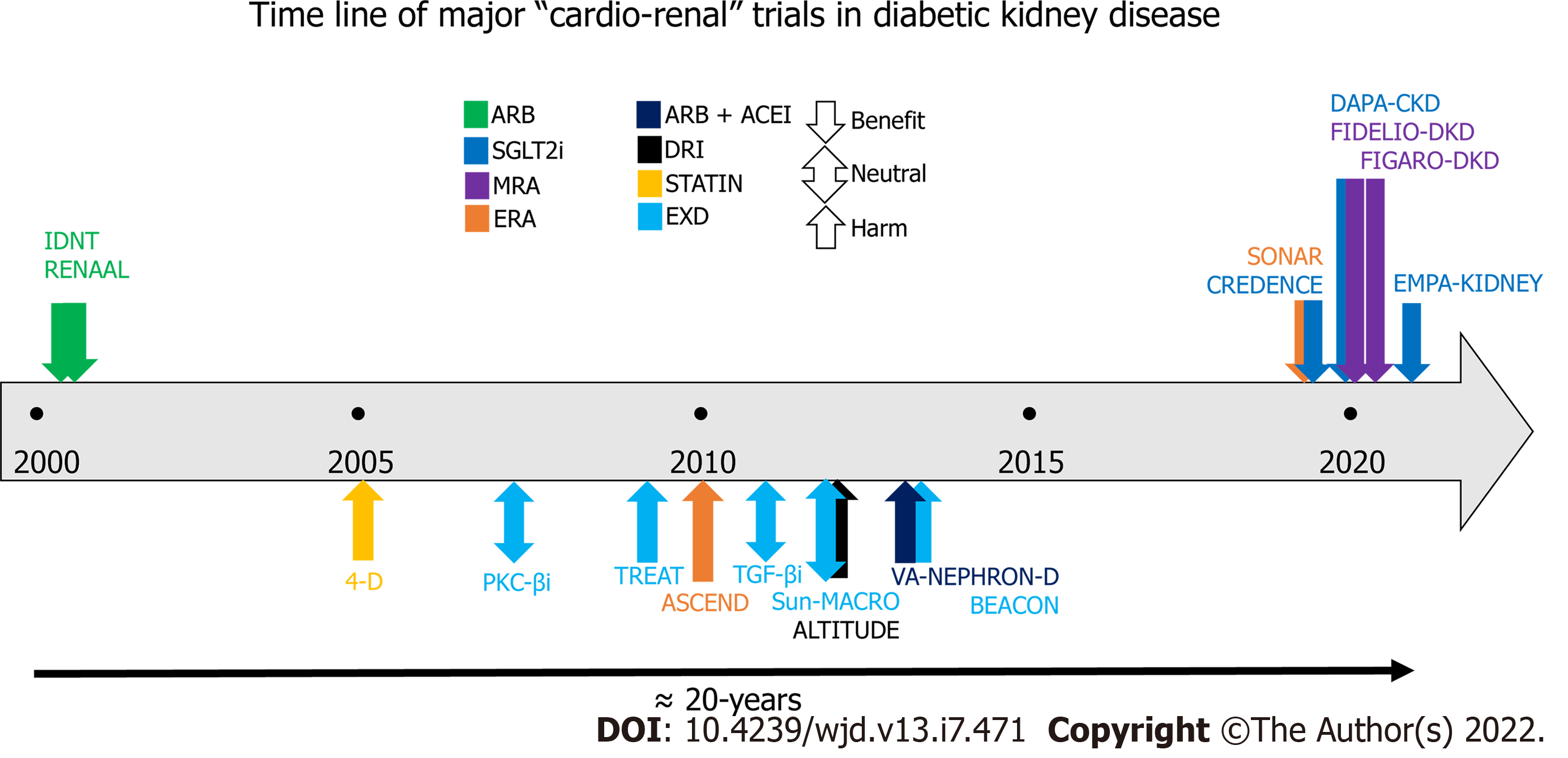Published online Jul 15, 2022. doi: 10.4239/wjd.v13.i7.471
Peer-review started: March 11, 2022
First decision: April 18, 2022
Revised: April 19, 2022
Accepted: June 18, 2022
Article in press: June 18, 2022
Published online: July 15, 2022
Processing time: 121 Days and 15.2 Hours
Several pharmacological agents to prevent the progression of diabetic kidney disease (DKD) have been tested in patients with type 2 diabetes mellitus (T2DM) in the past two decades. With the exception of renin-angiotensin system blockers that have shown a significant reduction in the progression of DKD in 2001, no other pharmacological agent tested in the past two decades have shown any clinically meaningful result. Recently, the sodium-glucose cotransporter-2 inhibitor (SGLT-2i), canagliflozin, has shown a significant reduction in the composite of hard renal and cardiovascular (CV) endpoints including progression of end-stage kidney disease in patients with DKD with T2DM at the top of renin-angiotensin system blocker use. Another SGLT-2i, dapagliflozin, has also shown a significant reduction in the composite of renal and CV endpoints including death in patients with chronic kidney disease (CKD), regardless of T2DM status. Similar positive findings on renal outcomes were recently reported as a top-line result of the empagliflozin trial in patients with CKD regardless of T2DM. However, the full results of this trial have not yet been published. While the use of older steroidal mineralocorticoid receptor antagonists (MRAs) such as spironolactone in DKD is associated with a significant reduction in albuminuria outcomes, a novel non-steroidal MRA finerenone has additionally shown a significant reduction in the composite of hard renal and CV endpoints in patients with DKD and T2DM, with reasonably acceptable side effects.
Core Tip: Angiotensin receptor blockers were the first drug class to show a conclusive benefit in preventing diabetic kidney disease (DKD) progression through two randomized trials IDNT and RENAAL in 2001. Several newer pharmacological agents have been tested in DKD in the past 20 years without much success. Notably, recently conducted renal outcome trials of sodium-glucose cotransporter-2 inhibitors in patients with DKD such as CREDENCE, DAPA-CKD, and EMPA-KIDNEY have shown significant improvement in disease progression. Similarly, recent trials of the non-steroidal mineralocorticoid receptor antagonist finerenone (FIDELIO-DKD and FIGARO-DKD) have shown significant improvement in both renal and cardiac endpoints in DKD.
