part. Furthermore, no sodium-glucose cotransporter (SGLT)2 protein expression was detected in cardiomyocytes.
Empagliflozin improves mitochondrial injury and cardiac dysfunction in db/db mice, and prevents HG-induced oxidative stress and cardiomyocyte apoptosis in vitro at least partially by activating AMPK/PGC-1α and inhibiting the RhoA/ROCK pathway independent of SGLT2.
Next step, we will establish a positive drug control in vivo and further clarify the effects of empagliflozin on DCM, with an objective of providing a new strategy for the prevention and treatment of DCM.
The authors thank professor Rong Zhang at Hebei Medical University for providing valuable suggestions.
| 1. | Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018;122:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1366] [Article Influence: 195.1] [Reference Citation Analysis (0)] |
| 2. | Jankauskas SS, Kansakar U, Varzideh F, Wilson S, Mone P, Lombardi A, Gambardella J, Santulli G. Heart failure in diabetes. Metabolism. 2021;125:154910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 3. | Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 734] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 4. | Habotta OA, Abdeen A, Roomi AB, Elgndy AI, Sorour SM, Morsi MH, Kamal KM, Ibrahim SF, Abdelrahaman D, Fericean L, Banatean-Dunea I, Ghamry HI, El-Nablaway M, Atawia RT, Abdelhady D. Nootkatone Mitigated Melamine-Evoked Hepatotoxicity by Featuring Oxidative Stress and Inflammation Interconnected Mechanisms: In Vivo and In Silico Approaches. Toxics. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Peng ML, Fu Y, Wu CW, Zhang Y, Ren H, Zhou SS. Signaling Pathways Related to Oxidative Stress in Diabetic Cardiomyopathy. Front Endocrinol (Lausanne). 2022;13:907757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 6. | Wei J, Zhao Y, Liang H, Du W, Wang L. Preliminary evidence for the presence of multiple forms of cell death in diabetes cardiomyopathy. Acta Pharm Sin B. 2022;12:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 7. | Tang Z, Wang P, Dong C, Zhang J, Wang X, Pei H. Oxidative Stress Signaling Mediated Pathogenesis of Diabetic Cardiomyopathy. Oxid Med Cell Longev. 2022;2022:5913374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8748] [Article Influence: 795.3] [Reference Citation Analysis (2)] |
| 9. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5669] [Article Influence: 629.9] [Reference Citation Analysis (0)] |
| 10. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4535] [Article Influence: 647.9] [Reference Citation Analysis (0)] |
| 11. | Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, Rodriguez-Cordero A, Zafar MU, Fergus I, Atallah-Lajam F, Contreras JP, Varley C, Moreno PR, Abascal VM, Lala A, Tamler R, Sanz J, Fuster V, Badimon JJ; EMPA-TROPISM (ATRU-4) Investigators. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2021;77:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 376] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 12. | Dhingra NK, Mistry N, Puar P, Verma R, Anker S, Mazer CD, Verma S. SGLT2 inhibitors and cardiac remodelling: a systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail. 2021;8:4693-4700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Zhang N, Wang Y, Tse G, Korantzopoulos P, Letsas KP, Zhang Q, Li G, Lip GYH, Liu T. Effect of sodium-glucose cotransporter-2 inhibitors on cardiac remodelling: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;28:1961-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Salah HM, Verma S, Santos-Gallego CG, Bhatt AS, Vaduganathan M, Khan MS, Lopes RD, Al'Aref SJ, McGuire DK, Fudim M. Sodium-Glucose Cotransporter 2 Inhibitors and Cardiac Remodeling. J Cardiovasc Transl Res. 2022;15:944-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Packer M. Critical examination of mechanisms underlying the reduction in heart failure events with SGLT2 inhibitors: identification of a molecular link between their actions to stimulate erythrocytosis and to alleviate cellular stress. Cardiovasc Res. 2021;117:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Hu Y, Chen H, Zhang L, Lin X, Li X, Zhuang H, Fan H, Meng T, He Z, Huang H, Gong Q, Zhu D, Xu Y, He P, Li L, Feng D. The AMPK-MFN2 axis regulates MAM dynamics and autophagy induced by energy stresses. Autophagy. 2021;17:1142-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 17. | Koyani CN, Plastira I, Sourij H, Hallström S, Schmidt A, Rainer PP, Bugger H, Frank S, Malle E, von Lewinski D. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol Res. 2020;158:104870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 18. | Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 464] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 19. | Xue F, Cheng J, Liu Y, Cheng C, Zhang M, Sui W, Chen W, Hao P, Zhang Y, Zhang C. Cardiomyocyte-specific knockout of ADAM17 ameliorates left ventricular remodeling and function in diabetic cardiomyopathy of mice. Signal Transduct Target Ther. 2022;7:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 20. | Chen L, Qin Y, Liu B, Gao M, Li A, Li X, Gong G. PGC-1α-Mediated Mitochondrial Quality Control: Molecular Mechanisms and Implications for Heart Failure. Front Cell Dev Biol. 2022;10:871357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 21. | Zhou H, Sun Y, Zhang L, Kang W, Li N, Li Y. The RhoA/ROCK pathway mediates high glucose-induced cardiomyocyte apoptosis via oxidative stress, JNK, and p38MAPK pathways. Diabetes Metab Res Rev. 2018;34:e3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Zhou H, Li YJ, Wang M, Zhang LH, Guo BY, Zhao ZS, Meng FL, Deng YG, Wang RY. Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol Sin. 2011;32:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Cai A, Li L, Zhou Y. Pathophysiological effects of RhoA and Rho-associated kinase on cardiovascular system. J Hypertens. 2016;34:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Lew JK, Pearson JT, Saw E, Tsuchimochi H, Wei M, Ghosh N, Du CK, Zhan DY, Jin M, Umetani K, Shirai M, Katare R, Schwenke DO. Exercise Regulates MicroRNAs to Preserve Coronary and Cardiac Function in the Diabetic Heart. Circ Res. 2020;127:1384-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Hammoudi N, Jeong D, Singh R, Farhat A, Komajda M, Mayoux E, Hajjar R, Lebeche D. Empagliflozin Improves Left Ventricular Diastolic Dysfunction in a Genetic Model of Type 2 Diabetes. Cardiovasc Drugs Ther. 2017;31:233-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Wang J, Huang X, Liu H, Chen Y, Li P, Liu L, Li J, Ren Y, Huang J, Xiong E, Tian Z, Dai X. Empagliflozin Ameliorates Diabetic Cardiomyopathy via Attenuating Oxidative Stress and Improving Mitochondrial Function. Oxid Med Cell Longev. 2022;2022:1122494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail. 2020;22:618-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Li N, Zhou H. SGLT2 Inhibitors: A Novel Player in the Treatment and Prevention of Diabetic Cardiomyopathy. Drug Des Devel Ther. 2020;14:4775-4788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Di Franco A, Cantini G, Tani A, Coppini R, Zecchi-Orlandini S, Raimondi L, Luconi M, Mannucci E. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: A new potential pharmacological target. Int J Cardiol. 2017;243:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Sag CM, Lebek S, Tarnowski D, Reinders J, Perbellini F, Terracciano C, Schmid C, Schopka S, Hilker M, Zausig Y, Pabel S, Sossalla ST, Schweda F, Maier LS, Wagner S. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail. 2018;5:642-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 31. | Tsushima K, Bugger H, Wende AR, Soto J, Jenson GA, Tor AR, McGlauflin R, Kenny HC, Zhang Y, Souvenir R, Hu XX, Sloan CL, Pereira RO, Lira VA, Spitzer KW, Sharp TL, Shoghi KI, Sparagna GC, Rog-Zielinska EA, Kohl P, Khalimonchuk O, Schaffer JE, Abel ED. Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of AKAP121, DRP1, and OPA1 That Promote Mitochondrial Fission. Circ Res. 2018;122:58-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 273] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 32. | Pereira RO, Tadinada SM, Zasadny FM, Oliveira KJ, Pires KMP, Olvera A, Jeffers J, Souvenir R, Mcglauflin R, Seei A, Funari T, Sesaki H, Potthoff MJ, Adams CM, Anderson EJ, Abel ED. OPA1 deficiency promotes secretion of FGF21 from muscle that prevents obesity and insulin resistance. EMBO J. 2017;36:2126-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 33. | Mizuno M, Kuno A, Yano T, Miki T, Oshima H, Sato T, Nakata K, Kimura Y, Tanno M, Miura T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6:e13741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 34. | Tan Y, Yu K, Liang L, Liu Y, Song F, Ge Q, Fang X, Yu T, Huang Z, Jiang L, Wang P. Sodium-Glucose Co-Transporter 2 Inhibition With Empagliflozin Improves Cardiac Function After Cardiac Arrest in Rats by Enhancing Mitochondrial Energy Metabolism. Front Pharmacol. 2021;12:758080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Song Y, Huang C, Sin J, Germano JF, Taylor DJR, Thakur R, Gottlieb RA, Mentzer RM Jr, Andres AM. Attenuation of Adverse Postinfarction Left Ventricular Remodeling with Empagliflozin Enhances Mitochondria-Linked Cellular Energetics and Mitochondrial Biogenesis. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Kubli DA, Gustafsson ÅB. Unbreak my heart: targeting mitochondrial autophagy in diabetic cardiomyopathy. Antioxid Redox Signal. 2015;22:1527-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Wei J, Yan T, Liang Y. Targeting TRAF3IP2 alleviates high glucose-induced cardiomyocyte inflammation and apoptosis. Drug Dev Res. 2022;83:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Kim M, Tian R. Targeting AMPK for cardiac protection: opportunities and challenges. J Mol Cell Cardiol. 2011;51:548-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P, Mao X, Huang K, Xie Z, Zou MH. Hyperglycemia-Driven Inhibition of AMP-Activated Protein Kinase α2 Induces Diabetic Cardiomyopathy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes In Vivo. Circulation. 2019;139:1913-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 40. | Guo Z, Tuo H, Tang N, Liu FY, Ma SQ, An P, Yang D, Wang MY, Fan D, Yang Z, Tang QZ. Neuraminidase 1 deficiency attenuates cardiac dysfunction, oxidative stress, fibrosis, inflammatory via AMPK-SIRT3 pathway in diabetic cardiomyopathy mice. Int J Biol Sci. 2022;18:826-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 41. | Al-Damry NT, Attia HA, Al-Rasheed NM, Mohamad RA, Al-Amin MA, Dizmiri N, Atteya M. Sitagliptin attenuates myocardial apoptosis via activating LKB-1/AMPK/Akt pathway and suppressing the activity of GSK-3β and p38α/MAPK in a rat model of diabetic cardiomyopathy. Biomed Pharmacother. 2018;107:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Cao X, Luo T, Luo X, Tang Z. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens Res. 2014;37:803-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Zhou H, Li Y. Long-term diabetic complications may be ameliorated by targeting Rho kinase. Diabetes Metab Res Rev. 2011;27:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Preau S, Delguste F, Yu Y, Remy-Jouet I, Richard V, Saulnier F, Boulanger E, Neviere R. Endotoxemia Engages the RhoA Kinase Pathway to Impair Cardiac Function By Altering Cytoskeleton, Mitochondrial Fission, and Autophagy. Antioxid Redox Signal. 2016;24:529-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Gao H, Hou F, Dong R, Wang Z, Zhao C, Tang W, Wu Y. Rho-Kinase inhibitor fasudil suppresses high glucose-induced H9c2 cell apoptosis through activation of autophagy. Cardiovasc Ther. 2016;34:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017-12022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2011] [Cited by in RCA: 2007] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 47. | Yang X, Liu Q, Li Y, Tang Q, Wu T, Chen L, Pu S, Zhao Y, Zhang G, Huang C, Zhang J, Zhang Z, Huang Y, Zou M, Shi X, Jiang W, Wang R, He J. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte. 2020;9:484-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 48. | Ahmed LA, Darwish HA, Abdelsalam RM, Amin HA. Role of Rho Kinase Inhibition in the Protective Effect of Fasudil and Simvastatin Against 3-Nitropropionic Acid-Induced Striatal Neurodegeneration and Mitochondrial Dysfunction in Rats. Mol Neurobiol. 2016;53:3927-3938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 50. | Yao J, Li Y, Jin Y, Chen Y, Tian L, He W. Synergistic cardioptotection by tilianin and syringin in diabetic cardiomyopathy involves interaction of TLR4/NF-κB/NLRP3 and PGC1a/SIRT3 pathways. Int Immunopharmacol. 2021;96:107728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai L, United States; Roomi AB, Iraq; Yan LJ, United States S-Editor: Lin C L-Editor: Wang TQ P-Editor: Xu ZH