Published online Aug 15, 2022. doi: 10.4239/wjd.v13.i8.613
Peer-review started: December 15, 2021
First decision: January 12, 2022
Revised: January 24, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 15, 2022
Processing time: 240 Days and 10.9 Hours
Glucagon-like peptide-1 (GLP1) is an endogenous peptide that regulates blood glucose level. But its susceptibility to rapid metabolic degradation limits its therapeutic use.
To prepare GLP1-encapsulated nanosize particle with controlled release property to improve the systemic half-life of GLP1.
GLP1 nanoparticles were prepared by complexation of GLP1 with carbonate apatite nanoparticles (CA NPs). The physicochemical properties of the CA NPs, the effects of GLP1-loaded CA NPs on cell viability, and the systemic bioavailability of GLP1 after CA NPs administration were determined.
The GLP1-loaded CA NPs was within 200 nm in size and stable in fetal bovine serum. The formulation did not affect the viability of human cell lines suggesting that the accumulation of CA NPs in target tissues is safe. In Sprague Dawley rats, the plasma GLP1 Levels as measured from the GLP1-loaded CA NPs-treated rats, were significantly higher than that of the control rats and free GLP1-treated rats at 1 h post-treatment (P < 0.05), and the level remained higher than the other two groups for at least 4 h.
The GLP1-loaded CA NPs improved the plasma half-life of GLP1. The systemic bioavailability of GLP1 is longer than other GLP1 nanoparticles reported to date.
Core Tip: Glucagon-like peptide-1 (GLP1), owing to its physiological properties, is a promising peptide in the treatment of obesity and diabetes. Due to the short half-life of GLP1 and in order to improve GLP1 therapeutic use, GLP1 receptor agonists (GLP1-RAs) have been widely synthesised and encapsulated into nanocarriers for targeted delivery. But the use of GLP1-RAs is associated with unwanted side effects and risks. In the present study, we synthesised a new nanocarrier for native GLP1 - the GLP1 carbonate apatite nanoparticles. The nanocarrier appears comparable if not significantly better than other GLP1 nanoparticles, which have shown promising features as therapeutic agents.
- Citation: Ibnat N, Zaman R, Uddin MB, Chowdhury E, Lee CY. Improved systemic half-life of glucagon-like peptide-1-loaded carbonate apatite nanoparticles in rats. World J Diabetes 2022; 13(8): 613-621
- URL: https://www.wjgnet.com/1948-9358/full/v13/i8/613.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i8.613
The incretin hormone glucagon-like peptide-1 (GLP1) is a peptide secreted from the L cells of the distal ileum and colon in response to nutrient to stimulate insulin secretion. However, as with other peptides, GLP1 is rapidly broken down by circulating enzyme dipeptidyl peptidase-IV (DPP-IV). The poor bioavailability of peptide-based therapeutics is the main challenge of achieving maximum benefit from the drugs.
The metabolic instability of GLP1 has led to the development of GLP1 receptor agonists (GLP1-RAs), which are now widely used in diabetic treatments. GLP1-RAs are generally delivered as payload of drug carriers in injectable formulation. Besides retaining the physiological functions of GLP1, GLP1-RAs have shown additional effects such as regulation of body weight, blood pressure, and cholesterol level[1,2]. But the use of GLP1-RAs was accompanied by side effects such as nausea, vomiting, adverse injection-site reaction, and has posed the risks for pancreatitis and thyroid cell carcinomas[2]. Another aspect of the GLP1-RAs preparations that is worth considering is their pharmacokinetics profile, which essentially is determined by the properties of the drug carriers. Systemic bioavailability of the same drug varies depending on the route of administration that the drug carriers are designed for.