- Citation: Singh AK, Singh R. Renin-angiotensin system blockers-SGLT2 inhibitors-mineralocorticoid receptor antagonists in diabetic kidney disease: A tale of the past two decades! World J Diabetes 2022; 13(7): 471-481
- URL: https://www.wjgnet.com/1948-9358/full/v13/i7/471.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i7.471
Type 2 diabetes mellitus (T2DM) remains the leading cause of both chronic kidney disease (CKD) and end-stage kidney disease (ESKD) worldwide[1]. The exact incidence and prevalence of CKD and ESKD from T2DM is difficult to assess due to infrequently performed invasive procedure of kidney biopsies (the gold standard for diagnosis of diabetic kidney disease [DKD]); and because most patients with DKD die before requiring renal replacement therapy. However, DKD affects nearly 20% of patients with T2DM[2-4]. Several factors that may lead to DKD include: the formation of advanced glycation end-products; generation of reactive oxygen species; activation of intercellular signals for proinflammatory and profibrotic gene expression causing cellular inflammation, injury, and fibrosis; alterations in glomerular hemodynamics; and associated hyperinsulinemia and insulin resistance further activating these pathogenic mechanisms[5]. Although the time to development of DKD in T2DM depends on multiple risk factors, its incidence is about 2% of patients per year and affects nearly 25% of patients within 10 years of diagnosis[6]. Classically, DKD progresses from three stages of albuminuria based on urinary albumin excretion: normal to mildly increased (< 30 mg/d or albumin/creatinine ratio [ACR] of < 30 mg/g), moderately increased (formerly called microalbuminuria-30 to 300 mg per day or ACR 30-300 mg/g), and severely increased (formerly called macroalbuminuria-> 300 mg per day or ACR > 300 mg/g) albuminuria. Importantly, the presence of severe albuminuria increases the annual risk of mortality by 4.6% compared with the risk of progression to ESKD (by 2.3%)[6]. These findings necessitate the role of pharmacological agents other than glycemic control in the management of DKD in patients with T2DM.
The general approach to managing DKD is similar to that in all patients with T2DM, which includes smoking cessation, weight loss, regular exercise, individualized glycemic targets, and statins. However, certain specific considerations are additionally needed in DKD which include: more intensive blood pressure lowering to prevent ESKD and cardiovascular (CV) morbidity in patients with severe albuminuria and to reduce mortality; and mandatory use of renin-angiotensin system blockers (RASBs), either angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), but not both. Since most individuals with DKD and hypertension require combination therapy, either a combination of an ACEI or ARB plus a dihydropyridine calcium channel blocker is the preferred regimen, except in patients with severe albuminuria where either a non-dihydropyridine CCB or a diuretic may be more suitable with RASB[7].
Although there are several randomized controlled trials (e.g., landmark studies: MICRO-HOPE, IRMA-2, and ADVANCE), which showed that RASB prevented progression from normal to microalbuminuria and micro- to macro-albuminuria in T2DM, reduction of albuminuria has generally been considered only a soft renal surrogate endpoint[8-10]. The first convincing evidence suggesting that RASB can significantly reduce hard renal endpoints and prevent the progression of CKD to ESKD in patients with T2DM with severe albuminuria dates back to 2001. The Irbesartan Diabetic Nephropathy Trial (IDNT) randomized 1715 T2DM patients (having urine protein excretion ≥ 0.9 g/d and mean serum creatinine of 1.7 mg/dL) to either irbesartan or amlodipine or placebo. At 2.6 years, the primary composite renal outcome (doubling of serum creatinine, development of ESKD or death from any cause) with irbesartan was 20% lower than placebo (hazard ratio [HR], 0.80; 95% confidence interval [CI]: 0.66-0.97; P = 0.02) and 23% lower than amlodipine (HR: 0.77; 95%CI: 0.63-0.93; P = 0.006). However, neither any significant reduction in secondary CV endpoint (CV death, non-fatal myocardial infarction [MI], non-fatal stroke, heart failure hospitalization [HHF], or lower limb amputation) nor any reduction in all-cause death was noted with irbesartan, compared to either placebo or amlodipine[11]. The Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) trial randomized 1513 T2DM patients (having albuminuria > 300 mg/d and mean serum creatinine of 1.9 mg/dL) to either losartan or placebo or both, in addition to conventional antihypertensive drugs (but not ACEI). At 3.4 years, the primary outcome (doubling of serum creatinine, development of ESKD, or death from any cause) was reduced by 16% (HR: 0.84; 95%CI: 0.72-0.98; P = 0.020) in losartan vs the placebo group. However, no reduction in all-cause death was noted between losartan vs placebo[12]. Importantly, despite the positive renal outcomes with ARBs, a substantial residual risk did remain in both IDNT (residual risk-32.6%) and RENAAL trials (residual risk-43.5%). These findings necessitate additional safe pharmacological agents along with RASBs to further reduce the remaining residual risks in patients with DKD.
From 2001 until 2018, several combinations of RASB (ACEI plus ARB such as lisinopril plus losartan [VA NEPHRON-D trial] or telmisartan plus ramipril [ONTARGET trial]) were tried without any success. Few older agents such as atorvastatin (4D trial) and several newer novel pharmacological agents (e.g., protein kinase C β [PKC-β] inhibitor: ruboxistaurin; darbepoetin-alfa; non-selective endothelin A receptor antagonist: avosentan; tumor growth factor-β [TGF-β] inhibitor: pirfenidine; pyridoxamine; a mixture of natural glycosaminoglycans polysaccharide: sulodexide; direct renin inhibitor: aliskiren; nuclear factor erythroid 2-related factor 2 [NRF-2] activator: bardoxolone methyl; and pentoxyphylline) were also tried in DKD with T2DM, without much success. Indeed, some of these studies showed harm and were stopped prematurely (Avosentan [ASCEND trial], Aliskiren [ALTITUDE trial], VA NEPHRON-D trial, and Bardoxolone [BEACON trial])[13-25].
