Published online Mar 15, 2022. doi: 10.4239/wjd.v13.i3.260
Peer-review started: May 28, 2021
First decision: June 24, 2021
Revised: July 8, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 15, 2022
Processing time: 290 Days and 22.5 Hours
The diagnosis of type 2 diabetes (T2D) in younger adults, an increasingly common public health issue, is associated with a higher risk of cardiovascular complications and mortality, which may be due to a more adverse cardiovascular risk profile in individuals diagnosed at a younger age.
To investigate the association between age at diagnosis and the cardiovascular risk profile in adults with T2D.
A pooled dataset was used, comprised of data from five previous studies of adults with T2D, including 1409 participants of whom 196 were diagnosed with T2D under the age of 40 years. Anthropometric and blood biomarker measurements included body weight, body mass index (BMI), waist circumference, body fat percentage, glycaemic control (HbA1c), lipid profile and blood pressure. Univariable and multivariable linear regression models, adjusted for diabetes duration, sex, ethnicity and smoking status, were used to investigate the association between age at diagnosis and each cardiovascular risk factor.
A higher proportion of participants diagnosed with T2D under the age of 40 were female, current smokers and treated with glucose-lowering medications, compared to participants diagnosed later in life. Participants diagnosed with T2D under the age of 40 also had higher body weight, BMI, waist circumference and body fat percentage, in addition to a more adverse lipid profile, compared to participants diagnosed at an older age. Modelling results showed that each one year reduction in age at diagnosis was significantly associated with 0.67 kg higher body weight [95% confidence interval (CI): 0.52-0.82 kg], 0.18 kg/m2 higher BMI (95%CI: 0.10-0.25) and 0.32 cm higher waist circumference (95%CI: 0.14-0.49), after adjustment for duration of diabetes and other confounders. Younger age at diagnosis was also significantly associated with higher HbA1c, total cholesterol, low-density lipoprotein cholesterol and triglycerides.
The diagnosis of T2D earlier in life is associated with a worse cardiovascular risk factor profile, compared to those diagnosed later in life.
Core Tip: The diagnosis of type 2 diabetes (T2D) in younger adults, an increasingly common public health issue, is associated with a higher risk of cardiovascular complications and mortality, which may be due to a more adverse cardiovascular risk profile in individuals diagnosed at a younger age. This analysis demonstrates the adverse effect of younger diagnosis of T2D on cardiovascular risk factors, highlighting the need for targeted multifactorial age-appropriate interventions in order to improve the cardiovascular risk factor profile of younger adults with T2D and reduce their subsequent risk of cardiovascular complications and mortality.
- Citation: Barker MM, Zaccardi F, Brady EM, Gulsin GS, Hall AP, Henson J, Htike ZZ, Khunti K, McCann GP, Redman EL, Webb DR, Wilmot EG, Yates T, Yeo J, Davies MJ, Sargeant JA. Age at diagnosis of type 2 diabetes and cardiovascular risk factor profile: A pooled analysis. World J Diabetes 2022; 13(3): 260-271
- URL: https://www.wjgnet.com/1948-9358/full/v13/i3/260.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i3.260
Type 2 diabetes (T2D), a significant and increasing public health issue, was tradi-tionally considered a disease of mid- to late adulthood[1]. However, the prevalence of T2D among younger adults (e.g., diagnosed < 40 years of age; “early-onset adult T2D”) has rapidly increased over the last few decades, now constituting between 15%-20% of all adults with T2D worldwide[2-4].The diagnosis of T2D at an earlier age is associated with an increased relative risk of mortality and of both microvascular and macrovascular complications, as highlighted by a recent meta-analysis of 26 studies[5]. Previous studies have suggested that a more adverse cardiovascular risk profile in adults diagnosed with T2D at a younger age [including higher glycaemic control (HbA1c), higher prevalence of obesity and a worse lipid profile] may explain some of the increased risk of mortality and complications observed among younger adults with T2D[3,6-9]. However, some conflicting results emerged within these studies[3,6,8,9], whilst most have investigated the effect of age at diagnosis as a categorical variable (early- vs later-onset T2D). Consequently, estimates for the difference in risk factors incurred by each one year reduction in age at diagnosis are sparse[3].
Given the increase in prevalence of early-onset T2D and the higher risk of mortality and cardiovascular complications observed in younger adults with T2D, a comprehensive understanding of the effect of diagnostic age on cardiovascular risk factor profile is crucial. This analysis aimed to investigate the association between age at diagnosis, as a continuous variable, and the cardiovascular risk profile of adults with T2D, including measures of adiposity, HbA1c, lipid metabolism and blood pressure, using a pooled dataset of research trial data from multi-ethnic study populations in the United Kingdom.
