Published online Aug 15, 2021. doi: 10.4239/wjd.v12.i8.1304
Peer-review started: February 23, 2021
First decision: March 30, 2021
Revised: April 9, 2021
Accepted: July 12, 2021
Article in press: July 12, 2021
Published online: August 15, 2021
Processing time: 167 Days and 0.9 Hours
It has been reported that vascular endothelial growth factor (VEGF) is a susceptibility gene for both type 2 diabetes mellitus (T2DM) and diabetic retinopathy (DR). In response to hypoxia, VEGF mRNA levels are increased, which is mainly mediated by the binding of hypoxia-inducible factor-1 (HIF-1) and hypoxia response element upstream of the transcriptional start site of VEGF. Therefore, HIF-1a is supposed to be involved in pathology of DR.
To investigate whether the polymorphisms in HIF-1a gene are associated with DR.
Two hundred and ninety-nine type 2 diabetic patients (128 males and 171 females) and 144 healthy volunteers were recruited. Mean age was 56.04 ± 21.05 years. According to the results of fundus fluorescein angiography and exami
The mean age of the cases with diabetes was 55.84 ± 3.66 years, the mean age of the cases with DR was 55.97 ± 4.66 years and that of controls was 56.32 ± 4.70 years. Two variations were found in 76 patients. Rs11549465 is the change of C-T base, rs11549467 is the change of G-A base. The rs11549467 G/A genotype was 5.33% in diabetes and 6.04% in DR patients, respectively. The rs11549465 C/T genotype was 10% and 12.75% in patients with diabetes and DR. The rs11549467 A allele frequencies and rs11549465 T frequencies was similar to that of controls. Paired SNP linkage disequilibrium analysis indicated that rs11549467 was in linkage disequilibrium with rs11549465. Haplotype association analysis denoted that the haplotype association exhibited similar distribution in the patients compared to the normal controls.
This study suggests that there is no relationship between the genetic variations of HIF1a and diabetes or DR.
Core Tip: Vascular endothelial growth factor (VEGF), a potent endothelial cell mitogen, plays a crucial role in neovascularization of proliferative diabetic retinopathy (DR). The human VEGF gene encoding VEGF was identified to be the first target genes of hypoxia-inducible factor-1 (HIF-1). Therefore, HIF-1a is supposed to be involved in pathology of DR. Here, we investigate whether the polymorphisms in HIF-1a gene are associated with DR.
- Citation: Liu YH, Guo C, Sun YQ, Li Q. Polymorphisms in HIF-1a gene are not associated with diabetic retinopathy in China. World J Diabetes 2021; 12(8): 1304-1311
- URL: https://www.wjgnet.com/1948-9358/full/v12/i8/1304.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i8.1304
Microvascular complications are the principal causes of early mortality in diabetes[1,2]. Most diabetic patients develop diabetic retinopathy (DR) with the progression of diabetes, which remains the major cause of new-onset blindness among diabetic adults[3]. Long duration of diabetes and hypertension along with hyperglycemia are widely considered as main risk factors for the development of DR[4,5]. However, accumu
DR is characterized by increased vascular permeability, ischemia and neovascularization[12]. Vitreous hemorrhage and fibrosis, as well as tractional retinal detachment led to a high risk of blindness in the course of neovascularization. Vascular endothelial growth factor (VEGF), a key factor in angiogenesis, can facilitate angiogenesis, increase the permeability of microvessels and promote the formation of collateral vessels[13]. Meanwhile, VEGF plays an essential role in determining mass and property of ß-cell by stimulating angiogenesis of islets in the developmental stage of pancreas[14]. The expression of VEGF mRNA is increased with the activation of transcription in response to hypoxia. This increase is mainly mediated by the binding of hypoxia-inducible factor-1 (HIF-1) and a hypoxia response element upstream of the transcriptional initiation site of VEGF[15,16]. It has been reported that VEGF is a susceptibility gene for both type 2 diabetes mellitus (T2DM) and DR[12].