Nevertheless, after failure of any favorable outcomes for nearly two decades, the year 2019 ushered a new hope for the management of DKD. A series of recent trials have shown a positive renal outcome including a reduction of death in patients with CKD and T2DM, at the top of RASB use. The SONAR (Study of Diabetic Nephropathy with Atrasentan [a selective endothelin A receptor antagonist]), randomized 2648 patients of CKD (eGFR 25-75 mL/min/1.73 m2 and urinary ACR of 300-5000 mg/g) with T2DM who were receiving a maximum tolerated dose of RASB to either atrasentan 0.75 mg daily or placebo. At a median follow-up of 2.2 years, the primary composite renal endpoint (doubling of serum creatinine or ESKD) was reduced by 35% (HR: 0.65; 95%CI: 0.49-0.88; P = 0.005) in atrasentan vs placebo. However, a higher frequency of HHF (33%) and death (9%) was also noted with atrasentan compared to the placebo[26]. Meanwhile, several cardiovascular outcome trials (CVOTs) conducted with SGLT-2 inhibitors (SGLT-2i) in patients with T2DM, with or without DKD (EMPA-REG, CANVAS Program, and DECLARE-TIMI conducted with empagliflozin, canagliflozin, and dapagliflozin, respectively), have also shown a significant reduction in prespecified renal composite endpoints including progression to ESKD, albeit the renal outcomes were exploratory in nature in all these studies[27-29]. Similarly, studies conducted with non-selective steroidal mineralocorticoid receptor antagonists (MRAs) such as spironolactone and eplerenone have shown a significant reduction in proteinuria in patients with CKD although no conclusive evidence is yet available suggesting that these drugs prevent the progression of DKD. While a meta-analysis of 16 RCTs conducted with spironolactone in CKD at the top of RASB showed a significant reduction in proteinuria (although at the increased risk of hyperkalemia[30], a recent (2020) proteomic prediction and renin-angiotensin-aldosterone system inhibition prevention of early diabetic nephropathy in type 2 diabetic patients with normoalbuminuria study failed to show prevention of progression to microalbuminuria with spironolactone, at the end of 2.5 years of follow-up[31]. Another recently updated (2020) Cochrane meta-analysis involving 44 trials of steroidal MRA (spironolactone and eplerenone) in early stage-CKD (mild-to-moderate proteinuria) showed a significant reduction in proteinuria but an increased risk of hyperkalemia (2.17-fold), acute kidney injury (2.04-fold) and gynecomastia (5.14-fold) was noted with spironolactone[32]. Moreover, the latest (2021) Cochrane meta-analysis of 16 trials of steroidal MRA in late-stage CKD requiring dialysis showed a significant reduction in CV- and all-cause mortality but with a significant 6-fold increased risk of gynecomastia and 1.4-fold increased trend of hyperkalemia[33]. However, the major limitations of these meta-analyses include smaller numbers, shorter duration of studies, and potential risk of bias. Indeed, one RCT of spironolactone (Mineralocorticoid Receptor Antagonists in End-Stage Renal Disease trial, commonly known as MiREnDa) that assessed the safety and CV outcomes with spironolactone and another RCT (Spironolactone in Dialysis-Dependent ESRD, commonly known as SPin-D)-both failed to show any benefit on the left ventricular mass index (LVMI) over 40 wk, or diastolic function or LVMI over 36-wk, respectively along with a dose-dependent increased risk of hyperkalemia[34,35]. Similarly, an eplerenone pilot trial PHASE (Hemodialysis patients undergoing Aldosterone Antagonism with Eplerenone) failed to show any CV benefit and had a 4.5-fold increased risk of hyperkalemia against placebo[36].
While SGLT-2i indicated improved renal outcomes in CVOTs of empagliflozin, canagliflozin, and dapagliflozin (EMPA-REG, CANVAS Program, and DECLARE-TIMI, respectively), the results of the first dedicated renal outcome study CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) in patients with DKD became available in the year 2019. CREDENCE trial randomized 4402 patients with CKD (eGFR 30 to < 90 mL/min/1.75 m2 and urinary ACR 300-5000 mg/g) and T2DM already receiving RASB, to either canagliflozin 100 mg daily or placebo. At a median follow up of 2.62 years, the relative risk reduction of primary composite outcome (composite of ESKD, a doubling of the serum creatinine level, or death from renal or CV causes) was 30% (HR: 0.70; 95%CI: 0.59-0.82; P = 0.00001) lower with canagliflozin compared to placebo. ESKD reduced by 31% (HR 0.68; 95%CI: 0.54-0.86; P = 0.002) with canagliflozin compared to placebo. The secondary CV outcome, a composite of 3P-MACE (CV death, non-fatal MI and non-fatal stroke) was found to reduce by 20% (HR: 0.80; 95%CI: 0.67-0.95; P = 0.01), while HHF reduced by 39% (HR: 0.61; 95%CI: 0.47-0.80; P < 0.001) with canagliflozin when compared to placebo[37]. The results of the second kidney outcome trial (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease [DAPA-CKD]) was published in the year 2020. DAPA-CKD randomized 4304 patients with CKD (eGFR 25 to 75 mL/min/1.73 m2 and urinary ACR of 200 to 5000 mg/g) having 2906 patients with T2DM, to either dapagliflozin 10 mg or placebo. Over a median of 2.4 years, the primary outcome (composite of the sustained decline of eGFR of at least 50%, ESKD, or death from renal or cardiovascular cause) was 39% (HR: 0.61; 95%CI: 0.51-0.72; P < 0.001) lower with dapagliflozin compared to placebo. Reduction in primary renal composite was similar in patients both with (HR: 0.64; 95%CI: 0.52-0.79) or without (HR: 0.50; 95%CI: 0.35-0.72) T2DM with dapagliflozin vs placebo. The secondary CV endpoints (composite of CV death or HHF) were reduced by 29% (HR: 0.71; 95%CI: 0.55-0.92; P = 0.009), while all-cause death was reduced by 31% (HR: 0.69; 95%CI: 0.53-0.88; P = 0.004) with dapagliflozin compared to placebo[38]. Ongoing empagliflozin renal outcome trial (EMPA-KIDNEY) in patients with CKD due to either T2DM or non-diabetic cause has been recently (March 16, 2022) stopped owing to the positive results which met the prespecified threshold for early termination against placebo[39]. It should be noted however that the residual risk of CKD progression or kidney failure was still evident in CREDENCE and DAPA-CKD in about 10% of patients despite a full dose of concomitant RASB use after a median follow-up of nearly 2.5 years[37,38]. This necessitates further strategies to combat the progression of DKD in patients with T2DM.
