Published online Nov 15, 2022. doi: 10.4239/wjd.v13.i11.972
Peer-review started: August 16, 2022
First decision: September 4, 2022
Revised: September 15, 2022
Accepted: October 11, 2022
Article in press: October 11, 2022
Published online: November 15, 2022
Processing time: 87 Days and 8.5 Hours
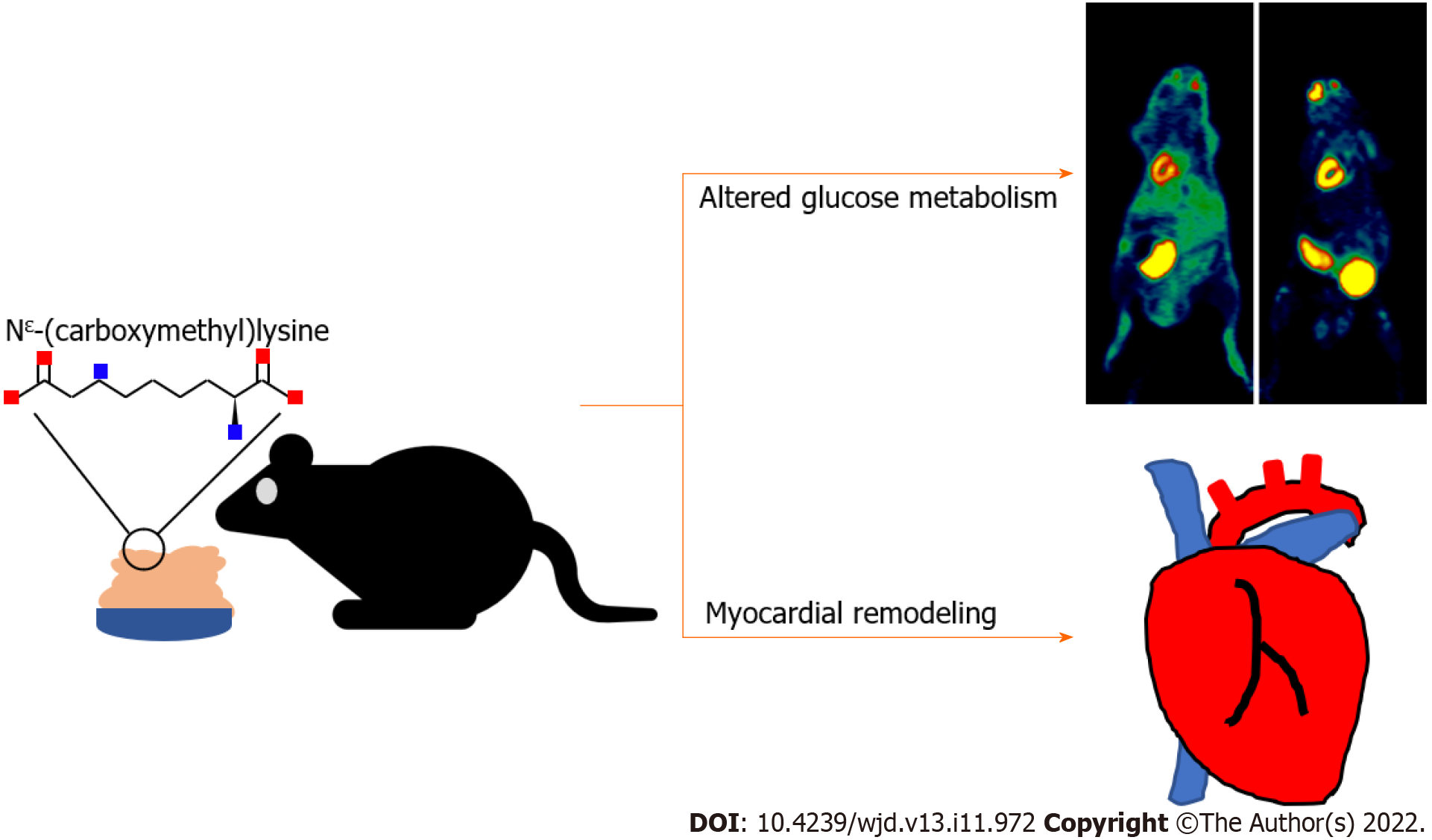
Myocardial remodeling is a key factor in the progression of cardiovascular disease to the end stage. In addition to myocardial infarction or stress overload, dietary factors have recently been considered associated with myocardial remodeling. Nε-(carboxymethyl)lysine (CML) is a representative foodborne toxic product, which can be ingested via daily diet. Therefore, there is a marked need to explore the effects of dietary CML on the myocardium.
To explore the effects of dietary CML (dCML) on the heart.
C57 BL/6 mice were divided into a control group and a dCML group. The control group and the dCML group were respectively fed a normal diet or diet supplemented with CML for 20 wk. Body weight and blood glucose were recorded every 4 wk. 18F-fluorodeoxyglucose (FDG) was used to trace the glucose uptake in mouse myocardium, followed by visualizing with micro-positron emission tomography (PET). Myocardial remodeling and glucose metabolism were also detected. In vitro, H9C2 cardiomyocytes were added to exogenous CML and cultured for 24 h. The effects of exogenous CML on glucose metabolism, collagen I expression, hypertrophy, and apoptosis of cardiomyocytes were analyzed.
Our results suggest that the levels of fasting blood glucose, fasting insulin, and serum CML were significantly increased after 20 wk of dCML. Micro-PET showed that 18F-FDG accumulated more in the myocardium of the dCML group than in the control group. Histological staining revealed that dCML could lead to myocardial fibrosis and hypertrophy. The indexes of myocardial fibrosis, apoptosis, and hypertrophy were also increased in the dCML group, whereas the activities of glucose metabolism-related pathways and citrate synthase (CS) were significantly inhibited. In cardiomyocytes, collagen I expression and cellular size were significantly increased after the addition of exogenous CML. CML significantly promoted cellular hypertrophy and apoptosis, while pathways involved in glucose metabolism and level of Cs mRNA were significantly inhibited.
This study reveals that dCML alters myocardial glucose metabolism and promotes myocardial remodeling.
Core Tip: Nε-(carboxymethyl)lysine (CML) exists in daily diet and is harmful to health. We established in vitro and in vivo models to investigate the effects of dietary CML (dCML) on the heart. We found that long-term dCML induced insulin resistance and elevated serum CML level. 18F-fluorodeoxyglucose imaging indicated that dCML promoted myocardial glucose uptake, but the glucose metabolism was disrupted. Myocardial fibrosis, apoptosis, and hypertrophy were significantly enhanced by dCML. In the cell model, CML supplementation promoted cardiomyocyte apoptosis, cellular hypertrophy, and collagen I expression, and also inhibited pathways involved in glucose metabolism.
- Citation: Wang ZQ, Sun Z. Dietary Nε-(carboxymethyl) lysine affects cardiac glucose metabolism and myocardial remodeling in mice. World J Diabetes 2022; 13(11): 972-985
- URL: https://www.wjgnet.com/1948-9358/full/v13/i11/972.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i11.972
Cardiovascular disease is the leading cause of mortality worldwide[1]. Myocardial remodeling can lead to decreased cardiac function, which is an important factor in increasing mortality due to cardiovascular disease[2]. Cardiac remodeling is characterized by cardiomyocyte hypertrophy, apoptosis, fibrosis, and increased fibrocollagen deposition[3]. Short-term compensatory remodeling increases cardiac contractility, whereas long-term sustained pathological remodeling leads to a decline in cardiac function or even heart failure[4]. Cardiac remodeling can be caused by myocardial infarction, stress overload, inflammatory cardiomyopathy, idiopathic dilated cardiomyopathy, or diabetes[5]. Although these causes differ, they share similar mechanisms such as oxidative stress, endoplasmic reticulum stress, and inflammatory response[6]. Attempts have been made to improve myocardial remodeling with drugs or other interventions, albeit with unsatisfactory results[7].
