Published online Jul 15, 2022. doi: 10.4239/wjd.v13.i7.543
Peer-review started: December 8, 2021
First decision: April 18, 2022
Revised: April 29, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: July 15, 2022
Processing time: 214 Days and 14.9 Hours
The association between blood levels of fructosamine (FMN) and recurrent coronavirus disease 2019 (COVID-19) is currently unclear.
To investigate a prospective relationship between blood levels of FMN and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reinfection.
A total of 146 Chinese hospitalized patients infected with SARS-CoV-2 were consecutively collectively recruited and followed from January 2020 to May 2021. Diagnosis of COVID-19 and SARS-CoV-2 reinfection was based on the diagnostic criteria and treatment protocol in China. The levels of FMN were determined in blood and divided into tertiles based on their distribution in the cohort of COVID-19 patients. Multivariate-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated for SARS-CoV-2 reinfection across the tertiles of FMN levels. A Cox regression model was used to generate the HR for SARS-CoV-2 reinfection in the participants in the top tertile of FMN levels compared with those at the bottom. Disease-free survival was used as the time variable, and relapse was used as the state variable, adjusted for age, gender, influencing factors such as diabetes mellitus, hypertension, and corticosteroid therapy, and clinical indexes such as acute liver failure, acute kidney failure, white blood cell (WBC) count, C-reactive protein, prognostic nutritional index (PNI), and blood lipids. Kaplan-Meier analysis with log-rank tests was used to compare the survival rate between patients with elevated FMN levels (FMN > 1.93 mmol/L, the top tertile) and those with nonelevated levels.
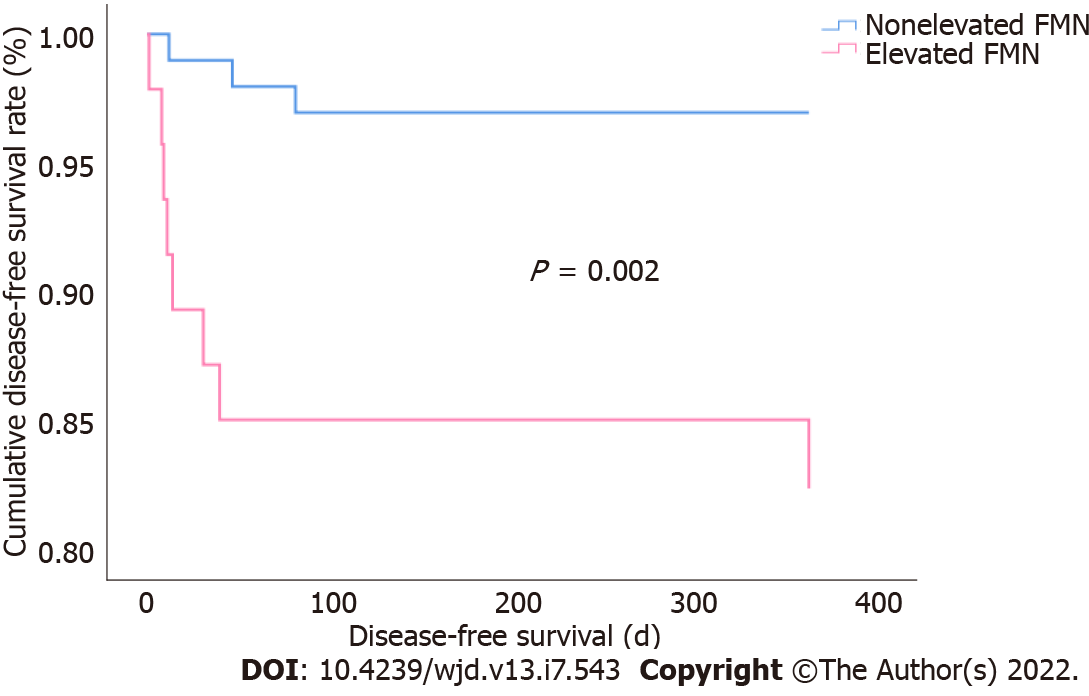
Clinical data for the 146 patients with confirmed COVID-19 [age 49 (39-55) years; 49% males] were analyzed. Eleven patients had SARS-CoV-2 reinfection. The SARS-CoV-2 reinfection rate in patients with elevated FMN levels was significantly higher than that in patients with nonelevated FMN (17% vs 3%; P = 0.008) at the end of the 12-mo follow-up. After adjustments for gender, age, diabetes mellitus, hypertension, corticosteroid therapy, WBC count, PNI, indexes of liver and renal function, and blood lipids, patients with nonelevated FMN levels had a lower risk of SARS-CoV-2 reinfection than those with elevated FMN levels (HR = 6.249, 95%CI: 1.377-28.351; P = 0.018). Kaplan-Meier analysis showed that the cumulative survival rate of patients infected with SARS-CoV-2 was higher in patients with nonelevated FMN levels than in those with elevated FMN levels (97% vs 83%; log rank P = 0.002).
Elevated levels of FMN are independently associated with SARS-CoV-2 reinfection, which highlights that patients with elevated FMN should be cautiously monitored after hospital discharge.
Core Tip: Diabetes is a risk factor for coronavirus disease 2019 (COVID-19), which results in increased severity and mortality but has no relationship with reinfection. The present study, for the first time, reported the relationship between severe acute respiratory syndrome coronavirus 2 reinfection and blood levels of fructosamine (FMN), an index reflecting recent glycemic control. Our results demonstrated that FMN levels may influence the prognosis of patients with COVID-19, and patients with high FMN levels should be followed closely to monitor reinfection.
- Citation: Huang XY, Yang LJ, Hu X, Zhang XX, Gu X, Du LJ, He ZY, Gu XJ. Elevated levels of fructosamine are independently associated with SARS-CoV-2 reinfection: A 12-mo follow-up study. World J Diabetes 2022; 13(7): 543-552
- URL: https://www.wjgnet.com/1948-9358/full/v13/i7/543.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i7.543
Coronavirus disease 2019 (COVID-19) was identified as an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019. It is mainly transmitted by droplets, contact, and aerosols in confined spaces[1,2]. It is highly infectious and widespread[3,4], with more than 505 million patients infected globally, with a cumulative mortality rate of 1.2%[5]. Diabetes is a risk factor for COVID-19, which results in increased severity and mortality[6-8]. A previous study found that of the 570 patients who died or were discharged from hospital, the mortality rate was 6.2% (of 386) for patients without diabetes or hyperglycemia, compared to 28.8% (of 184) for patients who had diabetes and/or uncontrolled hyperglycemia[9]. Hyperglycemia is considered a factor for severity of infection, including severe pneumonia, multiple organ failure, and death. In addition, hemoglobin (Hb)A1c level is an independent risk factor for death and a predictor of COVID-19 severity in patients with diabetes mellitus[10,11].
Fructosamine (FMN) reflects the overall glycemic control for the past 2-3 wk[12] and is strongly correlated with glucose and HbA1c levels[13,14]. HbA1c reflects overall glycemic control over the past 2-3 mo, and general blood glucose monitoring reflects glucose levels at the point. General blood glucose monitoring and HbA1c levels cannot accurately contribute to a prediction index for recent glycemic control. FMN level can be determined rapidly and better reflects recent glycemic control. It has also been associated with diabetic retinopathy, diabetic nephropathy, and long-term cardiovascular outcomes[15]. In addition, FMN levels are positively associated with the risk of periprosthetic joint infection and negatively associated with cancer risk. A previous study also demonstrated that FMN is a valuable marker for predicting adverse outcomes following total hip arthroplasty[16]. Hence, FMN correlates with diabetic complications, inflammation, and cancer. However, to date, no studies have demonstrated its association with COVID-19 risk or SARS-CoV-2 reinfection. The objective of the present study was to determine whether there is an association between FMN levels and COVID-19 risk and SARS-CoV-2 reinfection. This may provide a theoretical basis for the clinical treatment and prognosis of SARS-CoV-2 reinfection.
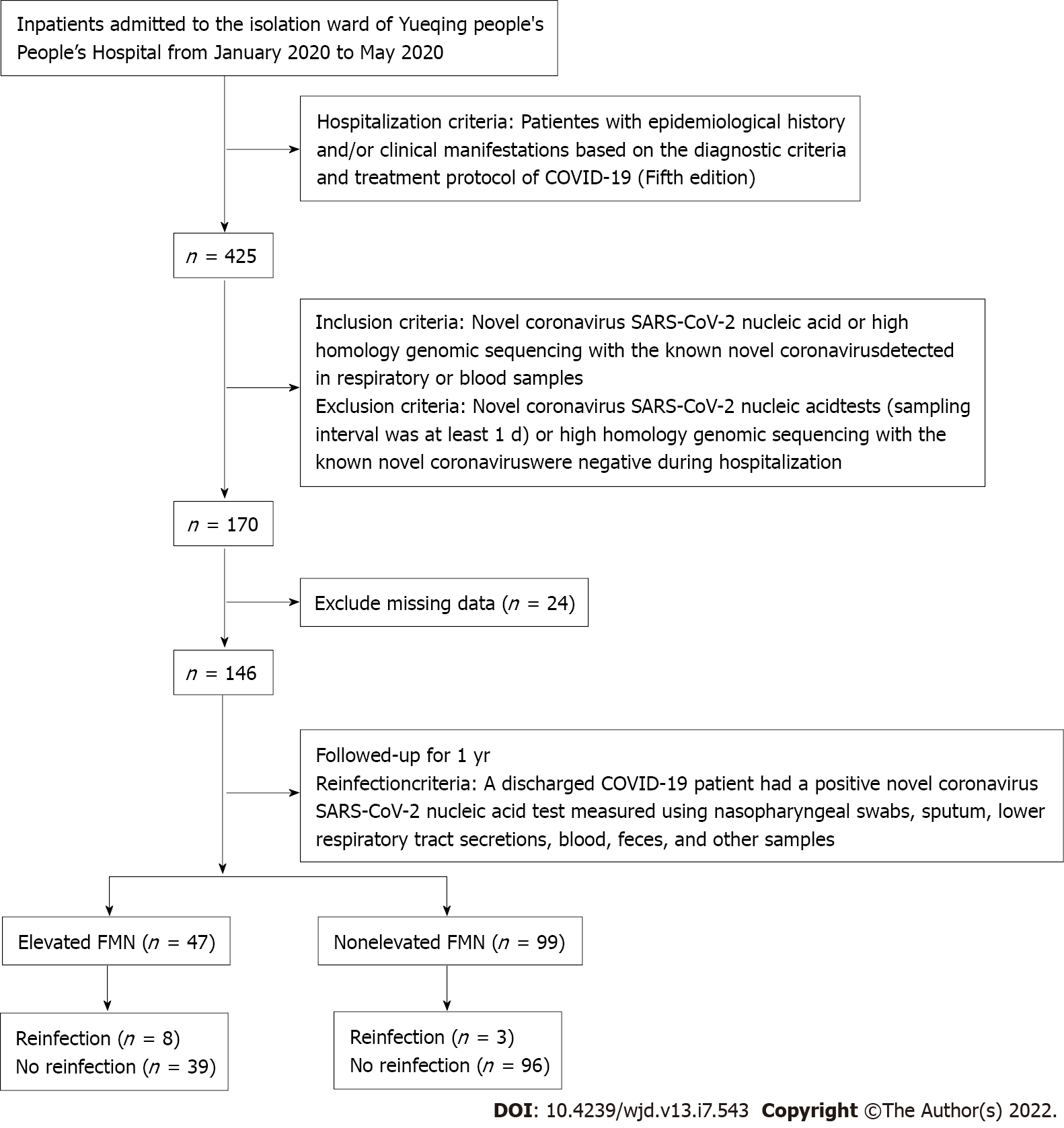
Between January and May 2020, we enrolled 146 patients from the isolation ward of Yueqing People’s Hospital, a designated isolation hospital for COVID-19. All the patients met the diagnostic criteria and treatment protocol for COVID-19 (5th edition). Elevated FMN was defined as levels higher than the upper tertile value of 1.93 mmol/L. The study cohort was divided into two groups based on FMN levels (Figure 1), i.e., elevated FMN group (> 1.93 mmol/L; n = 47) and nonelevated FMN group (≤ 1.93 mmol/L; n = 99). All patients were followed from January 2020 to May 2021, with an average follow-up period of 1 year. The study protocol was approved by the Ethics Committee of Yueqing People’s Hospital, Affiliated Hospital of Wenzhou Medical University (No. YQYY202100033).
Venous blood samples were collected after an overnight fast of ≥ 8 h. All laboratory data were obtained from the first serum collection during hospitalization. The absolute value of peripheral white blood cells (WBCs), lymphocytes, serum creatinine, liver function indexes (alanine and aspartate aminotransferases), lipid profiles (total cholesterol, triacylglycerol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol), and albumin were measured using standard methods. FMN levels were measured using the Roche automatic biochemical analyzer (Basel, Switzerland) and high performance liquid chromatography (Roche). The reference range for FMN was 1.15-2.25 mmol/L. Prognostic nutritional index (PNI) reflects the immune-nutritional status of patients and was determined by calculating serum albumin levels plus a fivefold total number of lymphocytes. PNI is associated with various cancers, such as lung, breast, and gynecological cancers[17].
The patients were diagnosed according to the Chinese Diagnostic Criteria and Treatment Protocol for COVID-19 (5th edition)[18].
Suspected cases: The patients were suspected to have COVID-19 based on a comprehensive analysis in combination with epidemiological history and clinical manifestations. The epidemiological history included: History of travel or residence in Wuhan and surrounding areas, or other communities where cases have been reported within 14 d before onset of illness; history of contact with a SARS-CoV-2-infected patient (positive for nucleic acid test) within 14 d before onset of illness; history of contact with patients with fever or respiratory symptoms from Wuhan and surrounding areas, or from communities where cases have been reported, within 14 d before onset of illness; and aggregation onset. Clinical manifestations included: Fever and/or respiratory symptoms; imaging features of SARS-CoV-2 pneumonia; normal or reduced total WBC count, or reduced lymphocyte count during the early stages of the disease. An individual with an epidemiological history and any two of the clinical manifestations were regarded as a suspected case. If there was no clear epidemiological history, three of the clinical manifestations should be satisfied.
Confirmed cases: Suspected cases with one of the following two tests being positive were regarded as confirmed cases: SARS-CoV-2 nucleic acid detected by real-time reverse transcription polymerase chain reaction in respiratory tract specimens or blood samples, and genomic sequencing of the respiratory or blood samples showing high homology with SARS-CoV-2.
A patient was discharged from isolation and transferred to other wards if his/her body temperature returned to normal and was stable for 3 d, respiratory symptoms improved significantly, lung imaging showed obvious improvement, and two nucleic acid tests were negative (sampling interval was at least 1 d).
SARS-CoV-2 reinfection was defined when a discharged patient had a positive result on the SARS-CoV-2 nucleic acid test measured using nasopharyngeal swabs, sputum, lower respiratory tract secretions, blood, feces, and other samples.
IBM SPSS Statistics version 26 (IBM, Armonk, NY, United States) was used for statistical analyses. Normality of data distribution was determined by one-sample Kolmogorov-Smirnov test. Normally distributed data are expressed as the mean ± SD, and were determined using an independent group t-test. Non-normally distributed data are expressed as the median and interquartile range and were analyzed using the Mann-Whitney U test. The c2 test was used for intergroup comparisons of categorical variables. Cox regression was used to determine the hazard ratio (HR) with 95% confidence interval (CI) for the positive reinfection across the tertiles of FMN levels, with the bottom tertile group as a reference. Kaplan-Meier analysis was used to determine the cumulative survival rate in patients with an FMN level higher than the top tertile compared with that in patients with nonelevated levels, tested using log-rank test. A two-sided P value < 0.05 was considered statistically significant.
Of the 146 patients with COVID-19, 72 were male (49%) and 74 were female (51%), with an average age of 49 years. Comparison between the nonelevated FMN and elevated FMN groups showed no significant difference in gender, respiratory failure, WBC count, C-reactive protein, PNI, alanine transferase, aspartate aminotransferase, serum creatinine, triglyceride, total cholesterol, high-density lipoprotein, or low-density lipoprotein (P > 0.05) (Table 1). The average age of patients with elevated FMN was higher than that of patients in the nonelevated FMN group [53 (43-58) years vs 47 (35-53) years, P = 0.008] (Table 1). There were significant differences in diabetes mellitus, hypertension, and corticosteroid therapy between the two groups (P < 0.05) (Table 1).
| Variable | Total | Elevated FMN1 | Nonelevated FMN2 | P value |
| Patients, n (%) | 146 | 47 (68) | 99 (32) | |
| Gender, n (%) | 0.319 | |||
| Male | 72 (49) | 26 (36) | 46 (64) | |
| Female | 74 (51) | 21 (28) | 53 (72) | |
| Age (yr) | 49 (39-55) | 53 (43-58) | 47 (35-53) | 0.008 |
| Diabetes mellitus, n (%) | 17 (12) | 14 (82) | 3 (18) | 0.000 |
| Hypertension, n (%) | 18 (12) | 8 (44) | 10 (56) | 0.023 |
| Respiratory failure, n (%) | 12 (8) | 6 (50) | 6 (50) | 0.291 |
| Corticosteroid therapy, n (%) | 30 (21) | 5 (17) | 25 (83) | 0.041 |
| WBC [(4.0 × 109/L)-(10.0 × 109/L)] | 4.64 (3.63-5.82) | 5.06 (3.85-6.45) | 4.58 (3.45-5.40) | 0.067 |
| CRP (< 5 mg/L) | 7.30 (5.0-25.60) | 6.80 (5.0-34.90) | 7.80 (5.0-23.60) | 0.320 |
| PNI | 47.80 (44.26-50.58) | 49.55 (46.05-50.95) | 47.05 (44.05-49.55) | 0.061 |
| ALT (0-55 U/L) | 20.50 (14.0-29.0) | 22.00 (15.0-31.0) | 19.00 (13.0-28.0) | 0.138 |
| AST (0-55 U/L) | 23.00 (18.0-31.0) | 25.00 (19.0-32.0) | 22.00 (18.0-30.0) | 0.016 |
| SCR (45-84 μmol/L) | 62 (50-74) | 64 (55-73) | 61 (50-75) | 0.460 |
| TC (3.60-5.70 mmol/L) | 4.24 ± 0.77 | 4.11 ± 0.79 | 4.30 ± 0.76 | 0.176 |
| TG (0.60-1.70 mmol/L) | 1.16 (0.86-1.69) | 1.22 (0.88-1.77) | 1.14 (0.86-1.66) | 0.239 |
| HDL-C (1.09-2.27 mmol/L) | 0.95 (0.80-1.16) | 0.92 (0.76-1.16) | 0.98 (0.83-1.16) | 0.314 |
| LDL-C (1.30-3.37 mmol/L) | 2.30 (1.94-2.91) | 2.22 (1.90-2.78) | 2.32 (1.98-2.92) | 0.242 |
| Reinfection case, n (%) | 11 (7.5) | 8 (73) | 3 (17) | 0.008 |
The SARS-CoV-2 reinfection rate was significantly higher in patients in the elevated FMN group than in those in the nonelevated FMN group (17% vs 3%, P = 0.008) (Table 1). In the Cox regression model, disease-free survival (DFS) was used as the time variable, and reinfection was used as the state variable. After full adjustment, the elevated FMN group showed an increased risk of reinfection (HR = 6.249, 95%CI: 1.377-28.351, P = 0.018; P for trend < 0.05) (Table 2).
| FMN dichotomy | B | SE | HR | 95%CI | P value |
| Model 1 | 1.827 | 0.677 | 6.214 | 1.647-23.438 | 0.007 |
| Model 2 | 1.898 | 0.759 | 6.674 | 1.507-29.544 | 0.012 |
| Model 3 | 1.832 | 0.772 | 6.249 | 1.377-28.351 | 0.018 |
Kaplan-Meier survival analysis showed that the cumulative DFS rate in the elevated FMN group was lower compared to that of the nonelevated FMN group (83% vs 97%, P = 0.002) (Figure 2). The survival rate was determined using the log-rank test, and the P for trend was < 0.05.
We found that patients with elevated FMN levels were older compared to patients in the nonelevated FMN group. Elevated FMN levels were positively associated with reinfection rate as well as HR for reinfection, while the cumulative DFS rate was lower in patients in the elevated FMN group. These results demonstrate that FMN levels may influence the prognosis of patients with COVID-19. COVID-19 is an acute inflammatory disease. Previous studies have demonstrated that patients with diabetes and severe disease were less likely to experience recurrence of SARS-CoV-2 infection[19]; however, patients with uncontrolled diabetes had an increased risk of reinfection[20]. Blood glucose monitoring reflects glucose levels at the point of testing and does not reflect overall blood glucose control. Compared to HbA1c, FMN can reflect blood glucose changes more recently. Previous studies have demonstrated that FMN is a good predictor of adverse events following total knee arthroplasty. Patients with high FMN levels were more likely to develop prosthetic joint infections compared to patients with low FMN levels. Unlike FMN, HbA1c does not show a significant association with complications[6]. FMN but not HbA1c is a significant predictor of infection in hemodialysis and diabetes patients with acute infections[21]. In our study, we found that FMN was associated with SARS-CoV-2 reinfection. Compared to patients with low FMN levels, patients with high FMN levels were found to have a higher reinfection rate. Patients with high FMN levels had a higher HR for reinfection, while patients with low FMN levels had higher cumulative DFS rates. It appears that FMN levels may predispose individuals to reinfection. Thus, the clinical focus should be on maintaining consistent euglycemia, using standard point-of-care glucose checks.
FMNs are advanced glycation end products (AGEs) generated when glucose reacts reversibly with amino groups in proteins. Reversible aldehyde imine intermediate is formed by the aldehyde group of carbohydrates and the N-terminal amino acids of proteins. However, irreversible AGEs are generated through a Maillard reaction[22]. Maillard reactions have been shown to impair cellular function[23]. FMN-3 kinase-related protein, designated as a potential longevity protein[24], can catalyze deglycation of Maillard intermediates directly downstream from FMN, thereby reducing AGE levels[25,26]. Several studies have demonstrated that AGE levels increase with age[27]. In our study, we found that older patients had higher FMN levels.
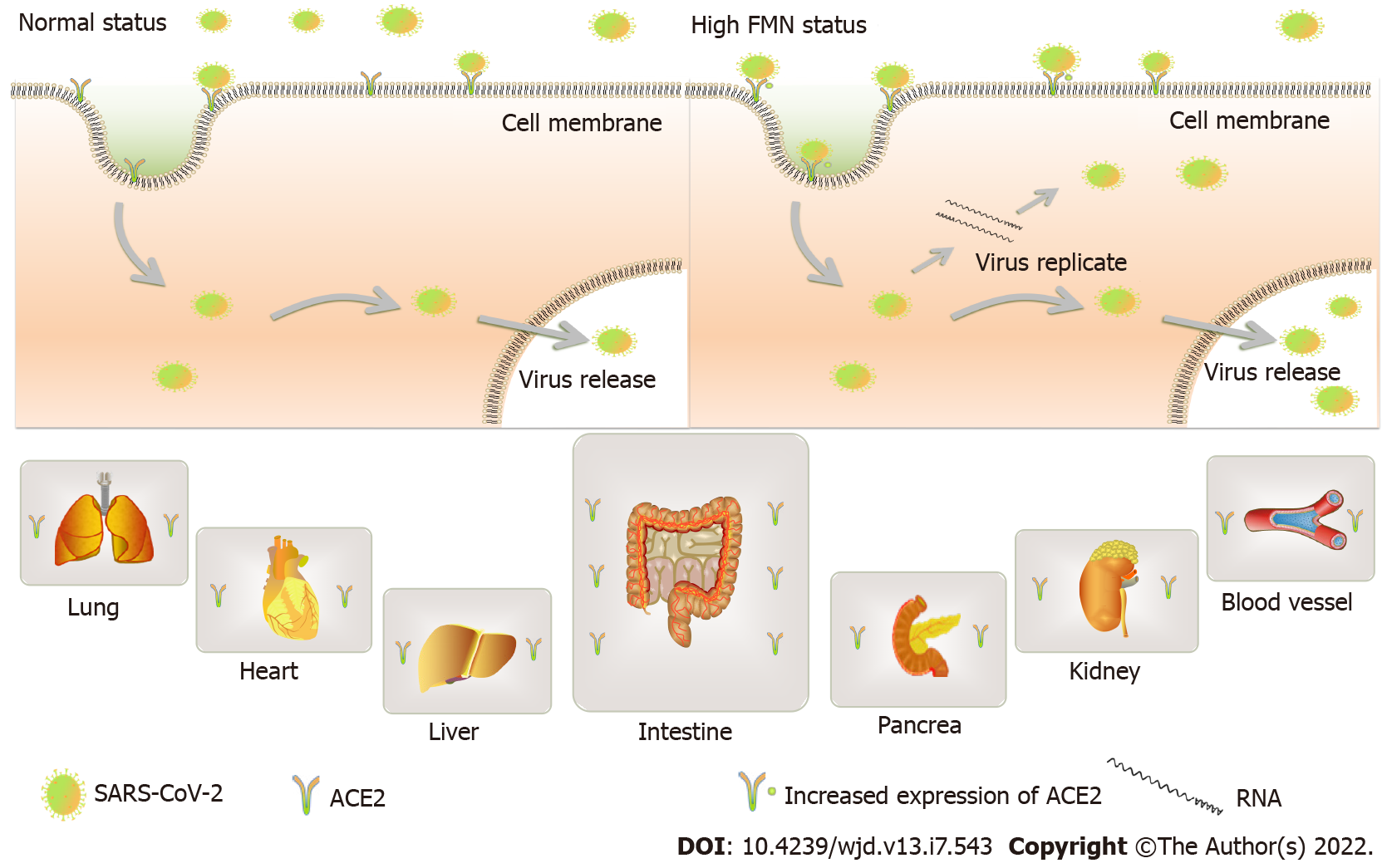
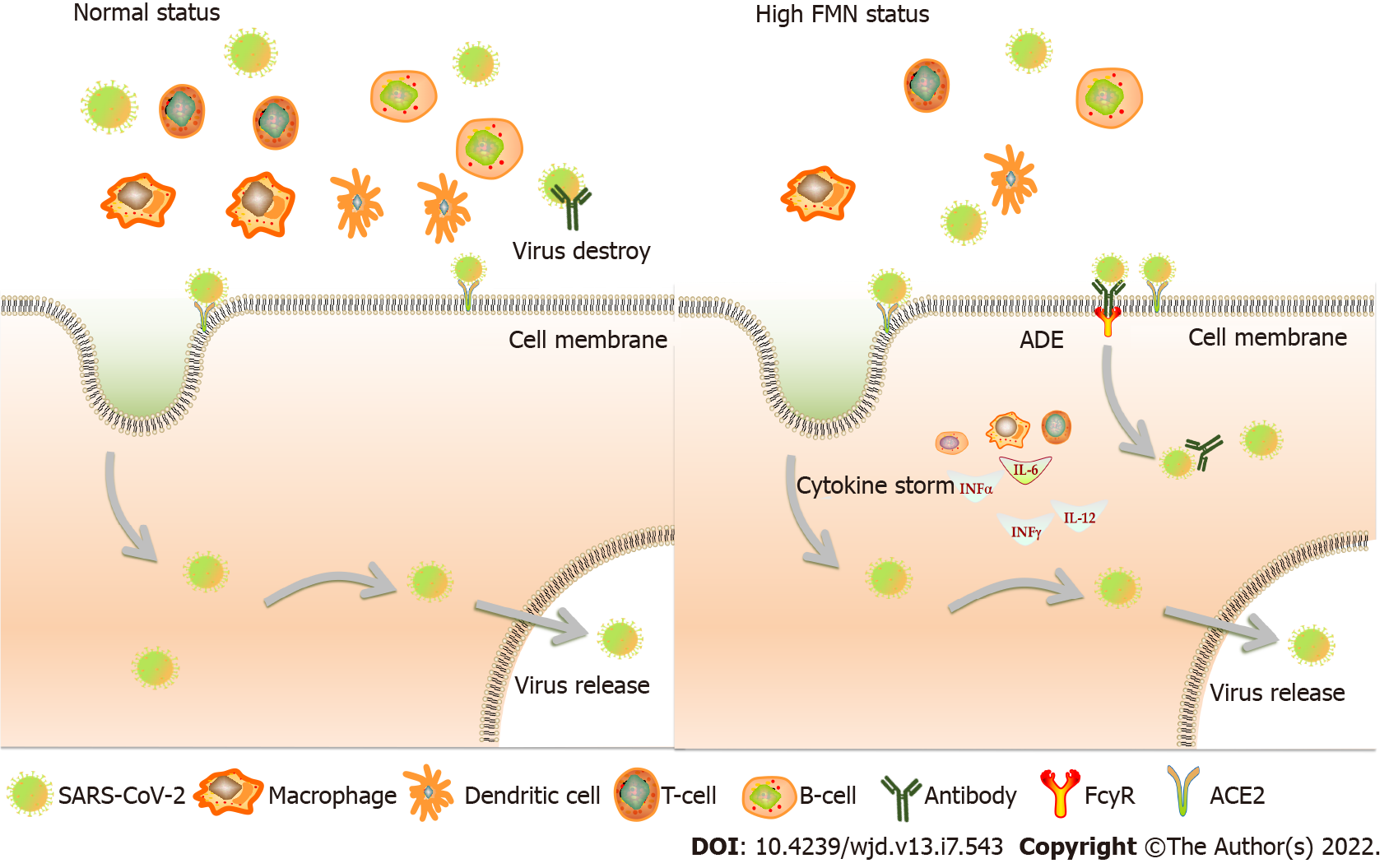
High FMN levels usually reflect hyperglycemia, which may lead to poor outcomes. Hyperglycemia enhances the expression of angiotensin-converting enzyme (ACE)2, which is the major cell entry receptor for SARS-CoV-2. ACE2 is widely expressed in the kidneys, lungs, and intestinal mucosal cells. SARS-CoV-2 can replicate abundantly in these sites and may contribute to reinfection[28] (Figure 3). Physiologically, hyperglycemia leads to a significant decrease in lymphocyte count, i.e., CD3+ and CD4+ T cells, which in turn reduces humoral immunity mediated by macrophages and dendritic cells, and induces interleukin-6, tumor necrosis factor α, etc. to induce a cytokine storm[29]. This immunological disorder may increase the occurrence of antibody-dependent enhancement (ADE). In patients who are positive for coronavirus-specific antibodies or are infected by different virus strains, their antibodies may not neutralize the infection, but instead trigger FCγ receptor-mediated uptake of the virus, leading to an increase in virus numbers in the body[30,31] (Figure 4). Hence, ADE may be another pathological mechanism of positive securement of SARS-CoV-2.
There were some limitations to the present study, which may have introduced potential bias. First, the study was a prospective, single center, small cohort study. Additional multicenter studies using larger patient cohorts should be performed to validate our findings. Second, HbA1c data for some of the patients were not available, which affected our comparative analysis of HbA1c and FMN levels. Third, diabetes was not excluded in the inclusion criteria, but we adjusted for diabetes.
Elevated FMN levels were found to predispose COVID-19 patients to reinfection and hence should be followed closely to monitor reinfection.
Diabetes is a risk factor for coronavirus disease 2019 (COVID-19) which results in increased severity and mortality but has no relationship with COVID-19 reinfection. No study has reported the relationship between COVID-19 reinfection and blood levels of fructosamine (FMN). The present study for the first time reported this relationship.
We mainly investigate the relationship between blood levels of FMN and COVID-19 reinfection.
We found that FMN levels may influence the prognosis of patients infected with COVID-19, which highlight that the hospitalization patients with elevated levels of FMN should be cautiously monitored at post discharge.
A total of 146 inpatients from the designated isolation hospital for COVID-19 patients, who were satisfied based on the diagnostic criteria and treatment protocol of COVID-19 (Fifth edition). The study cohort was divided into two groups based on FMN levels, elevated FMN was defined as levels higher than its upper tertile value, with the average follow-up period being one year. Cox regression was used to determine the hazard ratios (HRs) with 95% confidence intervals for the positive reinfection across the tertiles of FMN levels. Kaplan-Meier analysis was used to determine the cumulative survival rate in the patients with higher than the top tertiles of FMN levels compared with those with non-elevated levels, tested using log-rank.
We found that patients with elevated FMN levels were older than the non-elevated FMN group. Elevated FMN levels were positively associated with reinfection rate as well as HR for reinfection, while the cumulative disease-free survival rate was lower for patients in the elevated FMN group. These results demonstrate that FMN levels may influence the prognosis of patients infected with COVID-19.
Elevated levels of FMN are independently associated with COVID-19 reinfection, which highlight that the COVID-19 patients with elevated levels of FMN should be followed up closely to monitor reinfection.
Additional multicenter, hemoglobin A1c data available studies using larger patient cohorts should be performed to validate our findings.
The authors would like to thank Yueqing People’s Hospital, Affiliated Hospital of Wenzhou Medical University for the data support and Dr. Bo Yang for his careful statistical assistance.
| 1. | Naqvi AAT, Fatima K, Mohammad T, Fatima U, Singh IK, Singh A, Atif SM, Hariprasad G, Hasan GM, Hassan MI. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 701] [Article Influence: 116.8] [Reference Citation Analysis (1)] |
| 2. | Huang YN, Wang L, Cao MX, Huang ZC, Du R; Hu LG; Qu GB; Liang Y; Wang P. A review on the environmental transmission of novel coronavirus (SARS-CoV-2). Environ Chem. 2021;40:1945-1957. [DOI] [Full Text] |
| 3. | Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1670] [Cited by in RCA: 1781] [Article Influence: 296.8] [Reference Citation Analysis (1)] |
| 4. | Kirtipal N, Bharadwaj S, Kang SG. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect Genet Evol. 2020;85:104502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. WHO COVID-19 Dashboard, 2020. [cited 24 April 2022]. Available from: https://covid19.who.int/. |
| 6. | Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 482] [Article Influence: 80.3] [Reference Citation Analysis (1)] |
| 7. | Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? Diabetes Metab Syndr. 2020;14:535-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 466] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 8. | Ceriello A, Standl E, Catrinoiu D, Itzhak B, Lalic NM, Rahelic D, Schnell O, Škrha J, Valensi P; Diabetes and Cardiovascular Disease (D&CVD) EASD Study Group. Issues of Cardiovascular Risk Management in People With Diabetes in the COVID-19 Era. Diabetes Care. 2020;43:1427-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, Klonoff DC. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 494] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 10. | Liu W, Zhou Y, Song L, Zhou L, Hu Y. Analysis, clinical characteristics and death-related factors in patients with COVID-19 and diabetes. J Med University of Chongqing. 45:911-915. [DOI] [Full Text] |
| 11. | Merzon E, Green I, Shpigelman M, Vinker S, Raz I, Golan-Cohen A, Eldor R. Haemoglobin A1c is a predictor of COVID-19 severity in patients with diabetes. Diabetes Metab Res Rev. 2021;37:e3398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Cefalu WT, Bell-Farrow AD, Petty M, Izlar C, Smith JA. Clinical validation of a second-generation fructosamine assay. Clin Chem. 1991;37:1252-1256. [PubMed] |
| 13. | Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Choi R, Park MJ, Lee S, Lee SG, Lee EH. Association Among Glycemic Biomarkers in Korean Adults: Hemoglobin A1c, Fructosamine, and Glycated Albumin. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Rooney MR, Tang O, Pankow JS, Selvin E. Glycaemic markers and all-cause mortality in older adults with and without diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2021;64:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Shohat N, Goswami K, Breckenridge L, Held MB, Malkani AL, Shah RP, Schwarzkopf R, Parvizi J. Fructosamine is a valuable marker for glycemic control and predicting adverse outcomes following total hip arthroplasty: a prospective multi-institutional investigation. Sci Rep. 2021;11:2227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Hu X, Deng H, Wang Y, Chen L, Gu X, Wang X. Predictive value of the prognostic nutritional index for the severity of coronavirus disease 2019. Nutrition. 2021;84:111123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | China Bureau of Disease Control And Prevention. The diagnostic criteria and treatment protocol for COVID-19 (5th edition), 2020 Feb 21. [cited 2022 April 24]. Available from: http://www.nhc.gov.cn/jkj/s3577/202002/a5d6f7b8c48c451c87dba14889b30147.shtml. |
| 19. | Azam M, Sulistiana R, Ratnawati M, Fibriana AI, Bahrudin U, Widyaningrum D, Aljunid SM. Recurrent SARS-CoV-2 RNA positivity after COVID-19: a systematic review and meta-analysis. Sci Rep. 2020;10:20692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Pal R, Banerjee M. Are people with uncontrolled diabetes mellitus at high risk of reinfections with COVID-19? Prim Care Diabetes. 2021;15:18-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Burekovic A, Dizdarevic-Bostandzic A, Godinjak A. Poorly regulated blood glucose in diabetic patients--predictor of acute infections. Med Arh. 2014;68:163-166. [PubMed] |
| 22. | Gounden V, Ngu M, Anastasopoulou C, Jialal I. Fructosamine. 2021 Aug 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. [PubMed] |
| 23. | Voyer LE, Alvarado C. [Maillard reaction. Pathogenic effects]. Medicina (B Aires). 2019;79:137-143. [PubMed] |
| 24. | Torres GG, Nygaard M, Caliebe A, Blanché H, Chantalat S, Galan P, Lieb W, Christiansen L, Deleuze JF, Christensen K, Strauch K, Müller-Nurasyid M, Peters A, Nöthen MM, Hoffmann P, Flachsbart F, Schreiber S, Ellinghaus D, Franke A, Dose J, Nebel A. Exome-Wide Association Study Identifies FN3KRP and PGP as New Candidate Longevity Genes. J Gerontol A Biol Sci Med Sci. 2021;76:786-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Szwergold B. A Hypothesis: Fructosamine-3-Kinase-Related-Protein (FN3KRP) Catalyzes Deglycation of Maillard Intermediates Directly Downstream from Fructosamines. Rejuvenation Res. 2021;24:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Szwergold BS, Bunker RD, Loomes KM. The physiological substrates of fructosamine-3-kinase-related-protein (FN3KRP) are intermediates of nonenzymatic reactions between biological amines and ketose sugars (fructation products). Med Hypotheses. 2011;77:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Schulze C, Wetzel F, Kueper T, Malsen A, Muhr G, Jaspers S, Blatt T, Wittern KP, Wenck H, Käs JA. Stiffening of human skin fibroblasts with age. Clin Plast Surg. 2012;39:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Kumar A, Faiq MA, Pareek V, Raza K, Narayan RK, Prasoon P, Kumar P, Kulandhasamy M, Kumari C, Kant K, Singh HN, Qadri R, Pandey SN, Kumar S. Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Med Hypotheses. 2020;144:110271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Chee YJ, Tan SK, Yeoh E. Dissecting the interaction between COVID-19 and diabetes mellitus. J Diabetes Investig. 2020;11:1104-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Ulrich H, Pillat MM, Tárnok A. Dengue Fever, COVID-19 (SARS-CoV-2), and Antibody-Dependent Enhancement (ADE): A Perspective. Cytometry A. 2020;97:662-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 31. | Karthik K, Senthilkumar TMA, Udhayavel S, Raj GD. Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19. Hum Vaccin Immunother. 2020;16:3055-3060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ford J, United States; Gluvic Z, Serbia; Guven M, Turkey A-Editor: Liu X, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