While several studies of steroidal MRA (spironolactone and eplerenone) have shown a significant reduction in soft surrogates of proteinuria in patients with DKD albeit, at increased risk of hyperkalemia and gynecomastia (spironolactone), no conclusive evidence of benefit is yet available with these MRAs concerning prevention of ESKD progression. Two ongoing phase 3b RCTs of spironolactone are currently evaluating the CV effect in patients with CKD on dialysis. While the ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial (commonly known as ALCHEMIST; NCT01848639) is evaluating the primary composite endpoint of non-fatal MI, acute coronary syndrome, HHF, nonfatal stroke, or CV death; the Aldosterone bloCkade for Health Improvement EValuation in End-stage Renal Disease (commonly known as ACHIEVE; NCT03020303) trial is evaluating the composite of CV death or HHF, in patients on maintenance dialysis. The results of both studies are expected in 2023[40].
Meanwhile, several newer, selective, non-steroidal MRA such as finerenone, esaxerenone, and apararenone have also been tried in DKD. The Mineralocorticoid Receptor Antagonist Tolerability Study in Diabetic Nephropathy (ARTS-DN) study, which evaluated various doses of finerenone, showed a dose-dependent significant reduction in UACR (24% and 38% reduction with 10 and 20 mg, respectively) in patients with T2DM having albuminuria (UACR ≥ 30 mg/g) and eGFR of > 30 mL/min/1.73 m2 at the top of RASB use, although no difference in ≥ 30% decline in eGFR (secondary outcome) was noted against placebo[41]. Significant reduction in proteinuria was also exhibited by esaxerenone in the Esaxerenone in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN) study and apararenone study in patients with DKD and T2DM[42,43]. Nevertheless, the conclusive evidence to prevent progression of DKD with MRA was first noted only with finerenone in The Finerenone in Reducing Kidney Failure and Disease Progression in DKD (FIDELIO-DKD) trial that become available in the year 2020. FIDELIO-DKD randomized 5734 patients with CKD (eGFR 25 to < 60 mL/min/1.73 m2, urinary ACR of 30 to < 300 mg/g and diabetic retinopathy, or urinary ACR 300-5000 mg/g and eGFR 25 to < 75 mL/min/1.73 m2) and T2DM on maximum licensed dose of RASB, to either finerenone 10 mg (< 60 mL/min/1.73 m2) or 20 mg (≥ 60 mL/min/1.73 m2) once daily, or placebo. At the median follow-up of 2.6 years, FIDELIO-DKD showed an 18% reduction (HR: 0.82; 95%CI: 0.73-0.93; P = 0.001) in primary renal outcome (composite of kidney failure, a sustained decrease of at least 40% in the eGFR from baseline, or death from renal causes) with finerenone compared to placebo. A significant reduction of 14% (HR: 0.86; 95%CI: 0.75-0.99; P = 0.03) in secondary CV outcome (composite of CV death, nonfatal MI, and nonfatal stroke, or HHF) was also shown with finerenone compared to placebo. Although adverse events were similar in both arms, hyperkalemia-related drug discontinuation was 2.5 times higher with finerenone (2.3%) compared to placebo (0.9%)[44]. Another study conducted with Finerenone FIGARO-DKD (Reducing Cardiovascular Mortality and Morbidity in DKD) has been published recently in 2021. FIGARO-DKD trial randomized 7437 patients with CKD (eGFR 25 to 90 mL/min/1.73 m2 and urinary ACR of 30 to < 300 mg/g, or urinary ACR 300 to 500 mg/g and eGFR ≥ 60 mL/min/1.73 m2) and T2DM to either finerenone 10 mg (25 to < 60 mL/min/1.73 m2) or 20 mg (≥ 60 mL/min/1.73 m2) once daily, or placebo on the maximum licensed dose of RASB. On a median follow-up of 3.4 years, the primary CV outcome (composite of CV death, nonfatal MI, and nonfatal stroke, or HHF) was significantly reduced by 13% (HR: 0.87; 95%CI: 0.76-0.98; P = 0.03) primarily driven by 29% reduction (HR: 0.71; 95%CI: 0.56-0.90) in HHF with finerenone compared to placebo. Interestingly, no significant difference (HR: 0.87; 95%CI: 0.76-1.01) was noted in secondary renal outcome (composite of kidney failure, a sustained decrease of at least 40% in the eGFR from baseline, or death from renal cause) with finerenone compared to placebo. Overall, no difference in the adverse events was noted in the two arms, however hyperkalemia-related drug discontinuation was 3-times higher with finerenone (1.2%) compared to placebo (0.4%)[45]. Table 1 summarizes the results from all these studies (in chronological order) which have been conducted in patients with T2DM having CKD that evaluated hard renal or cardiovascular composite endpoint as the primary objective[11-26,37,38,44,45]. Figure 1 is a schematic representation of timelines and outcomes from all these cardio-renal outcome trials.