Recent studies have shown that in addition to diseases such as diabetes and coronary heart disease, foodborne factors are also associated with myocardial remodeling[8]. Nakamura et al[9] found that carbohydrate content in the diet could affect myocardial remodeling. Zeng et al[10] reported that a high-fat diet promoted myocardial remodeling. Therefore, it is important to identify the key pathogenic components in the diet for the prevention and treatment of myocardial remodeling.
Advanced glycation end products (AGEs) are a class of heterogeneous irreversible products formed by non-enzymatic reactions[11]. AGEs can accumulate in various tissues resulting in adverse health effects by increasing disease pathogenesis[12,13]. AGEs can accumulate via endogenous and exogenous mechanisms. Food is the main source of exogenous AGEs[14]. Nε-(carboxymethyl)lysine (CML) is considered a representative of food-derived AGEs[15]. CML has been found in a variety of foods such as milk, bakery products, and coffee. Ahmed et al[16] reported that the concentration of CML is 877 ± 47 nM in pasteurized milk. Assar et al[17] reported that bread crust contains 46.1 mg/kg of CML. Ingestion of CML via routine diet is substantially higher than the level of CML in plasma and tissues[18]. Daily exposure to high levels of CML is a health risk for humans[19]. The dietary intake of CML is positively correlated with prevalent vertebral fractures[20]. Studies have also shown that foodborne CML can accelerate the progression of atherosclerosis, Alzheimer’s disease, and other diseases[21]. Our previous studies reported that long-term exposure to CML leads to osteogenic differentiation of vascular smooth muscle cells and calcification in diabetic plaques[12]. Exogenous CML can lead to the continuous evolution of atherosclerotic plaques[22]. However, it is currently unknown whether CML intake affects myocardial remodeling.
Glucose metabolism is an important energy source for heart activities. Physiologically, cardio-myocytes uptake and transport glucose via the glucose transporter (Glut) family and obtain energy via aerobic glucose oxidation[23]. Under pathological conditions such as myocardial infarction, glucose metabolism is altered and glucose uptake is increased to counteract the decline in cardiac function[24]. Impaired glucose metabolism is an independent risk factor for the progression of heart failure[25]. However, whether foodborne factors have an impact on myocardial glucose metabolism needs further study.
In the present study, we hypothesized that dietary CML (dCML) can lead to myocardial glucose metabolism dysfunction and myocardial remodeling. We fed mice a CML-supplemented diet and used 18F-fluorodeoxyglucose (FDG) to track the glucose uptake by the mouse myocardium in vitro and in vivo. Our study provides new insights into the relationship between dCML and myocardial remodeling.
All animal experiments were approved by the Experimental Animal Use Ethics Committee of Jiangsu University and followed the ARRIVE guidelines. Ten-wk-old male C57 BL/6 mice (Cavens, ChangZhou, China) were stored in a light:dark (12:12) cycle environment at a temperature of 26 °C and humidity of 70%. Mice were randomly divided into two groups: control (Ctrl) group and dCML group (n = 25). Mice in the Ctrl group were freely administered a standard pelleted diet (XieTong, Nanjing, China). The dCML group received a daily standard pelleted diet mixed with CML (1 g/kg)[26]. The body weight and blood glucose of mice were measured every 4 wk. Blood glucose was detected with a glucose meter (Roche, Basel, Switzerland). After 20 wk of feeding, the fasting insulin levels of the mice were detected via the enzyme-linked immunoassay (ELISA) (Mercodia, Sweden), and the homeostatic model assessment insulin resistance (HOMA-IR) was calculated [HOMA-IR = glucose (mmol/L) × insulin (mIU/L)]/22.5. Then the mice were subjected to the oral glucose tolerance test (OGTT)[27]. Each mouse was fasted for 8 h prior to the tests. During fasting, mice had access to adequate water. The serum CML level in mice was detected via ELISA (Cloud-Clone, Wuhan, China). The operation steps followed the instructions. The citrate synthase (CS) in mouse tissues was detected with the Citrate Synthase Activity Detection Kit (Solarbio, Beijing, China). The mouse tissues were homogenized and centrifuged at 11000 g for 10 min. The protein concentration of supernatants was measured using the bicinchoninic acid assay. The samples were reacted with the detection reagent for 10 s or 2 min, and the absorbance at 412 nm was measured. Then the activity was calculated and expressed as nmol/min/mg protein.
Mice were euthanized with carbon dioxide (CO2). Mouse hearts were isolated and the left ventricles were used for subsequent analysis. Masson’s trichrome staining was performed to assess the degree of myocardial fibrosis in mice using an appropriate kit (Solarbio). Myocardial glycogen accumulation was measured using the Glycogen Periodic Acid Schiff (PAS) Stain Kit (Solarbio)[28]. The cytoplasm and nuclei were stained with hematoxylin and eosin (H&E) dye according to the manufacturer’s instructions (Solarbio), followed by imaging using a microscope (Olympus, Tokyo, Japan). Areas testing positive with Masson’s trichrome and PAS stains were measured using ImageJ software.
To assess glucose uptake in the mouse myocardium in vivo, 18F-FDG was used to trace the glucose metabolism and visualized with micro-positron emission tomography (PET) (Inveon; Siemens, Munich, Germany)[29]. Mice were fasted for 12 h before scanning. The mice were weighed and anesthetized with isoflurane/oxygen mixture (15-20 mL/L). 18F-FDG (7.4 MBq, 150 μL) was injected through the tail vein. Micro-PET scanning was performed 2 h after injection for 10 min. The scanned images were iteratively reconstructed with ordered set expectation maximization three-dimensional software. The average level of radioactive material uptake in the cardiac region was analyzed using ASIProVM software. The mean of standard uptake value (SUVmean) was calculated according to the formula: SUVmean = cardiac radioactive material uptake (μCi/g)/[total injected dose (μCi)/body weight (g)]. Similarly, the in vitro micro-PET scanning of mouse hearts was also performed[30]. Two hours after the injection of 18F-FDG, the mice were euthanized with CO2 and the hearts were isolated. The isolated heart was placed in a tube filled with ultrasound gel and scanned via micro-PET. The calculation of SUVmean of isolated mouse hearts was the same as described above.
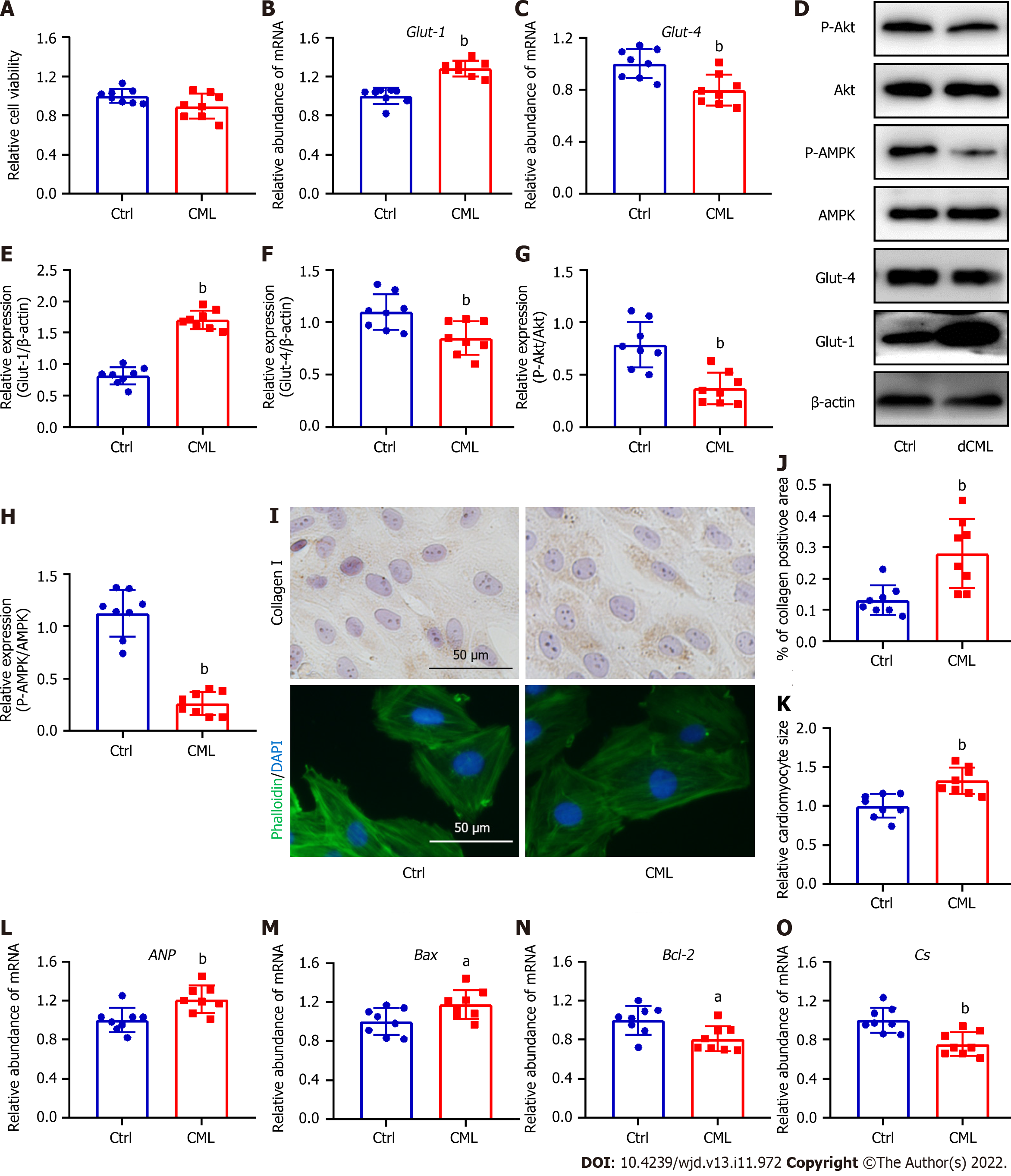
H9C2 cells (Procell, Wuhan, China) were cultured in Dulbecco’s Modified Eagle Medium supplemented with 100 mL/L fetal bovine serum[31]. Cells were divided into a Ctrl group and a CML group, followed by seeding of 2 × 105 cells and addition of 10 mmol/L CML to the CML group, and incubated for 24 h. Cell viability was detected with Cell Counting Kit-8 (C0037; Beyotime, Shanghai, China). All cells were cultured at 37 °C with 50 mL/L CO2. Collagen I content in H9C2 cells was assessed via immunocytochemical staining. The SP Rabbit & Mouse HRP Kit (CoWin Century, Beijing, China) was used for immunocytochemical staining. Briefly, after fixing in 40 g/L paraformaldehyde for 30 min, the cell samples were washed with phosphate-buffered saline. The samples were incubated with 100 mL/L goat serum and 2.5 mL/L Triton X-100 at room temperature, followed by incubation with primary and secondary antibodies. The primary antibody used for the staining was: anti-collagen I (1:500, 14695-1-AP; Proteintech, Rosemont, IL, United States). To assess the cardiomyocyte area, cells were labeled with Phalloidin (P5282; Sigma-Aldrich, St. Louis, MO, United States). After fixing in 40 g/L paraformaldehyde, the cells were incubated with 2.5 mL/L Triton X-100 for 15 min, followed by labeling with Phalloidin (5 μmol/L) to analyze cell morphology. The nuclei were subsequently stained with DAPI. All stained images were acquired under a microscope (Olympus) and quantified with a computer-assisted image analysis system. Six high-resolution fields in each independent experiment were randomly selected and the area of at least 100 cells was calculated.
The experimental steps were performed as previously described[32]. Protein samples were prepared using RIPA lysis buffer supplemented with protease and phosphatase inhibitors (Beyotime). Proteins were transferred to PVDF membranes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After blocking in 50 g/L milk powder for 1 h, the membranes were incubated with suitable diluted primary antibodies overnight. The membranes were incubated with horseradish peroxidase-labeled secondary antibodies and imaged using a chemiluminescence system (Amersham Imager 600; GE Healthcare, Chicago, IL, United States). The acquired images were analyzed using ImageJ software. Primary antibodies used for western blotting were anti-CML (1:1000, ab125145; Abcam, Cambridge, United Kingdom), anti-collagen I (1:500, 14695-1-AP; Proteintech), anti-Glut-1 (1:2000, 21829-1-AP; Proteintech), anti-Glut-4 (1:2000, 66846-1-Ig; Proteintech), anti-Akt (1:5000, 10176-2-AP; Proteintech), anti-B-cell leukemia/lymphoma 2 (Bcl-2) (1:2000, 26593-1-AP; Proteintech), anti-Bcl-2-associated X, apoptosis regulator (BAX) (1:2000, 50599-2-Ig; Proteintech), anti-Akt (phospho-Ser473; 1:5000, 66444-1-Ig; Proteintech), anti-AMP-activated protein kinase (AMPK) (1:5000, 66536-1-Ig; Proteintech), anti-phospho-AMPK (phospho-Thr183 and Thr172; 1:5000, ab133448; Abcam) and anti-β-actin (1:2000, ET1702-67; HUABIO, Hangzhou, China).
Total RNA from tissues/cells was obtained with the RNA-Quick Purification Kit (ES-RN001; YISHAN BIOTECH, Shanghai, China). The mRNA was reverse-transcribed into cDNA using a reverse transcriptase kit (R222; Vazyme Biotech, Nanjing, China)[33]. The SYBR qPCR Master Mix Kit (Q311, Vazyme Biotech) was used to detect the level of each gene. All experimental operations were carried out according to the manufacturer’s instructions. The primer sequences are shown in Table 1.
| Gene | Forward, 5’-3’ | Reverse, 5’-3’ |
| Bax (mouse) | GAACAGATCATGAAGACAGGG | CAGTTCATCTCCAATTCGCC |
| Bcl-2 (mouse) | AGGGGGAAACACCAGAATC | GGTAGCGACGAGAGAAGT |
| ANP (mouse) | CCTGTGTACAGTGCGGTGTC | CCTAGAAGCACTGCCGTCTC |
| Glut-1 (mouse) | ACGCCCCCCAGAAGGTTAT | GCGTGGTGAGTGTGGTGGAT |
| Glut-4 (mouse) | TTGCACACGGCTTCCGAACG | GATCTGCTGGAAACCCGACGG |
| β-actin (mouse) | TCTTGGGTATGGAATCCTGTG | ATCTCCTTCTGCATCCTGTCA |
| Bax (rat) | TGCAGAGGATGATTGCTGAC | GATCAGCTCGGGCACTTTAG |
| Bcl-2 (rat) | AGTGGGATGCGGGAGATGTG | GGGGCCGTACAGTTCCACAA |
| ANP (rat) | CCGTATACAGTGCGGTGTCCAAC | TCATCGGTCTGCTCGCTCAGG |
| Glut-1 (rat) | GCCTGAGACCAGTTGAAAGCAC | CTGCTTAGGTAAAGTTACAGGAG |
| Glut-4 (rat) | AGGCACCCTCACTACCCTTT | AGCATAGCCCTTTTCCTTCC |
| Cs (rat) | GGAACACACTCAACTCGGGA | ACCCCACTGTGAGCATCTACG |
| β-actin (rat) | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
All data were presented as the mean ± SD. The differences between the two groups were analyzed with the Student’s t-test. Statistical significance was set at P < 0.05. All data were analyzed using SPSS 22.0 software.
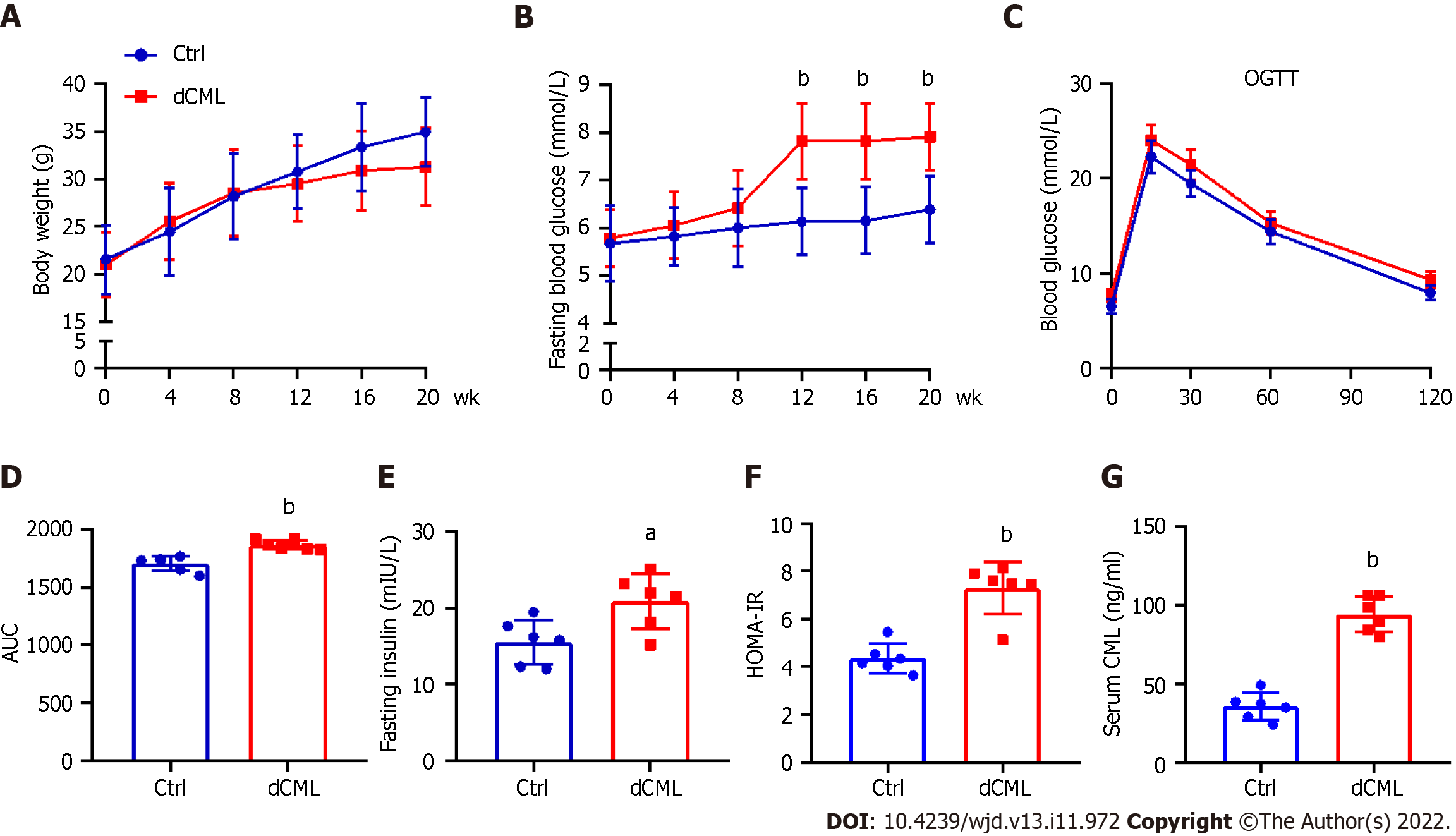
After feeding the mice a diet supplemented with CML, we assessed their body weight and blood glucose every 4 wk until week 20. Body weight of the Ctrl group increased with feeding time, whereas the weight gain of the dCML group slowed down from the 8th wk of feeding (Figure 1A). There was no significant difference in body weight between the Ctrl group and the dCML group. From the 12th wk of feeding, the fasting blood glucose of mice in the dCML group was significantly higher than in the Ctrl group (Figure 1B). Further, we detected the glucose tolerance of mice. The OGTT test showed that the blood glucose AUC of the dCML group was significantly increased compared with the Ctrl group, suggesting impaired glucose tolerance of the dCML group (Figure 1C and D). There were significant differences in fasting insulin and HOMA-IR values between the two groups of mice. The fasting insulin and HOMA-IR levels of the dCML group were higher than those of the Ctrl group, suggesting insulin resistance in the dCML group (Figure 1E and F). Similarly, the serum CML level of mice in the dCML group was also significantly increased due to the consumption of CML, which was 2.6-fold higher than that in the Ctrl group (Figure 1G).
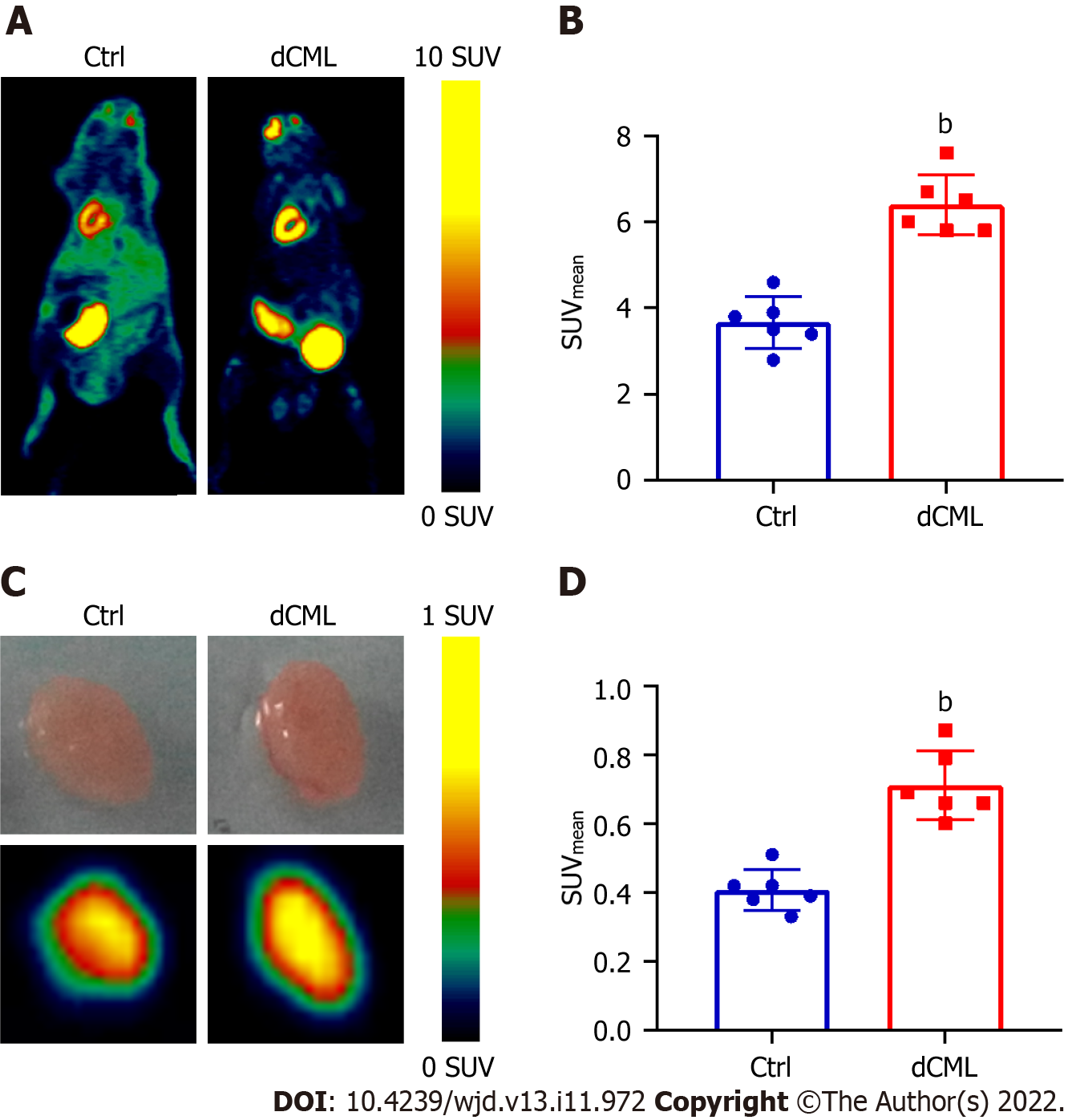
18F-FDG is a glucose analog that can be used to track the glucose uptake in tissues and organs. Micro-PET scanning was performed to detect the uptake of 18F-FDG in the mouse myocardium. Compared with the Ctrl group, the myocardial SUVmean of the dCML group was significantly increased (6.40 ± 0.70 vs 3.67 ± 0.60; P < 0.01) (Figure 2A and B). Then the mouse hearts were isolated and detected via micro-PET scanning in vitro. The SUVmean of hearts in the dCML group was still significantly higher than that in the Ctrl group (0.71 ± 0.10 vs 0.41 ± 0.06; P < 0.01) (Figure 2C and D), suggesting that the glucose uptake of the myocardium was increased after supplementation with the CML diet.
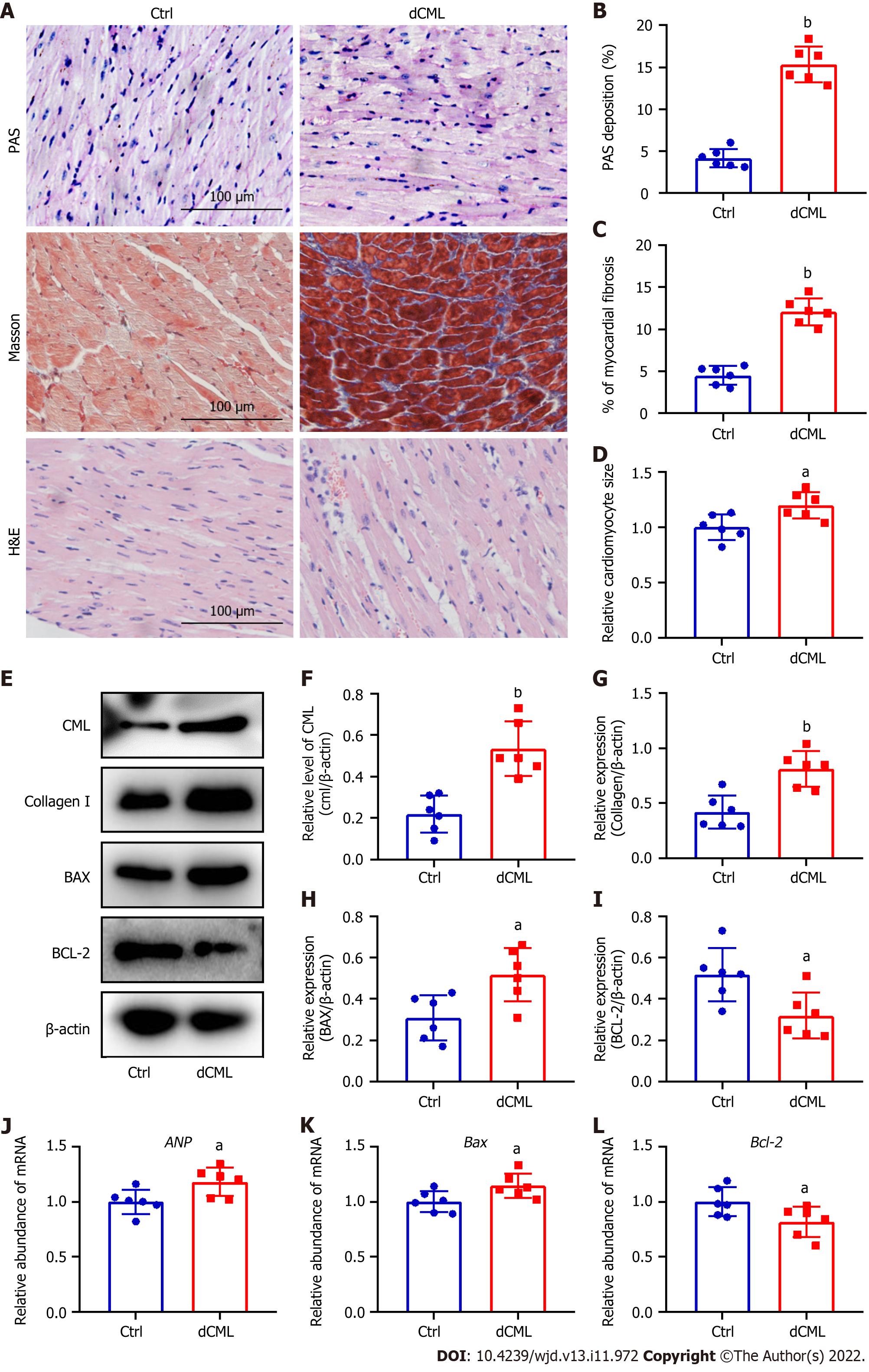
Histopathological changes in the myocardium of mice were detected after 20 wk of dCML. Glycogen PAS staining showed a significant increase in the accumulation of glycogen in the myocardium of the dCML group, which was 3.7-fold higher than that of the Ctrl group, suggesting impaired glucose metabolism (Figure 3A and B). Masson staining indicated that myocardial fibrosis was aggravated in the dCML group (Figure 3A and C). H&E staining showed that the cell area in the myocardium of the dCML group was 1.32-fold higher than that of the Ctrl group (Figure 3A and D). The level of CML in the myocardium of the dCML group also significantly increased (Figure 3E and F). Protein levels of collagen I and the apoptosis regulator BAX were significantly upregulated, whereas the anti-apoptotic factor Bcl-2 was significantly inhibited (Figure 3E, G, H and I). The mRNA level of the cardiac hypertrophy indicator atrial natriuretic peptide (ANP) was significantly increased in the dCML group (Figure 3J). Compared with the Ctrl group, Bax mRNA was significantly upregulated, whereas Bcl-2 was significantly downregulated in the dCML group, suggesting increased myocardial apoptosis in the dCML group (Figure 3K and L).
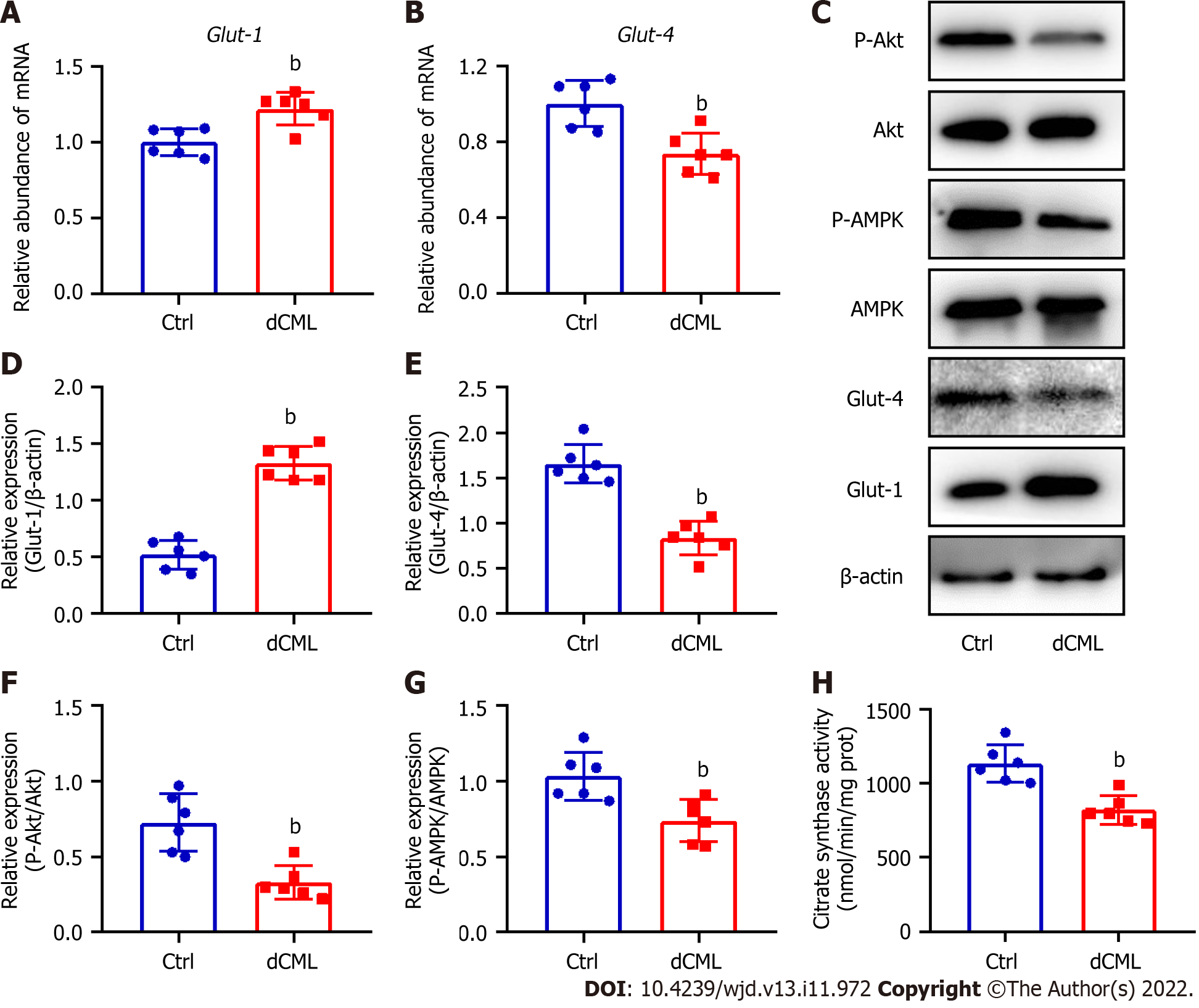
Glut-1 and Glut-4 play a key roles in myocardial glucose transport[34]. Glut-1 mRNA and protein levels were significantly increased in the mouse myocardium of the dCML group, whereas Glut-4 was significantly decreased (Figure 4A-E). Akt and AMPK signaling are key regulatory pathways in glucose metabolism[34,35]. Compared with the Ctrl group, the dCML group showed significant inhibition of Akt and AMPK activities of the myocardium (Figure 4C, F and G). These results suggest that the myocardial glucose metabolism of mice is impaired after dCML. CS is the rate-limiting enzyme in the aerobic oxidation of glucose. The activity of CS was significantly inhibited in the dCML group (Figure 4H).
Given that dCML inhibits myocardial glucose metabolism and promotes myocardial remodeling in mice, we further investigated the direct effects of CML in vitro. Exogenous stimulation with 10 mmol/L CML did not affect the viability of H9C2 cells (Figure 5A). Exogenous CML stimulation significantly decreased Glut-4 expression and significantly reduced the levels of Akt and AMPK phosphorylation in H9C2 cardiomyocytes, whereas the mRNA and protein levels of Glut-1 were significantly increased (Figure 5B-H). Immunocytochemical staining indicated that CML increased the content of collagen I in cardiomyocytes (Figure 5I and J). Phalloidin staining showed that the cardiomyocyte area in the CML group was significantly increased, and the expression of cardiac hypertrophy marker ANP was also significantly upregulated (Figure 5I, K and L). Meanwhile, the mRNA level of the apoptosis indicator Bax was increased in the CML group, and the level of the anti-apoptotic marker Bcl-2 was significantly downregulated, suggesting that CML induced cardiomyocyte apoptosis (Figure 5M and N). The Cs mRNA level was also decreased in the CML group (Figure 5O).
This study investigated the effects of dCML on myocardial glucose metabolism and myocardial remodeling using experimental mouse models and cells. In the in vivo model, myocardial glucose uptake was tracked using 18F-FDG micro-PET scans. The glucose uptake in the dCML group was significantly increased. Histological staining and detection of related molecular indicators suggested that dCML inhibited glucose metabolism in the myocardium and promoted myocardial fibrosis, cardiac hypertrophy, and apoptosis (Figure 6). In vitro, H9C2 cardiomyocytes were treated with exogenous CML to analyze changes in cardiac remodeling and glucose metabolism. Consistent with the in vivo evidence, CML inhibited glucose metabolism and promoted hypertrophy, collagen I expression, and apoptosis of cardiomyocytes. Our study may reveal new clues for underlying foodborne factors associated with myocardial injury and provide new ideas for the prevention and treatment of myocardial remodeling.
AGEs are a class of non-enzymatic reaction products composed of complex components, and the pathogenic role of AGEs has been previously reported[11,12]. The effects of AGEs may be receptor-dependent or receptor-independent. In the receptor-independent pathway, AGEs cross-link with the extracellular matrix and change the physicochemical properties, which affects cell physiology and tissue function[36]. In the receptor-dependent pathway, AGEs bind to cell surface receptors, change the original signal transmission pathway, and lead to pathological outcomes[37]. In addition to AGEs, the precursors of AGEs, such as methylglyoxal, also accumulate in the body. These precursors can play a direct pathogenic role or continue to form AGEs[38]. Adverse effects of dietary AGEs have been previously reported. Wang et al[39] found that dietary AGEs disrupted gut microbiota and induced insulin resistance. Thornton et al[40] suggested that dietary AGEs affected ovarian function. A western diet rich in AGEs can also induce changes in the cardiovascular system[41]. Given that AGEs are multi-component, but whether each component has a similar effect is unclear. It is still unknown which component plays the most critical role. CML is one of the most active components of AGEs. Due to its ease of formation, CML is also found in high concentrations in food[42]. Therefore, our study explored the role of dCML, and showed that dCML can lead to disorders of myocardial glucose metabolism and myocardial remodeling. This finding may provide more evidence underlying the negative effects of foodborne AGEs.
Some studies have also reported the limited pathogenic role of dietary AGEs. Koyama et al[43] found no significant association between dietary AGEs and all-cause mortality in adults with diabetes. The double blind parallel study by Linkens et al[44] suggested that a short-term AGE diet did not affect the sensitivity, secretion and clearance of insulin, vascular function, and overall inflammation in individuals with abdominal obesity. The Maastricht Study also revealed that dietary AGEs are not associated with stiffness of the aorta or carotid arteries[45]. These studies are population-based, suggesting additional confounding factors than in animal models. Moreover, surveys of dietary structure or dietary interventions in these subjects were conducted for relatively short periods of time, which may not be sufficient to represent the long-term dietary habits of individuals. In our study, we focused on myocardial glucose uptake and its remodeling and found adverse effects of dCML. We also evaluated the systemic effects of dCML on mice. Exposure to 20 wk of dCML resulted in glucose intolerance and insulin resistance, but the fasting glucose did not reach the level of diabetes, suggesting a progressive pathogenic effect of dCML. It may take more than 20 wk of a CML diet to further increase blood glucose and worsen insulin resistance. Also, the weight gain of dCML mice became more obvious starting from the 12th wk. Prolonged dCML time may induce significant changes in mouse body weight.
Myocardium is one of the most energy-consuming organs, with 70% of the energy supply of adult myocardium derived from ATP produced by fatty acid oxidation, and glucose metabolism plays a secondary but important role[46]. Under physiological conditions, glucose is converted to pyruvate. ATP is produced via tricarboxylic acid cycle and respiratory chain. Under specific pathological conditions, glucose is the main energy substrate as a result of the reorganization of enzymes involved in energy metabolism. However, glycolysis is the main energy source rather than aerobic oxidation. The energy provided by glycolysis does not meet the long-term needs of myocardial activity. The overall cardiac metabolic activity is subsequently reduced, eventually leading to cardiomyocyte apoptosis and malignant remodeling of the myocardium[47]. However, an increase or decrease in myocardial glucose metabolism is also associated with specific pathological changes. For example, in diabetic cardiomyopathy, the lipotoxicity caused by diabetes increases the fatty acid metabolism in the heart, thus inhibiting glucose metabolism[48]. The interaction between fatty acids and glucose metabolism is also known as Randle Cycle[49]. However, the relationship between myocardial metabolic reprogramming and myocardial pathological remodeling is unclear. Whether myocardial metabolic disturbance is a cause or a consequence of myocardial remodeling is still inconclusive. In this study, we observed impaired myocardial glucose metabolism but increased glucose uptake after long-term dCML in mice. The glucose metabolic pathways Akt and AMPK were significantly inhibited. The long-term dCML may alter the metabolic substrates for myocardial energy supply. CS, an enzyme initiating the tricarboxylic acid cycle, was also inhibited after exposure to dCML. Therefore, the myocardium has to absorb more glucose to provide adequate substrates for energy metabolism. The specific mechanism will be further explored in future studies.
To analyze the glucose uptake in the myocardium, we used micro-PET imaging based on the 18F-FDG probe. Since 18F-FDG was synthesized in 1969, it has been widely used in the diagnosis, staging and prognostic assessment of clinical diseases[50]. FDG is a glucose analog and is therefore involved in glucose processing in vivo. Under pathological conditions, inflammation or hypoxia can lead to impaired glucose metabolism but increased glucose uptake to provide adequate energy. Therefore, 18F-FDG usually accumulates in the lesions. In the study of cardiovascular disease, 18F-FDG imaging also plays an important role. 18F-FDG is the reference standard for molecular imaging of myocardial inflammation[51]. Our study found an increased 18F-FDG uptake but impaired glucose transport and metabolism in the myocardium of dCML mice, which may be related to the elevated levels of inflammation. Consistent with our study, in the spontaneously hypertensive rat model, myocardial 18F-FDG imaging SUV was elevated, whereas glucose aerobic oxidation-related transporters and metabolic pathways were significantly inhibited[29].
We investigated the detrimental effects of dietary CML on myocardial remodeling to draw attention to the CML content in the diet. However, this study has some limitations. The exploration of specific mechanisms needs to be further continued in the future, and clinical evidence is also needed. We speculate that dietary CML may also promote myocardial remodeling through non-receptor and receptor approaches. CML could increase collagen cross-linking in the extracellular matrix and bind to its receptors to activate related signals. In the future, we will further explore the mechanism of cardiac remodeling induced by dietary CML to identify effective targets for intervention. There is an endogenous CML generation system in human body. Compared with reducing endogenous CML, reducing exogenous CML from dietary sources appears to be more controllable. Previous studies have also reported some CML inhibitors, such as antioxidants and aminoguanidine[15]. However, these additives may change the original methods of food production, and their high cost and unclear safety also limit the application. Therefore, in the future, we will also focus on strategies to inhibit CML in diet preparation.
Our study focused on the adverse effects of food-derived CML on myocardial glucose metabolism and remodeling. Long-term dCML leads to impaired myocardial glucose metabolism and induces myocardial hypertrophy, fibrosis, and apoptosis. This study offers new clues associated with myocardial remodeling and also provides an experimental basis for dietary planning to prevent cardiovascular disease prevention.
Nε-(carboxymethyl)lysine (CML), a major component of advanced glycation end products, exists in the daily diet and poses a threat to health after ingestion. It is necessary to evaluate the effect of dietary CML on the heart.
Previous studies have confirmed that the toxic metabolite CML can cause pathological changes in a variety of tissues such as blood vessels and bones. Foodborne CML, as the main source of CML, may lead to cardiac injuries.
To investigate the effects of dietary CML on cardiac remodeling and glucose metabolism.
C57 BL/6 mice received a 20-wk CML diet (1 g/kg). The body weight, fasting blood glucose, fasting insulin and serum CML levels of mice were recorded. Exogenous CML was given to establish an in vitro H9C2 cell model. Micro-positron emission tomography was used to evaluate the glucose uptake of the mouse heart. Myocardial remodeling and glucose metabolism were detected by histological/cytological staining, Western blotting, and polymerase chain reaction.
The 20 wk of CML diet could cause insulin resistance in mice and increase CML levels in serum and heart. Myocardial fibrosis, hypertrophy and apoptosis in mice were significantly aggravated after dietary CML. Moreover, dietary CML increased myocardial glucose uptake but disrupted glucose metabolism. In vitro, exogenous CML inhibited glucose metabolism-related signaling pathways and promoted H9C2 cell hypertrophy, apoptosis and collagen I expression.
Dietary CML promoted cardiac remodeling and abnormal glucose metabolism.
This study emphasizes the cardiac hazards of dietary CML and provides new suggestions for the diet preparation in the prevention and treatment cardiovascular diseases.
We thank the Experimental Animal Center of Jiangsu University for guiding animal experiments.
| 1. | Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254-e743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 3872] [Article Influence: 774.4] [Reference Citation Analysis (0)] |
| 2. | Alam P, Maliken BD, Jones SM, Ivey MJ, Wu Z, Wang Y, Kanisicak O. Cardiac Remodeling and Repair: Recent Approaches, Advancements, and Future Perspective. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1823] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 4. | Li G, Shao Y, Guo HC, Zhi Y, Qiao B, Ma K, Du J, Lai YQ, Li Y. MicroRNA-27b-3p down-regulates FGF1 and aggravates pathological cardiac remodelling. Cardiovasc Res. 2022;118:2139-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Xiong R, Li N, Chen L, Wang W, Wang B, Jiang W, Geng Q. STING protects against cardiac dysfunction and remodelling by blocking autophagy. Cell Commun Signal. 2021;19:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Feng Y, Zhang Y, Xiao H. AMPK and cardiac remodelling. Sci China Life Sci. 2018;61:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 514] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 8. | Mellor KM, Ritchie RH, Davidoff AJ, Delbridge LM. Elevated dietary sugar and the heart: experimental models and myocardial remodeling. Can J Physiol Pharmacol. 2010;88:525-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Nakamura M, Odanovic N, Nakada Y, Dohi S, Zhai P, Ivessa A, Yang Z, Abdellatif M, Sadoshima J. Dietary carbohydrates restriction inhibits the development of cardiac hypertrophy and heart failure. Cardiovasc Res. 2021;117:2365-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Zeng H, Vaka VR, He X, Booz GW, Chen JX. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med. 2015;19:1847-1856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Plemmenos G, Piperi C. Pathogenic Molecular Mechanisms in Periodontitis and Peri-Implantitis: Role of Advanced Glycation End Products. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Jiang Y, Liu N, Ren L, Zhu Y, An Y, Chen D. Advanced glycation end-product Nε-carboxymethyl-Lysine accelerates progression of atherosclerotic calcification in diabetes. Atherosclerosis. 2012;221:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Wang ZQ, Jing LL, Yan JC, Sun Z, Bao ZY, Shao C, Pang QW, Geng Y, Zhang LL, Li LH. Role of AGEs in the progression and regression of atherosclerotic plaques. Glycoconj J. 2018;35:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Chen Y, Guo TL. Dietary advanced glycation end-products elicit toxicological effects by disrupting gut microbiome and immune homeostasis. J Immunotoxicol. 