This analysis used a pooled dataset, comprising data from five previous or ongoing studies of adults with T2D in the United Kingdom: Chronotype of Patients with Type 2 Diabetes and Effect on Glycaemic Control (CODEC)[10], Effects of Liraglutide in Young Adults With Type 2 Diabetes (LYDIA), Early Detection of Cardiac Dysfunction and Health Behaviours in the Young with Type 2 Diabetes (EXPEDITION)[11], Diabetes Interventional Assessment of Slimming or Training to Lessen Inconspicuous Cardiovascular Dysfunction (DIASTOLIC)[12] and Prevalence and Determinants of Subclinical Cardiovascular Dysfunction in Adults with Type 2 Diabetes Mellitus (PREDICT)[13]. The rationale, design and eligibility criteria of these studies have been published previously, in addition to the main outcomes of the completed trials (LYDIA, EXPEDITION, DIASTOLIC)[10-14]. The aims, eligible age ranges and progress of each study are described in Table 1. Each study received ethical approval and all participants provided written informed consent. The pooled dataset used in the current analysis included all participants diagnosed at 16 years or older.
| Study name | Aim | Eligible age range (yr) | Exclusion criteria1 | Clinical Trials.gov Registration Number | Ongoing/completed |
| CODEC | Observational study to investigate the effect of chronotype on glycaemic controls in adults with T2DM | 18-75 | N/A | NCT02973412 | Ongoing |
| LYDIA | Randomised active-comparator trial to investigate the effect of liraglutide compared to sitagliptin on cardiac structure and function in younger adults with T2DM | 18-60 | Treatment with insulin, SGLT-2 inhibitors, GLP-1 receptor agonists of DPP-4 inhibitors; Active cardiovascular disease, including history of myocardial infarction within the past 6 mo and/or heart failure | NCT02043054 | Completed |
| EXPEDITION | Observational study to phenotype younger adults with T2DM | 18-40 | N/A | N/A | Completed |
| DIASTOLIC | Randomised controlled trial to compare diet and exercise interventions to standard care in adults with T2DM | 18-65 | Current treatment with more than three glucose-lowering medications or insulin; Stroke, peripheral vascular disease, atrial fibrillation, heart failure/disease, angina | NCT02590822 | Completed |
| PREDICT | Observational study to investigate the prevalence and determinants of subclinical cardiovascular dysfunction in adults with T2DM | 18-75 | Stroke, symptomatic peripheral vascular disease, atrial fibrillation, history of heart failure, history of myocardial infarction, moderate or severe heart valve disease, angina | NCT03132129 | Ongoing |
Outcome data used in this analysis were collected during baseline assessments within the pooled studies. During these baseline visits, information on demographics (including current age at visit), medical history (including age at T2D diagnosis) and medication use were collected. Anthropometric [including body weight, body mass index (BMI), waist circumference, and body fat percentage] and blood pressure measurements were collected using standardised procedures, and a blood sample was taken for measurement of routine circulating biomarkers (performed by accredited NHS clinical pathology laboratories using quality controlled enzymatic assays).
In order to compare demographic variables, cardiovascular complications, medication use, and cardiovascular risk factors [body weight, BMI, waist circumference, body fat percentage, HbA1c, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, systolic and diastolic blood pressure] by age at diagnosis, all data were first presented as median [interquartile range (IQR)] or percentages, as appropriate, using three diagnostic age categories: Diagnosed under 40 years of age, diagnosed between 40-59 years and diagnosed aged 60 years or older. Linear regression models were then used to investigate the association of age at diagnosis, used as a continuous variable, and each cardiova-scular risk factor. In order to assess the possibility of deviations from linearity, models were also conducted using a spline transformation of age at diagnosis for each cardiovascular risk factor. These models were compared to the linear models using Bayesian Information Criterion scores. For all models, there was no evidence of a significant difference between the spline and the linear models, therefore linear regression was used for the analyses.
As younger diagnosis may often predispose individuals to longer duration of T2D, it was important to assess whether any association between age at diagnosis and cardiovascular risk factors remained once diabetes duration was controlled for, as well as after adjustment for other important confounding variables. Therefore, three models were constructed for each cardiovascular risk factor: Model 1 (unadjusted univariable model), Model 2 (adjusted for duration of T2D alone), Model 3 (adjusted for duration of T2D, sex, ethnicity and smoking status). Robust standard errors were used to account for the clustering of data from the different studies.