| Ref. | n | Comparator | Duration (mean/median) | Primary endpoints | Results | Remarks |
| Lewis et al[11], 2001, IDNT | 1715 | Irbesartan 75-300 mg vs Amlodipine 2.5-10 mg vs PBO | 2.6 yr | Composite of doubling of serum Cr, development of ESKD or death from any cause | The primary outcome with IRBE was 20% lower than PBO and 23% lower than AMLO. Doubling of Cr was significantly 33% lower in IRBE vs PBO (P = 0.003) and 37% lower with IRBE vs AMLO (P < 0.001). The risk of ESKD was non-significantly 23% lower vs both PBO and AMLO (P = 0.07, for both comparisons). No difference in CV- or all-cause death was noted | Similar BP control with IRBE and AMLO. Protection is independent of reduction in BP |
| Brenner et al[12], 2001, RENAAL | 1513 | Losartan 50-100 mg vs PBO | 3.4 yr | Composite of doubling of serum Cr, development of ESKD or death from any cause | Primary outcome reduced by 16% risk in LOSA vs PBO (P = 0.020). LOSA reduced the doubling of Cr by 25% (P = 0.006) and ESKD by 28% (P = 0.002) vs PBO but no effect on death was noted. HHF was reduced by 32% in LOSA (P = 0.005) while proteinuria was reduced by 35% (P < 0.001) vs PBO | There was no active comparator, and the mean blood pressure throughout the study was lower among those assigned losartan |
| Wanner et al[13], 2005, 4D | 1255 | Atorvastatin 20 mg vs PBO (receiving hemodialysis) | 4.0 yr | Composite of 3P-MACE (death from CV causes, nonfatal MI, and stroke) | No benefit in 3P-MACE (RR: 0.92; 95%CI: 0.77-1.10; P = 0.37) but significant increase in fatal stroke (RR: 2.03; P = 0.04) | An increase in stroke could be a chance finding, given the data from the CARDS trial that showed atorvastatin lowers the incidence of stroke (HR: 0.52; 95%CI: 0.31-0.89) |
| Tuttle et al[14], 2007, PKC-DRS, PKC-DMES and PKC-DRS 2 study | 1157 | Ruboxistaurin vs PBO | 33-39 mo | Composite of doubling of serum Cr, development of advanced chronic kidney disease (stages 4 to 5), and death | No difference between the two group | - |
| Pfeffer et al[15], 2009, TREAT | 4038 | Darbepoetin alfa vs PBO | 4.0 yr | Composite outcomes of death or a CV event (nonfatal MI, CHF, stroke, or hospitalization for myocardial ischemia) and of death or ESKD | No difference in the composite of death or ESKD (HR: 1.06; 95%CI: 0.95-1.19; P = 0.29) or ESKD (HR: 1.02; 95%CI: 0.87-1.18; P = 0.83) between darbepoetin alfa and PBO. An increase in stroke (fatal or nonfatal stroke) occurred in darbepoetin alfa compared with PBO (HR: 1.92; 95%CI: 1.38-2.68; P < 0.001) | - |
| Mann et al[16], 2010, ASCEND | 1392 | Avosentan 25/50 mg vs PBO | 4 mo | Composite of doubling of serum Cr, ESKD, or death | No difference in primary outcome (25 mg 8.1% vs PBO 9.6%; P = 0.46; 50 mg 8.6% vs PBO 9.6%; P = 0.79) but a significant increase in CHF with avosentan (25 mg 5.9% vs PBO 2.2%; P = 0.008; 50 mg 6.1% vs PBO 2.2%; P = 0.05) compared with PBO | The trial terminated prematurely after a median follow-up of 4 mo (maximum 16 mo) because of an excess of CV events with avosentan |
| Sharma et al[17], 2011 | 77 | Pirfenidone 1200/2400 mg vs PBO | 1 yr | Change in eGFR | Mean eGFR significantly increased the pirfenidone 1200-mg/d group vs PBO (+3.3 vs -2.2 mL/min; P = 0.03) but no improvement in 2400-mg/d group | - |
| Pergola et al[18], 2011, BEAM | 227 | Bardoxolone 25/75/150 mg OD vs PBO | 12 mo | Change in eGFR at 6 mo | Significant increase in mean eGFR both at 6-mo (8.2-11.4 mL/min; P < 0.001) and 12-mo (5.8-10.5 mL/min) against PBO | Muscle spasms were the MC observed S/E with BDX |
| Lewis et al[19], 2012 | 317 | Pyridoxamine 150/300 mg BID vs PBO | 52-wk | Change in serum Cr | No difference in outcome observed | - |
| Packham et al[20], 2012, Sun-MACRO | 1248 | Sulodexide vs PBO | 11 mo | Composite of a doubling of serum Cr, development of ESKD, or serum Cr ≥6.0 mg/dl | No difference in the outcome | The trial was stopped prematurely due to futility |
| Parving et al[21], 2012, ALTITUDE | 8561 | Aliskiren vs PBO | 32.9 mo | Composite of CV death or the first occurrence of cardiac arrest with resuscitation; nonfatal MI; nonfatal stroke; unplanned HHF; ESKD, death attributable to kidney failure, or the need for RRT with no dialysis or transplantation available or initiated; or doubling of Cr level | Results of the primary endpoint were no different between the two arms (HR: 1.08; 95% 0.98-1.20; P = 0.12) | The trial was stopped prematurely after the second interim efficacy analysis because of significantly higher (11.2% vs 7.2%; P < 0.001) hyperkalemia (Serum K level ≥ 6 mmol/L) and hypotension (12.1% vs 8.3%; P < 0.001 in the aliskiren group vs PBO |
| Fried et al[22], 2013, VA NEPHRON-D | 1448 | Losartan plus lisinopril vs losartan plus PBO | 2.2 yr | Composite of change in the eGFR, ESKD, or death | No difference in outcome (HR: 0.88; 95%CI: 0.70 to 1.12; P = 0.30). Combination therapy increased the risk of hyperkalemia (P < 0.001) and acute kidney injury (P < 0.001) compared to monotherapy | The trial was stopped prematurely |
| Mann et al[23], 2013, ONTARGET | 3163 | Ramipril 10 mg vs telmisartan 80 mg vs ramipril plus telmisartan (10 + 80) | 56-mo | Composite of dialysis, doubling of serum Cr, and death | Combination therapy was associated with a non-significantly higher ESKD or doubling of serum creatinine (5.3% vs 4.8 %), but a similar death rate (2.3% vs 2.2 %) vs monotherapy. Combination therapy had higher rates of acute kidney injury requiring dialysis (1.4% vs 0.8 %) | This is the data of 3163 people having DKD from a total of 9628 patients with diabetes |
| de Zeeuw et al[24], 2013, BEACON | 2185 | Bardoxolone 20 mg OD vs PBO | 9.0 mo | Composite of ESKD or CV death | No difference (HR: 0.98; 95%CI: 0.70-1.37; P = 0.92). Significant increase in HHF and death due to HHF with bardoxolone (HR: 1.83; 95%CI: 1.32-2.55; P < 0.001) vs PBO | The trial was stopped prematurely |
| Navarro-Gonzalez et al[25], 2015, PREDIAN | 169 | Pentoxyphylline 600 mg BID vs PBO | 2-yr | Change in eGFR | Significant less decrease in eGFR in PTF vs PBO (-2.1 vs -6.5 mL/min; Group difference -4.3 mL/min; P < 0.001) | Open-label design and envelope (rather than computer-generated) randomization could have biased the results |