Frequent parenteral administration of therapeutic peptides may lead to a lower patient compliance as compared to oral administration, and also increase the chance of side effects. Moreover, systemically administered peptide drugs have very short half-lives owing to renal clearance and their interaction with the host immune system[3], thus necessitating multiple administration over the course of treatment, which may in turn causes systemic toxicity and undesirable off-target effects. The drawback of GLP1 has also led to the common strategies of conjugating GLP1 or its analogues to polyethylene-glycol (PEG)[4,5] and albumin[6,7] with the aim to extend the peptide’s half-life. However, the efficiency and long-term safety of these conjugates have limited their use[6]. Of note, most of these chemical conjugations was done on GLP1-RAs.
Controlled release formulation is a promising approach as it releases the peptide molecule depending on the needs. Such formulation is therefore able to maintain the circulating level of the peptide and prolong therapeutic activities[8-10]. Previously, we demonstrated that pH sensitive inorganic carbonate apatite nanoparticles (CA NPs) were excellent carriers for intracellular delivery of deoxyribonucleic acid (DNA). We have reported the properties of the CA NPs including their sizes, distribution and zeta potential, measurements from the fourier transform-infrared spectroscopy (FT-IR) and x-ray diffraction and dissolution studies, and effects on crystal growth kinetics[11].
In this study, GLP1-loaded CA NPs was formulated, and the in vitro and in vivo properties of the NPs, specifically their ability to improve the systemic half-life of GLP1 were assessed. We asked the following questions: (1) Does GLP1-CA NPs increase the systemic bioavailability of GLP1 as compared to free GLP1? And (2) Will GLP1-CA NPs with controlled release properties improve the systemic half-life of GLP1 to a similar extent to that of GLP1-RAs?
The CA NPs was prepared by dissolving 44 mmol/L of sodium bicarbonate and Dulbecco’s Modified Eagle Medium (DMEM) powder in mili Q water (pH adjusted to 7.4). The DMEM solution was then mixed with 7 mmol/L concentration of calcium chloride (CaCl2), followed by 30 min incubation at 37°C. For the complexation of GLP1 with CA NPs, a series of GLP1 concentrations ranging from 10 μg to 2 mg, was added to the DMEM solution prior to the addition of 7 mmol/L CaCl2, and the preparations were incubated at 37°C for 30 min.
Turbidity measurement was carried out to determine the growth of the particles. The CA NPs and the GLP1-loaded CA NPs were formulated, as described above. The turbidity of the particle suspensions was assessed using ultraviolet (UV) spectrophotometer at 320 nm absorbance wavelength (UV 1800 Spectrophotometer, Shimadzu, Japan).
The field emission-scanning electron microscope (FE-SEM) was used to observe the morphology of the NPs. The GLP1-CA NPs samples were prepared by adding GLP1 (1 and 10 μg) along with 4 mmol/L CaCl2 to 1 mL bicarbonate-buffered medium containing inorganic phosphate. One drop of the complex particle suspension was dried on a glass slide at 37°C for 1 h. The slide was placed onto a carbon tape-coated sample holder. The dried samples underwent platinum sputtering with 30 mA sputter current at 2.30 tooling factor for 70 s, and the sputtered particles visualised at 5.00 kV (Hitachi/SU8010, Tokyo, Japan).
Fetal bovine serum (FBS, 1%) was used as a source of serum protein to test the binding affinity of GLP1 to CA NPs. The CA NPs containing 5 mmol/L CaCl2 was prepared under the same condition as mentioned above. The CA NPs was then added with 1% FBS and incubated for 10 min. The NPs, coupled with different concentrations of GLP1 - 500 μg, 1 mg and 2 mg were incubated for 30 min at 37°C. These samples together with the free GLP1 were centrifuged at 13000 rpm for 10 min, where the supernatant was discarded. The pellet was washed with 1 mL DMEM before being dissolved with ethylenediaminetetraacetic acid (EDTA) in phosphate-buffered saline (50 mmol/L). The samples were then subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) in 1% agarose gel. The gel was fixed in a fixing solution for 1 h, stained with Coomassie Blue for 20 min with gentle agitation, and de-stained in a de-staining solution. The image of the de-stained gel was captured using the gel documentation system from Bio-Rad (The United States of America, United States).