2021;18:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Han L, Li L, Li B, Zhao D, Li Y, Xu Z, Liu G. Review of the characteristics of food-derived and endogenous ne-carboxymethyllysine. J Food Prot. 2013;76:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ahmed N, Mirshekar-Syahkal B, Kennish L, Karachalias N, Babaei-Jadidi R, Thornalley PJ. Assay of advanced glycation endproducts in selected beverages and food by liquid chromatography with tandem mass spectrometric detection. Mol Nutr Food Res. 2005;49:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Assar SH, Moloney C, Lima M, Magee R, Ames JM. Determination of Nepsilon-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids. 2009;36:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Henle T. AGEs in foods: do they play a role in uremia? Kidney Int Suppl. 2003;S145-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Liu X, Zheng L, Zhang R, Liu G, Xiao S, Qiao X, Wu Y, Gong Z. Toxicological evaluation of advanced glycation end product Nε-(carboxymethyl)lysine: Acute and subacute oral toxicity studies. Regul Toxicol Pharmacol. 2016;77:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Waqas K, Chen J, van der Eerden BCJ, Ikram MA, Uitterlinden AG, Voortman T, Zillikens MC. Dietary Advanced Glycation End-Products (dAGEs) Intake and Bone Health: A Cross-Sectional Analysis in the Rotterdam Study. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Delgado-Andrade C, Fogliano V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of their Intake. Annu Rev Food Sci Technol. 2018;9:271-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 22. | Wang Z, Bao Z, Ding Y, Xu S, Du R, Yan J, Li L, Sun Z, Shao C, Gu W. Nε-carboxymethyl-lysine-induced PI3K/Akt signaling inhibition promotes foam cell apoptosis and atherosclerosis progression. Biomed Pharmacother. 2019;115:108880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Tardy-Cantalupi I, Montessuit C, Papageorgiou I, Remondino-Müller A, Assimacopoulos-Jeannet F, Morel DR, Lerch R. Effect of transient ischemia on the expression of glucose transporters GLUT-1 and GLUT-4 in rat myocardium. J Mol Cell Cardiol. 1999;31:1143-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Tran DH, Wang ZV. Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J Am Heart Assoc. 2019;8:e012673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 25. | Marsico F, Gargiulo P, Marra AM, Parente A, Paolillo S. Glucose Metabolism Abnormalities in Heart Failure Patients: Insights and Prognostic Relevance. Heart Fail Clin. 2019;15:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Sowndhar Rajan B, Manivasagam S, Dhanusu S, Chandrasekar N, Krishna K, Kalaiarasu LP, Babu AA, Vellaichamy E. Diet with high content of advanced glycation end products induces systemic inflammation and weight gain in experimental mice: Protective role of curcumin and gallic acid. Food Chem Toxicol. 2018;114:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Small L, Ehrlich A, Iversen J, Ashcroft SP, Trošt K, Moritz T, Hartmann B, Holst JJ, Treebak JT, Zierath JR, Barrès R. Comparative analysis of oral and intraperitoneal glucose tolerance tests in mice. Mol Metab. 2022;57:101440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | Zhang L, Ye K, Xiaokereti J, Ma M, Guo Y, Zhou X, Tang B. Histopathological substrate of the atrial myocardium in the progression of obstructive sleep apnoea-related atrial fibrillation. Sleep Breath. 2021;25:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Wang J, Li Z, Wang Y, Zhang J, Zhao W, Fu M, Han X, Zhou J, Ge J. Qiliqiangxin Enhances Cardiac Glucose Metabolism and Improves Diastolic Function in Spontaneously Hypertensive Rats. Evid Based Complement Alternat Med. 2017;2017:3197320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Hellberg S, Silvola JM, Kiugel M, Liljenbäck H, Metsälä O, Viljanen T, Metso J, Jauhiainen M, Saukko P, Nuutila P, Ylä-Herttuala S, Knuuti J, Roivainen A, Saraste A. Type 2 diabetes enhances arterial uptake of choline in atherosclerotic mice: an imaging study with positron emission tomography tracer ¹⁸F-fluoromethylcholine. Cardiovasc Diabetol. 2016;15:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Sun Z, Zhang L, Li L, Shao C, Liu J, Zhou M, Wang Z. Galectin-3 mediates cardiac remodeling caused by impaired glucose and lipid metabolism through inhibiting two pathways of activating Akt. Am J Physiol Heart Circ Physiol. 2021;320:H364-H380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Sun Z, Wang Z, Li L, Yan J, Shao C, Bao Z, Jing L, Pang Q, Geng Y, Zhang L. RAGE/galectin-3 yields intraplaque calcification transformation via sortilin. Acta Diabetol. 2019;56:457-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Sun Z, Li L, Yan Z, Zhang L, Zang G, Qian Y, Wang Z. Circadian rhythm disorders elevate macrophages cytokines release and promote multiple tissues/organs dysfunction in mice. Physiol Behav. 2022;249:113772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail. 2011;13:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Entezari M, Hashemi D, Taheriazam A, Zabolian A, Mohammadi S, Fakhri F, Hashemi M, Hushmandi K, Ashrafizadeh M, Zarrabi A, Ertas YN, Mirzaei S, Samarghandian S. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed Pharmacother. 2022;146:112563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 260] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 36. | Olson LC, Redden JT, Schwartz Z, Cohen DJ, McClure MJ. Advanced Glycation End-Products in Skeletal Muscle Aging. Bioengineering (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Ajith TA, Vinodkumar P. Advanced Glycation End Products: Association with the Pathogenesis of Diseases and the Current Therapeutic Advances. Curr Clin Pharmacol. 2016;11:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018;93:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 299] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 39. | Wang J, Cai W, Yu J, Liu H, He S, Zhu L, Xu J. Dietary Advanced Glycation End Products Shift the Gut Microbiota Composition and Induce Insulin Resistance in Mice. Diabetes Metab Syndr Obes. 2022;15:427-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Thornton K, Merhi Z, Jindal S, Goldsammler M, Charron MJ, Buyuk E. Dietary Advanced Glycation End Products (AGEs) could alter ovarian function in mice. Mol Cell Endocrinol. 2020;510:110826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Peppa M, Mavroeidi I. Experimental Animal Studies Support the Role of Dietary Advanced Glycation End Products in Health and Disease. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Chen G. Dietary N-epsilon-carboxymethyllysine as for a major glycotoxin in foods: A review. Compr Rev Food Sci Food Saf. 2021;20:4931-4949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Koyama AK, Pavkov ME, Wu Y, Siegel KR. Is dietary intake of advanced glycation end products associated with mortality among adults with diabetes? Nutr Metab Cardiovasc Dis. 2022;32:1402-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Linkens AM, Houben AJ, Niessen PM, Wijckmans NE, de Goei EE, Van den Eynde MD, Scheijen JL, van den Waarenburg MP, Mari A, Berendschot TT, Streese L, Hanssen H, van Dongen MC, van Gool CC, Stehouwer CD, Eussen SJ, Schalkwijk CG. A 4-week high-AGE diet does not impair glucose metabolism and vascular function in obese individuals. JCI Insight. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Linkens AM, Eussen SJ, Houben AJ, Kroon AA, Schram MT, Reesink KD, Dagnelie PC, Henry RM, van Greevenbroek M, Wesselius A, Stehouwer CD, Schalkwijk CG. Habitual Intake of Dietary Advanced Glycation End Products Is Not Associated with Arterial Stiffness of the Aorta and Carotid Artery in Adults: The Maastricht Study. J Nutr. 2021;151:1886-1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Zhang Q, Shao M, Zhang X, Wang Q, Guo D, Yang X, Li C, Wang Y. The Effect of Chinese Medicine on Lipid and Glucose Metabolism in Acute Myocardial Infarction Through PPARγ Pathway. Front Pharmacol. 2018;9:1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Tuomainen T, Tavi P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Exp Cell Res. 2017;360:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 48. | Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014;171:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 49. | RANDLE PJ, GARLAND PB, HALES CN, NEWSHOLME EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3466] [Cited by in RCA: 3382] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 50. | Feng H, Wang X, Chen J, Cui J, Gao T, Gao Y, Zeng W. Nuclear Imaging of Glucose Metabolism: Beyond 18F-FDG. Contrast Media Mol Imaging. 2019;2019:7954854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Kircher M, Lapa C. Novel Noninvasive Nuclear Medicine Imaging Techniques for Cardiac Inflammation. Curr Cardiovasc Imaging Rep. 2017;10:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jovandaric MZ, Serbia; Mai S, Italy S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YX