| Heerspink et al[26], 2019, SONAR | 2648 | Atrasentan 0.75 mg vs PBO | 2.2 yr (Median) | Composite of doubling of serum Cr or ESKD or death from kidney failure | 35% reduction in primary composite renal endpoint event (HR: 0.65; 95%CI: 0.49-0.88; P = 0.005) | HHF was insignificantly higher in atrasentan (HR: 1.33; 95%CI: 0.85-2.07; P = 0.208) vs PBO |
| Perkovic et al[37], 2019, CREDENCE | 4401 | Canagliflozin 100 mg vs PBO | 2.6 yr | Composite of ESKD, doubling of serum Cr, or death from renal or CV causes | 30% reduction in primary composite (HR: 0.70; 95%CI: 0.59-82; P = 0.00001), 34% reduction (HR: 0.66; 95%CI: 0.53-0.81; P < 0.001) in renal-specific composite (of ESKD, doubling of Cr, renal death) and 32% reduction in ESKD (HR: 0.68; 95%CI: 0.54-0.86; P = 0.002) in CANA vs PBO. Reduction in composite of 3P-MACE was 20% (HR: 0.80; 95%CI: 0.67-0.95; P = 0.01) while HHF reduced by 39% (HR: 0.61; 95%CI: 0.47-0.80; P < 0.001) in CANA arm vs PBO | The trial was stopped prematurely due to efficacy |
| Heerspink et al[38], 2020, DAPA-CKD | 4304 | Dapagliflozin 10 mg vs PBO | 2.4 yr | Composite of ESKD, sustained decline in eGFR of at least 50%, or death from renal or CV causes | 39% reduction in primary composite (HR: 0.61; 95%CI: 0.51-0.72; P < 0.001), 44% reduction (HR: 0.56; 95%CI: 0.45-0.68; P < 0.001) in renal-specific composite (of ESKD, decline in eGFR of at least 50%, or renal death), 29% reduction (HR: 0.71; 95%CI: 0.55-0.92; P = 0.009) in composite of CV death of HHF and 31% reduction in death (HR: 0.69; 95%CI: 0.53-0.88; P = 0.004) in DAPA arm vs PBO | The trial stopped prematurely due to efficacy |
| Bakris et al[44], 2020, FIDELIO-DKD | 5734 | Finerenone 10/20 mg vs PBO | 2.6 yr | Composite of kidney failure, sustained decrease of at least 40% in the eGFR from baseline, or death from renal causes | 18% reduction (HR: 0.82; 95%CI: 0.73-0.93; P = 0.001) in primary composite in FINE vs PBO arm. 14% reduction (HR: 0.86; 95%CI: 0.75-0.99; P = 0.03) in secondary outcome composite (CV death, non-fatal MI, non-fatal stroke, or HHF) in FINE vs PBO arm | Hyperkalemia-related discontinuation of the drug was higher in the FINE vs PBO (2.3% vs 0.9%) arm |
| Pitt et al[45], 2021, FIGARO-DKD | 7437 | Finerenone 10/20 mg vs PBO | 3.4 yr | Composite of CV death, nonfatal MI, nonfatal stroke, or HHF. The secondary outcome was a composite of a decrease of eGFR by at least 40%, ESKD, or death from renal causes | 13% reduction (HR: 0.87; 95%CI: 0.76-0.98; P = 0.03) in primary cardiac composite primarily driven due to 29% reduction (HR: 0.71; 95%CI: 0.56-0.90) in HHF with FINE vs PBO. Non-significant 13% reduction (HR: 0.87; 95%CI: 0.76-1.01) in secondary renal composite with FINE vs PBO | Hyperkalemia-related discontinuation of the drug was higher in the FINE vs PBO (1.2% vs 0.4%) arm |
In summary, several agents have been tried in the past two decades in patients with DKD and T2DM, but only three drug classes (RASB, SGLT-2i, and MRA especially finerenone) have conclusively shown both ≥ 30% reduction in albuminuria and a significant lowering in renal disease progression. It should be recalled that a cut-off of 30% geometric mean albuminuria reduction within 6 mo or an eGFR slope reduction of 0.5-1.0 mL/min/1.73 m2/year over 2-3 years has been adopted as a surrogate renal endpoint for CKD progression for clinical trials by National Kidney Foundation, European Medicines Agency, and US Food and Drug Administration in the year 2020[46]. This cut-off seems to have primarily originated from at least two meta-analyses[47,48]. While the Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium showed each 30% reduction in albuminuria lowered the risk of ESKD by 24%, a meta-analysis of observational studies involving nearly 700000 individuals found that a 30% reduction of albuminuria over 2 years lowered ESKD by 22%, regardless of drug class tested[47,48]. However, the pressing question which remains unanswered conclusively is whether the addition of MRA including finerenone to the patients who are already receiving SGLT-2i and RASB would help prevent further progression of kidney disease[49]. Mechanistically, the action of both SGLT-2i and MRA including finerenone appears to be complementary due to the following: (1) The differential mechanism of action. While SGLT-2i reduces glomerular hyperfiltration and could have direct beneficial cellular and metabolic effect, finerenone reduces inflammation and fibrosis by inhibiting mineralocorticoid receptor pathway; and (2) Hyperkalemia induced by finerenone (the commonest reason for drug discontinuation) can be counterbalanced by SGLT-2i. A recent meta-analysis from the pooled data of five RCTs (n = 8296) in patients with reduced ejection fraction showed SGLT-2i plus MRA to significantly reduce both cardiovascular composite of CV death or HHF (HR: 0.73; 95%CI: 0.66-0.80; P < 0.00001) and composite renal endpoints (HR 0.56; 95%CI: 0.39-0.81; P = 0.002) but with a significantly lower risk of hyperkalemia (HR 0.60; 95%CI: 0.42-0.87; P = 0.007), compared to MRA alone[50]. However, renal outcomes were exploratory endpoints in these RCTs included in this meta-analysis. In FIDELIO-DKD, 4.6% (259/5674) patients were receiving SGLT-2i at the baseline and reduction in primary renal composite was similar (PInteraction = 0.21), regardless of the SGLT-2i use (SGLT-2i users: HR, 1.38; 95%CI: 0.61-3.10; SGLT-2i non-users: HR, 0.82; 95%CI: 0.72-0.92). Similarly, in FIGARO-DKD, 8.4% patients (618/7352) were receiving SGLT-2i at baseline and benefit in primary CV composite was similar, regardless of SGLT-2i use (SGLT-2i users: HR, 0.49; 95%CI: 0.28-0.86; SGLT-2i non-users: HR, 0.89; 95%CI: 0.78-1.01). Importantly, a recent subgroup analysis of FIDELIO-DKD found that finerenone caused a 25% reduction in UACR in patients receiving SGLT-2i at the baseline, and patients on SGLT-2i also had fewer hyperkalemia events. Indeed, this subgroup analysis stratified on the baseline SGLT-2i use reported a lesser episode of treatment-emergent hyperkalemia of both moderate (> 5.5 mmol/L) and severe (> 6.0 mmol/L) nature in combined SGLT-2i plus finerenone users (7% and 0%, respectively), compared with finerenone alone (22% and 5%, respectively)[51]. Notably, a recent meta-analysis of six cardio-renal trials involving 49875 individuals has found a 16% lower risk (HR: 0.84; 95%CI: 0.76-0.93) of serious hyperkalemia (> 6.0 mmol/L) with SGLT-2i without any higher risk of hypokalemia[52]. Collectively, these finding hints that combination therapy of SGLT-2i and finerenone would likely reduce the risk of hyperkalemia. Whether combining MRA to SGLT-2i would enhance the CV or renal outcome is not clearly known due to: (1) Low number of events in a small population of baseline SGLT-2i users in both FIDELIO-DKD and FIGARO-DKD trial (number of events 24 and 61, respectively); and (2) Absence of any dedicated RCT that has assessed the renal or CV outcome with the combination therapy in patients with CKD and T2DM. Efficacy and safety of finerenone plus empagliflozin compared with either finerenone or empagliflozin in 807 participants with CKD and T2DM (CONFIDENCE Trial, NCT05254002) is currently planned and expected to be complete by end of 2023[53].