The human Michigan Cancer Foundation-7 (MCF-7) cell line, which is a breast cancer cell line, was grown in DMEM supplemented with 10% FBS and 1% penicillin and streptomycin antibiotic in a 25 mm3 culture flask. One day before the treatment, the exponentially growing cells were trypsinised, centrifuged and re-suspended using DMEM. Cells were counted under the optical microscope using a haemocytometer and seeded on a 24-well plate with cell density of 50000 cells per well. The cells were allowed to attach overnight at 37°C with 5% carbon dioxide (CO2). Cells were then treated with either free GLP1 or GLP1-CA NPs in the presence or absence of 10% FBS, and for different length of time prior to the cytotoxicity study using 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT). In the MTT assay, 50 µL MTT (5 mg/mL in phosphate buffered saline) was added to each well and incubated for 4 h. The formazan products were dissolved with 300 μL dimethyl sulfoxide, and the absorbance measured at 595 nm wavelength with reference to 630 nm on a microplate reader (Dynex Opsys MR, United States).
The experiment was approved by the Monash University Animal Ethics Committee (MARP/2016/008). Male Sprague Dawley rats (n = 18, 6 wk old) were obtained from the Monash Animal Facility and handled according to the appropriate animal care guidelines. Each rat was housed in an individually ventilated cage, in a temperature- and humidity-controlled room with a 12 h light-dark cycle (lights on 06:00-18:00), and was allowed free access to control diet (Gold Coin Sdn. Bhd.) and water. After 7 d of acclimatisation period, the rats were divided into three groups (n = 6 per group), and administered via the tail vein, one of the following preparations: CA NPs only, 1 mg/kg free GLP1, and GLP1-loaded CA NPs containing 1 mg/kg GLP1. Approximately 300 μL of blood sample was collected from the tail vein and transferred to a sterile 1.5 mL EDTA-coated tube. Samples were collected at 0 h (pre-treatment), and 1, 2, 4, and 24 h post-treatment, and the blood was centrifuged at 3000 rpm for 15 min at 4°C to separate out the plasma, which was then stored at -20°C. The plasma levels of GLP1 were measured using a commercially available GLP1 enzyme-linked immunosorbent assay (ELISA) kit (Millipore, United States).
Results were presented as mean ± standard error of the mean (SEM). The statistical significance of the treatment groups compared to the control was analysed using the Student’s t test. Student’s t test is a well-established parametric statistical method that compares the means of two independent groups and is more appropriate to be used when sample size is small. A P value of less than 0.05 was considered statistically significant[12].
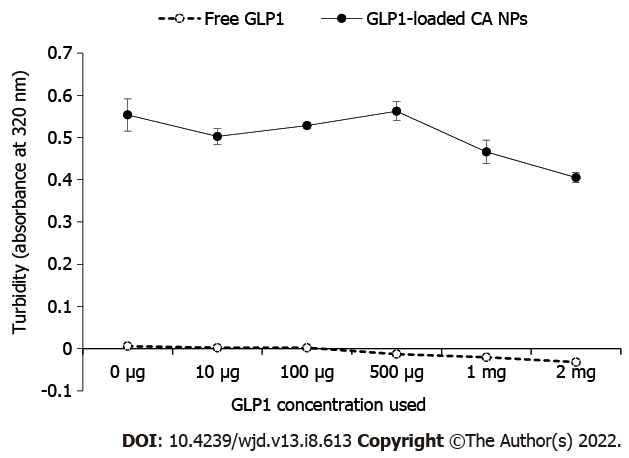
As illustrated in Figure 1, increasing the concentration of free GLP1 in the DMEM solution did not change the absorbance intensity of GLP1. An inverse relationship between GLP1 concentrations (ranged from 10 μg to 2 mg) and the formation of CA NPs was found. This indicates that GLP1 might interact with the growing CA NPs and modulate the NPs growth kinetics.
As shown in Figure 2A, CA NPs was approximate 200 nm in sizes. The presence of GLP1 (1 and 10 μg) along with 4 mmol/L CaCl2 yielded particles of heterogeneous sizes. Accordingly, 1 μg GLP1 gave rise to particle sizes ranging from approximate 15 to 200 nm (Figure 2B), while 10 μg GLP1 produced particles with intermediate sizes ranging from approximate 60 to 70 nm (Figure 2C). Energy Dispersive X-Ray Analysis-based elemental analysis showed that particles formed with 10 μg GLP1 contained more carbon than particles formed with 1 μg GLP1, which may be explained by the presence of higher amount of hydrocarbon in the particles formed with 10 µg GLP1. In addition, the calcium/phosphate (Ca2+/P) ratio was found to be much higher in particles fabricated with higher amount of GLP1, probably as a result of phase transformation (data not shown).
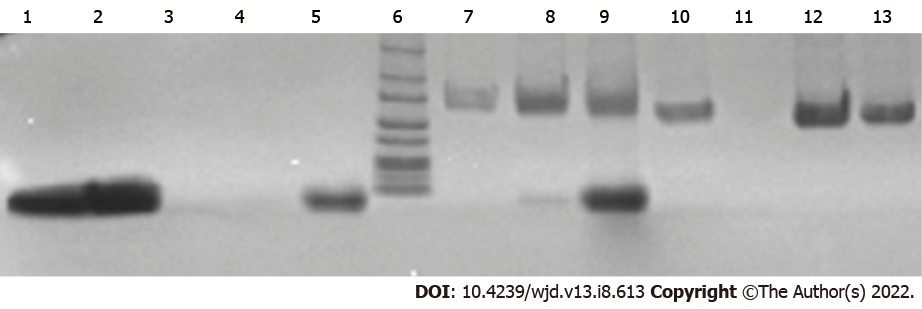
In SDS-PAGE, a GLP1 band along with a FBS band was observed for CA NPs prepared with 1 mg and 2 mg GLP1. A more prominent GLP1 band was seen from the latter (Figure 3). Results confirmed that there was sufficiently stable complex formation between the NPs and GLP1. Serum proteins did not trigger the dissociation of GLP1 from the CA NPs.
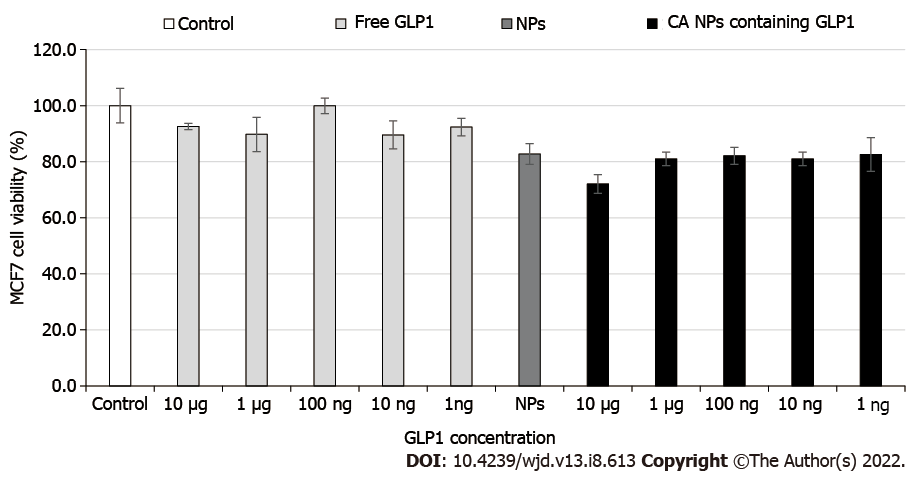
In the MTT assay, both the free GLP1 and CA NPs-bound GLP1 did not exert noticeable effects on the viability of the human cell line (Figure 4). This suggests that the GLP1-loaded CA NPs are likely to be safe for clinical application. More pre-clinical studies are needed to confirm this.
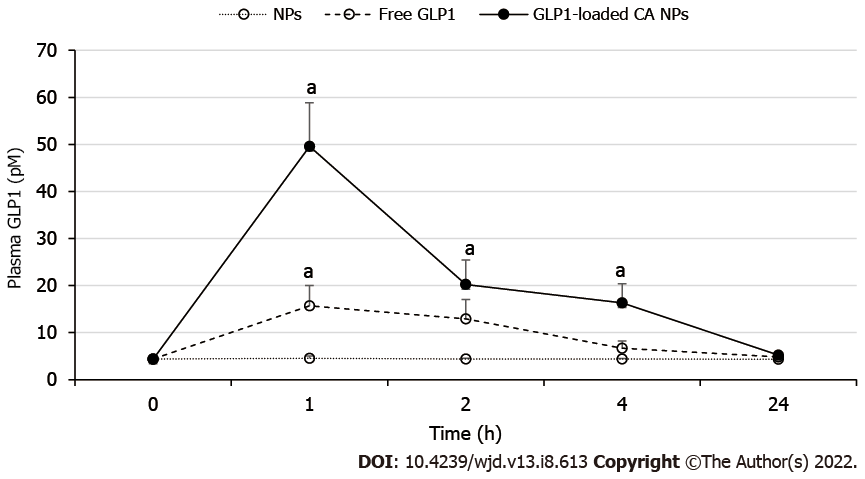
The plasma levels of GLP1 in rats treated with GLP1-CA NPs were significantly higher than control rats at 1 h (49.61 ± 9.24 picomolar (pM), P < 0.05), 2 h (20.22 ± 5.20 pM, P < 0.05), and 4 h (16.32 ± 4.01 pM, P < 0.05) (Figure 5). The increased plasma GLP1 Level at 1 h in GLP1-CA NPs treated rats was also significantly higher than the levels measured from rats administered with free GLP1 (15.68 ± 4.34 pM, P < 0.05).
Therapeutic drug with a high tendency of reaching its target site may have increased efficiency and limited side effects. Nanosize particle has the advantage in this aspect as it allows targeted drug delivery. In the present study, a new GLP1-loaded nanosize particle with controlled release property was successfully developed. The formulation adds to the list of GLP1 nanoparticles reported to date, which has made little progress since our review of clinically available GLP1 NPs for diabetic treatment 5 years ago[13].
The slow progress or lack of interest in developing particles that encapsulate native GLP1 could be attributed to the rapid metabolism of GLP1, and as such, making GLP1-RAs a potentially more viable option. The only NPs preparation for intravenous GLP1 administration, a study design closest to the present study, was reported more than a decade ago[14]. The preparation, which used liposome as the drug carrier, produced a 3.6-fold higher serum GLP1 Level than that of free GLP1 at 15 min post-treatment. But the elevated GLP1 Level decreased rapidly thereafter. Another preparation, which was comparable to the present study in terms of the administrative dose and test subject, and involved a silica-based pH sensitive nanomatrix system, showed a burst release of GLP1 during the first hour. The plasma level of GLP1 however, returned to basal level at 4 h[15].
The GLP1-CA NPs have improved the systemic bioavailability of GLP1, showing better sustained release properties than the liposomal carrier and pH sensitive nanomatrix system. The plasma GLP1 Level of the CA NPs-treated rats was 3.2-fold higher compared to the free GLP1-treated rats at 1 h, and the level was sustained for at least 4 h post-treatment. Although carbonate apatite was reported to have strong affinity toward bovine serum albumin at physiological pH[16], the observed high binding affinity of GLP1 towards CA NPs may have negated the potential interaction between CA NPs and various blood proteins.