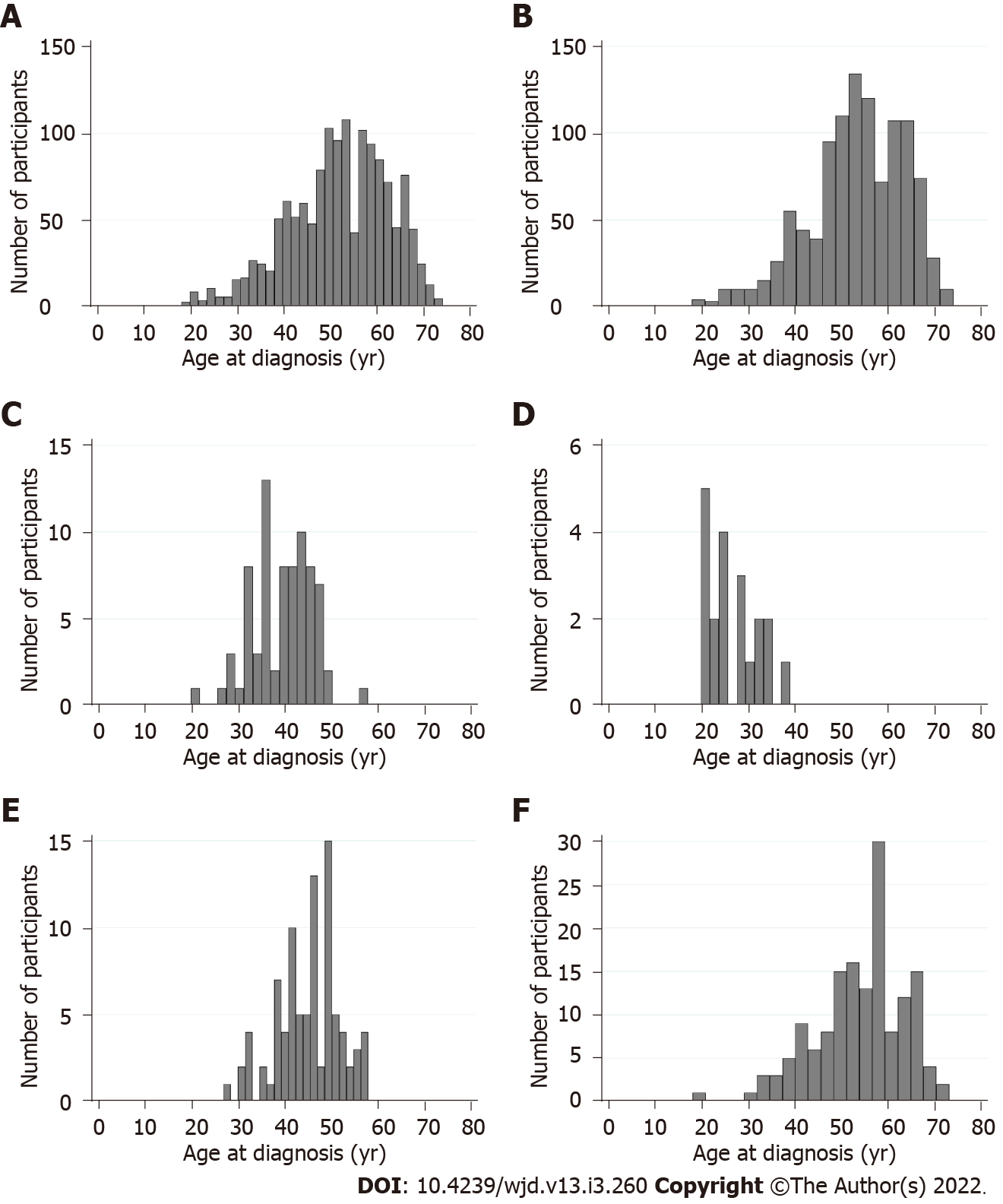
In total, 1409 participants were included in the pooled dataset, of whom 196 (13.9%) were diagnosed with T2D under the age of 40 years, 846 (60.0%) were diagnosed between 40-59 years, and 367 (26.1%) were diagnosed aged 60 years or older. The age at which participants were diagnosed with T2D ranged from 18 to 74 years (Figure 1). Table 2 presents participant characteristics by diagnostic age categories. Participants who were diagnosed under 40 years had a median current age of 46 years (IQR: 38-55) at study entry compared to 71 years (IQR: 68-73) among participants diagnosed at 60 years or over. As expected, median T2D duration was highest among participants who were diagnosed under the age of 40 years (11 years, IQR: 5-21) and lowest in parti-cipants diagnosed at 60 years or over (5 years, IQR: 3-8 years).
| Age at T2DM diagnosis | Total sample (n = 1409) | |||
| Under 40 yr (n = 196) | 40-59 yr (n = 846) | 60 yr or over (n = 367) | ||
| Number of participants from each dataset, n (%) | ||||
| CODEC | 111 (56.6) | 636 (75.2) | 326 (88.8) | 1073 (76.2) |
| LYDIA | 35 (17.9) | 41 (4.9) | 0 (0.0) | 76 (5.4) |
| EXPEDITION | 20 (10.2) | 0 (0.0) | 0 (0.0) | 20 (1.4) |
| DIASTOLIC | 17 (8.7) | 72 (8.5) | 0 (0.0) | 89 (6.3) |
| PREDICT | 13 (6.6) | 97 (11.5) | 41 (11.2) | 151 (10.7) |
| Current age, yr (n = 1408) | 46 (38-55) | 61 (56-67) | 71 (68-73) | 63 (55-69) |
| Diabetes duration, yr (n = 1408) | 11 (5-21) | 10 (5-15) | 5 (3-8) | 8 (4-14) |
| Sex, n (%) | ||||
| Male | 100 (51.0) | 565 (66.8) | 243 (66.2) | 908 (64.4) |
| Female | 96 (49.0) | 281 (33.2) | 124 (33.8) | 501 (35.6) |
| Ethnicity, n (%) | ||||
| White | 125 (63.8) | 665 (78.6) | 333 (90.7) | 1123 (79.7) |
| Asian | 55 (28.1) | 145 (17.1) | 28 (7.6) | 228 (16.2) |
| Other | 6 (3.1) | 33 (3.9) | 5 (1.4) | 44 (3.1) |
| Unknown | 10 (5.1) | 3 (0.4) | 1 (0.3) | 14 (1.0) |
| Smoking status, n (%) | ||||
| Current smoker | 24 (12.2) | 66 (7.8) | 20 (5.5) | 110 (7.98) |
| Ex-smoker | 58 (29.6) | 367 (43.4) | 182 (49.6) | 607 (43.1) |
| Never smoked | 114 (58.2) | 413 (48.8) | 165 (45.0) | 692 (49.1) |
| Family history of T2D, n (%) | ||||
| Yes | 90 (45.9) | 326 (38.5) | 131 (35.7) | 547 (38.8) |
| No | 37 (18.9) | 264 (31.2) | 169 (46.1) | 470 (33.3) |
| Unknown | 69 (35.2) | 256 (30.3) | 67 (18.3) | 393 (27.9) |
| Cardiovascular complications, n (%) | ||||
| Myocardial infarction (n = 1233) | 7 (4.3) | 60 (8.0) | 36 (11.1) | 103 (8.4) |
| Heart failure (n = 1229) | 4 (2.5) | 12 (1.6) | 9 (2.8) | 25 (2.0) |
| Heart valve disease (n = 1228) | 3 (1.8) | 22 (3.0) | 11 (3.4) | 36 (2.9) |
| Atrial fibrillation (n = 1223) | 2 (1.2) | 42 (5.7) | 20 (6.2) | 64 (5.2) |
| Peripheral vascular disease (n = 1227) | 7 (4.4) | 43 (5.8) | 21 (6.5) | 71 (5.8) |
| Stroke (n = 1235) | 3 (1.9) | 31 (4.1) | 24 (7.4) | 58 (4.7) |
| Angina (n = 1230) | 5 (3.1) | 60 (8.1) | 33 (10.2) | 98 (8.0) |