While optimal glucose control, intensive blood pressure control, and use of RASB have been the traditional foundation of treatment in slowing the progression of kidney disease in patients with albuminuria and T2DM for the past two decades, the addition of SGLT-2i to this foundational treatment has further shown to reduce the disease progression including death (DAPA-CKD). Finerenone would be a welcome addition to the list of novel drugs that have been able to reduce the progression of CKD successfully in patients with T2DM along with RASB. It is also possible that finerenone plus SGLT-2i combination can further prevent the progression of DKD in T2DM but that has to be proved through dedicated RCTs.
| 1. | International Diabetes Federation. IDF Diabetes Atlas, 8th end. Brussels: International Diabetes Federation, 2017. |
| 2. | Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 966] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 3. | Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Trends in Prevalence of Chronic Kidney Disease in the United States. Ann Intern Med. 2016;165:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 449] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 4. | Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 5. | Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol. 2017;312:F716-F731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 6. | Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1157] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 7. | Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1515] [Cited by in RCA: 1434] [Article Influence: 79.7] [Reference Citation Analysis (1)] |
| 8. | Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253-259. [PubMed] |
| 9. | Patel A; ADVANCE Collaborative Group, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1450] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 10. | Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2280] [Cited by in RCA: 2130] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 11. | Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4068] [Cited by in RCA: 4077] [Article Influence: 163.1] [Reference Citation Analysis (1)] |
| 12. | Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5059] [Cited by in RCA: 5188] [Article Influence: 207.5] [Reference Citation Analysis (0)] |
| 13. | Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1926] [Cited by in RCA: 1891] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 14. | Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW; PKC-DRS, PKC-DMES, and PKC-DRS 2 Study Groups. Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol. 2007;2:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1506] [Cited by in RCA: 1526] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 16. | Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G; ASCEND Study Group. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 396] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, Donohue M, Ramachandrarao S, Xu R, Fervenza FC, Kopp JB. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG; BEAM Study Investigators. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 730] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 19. | Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, Pohl MA, Rohde RD, Raz I, Yerushalmy Y, Yagil Y, Herskovits T, Atkins RC, Reutens AT, Packham DK, Lewis JB; Collaborative Study Group. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Packham DK, Wolfe R, Reutens AT, Berl T, Heerspink HL, Rohde R, Ivory S, Lewis J, Raz I, Wiegmann TB, Chan JC, de Zeeuw D, Lewis EJ, Atkins RC; Collaborative Study Group. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 918] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 22. | Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 824] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 23. | Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Rydén L, Sleight P, Teo KK, Yusuf S; ONTARGET investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens. 2013;31:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 24. | de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM; BEACON Trial Investigators. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 830] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 25. | Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, Macía M, del Castillo N, Rivero A, Getino MA, García P, Jarque A, García J. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015;26:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Melnick JZ, Miller MG, Pergola PE, Perkovic V, Tobe S, Yi T, Wigderson M, de Zeeuw D; SONAR Committees and Investigators. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 487] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 27. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8755] [Article Influence: 795.9] [Reference Citation Analysis (2)] |
| 28. | Neal B, Perkovic V, Matthews DR. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 29. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4538] [Article Influence: 648.3] [Reference Citation Analysis (0)] |
