Published online Mar 15, 2022. doi: 10.4239/wjd.v13.i3.161
Peer-review started: September 11, 2021
First decision: December 4, 2021
Revised: December 8, 2021
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: March 15, 2022
Processing time: 184 Days and 17.6 Hours
The magnitude of diabetes mellitus (DM) has increased in recent decades, where the number of cases and the proportion of the disease have been gradually increasing over the past few decades. The chronic complications of DM affect many organ systems and account for the majority of morbidity and mortality associated with the disease. The prevalence of type 1 DM (T1DM) is increasing globally, and it has a very significant burden on countries and at an individual level. T1DM is a chronic illness that requires ongoing medical care and patient self-management to prevent complications. This study aims to discuss the health benefits of physical activity (PA) in T1DM patients. The present review article was performed following a comprehensive literature search. The search was conducted using the following electronic databases: “Cochrane Library”, Web of Science, PubMed, HINARI, EMBASE, Google for grey literature, Scopus, African journals Online, and Google Scholar for articles published up to June 21, 2021. The present review focused on the effects of PA on many outcomes such as blood glucose (BG) control, physical fitness, endothelial function, insulin sensitivity, well-being, the body defense system, blood lipid profile, insulin resistance, cardiovascular diseases (CVDs), insulin requirements, blood pressure (BP), and mortality. It was found that many studies recommended the use of PA for the effective management of T1DM. PA is a component of comprehensive lifestyle modifications, which is a significant approach for the management of T1DM. It provides several health benefits, such as improving BG control, physical fitness, endothelial function, insulin sensitivity, well-being, and the body defense system. Besides this, it reduces the blood lipid profile, insulin resistance, CVDs, insulin requirements, BP, and mortality. Overall, PA has significant and essential protective effects against the health risks associated with T1DM. Even though PA has several health benefits for patients with T1DM, these patients are not well engaged in PA due to barriers such as a fear of exercise-induced hypoglycemia in particular. However, several effective strategies have been identified to control exercise-induced hypoglycemia in these patients. Finally, the present review concludes that PA should be recommended for the management of patients with T1DM due to its significant health benefits and protective effects against associated health risks. It also provides suggestions for the future direction of research in this field.
Core Tip: Diabetes mellitus (DM) is a group of chronic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The impairment of beta-cell function is an ancient feature of disease pathogenesis, while a significant reduction in beta-cell mass is closely associated with clinical manifestations in type 1 DM and type 2 DM. Physical activity (PA) is good for almost every individual. PA is a significant mediator of glycemic control and prevents pathologies related to increased postprandial glucose. Its significant role in the prevention and management of noncommunicable diseases is extensively understood. PA is widely known to be an effective approach for the prevention and management of numerous chronic diseases.
- Citation: Wake AD. Protective effects of physical activity against health risks associated with type 1 diabetes: “Health benefits outweigh the risks”. World J Diabetes 2022; 13(3): 161-184
- URL: https://www.wjgnet.com/1948-9358/full/v13/i3/161.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i3.161
Diabetes mellitus (DM) is a group of chronic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both[1]. The loss of beta-cells (β-cells) is a determinant factor for the development of type 1 DM (T1DM)[2]. In T1DM and type 2 DM (T2DM), the impairment of β-cell function is an early feature of disease pathogenesis, while a significant reduction in β-cell mass is closely associated with clinical manifestations[3]. Reduced functional β-cell mass is the hallmark of both T1DM and T2DM and this triggers absolute or relative insulin insufficiency in both circumstances[4]. T1DM is characterized by an immune-mediated depletion of β-cells that results in a lifelong dependence on exogenous insulin[5-9]. Despite all the efforts made to identify efficient therapeutic methods for T1DM, insulin is the only effective treatment[2].
However, even modest levels of β-cell activity were associated with a reduction in the incidence of retinopathy and nephropathy in T1DM individuals[10]. Even though the factors that predict the occurrence and rapidity of the decrease in β-cell function are still largely unknown, evidence has identified islet cell autoantibodies as predictors. Historical as well as recent clinical experience has underlined the significance of residual insulin production for glycemic control and to avoid end-organ complications[11]. T1DM can arise at any age while a peak in incidence is seen around puberty[12]. Overweight and obesity are highly prevalent among young people and adults with T1DM which is (25%–35%) and (37% to nearly 80%), respectively. Obesity raises the risk of developing T1DM and may lead to an earlier age at diagnosis. Also, obesity may raise the risk of macrovascular disease, retinopathy, and metabolic syndrome among these patients[13]. The chronic hyperglycemia of diabetes is linked to long-term damage, dysfunction, and failure of different organs, particularly the eyes, kidneys, nerves, heart, and blood vessels[1].
Generally, diabetes-related complications can be divided into macrovascular and microvascular complications. Stroke, coronary artery disease, and peripheral arterial disease are included under macrovascular complications and microvascular complications comprise, retinopathy, diabetic nephropathy, and neuropathy[14]. In addition, T1DM is linked with premature cardiovascular disease (CVD)[15]. In T1DM individuals, the absolute and relative risks of CVD remain very high[16]. When women with T1DM are compared to men with T1DM, women have nearly 40% more excess risk of all-cause mortality and twice the excess risk of fatal and nonfatal vascular events[17]. The age at onset and the duration of T1DM seem to be significant determinants of survival and all cardiovascular outcomes. Early onset is associated with up to a 30-fold augmented risk of serious cardiovascular outcomes, with risk levels being 90-fold greater for women with early-onset diabetes, and who die approximately 18 years earlier than nondiabetics[18]. Vascular complications are a significant cause of morbidity and mortality in individuals with T1DM and T2DM[19].
The magnitude of DM has increased in recent decades[20], where the number of cases and the proportion of the disease have been gradually increasing over the past few decades[21]. The incidence of childhood T1DM is significantly increasing globally[22]. It is the epidemic of the century and without effective diagnostic approaches at an early stage, diabetes will continue to increase[23]. The global estimates for the prevalence of diabetes for 2015 and 2040 were 8.8% and 10.4%, respectively[24]. Whereas it was 8.8% and 9.9% of the world population in 2017 and by 2045, respectively[25].
In recent decades, a significant increase in the proportion of DM has been evidenced in nearly all regions of the world. The increase in the number of subjects with the disease is possibly due to a change in the disease profile in numerous populations around the world and this is primarily because of a larger incidence of diabetes-related complications such as kidney failure and peripheral arterial disease[26]. It is a significant public health problem and is one of the four ranked noncommunicable diseases targeted for action by world leaders[21]. T1DM is associated with an increased risk of CVDs and all-cause mortality in insulin-treated patients with diabetes while the connection between hypoglycemia and cardiovascular consequences and mortality exists over a long period[27]. Whereas, more than the general population, elderly individuals with diabetes have higher all-cause mortality rates[28]. The excess mortality observed in T1DM is almost totally associated with diabetes and its related comorbidities[29].
The increasing disease burden of DM globally is a major public health priority, placing a fluctuating need on the patients, their careers, health systems, and society[30]. Diabetes is one of the leading and rising causes of hospital admission and disability due to other diseases[31]. This high magnitude of the disease has a greater social and financial burden[24]. It imposes a rising economic burden on national health care systems globally[32]. The global costs of DM and its significance are huge and will markedly rise by 2030[33]. Even though the present data found an increase in the magnitude of diabetes, the recent understanding of the international burden of and variation in the disease linked with complications is poor worldwide[26].
Diabetes is a chronic illness that requires ongoing medical care and patient self-management to prevent complications. Diabetes care is complex, requires numerous issues to be addressed, and it is more than glycemic control[34]. T1DM is a chronic disease with severe complications due to its mismanagement. The health professionals should be equipped with suitable evidence based on multiple management approaches to those individuals to support patient-centered care and improve their capacity for problem-solving and self-management[35]. Maintaining the long-term integration of lifestyle changes and medical management is crucial to accomplish good metabolic control in diabetes subjects[14,36].
Self-management participation could lead to clinically associated progress in the behavior and clinical parameters[37] and those individuals who participate in self-management can be considered volunteers in the majority of cases where they have either wanted an intervention or decided to take part[38]. Physical activity (PA) and nutrition are significant components of a healthy lifestyle and treatment of diabetes[39]. The benefit of exercise in T1DM remains a significant component of its treatment[40]. Therefore, the adoption and maintenance of PA are crucial for the management of glycemia and the entire health of individuals with diabetes and prediabetes[41]. Exercise is a cornerstone in the lifestyle of nearly all individuals with T1DM[42]. As the patients may be more agreeable to lifestyle changes, exercise should be encouraged from diagnosis. In addition, to improve patient confidence in managing their diabetes with exercise, standard advice on exercise and diabetes needs to be made available to health professionals and subjects with diabetes[43].
With regard to PA, even though, the term PA and exercise are not synonymous they are often used interchangeably[44,45]. However, the term "PA" should not be mistaken with "exercise", as exercise is a subgroup of PA[46]. Due to this, it is recommended that they should not be used interchangeably[47]. PA can be defined as any bodily movement formed by the skeletal muscles that result in energy expenditure above resting levels[46,48-50]. While exercise is defined as a planned, structured PA typically performed with the intent of improving health and/or fitness[46,48]. The term PA is broadly comprised of exercise and sport, and PA is performed as a part of daily living, occupation, leisure, and active sport[46,48]. Exercise can be classified as aerobic and resistance exercise[51]. Aerobic exercise involves the repeated and continuous movement of huge muscle groups[52]. Anaerobic exercise comprises activities such as walking, cycling, jogging, and swimming. Resistance exercise includes activities such as free weights, weight machines, bodyweight, or elastic resistance bands[51].
What are the protective effects of physical activity against the health risks associated with T1DM?
The present review article includes all studies conducted in various countries globally.
The present review article was carried out using a comprehensive literature search. The search was performed using the following electronic databases: “Cochrane Library”, Web of Science, PubMed, HINARI, EMBASE, Google for grey literature, Scopus, African journals Online, and Google Scholar. The search was conducted using the following search terms; “diabetes mellitus”, “type 1 diabetes”, “T1DM”, “complications”, “insulin-dependent diabetes mellitus”, “IDDM”, “physical activity”, “exercise”, “Glycemic Control”, “Physical Fitness”, “Blood Lipids Profile”, “Endothelial Function”, “Insulin Resistance”, “Insulin Sensitivity”, “Insulin Requirement”, “Cardiovascular Diseases”, “Blood Pressure”, “Well-being”, “Body’s Defense Systems”, “Mortality”, “barriers”, “factors”, “strategy”, and “hypoglycemia”. The Boolean operators; “AND” and “OR” were used to integrate them during the search.
The inclusion criteria for the present review were; Articles on this topic globally, published in the English language, quality articles, and those with outcome variables well defined and measured, and articles published up to June 21, 2021. The exclusion criteria for the present review article were: articles of poor quality and articles in which the outcome variable was not clearly defined and measured.
Overall, PA is good for almost every individual[52]. It provides numerous health benefits mainly for obese individuals[53]. Even among healthy individuals, daily PA is a significant mediator of glycemic control and enhances the prevention of pathologies related to increased postprandial glucose[54]. Its significant role in the prevention and management of noncommunicable diseases is extensively understood[55]. Exercise is largely known as an effective approach for the prevention and management of many chronic diseases[56]. It is essential in the primary and secondary prevention of chronic diseases such as CVD, diabetes, cancer, hypertension, obesity, depression and osteoporosis, and premature death[57]. The effects of exercise also include the management of many metabolic syndromes as well as improved mood and quality of life[58].
Exercise training leads to improved body composition, cardiovascular, and metabolic outcomes in subjects with metabolic syndrome[59]. Moreover, PA helps to decrease all causes of morbidity and improves the quality of life in people of all age groups[60,61]. It also benefits principally older adults by protecting against and ameliorating several diseases, the achievement and maintenance of a healthy body weight, improved mental health and well-being, and musculoskeletal health. The amelioration of disease risk factors, the achievement of peak bone mass, and maintenance of healthy body weight were the benefits of PA in children[62].
Activity may play a protective role due to the consistent relationship between historical PA and the development of complications in insulin-dependent DM (IDDM)[63]. Exercise also has many significant health merits in both T1DM and T2DM subjects[64] and many exercises are supportive in these subjects[65]. The findings from randomized trials support the role of resistance training as an adjunctive mode of management in T1DM patients[66]. Both aerobic and resistance exercises are excellent for patients with T1DM[67]. In addition, regular moderate to vigorous PA was linked with many health benefits in adolescents with T1DM[68]. Exercise decreases the rate of diabetes-related complications in T1DM subjects[69].
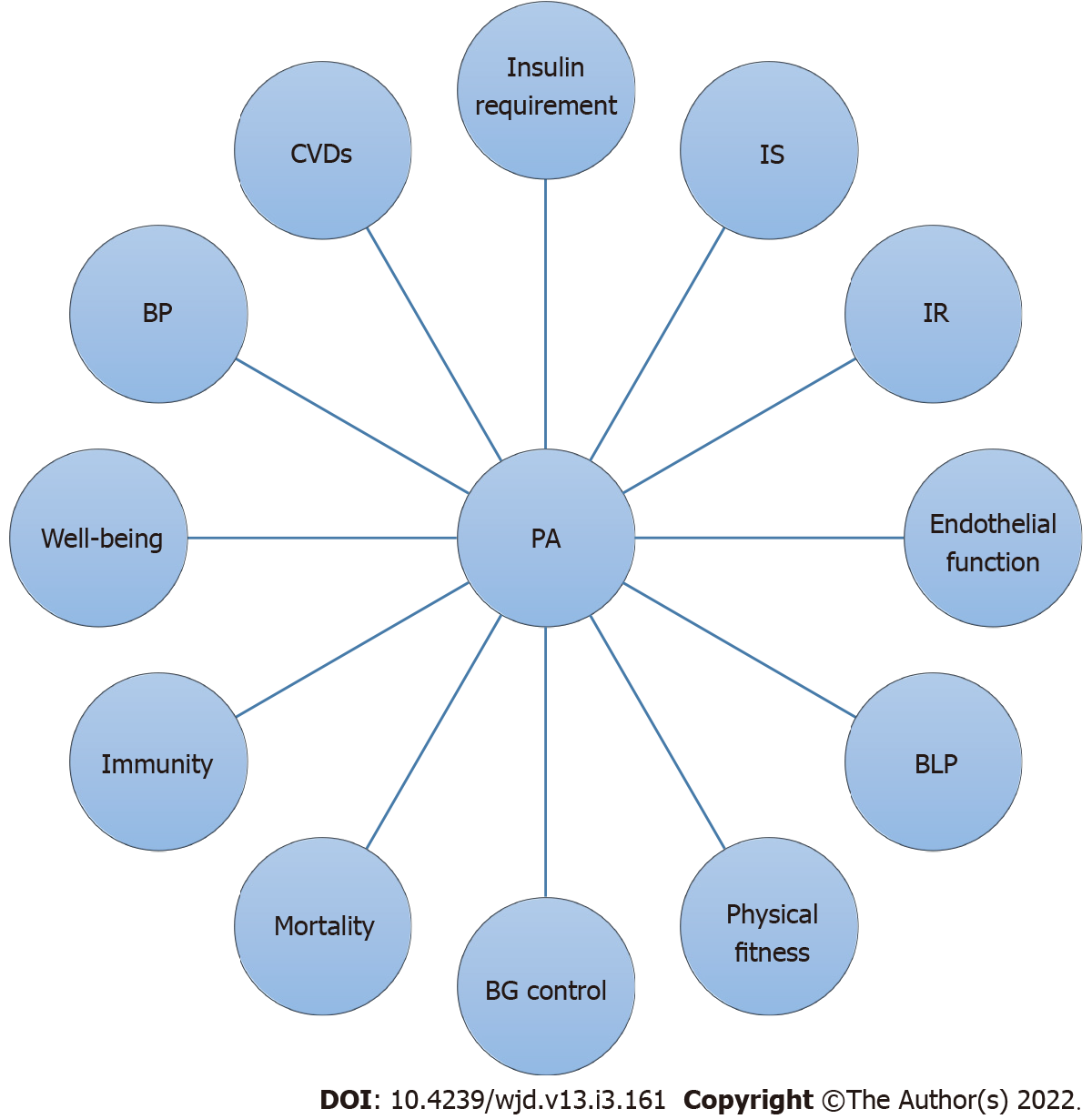
Vigorous-intensity PA has a role in metabolic control in T1DM patients[70]. Consistent regular PA can improve metabolic control in these patients[71] and is significant for best physical and psychological development during childhood, and it improved glycemic control, cardiovascular function, blood lipid profiles, and psychological well-being[72]. Consistent PA has a beneficial effect on glycemic control, diabetes-related comorbidities, and cardiovascular risk factors without the risk of adverse events[73]. A summary of the effects of PA on many health outcomes such as blood glucose (BG) control, physical fitness, endothelial function, insulin sensitivity, well-being, body defense system, blood lipid profile, insulin resistance, CVDs, insulin requirements, BP, and mortality is shown in Figure 1.
Discrepancies have been observed in the literature regarding the role of PA in T1DM glycemic control. For instance, in T1DM female individuals, daily physical training for several months did not improve glycemic control[74]. In addition, Yki-Jarvinen et al[75] demonstrated that a controlled physical training program in pump-treated T1DM subjects did not change an already near-normal glycemic control. Furthermore, Zinman et al[76] found that, although plasma glucose declines acutely with exercise, an augmented caloric intake on exercising days obviates the long-term effect of training on glucose control. Similarly, glycemic control did not significantly improve in pregnant women with T1DM during postprandial walking exercise[77]. Moreover, glycemic control was not found to be associated with long-term PA in T1DM subjects and PA did not negatively affect long-term glycemic control[78].
Several studies have found that PA improved glycemic control in individuals with T1DM[69,71,79-113]. Regular PA can lead to decreased BG level among these patients, it is safe and does not result in more hypoglycemic episodes[91]. A systematic review showed that PA had a positive impact on glycemic control in children and adolescents with T1DM[97]. Similarly, regular PA enhances BG control in children with T1DM[98]. Prolonged moderate aerobic exercise leads to a consistent decrease in plasma glucose but frequent hypoglycemia can occur when pre-exercise glucose concentrations are < 120 mg/dL in young people with T1DM[100]. Increased leisure-time PA (LTPA) between the ages of 50 and 70 years in the absence of active intervention was also found to be associated with improved glucose in men[101]. Campaign et al[71] demonstrated that regular high-intensity PA can improve metabolic control in young children with IDDM. In addition, combined exercise training (endurance training and resistance training) improves glycemic control to a better extent than endurance or resistance training alone, under moderate-intensive training situations with equal training durations[82]. Supervised strength training in T1DM male patients was associated with significant changes in glycemic control[93]. Marrone et al[95] found that free-play PA has a crucial role in helping to maintain BG levels in children with T1DM. Furthermore, anaerobic circuit training was found to improve glucose regulation in adolescents with IDDM[96]. Regular participation in moderate to intense PA or sports improves metabolic control in T1DM subjects[103]. High-intensity training (HIT)[105] and resistance training[106] improve plasma glucose in T1DM patients. Generally, an enhanced skeletal muscle, by either an intrinsic mechanism or PA, provides better advantages and benefits in facilitating glucose regulation[86] as peripheral glucose utilization rises during exercise, despite a reduction in circulating insulin levels[85]. During PA, muscle glucose uptake also rises and can reach values that are 30-50 times greater than at rest[87].
PA decreased glycosylated hemoglobin (HbA1c) in T1DM patients[81,89,90,111-113]. This effect is acceptable since the HbA1c level is increased following PA cessation[89]. This shows that the reduction of HbA1c level is a major sign of glycemic control. This is because the amount of glucose that combines with HbA1c is directly proportional to the total amount of glucose within a system. This means, if the BG levels have been high in current weeks, the HbA1c level will also increase. This could be evidence of PA reducing BG level, and was proved by the decrease in this biomarker of glycemic control.
Physical fitness is defined as a set of attributes that are either health- or skill-related and the extent to which individuals have these attributes can be measured with specific tests[50]. Evidence shows that patients with T1DM have reduced physical fitness[114]. Furthermore, children with T1DM presenting with poor glycemic control had lower aerobic fitness compared to those with good glycemic control[115]. In addition, lower cardiorespiratory fitness in children with T1DM is associated with poor glycemic control[116].
However, numerous studies have shown that PA improves physical fitness in individuals with T1DM[92,93,96,97,117,118]. Supervised strength training in male patients was associated with augmented strength[93]. Also, exercise training among adolescents with T1DM leads to improved physical fitness[92]. Even, a training program of 1 h per week for 3 mo was found to improve physical fitness[117]. Anaerobic circuit training improved muscle strength in adolescents with IDDM[96]. Furthermore, a systematic review showed that PA improved physical fitness in children and adolescents with T1DM[97]. Moreover, a randomized trial demonstrated that combined exercise training appeared to improve physical fitness in adolescents with T1DM[118]. Regular PA also improved cardiovascular fitness[71,98,102,107] and increased lean mass in these patients[98].
A study found that youths with T1DM have abnormal lipid levels and atherogenic changes in lipoprotein composition, even after a relatively short disease duration, and glycemic control is a significant mediator of these abnormalities[119]. In normal-weight T1DM youths, mainly females had more atherogenic low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C) distributions which are associated with lower insulin sensitivity[120]. Dyslipidemia is significantly more frequent in children and adolescents with T1DM compared to non-diabetic peers whilst high LDL-C and low HDL-C were the most frequent type of dyslipidemia in the dyslipidemic group[121]. Similarly, dyslipidemia is frequently found in T1DM and appears to be associated with glycemic control. It is a major risk factor for coronary heart disease, and one of the most significant and frequent complications with a high premature mortality and morbidity rate[122]. In addition, apolipoprotein B is consistently associated with an increased risk of mortality in T1DM due to all causes as well as in cardiac disease and ischemic heart disease[123].
Several studies have proved that PA decreases the blood lipids profile in individuals with T1DM[69,74,80,93,98,101-103,110,111,124-128]. Laaksonen et al[125] found that endurance training improved the lipid profile in physically active T1DM men. Anaerobic circuit training also improved the lipid profile in adolescents with IDDM[96]. Similarly, regular PA improved the blood lipid profile and reduced body adiposity in children with T1DM[98]. In addition, there is a linear dose-response relationship between augmented PA and loss of abdominal fat in T1DM[91]. Regular exercise had beneficial effects on body fat content and the lipoprotein profile in subjects with T1DM by decreasing high plasma lipoprotein(a) concentrations[128]. Similarly, PA improved the lipoprotein profile in T1DM patients[102]. Increased PA in children with T1DM is related to a lower lipoprotein level[80]. Daily training for a number of months in T1DM females had a significant effect on the HDL3-C subfraction but led to minor changes in serum lipoprotein profiles[74]. Austin et al[127] demonstrated that the state of physical fitness was significantly correlated to lipid levels and lipoprotein(a) in adolescents with IDDM, where higher physical fitness levels decreased lipid levels. Total cholesterol (TC) levels significantly declined after an exercise intervention[124]. Consistent supervised strength training among male patients with T1DM was associated with a reduced TC level[93]. A systematic review also showed that PA reduced the TC level in children and adolescents with T1DM[97]. In addition, physical training in IDDM leads to reduced TC levels[126]. Yki-Jarvinen et al[75] found that a controlled physical training program in pump-treated diabetic patients increased the HDL-C to TC ratio in T1DM subjects. Postprandial walking exercise in pregnant women with T1DM was associated with significantly lower fasting plasma triglyceride levels in an intensive perinatal diabetes program[77]. Regular moderate to intense PA or sports participation improved the lipid profile in T1DM subjects[103]. Also, augmented LTPA between the ages of 50 and 70 years in the absence of active intervention was associated with improved lipid metabolism in men[101].
Evidence shows that endothelial dysfunction is common in adolescents with T1DM[129] and it is a predictor of CVDs in these patients[130]. Studies have found that PA improves endothelial function in individuals with T1DM[110,131,132]. Anaerobic exercise training can improve endothelial function in different vascular beds in individuals with long-standing T1DM who are at substantial risk of diabetic angiopathy[131]. However, regular exercise training involving the lower extremities did not improve endothelial function in the micro- and macro-circulation of the non-exercised upper extremity in T1DM individuals[132].
T1DM subjects are insulin resistant compared with nondiabetic subjects[133], and insulin resistance in the liver and skeletal muscle was found to be a significant characteristic in T1DM[134,135]. Youths with T1DM have adipose, hepatic, and peripheral insulin resistance[136]. This insulin resistance is an independent risk factor for the development of macro-and microvascular complications and may also contribute to the development of the disease[137]. Insulin resistance could also impact the length of the honeymoon period, diabetic control and patterns of growth during puberty, insulin requirements and BG control at any time, the birth weight of infants born to diabetic mothers, lipid metabolism, hypertension, rates of progression to insulin dependence and eventually contribute to excess mortality[138].
Insulin resistance is linked with a greater atherogenic lipoprotein cholesterol distribution in all men and women with T1DM[139]. Gender differences in insulin resistance-associated fat distribution may clarify why T1DM increases coronary calcification in women more than in men[140]. Greater insulin resistance was found in a group of premenopausal women with T1DM compared with nondiabetic subjects which was not related to abdominal adiposity, lipids, or androgens[141].
Studies have shown that PA improves insulin resistance in patients with T1DM[142,143]. For instance, aerobic exercise decreases waist circumference which is related to a tendency for raised HDL-C levels, and this may indicate a decrease in visceral fat with an improvement in insulin resistance[143]. This finding is also supported by the trial conducted by Dotzert et al[144] where aerobic exercise training was found to improve insulin resistance in insulin-resistant T1DM rats. The capacity of exercise to increase insulin-stimulated glucose uptake in vivo was decreased in subjects with insulin-resistant T1DM compared with normal individuals, and this could have been due to either separate or common defects in exercise- and insulin-stimulated pathways[142]. In β-cell transplanted recipients, endurance training may be helpful and preventive by counteracting graft dysfunction, alleviating the side effects of immunosuppressive drugs, and conserving insulin independence after islet transplantation[145].
T1DM adolescents have significantly reduced insulin sensitivity compared with nondiabetic adolescents[146]. Several studies have verified that PA improves insulin sensitivity in patients with T1DM[39,75,83,84,92,98,101,126,147,148]. Augmented LTPA levels were associated with raised insulin sensitivity[84,101]. Regular PA enhanced insulin sensitivity in children with T1DM[98]. Also, a controlled physical training program in pump-treated T1DM subjects improved body sensitivity to insulin[75]. Exercise training in adolescents with T1DM can lead to improved insulin sensitivity[92]. Physical training in IDDM also leads to raised peripheral insulin sensitivity[126]. Regular moderate-intensity PA can improve insulin sensitivity in T1DM individuals[39]. The findings from the trial, where exercise led to improved insulin sensitivity and responsiveness by different mechanisms in rats[149] also supports these studies.
PA also decreases insulin requirements in subjects with T1DM[75,84,128]. A controlled physical training program in pump-treated T1DM subjects decreased insulin requirements[75]. In addition, male patients with T1DM appeared to use less insulin when they were physically active[84]. Furthermore, regular exercise[128] and combined exercise training (aerobic and resistance) appears to lower daily insulin requirements in patients with T1DM[118]. Similarly, a study showed that exercise can improve insulin requirements in T1DM rats[150].
Normal weight adolescents with T1DM have impaired autonomic function and augmented energy expenditure and fat oxidation compared to individuals without diabetes who have similar levels of fitness and PA[151]. T1DM adolescents had significantly reduced peak oxygen consumption (VO2peak), and peak work rate compared with nondiabetics. They also had decreased vascular reactivity, diastolic dysfunction, and left ventricular hypertrophy[146]. Maximal workload and oxygen uptake were markedly diminished in chronically hyperglycemic IDDM subjects and physiologically significant cardiopulmonary dysfunction developed in asymptomatic patients with long-standing disease[152]. Insulin resistance in T1DM may contribute to the augmented CVD burden[120]. In T1DM, heart rate variability and arterial wall stiffness are linked to each other where the autonomic nervous system could be a connection between diabetes and vascular disease[153]. In African Americans with T1DM, high plasma interferon-inducible protein 10 was found to be an independent predictor of incident CVD[154].
Studies have confirmed that PA decreases the risk of CVDs in T1DM patients[39,80,91,104,110,112,124,127,128,143,155-159]. Increased PA in children with T1DM has a beneficial effect on the CV risk profile[80]. There is a linear dose-response relationship between augmented PA and a decrease in lipid-related CV risk factors, with a preferential rise in the HDL3-C subfraction in patients with T1DM[91]. Exercise also improves diabetic complications such as subclinical autonomic neuropathy and CVD risk in children with T1DM[124]. Higher physical fitness levels due to exercise decrease lipid levels and this, in turn, may reduce the risk of CVD[127]. In addition, regular exercise may decrease CV risk in T1DM patients by decreasing high plasma lipoprotein Lp(a) concentrations[128]. Aerobic exercise decreases waist circumference which is related to a tendency for raised HDL-C levels, and this may indicate a decrease in visceral fat with an improvement in insulin resistance which could have an influence on reducing CV risk in these patients[143]. An inverse association was found between PA and the incidence of CVD in women with T1DM[155]. Also, high frequency and high-intensity exercise may decrease the risk of CVD in individuals with T1DM[156]. Regular moderate-intensity PA can decrease the risk of CVD in T1DM individuals[39]. Furthermore, PA has the potential to delay CVD in T1DM as it reduces the risk of CVD[104,112,157]. Integrated with diet, it can also influence lipid-related CV risk factors independent of changes in insulin treatment[158]. T1DM subjects who are physically more active have a lower overall risk of CV events than their sedentary counterparts[159]. Moreover, the study showed that aerobic circuit training was found to improve cardiorespiratory endurance[96] and regular PA improved vascular health in those subjects[160].
PA also improved blood pressure (BP) in patients with T1DM[80,91,102,124,128]. A study showed that regular exercise was found to have beneficial effects on BP in these patients[128]. Increased PA in children with T1DM leads to improved diastolic BP (DBP)[80]. A linear dose-response relationship between increased regular PA and decreased BP in patients with T1DM was also found[91]. DBP and heart failure were significantly correlated with lower TC levels which were lowered by performing exercise[124]. In addition, adolescents with T1DM have been associated with reduced stroke volume during exercise[161]. T1DM adolescent girls showed decreased sympathetic activity, although this was possibly compensated by higher adrenomedullary responsiveness or sensitivity, and did not affect their heart rate adaptation to exercise[162].
Studies have also found that PA improves the well-being of individuals with T1DM[39,69,97,98,118]. A systematic review showed that PA improved the well-being and psychological health of children and adolescents with T1DM[97]. Regular PA enhanced psychosocial well-being in children with T1DM[98]. A randomized trial demonstrated that combined exercise training (aerobic and resistance) appeared to lead to better well-being in adolescents with T1DM[118]. Also, regular moderate-intensity PA can improve the psychological well-being of these individuals[39]. Furthermore, Brazeau et al[163] demonstrated an association between greater PA and a better body mass index, body composition, and more favorable health status in individuals with T1DM similar to individuals without diabetes.
T1DM individuals have higher levels of free radicals and may, as a result, be at augmented risk of developing complications related to T1DM[164]. PA has been found to protect against protein denaturation[165]. Aerobic training improves oxidative stress in individuals with diabetes[110]. Acute exercise is an immune system adjuvant that improves defense activity and metabolic health. In addition, there is a clear inverse relationship between moderate exercise training and illness risk[166]. Farinha et al[9] demonstrated that exercise training improves the body’s defense systems and metabolic health in T1DM patients and induces numerous benefits by decreasing inflammation and improving antioxidant defenses.
Mortality rates in the past decade continue to be much larger in individuals with T1DM than in those without diabetes despite advances in inpatient care[167]. The risk of death rises with less favorable glycemic status and impaired carbohydrate metabolism contributes to mortality from any cause[168]. The mortality rate due to ischemic cardiac disease is greater in T1DM patients compared with the general population[169]. In these patients, the presence of metabolic syndrome is frequent, and it is linked with an augmented incidence of chronic complications and mortality[170]. Macrovascular and microvascular disease are the main causes of mortality in T1DM[130]. In addition, physical inactivity has been found to contribute to a substantial number of deaths in those with DM[171]. A significantly increased mortality risk due to diabetes was associated with decreased health-related quality of life in subjects who reported no LTPA[172].
Many studies have shown that PA decreases mortality in subjects with T1DM[93,143,155,172-175]. Supervised strength training in men with T1DM was related with no morbidity[93]. An inverse association was found between PA and all-cause mortality in both genders with T1DM[155]. Participating in LTPA may be linked with improved survival in patients with diabetes[172]. Furthermore, exercise is associated with a lower risk of premature all-cause mortality such as CVD and chronic kidney disease in patients with T1DM[173], and PA has been found to decrease CV mortality in these subjects[143,174]. Moreover, PA offers a beneficial effect in terms of long life in IDDM patients[175]. In African Americans with T1DM, low plasma stromal-derived factor-1 was found to be an independent predictor of mortality[154].
Even though several literature reports have supported the utilization of PA for individuals with T1DM, most of the patients did not engage in regular exercise due to various obstacles. For instance, the fear of exercise-induced hypoglycemia is the strongest barrier to regular PA in adults with T1DM[39,176-180]. Although technological advances have permitted exercisers with diabetes to progress toward more successful management of their BG levels during various types of PA, technology is still far from fully avoiding the fear of hypoglycemia in T1DM subjects[176]. Glucoregulatory failure may cause hypoglycemia in IDDM individuals during and after exercise. This could be due to hypoglycemic episodes which blunt the glucoregulatory response to subsequent exercise while exercise blunts the glucoregulatory response to subsequent insulin excess[181]. Even though regular PA was found to improve glycemic control, its frequency is a major factor affecting the control of glycemia without raising the risk of severe hypoglycemia in pediatric patients with T1DM[94]. This may be supported by a study, where low levels of LTPA were associated with poor glycemic control in T1DM women[84].
Adolescents with T1DM who participate in moderate-intensity exercise in the afternoon have augmented glucose needs at the time of and shortly after the completion of exercise. In addition, the reduced counter-regulatory responses to hypoglycemia post-exercise may lead to a higher risk of hypoglycemia overnight[182]. Antecedent hypoglycemia induces acute counter-regulatory failure both during subsequent hypoglycemia and moderate exercise in T1DM. This acute state of counter-regulatory impairment may be one cause of exercise-associated hypoglycemia in these individuals[183]. Anaerobic exercise usually causes BG concentration to reduce quickly, whereas anaerobic exercise may cause it to increase, making glycemic control challenging for patients with T1DM[184]. Prolonged exercise could lead to hypoglycemia even in normal male individuals[185]. Even though the risk of exercise-induced hypoglycemia is a great challenge for these patients, the glycemic response to exercise depends upon several factors concerning the patient him/herself such as therapy, glycemic control, training level, and the characteristics of the exercise performed[186].
Also, evidence shows that there are sex-related differences in exercise responses that might affect BG levels during exercise in patients with T1D[187,188]. Marked sexual dimorphism occurs in the pattern of counter-regulatory responses to moderate, prolonged euglycemic exercise in subjects with T1DM. Despite decreased plasma levels of epinephrine, norepinephrine, and growth hormone, T1DM women have a higher lipolytic response, which probably reflects greater tissue sensitivity to one of these hormones during exercise[188]. When compared with men, women with T1DM are more resistant to the blunting effects of antecedent hypoglycemia on neuroendocrine and metabolic responses to subsequent moderate exercise[189].
A study showed that patients with T1DM have a variable glycemic response to prolonged aerobic exercise, and this variability is partially explained by their pre-exercise BG levels[190]. High-intensity interval training (HIIT) has been found to improve anaerobic capacity without a detrimental decrease in BG in these patients[191]. However, another study showed that HIIT in fasting individuals with T1D produces a large and consistent hyperglycemic response instantly post-exercise[192]. Also, Fahey et al[193] demonstrated that a sprint as short as 10 s can raise plasma glucose levels in nondiabetic and T1DM subjects, with this increase resulting from a transient decrease in glucose rate of disappearance (Rd) rather than from a disproportionate rise in glucose rate of appearance (Ra) relative to glucose Rd as reported with intense aerobic exercise. Furthermore, other identified barriers were lack of time and work-related factors, access to facilities, lack of motivation, embarrassment and body image, weather, and having low levels of knowledge about managing diabetes and its complications in relation to exercise as the main barriers to perform exercise[194].
The effective management of T1DM desires a multidisciplinary combined method to develop individualized programs, attention to all factors that may influence the result, and the expectations of those with TIDM should be paramount in the strategy adopted by the diabetes care team[195]. It is essential to know that both hypoglycemia and hyperglycemia can arise during exercise; however, strategies are available to deal with these challenges[64]. In T1DM, due to the potential risk of hypoglycemia, the patients must be carefully educated about the consequences of PA on their BG levels and the modifications of diet and insulin therapy before starting exercise sessions[196].
It is supportive for subjects to monitor their BG levels before, during, and after exercise, to avoid T1DM complications and to identify when changes in insulin or food intake are essential. In particular, individuals who experience late or nocturnal hypoglycemia should have a snack after exercise and/or before going to sleep[197]. It is suggested that the personalized exercise carbohydrate requirement estimation system can be used for the management of exercise-related glycemic imbalances in T1DM[198]. In individuals with T1DM being treated with intensive insulin therapy containing the basal-bolus (NPH-human regular) insulin regimen, walking after meals improves glycemic control[199]. Also, performing resistance exercise before aerobic exercise improves glycemic stability throughout exercise and decreases the duration and severity of post-exercise hypoglycemia in subjects with T1DM[200]. Performing a morning resistance exercise session after an overnight fast and omission of pre-exercise rapid-acting insulin does not induce acute post-exercise hypoglycemia or increase the marker of muscle damage in T1DM patients[201]. In addition, morning exercise reduces the risk of late-onset hypo
Furthermore, a larger insulin basal rate decrease and supplemental carbohydrates during exercise may be essential to avoid hypoglycemia[206]. In addition, a combination of ideal glycemic control, empirical adjustments of insulin administration at the time of exercise, and ingestion of carbohydrate supplements tailored to the type, intensity, and duration of an exercise also help to prevent hypoglycemia[207]. Exercise has a role in insulin pump therapy and improves metabolic control in patients with T1DM[208]. A reduction in the basal rate during fasting exercise in continuous subcutaneous insulin infusion-treated individuals seems to be a reasonable step in the maintenance of near-normoglycemia in individuals in whom this occurs[209].
However, reducing the basal insulin infusion rate by 80% up to 40 min pre-exercise onset was found to be insufficient to decrease exercise-induced hypoglycemia[210]. In children, even discontinuing basal insulin during exercise is an effective approach for reducing hypoglycemia in children with T1DM, but the risk of hyperglycemia is increased[211]. Another approach is short-time hypoxia together with graded exercise, which increases cardiorespiratory adaptation to exercise and permits more effective control of glucose homeostasis in T1DM[88]. Combining exercise with hypoxia may permit more effective short-term glycemic control in these patients[212]. Besides, home-HIT appears to provide a strategy to decrease the fear of hypoglycemia, while simultaneously eliminating other identified barriers in individuals with T1DM from performing exercise such as being time-efficient, no travel time or costs related to gym memberships, and providing them with the chance to exercise in their chosen environment, decreasing embarrassment experienced by some when exercising in public[213].
Technology such as continuous glucose monitoring (CGM) is a strategy to control hypoglycemia during exercise in T1DM, and it allows individuals to see the trends in glycemic fluctuations when exercising and in the subsequent night to deal pre-emptively with hypoglycemic risks and treat hypoglycemic episodes in a timely way[214]. Using CGM during exercise may avoid exercise-induced hypoglycemia, but usual BG control should be carried out during intensive exercise[215]. High-intensity exercise leads to delayed nocturnal hypoglycemia and CGM is a useful approach in T1DM subjects who undergo an exercise program[216].
Using CGM trends and carbohydrate intake based on standard exercise carbohydrate intake guidelines to facilitate exercise in children with T1DM is effective as both were found to minimize hypoglycemia and maintained euglycemia during exercise in young children with T1DM[217]. In addition to this, real-time continuous glucose monitoring (RT-CGM) can be recommended as an extra tool that offers T1DM adolescents a rapid reaction to decrease glycemic variability within a short time[218]. Besides, RT-CGM with a carbohydrate intake algorithm may avoid hypoglycemia and maintain euglycemia during exercise, mainly if the subject consumes carbohydrates when trend arrows alert them to a drop in glycemia[219].
Furthermore, closed-loop insulin delivery also offers an effective means to decrease the risk of nocturnal hypoglycemia while increasing the percentage of time spent in the target range, irrespective of activity level during mid-afternoon. This could benefit these patients even if it is limited to the overnight period[220]. The hybrid closed-loop systems are another approach that help to avoid hypoglycemia, relying on accurate carbohydrate ratios and carbohydrate counting, and the algorithm that was tested against moderate exercise and an over-reading glucose sensor performed well in terms of hypoglycemia prevention[221]. Moreover, a study demonstrated that the heart rate–enhanced artificial pancreas system improved protection against hypoglycemia during exercise in T1DM[222]. Several studies have shown different strategies that could be integrated with exercise as a means of glycemic control in individuals with T1DM (Table 1)[223-240].
| Ref. | Year | Intervention | Findings |
| Sonnenberg et al[223] | 1990 | CSII during exercise | Hypoglycemia could only be avoided when the premeal insulin bolus was decreased by 50% and discontinuation of the basal insulin infusion during exercise |
| Rabasa-Lhoret et al[224] | 2001 | Premeal insulin dose reductions forpost-prandial exercises | Minimized risk of hypoglycemia during postprandial exercises of different intensities and different durations by a suitable decrease in premeal insulin lispro |
| Dubé et al[225] | 2005 | Glucose supplement during exercise in subjects using N-lispro | For 60 min of late post-prandial exercise followed by 60 min of recovery, an estimated 40 g of a liquid glucose supplement, ingested 15 min before exercise was good for BG control |
| Diabetes Research in Children Network (DirecNet) Study Group et al[211] | 2006 | Suspension of basal insulin during exercise | Basal insulin suspension decreases hypoglycemia from 43% to 16% in individuals, but hyperglycemia 45 min after exercise was more frequent |
| Bussau et al[226] | 2006 | Ten-second sprint after moderate-intensity exercise | This avoided early post-moderate intensity exercise hypoglycemia |
| Bussau et al[227] | 2007 | Ten-second sprint before moderate-intensity exercise | Prevented hypoglycemia during early recovery from moderate-intensity exercise |
| West et al[228] | 2010 | Reductions in pre-exercise rapid-acting insulin by 75%, 50%, or 25% | A 75% reduction in pre-exercise insulin resulted in the greatest preservation of BG, and a decreased dietary intake, for 24 h after running |
| Taplin et al[229] | 2010 | 20% reduction of basal rate overnight | Was safe and effective in preventing nocturnal hypoglycemia |
| 2.5 mg bedtime dose of oral terbutaline | Effective at avoiding hypoglycemia, but linked with hyperglycemia | ||
| Riddell et al[219] | 2011 | RT-CGM and carbohydrate intake algorithm (8-20 g), depending on the concentration of glucose at the time of RT-CGM alert and rates of change in glycemia | The coupled carbohydrate intake algorithm with RT-CGM avoided hypoglycemia and maintained euglycemia during exercise |
| Garg et al[230] | 2012 | An automatic suspension of insulin delivery when BG ≤ 70 mg/dL during or after exercise | This significantly decreased the duration and severity of induced hypoglycemia without causing rebound hyperglycemia |
| Yardley et al[200] | 2012 | Resistance exercise before aerobic exercise | Performing resistance first improved glycemic stability throughout the exercise and decreased the duration and severity of post-exercise hypoglycemia |
| Yardley et al[231] | 2013 | Resistance vs aerobic exercise | Resistance caused a less initial decline in BG but prolonged decreases in post-exercise glycemia than aerobic exercise |
| Campbell et al[232] | 2013 | Pre- and post-exercise rapid-acting insulin reductions | 25% pre-exercise and 50% post-exercise rapid-acting insulin dose preserved glycemia and protected patients against early-onset hypoglycemia (8 h) |
| Schiavon et al[233] | 2013 | In silico optimization of basal insulin infusion rate during exercise | A decrease in basal insulin by 50% starting 90 min before exercise and by 30% during exercise is safe and effective for glucose control |
| Danne et al[234] | 2014 | PLGM (suspension of insulin delivery based on predicted sensor glucose values) | PLGM may decrease the severity of hypoglycemia above that already established for algorithms that use a threshold-based suspension |
| Campbell et al[235] | 2015 | Combined basal-bolus insulin dose reduction and carbohydrate feeding strategy following exercise | Reducing basal-bolus insulin by 20% (80%) protected from nocturnal hypoglycemia for 24 h post-exercise |
| Cherubini et al[236] | 2019 | PLGM system during exercise | Effective for avoiding hypoglycemia during and after exercise, regardless of the thresholds of PLGM used |
| Moser et al[237] | 2019 | Oral administration of carbohydrates during moderate-intensity exercise | Pre-exercise BG levels determine the amount of orally administered carbohydrates during exercise to maintain euglycemia |
| Zaharieva et al[238] | 2019 | Basal rate reductions set 90 min pre-exercise vs pump suspension at exercise onset | 50%-80% Basal rate reductions set 90 min pre-exercise improved BG control and reduced hypoglycemia risk during exercise better than pump suspension at exercise onset |
| Moser et al[239] | 2019 | Reduction in insulin degludec dose (75% IDeg dose vs 100% IDeg dose) | Reducing the usual IDeg dose by 25% led to more time spent in euglycemia with small effects on time spent in hypo- and hyperglycemia |
| Zaharieva et al[240] | 2020 | Insulin pump connected (pump on) vs pump disconnected (pump off) during high-intensity exercise | No significant differences in BG concentrations during 40 min of intermittent high-intensity exercise |
It is essential to balance the risks of insulin-induced hypoglycemia with the risks related to poorly controlled diabetes and poor physical fitness in individuals with T1DM[241]. Considering the risk-benefit ratio, several studies have recommended PA in patients with T1DM[9,71,79,92,97,113,124,158,191,207,214,231,242-248]. Regular PA should be a routine aim in these subjects, for various health and fitness reasons. However, considerable challenges remain for these patients, and their healthcare team, in the management of exercise and sports[244]. Exercise is highly recommended for patients with T1DM as it has several beneficial health effects, with the prevention of long-term cardiovascular complications being dominant[207,214].
With regard to exercise, regular moderate-to-vigorous exercise should become a central part of the management of subjects with T1DM, in the absence of contraindications and accompanied by all desirable educational support for optimal diabetes management[245]. Children with IDDM can be engaged in regular vigorous PA (with minimal risks)[71] and regular exercise[124]. A combined exercise of strength training (ST) and HIIT for at least 2 mo, 3 times per week, will provide many health benefits for T1DM subjects[9].
Another study showed that HIIT sessions reduced glycemia to a greater degree than ST or ST+HIIT sessions over 10 wk in real-life situations. Because of this, T1DM individuals who develop severe exercise-associated hypoglycemia and/or present pre-exercise capillary glucose levels close to 5.5 mmol/L are recommended to carry out ST or HIIT after ST as the preferred option[247]. Resistance exercise was also found to have several benefits and should be recommended as a significant activity for health and well-being in these individuals, although caution with regard to BG levels will always be essential while performing resistance exercise[231]. However, HIIT may be the chosen training approach for some individuals with T1DM as it has been found to improve anaerobic capacity without a detrimental decrease in BG in these patients[191]. For T1DM patients using ultra-long-acting insulin, both aerobic high-intensity interval exercise and moderate continuous exercise can be safely performed[67].
In addition, high-intensity exercise does not raise the risk of early post-exercise hypoglycemia in patients with T1DM[249]. High-intensity bouts linked with high-intensity exercise result in a more rapid and higher increase in endogenous glucose production during exercise than moderate-intensity exercise alone. During early recovery from exercise, glucose use reduces following high-intensity exercise, while leftovers raised after moderate-intensity exercise despite the performance of more whole work[250]. In pediatric patients with T1DM, the frequency of regular PA was the main factor that affected the control of BG without raising the risk of severe hypoglycemia and this is why it is recommended in these patients[94].
Moreover, for subjects with T1DM, the emphasis must be on adjusting the therapeutic regimen to allow safe participation in all forms of PA consistent with an individual’s desires and goals. Eventually, all subjects with diabetes should have the chance to benefit from the valuable effects of PA[248]. Daily PA should be recommended in these patients as part of their management[113] and could be used as an adjunct in glycemic control[92]. Diet and PA can influence glycemic control in IDDM independent of changes in insulin treatment[158].
Besides exogenous insulin therapy and CGM, exercise is recommended in adults with T1DM to improve the entire health of individuals[79]. However, to perform regular PA, the patient and those who supervise them should be aware of disease-specific recommendations and contraindications[242]. Evidence recommends that even individuals with ketonemia may engage in intensive physical training, provided this is part of a program including adequate insulin dosage, dietary advice, and close supervision with multiple daily BG measurements[246]. Evidence suggests that it is significant to consider the needs of the wider support network, as well as the child’s or adolescent’s concerns and preferences, during the development of new or existing strategies and programs to promote PA in children and adolescents with T1DM[251]. Finally, during PA, the parameters such as supervision, duration, frequency of sessions, protocols with mixed PA may positively affect the metabolic outcome of patients with T1DM[97].
As T1DM is rising globally, PA is recommended as evidence shows that PA can control the burden of disease. The benefit of PA in T1DM is a significant component in its management. It is recognized as having beneficial effects and is key to a healthy lifestyle as well as the management of T1DM. PA can be considered an efficient and inexpensive non-pharmacologic tool for the management of T1DM in addition to insulin therapy. It has significant health benefits such as improves BG control, physical fitness, endothelial function, insulin sensitivity, well-being, and the body defense system. In addition, it reduces the blood lipid profile, insulin resistance, CVDs, insulin requirements, BP, and mortality associated with T1DM.
Overall, ideal glucose control is of principal significance in T1DM including during PA. Previously, it is challenging to prevent the hazards associated with PA in these individuals. Hypoglycemia and fear of hypoglycemia are the greatest challenges in T1DM individuals engaging in PA and can limit suitable glycemic control in these patients. However, a better understanding of energy metabolism and homeostasis has made it possible for individuals with diabetes to take part in exercise. In addition, several strategies have been identified to make PA more suitable for T1DM individuals. In particular, improvements in glucose monitoring technology and the availability of other interventional approaches during PA have further contributed to the feasibility of exercise programs for these subjects. There are also strategies for preventing exercise-induced hypoglycemia during and after exercise.
The present review may help health professionals to encourage PA as part of the management of individuals with T1DM. It is also recommended that PA can be performed carefully with reference to diabetes guidelines. Therefore, health professionals in clinical practice should inform and encourage patients with T1DM to manage exercise-induced hypoglycemia. Furthermore, in-depth knowledge of factors such as gender, therapy, glycemic control, training level and characteristics of the exercise performed will allow the development of individualized strategies to minimize the risk of hypoglycemia as the glycemic response to exercise depends upon these factors.
Moreover, health professionals should distinguish between hyperglycemia induced by HIIT and the concern of hypoglycemia-related to less intense forms of exercise during patient counseling for T1DM. Also, this should be clearly set out in practice guidelines. Patients and health professionals should be aware of the degree and duration of post-HIIT hyperglycemia and the potential benefit of an insulin correction bolus. Support for patients on how better to control their BG after exercise could encourage these patients to be less fearful of exercise-induced hypoglycemia and participate in regular PA. Clear-cut quantitative approaches to prevent exercise-induced hypoglycemia are desired to allow patients to engage in regular PA and enjoy its beneficial aspects. It is essential to consider the potential alterations in exercise responses that may occur in T1DM and not to judge this activity as harmful, but these alterations can reduce its full beneficial health effects. Lastly, the present review also provides suggestions for the future direction of research on the types of exercise, duration, and intensity recommended for T1DM patients, considering the individual’s factors that could be detrimental to the response that occurs during and after exercise.
| 1. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3654] [Cited by in RCA: 4362] [Article Influence: 272.6] [Reference Citation Analysis (0)] |
| 2. | Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, Schatz DA, Moran AM, Lachin JM, Skyler JS; Type 1 Diabetes TrialNet MMF/DZB Study Group. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes. Diabetes Care. 2010;33:826-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab. 2017;6:943-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 4. | Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034-2054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 738] [Cited by in RCA: 642] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 6. | Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark Å. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:17016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 862] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 7. | Authors/Task Force Members, Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL; ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schächinger V, Scheen A, Schirmer H, Strömberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 1465] [Article Influence: 112.7] [Reference Citation Analysis (5)] |
| 8. | American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S90-S102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 605] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 9. | Farinha JB, Krause M, Rodrigues-Krause J, Reischak-Oliveira A. Exercise for type 1 diabetes mellitus management: General considerations and new directions. Med Hypotheses. 2017;104:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 551] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 11. | Sherry NA, Tsai EB, Herold KC. Natural history of beta-cell function in type 1 diabetes. Diabetes. 2005;54 Suppl 2:S32-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Tuomilehto J. The emerging global epidemic of type 1 diabetes. Curr Diab Rep. 2013;13:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2015;22:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. |
Fowler MJ.
Microvascular and Macrovascular Complications of Diabetes |
| 15. | Schofield J, Ho J, Soran H. Cardiovascular Risk in Type 1 Diabetes Mellitus. Diabetes Ther. 2019;10:773-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Huxley RR, Peters SA, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women vs men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198-206. [RCA] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 18. | Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjörnsdottir S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 551] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 19. | Domingueti CP, Dusse LM, Carvalho Md, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 20. | Heydari I, Radi V, Razmjou S, Amiri A. Chronic complications of diabetes mellitus in newly diagnosed patients. International Journal of Diabetes Mellitus. 2010;2:61-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | World Health Organization. Global report on diabetes. World Health Organization, 2016. |
| 22. | Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 654] [Article Influence: 25.2] [Reference Citation Analysis (1)] |
| 23. | Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes. 2015;6:850-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 584] [Cited by in RCA: 588] [Article Influence: 53.5] [Reference Citation Analysis (47)] |
| 24. | Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2595] [Article Influence: 288.3] [Reference Citation Analysis (5)] |
| 25. | Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur J Prev Cardiol. 2019;26:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 26. | Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 1029] [Article Influence: 147.0] [Reference Citation Analysis (1)] |
| 27. | Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 28. | Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care. 2002;25:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59:3216-3222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Naslafkih A, Sestier F. Diabetes mellitus related morbidity, risk of hospitalization and disability. J Insur Med. 2003;35:102-113. [PubMed] |
| 32. | Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 696] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 33. | Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, Davies J, Vollmer S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care. 2018;41:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 708] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 34. | American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S33-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 401] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 35. | Turton JL, Raab R, Rooney KB. Low-carbohydrate diets for type 1 diabetes mellitus: A systematic review. PLoS One. 2018;13:e0194987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7:354-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 380] [Cited by in RCA: 384] [Article Influence: 38.4] [Reference Citation Analysis (19)] |
| 37. | Heinrich E, Schaper N, de Vries N. Self-management interventions for type 2 diabetes: a systematic review. European Diabetes Nursing. 2010;7:71-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 38. | Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1837] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 39. | Ajčević M, Francescato MP, Geat M, Accardo A. Comparison of ECRES Algorithm with Classical Method in Management of Diabetes Type 1 Exercise-Related Imbalances. In: Lhotska L, Sukupova L, Lacković I, Ibbott GS. World Congress on Medical Physics and Biomedical Engineering 2018. Singapore: Springer Singapore, 2019: 803–806. |
| 40. | Kennedy A, Nirantharakumar K, Chimen M, Pang TT, Hemming K, Andrews RC, Narendran P. Does exercise improve glycaemic control in type 1 diabetes? PLoS One. 2013;8:e58861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1750] [Article Influence: 175.0] [Reference Citation Analysis (1)] |
| 42. | Codella R, Terruzzi I, Luzi L. Why should people with type 1 diabetes exercise regularly? Acta Diabetol. 2017;54:615-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Kennedy A, Narendran P, Andrews RC, Daley A, Greenfield SM; EXTOD Group. Attitudes and barriers to exercise in adults with a recent diagnosis of type 1 diabetes: a qualitative study of participants in the Exercise for Type 1 Diabetes (EXTOD) study. BMJ Open. 2018;8:e017813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Taylor HL. Physical activity: is it still a risk factor? Prev Med. 1983;12:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, ninth edition. Philadelphia: Lippincott Williams and Wilkins, 2014: 482. |
| 46. | World Health Organization. Physical Activity. [cited 25 Apr 2020]. Available from: http://www.who.int/dietphysicalactivity/pa/en/. |
| 47. | Dasso NA. How is exercise different from physical activity? Nurs Forum. 2019;54:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 48. | Diabetes Canada Clinical Practice Guidelines Expert Committee, Sigal RJ, Armstrong MJ, Bacon SL, Boulé NG, Dasgupta K, Kenny GP, Riddell MC. Physical Activity and Diabetes. Can J Diabetes. 2018;42 Suppl 1:S54-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 49. | Corbin CB, Pangrazi RP, Franks BD. Definitions: Health, Fitness, and Physical Activity. Washington (DC): President’s Council on Physical Fitness and Sports, 2000. |
| 50. | Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126-131. [PubMed] |
| 51. | Herriott MT, Colberg SR, Parson HK, Nunnold T, Vinik AI. Effects of 8 weeks of flexibility and resistance training in older adults with type 2 diabetes. Diabetes Care. 2004;27:2988-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: Department of Health and Human Services, 2008: 683. |
| 53. | Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 529] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 54. | Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc. 2012;44:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Lobelo F, Rohm Young D, Sallis R, Garber MD, Billinger SA, Duperly J, Hutber A, Pate RR, Thomas RJ, Widlansky ME, McConnell MV, Joy EA; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Cardiovascular Surgery and Anesthesia; and Stroke Council. Routine Assessment and Promotion of Physical Activity in Healthcare Settings: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e495-e522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 56. | Seo DY, Ko JR, Jang JE, Kim TN, Youm JB, Kwak HB, Bae JH, Kim AH, Ko KS, Rhee BD, Han J. Exercise as A Potential Therapeutic Target for Diabetic Cardiomyopathy: Insight into the Underlying Mechanisms. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4069] [Cited by in RCA: 4283] [Article Influence: 214.2] [Reference Citation Analysis (0)] |
| 58. | Montesi L, Moscatiello S, Malavolti M, Marzocchi R, Marchesini G. Physical activity for the prevention and treatment of metabolic disorders. Intern Emerg Med. 2013;8:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Ostman C, Smart NA, Morcos D, Duller A, Ridley W, Jewiss D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 60. | King AC, Guralnik JM. Maximizing the potential of an aging population. JAMA. 2010;304:1944-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Nicklett EJ, Semba RD, Xue QL, Tian J, Sun K, Cappola AR, Simonsick EM, Ferrucci L, Fried LP. Fruit and vegetable intake, physical activity, and mortality in older community-dwelling women. J Am Geriatr Soc. 2012;60:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Stanner S. At Least Five a Week- a summary of the report from the Chief Medical Officer on physical activity. Nutr Bulletin. 2004;29:350-352. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Kriska AM, LaPorte RE, Patrick SL, Kuller LH, Orchard TJ. The association of physical activity and diabetic complications in individuals with insulin-dependent diabetes mellitus: the Epidemiology of Diabetes Complications Study--VII. J Clin Epidemiol. 1991;44:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Lumb A. Diabetes and exercise. Clin Med (Lond). 2014;14:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Kourtoglou GI. Insulin therapy and exercise. Diabetes Res Clin Pract. 2011;93 Suppl 1:S73-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Yardley JE, Kenny GP, Perkins BA, Riddell MC, Goldfield GS, Donovan L, Hadjiyannakis S, Wells GA, Phillips P, Sigal RJ; READI trial investigators. Resistance Exercise in Already-Active Diabetic Individuals (READI): study rationale, design and methods for a randomized controlled trial of resistance and aerobic exercise in type 1 diabetes. Contemp Clin Trials. 2015;41:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Moser O, Tschakert G, Mueller A, Groeschl W, Pieber TR, Obermayer-Pietsch B, Koehler G, Hofmann P. Effects of High-Intensity Interval Exercise versus Moderate Continuous Exercise on Glucose Homeostasis and Hormone Response in Patients with Type 1 Diabetes Mellitus Using Novel Ultra-Long-Acting Insulin. PLoS One. 2015;10:e0136489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 68. | Aljawarneh YM, Wardell DW, Wood GL, Rozmus CL. A Systematic Review of Physical Activity and Exercise on Physiological and Biochemical Outcomes in Children and Adolescents With Type 1 Diabetes. J Nurs Scholarsh. 2019;51:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | Lu X, Zhao C. Exercise and Type 1 Diabetes. Adv Exp Med Biol. 2020;1228:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Yardley J, Mollard R, MacIntosh A, MacMillan F, Wicklow B, Berard L, Hurd C, Marks S, McGavock J. Vigorous intensity exercise for glycemic control in patients with type 1 diabetes. Can J Diabetes. 2013;37:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Campaigne BN, Gilliam TB, Spencer ML, Lampman RM, Schork MA. Effects of a physical activity program on metabolic control and cardiovascular fitness in children with insulin-dependent diabetes mellitus. Diabetes Care. 1984;7:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Chetty T, Shetty V, Fournier PA, Adolfsson P, Jones TW, Davis EA. Exercise Management for Young People With Type 1 Diabetes: A Structured Approach to the Exercise Consultation. Front Endocrinol (Lausanne). 2019;10:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 73. | Bohn B, Herbst A, Pfeifer M, Krakow D, Zimny S, Kopp F, Melmer A, Steinacker JM, Holl RW; DPV Initiative. Impact of Physical Activity on Glycemic Control and Prevalence of Cardiovascular Risk Factors in Adults With Type 1 Diabetes: A Cross-sectional Multicenter Study of 18,028 Patients. Diabetes Care. 2015;38:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 74. | Wallberg-Henriksson H, Gunnarsson R, Rössner S, Wahren J. Long-term physical training in female type 1 (insulin-dependent) diabetic patients: absence of significant effect on glycaemic control and lipoprotein levels. Diabetologia. 1986;29:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Yki-Järvinen H, DeFronzo RA, Koivisto VA. Normalization of insulin sensitivity in type I diabetic subjects by physical training during insulin pump therapy. Diabetes Care. 1984;7:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Zinman B, Zuniga-Guajardo S, Kelly D. Comparison of the acute and long-term effects of exercise on glucose control in type I diabetes. Diabetes Care. 1984;7:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Hollingsworth DR, Moore TR. Postprandial walking exercise in pregnant insulin-dependent (type I) diabetic women: reduction of plasma lipid levels but absence of a significant effect on glycemic control. Am J Obstet Gynecol. 1987;157:1359-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Ligtenberg PC, Blans M, Hoekstra JB, van der Tweel I, Erkelens DW. No effect of long-term physical activity on the glycemic control in type 1 diabetes patients: a cross-sectional study. Neth J Med. 1999;55:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Moser O, Eckstein ML, West DJ, Goswami N, Sourij H, Hofmann P. Type 1 Diabetes and Physical Exercise: Moving (forward) as an Adjuvant Therapy. Curr Pharm Des. 2020;26:946-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Herbst A, Kordonouri O, Schwab KO, Schmidt F, Holl RW; DPV Initiative of the German Working Group for Pediatric Diabetology Germany. Impact of physical activity on cardiovascular risk factors in children with type 1 diabetes: a multicenter study of 23,251 patients. Diabetes Care. 2007;30:2098-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Qadir KJ, Zangana KO. Effect of swimming program on glycemic control in male adolescents with type 1 diabetes mellitus. J Sports Med Phys Fitness. 2020;60:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Röhling M, Herder C, Roden M, Stemper T, Müssig K. Effects of Long-Term Exercise Interventions on Glycaemic Control in Type 1 and Type 2 Diabetes: a Systematic Review. Exp Clin Endocrinol Diabetes. 2016;124:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Leclair E, de Kerdanet M, Riddell M, Heyman E. Type 1 Diabetes and Physical Activity in Children and Adolescents. J Diabetes Metab. 2013;1. |
| 84. | Wadén J, Tikkanen H, Forsblom C, Fagerudd J, Pettersson-Fernholm K, Lakka T, Riska M, Groop PH; FinnDiane Study Group. Leisure time physical activity is associated with poor glycemic control in type 1 diabetic women: the FinnDiane study. Diabetes Care. 2005;28:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971;50:2715-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 397] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 86. | Yang J. Enhanced skeletal muscle for effective glucose homeostasis. Prog Mol Biol Transl Sci. 2014;121:133-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 88. | Żebrowska A, Hall B, Kochańska-Dziurowicz A, Janikowska G. The effect of high intensity physical exercise and hypoxia on glycemia, angiogenic biomarkers and cardiorespiratory function in patients with type 1 diabetes. Adv Clin Exp Med. 2018;27:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Ruzic L, Sporis G, Matkovic BR. High volume-low intensity exercise camp and glycemic control in diabetic children. J Paediatr Child Health. 2008;44:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Sideraviciūte S, Gailiūniene A, Visagurskiene K, Vizbaraite D. The effect of long-term swimming program on glycemia control in 14-19-year aged healthy girls and girls with type 1 diabetes mellitus. Medicina (Kaunas). 2006;42:513-518. [PubMed] |
| 91. | Lehmann R, Kaplan V, Bingisser R, Bloch KE, Spinas GA. Impact of physical activity on cardiovascular risk factors in IDDM. Diabetes Care. 1997;20:1603-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 92. | Landt KW, Campaigne BN, James FW, Sperling MA. Effects of exercise training on insulin sensitivity in adolescents with type I diabetes. Diabetes Care. 1985;8:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Durak EP, Jovanovic-Peterson L, Peterson CM. Randomized crossover study of effect of resistance training on glycemic control, muscular strength, and cholesterol in type I diabetic men. Diabetes Care. 1990;13:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Herbst A, Bachran R, Kapellen T, Holl RW. Effects of regular physical activity on control of glycemia in pediatric patients with type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2006;160:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Marrone S, Plume JW, Kerr P, Pignol A, Vogeltanz-Holm N, Holm J, Larsen MA. The role of free-play physical activity in healthy blood glucose maintenance in children with type 1 diabetes mellitus. Psychol Health Med. 2009;14:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 96. | Mosher PE, Nash MS, Perry AC, LaPerriere AR, Goldberg RB. Aerobic circuit exercise training: effect on adolescents with well-controlled insulin-dependent diabetes mellitus. Arch Phys Med Rehabil. 1998;79:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Absil H, Baudet L, Robert A, Lysy PA. Benefits of physical activity in children and adolescents with type 1 diabetes: A systematic review. Diabetes Res Clin Pract. 2019;156:107810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 98. | Riddell MC, Iscoe KE. Physical activity, sport, and pediatric diabetes. Pediatr Diabetes. 2006;7:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Lopes Souto D, Paes de Miranda M. Physical excercises on glycemic control in type 1 diabetes mellitus. Nutr Hosp. 2011;26:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 100. | Tansey MJ, Tsalikian E, Beck RW, Mauras N, Buckingham BA, Weinzimer SA, Janz KF, Kollman C, Xing D, Ruedy KJ, Steffes MW, Borland TM, Singh RJ, Tamborlane WV; Diabetes Research in Children Network (DirecNet) Study Group. The effects of aerobic exercise on glucose and counterregulatory hormone concentrations in children with type 1 diabetes. Diabetes Care. 2006;29:20-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Byberg L, Zethelius B, McKeigue PM, Lithell HO. Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia. 2001;44:2134-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Iughetti L, Gavioli S, Bonetti A, Predieri B. Effects of Exercise in Children and Adolescent with Type 1 Diabetes Mellitus. Health. 2015;7:1357-1365. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 103. | Valerio G, Spagnuolo MI, Lombardi F, Spadaro R, Siano M, Franzese A. Physical activity and sports participation in children and adolescents with type 1 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2007;17:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 104. | Wróbel M, Rokicka D, Czuba M, Gołaś A, Pyka Ł, Greif M, Szymborska-Kajanek A, Strojek K, Gąsior M. Aerobic as well as resistance exercises are good for patients with type 1 diabetes. Diabetes Res Clin Pract. 2018;144:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Harmer AR, Chisholm DJ, McKenna MJ, Morris NR, Thom JM, Bennett G, Flack JR. High-intensity training improves plasma glucose and acid-base regulation during intermittent maximal exercise in type 1 diabetes. Diabetes Care. 2007;30:1269-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | Reddy R, Wittenberg A, Castle JR, El Youssef J, Winters-Stone K, Gillingham M, Jacobs PG. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults With Type 1 Diabetes. Can J Diabetes. 2019;43:406-414.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 107. | Yardley JE, Hay J, Abou-Setta AM, Marks SD, McGavock J. A systematic review and meta-analysis of exercise interventions in adults with type 1 diabetes. Diabetes Res Clin Pract. 2014;106:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 108. | Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974;53:1080-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 527] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 109. | Gomez AM, Gomez C, Aschner P, Veloza A, Muñoz O, Rubio C, Vallejo S. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. J Diabetes Sci Technol. 2015;9:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 110. | Miele EM, Headley SAE. The Effects of Chronic Aerobic Exercise on Cardiovascular Risk Factors in Persons with Diabetes Mellitus. Curr Diab Rep. 2017;17:97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 111. | Aouadi R, Khalifa R, Aouidet A, Ben Mansour A, Ben Rayana M, Mdini F, Bahri S, Stratton G. Aerobic training programs and glycemic control in diabetic children in relation to exercise frequency. J Sports Med Phys Fitness. 2011;51:393-400. [PubMed] |
| 112. | Salem MA, AboElAsrar MA, Elbarbary NS, ElHilaly RA, Refaat YM. Is exercise a therapeutic tool for improvement of cardiovascular risk factors in adolescents with type 1 diabetes mellitus? Diabetol Metab Syndr. 2010;2:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 113. | Beraki Å, Magnuson A, Särnblad S, Åman J, Samuelsson U. Increase in physical activity is associated with lower HbA1c levels in children and adolescents with type 1 diabetes: results from a cross-sectional study based on the Swedish pediatric diabetes quality registry (SWEDIABKIDS). Diabetes Res Clin Pract. 2014;105:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 114. | Lukács A, Mayer K, Juhász E, Varga B, Fodor B, Barkai L. Reduced physical fitness in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2012;13:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 115. | Nguyen T, Obeid J, Walker RG, Krause MP, Hawke TJ, McAssey K, Vandermeulen J, Timmons BW. Fitness and physical activity in youth with type 1 diabetes mellitus in good or poor glycemic control. Pediatr Diabetes. 2015;16:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 116. | Williams BK, Guelfi KJ, Jones TW, Davis EA. Lower cardiorespiratory fitness in children with Type 1 diabetes. Diabet Med. 2011;28:1005-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Huttunen NP, Länkelä SL, Knip M, Lautala P, Käär ML, Laasonen K, Puukka R. Effect of once-a-week training program on physical fitness and metabolic control in children with IDDM. Diabetes Care. 1989;12:737-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | D'hooge R, Hellinckx T, Van Laethem C, Stegen S, De Schepper J, Van Aken S, Dewolf D, Calders P. Influence of combined aerobic and resistance training on metabolic control, cardiovascular fitness and quality of life in adolescents with type 1 diabetes: a randomized controlled trial. Clin Rehabil. 2011;25:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 119. | Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, D'Agostino R Jr, Marcovina S, Dabelea D. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for Diabetes in Youth case-control study. Diabetes Care. 2009;32:416-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 120. | Ercan O, Hatemi S, Kutlu E, Yüksel L, Koçer N. "Diabetes insipidus associated with Langerhans cell histiocytosis: is it reversible? Med Pediatr Oncol. 1998;30:197-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 121. | Mona HM, Sahar SA, Hend SM, Nanees A-WA. Dyslipidemia in type 1 diabetes mellitus: Relation to diabetes duration, glycemic control, body habitus, dietary intake and other epidemiological risk factors. Egyptian Pediatric Association Gazette. 2015;63:63-68. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 122. | Ladeia AM, Adan L, Couto-Silva AC, Hiltner A, Guimarães AC. Lipid profile correlates with glycemic control in young patients with type 1 diabetes mellitus. Prev Cardiol. 2006;9:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Stettler C, Suter Y, Allemann S, Zwahlen M, Christ ER, Diem P. Apolipoprotein B as a long-term predictor of mortality in type 1 diabetes mellitus: a 15-year follow up. J Intern Med. 2006;260:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Shin KO, Moritani T, Woo J, Jang KS, Bae JY, Yoo J, Kang S. Exercise training improves cardiac autonomic nervous system activity in type 1 diabetic children. J Phys Ther Sci. 2014;26:111-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 125. | Laaksonen DE, Atalay M, Niskanen LK, Mustonen J, Sen CK, Lakka TA, Uusitupa MI. Aerobic exercise and the lipid profile in type 1 diabetic men: a randomized controlled trial. Med Sci Sports Exerc. 2000;32:1541-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 126. | Wallberg-Henriksson H, Gunnarsson R, Henriksson J, DeFronzo R, Felig P, Ostman J, Wahren J. Increased peripheral insulin sensitivity and muscle mitochondrial enzymes but unchanged blood glucose control in type I diabetics after physical training. Diabetes. 1982;31:1044-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 127. | Austin A, Warty V, Janosky J, Arslanian S. The relationship of physical fitness to lipid and lipoprotein(a) levels in adolescents with IDDM. Diabetes Care. 1993;16:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 128. | Rigla M, Sánchez-Quesada JL, Ordóñez-Llanos J, Prat T, Caixàs A, Jorba O, Serra JR, de Leiva A, Pérez A. Effect of physical exercise on lipoprotein(a) and low-density lipoprotein modifications in type 1 and type 2 diabetic patients. Metabolism. 2000;49:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 129. | Scaramuzza AE, Redaelli F, Giani E, Macedoni M, Giudici V, Gazzarri A, Bosetti A, De Angelis L, Zuccotti GV. Adolescents and young adults with type 1 diabetes display a high prevalence of endothelial dysfunction. Acta Paediatr. 2015;104:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 130. | Bertoluci MC, Cé GV, da Silva AM, Wainstein MV, Boff W, Puñales M. Endothelial dysfunction as a predictor of cardiovascular disease in type 1 diabetes. World J Diabetes. 2015;6:679-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 131. | Fuchsjäger-Mayrl G, Pleiner J, Wiesinger GF, Sieder AE, Quittan M, Nuhr MJ, Francesconi C, Seit HP, Francesconi M, Schmetterer L, Wolzt M. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25:1795-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 132. | Veves A, Saouaf R, Donaghue VM, Mullooly CA, Kistler JA, Giurini JM, Horton ES, Fielding RA. Aerobic exercise capacity remains normal despite impaired endothelial function in the micro- and macrocirculation of physically active IDDM patients. Diabetes. 1997;46:1846-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 133. | Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 134. | Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Eckel RH, Perreault L, Rewers M. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab. 2012;97:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 135. | Kaul K, Apostolopoulou M, Roden M. Insulin resistance in type 1 diabetes mellitus. Metabolism. 2015;64:1629-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 136. | Cree-Green M, Stuppy JJ, Thurston J, Bergman BC, Coe GV, Baumgartner AD, Bacon S, Scherzinger A, Pyle L, Nadeau KJ. Youth With Type 1 Diabetes Have Adipose, Hepatic, and Peripheral Insulin Resistance. J Clin Endocrinol Metab. 2018;103:3647-3657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 137. | Pang TT, Narendran P. Addressing insulin resistance in Type 1 diabetes. Diabet Med. 2008;25:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 138. | Leslie RD, Taylor R, Pozzilli P. The role of insulin resistance in the natural history of type 1 diabetes. Diabet Med. 1997;14:327-331. [PubMed] [DOI] [Full Text] |
| 139. | Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 836] [Cited by in RCA: 762] [Article Influence: 47.6] [Reference Citation Analysis (5)] |
| 140. | Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M; Coronary Artery Calcification in Type 1 Diabetes Study. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? Diabetes. 2003;52:2833-2839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 141. | Greenfield JR, Samaras K, Chisholm DJ. Insulin resistance, intra-abdominal fat, cardiovascular risk factors, and androgens in healthy young women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2002;87:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 142. | Peltoniemi P, Yki-Järvinen H, Oikonen V, Oksanen A, Takala TO, Rönnemaa T, Erkinjuntti M, Knuuti MJ, Nuutila P. Resistance to exercise-induced increase in glucose uptake during hyperinsulinemia in insulin-resistant skeletal muscle of patients with type 1 diabetes. Diabetes. 2001;50:1371-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Ramalho AC, de Lourdes Lima M, Nunes F, Cambuí Z, Barbosa C, Andrade A, Viana A, Martins M, Abrantes V, Aragão C, Temístocles M. The effect of resistance versus aerobic training on metabolic control in patients with type-1 diabetes mellitus. Diabetes Res Clin Pract. 2006;72:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 144. | Dotzert MS, Murray MR, McDonald MW, Olver TD, Velenosi TJ, Hennop A, Noble EG, Urquhart BL, Melling CW. Metabolomic Response of Skeletal Muscle to Aerobic Exercise Training in Insulin Resistant Type 1 Diabetic Rats. Sci Rep. 2016;6:26379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 145. | Codella R, Adamo M, Maffi P, Piemonti L, Secchi A, Luzi L. Ultra-marathon 100 km in an islet-transplanted runner. Acta Diabetol. 2017;54:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 146. | Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 147. | Robertson K, Adolfsson P, Scheiner G, Hanas R, Riddell MC. Exercise in children and adolescents with diabetes. Pediatr Diabetes. 2009;10 Suppl 12:154-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 148. | Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol (1985). 1998;85:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 149. | Zorzano A, Balon TW, Goodman MN, Ruderman NB. Glycogen depletion and increased insulin sensitivity and responsiveness in muscle after exercise. Am J Physiol. 1986;251:E664-E669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 150. | Hall KE, McDonald MW, Grisé KN, Campos OA, Noble EG, Melling CW. The role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced Type 1 diabetic rodents. Metabolism. 2013;62:1485-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 151. | Ansell SKD, Jester M, Tryggestad JB, Short KR. A pilot study of the effects of a high-intensity aerobic exercise session on heart rate variability and arterial compliance in adolescents with or without type 1 diabetes. Pediatr Diabetes. 2020;21:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 152. | Niranjan V, McBrayer DG, Ramirez LC, Raskin P, Hsia CC. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am J Med. 1997;103:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 153. | Jensen-Urstad K, Reichard P, Jensen-Urstad M. Decreased heart rate variability in patients with type 1 diabetes mellitus is related to arterial wall stiffness. J Intern Med. 1999;245:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 154. | Roy MS, Janal MN, Crosby J, Donnelly R. Plasma markers of inflammation and prediction of cardiovascular disease and mortality in African Americans with type 1 diabetes. Diabetes Res Clin Pract. 2016;114:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Tielemans SM, Soedamah-Muthu SS, De Neve M, Toeller M, Chaturvedi N, Fuller JH, Stamatakis E. Association of physical activity with all-cause mortality and incident and prevalent cardiovascular disease among patients with type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetologia. 2013;56:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 156. | Tikkanen-Dolenc H, Wadén J, Forsblom C, Harjutsalo V, Thorn LM, Saraheimo M, Elonen N, Rosengård-Bärlund M, Gordin D, Tikkanen HO, Groop PH; FinnDiane Study Group. Frequent and intensive physical activity reduces risk of cardiovascular events in type 1 diabetes. Diabetologia. 2017;60:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 157. | Quirk H, Blake H, Tennyson R, Randell TL, Glazebrook C. Physical activity interventions in children and young people with Type 1 diabetes mellitus: a systematic review with meta-analysis. Diabet Med. 2014;31:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 158. | Perry TL, Mann JI, Lewis-Barned NJ, Duncan AW, Waldron MA, Thompson C. Lifestyle intervention in people with insulin-dependent diabetes mellitus (IDDM). Eur J Clin Nutr. 1997;51:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 159. | Burr JF, Shephard RJ, Riddell MC. Physical activity in type 1 diabetes mellitus: assessing risks for physical activity clearance and prescription. Can Fam Physician. 2012;58:533-535. [PubMed] |
| 160. | Mason NJ, Jenkins AJ, Best JD, Rowley KG. Exercise frequency and arterial compliance in non-diabetic and type 1 diabetic individuals. Eur J Cardiovasc Prev Rehabil. 2006;13:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 161. | Gusso S, Pinto TE, Baldi JC, Robinson E, Cutfield WS, Hofman PL. Diastolic function is reduced in adolescents with type 1 diabetes in response to exercise. Diabetes Care. 2012;35:2089-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 162. | Heyman E, Delamarche P, Berthon P, Meeusen R, Briard D, Vincent S, DeKerdanet M, Delamarche A. Alteration in sympathoadrenergic activity at rest and during intense exercise despite normal aerobic fitness in late pubertal adolescent girls with type 1 diabetes. Diabetes Metab. 2007;33:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 163. | Brazeau AS, Leroux C, Mircescu H, Rabasa-Lhoret R. Physical activity level and body composition among adults with type 1 diabetes. Diabet Med. 2012;29:e402-e408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 164. | Davison GW, George L, Jackson SK, Young IS, Davies B, Bailey DM, Peters JR, Ashton T. Exercise, free radicals, and lipid peroxidation in type 1 diabetes mellitus. Free Radic Biol Med. 2002;33:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 165. | Krause Mda S, de Bittencourt PI Jr. Type 1 diabetes: can exercise impair the autoimmune event? Cell Biochem Funct. 2008;26:406-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 166. | Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8:201-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 748] [Article Influence: 106.9] [Reference Citation Analysis (1)] |
| 167. | Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992-1999. Diabetologia. 2006;49:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 168. | Kohl HW, Gordon NF, Villegas JA, Blair SN. Cardiorespiratory fitness, glycemic status, and mortality risk in men. Diabetes Care. 1992;15:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 169. | Brindisi MC, Bouillet B, Vergès B, Halimi S. Cardiovascular complications in type 1 diabetes mellitus. Diabetes Metab. 2010;36:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 170. | Chillarón JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014;63:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 171. | Silva DAS, Naghavi M, Duncan BB, Schmidt MI, de Souza MFM, Malta DC. Physical inactivity as risk factor for mortality by diabetes mellitus in Brazil in 1990, 2006, and 2016. Diabetol Metab Syndr. 2019;11:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 172. | Li CL, Chang HY, Hsu CC, Lu JF, Fang HL. Joint predictability of health related quality of life and leisure time physical activity on mortality risk in people with diabetes. BMC Public Health. 2013;13:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 173. | Tikkanen-Dolenc H, Wadén J, Forsblom C, Harjutsalo V, Thorn LM, Saraheimo M, Elonen N, Tikkanen HO, Groop PH; FinnDiane Study Group. Physical Activity Reduces Risk of Premature Mortality in Patients With Type 1 Diabetes With and Without Kidney Disease. Diabetes Care. 2017;40:1727-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 174. | Ramalho AC, Soares S. [The role of exercise in the treatment of type 1 diabetes]. Arq Bras Endocrinol Metabol. 2008;52:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 175. | Moy CS, Songer TJ, LaPorte RE, Dorman JS, Kriska AM, Orchard TJ, Becker DJ, Drash AL. Insulin-dependent diabetes mellitus, physical activity, and death. Am J Epidemiol. 1993;137:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 176. | Colberg SR, Laan R, Dassau E, Kerr D. Physical activity and type 1 diabetes: time for a rewire? J Diabetes Sci Technol. 2015;9:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 177. | Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31:2108-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 425] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 178. | Liese AD, Ma X, Maahs DM, Trilk JL. Physical activity, sedentary behaviors, physical fitness, and their relation to health outcomes in youth with type 1 and type 2 diabetes: A review of the epidemiologic literature. J Sport Health Sci. 2013;2:21-38. [DOI] [Full Text] |
| 179. | McGill DE, Levitsky LL. Management of Hypoglycemia in Children and Adolescents with Type 1 Diabetes Mellitus. Curr Diab Rep. 2016;16:88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 180. | Cockcroft EJ, Narendran P, Andrews RC. Exercise-induced hypoglycaemia in type 1 diabetes. Exp Physiol. 2020;105:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 181. | Camacho RC, Galassetti P, Davis SN, Wasserman DH. Glucoregulation during and after exercise in health and insulin-dependent diabetes. Exerc Sport Sci Rev. 2005;33:17-23. [PubMed] |
| 182. | McMahon SK, Ferreira LD, Ratnam N, Davey RJ, Youngs LM, Davis EA, Fournier PA, Jones TW. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab. 2007;92:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 183. | Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of antecedent hypoglycemia on counterregulatory responses to subsequent euglycemic exercise in type 1 diabetes. Diabetes. 2003;52:1761-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 184. | Riddell M, Perkins BA. Exercise and glucose metabolism in persons with diabetes mellitus: perspectives on the role for continuous glucose monitoring. J Diabetes Sci Technol. 2009;3:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 185. | Felig P, Cherif A, Minagawa A, Wahren J. Hypoglycemia during prolonged exercise in normal men. N Engl J Med. 1982;306:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 140] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 186. | Francescato MP, Ajčević M, Accardo A. Carbohydrate Requirement for Exercise in Type 1 Diabetes: Effects of Insulin Concentration. J Diabetes Sci Technol. 2020;14:1116-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 187. | Brockman NK, Yardley JE. Sex-related differences in fuel utilization and hormonal response to exercise: implications for individuals with type 1 diabetes. Appl Physiol Nutr Metab. 2018;43:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 188. | Galassetti P, Tate D, Neill RA, Morrey S, Davis SN. Effect of gender on counterregulatory responses to euglycemic exercise in type 1 diabetes. J Clin Endocrinol Metab. 2002;87:5144-5150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 189. | Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of sex on counterregulatory responses to exercise after antecedent hypoglycemia in type 1 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E16-E24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 190. | Riddell MC, Zaharieva DP, Tansey M, Tsalikian E, Admon G, Li Z, Kollman C, Beck RW. Individual glucose responses to prolonged moderate intensity aerobic exercise in adolescents with type 1 diabetes: The higher they start, the harder they fall. Pediatr Diabetes. 2019;20:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 191. | Scott SN, Cocks M, Andrews RC, Narendran P, Purewal TS, Cuthbertson DJ, Wagenmakers AJM, Shepherd SO. High-Intensity Interval Training Improves Aerobic Capacity Without a Detrimental Decline in Blood Glucose in People With Type 1 Diabetes. J Clin Endocrinol Metab. 2019;104:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 192. | Aronson R, Brown RE, Li A, Riddell MC. Optimal Insulin Correction Factor in Post-High-Intensity Exercise Hyperglycemia in Adults With Type 1 Diabetes: The FIT Study. Diabetes Care. 2019;42:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 193. | Fahey AJ, Paramalingam N, Davey RJ, Davis EA, Jones TW, Fournier PA. The effect of a short sprint on postexercise whole-body glucose production and utilization rates in individuals with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:4193-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 194. | Lascar N, Kennedy A, Hancock B, Jenkins D, Andrews RC, Greenfield S, Narendran P. Attitudes and barriers to exercise in adults with type 1 diabetes (T1DM) and how best to address them: a qualitative study. PLoS One. 2014;9:e108019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 195. | Sinclair AJ, Dunning T, Dhatariya K; an International Group of Experts. Clinical guidelines for type 1 diabetes mellitus with an emphasis on older adults: an Executive Summary. Diabet Med. 2020;37:53-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 196. | Santeusanio F, Di Loreto C, Lucidi P, Murdolo G, De Cicco A, Parlanti N, Piccioni F, De Feo P. Diabetes and exercise. J Endocrinol Invest. 2003;26:937-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 197. | Bernardini AL, Vanelli M, Chiari G, Iovane B, Gelmetti C, Vitale R, Errico MK. Adherence to physical activity in young people with type 1 diabetes. Acta Biomed. 2004;75:153-157. [PubMed] |