HIF-1, a transcription factor found in mammalian cells cultured in hypoxic environment, is a heterodimer composed of a HIF-1a subunit complexed with a HIF-1ß subunit[17]. The HIF-1a gene is located at chromosome 14q21-q24[18]. The transcriptional activity and protein expression of HIF-1a are strictly regulated in response to oxygen concentration. Thus, oxygen-regulated expression of the HIF-1a controlled the activity of HIF-1. HIF-1 plays an important role in systemic homeostatic and the increase in expression of VEGF in response to hypoxia.
Therefore, the purpose of the present study was to examine whether there is association of HIF-1a polymorphisms in DR and to assess whether the combined effects of these genetic variations have an influence on the risk for DR.
The study was approved by the Clinical Research Ethics Committee of Harbin Medical University and conducted according to the principles in the Declaration of Helsinki. All subjects gave written informed consent. Two hundred and ninety-nine patients with T2DM who were enrolled consecutively between January 2012 and August 2013 were recruited from the Second Affiliated Hospital Ophthalmic Clinic of Harbin Medical University. Of this group, 149 patients were diagnosed with DR. Diabetes was diagnosed according to American Diabetes Association criteria. Control subjects (n = 144) were healthy volunteers with negative family history of diabetes and normal fasting glucose (Table 1). Briefly, the patients received a standard questionnaire that include questions about family history, the age of diabetes diagnosis, treatment methods, smoking status and other medical issues. All patients received a complete ophthalmic examination at least once a year, including fundus photography, ophthalmoscopy and corrected visual acuity. Retinopathy was diagnosed based on the Early Treatment Diabetic Retinopathy Study (ETDRS) criteria[19]. Fundoscopic findings were assessed by retinal specialists. In this study, patients were not stratified according to the severity of retinopathy. Patients and controls were matched on gender and age (χ2 test, P = 0.84).
| Group | Total | Male (%) | Female (%) | Mean age (yr) |
| Diabetes | 150 | 66 | 84 | 55.84 ± 3.66 |
| Diabetic retinopathy | 149 | 62 | 87 | 55.97 ± 4.66 |
| Controls | 144 | 67 | 77 | 56.32 ± 4.70 |
| P = 0.84 |
Fasting blood samples were collected in the morning between 9:00 and 10:00 h after overnight fasting. Serum insulin and C-peptide were measured by Commercial RIA kits. Autoanalyzer was used to measure the level of glucose and lipids.
Ten milliliter samples of venous blood were collected from the control subjects and the patients with T2DM in EDTA vacutainers (BD, San Jose, CA, United States). Blood Genome DNA Extraction Kits (TAKARA BIOTECHNOLOGY, DALIAN, China ) was used to extract genome DNA from peripheral blood leukocytes. The primers (Table 1) for polymerase chain reaction were designed according to the reference sequences in NCBI Gene database by using Primer 5. Detection of SNP gene of the selected genotypes using matrix - assisted laser desorption / Ionization time of flight mass spectrometry (MALDI-TOF-MS) method (Beijing bo’ao biotechnology company).
Data of quantitative characteristics are represented as means ± SD. Data of qualitative characteristics are showed as absolute numbers or percent values. Comparisons between groups were carried out χ2 test (nominal data) or Student’s t-test (interval data). The value of P < 0.05 was considered as statistical significant. Hardy–Weinberg equilibrium was conducted by the χ2 method. Univariate and multivariable logistic regression for the association with DR was performed using the SAS program.
One hundred and fifty healthy controls, 144 patients with DR and 149 patients with diabetes were recruited in this study. The mean age of the cases with diabetes was 55.84 ± 3.66 years, the mean age of the cases with DR was 55.97 ± 4.66 years and that of controls was 56.32 ± 4.70 years. In addition, the patients and controls were matched on gender (Table 1). Age differences were adjusted with logistic regression.
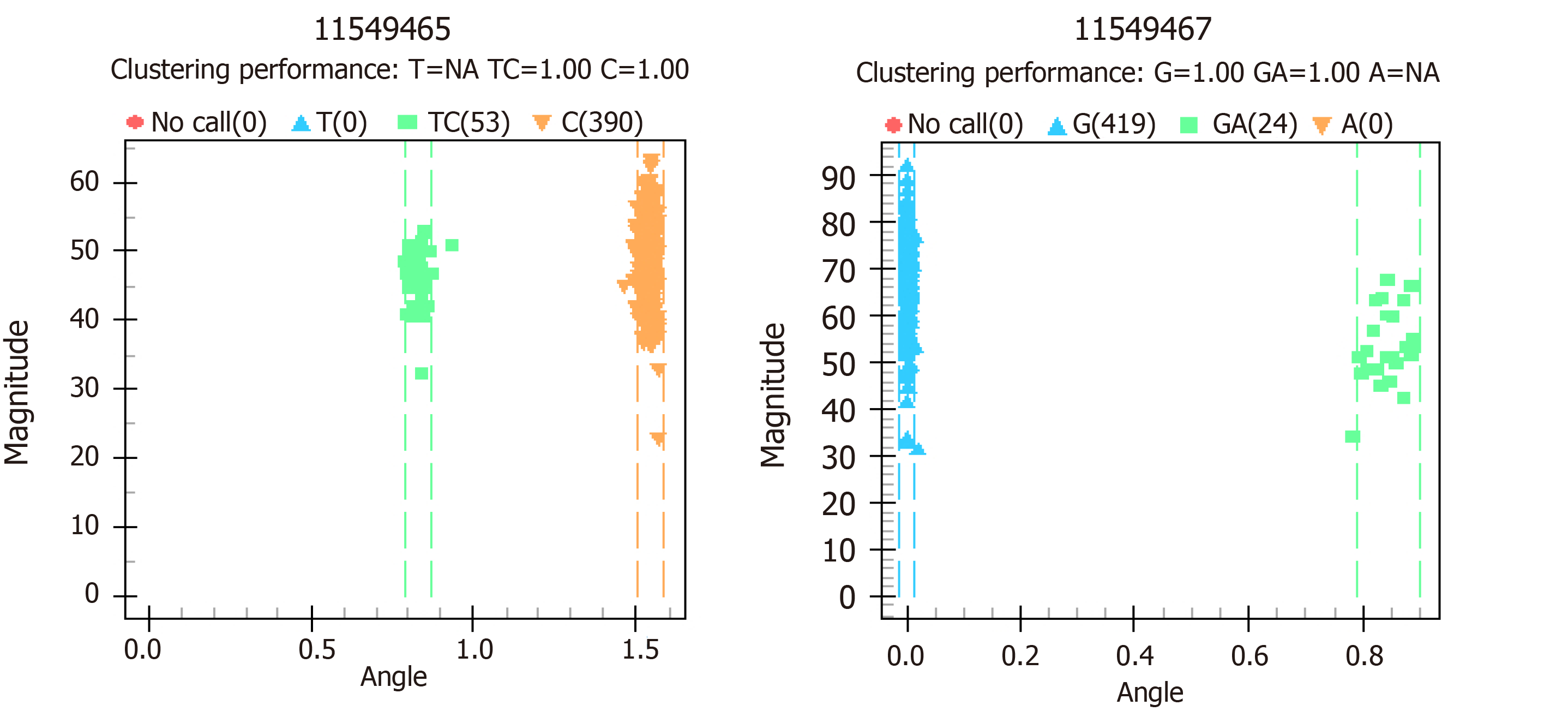
Two novel nucleotide changes were detected. Rs11549465 is the change of C-T base, rs11549467 is the change of G-A base. These two loci are identical with SNP (rs11549465 and rs11549467) of HIF1A gene in NCBI gene bank (Figure 1). The distributions of all SNPs were in accordance with the Hardy-Weinberg equilibrium (HWE) in the control group (Table 2). Compared with the healthy controls, the rs11549467 G/G genotype and G/A allele was showed with slightly lower frequency in patients with diabetes and DR, while this decrease failed to achieve statistical significance. The rs11549465 C/C genotype and C/T was found with mildly higher frequencies in the patients with diabetes and DR, however, this was not significant different. Table 3 exhibits the distribution of the genotype and allele frequency of rs11549467 and rs11549465.