| 30. | Hou J, Xiong W, Cao L, Wen X, Li A. Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis. Clin Ther. 2015;37:2086-2103.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Tofte N, Lindhardt M, Adamova K, Bakker SJL, Beige J, Beulens JWJ, Birkenfeld AL, Currie G, Delles C, Dimos I, Francová L, Frimodt-Møller M, Girman P, Göke R, Havrdova T, Heerspink HJL, Kooy A, Laverman GD, Mischak H, Navis G, Nijpels G, Noutsou M, Ortiz A, Parvanova A, Persson F, Petrie JR, Ruggenenti PL, Rutters F, Rychlík I, Siwy J, Spasovski G, Speeckaert M, Trillini M, Zürbig P, von der Leyen H, Rossing P; PRIORITY investigators. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 32. | Chung EY, Ruospo M, Natale P, Bolignano D, Navaneethan SD, Palmer SC, Strippoli GF. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020;10:CD007004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 33. | Hasegawa T, Nishiwaki H, Ota E, Levack WM, Noma H. Aldosterone antagonists for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2021;2:CD013109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Hammer F, Malzahn U, Donhauser J, Betz C, Schneider MP, Grupp C, Pollak N, Störk S, Wanner C, Krane V; MiREnDa Study Group. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int. 2019;95:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 35. | Charytan DM, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, Anderson AH, Hung AM, Mehrotra R, Sharma S, Weiner DE, Williams M, DiCarli M, Skali H, Kimmel PL, Kliger AS, Dember LM; Hemodialysis Novel Therapies Consortium. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int. 2019;95:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Walsh M, Manns B, Garg AX, Bueti J, Rabbat C, Smyth A, Tyrwhitt J, Bosch J, Gao P, Devereaux PJ, Wald R. The Safety of Eplerenone in Hemodialysis Patients: A Noninferiority Randomized Controlled Trial. Clin J Am Soc Nephrol. 2015;10:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 4332] [Article Influence: 618.9] [Reference Citation Analysis (0)] |
| 38. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3629] [Article Influence: 604.8] [Reference Citation Analysis (1)] |
| 39. | Zoler ML. Empagliflozin Scores Topline Win in EMPA-KIDNEY Trial. Mar 17, 2022. [cited 17 March 2022]. Available from: https://www.medscape.com/viewarticle/970434. |
| 40. | Georgianos PI, Vaios V, Eleftheriadis T, Zebekakis P, Liakopoulos V. Mineralocorticoid Antagonists in ESRD: An Overview of Clinical Trial Evidence. Curr Vasc Pharmacol. 2017;15:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA. 2015;314:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 533] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 42. | Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, Sawanobori T. Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN): Phase 3 Randomized Controlled Clinical Trial. Clin J Am Soc Nephrol. 2020;15:1715-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 43. | Wada T, Inagaki M, Yoshinari T, Terata R, Totsuka N, Gotou M, Hashimoto G. Apararenone in patients with diabetic nephropathy: results of a randomized, double-blind, placebo-controlled phase 2 dose-response study and open-label extension study. Clin Exp Nephrol. 2021;25:120-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383:2219-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1696] [Cited by in RCA: 1794] [Article Influence: 299.0] [Reference Citation Analysis (1)] |
| 45. | Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD Investigators. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021;385:2252-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 1063] [Article Influence: 212.6] [Reference Citation Analysis (0)] |
| 46. | Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, Greene T, Tighiouart H, Matsushita K, Ballew SH, Sang Y, Vonesh E, Ying J, Manley T, de Zeeuw D, Eckardt KU, Levin A, Perkovic V, Zhang L, Willis K. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75:84-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 443] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 47. | Heerspink HJ, Kröpelin TF, Hoekman J, de Zeeuw D; Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium. Drug-Induced Reduction in Albuminuria Is Associated with Subsequent Renoprotection: A Meta-Analysis. J Am Soc Nephrol. 2015;26:2055-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 48. | Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, Black C, Brunskill NJ, Carrero JJ, Feldman HI, Fox CS, Inker LA, Ishani A, Ito S, Jassal S, Konta T, Polkinghorne K, Romundstad S, Solbu MD, Stempniewicz N, Stengel B, Tonelli M, Umesawa M, Waikar SS, Wen CP, Wetzels JFM, Woodward M, Grams ME, Kovesdy CP, Levey AS, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 256] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 49. | Neuen BL, Jardine MJ. SGLT2 inhibitors and finerenone: one or the other or both? Nephrol Dial Transplant. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Cordovez RA, Rivera K, Denila RA, Patricio M. Cardiorenal Effects of Sodium-Glucose Co-Transporter-2 Inhibitors in Combination with Mineralocorticoid Receptor Antagonist for Heart Failure with Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. JACC. 2022;79:340. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Rossing P, Filippatos G, Agarwal R, Anker SD, Pitt B, Ruilope LM, Chan JCN, Kooy A, McCafferty K, Schernthaner G, Wanner C, Joseph A, Scheerer MF, Scott C, Bakris GL; FIDELIO-DKD Investigators. Finerenone in Predominantly Advanced CKD and Type 2 Diabetes With or Without Sodium-Glucose Cotransporter-2 Inhibitor Therapy. Kidney Int Rep. 2022;7:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 52. | Neuen BL, Oshima M, Agarwal R, Arnott C, Cherney DZ, Edwards R, Langkilde AM, Mahaffey KW, McGuire DK, Neal B, Perkovic V, Pong A, Sabatine MS, Raz I, Toyama T, Wanner C, Wheeler DC, Wiviott SD, Zinman B, Heerspink HJL. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Hyperkalemia in People With Type 2 Diabetes: A Meta-Analysis of Individual Participant Data From Randomized, Controlled Trials. Circulation. 2022;145:1460-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 53. | A Study to Learn How Well the Treatment Combination of Finerenone and Empagliflozin Works and How Safe it is Compared to Each Treatment Alone in Adult Participants With Long-term Kidney Disease (Chronic Kidney Disease) and Type 2 Diabetes. (Accessed on April 11, 2022). In: ClinicalTrials.gov [Internet]. Bethesda (MD): US. National Library of Medicine. Available from: http://clinicaltrials.gov/ct2/show/NCT05254002. ClinicalTrials.gov Identifier: NCT05254002. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Techane T; Wang QY, China S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL