Published online Sep 15, 2022. doi: 10.4239/wjd.v13.i9.752
Peer-review started: March 11, 2022
First decision: April 25, 2022
Revised: May 13, 2022
Accepted: August 22, 2022
Article in press: August 22, 2022
Published online: September 15, 2022
Processing time: 181 Days and 22.2 Hours
Benzylamine (Bza) oral administration delays the onset of hyperglycemia in insulin-resistant db-/- mice; a genetic model of obesity and type 2 diabetes.
To extend the antihyperglycemic properties of oral benzylamine to a model of insulin-deficient type 1 diabetes.
Male Swiss mice were rendered diabetic by streptozotocin treatment (STZ) and divided in two groups: one received 0.5% Bza as drinking solution for 24 d (STZ Bza-drinking) while the other was drinking water ad libitum. Similar groups were constituted in age-matched, nondiabetic mice. Food intake, liquid intake, body weight gain and nonfasting blood glucose levels were followed during treatment. At the end of treatment, fasted glycemia, liver and white adipose tissue (WAT) mass were measured, while glucose uptake assays were performed in adipocytes.
STZ diabetic mice presented typical features of insulin-deficient diabetes: reduced body mass and increased blood glucose levels. These altered parameters were not normalized in the Bza-drinking group in spite of restored food and water intake. Bza consumption could not reverse the severe fat depot atrophy of STZ diabetic mice. In the nondiabetic mice, no difference was found between control and Bza-drinking mice for any parameter. In isolated adipocytes, hexose uptake was partially activated by 0.1 mmol/L Bza in a manner that was obliterated in vitro by the amine oxidase inhibitor phenelzine and that remained unchanged after Bza supplementation. Oxidation of 0.1 mmol/L Bza in WAT was lower in STZ diabetic than in normoglycemic mice.
Bza supplementation could not normalize the altered glucose handling of STZ diabetic mice with severe WAT atrophy. Consequently, its antidiabetic potential in obese and diabetic rodents does not apply to lipoatrophic type 1 diabetic mice.
Core Tip: In adipocytes, benzylamine (Bza) is oxidized by amine oxidases and stimulates glucose uptake. Bza oral administration alleviates insulin-resistant diabetes in obese and diabetic mice. It was investigated whether Bza was also antihyperglycemic in insulin-deficient type 1 diabetes. To this aim, a 0.5% Bza drinking solution was given to streptozotocin-induced diabetic mice. Oral Bza did not recover hyperglycemia and reduced adiposity of lipoatrophic and diabetic mice. A minimal level of adiposity was required to support benzylamine oxidation and to improve glucose utilization. Thus, the antidiabetic properties of Bza in obese and diabetic models, do not apply for diabetes with severe lipoatrophy.
- Citation: Carpéné C, Stiliyanov Atanasov K, Les F, Mercader Barcelo J. Hyperglycemia and reduced adiposity of streptozotocin-induced diabetic mice are not alleviated by oral benzylamine supplementation. World J Diabetes 2022; 13(9): 752-764
- URL: https://www.wjgnet.com/1948-9358/full/v13/i9/752.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i9.752
A recent study indicates that orally given benzylamine (Bza) delays the onset of diabetes in obese and insulin-resistant db-/- mice[1]. Supplementation with 0.5% Bza (5 g/L) in the drinking water impaired the increase in blood glucose, water intake and urine emission that occurs after weaning in this mouse model of insulin-resistant type 2 diabetes. The proposed mechanism of action for ingested Bza, which is naturally present in vegetables and edible plants, relies on its oxidation by an amine oxidase, which is a copper-containing enzyme highly expressed in fat cells[2,3]: The semicarbazide-sensitive amine oxidase (SSAO)[4] also known as amine oxidase copper containing 3[5] and identical to vascular adhesion protein (VAP-1)[6]. More precisely, it is hydrogen peroxide, one of the products of amine oxidation, and known from decades to stimulate glucose uptake in fat cells[7], that supports the insulin-mimetic actions of Bza in adipocytes, either in rodents[8] or in humans[9]. The in vitro insulin-like actions of Bza encompass activation of glucose uptake[10], induction of adipogenesis[11] , stimulation of lipogenesis[12], and inhibition of lipolysis. They occur even in the absence of insulin[8]. It was therefore of interest to investigate whether an oral treatment with Bza is capable of alleviating the impaired glucose handling of insulin-deficient, type 1 diabetic states.
Type 1 diabetes is characterized by a deficiency in insulin resulting from endocrine pancreas injury. To treat this disease, it is necessary to permanently normalize the altered blood glucose homeostasis. Since insulin is the major regulator of blood glucose levels, many therapeutic beneficial approaches have consisted in providing this pancreatic hormone, via repeated injections, or even by more sophisticated administration modes using biotechnologies, islet transplants or cell therapies[13]. Whatever the mode of supply, insulin overdose has to be avoided to prevent the risk of fatal hypoglycemia and to limit the onset of insulin resistance. Of note, various pharmacological agents or naturally occurring molecules can act as insulin-like factors on the glucose utilization by peripheral tissues[14]. In this view, testing the putative antihyperglycemic effect of Bza in type 1 diabetic rodents remains a preclinical step that deserves descriptive studies.
Alongside its capacity to oxidize Bza[1], fat tissue is not quantitatively but qualitatively of paramount importance in the regulation of glucose disposal. Adipose tissue uses glucose for accumulating lipid stores, and it also acts as an endocrine organ secreting a variety of adipokines with hyperglycemic or hypoglycemic properties, even in the absence of exogenous insulin[15]. The lack of adipose tissue (lipoatrophy), such as that obtained in several genetically modified mice, is accompanied with altered glucose homeostasis[16,17]. Similarly, diabetic type 1 models, such as streptozotocin (STZ) diabetic rodents, with destroyed endocrine pancreas, exhibit reduced fat stores[18,19]. In humans, successful treatment of type 1 diabetes is concomitant with both restoration of normal glucose levels and adipose tissue recovery[20].
More importantly, diabetic phenotypes of diverse animal models have been ameliorated when white adipose tissue (WAT) or brown adipose tissue (BAT) has been reintroduced in these models, irrespective of the method used. Nowadays, it is suggested that adipose tissue contributes to the correction of type 1 diabetes, since hyperglycemia was lowered in diabetic mice treated by conditioned media from adipose-derived stem cells[21], and since mitigation of diabetes was observed in STZ diabetic mice receiving BAT transplantation[19]. To date, the beneficial effects of ingested Bza on glucose and lipid handling have been studied in obese rodents only[1,22]. These studies have suggested that enhanced fat deposition contribute to the insulin-like effects observed in vivo. Again, these considerations reinforced our interest in investigating the effects of Bza in a lipoatrophic model of type 1 diabetes.
The capacity of Bza to activate glucose transport in rat or mouse adipocytes is potentiated by the presence of vanadium[10,23], a widely recognized insulin-like agent[24,25]. Accordingly, it has been already demonstrated that in vivo treatments with a combination of amine oxidase substrates and vanadium exert antidiabetic effects in diverse diabetic rodents, including the STZ diabetic rats[10,26]. However, we demonstrated in recent studies that Bza[9] or catecholamines[27] are capable of activating glucose transport in human adipocytes, even in the absence of vanadium, and that the synergism vanadate/amine is much more weak in human adipocytes than in the murine ones. All these observations prompted us to examine for the first time the influence of prolonged oral administration of Bza alone-without any added vanadate—in a model of type 1 diabetes, which is nonobese and insulin-deficient; the STZ-induced diabetic mouse.
We investigated whether Bza alone was able, via oral consumption, to improve glucose handling in insulin-deficient STZ mice. The following results do not confirm our assumption, although they suggest that Bza action on glucose disposal requires a minimal amount of adipocytes prone to increase their glucose consumption when oxidizing this SSAO substrate.
Benzylamine hydrochloride, STZ, bovine insulin, phenelzine, collagenase A, and most of the other reagents were from Sigma–Aldrich–Merck (Saint Quentin Fallavier, France). [3H]-2-Deoxyglucose (2-DG) was from Perkin Elmer (Boston, MA, USA). The glucometers and consumables for follow-up of fed blood glucose were provided by Pr. Valet P. (Univ Toulouse, France), and used as previously described[28].
Male Swiss mice obtained from Charles River Laboratories (L’arbresle, France) were housed at constant temperature (20–22°C) and with a 12-h light–dark cycle. At the age of 2 mo, they received an intraperitoneal injection of STZ (40 mg/kg) diluted in citrate buffer (0.05 mmol/L, pH 4.5) for four consecutive days, as described previously[21]. A week later, mice receiving only citrate buffer (nondiabetic) and treated mice exhibiting blood glucose ≥ 300 mg/100 mL (STZ diabetic) were subdivided into four groups of eight males, with either free access to water (control) or a 0.5% Bza solution as drinking liquid (Bza-drinking) for 24 d. To measure plasma insulin levels at the beginning of treatment, blood samples were withdrawn from tail vein then centrifuged and analyzed using Ultrasensitive insulin-ELISA kit (Mercodia, Uppsala, Sweden). All the mice had free access to food and water and were treated in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments)[29]. During this period, nonfasting blood glucose levels were determined every 3 d at 12:00 h (equivalent in the used circadian rhythm to 4 h after lights turned on) using an Accu-Check glucometer (Roche Diagnostics) on a blood drop withdrawn from the tail vein. Mice were killed after overnight fasting at the end of treatment and organs were collected and weighed.
Adipocyte preparations were obtained by collagenase digestion of WAT immediately after removal from the epididymal, intra-abdominal and inguinal anatomical locations. WAT was cut into small pieces, digested at 37°C by collagenase under agitation in Krebs–Ringer buffered at pH 7.5 with 15 mmol/L sodium bicarbonate, 10 mmol/L HEPES, supplemented with 3.5% of bovine serum albumin, as previously described[1]. Preparations of buoyant adipocytes were isolated from the digested WAT by filtration through nylon stockings and two gentle buffer washes, as described previously[10]. In our digestion process, approximately 1 g WAT was necessary to obtain sufficient functional adipocytes for the subsequent hexose uptake assays. When total amount of dissected WAT exceeded 1 g, excess samples were snap-frozen at -80°C. This occurred for each of the normoglycemic mice but not for the lipoatrophic STZ-treated mice. In this case, pools of two mice were used to freeze approximately 200 mg WAT.
The nonmetabolizable analog [3H]-2-DG was the only source of hexose for the cell preparations during glucose transport assays. It was added at a final concentration of 0.1 mmol/L after 45 min incubation of the fat cell suspension with the tested agents, as previously described[10]. Pyruvate (2 mmol/L) was also present in the medium throughout the experiments for energy supply. Radioactive 2-DG (100 μL; approximately 1300000 dpm/vial) was added to 400 μL fat cell suspension, and hexose uptake assays were stopped 10 min later with 100 μL 100 μmol/L cytochalasin B. Cell suspensions (200 μL) were immediately transferred to plastic centrifugation microtubes prefilled with dinonyl-phthalate (density 0.98 g/mL), then subjected to a 30 s spin. The upper part of the tubes, containing radiolabelled hexose internalized in intact fat cells floating above the silicon layer was counted in scintillation vials, as described previously[10]. The extracellular [3H]-2-DG present in the upper part of the tubes was determined in tubes receiving cytochalasin B prior to 2-DG. It averaged 1%–5% of the radioactivity found in control uptake, and was subtracted from all assays, as described previously[9].
Amine oxidase activity was determined at 37°C using [14C]-Bza as substrate, in homogenates of thawed WAT samples, as previously described[10]. Isotopic dilution of [14C]-Bza (final concentration: 0.1 mmol/L) was incubated for 30 min in 200 μL 200 mmol/L phosphate buffer with approximately 50 μg proteins, then the radiolabeled oxidation products were immediately extracted in toluene/ethyl acetate and counted as previously specified[9]. Results were expressed as nmol of deamination products/mg protein/min.
Results are presented as means ± SEM of (n) observations. All the statistical analyses for comparisons between parameters used ANOVA followed by post hoc Dunnett’s multiple comparisons test, analyzed with Prism 6 for Mac OS X (GraphPad Software). Relative EC50 values were calculated by nonlinear regression.
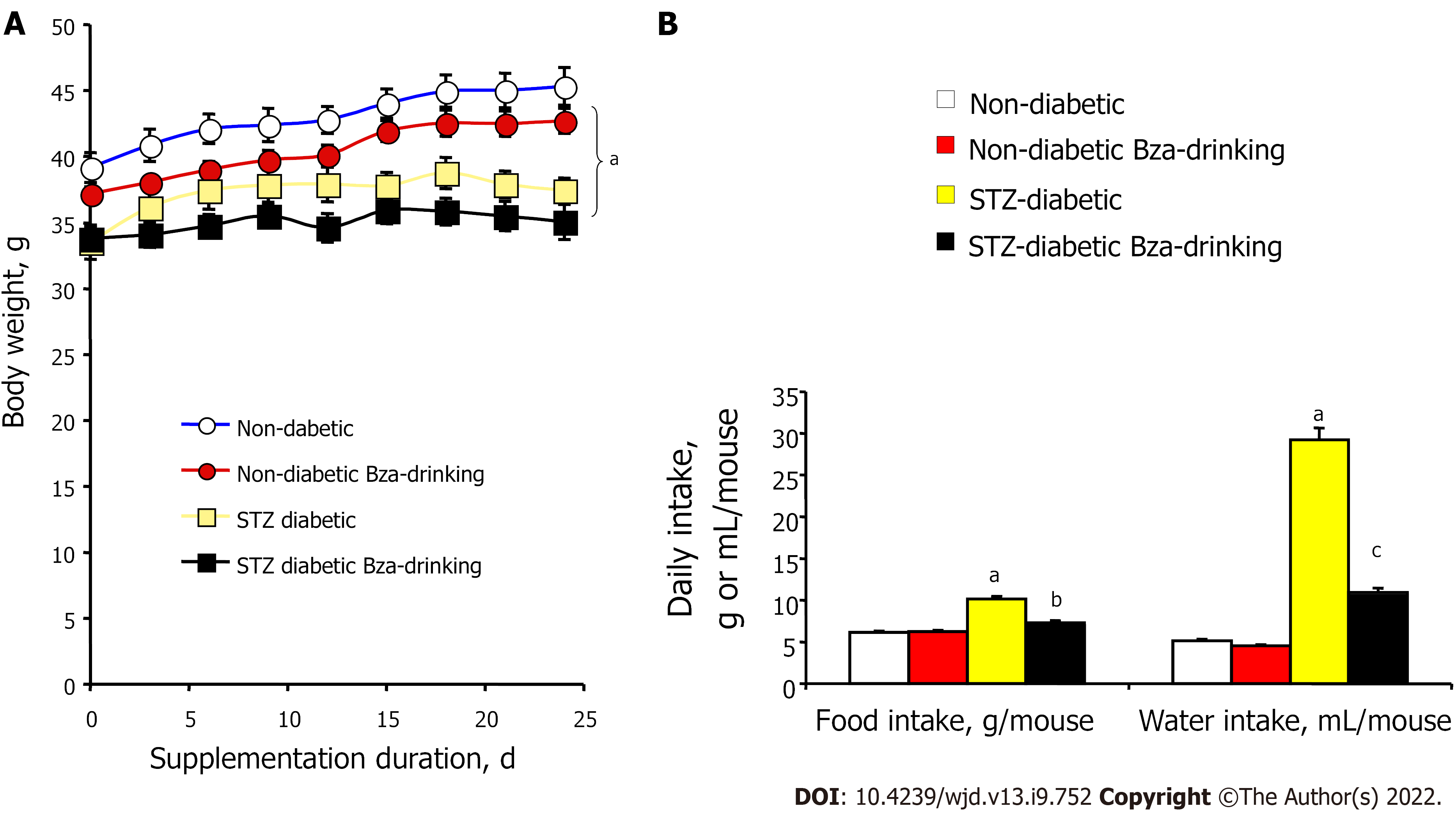
At the start of the experiment, the STZ-induced diabetic mice exhibited lower body weight when compared to age-matched normoglycemic mice (Figure 1). The body weight gain of the insulin-deficient mice was also limited during the treatment period and was not corrected by Bza supplementation. At the end of the experiment, the mean body weight of STZ mice remained lower than that of normoglycemic mice. Hence, Bza supplementation tended to limit body weight gain in both groups, but this trend did not reach significance (Figure 1A). No significant decrease in food consumption was found in the Bza-drinking normoglycemic mice. By contrast, the hyperphagic status of the STZ mice was alleviated by Bza supplementation (Figure 1B). A similar influence of Bza supplementation was found for water consumption. An almost normalization of the elevated daily water intake of STZ diabetic mice occurred in the group subjected to Bza drinking (Figure 1B).
Figure 1 also shows that the characteristic polydipsic feature that occurs in STZ-induced type 1 diabetes was of greater magnitude than the hyperphagy triggered by the noxious diabetogenic agent. The exaggerated liquid consumption of the diabetic group was increased by 5.7 times when compared to normoglycemic control while this increase only reached 1.7 times for food intake. The former defect was expected to traduce glycosuria[19,30], while the second likely corresponded to a lowered efficiency of the ingested carbohydrates that accompanies insulin deficiency[31].
In view of these alterations of food and water intake in STZ diabetic mice and their recovery after Bza drinking, the influence of Bza supplementation on blood glucose levels was examined in both fed and fasted conditions.
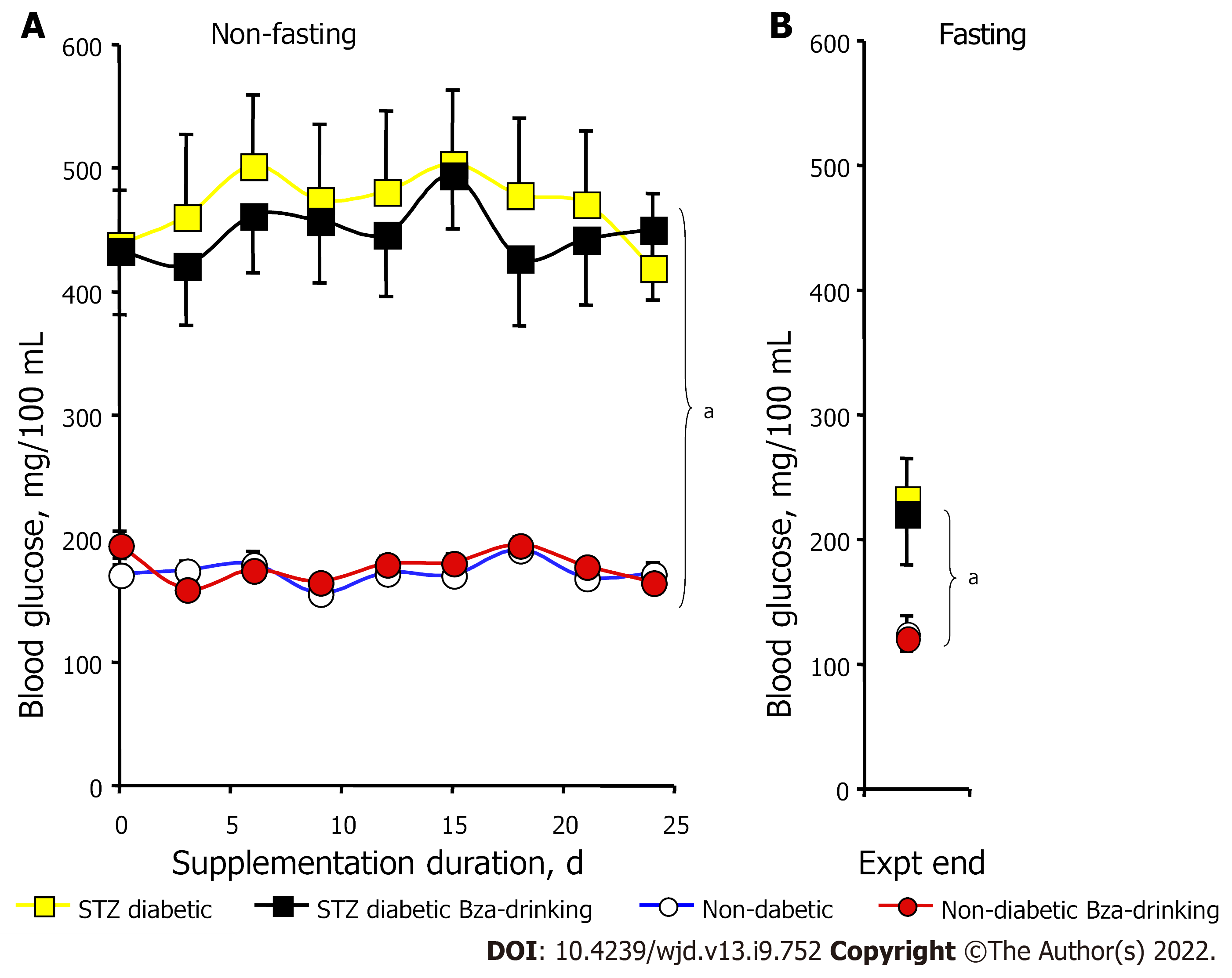
Figure 2 shows the pattern of nonfasting glycemia during the treatment period for the four experimental groups. The unfasted blood glucose levels of the mice previously challenged with STZ were at least twice higher than those of the controls throughout the study (Figure 2A). Such strong hyperglycemia was mainly a consequence of the low circulating levels of insulin found at the start of treatment in the two groups of STZ diabetic mice (0.40 ± 0.04 and 0.38 ± 0.05 ng/mL) when compared to the nondiabetic mice (1.26 ± 0.14 and 1.35 ± 0.09 ng/mL, n = 8; P < 0.001). In the STZ diabetic mice, the blood glucose levels remained elevated in both Bza-drinking and water-drinking groups (Figure 2A). In the normoglycemic mice, the nonfasting blood glucose was superimposed in the control and Bza-drinking groups and remained below 200 mg/100 mL. Thus, blood glucose levels were not significantly influenced by repeated Bza consumption.
To avoid any alteration in body weight gain and in glucose handling, the mice were subjected to overnight fasting only once, at the end of experiment. Fasting blood levels were expectedly lower than nonfasting blood glucose (Figure 2B). Again, the fasting values were superimposable in Bza-drinking mice and their respective controls, while the fasting blood glucose of STZ diabetic mice was higher than that in nondiabetic groups (Figure 2B). Thus, Bza supplementation did not exhibit any hypoglycemic or antihyperglycemic action in this animal model of severe type 1 diabetes.
These findings contrasted with the capacity of Bza to delay the onset of diabetes in the genetically obese and diabetic db-/- mice[1]. Given the unexpected lack of efficiency of Bza consumption on glucose handling, it was poorly appropriate to delineate its putative mechanisms of action or to further examine other surrogate makers of diabetic state, as reported previously[1]. Instead, we verified whether the dose of Bza ingested was similar in the two diabetic models. Considering the daily liquid intake and the body mass of the STZ mice, it was calculated that these type 1 diabetic mice ingested 10850 ± 598 μmol/kg bw/d Bza throughout the treatment. This dose was similar to that used for Bza supplementation in young type 2 diabetic db-/- mice[1], which ranged between 9300 and 10 100 μmol/kg bw/d. However, another difference between type 2 (insulin-resistant) and type 1 (insulin-deficient) diabetic mouse models lies in the occurrence of excessive fat depots in the former and a clearly emaciated state in the latter. Therefore, attention was focused on WAT in the STZ mice and their controls.
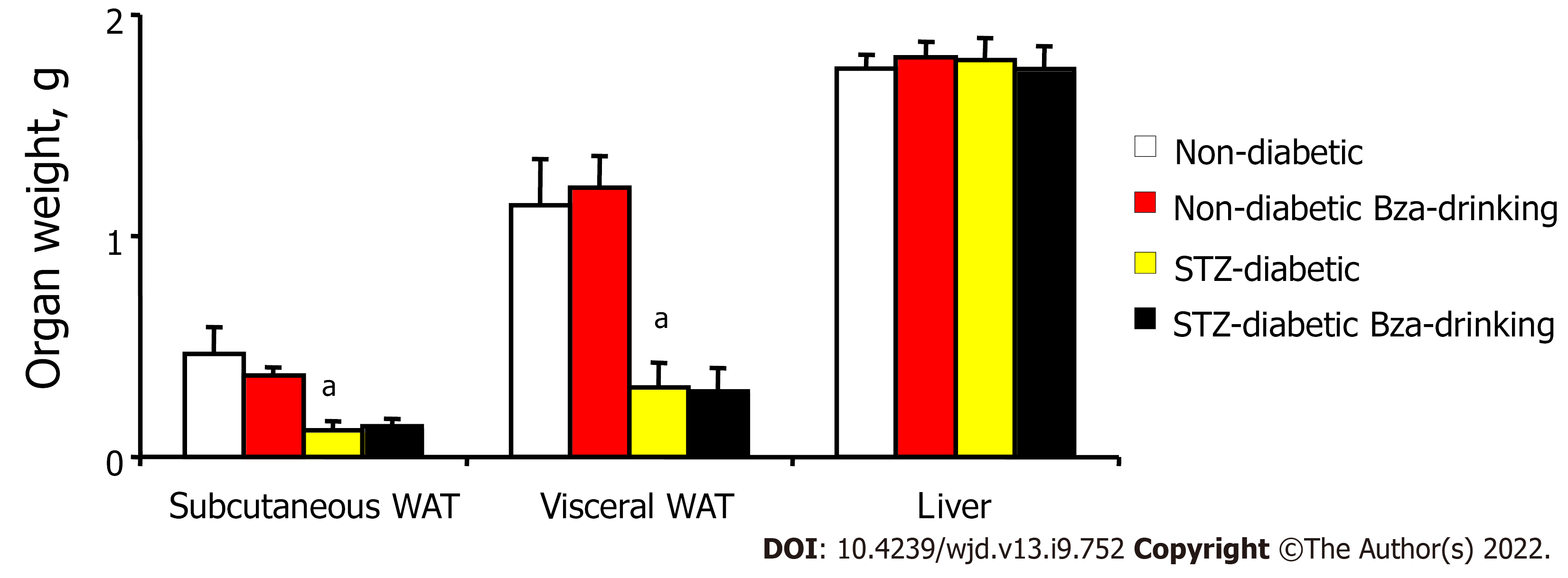
Smaller mass of subcutaneous and visceral WAT was a typical feature of STZ-induced diabetic mice when compared to normoglycemic controls (Figure 3). In the STZ diabetic mice, the low mass of fat pads was not modified by Bza drinking, whatever their anatomical location. Similarly, the normal adiposity of the nondiabetic mice was not modified after oral Bza supplementation.
When the mass of the dissected fat depots was normalized as percentage of body weight, such adiposomatic index[22] was significantly lower in diabetic than in nondiabetic mice (1.2 ± 0.4% vs 3.7 ± 0.6%, P < 0.001). Again, Bza supplementation did not modify adiposomatic index: 1.3 ± 0.4% and 3.9 ± 0.4%, in Bza-drinking diabetic and nondiabetic groups, respectively.
In contrast, the weight of the liver was identical in the four experimental groups (Figure 3). However, when liver mass was expressed as ratio to body weight, the difference that appeared between diabetic and nondiabetic animals was opposite to that of the adiposomatic index. The liver represented 5.3 ± 0.2% of body mass in both STZ diabetic and STZ diabetic Bza-drinking mice (NS, n = 8). This proportion was smaller in nondiabetic mice (4.2 ± 0.1%, P < 0.001), even after Bza drinking (4.5 ± 0.2%).
These observations indicated that the STZ-induced diabetic mice did not normalize their reduced fat deposition and body weight gain after Bza supplementation, in spite of partial recovery of their altered food intake. Moreover, Bza supplementation was not efficient in normalizing the altered blood glucose control or relative hepatomegaly of the STZ mice, although limiting polydipsia. We have previously proposed that Bza oxidation occurring in the hypertrophied WAT of obese and diabetic db-/- mice supports its insulin-like in vitro effects by facilitating glucose utilization in adipocytes and contributes to its antihyperglycemic action[1]. Therefore, such in vitro effects were examined.
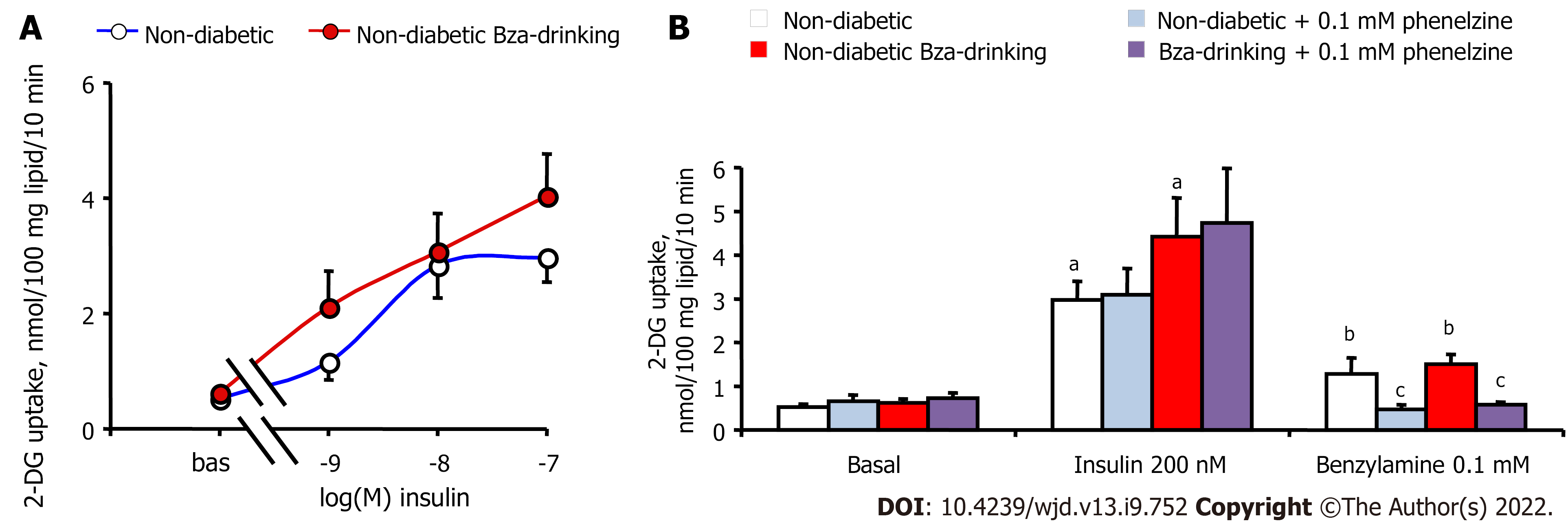
Unfortunately, the WAT atrophy of the STZ diabetic mice did not allow the preparation of sufficient biological material for exploring the activation of 2-DG uptake in functional adipocytes from diabetic and Bza-drinking diabetic mice. There was only a pool of around 400 mg of WAT dissected from different anatomical locations in each STZ mouse, while 1–2 g was removed from each nondiabetic mouse. Consequently, sufficient adipocytes could be isolated from the latter samples only, and the subsequent hexose uptake assays were performed with adipocyte preparations that contained 18.0 ± 2.8 and 19.0 ± 2.5 mg lipid/400 μL in normoglycemic Bza-drinking and control mice, respectively. Thus, Figure 4A shows insulin stimulation of 2-DG uptake in nondiabetic mice only. As expected, insulin dose-dependently activated hexose uptake in adipocytes from control mice, and a tendency to improve insulin maximal effect was detected in Bza-drinking mice. EC50 values of insulin were 0.4 and 2.3 nmol/L for Bza-drinking and control mice, respectively, without showing a significant difference between them. Figure 4B indicates that 0.1 mmol/L benzylamine was capable of reproducing one-third of the maximal insulin stimulation, in a manner that was blunted by the amine oxidase inhibitor phenelzine, which was inactive on basal or insulin-stimulated hexose uptake. The amine-oxidase-dependent insulin-like effect of 0.1 mmol/L Bza was similar in control and Bza-drinking nondiabetic mice. There was no influence of oral Bza supplementation on the capacity of phenelzine to inhibit in vitro the insulin-like action of the amine (Figure 4B).
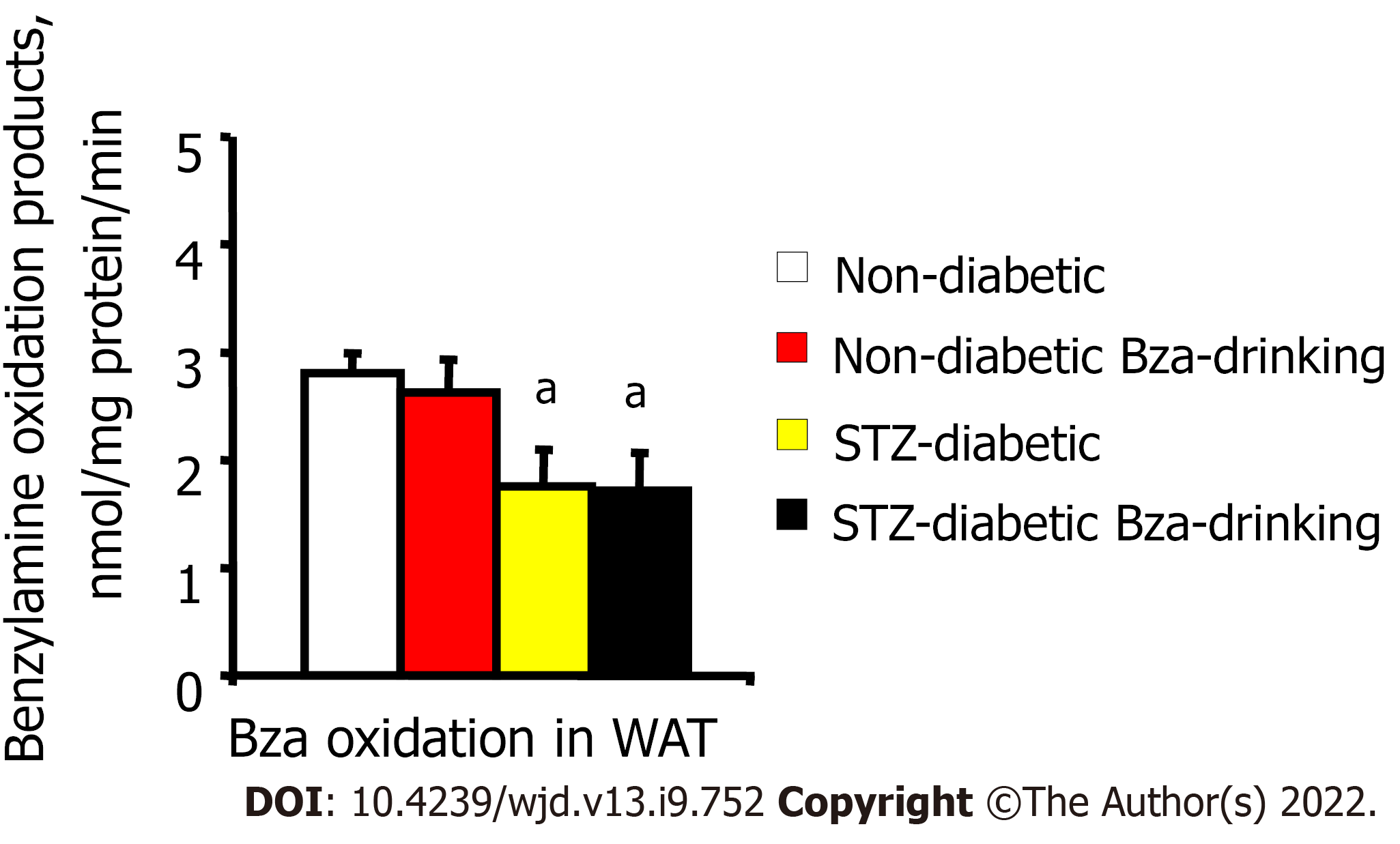
Amine oxidase activity was determined in homogenates from thawed WAT samples by measuring their capacity to oxidize 0.1 mmol/L [14C]-Bza. When expressed as nmol amine oxidized/mg protein/min, the activity was limited in WAT from STZ diabetic mice compared to normoglycemic ones, whether in the control or Bza-drinking groups (Figure 5). The reduced amount of WAT and its limited amine oxidase activity did not argue for a strong contribution of fat stores to the biotransformation of the Bza ingested by STZ diabetic mice.
At the first glance, the lack of antihyperglycemic effect of Bza drinking described here in STZ diabetic mice contrasts with its antidiabetic action observed in obese and diabetic db-/- mice[1]. As discussed below, all these findings converge to propose that the difference in Bza-drinking efficiency between the models of type 1 and type 2 diabetes is not related to insulin deficiency versus resistance, but rather to a difference in adiposity between the murine models.
Alongside bearing dramatically larger fat depots than their lean counterparts, the obese and diabetic db-/- mice also possess higher levels of SSAO activity in their fat cells[1,32]. Thus, the antihyperglycemic effect of oral Bza reported for db-/- mice, and not for their lean littermates, could be related to the elevated amine oxidase activity found in the hypertrophied WAT of obese and diabetic animals[1]. In contrast, STZ diabetic rats exhibit lower monoamine oxidase (MAO) and SSAO activities in WAT than their normoglycemic controls[18]. The lack of antihyperglycemic effect of Bza supplementation in STZ mice reported here resembles the weak antidiabetic effect of prolonged administration of tyramine in STZ rats[18]. Tyramine, which is a substrate of both MAO and SSAO, can limit the hyperglycemic responses to a glucose load during a glucose tolerance test but cannot normalize the elevated fasting blood levels of these insulin-deficient rats. Tyramine or Bza can lower the elevated blood glucose of STZ-induced diabetic rats only when combined with vanadium[10,18,33].
Particular attention has been paid to studying the potential antidiabetic effects of amines alone since the synergism between vanadium and biogenic amines on the activation of glucose transport does not work well in human adipocytes[9,27]. Moreover, the potential antidiabetic use of vanadium derivatives is still limited by toxicological aspects. Several observations suggest that the beneficial effects of dietary amines on glucose handling in diabetic rodents (even when not combined with vanadium) rely upon the amount of SSAO present in WAT. The supplementation of drinking water with 0.4% methylamine (another SSAO substrate) has been reported to increase epididymal WAT mass and to improve glucose tolerance in transgenic mice overexpressing a human form of SSAO/VAP-1, while it is inefficient in nontransgenic mice[34]. Oral Bza also improves glucose handling in high-fat diet fed mice, characterized by increased adiposity[22]. Here, we suppose that it is the lipoatrophy of STZ diabetic mice (and not their lack of insulin) that prevented the occurrence of an antihyperglycemic action of Bza.
The sole beneficial effect of Bza drinking seen in the STZ diabetic mice was an almost total recovery of their characteristic hyperphagic and polydipsic behavior[31]. It could be supposed that urinary glucose leak of STZ mice was partially rescued by Bza drinking. Unfortunately, individual metabolic cages were not available for this study and we could not determine daily urine emission or glucosuria. However, water intake reduction occurred without correction of hyperglycemia. This indicated that renal glucose leak, if any, was not sufficiently rescued by Bza drinking to influence the overall glucose homeostasis, while this was the case for db-/- mice[1]. Food intake was also reduced in Bza-drinking STZ diabetic mice, but without notable decrease in body weight gain. Thus, food efficiency was increased by Bza drinking. However, we cannot propose any underlying mechanism for this effect.
Indeed, it cannot be excluded that mechanisms other than oxidation by amine oxidases might be involved in the in vivo effect of Bza on food and water intake. Raimondi and coworkers have reported that Bza, like methylamine, rapidly induces hypophagia in mice via a modulation of neuronal channels, which is reinforced by SSAO inhibition[35,36]. This suggests that adipose SSAO is likely not the sole target of ingested Bza. Regarding activation of glucose uptake in adipocytes, the effect of Bza is impaired when its oxidation by SSAO is blocked. Surprisingly, the opposite occurred regarding its central effects on food and water intake. When Bza degradation by SSAO is blocked, its half-life is increased and its capacity to modulate the neuronal channels depicted by the group of Raimondi is improved[35,36]. Since there is practically no WAT in the STZ-diabetic mice, and since they have little adipose SSAO, we propose that the limitation of hyperphagia and polydipsia observed in these animals is likely due to a central effect distinct from oxidation by peripheral tissues.
Although the liver is another of the organs reached by ingested Bza, it is not a major site for its biotransformation or detoxification because Bza is metabolized to only a small extent by hepatic subcellular fractions, as observed by Mutlib et al [37]. By contrast, these authors reported that, when orally given to rats, Bza undergoes oxidative deamination and generates benzaldehyde, then hippuric acid, which is the major metabolite. These authors also observed that Bza was fairly stable in rat plasma despite of the presence of a soluble form of SSAO. Although circulating SSAO activity is known to increase with diabetes[18,38-40], it is low when compared to the levels of SSAO found in WAT[1]. A putative mediation of the amine effects via modulation of insulin secretion can be ruled out because, in another model of insulin-deficient diabetes, the alloxan-injected rat, oral administration of tyramine reduced the hyperglycemia by 35%–43% in a manner that was more dependent on insulin-like than on insulin-releasing actions[41].
A limitation of the study was that insulin plasma levels were not determined throughout the treatment since such measurements were performed only at the beginning. However, since circulating insulin was dramatically decreased by STZ challenge, and since the overt hyperglycemia was not corrected by Bza drinking, it was hypothesized that pancreatic injury was not recovered. The hyperinsulinemic levels of the insulin-resistant db-/- mice remained unchanged after Bza supplementation[1]. Similarly, no change in plasma insulin was found in the db+/+ lean control after Bza drinking. Nonetheless, it has been reported that methylamine (another SSAO substrate) limits the insulin degradation by adipocytes[42]. If one supposes that increasing the ability of insulin to stimulate glucose transport is one of the mechanisms involved in the antidiabetic effect of Bza, this can explain why Bza was active in insulin-resistant but not in insulin-deficient diabetes models. Such a paradigm of insulin-sensitizer capacity might provide an alternative to our interpretations based on the necessary abundance of SSAO and WAT to support peripheral glucose disposal. However, it requires to be demonstrated by further investigations, while we report in the current study that Bza alone activated 2-DG uptake in adipocytes, being therefore able to act as an insulin mimicker even in the absence of insulin.
Whether the in vitro SSAO-mediated insulin-like effect of Bza is solely responsible for the antihyperglycemic effect of Bza drinking is far from being demonstrated here. However, this assumption agrees with the conclusions of independent studies showing that treatment of diabetic rodents with SSAO inhibitors prevents diabetic complications but is not antihyperglycemic at all[43-45]. All these observations bring evidence that adipose cells are predominantly involved in Bza oxidation, as a consequence of their high SSAO expression[3], although they do not rule out other concomitant mechanisms.
We designed the current study to achieve a similar daily amount of Bza ingested by the STZ diabetic mice to that ingested by the obese and type 2 diabetic db-/- mice[1]. The results showed that such an objective was reached. However, similar amine intake did not result in a similar beneficial influence on glucose disposal in the two models. In the STZ diabetic mice, the lipoatrophy and lower richness of WAT in amine oxidase activity gave less probability for an adipocyte-dependent metabolism of the ingested amine and subsequent insulin-like actions. Another apparent weakness of the present study was that the nondiabetic Swiss mice did not ingest the same daily amount of Bza than those subjected to the STZ diabetogenic challenge. Our experiments showed that the polydipsia of the STZ diabetic mice was early rescued, after the first week of Bza supplementation. They also showed that, among the Bza-drinking groups, the accumulated fluid intake of the STZ diabetic mice was about twice that of the normoglycemic mice. It could be easily justified post hoc that, considering the initial polydipsia of diabetic mice, it would have been preferable to double the Bza concentration in the solution given to the Bza-drinking nondiabetic group. Hence, it cannot be excluded that such a high dose of Bza would have reduced liquid consumption in the nondiabetic mice also. By assumption, such an adverse effect on liquid consumption remains unlikely since, as with other organic amines, Bza has a taste varying from almond to fish waste[46], which is not supposed to be repellent for rodents. In reality, achieving exactly the same oral dose of Bza for diabetic and nondiabetic animals would have required weekly pair-adjustments, which are difficult to achieve, and would not have yielded more information about the mechanisms of action. The unchanged lipoatrophy, together with the early recovery of polydipsia in the Bza-drinking group, converge to indicate that the antipolydipsic effect of the amine is mediated by a central effect, distinct from that observed in adipocytes.
The in vitro insulin-like effect of submillimolar dose of Bza on glucose transport in adipocytes, and its blockade by phenelzine, reinforced our hypothesis of enhancement of peripheral glucose disposal, although it could not be evidenced in lipoatrophic Bza-drinking STZ mice. Phenelzine, which is a combined MAO and SSAO inhibitor, was used because both MAO and SSAO substrates mimic insulin-like effects in adipocytes[33]. It blocked Bza-stimulated hexose uptake, but not basal or insulin-stimulated hexose uptake. No resistance to the selective blockade by phenelzine appeared in the fat cells from Bza-drinking nondiabetic mice, indicating that continuous supplementation with the substrate did not dramatically downregulate the amine oxidase activities. These hexose uptake assays, which could be performed on nondiabetic mice only, confirmed that, even in the absence of insulin, Bza oxidation activates hexose uptake in adipocytes from Swiss white mice as well as in other rodents[43]. According to the literature, the increase of glucose transport by SSAO activation is limited to adipocytes, and only rare reports have extended this hydrogen-peroxide-dependent insulin-like action to other cell types[47]. Unfortunately, the insufficient number of adipocytes isolated from the atrophied WAT of STZ mice hampered the verification of glucose transport responsiveness to insulin and Bza in the type 1 diabetic state. Even if such insulin mimicry also occurred in adipocytes from insulin-deficient mice, it was too limited to modify the glucose handling, when considering the low mass of WAT, as attested by the significantly lower adiposomatic index found in STZ-treated mice. The limited oxidative metabolism of Bza found in WAT of STZ mice was likely unable to contribute to a replenishment of the atrophied fat depots via the increase of glucose utilization demonstrated in adipocytes of the normoglycemic controls.
Being poorly biotransformed by the limited fat stores of STZ diabetic mice, the ingested Bza could not increase glucose entry in adipocytes and thereby did not contribute to glucose disposal. We presume that such a lack of Bza action explains how its consumption did not decrease elevated blood glucose. Such inefficiency does not preclude future improvements of the antidiabetic therapeutic applications of other amine substrates. However, our findings limit the relevance of Bza consumption to alleviate the complications of type 1 diabetes, especially when accompanied with lipoatrophy. Nevertheless, Bza and its derivatives remain potential antihyperglycemic agents since a recent integrated network pharmacology analysis has revealed that Bza derivatives contribute to the anti-insulin resistance effects of Moringa oleifera[48], one of the most potent antidiabetic medicinal plants[30,49,50].
Although Bza drinking is devoid of beneficial in vivo effects on the type 1 diabetes at doses that limit the onset of type 2 diabetes in genetically obese db-/- mice, the present findings reinforce the hypothesis that oxidation of Bza at the level of adipocytes contributes to peripheral glucose uptake and improves glucose homeostasis. When no sufficient WAT is present (in STZ diabetic mice), the antihyperglycemic effect of Bza is hampered. In contrast, when Bza can be readily oxidized in WAT, it improves glucose tolerance at the expense of an enlargement of fat stores (in db-/- mice). The in vitro experiments confirmed the capacity of submillimolar doses of Bza to activate glucose transport in adipocytes. They also show that such SSAO-dependent insulin mimicry is not altered by chronic administration of the substrate.
Oral administration of benzylamine (Bza) exerts antihyperglycemic effects in obese and diabetic rodent models. This effect has been proposed to depend on the insulin-like action of Bza in adipose cells. The amine oxidation catalyzed by amine oxidases abundantly present in adipocytes generates hydrogen peroxide, which activates glucose transport.
To extrapolate the potential antihyperglycemic properties of Bza found in obese and diabetic models to the treatment of insulin-deficient type 1 diabetic states. Bza administration might facilitate glucose utilization to increase lipogenic and adipogenic activities in the adipose tissue and thereby improve glucose disposal even in the absence of insulin.
To evaluate the impact of Bza supplementation on hyperglycemia, polydipsia and hyperphagia in type 1 diabetic mouse, and to demonstrate that Bza metabolism by adipose tissue supports these antidiabetic effects.
Bza solution (5 g/L, Bza-drinking) replaced drinking water in streptozotocin (STZ)-induced, insulin-deficient diabetic mice. Similar comparison between control and Bza-drinking groups was performed in normoglycemic mice. Nonfasting blood glucose, water and food intake were periodically recorded in the four groups. Adiposity was determined at the end of a 24-d treatment. Glucose transport in freshly isolated adipocytes was assessed ex vivo by determining the uptake of the nonmetabolizable radiolabeled 2-deoxyglucose.
Chronic Bza intake did not normalize hyperglycemia in STZ diabetic mice, despite it alleviating excessive water and food consumption. Bza intake had no effect on the limited body weight of the STZ diabetic mice and could not restore their dramatically reduced adipose tissue mass. In normoglycemic mice, the Bza-drinking group did not show altered body weight, or food or water consumption. However, when directly given in vitro to adipocytes isolated from nondiabetic mice, Bza was efficient in activating glucose uptake in both control and Bza-drinking groups.
The capacity of Bza supplementation to reduce hyperglycemia, previously reported in obese and diabetic rodents, was not detectable in the emaciated and insulin-deficient STZ diabetic mice. However, the capacity of Bza to activate glucose transport in adipocytes was confirmed in nonobese, nondiabetic mice. It is likely that the adipose tissue atrophy induced by STZ challenge hampered the lipogenic and adipogenic action of Bza in this severe model of lipoatrophic, insulin-deficient diabetes.
The current findings and their interpretations considerably limit the field of applications of oral Bza since this molecule did not work as an antidiabetic agent in rodents with reduced adiposity, as it is the case in type 1 STZ diabetic and lipoatrophic mice. Nevertheless, since SSAO substrates exhibit a direct action on glucose handling by fat cells, they still have potential interest for therapeutic use to combat other diabetic states.
We thank Thomas Cadoudal (I2MC, Toulouse, France) for managing STZ treatment and subsequent hyperglycemic and insulin deficiency screening. The authors also thank Ana Gomez-Ruiz and Sophie Fonvieille (I2MC, Toulouse, France) for help in the follow-up, and for disposable plastic supply, respectively. Special thanks to Philippe Valet and Anne Bouloumié (Univ. Toulouse) for sharing their invaluable knowledge about obese and diabetic rodents.
| 1. | Iffiú-Soltesz Z, Wanecq E, Tóthfalusi L, Szökő É, Carpéné C. Oral Supplementation with Benzylamine Delays the Onset of Diabetes in Obese and Diabetic db-/- Mice. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Moldes M, Fève B, Pairault J. Molecular cloning of a major mRNA species in murine 3T3 adipocyte lineage. differentiation-dependent expression, regulation, and identification as semicarbazide-sensitive amine oxidase. J Biol Chem. 1999;274:9515-9523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Shen SH, Wertz DL, Klinman JP. Implication for functions of the ectopic adipocyte copper amine oxidase (AOC3) from purified enzyme and cell-based kinetic studies. PLoS One. 2012;7:e29270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Olivieri A, Tipton KF, O'Sullivan J. Characterization of the in vitro binding and inhibition kinetics of primary amine oxidase/vascular adhesion protein-1 by glucosamine. Biochim Biophys Acta. 2012;1820:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Romauch M. Zinc-α2-glycoprotein as an inhibitor of amine oxidase copper-containing 3. Open Biol. 2020;10:190035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Salmi M, Jalkanen S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid Redox Signal. 2019;30:314-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Czech MP. Differential effects of sulfhydryl reagents on activation and deactivation of the fat cell hexose transport system. J Biol Chem. 1976;251:1164-1170. [PubMed] |
| 8. | Fontana E, Boucher J, Marti L, Lizcano JM, Testar X, Zorzano A, Carpéné C. Amine oxidase substrates mimic several of the insulin effects on adipocyte differentiation in 3T3 F442A cells. Biochem J. 2001;356:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Morin N, Lizcano JM, Fontana E, Marti L, Smih F, Rouet P, Prévot D, Zorzano A, Unzeta M, Carpéné C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J Pharmacol Exp Ther. 2001;297:563-572. [PubMed] |
| 10. | Marti L, Abella A, Carpéné C, Palacín M, Testar X, Zorzano A. Combined treatment with benzylamine and low dosages of vanadate enhances glucose tolerance and reduces hyperglycemia in streptozotocin-induced diabetic rats. Diabetes. 2001;50:2061-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Mercier N, Moldes M, El Hadri K, Fève B. Semicarbazide-sensitive amine oxidase activation promotes adipose conversion of 3T3-L1 cells. Biochem J. 2001;358:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Yang H, Ralle M, Wolfgang MJ, Dhawan N, Burkhead JL, Rodriguez S, Kaplan JH, Wong GW, Haughey N, Lutsenko S. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLoS Biol. 2018;16:e2006519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Paget MB, Murray HE, Bailey CJ, Downing R. From insulin injections to islet transplantation: An overview of the journey. Diabetes Obes Metab. 2022;24 Suppl 1:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Khalivulla SI, Mohammed A, Mallikarjuna K. Novel Phytochemical Constituents and their Potential to Manage Diabetes. Curr Pharm Des. 2021;27:775-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci U S A. 2010;107:17391-17396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Savage DB. Mouse models of inherited lipodystrophy. Dis Model Mech. 2009;2:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Gao H, Guo Y, Yan Q, Yang W, Li R, Lin S, Bai X, Liu C, Chen D, Cao H, Xiao G. Lipoatrophy and metabolic disturbance in mice with adipose-specific deletion of kindlin-2. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Visentin V, Bour S, Boucher J, Prévot D, Valet P, Ordener C, Parini A, Carpéné C. Glucose handling in streptozotocin-induced diabetic rats is improved by tyramine but not by the amine oxidase inhibitor semicarbazide. Eur J Pharmacol. 2005;522:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, Orchard TJ. Adiposity and mortality in type 1 diabetes. Int J Obes (Lond). 2009;33:796-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Dias I, Pinheiro D, Ribeiro Silva K, Stumbo AC, Thole A, Cortez E, de Carvalho L, Carvalho SN. Secretome effect of adipose tissue-derived stem cells cultured two-dimensionally and three-dimensionally in mice with streptozocin induced type 1 diabetes. Curr Res Pharmacol Drug Discov. 2021;2:100069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Iffiú-Soltész Z, Wanecq E, Lomba A, Portillo MP, Pellati F, Szöko E, Bour S, Woodley J, Milagro FI, Alfredo Martinez J, Valet P, Carpéné C. Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacol Res. 2010;61:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Yraola F, García-Vicente S, Marti L, Albericio F, Zorzano A, Royo M. Understanding the mechanism of action of the novel SSAO substrate (C7NH10)6(V10O28).2H2O, a prodrug of peroxovanadate insulin mimetics. Chem Biol Drug Des. 2007;69:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Shechter Y, Karlish SJ. Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature. 1980;284:556-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 386] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Srivastava AK, Mehdi MZ. Insulino-mimetic and anti-diabetic effects of vanadium compounds. Diabet Med. 2005;22:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Zorzano A, Palacín M, Marti L, García-Vicente S. Arylalkylamine vanadium salts as new anti-diabetic compounds. J Inorg Biochem. 2009;103:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Carpéné C, Boulet N, Grolleau JL, Morin N. High doses of catecholamines activate glucose transport in human adipocytes independently from adrenoceptor stimulation or vanadium addition. World J Diabetes. 2022;13:37-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Rancoule C, Attané C, Grès S, Fournel A, Dusaulcy R, Bertrand C, Vinel C, Tréguer K, Prentki M, Valet P, Saulnier-Blache JS. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia. 2013;56:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3050] [Cited by in RCA: 3245] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 30. | Jaiswal D, Kumar Rai P, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol. 2009;123:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Granneman JG, Stricker EM. Food intake and gastric emptying in rats with streptozotocin-induced diabetes. Am J Physiol. 1984;247:R1054-R1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Cioni L, De Siena G, Ghelardini C, Sernissi O, Alfarano C, Pirisino R, Raimondi L. Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: interactive effects with methylamine and alpha-aminoguanidine. Eur J Pharmacol. 2006;529:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Marti L, Morin N, Enrique-Tarancon G, Prevot D, Lafontan M, Testar X, Zorzano A, Carpéné C. Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase-dependent generation of hydrogen peroxide. J Pharmacol Exp Ther. 1998;285:342-349. [PubMed] |
| 34. | Stolen CM, Madanat R, Marti L, Kari S, Yegutkin GG, Sariola H, Zorzano A, Jalkanen S. Semicarbazide sensitive amine oxidase overexpression has dual consequences: insulin mimicry and diabetes-like complications. FASEB J. 2004;18:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Banchelli G, Ghelardini C, Raimondi L, Galeotti N, Pirisino R. Selective inhibition of amine oxidases differently potentiate the hypophagic effect of benzylamine in mice. Eur J Pharmacol. 2001;413:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Pirisino R, Ghelardini C, Banchelli G, Galeotti N, Raimondi L. Methylamine and benzylamine induced hypophagia in mice: modulation by semicarbazide-sensitive benzylamine oxidase inhibitors and aODN towards Kv1.1 channels. Br J Pharmacol. 2001;134:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Mutlib AE, Dickenson P, Chen SY, Espina RJ, Daniels JS, Gan LS. Bioactivation of benzylamine to reactive intermediates in rodents: formation of glutathione, glutamate, and peptide conjugates. Chem Res Toxicol. 2002;15:1190-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Mészáros Z, Szombathy T, Raimondi L, Karádi I, Romics L, Magyar K. Elevated serum semicarbazide-sensitive amine oxidase activity in non-insulin-dependent diabetes mellitus: correlation with body mass index and serum triglyceride. Metabolism. 1999;48:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Salmi M, Stolen C, Jousilahti P, Yegutkin GG, Tapanainen P, Janatuinen T, Knip M, Jalkanen S, Salomaa V. Insulin-regulated increase of soluble vascular adhesion protein-1 in diabetes. Am J Pathol. 2002;161:2255-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Januszewski AS, Mason N, Karschimkus CS, Rowley KG, Best JD, O'Neal DN, Jenkins AJ. Plasma semicarbazide-sensitive amine oxidase activity in type 1 diabetes is related to vascular and renal function but not to glycaemia. Diab Vasc Dis Res. 2014;11:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Lino CdS, Sales TdP, Gomes PB, Amaral JFd, Alexandre FSA, Silveira ER, Ferreira JM, Daniel F. de Sousa, Queiroz MGRd, Sousa FCFd, Brito GAC, Brito SMRC, Viana GSB. Anti-diabetic activity of a fraction from Cissus verticillata and tyramine, its main bioactive constituent, in alloxan-induced diabetic rats. Am J Pharmacol Toxicol. 2007;2:178-188. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Kahn CR, Baird KL. Pathways of insulin degradation in isolated adipocytes: evaluation by gel filtration and differential precipitation. Metabolism. 1985;34:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Yu PH, Wang M, Fan H, Deng Y, Gubisne-Haberle D. Involvement of SSAO-mediated deamination in adipose glucose transport and weight gain in obese diabetic KKAy mice. Am J Physiol Endocrinol Metab. 2004;286:E634-E641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Papukashvili D, Rcheulishvili N, Deng Y. Beneficial Impact of Semicarbazide-Sensitive Amine Oxidase Inhibition on the Potential Cytotoxicity of Creatine Supplementation in Type 2 Diabetes Mellitus. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Tékus V, Horváth ÁI, Csekő K, Szabadfi K, Kovács-Valasek A, Dányádi B, Deres L, Halmosi R, Sághy É, Varga ZV, Adeghate E, Kőszegi T, Mátyus P, Gábriel R, Ferdinandy P, Pintér E, Helyes Z. Protective effects of the novel amine-oxidase inhibitor multi-target drug SZV 1287 on streptozotocin-induced beta cell damage and diabetic complications in rats. Biomed Pharmacother. 2021;134:111105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Olivieri A, Rico D, Khiari Z, Henehan G, O'Sullivan J, Tipton K. From caffeine to fish waste: amine compounds present in food and drugs and their interactions with primary amine oxidase. J Neural Transm (Vienna). 2011;118:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | El Hadri K, Moldes M, Mercier N, Andreani M, Pairault J, Feve B. Semicarbazide-sensitive amine oxidase in vascular smooth muscle cells: differentiation-dependent expression and role in glucose uptake. Arterioscler Thromb Vasc Biol. 2002;22:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Huang Q, Liu R, Liu J, Huang Q, Liu S, Jiang Y. Integrated Network Pharmacology Analysis and Experimental Validation to Reveal the Mechanism of Anti-Insulin Resistance Effects of Moringa oleifera Seeds. Drug Des Devel Ther. 2020;14:4069-4084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Kar A, Choudhary BK, Bandyopadhyay NG. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol. 2003;84:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 50. | Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, Gupta RS. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes. 2012;4:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balbaa ME, Egypt; Cheng JT, Taiwan; Zan-Chao L, China S-Editor: Chang KL L-Editor: Kerr C P-Editor: Chang KL