| Glucose-lowering medication use, n (%) | ||||
| Any glucose-lowering medication (n = 1403) | 185 (94.9) | 753 (89.5) | 257 (70.0) | 1195 (85.2) |
| Insulin | 74 (37.8) | 178 (21.0) | 24 (6.5) | 276 (19.6) |
| Metformin (n = 1407) | 157 (80.1) | 658 (78.0) | 234 (63.8) | 1049 (74.6) |
| Sulphonylurias (n = 1407) | 36 (18.4) | 205 (24.3) | 51 (13.9) | 292 (20.8) |
| DPP-4 inhibitors | 18 (9.2) | 139 (16.4) | 39 (10.6) | 196 (13.9) |
| GLP-1 agonists | 33 (16.8) | 63 (7.5) | 9 (2.5) | 105 (7.5) |
| SGLT2 inhibitors (n = 1389) | 27 (17.0) | 89 (11.5) | 15 (4.1) | 131 (10.1) |
| Other1 (n = 1390) | 3 (1.9) | 19 (2.4) | 4 (1.1) | 26 (2.0) |
| Lipid-lowering medication use, n (%) | ||||
| Any lipid-lowering medication (n = 1407) | 112 (57.4) | 583 (69.0) | 254 (69.2) | 949 (67.5) |
| Statins (n = 1408) | 108 (55.4) | 580 (68.6) | 251 (68.4) | 939 (66.7) |
| Fibrates (n = 1407) | 10 (5.1) | 23 (2.7) | 4 (1.1) | 37 (2.6) |
| Antihypertensive medication use, n (%) | ||||
| Any antihypertensive medication (n = 1389) | 97 (54.8) | 582 (68.8) | 252 (68.9) | 931 (67.0) |
| ACE inhibitors (n = 1390) | 68 (38.4) | 356 (42.1) | 125 (34.1) | 549 (39.5) |
| Alpha blockers (n = 1388) | 8 (4.6) | 86 (10.2) | 53 (14.5) | 147 (10.6) |
| Angiotensin receptor blockers (n = 1389) | 17 (9.7) | 134 (15.8) | 61 (16.6) | 212 (15.3) |
| Beta blockers (n = 1388) | 20 (11.4) | 157 (18.6) | 70 (19.1) | 247 (17.8) |
| Calcium channel blockers (n = 1389) | 31 (17.6) | 246 (29.1) | 103 (28.1) | 380 (27.4) |
| Diuretics (n = 1389) | 25 (14.2) | 122 (14.4) | 58 (15.8) | 205 (14.8) |
| Metabolic syndrome prevalence, n (%)2 (n = 1290) | 159 (94.1) | 697 (90.2) | 298 (85.6) | 1154 (89.5) |
A higher proportion of participants diagnosed under the age of 40 were female (49.0%) compared to those diagnosed between 40-59 years (33.2%) or over 60 years (33.8%). Although the most common ethnicity across all diagnostic age groups was white, there were proportionally more Asian participants in those diagnosed before the age of 40 (28.1%), compared to those diagnosed aged 40-59 years or aged 60 years or older (17.1% and 7.6%, respectively). Participants diagnosed under the age of 40 were also more likely to be current smokers (12.2%) and to have a family history of T2D (45.9%). The prevalence of all cardiovascular complications was lower at the point of study entry among participants diagnosed under the age of 40, compared to those diagnosed at the age of 40 or over. The prevalence of metabolic syndrome was higher among participants diagnosed under 40 years (94.1%) compared to those diagnosed between 40-59 years (90.2%) or at 60 years or over (85.6%). The proportion of participants using glucose-lowering medications was higher in participants diagnosed before 40 years (94.9%) compared to participants diagnosed between 40-59 years (89.5%) or 60 years or over (70.0%), whilst the opposite trend was observed for lipid-lowering or antihypertensive medications.
Table 3 displays participants’ cardiovascular risk factor profiles by diagnostic age categories. Participants diagnosed with T2D under the age of 40 had a higher body weight (95.2 kg, IQR: 82.5-108.9 kg) compared to participants diagnosed between the ages of 40-59 years (92.0 kg, IQR: 79.6-105.6 kg) or at 60 years or over (84.6 kg, IQR: 73.7-97.4 kg). BMI was also highest among participants diagnosed under 40 years (33.0 kg/m2, IQR: 29.0-36.8 kg/m2) compared to those diagnosed between 40-59 years (31.6 kg/m2, IQR: 28.0-35.3 kg/m2) or those diagnosed later than 60 years of age (29.2 kg/m2, IQR: 26.3-33.0 kg/m2). A similar trend was observed for waist circumference and body fat percentage.
| Age at T2DM diagnosis | Total sample (n = 1409) | |||
| Under 40 yr (n = 196) | 40-59 yr (n = 846) | 60 yr or over (n = 367) | ||
| Weight (kg) | 95.2 (82.5-108.9) | 92.0 (79.6-105.6) | 84.6 (73.7-97.4) | 90.6 (78.2-103.8) |
| BMI (kg/m2) | 33.0 (29.0-36.8) | 31.6 (28.0-35.3) | 29.2 (26.3-33.0) | 31.1 (27.5-35.0) |
| Waist circumference (cm), n = 1403 | 112.0 (102.2-119.1) | 109.0 (100.0-118.0) | 104.0 (96.8-113.5) | 108.0 (99.0-117.8) |
| Body fat (%), n = 1076 | 36.9 (29.5-44.5) | 34.0 (27.6-40.7) | 32.5 (26.3-41.0) | 33.8 (27.4-41.2) |
| HbA1c (%), n = 1370 | 7.5 (6.7-8.5) | 7.1 (6.4-7.9) | 6.5 (6.0-7.2) | 7.0 (6.3-7.8) |
| Total cholesterol (mmol/L), n = 1352 | 4.4 (3.8-5.2) | 4.2 (3.6-4.9) | 4.1 (3.5-4.8) | 4.2 (3.6-4.9) |
| LDL cholesterol (mmol/L), n = 1308 | 2.3 (1.8-2.9) | 2.1 (1.6-2.7) | 2.1 (1.6-2.6) | 2.1 (1.6-2.7) |
| HDL cholesterol (mmol/L), n = 1338 | 1.1 (1.0-1.4) | 1.2 (1.0-1.5) | 1.3 (1.1-1.5) | 1.2 (1.0-1.5) |
| Triglycerides (mmol/L), n = 1351 | 1.8 (1.2-2.7) | 1.7 (1.2-2.3) | 1.5 (1.1-2.1) | 1.6 (1.1-2.3) |
| Systolic blood pressure (mmHg), n = 1408 | 128.0 (119.0-140.0) | 135.0 (124.0-146.5) | 137.0 (125.5-148.7) | 135.0 (123.8-146.0) |
| Diastolic blood pressure (mmHg), n = 1408 | 83.0 (76.5-90.5) | 82.0 (76.0-89.0) | 79.5 (73.0-86.5) | 81.5 (75.0-88.6) |
Median HbA1c was also higher among participants diagnosed under the age of 40 (7.5%, IQR: 6.7%-8.5%) compared to those diagnosed between the age of 40-59 years (7.1%, IQR: 6.4%-7.9%) or at 60 years or over (6.5%, IQR: 6.0%-7.2%). Additionally, a marginally more adverse lipid profile was identified among participants diagnosed under the age of 40, showing higher total cholesterol, LDL cholesterol and triglycerides, and lower HDL cholesterol compared to participants diagnosed at 40 years or over.
As shown in Table 4, younger age at diagnosis of T2D was significantly associated with higher body weight, BMI, waist circumference and HbA1c. Results from Model 3 (adjusted for duration of T2D, sex, ethnicity and smoking status) showed that each one year reduction in age at diagnosis of T2D was significantly associated with 0.67 kg [95% confidence interval (CI): 0.52-0.82 kg] higher body weight, 0.18 kg/m2 (95%CI: 0.10-0.25 kg/m2) higher BMI, 0.32 cm (95%CI: 0.14-0.49 cm) higher waist circumference and 0.03% (95%CI: 0.03%-0.04%) higher HbA1c. Similarly, results from Model 3 indicate that each one year reduction in age at diagnosis was significantly associated with 0.01 mmol/L (95%CI: 0.01-0.02 mmol/L) higher total cholesterol, 0.01 mmol/L higher LDL cholesterol (95%CI: 0.01-0.02 mmol/L) and 0.02 mmol/L (95%CI: 0.01-0.03 mmol/L) higher triglycerides, after adjustment for the same covariates. Each one year reduction in diagnostic age was significantly associated with 0.22 mmHg (95%CI: 0.14-0.30 mmHg) higher diastolic blood pressure, but 0.24 mmHg (95%CI: 0.02-0.45 mmHg) lower systolic blood pressure.
| Model 1 | Model 2 | Model 3 | ||||
| Estimate | n | Estimate | n | Estimate | n | |
| Weight (kg) | -0.32 [(-0.51) to (-0.14)]a | 1409 | -0.45 [(-0.60) to (-0.31)]a | 1408 | -0.67 [(-0.82) to (-0.52)]a | 1394 |
| BMI (kg/m2) | -0.11 [(-0.19) to (-0.02)]b | 1409 | -0.15 [(-0.23) to (-0.07)]a | 1408 | -0.18 [(-0.25) to (-0.10)]a | 1394 |
| Waist circumference (cm) | -0.21 [(-0.32) to (-0.09)]a | 1403 | -0.23 [(-0.41) to (-0.05)]b | 1402 | -0.32 [(-0.49) to (-0.14)]a | 1388 |
| Body fat (%) | -0.10 (-0.80 to 0.60) | 1076 | -0.16 (-0.85 to 0.53) | 1075 | -0.11 (-0.43 to 0.20) | 1073 |
| HbA1c (%) | -0.04 [(-0.04) to (-0.03)]a | 1370 | -0.03 [(-0.04) to (-0.03)]a | 1369 | -0.03 [(-0.04) to (-0.03)]a | 1356 |
| Total cholesterol (mmol/L) | -0.01 (-0.01 to 0.00) | 1352 | -0.02 [(-0.02) to (-0.01)]a | 1351 | -0.01 [(-0.02) to (-0.01)]a | 1337 |
| LDL cholesterol (mmol/L) | -0.01 (-0.01 to 0.00)b | 1308 | -0.01 [(-0.02) to (-0.01)]a | 1307 | -0.01 [(-0.02) to (-0.01)]a | 1294 |
| HDL cholesterol (mmol/L) | 0.00 (0.00 to 0.01) | 1338 | 0.00 (0.00 to 0.01) | 1337 | 0.01 (0.00 to 0.01) | 1323 |
| Triglycerides (mmol/L) | -0.01 (-0.03 to 0.00) | 1351 | -0.02 [(-0.03) to (-0.01)]b | 1350 | -0.02 [(-0.03) to (-0.01)]a | 1336 |
| Systolic BP (mmHg) | 0.24 (0.14 to 0.35)a | 1408 | 0.31 (0.11 to 0.51)b | 1407 | 0.24 (0.02 to 0.45)b | 1393 |
| Diastolic BP (mmHg) | -0.10 (-0.21 to 0.02) | 1408 | -0.20 [(-0.29) to (-0.12)]a | 1407 | -0.22 [(-0.30) to (-0.14)]a | 1393 |
This analysis investigated the association between age at diagnosis of T2D and the cardiovascular risk profile among 1410 adults with T2D, using a diverse pooled dataset. The demographic characteristics of the participants included in our analysis varied by diagnostic age, with a higher proportion of females and people of Asian ethnicity among participants diagnosed earlier in life. These results are consistent with previous studies, which have also highlighted increased risk of microvascular and macrovascular complications, as well as incidence of certain co-morbidities, in these high risk subgroups[2,6,15-17]. In our analysis, younger age at diagnosis was also significantly associated with higher BMI, supporting findings from previous studies[3,7,9]. However, the current analysis adds to previous findings by quantifying the change in several cardiovascular risk factors, including body weight, BMI, waist circumference, HbA1c, lipids and blood pressure, for each year earlier diagnosis of T2D.
The association between younger age at diagnosis of T2D and poorer HbA1c identified in this analysis is supported by findings from previous studies[3,6-9]. One study from Asia investigated the association between HbA1c and age of diagnosis, analyzed as a continuous variable, reporting that each one year earlier age at diagnosis was significantly associated with 0.01% higher HbA1c. This is similar, albeit smaller in magnitude, to the results of our current analysis, which identified a 0.03% increase in HbA1c for each year earlier diagnosis.
In the current analysis, diagnosis of T2D at a younger age was significantly associated with higher total and LDL cholesterol and higher triglycerides, however no significant association was observed between age at diagnosis and HDL cholesterol. Similarly, previous studies have reported conflicting results for the relationship between age at diagnosis and lipid profile. For example, Yeung et al[3] reported a significant association between age at diagnosis and all lipid markers, whereas Huang et al[7] found participants with early-onset T2D had significantly higher total cholesterol and triglycerides compared to those with later-onset T2D, whilst no significant differences were observed from LDL or HDL cholesterol. Younger age at T2D diagnosis was significantly associated with higher diastolic blood pressure and lower systolic blood pressure in this analysis, which is also consistent with previous studies[3,8,9].
The adverse risk factor phenotype observed among younger adults with T2D may contribute to their significantly increased relative risk of microvascular and macrovascular disease and mortality. A recent meta-analysis of 26 studies found that for every one year increase in age at diagnosis, the risk of microvascular disease, macrovascular disease and all-cause mortality fell by 5%, 3% and 4%, respectively[5]. Research has also shown that the risk of cardiovascular complications and all-cause mortality can be reduced by the control of multiple cardiovascular risk factors, even among younger adults[18-20]. It is therefore imperative that adults with early-onset T2D have access to fit-for-purpose, multifactorial interventions to prevent long term complications, particularly given the global increase in the prevalence of early-onset T2D, and evidence that less than half of younger adults with T2D meet HbA1c targets[21]. Such interventions must also be tailored specifically to the co-occurring challenges that adults with early-onset T2D may face (e.g., early careers, ongoing education and young families), which may be different to older adults, and also allow for sex and cultural differences between individuals. Work to guide the development of such tailored approaches is urgently required, particularly given that adults with early-onset T2D are underrepresented in existing T2D trials[2,22].