The liposomal preparation[14], and the silica-based pH sensitive nanomatrix system[15] mentioned above reported significant reduction in the glucose level, and the liposomal GLP1 also significantly increased insulin secretion. Given that CA NPs showed superior plasma GLP1 profile to these preparations, it is reasonable to predict that GLP1-loaded CA NPs will exert similar insulinotropic and hypoglycaemic effects. Nonetheless, further studies are necessary to confirm the therapeutic effects of GLP1-CA NPs.
Despite having a short plasma half-life, GLP1 may still be preferred to GLP1 agonists for therapeutic use. This is evidenced in several studies which used nanomaterials without GLP1 or GLP1-RAs as payload to stimulate GLP1 secretion[5,17,18]. The surface-modified lipid-based nanocarriers not only increased GLP1 secretion significantly, but normalised plasma glucose levels, and reduced insulin resistance in obese/diabetic mice following a 4 wk treatment[5].
Liraglutide, exenatide and exendin-4 are GLP1-RAs widely used to improve the therapeutic effects of GLP1. It is therefore useful to understand the pharmacokinetic/pharmacodynamic profiles of GLP1-RA-loaded carrier systems so as to gain a better understanding of how significant these preparations had in improving GLP1 bioavailability when comparing with GLP1 nanoparticles, and this was reviewed recently[19]. In order to make comparison between GLP1-CA NPs and GLP1-RAs preparations for their systemic bioavailability and usefulness, only studies that used rats as test subjects and preparations meant for oral administration were discussed below.
Exendin-4 released from enteric-coated capsule containing pH responsive NPs showed a maximum plasma concentration at 5 h after treatment[20]. Another preparation that involved poly(lactic-co-glycolic acid) NPs conjugated with dextran and a C-terminal Src kinase peptide showed peak plasma exenatide level at 6 h post-administration. However, the elevated plasma exenatide level was not significantly different from rats receiving subcutaneous injection of exenatide solution, which was used as a positive control[21]. Zhang et al[22,23] who used functionalised NPs for oral exenatide delivery, reported maximum plasma exenatide level at 4 h and 6 h, respectively after administration. But similar to the study by Song et al[21], the exenatide level was not significantly different from that seen in rats administered subcutaneously with an exenatide solution. Overall, the sustainability of the plasma concentration of GLP1-RAs encapsulated in various types of nanomaterials was between 4-6 h, only slightly higher than that of GLP1-encapsulated CA NPs, although an oral formulation may produce better patient compliance than parenteral administration.
On this note, it is necessary to highlight that the GLP1 systemic bioavailability and/or the biological effects of the different nanomaterial preparations discussed above were cited from and compared between animal subjects. Preparations which are under pre-clinical testing stages, as per the present study, provide useful information on the potential usability and practicality of the preparations in human, and are instructive for future investigation on other animal species and subsequently, human subjects.
Liraglutide and semaglutide have been approved by the United States Food and Drug Administration as weight management agents, and semaglutide in obese or overweight adults with at least one weight-related condition including diabetes. Emerging evidences support the implementation of pharmacotherapy along with behavioural therapy and dietary intervention in optimising obesity treatment. The same approach may apply to diabetes management because consumption of food with antioxidant and anti-inflammatory properties could synergise the effects of GLP1 and GLP1-RAs[24]. If combination therapy is the recommendation for the treatment of metabolic syndrome, the overall cost of management should be taken into consideration.
This study was limited by the lack of chronic and sub-chronic in vivo study involving obese or diabetic animals. A longer duration of study and measurement of metabolic parameters will provide evidence of the effectiveness of GLP1 CA NPs in the long-term treatment of metabolic syndrome. Nevertheless, we showed that this new preparation has therapeutic potential. A second limitation is on the discussion of the data itself. In order to make valid and comparable comparison of the pharmacokinetics profiles between the different GLP1 nanocarriers, only studies that used the same animal species, and native GLP1 were included in the discussion. This means that not all GLP1 and GLP1 RAs nanocarriers that are reported to date have been included. The literature search has nevertheless implied that there is higher interest in GLP1-RAs than native GLP1 despite the high cost of producing GLP1-RAs.
In summary, we have developed a new nanocarrier for GLP1. In vitro, the GLP1-CA NPs are safe on human cell lines, and stable against dissociation from interaction with plasma proteins. In vivo, GLP1-CA NPs demonstrated sustained release properties by significantly improving the plasma half-life of GLP1. The preparation has resulted in a better GLP1 systemic bioavailability than previously reported GLP1-loaded nanocarriers, making it a potentially promising treatment option for metabolic syndrome.
Apart from Glucagon-like peptide-1 (GLP1) receptor agonists that are being widely used and studied, more effort should also be channeled to designing carrier with sustained release properties for native GLP1 because both approaches may be equally effective in improving the systemic half-life of GLP1.
The GLP1-carbonate apatite nanoparticles (CA NPs) overcome the short half-life of GLP1. The nanoparticles could be a potential therapeutic option for metabolic syndrome and warrant further investigation.
A stable GLP1-CA NPs was successfully fabricated. The NPs improved the systemic half-life of GLP1 as compared with free GLP1-treated rats. The increased plasma GLP1 Level was maintained for at least 4 h post-treatment.
The nanoparticles were fabricated through complexation between GLP1 and CA NPs. The GLP1-CA NPs was then evaluated for physicochemical properties, tested for their potential cytotoxic effects on human cell line, and finally measured for systemic bioavailability in rats through intravenous administration.
To fabricate GLP1-loaded carbonate apatite nanoparticles (GLP1-CA NPs), and improve the systemic half-life of GLP1 through GLP1-CA NPs.
pH sensitive inorganic carbonate apatite nanoparticles, which we have successfully formulated previously may be a potential carrier for GLP1.
GLP1 is an endogenous peptide with established glucose lowering property. Its therapeutic use however is limited due to it being rapidly degraded in the systemic circulation. Nanosize particles with sustained release property may protect as well as extend the plasma half-life of GLP1.
The authors would also like to thank Md. Karim E for his assistance in the in vivo study.
| 1. | Chatterjee S, Ghosal S, Chatterjee S. Glucagon-like peptide-1 receptor agonists favorably address all components of metabolic syndrome. World J Diabetes. 2016;7:441-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 3. | Patel A, Cholkar K, Mitra AK. Recent developments in protein and peptide parenteral delivery approaches. Ther Deliv. 2014;5:337-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | van Witteloostuijn SB, Pedersen SL, Jensen KJ. Half-Life Extension of Biopharmaceuticals using Chemical Methods: Alternatives to PEGylation. ChemMedChem. 2016;11:2474-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Xu Y, De Keersmaecker H, Braeckmans K, De Smedt S, Cani PD, Préat V, Beloqui A. Targeted nanoparticles towards increased L cell stimulation as a strategy to improve oral peptide delivery in incretin-based diabetes treatment. Biomaterials. 2020;255:120209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Bech EM, Pedersen SL, Jensen KJ. Chemical Strategies for Half-Life Extension of Biopharmaceuticals: Lipidation and Its Alternatives. ACS Med Chem Lett. 2018;9:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Park J, Bak M, Min K, Kim HW, Cho JH, Tae G, Kwon I. Effect of C-terminus Conjugation via Different Conjugation Chemistries on In Vivo Activity of Albumin-Conjugated Recombinant GLP-1. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Patil S, Vhora I, Amrutiya J, Lalani R, Misra A. Role of Nanotechnology in Delivery of Protein and Peptide Drugs. Curr Pharm Des. 2015;21:4155-4173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Lee PW, Pokorski JK. Poly(lactic-co-glycolic acid) devices: Production and applications for sustained protein delivery. Interdiscip Rev Nanomed Nanobiotechnol. 