| 198. | Ajčević M, Candido R, Assaloni R, Accardo A, Francescato MP. Personalized Approach for the Management of Exercise-Related Glycemic Imbalances in Type 1 Diabetes: Comparison with Reference Method. J Diabetes Sci Technol. 2021;15:1153-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 199. | Yamanouchi K, Abe R, Takeda A, Atsumi Y, Shichiri M, Sato Y. The effect of walking before and after breakfast on blood glucose levels in patients with type 1 diabetes treated with intensive insulin therapy. Diabetes Res Clin Pract. 2002;58:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 200. | Yardley JE, Kenny GP, Perkins BA, Riddell MC, Malcolm J, Boulay P, Khandwala F, Sigal RJ. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care. 2012;35:669-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 201. | Turner D, Luzio S, Gray BJ, Dunseath G, Rees ED, Kilduff LP, Campbell MD, West DJ, Bain SC, Bracken RM. Impact of single and multiple sets of resistance exercise in type 1 diabetes. Scand J Med Sci Sports. 2015;25:e99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 202. | Campbell MD, Walker M, Trenell MI, Stevenson EJ, Turner D, Bracken RM, Shaw JA, West DJ. A low-glycemic index meal and bedtime snack prevents postprandial hyperglycemia and associated rises in inflammatory markers, providing protection from early but not late nocturnal hypoglycemia following evening exercise in type 1 diabetes. Diabetes Care. 2014;37:1845-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 203. | Soon WHK, Guelfi KJ, Davis EA, Smith GJ, Jones TW, Fournier PA. Effect of combining pre-exercise carbohydrate intake and repeated short sprints on the blood glucose response to moderate-intensity exercise in young individuals with Type 1 diabetes. Diabet Med. 2019;36:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 204. | Dizon S, Malcolm J, Rowan M, Keely EJ. Patient Perspectives on Managing Type 1 Diabetes During High-Performance Exercise: What Resources Do They Want? Diabetes Spectr. 2019;32:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 205. | Iscoe KE, Riddell MC. Continuous moderate-intensity exercise with or without intermittent high-intensity work: effects on acute and late glycaemia in athletes with Type 1 diabetes mellitus. Diabet Med. 2011;28:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 206. | McAuley SA, Horsburgh JC, Ward GM, La Gerche A, Gooley JL, Jenkins AJ, MacIsaac RJ, O'Neal DN. Insulin pump basal adjustment for exercise in type 1 diabetes: a randomised crossover study. Diabetologia. 2016;59:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 207. | Galassetti P, Riddell MC. Exercise and Type 1 Diabetes (T1DM). In: Terjung R. Comprehensive Physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2013: c110040. |
| 208. | Adamo M, Codella R, Casiraghi F, Ferrulli A, Macrì C, Bazzigaluppi E, Terruzzi I, Inverardi L, Ricordi C, Luzi L. Active Subjects With Autoimmune Type 1 Diabetes Have Better Metabolic Profiles Than Sedentary Controls. Cell Transplant. 2017;26:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 209. | Schiffrin A, Parikh S, Marliss EB, Desrosiers MM. Metabolic response to fasting exercise in adolescent insulin-dependent diabetic subjects treated with continuous subcutaneous insulin infusion and intensive conventional therapy. Diabetes Care. 1984;7:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 210. | Roy-Fleming A, Taleb N, Messier V, Suppère C, Cameli C, Elbekri S, Smaoui MR, Ladouceur M, Legault L, Rabasa-Lhoret R. Timing of insulin basal rate reduction to reduce hypoglycemia during late post-prandial exercise in adults with type 1 diabetes using insulin pump therapy: A randomized crossover trial. Diabetes Metab. 2019;45:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 211. | Diabetes Research in Children Network (DirecNet) Study Group; Diabetes Research in Children Network (DirecNet) Study Group, Tsalikian E, Kollman C, Tamborlane WB, Beck RW, Fiallo-Scharer R, Fox L, Janz KF, Ruedy KJ, Wilson D, Xing D, Weinzimer SA. Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care. 2006;29:2200-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 212. | Hall B, Zebrowska A, Kaminski T, Stanula A, Robins A. Effects of Hypoxia during Continuous and Intermittent Exercise on Glycaemic Control and Selected Markers of Vascular Function in Type 1 Diabetes. Exp Clin Endocrinol Diabetes. 2018;126:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 213. | Scott SN, Shepherd SO, Strauss JA, Wagenmakers AJM, Cocks M. Home-based high-intensity interval training reduces barriers to exercise in people with type 1 diabetes. Exp Physiol. 2020;105:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 214. | Tagougui S, Taleb N, Rabasa-Lhoret R. The Benefits and Limits of Technological Advances in Glucose Management Around Physical Activity in Patients Type 1 Diabetes. Front Endocrinol (Lausanne). 2018;9:818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 215. | Moser O, Mader JK, Tschakert G, Mueller A, Groeschl W, Pieber TR, Koehler G, Messerschmidt J, Hofmann P. Accuracy of Continuous Glucose Monitoring (CGM) during Continuous and High-Intensity Interval Exercise in Patients with Type 1 Diabetes Mellitus. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 216. | Maran A, Pavan P, Bonsembiante B, Brugin E, Ermolao A, Avogaro A, Zaccaria M. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther. 2010;12:763-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 217. | Burckhardt MA, Chetty T, Smith GJ, Adolfsson P, de Bock M, Jones TW, Davis EA. Use of Continuous Glucose Monitoring Trends to Facilitate Exercise in Children with Type 1 Diabetes. Diabetes Technol Ther. 2019;21:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 218. | Jamiołkowska M, Jamiołkowska I, Łuczyński W, Tołwińska J, Bossowski A, Głowińska Olszewska B. Impact of Real-Time Continuous Glucose Monitoring Use on Glucose Variability and Endothelial Function in Adolescents with Type 1 Diabetes: New Technology--New Possibility to Decrease Cardiovascular Risk? J Diabetes Res. 2016;2016:4385312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 219. | Riddell MC, Milliken J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther. 2011;13:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 220. | Sherr JL, Cengiz E, Palerm CC, Clark B, Kurtz N, Roy A, Carria L, Cantwell M, Tamborlane WV, Weinzimer SA. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care. 2013;36:2909-2914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 221. | de Bock M, Dart J, Roy A, Davey R, Soon W, Berthold C, Retterath A, Grosman B, Kurtz N, Davis E, Jones T. Exploration of the Performance of a Hybrid Closed Loop Insulin Delivery Algorithm That Includes Insulin Delivery Limits Designed to Protect Against Hypoglycemia. J Diabetes Sci Technol. 2017;11:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 222. | Breton MD, Brown SA, Karvetski CH, Kollar L, Topchyan KA, Anderson SM, Kovatchev BP. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 223. | Sonnenberg GE, Kemmer FW, Berger M. Exercise in type 1 (insulin-dependent) diabetic patients treated with continuous subcutaneous insulin infusion. Prevention of exercise induced hypoglycaemia. Diabetologia. 1990;33:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 224. | Rabasa-Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care. 2001;24:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 225. | Dubé MC, Weisnagel SJ, Prud'homme D, Lavoie C. Exercise and newer insulins: how much glucose supplement to avoid hypoglycemia? Med Sci Sports Exerc. 2005;37:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 226. | Bussau VA, Ferreira LD, Jones TW, Fournier PA. The 10-s maximal sprint: a novel approach to counter an exercise-mediated fall in glycemia in individuals with type 1 diabetes. Diabetes Care. 2006;29:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 227. | Bussau VA, Ferreira LD, Jones TW, Fournier PA. A 10-s sprint performed prior to moderate-intensity exercise prevents early post-exercise fall in glycaemia in individuals with type 1 diabetes. Diabetologia. 2007;50:1815-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 228. | West DJ, Morton RD, Bain SC, Stephens JW, Bracken RM. Blood glucose responses to reductions in pre-exercise rapid-acting insulin for 24 h after running in individuals with type 1 diabetes. J Sports Sci. 2010;28:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 229. | Taplin CE, Cobry E, Messer L, McFann K, Chase HP, Fiallo-Scharer R. Preventing post-exercise nocturnal hypoglycemia in children with type 1 diabetes. J Pediatr. 2010;157:784-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 230. | Garg S, Brazg RL, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, Shin J, Welsh JB, Kaufman FR. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technol Ther. 2012;14:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 231. | Yardley JE, Sigal RJ, Perkins BA, Riddell MC, Kenny GP. Resistance exercise in type 1 diabetes. Can J Diabetes. 2013;37:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 232. | Campbell MD, Walker M, Trenell MI, Jakovljevic DG, Stevenson EJ, Bracken RM, Bain SC, West DJ. Large pre- and postexercise rapid-acting insulin reductions preserve glycemia and prevent early- but not late-onset hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2013;36:2217-2224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 233. | Schiavon M, Dalla Man C, Kudva YC, Basu A, Cobelli C. In silico optimization of basal insulin infusion rate during exercise: implication for artificial pancreas. J Diabetes Sci Technol. 2013;7:1461-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 234. | Danne T, Tsioli C, Kordonouri O, Blaesig S, Remus K, Roy A, Keenan B, Lee SW, Kaufman FR. The PILGRIM study: in silico modeling of a predictive low glucose management system and feasibility in youth with type 1 diabetes during exercise. Diabetes Technol Ther. 2014;16:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 235. | Campbell MD, Walker M, Bracken RM, Turner D, Stevenson EJ, Gonzalez JT, Shaw JA, West DJ. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2015;3:e000085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 236. | Cherubini V, Gesuita R, Skrami E, Rabbone I, Bonfanti R, Arnaldi C, D'Annunzio G, Frongia A, Lombardo F, Piccinno E, Schiaffini R, Toni S, Tumini S, Tinti D, Cipriano P, Minuto N, Lenzi L, Ferrito L, Ventrici C, Ortolani F, Cohen O, Scaramuzza A. Optimal predictive low glucose management settings during physical exercise in adolescents with type 1 diabetes. Pediatr Diabetes. 2019;20:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 237. | Moser O, Eckstein ML, Mueller A, Birnbaumer P, Aberer F, Koehler G, Sourij C, Kojzar H, Pferschy P, Dietz P, Bracken RM, Hofmann P, Sourij H. Pre-Exercise Blood Glucose Levels Determine the Amount of Orally Administered Carbohydrates during Physical Exercise in Individuals with Type 1 Diabetes-A Randomized Cross-Over Trial. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 238. | Zaharieva DP, McGaugh S, Pooni R, Vienneau T, Ly T, Riddell MC. Improved Open-Loop Glucose Control With Basal Insulin Reduction 90 Minutes Before Aerobic Exercise in Patients With Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion. Diabetes Care. 2019;42:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 239. | Moser O, Eckstein ML, Mueller A, Birnbaumer P, Aberer F, Koehler G, Sourij C, Kojzar H, Holler P, Simi H, Pferschy P, Dietz P, Bracken RM, Hofmann P, Sourij H. Reduction in insulin degludec dosing for multiple exercise sessions improves time spent in euglycaemia in people with type 1 diabetes: A randomized crossover trial. Diabetes Obes Metab. 2019;21:349-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 240. | Zaharieva DP, Cinar A, Yavelberg L, Jamnik V, Riddell MC. No Disadvantage to Insulin Pump Off vs Pump On During Intermittent High-Intensity Exercise in Adults With Type 1 Diabetes. Can J Diabetes. 2020;44:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 241. | Pivovarov JA, Taplin CE, Riddell MC. Current perspectives on physical activity and exercise for youth with diabetes. Pediatr Diabetes. 2015;16:242-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 242. | Williams JE, Helsel B, Nelson B, Eke R. Exercise Considerations for Type 1 and Type 2 Diabetes. ACSMʼs Health & Fitness Journal. 2018;22:10-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 243. | Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? Diabetologia. 2012;55:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 244. | Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, Kowalski A, Rabasa-Lhoret R, McCrimmon RJ, Hume C, Annan F, Fournier PA, Graham C, Bode B, Galassetti P, Jones TW, Millán IS, Heise T, Peters AL, Petz A, Laffel LM. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 593] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 245. | Pongrac Barlovic D, Tikkanen-Dolenc H, Groop PH. Physical Activity in the Prevention of Development and Progression of Kidney Disease in Type 1 Diabetes. Curr Diab Rep. 2019;19:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 246. | Baevre H, Søvik O, Wisnes A, Heiervang E. Metabolic responses to physical training in young insulin-dependent diabetics. Scand J Clin Lab Invest. 1985;45:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 247. | Farinha JB, Boff W, Dos Santos GC, Boeno FP, Ramis TR, Vieira AF, Macedo RCO, Rodrigues-Krause J, Reischak-Oliveira A. Acute glycemic responses along 10-week high-intensity training protocols in type 1 diabetes patients. Diabetes Res Clin Pract. 2019;153:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 248. | American Diabetes Association. Physical activity/exercise and diabetes. Diabetes Care. 2004;27 Suppl 1:S58-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 249. | Guelfi KJ, Jones TW, Fournier PA. Intermittent high-intensity exercise does not increase the risk of early postexercise hypoglycemia in individuals with type 1 diabetes. Diabetes Care. 2005;28:416-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 250. | Guelfi KJ, Ratnam N, Smythe GA, Jones TW, Fournier PA. Effect of intermittent high-intensity compared with continuous moderate exercise on glucose production and utilization in individuals with type 1 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E865-E870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 251. | Dash K, Goyder EC, Quirk H. A qualitative synthesis of the perceived factors that affect participation in physical activity among children and adolescents with type 1 diabetes. Diabet Med. 2020;37:934-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Ethiopia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Plagens-Rotman K, Shalaby MN S-Editor: Zhang H L-Editor: Webster JR P-Editor: Zhang H