This analysis has many strengths. Firstly, the pooled dataset used included a large sample of adults diagnosed with T2D over a wide age range (18-74 years of age) increasing the reliability and generalisability of the conclusions made. The use of age at diagnosis as a continuous variable in the analysis is another benefit, given that most previous literature has investigated age at diagnosis as a categorical variable, comparing people with ‘early-onset’ T2D to those with ‘later-onset’ T2D. Although such studies are valuable in assessing whether adults classified as ‘early onset’ have more cardiovascular risk factors, the range of ages included in the ‘early-onset’ and ‘late-onset’ categories are very wide and therefore it was previously unknown how diagnostic age was associated with cardiovascular risk factors within each of these categories. The current study has provided such insight by showing that each reduction of one year in age at diagnosis was significantly associated with a more adverse adiposity, HbA1c, and lipid profile, even after the adjustment for disease duration.
Limitations of the study must also be noted. As the information relating to diagnostic age was self-reported, some participants may not have accurately recalled the date at which they were diagnosed. However, there is evidence that self-reported age at diagnosis of T2D is fairly accurate and a valid measure of diagnostic age[23]. There is also the possibility of selection bias as the data used in this analysis is from volunteers who were motivated to undertake the research studies. In addition, it is possible that differences in recruitment rates by age at diagnosis and investigated risk factors acted to introduce a form of collider bias. Furthermore, only variables collected in the studies were available for adjustment in this analysis, therefore the effects of other covariates could not be assessed. As the cardiovascular risk factors investigated in this analysis were collected at study enrolment rather than at diagnosis of T2D, the results from this analysis do not indicate how the cardiovascular risk profile differs by diagnostic age at the time of diagnosis. Lastly, the age at diagnosis, age at enrolment and duration of diabetes are correlated and therefore disentangling the effect of one from the others is complex. Nevertheless, the results from the current analysis were unaffected by adjustment for diabetes duration.
In conclusion, this study supports previous literature demonstrating an association between younger diagnosis of T2D and a more adverse cardiovascular risk profile. This highlights the need for interventions targeting multiple risk factors in younger adults with T2D in order to reduce their risk of cardiovascular complications and mortality.
The prevalence of type 2 diabetes (T2D) among younger adults is increasing, and is associated with a higher relative risk of mortality and diabetes-related complications compared to older adults with T2D. This may be due to younger adults with T2D having a more adverse cardiovascular risk factor profile.
Although some research has observed a more adverse cardiovascular risk profile among younger adults with T2D, conflicting findings and methodological limitations have emerged within these studies.
To use a pooled dataset to investigate the association between age at diagnosis (as a continuous variable) and the cardiovascular risk factor profile of adults with T2D.
The pooled dataset used for this analysis included 1409 participants, 196 of whom were diagnosed with T2D under the age of 40 years. Descriptive analysis and both univariable and multivariable linear regression models were used to investigate the association between diagnostic age and cardiovascular risk factors [weight, body mass index (BMI), waist circumference, body fat percentage, glycaemic control (HbA1c), lipid profile and blood pressure].
Results from the analysis revealed that younger age at T2D diagnosis was significantly associated with higher weight, BMI, waist circumference, HbA1c and a more adverse lipid profile, even once confounding factors such as diabetes duration, sex and ethnicity were accounted for.
This analysis supports previous studies which demonstrate an association between younger age at T2D diagnosis and a worse cardiovascular risk factor profile.
The results from this analysis highlight the importance of multifactorial interventions targeting multiple risk factors in younger adults with T2D, in order to reduce their risk of mortality and cardiovascular complications.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Funding for the studies pooled was provided by the NIHR Leicester Biomedical Research Centre (CODEC), the Medical Research Council through an Interdisciplinary Bridging Award (EXPEDITION), NIHR Career Development Fellowship (DIASTOLIC) and Research Professorship (PREDICT), and the British Heart Foundation through a Clinical Research Training Fellowship (PREDICT). LYDIA was an investigator-initiated study funded by Novo Nordisk. The authors would also like to thank all participants who contributed to the research studies included in this pooled dataset.