2018;10:e1516. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Raza F, Zafar H, Zhu Y, Ren Y, -Ullah A, Khan AU, He X, Han H, Aquib M, Boakye-Yiadom KO, Ge L. A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers. Pharmaceutics. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Chowdhury EH, Maruyama A, Kano A, Nagaoka M, Kotaka M, Hirose S, Kunou M, Akaike T. pH-sensing nano-crystals of carbonate apatite: effects on intracellular delivery and release of DNA for efficient expression into mammalian cells. Gene. 2006;376:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Kim TK. T test as a parametric statistic. Korean J Anesthesiol. 2015;68:540-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 411] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 13. | Lee CY. Glucagon-Like Peptide-1 Formulation--the Present and Future Development in Diabetes Treatment. Basic Clin Pharmacol Toxicol. 2016;118:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hanato J, Kuriyama K, Mizumoto T, Debari K, Hatanaka J, Onoue S, Yamada S. Liposomal formulations of glucagon-like peptide-1: improved bioavailability and anti-diabetic effect. Int J Pharm. 2009;382:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Qu W, Li Y, Hovgaard L, Li S, Dai W, Wang J, Zhang X, Zhang Q. A silica-based pH-sensitive nanomatrix system improves the oral absorption and efficacy of incretin hormone glucagon-like peptide-1. Int J Nanomedicine. 2012;7:4983-4994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Hossain S, Stanislaus A, Chua MJ, Tada S, Tagawa Y, Chowdhury EH, Akaike T. Carbonate apatite-facilitated intracellularly delivered siRNA for efficient knockdown of functional genes. J Control Release. 2010;147:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Beloqui A, Alhouayek M, Carradori D, Vanvarenberg K, Muccioli GG, Cani PD, Préat V. A Mechanistic Study on Nanoparticle-Mediated Glucagon-Like Peptide-1 (GLP-1) Secretion from Enteroendocrine L Cells. Mol Pharm. 2016;13:4222-4230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Xu Y, Carradori D, Alhouayek M, Muccioli GG, Cani PD, Préat V, Beloqui A. Size Effect on Lipid Nanocapsule-Mediated GLP-1 Secretion from Enteroendocrine L Cells. Mol Pharm. 2018;15:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Eissa NG, Elsabahy M, Allam A. Engineering of smart nanoconstructs for delivery of glucagon-like peptide-1 analogs. Int J Pharm. 2021;597:120317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Nguyen HN, Wey SP, Juang JH, Sonaje K, Ho YC, Chuang EY, Hsu CW, Yen TC, Lin KJ, Sung HW. The glucose-lowering potential of exendin-4 orally delivered via a pH-sensitive nanoparticle vehicle and effects on subsequent insulin secretion in vivo. Biomaterials. 2011;32:2673-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Song Y, Shi Y, Zhang L, Hu H, Zhang C, Yin M, Chu L, Yan X, Zhao M, Zhang X, Mu H, Sun K. Synthesis of CSK-DEX-PLGA Nanoparticles for the Oral Delivery of Exenatide to Improve Its Mucus Penetration and Intestinal Absorption. Mol Pharm. 2019;16:518-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Zhang L, Shi Y, Song Y, Duan D, Zhang X, Sun K, Li Y. Tf ligand-receptor-mediated exenatide-Zn2+ complex oral-delivery system for penetration enhancement of exenatide. J Drug Target. 2018;26:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Zhang L, Shi Y, Song Y, Sun X, Zhang X, Sun K, Li Y. The use of low molecular weight protamine to enhance oral absorption of exenatide. Int J Pharm. 2018;547:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Lee CY. A Combination of Glucagon-Like Peptide-1 Receptor Agonist and Dietary Intervention Could Be a Promising Approach for Obesity Treatment. Front Endocrinol (Lausanne). 2021;12:748477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Turkey; Dziegielewska-Gesiak S, Poland S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