| 1. | Laakso M, Pyörälä K. Age of onset and type of diabetes. Diabetes Care. 1985;8:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 156] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 588] [Article Influence: 73.5] [Reference Citation Analysis (1)] |
| 3. | Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, Aravind SR, Sheu W, Nguyen TK, Ozaki R, Deerochanawong C, Tsang CC, Chan WB, Hong EG, Do TQ, Cheung Y, Brown N, Goh SY, Ma RC, Mukhopadhyay M, Ojha AK, Chakraborty S, Kong AP, Lau W, Jia W, Li W, Guo X, Bian R, Weng J, Ji L, Reyes-dela Rosa M, Toledo RM, Himathongkam T, Yoo SJ, Chow CC, Ho LL, Chuang LM, Tutino G, Tong PC, So WY, Wolthers T, Ko G, Lyubomirsky G, Chan JC. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2:935-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Centres for Disease Control and Prevention. National Diabetes Statistics Report, 2020. [cited 24 March 2021]. Available from: https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html. |
| 5. | Nanayakkara N, Curtis AJ, Heritier S, Gadowski AM, Pavkov ME, Kenealy T, Owens DR, Thomas RL, Song S, Wong J, Chan JC, Luk AO, Penno G, Ji L, Mohan V, Amutha A, Romero-Aroca P, Gasevic D, Magliano DJ, Teede HJ, Chalmers J, Zoungas S. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia. 2021;64:275-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 6. | Chan JC, Lau ES, Luk AO, Cheung KK, Kong AP, Yu LW, Choi KC, Chow FC, Ozaki R, Brown N, Yang X, Bennett PH, Ma RC, So WY. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med. 2014;127:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Huang JX, Liao YF, Li YM. Clinical Features and Microvascular Complications Risk Factors of Early-onset Type 2 Diabetes Mellitus. Curr Med Sci. 2019;39:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Huo X, Gao L, Guo L, Xu W, Wang W, Zhi X, Li L, Ren Y, Qi X, Sun Z, Li W, Ji Q, Ran X, Su B, Hao C, Lu J, Guo X, Zhuo H, Zhang D, Pan C, Weng J, Hu D, Yang X, Ji L. Risk of non-fatal cardiovascular diseases in early-onset vs late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 9. | Li L, Ji L, Guo X, Ji Q, Gu W, Zhi X, Li X, Kuang H, Su B, Yan J, Yang X. Prevalence of microvascular diseases among tertiary care Chinese with early vs late onset of type 2 diabetes. J Diabetes Complications. 2015;29:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Brady EM, Hall AP, Baldry E, Chatterjee S, Daniels LJ, Edwardson C, Khunti K, Patel MI, Henson JJ, Rowlands A, Smith AC, Yates T, Davies MJ. Rationale and design of a cross-sectional study to investigate and describe the chronotype of patients with type 2 diabetes and the effect on glycaemic control: the CODEC study. BMJ Open. 2019;9:e027773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Wilmot EG, Leggate M, Khan JN, Yates T, Gorely T, Bodicoat DH, Khunti K, Kuijer JP, Gray LJ, Singh A, Clarysse P, Croisille P, Nimmo MA, McCann GP, Davies MJ. Type 2 diabetes mellitus and obesity in young adults: the extreme phenotype with early cardiovascular dysfunction. Diabet Med. 2014;31:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Gulsin GS, Swarbrick DJ, Athithan L, Brady EM, Henson J, Baldry E, Argyridou S, Jaicim NB, Squire G, Walters Y, Marsh AM, McAdam J, Parke KS, Biglands JD, Yates T, Khunti K, Davies MJ, McCann GP. Effects of Low-Energy Diet or Exercise on Cardiovascular Function in Working-Age Adults With Type 2 Diabetes: A Prospective, Randomized, Open-Label, Blinded End Point Trial. Diabetes Care. 2020;43:1300-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Prevalence and Determinants of Subclinical Cardiovascular Dysfunction in Adults With Type 2 Diabetes Mellitus (PREDICT) 2020. [accessed 2021 Mar 24]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03132129 ClinicalTrials.gov Identifier: NCT03132129. |
| 14. | Webb DR, Htike ZZ, Swarbrick DJ, Brady EM, Gray LJ, Biglands J, Gulsin GS, Henson J, Khunti K, McCann GP, Waller HL, Webb MA, Sargeant JA, Yates T, Zaccardi F, Davies MJ. A randomized, open-label, active comparator trial assessing the effects of 26 wk of liraglutide or sitagliptin on cardiovascular function in young obese adults with type 2 diabetes. Diabetes Obes Metab. 2020;22:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Koye DN, Ling J, Dibato J, Khunti K, Montvida O, Paul SK. Temporal Trend in Young-Onset Type 2 Diabetes-Macrovascular and Mortality Risk: Study of U.K. Primary Care Electronic Medical Records. Diabetes Care. 2020;43:2208-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Shah A, Kanaya AM. Diabetes and associated complications in the South Asian population. Curr Cardiol Rep. 2014;16:476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Omar MA, Motala AA, Jialal I, Seedat MA. Microvascular complications and non-insulin-dependent diabetes of the young in South African Indians. Diabetes Res. 1986;3:483-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjörnsdottir S. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379:633-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 999] [Article Influence: 124.9] [Reference Citation Analysis (0)] |
| 19. | Gæde P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, Pedersen O. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016;59:2298-2307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 343] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 20. | Oellgaard J, Gæde P, Rossing P, Rørth R, Køber L, Parving HH, Pedersen O. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study. Diabetologia. 2018;61:1724-1733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368:1613-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 741] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 22. | Sargeant JA, Brady EM, Zaccardi F, Tippins F, Webb DR, Aroda VR, Gregg EW, Khunti K, Davies MJ. Adults with early-onset type 2 diabetes (aged 18-39 years) are severely underrepresented in diabetes clinical research trials. Diabetologia. 2020;63:1516-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Pastorino S, Richards M, Hardy R, Abington J, Wills A, Kuh D, Pierce M; National Survey of Health and Development Scientific and Data Collection Teams. Validation of self-reported diagnosis of diabetes in the 1946 British birth cohort. Prim Care Diabetes. 2015;9:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5429] [Article Influence: 258.5] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jialal I S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