| Group | SNP loci | Genotype | Actual cases | Examples of H-W equilibrium theory | P value |
| Diabetes | 11549467 | GG|GA|AA | 142|8|0 | 142.11|7.79|0.11 | 0.7372 |
| 11549465 | CC|CT|TT | 135|15|0 | 135.38|14.25|0.38 | 0.5192 | |
| Diabetic retinopathy | 11549467 | GG|GA|AA | 140|9|0 | 140.14|8.73|0.14 | 0.7038 |
| 11549465 | CC|CT|TT | 130|19|0 | 130.61|17.79|0.61 | 0.4058 | |
| Controls | 11549467 | GG|GA|AA | 137|7|0 | 137.09|6.83|0.09 | 0.7650 |
| 11549465 | CC|CT|TT | 125|19|0 | 125.63|17.75|0.63 | 0.3967 |
| SNP | Patient/control | Genotype, n (%) | Allele, n (%) | |||
| rs11549467 | G/G | G/A | A/A | G | A | |
| Diabetes | 142 (94.67) | 8 (5.33) | 0 (0) | 292 (97.33) | 8 (2.67) | |
| Diabetic retinopathy | 140 (93.96) | 9 (6.04) | 0 (0) | 289 (96.98) | 9 (3.02) | |
| Controls | 137 (95.14) | 7 (4.86) | 0 (0) | 281 (97.57) | 7 (2.43) | |
| P > 0.05 | ||||||
| rs11549465 | C/C | C/T | T/T | C | T | |
| Diabetes | 135 (90) | 15 (10) | 0 (0) | 285 (95) | 15 (5) | |
| Diabetic rentinopathy | 130 (87.25) | 19 (12.75) | 0 (0) | 279 (93.62) | 19 (6.38) | |
| Controls | 125 (86.81) | 19 (13.19) | 0 (0) | 269 (93.4) | 19 (6.6) | |
| P > 0.05 | ||||||
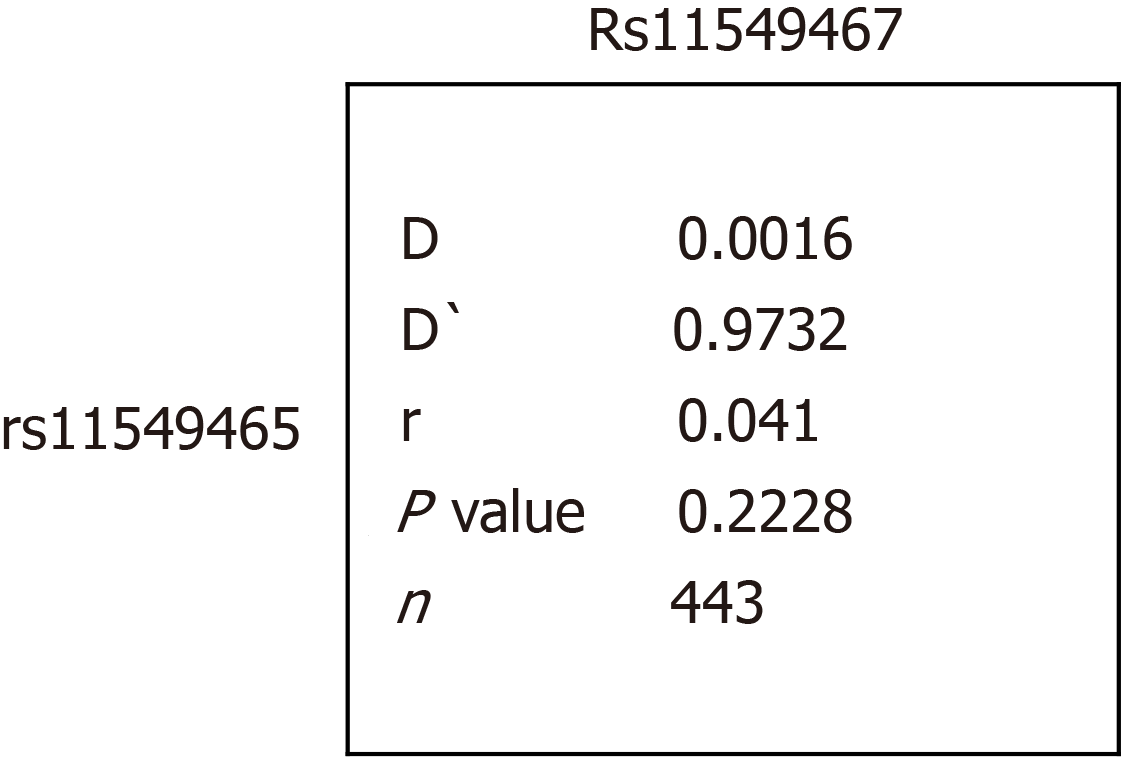
As shown in Figure 2, paired SNP linkage disequilibrium analysis demonstrated that rs11549467 was in linkage disequilibrium with rs11549465. The A allele of rs11549467 and the T allele of rs11549465 was found in one diabetic group, however, this failed to achieve statistically significant due to the low frequency. As shown in Table 4, haplotype association analysis denoted that the haplotype association exhibited similar distribution in the patients compared to the normal controls.
| rs11549467 | rs11549465 | Diabetes frequency | Diabetic retinopathy frequency | Control frequency | P value |
| G | C | 92.535 | 90.604 | 90.972 | - |
| G | T | 4.798 | 6.376 | 6.597 | 0.13 |
| A | C | 2.465 | 3.020 | 2.431 | 0.29 |
VEGF, a potent endothelial cell mitogen, plays an essential role in neovascularization in proliferative DR. The human VEGF gene encoding VEGF that is essential for the angiogenic process was identified to be the first target genes of HIF-1[20]. The level of VEGF mRNA increases due to the increased transcriptional activation. This increase is mainly mediated by the binding of HIF-1 to a hypoxia response element[21]. VEGF facilitates angiogenesis, increases the permeability of microvessels and promotes the formation of collateral vessels[13]. Besides, it has been reported that VEGF is a susceptible gene of T2DM and DR, leading us to evaluate the association between HIF-1 and T2DM as well as retinopathy[12].
HIF-1, a transcription factor found in mammalian cells cultured in hypoxic environment, act as a heterodimer comprised of a 120-kDa HIF-1a subunit complexed with a 91- to 94-kDa HIF-1β subunit[17]. oxygen-regulated expression of the HIF-1a controlled the activity of HIF-1[22]. HIF-1 plays an important role in systemic homeostatic and the increased expression of VEGF in response to hypoxia.
Expression of HIF-1 Leads to a variety of effects. Some of them may protect against peripheral artery disease, such as formation of collateral vessels or promotion of erythropoiesis. On the other hand, HIF-1 is involved in the vascularization and destabilization of atherosclerotic plaques and may be regarded as a potential risk factor for peripheral artery disease[23,24]. Subsequent gain- or loss-of-function experiments showed that the expression of other angiogenic growth factors, including platelet-derived growth factor B, stromal-derived factor 1, ANGPT2, and placental growth factor was also controlled by HIF-1[25]. Moreover, loss of HIF-1 blunts translocation of GLUT4 and leads to the decrease in glucose uptake by the skeletal muscle cells[26].
Indeed, there is a large number of evidences supporting that the deregulation of HIF-1 in diabetes is related to the failure of cellular adaptation to hypoxia and demonstrating that low oxygen levels by high glucose concentrations results in HIF-1 instability[27]. Catrina observed that high glucose reduced hypoxia-induced function and stabilization of HIF- 1a in human dermal microvascular endothelial cells in culture[28]. Another important founding is that HIF-1β mRNA level was decreased in pancreatic islets in patients with T2DM compared with non-diabetic patients, suggesting that dysfunction of HIF-1 is involved in the etiology of diabetes[29]. In the past years, it has demonstrated that human a HIF-1a P582S genetic polymorphism has protective effects on DR and delays the development of severe DR[30]. Although the molecular mechanisms underlying disorders of HIF-1 in diabetes are still unknown, some studies suppose that diabetes may result in the down-regulation of HIF-1[31]. Additionally, insulin can stimulate HIF- 1a expression through a signaling pathway dependent on the activation of PI3K and the target of rapamycin[32]. This could underlie the amelioration of diabetic complications arising from a blunt cellular response to low oxygen levels after insulin administration. Furthermore, Lin demonstrated that HIF-1a is a key mediator of the main pathological changes in DR, including vascular inflammation, vascular leakage and retinal neovascularization[33].
In this study, we investigate the relationship between HIF-1 genetic polymorphisms and the risk of diabetes and DR in the Chinese population. We did not find any difference in the HIF1 polymorphism frequencies between the diabetes, DR and normal controls. It suggested that functional polymorphisms in the HIF1 gene did not contribute to susceptibility to diabetes or DR. Meanwhile, paired SNP linkage disequilibrium analysis showed that rs11549467 was in linkage disequilibrium with rs11549465 and the haplotype association including the G allele of rs11549467 and the C allele of rs11549465, though exhibited similar distribution in patients with controls.
This study investigated the association between HIF-1, diabetes and DR in Chinese population. No effects of above two polymorphisms have been observed on diabetes nor DR. However, further studies are needed to elucidate the mechanism underlying HIF-1 deregulation in diabetes and DR.
Vascular endothelial growth factor (VEGF) mRNA levels are increased response to hypoxia, which is mainly mediated by the binding of hypoxia-inducible factor-1 (HIF-1) and hypoxia response element upstream of the transcriptional start site of VEGF. Therefore, HIF-1a is supposed to be involved in pathology of diabetic retinopathy (DR).
In order to find causes associated with DR.
In order to investigate whether the polymorphisms in HIF-1a gene are associated with DR.
Two hundred and ninety-nine type 2 diabetic patients (128 males and 171 females) and 144 healthy volunteers were recruited to 2 groups, DNR group (diabetes without retinopathy) and DR group (diabetes with retinopathy). There are 150 cases in DNR group and 149 cases in DR group. Two single nucleotide polymorphisms of the HIF-1a gene were tested using matrix-assisted laser desorption/Ionization time of flight mass spectrometry. The frequency of genotypes and alleles, and odds ratio were measured.
There is no difference in the HIF1 polymorphism frequencies between the diabetes, DR and normal controls. Haplotype association exhibited similar distribution in the patients compared to the normal controls.
This study suggests that there is no relationship between the genetic variations of HIF1a and diabetes or DR.
Further research is needed to illustrate the mechanism underlying deregulation in HIF-1 in diabetes.
| 1. | He Z, King GL. Microvascular complications of diabetes. Endocrinol Metab Clin North Am. 2004;33:215-238, xi-xii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Maranta F, Cianfanelli L, Cianflone D. Glycaemic Control and Vascular Complications in Diabetes Mellitus Type 2. Adv Exp Med Biol. 2021;1307:129-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, Simó R. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 383] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 4. | Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM; UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 674] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 6. | Han J, Lando L, Skowronska-Krawczyk D, Chao DL. Genetics of Diabetic Retinopathy. Curr Diab Rep. 2019;19:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Tang ZH, Wang L, Zeng F, Zhang K. Human genetics of diabetic retinopathy. J Endocrinol Invest. 2014;37:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Huang YC, Lin JM, Lin HJ, Chen CC, Chen SY, Tsai CH, Tsai FJ. Genome-wide association study of diabetic retinopathy in a Taiwanese population. Ophthalmology. 2011;118:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 9. | Cabrera AP, Monickaraj F, Rangasamy S, Hobbs S, McGuire P, Das A. Do Genomic Factors Play a Role in Diabetic Retinopathy? J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Fu YP, Hallman DM, Gonzalez VH, Klein BE, Klein R, Hayes MG, Cox NJ, Bell GI, Hanis CL. Identification of Diabetic Retinopathy Genes through a Genome-Wide Association Study among Mexican-Americans from Starr County, Texas. J Ophthalmol. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Hallman DM, Boerwinkle E, Gonzalez VH, Klein BE, Klein R, Hanis CL. A genome-wide linkage scan for diabetic retinopathy susceptibility genes in Mexican Americans with type 2 diabetes from Starr County, Texas. Diabetes. 2007;56:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Iwona BS. Growth Factors in the Pathogenesis of Retinal Neurodegeneration in Diabetes Mellitus. Curr Neuropharmacol. 2016;14:792-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Huang H, He J, Johnson D, Wei Y, Liu Y, Wang S, Lutty GA, Duh EJ, Semba RD. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1α-VEGF pathway inhibition. Diabetes. 2015;64:200-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 787] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 15. | Wang W, Lo ACY. Diabetic Retinopathy: Pathophysiology and Treatments. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 828] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 16. | Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5' enhancer. Circ Res. 1995;77:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 736] [Article Influence: 23.7] [Reference Citation Analysis (6)] |
| 17. | Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304-4308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1108] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 18. | Silander K, Scott LJ, Valle TT, Mohlke KL, Stringham HM, Wiles KR, Duren WL, Doheny KF, Pugh EW, Chines P, Narisu N, White PP, Fingerlin TE, Jackson AU, Li C, Ghosh S, Magnuson VL, Colby K, Erdos MR, Hill JE, Hollstein P, Humphreys KM, Kasad RA, Lambert J, Lazaridis KN, Lin G, Morales-Mena A, Patzkowski K, Pfahl C, Porter R, Rha D, Segal L, Suh YD, Tovar J, Unni A, Welch C, Douglas JA, Epstein MP, Hauser ER, Hagopian W, Buchanan TA, Watanabe RM, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A large set of Finnish affected sibling pair families with type 2 diabetes suggests susceptibility loci on chromosomes 6, 11, and 14. Diabetes. 2004;53:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786-806. [PubMed] |
| 20. | Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604-4613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2798] [Cited by in RCA: 2957] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 21. | Yamada N, Horikawa Y, Oda N, Iizuka K, Shihara N, Kishi S, Takeda J. Genetic variation in the hypoxia-inducible factor-1alpha gene is associated with type 2 diabetes in Japanese. J Clin Endocrinol Metab. 2005;90:5841-5847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510-5514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4392] [Cited by in RCA: 4786] [Article Influence: 154.4] [Reference Citation Analysis (0)] |
| 23. | Ferns GAA, Heikal L. Hypoxia in Atherogenesis. Angiology. 2017;68:472-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Hong M, Shi H, Wang N, Tan HY, Wang Q, Feng Y. Dual Effects of Chinese Herbal Medicines on Angiogenesis in Cancer and Ischemic Stroke Treatments: Role of HIF-1 Network. Front Pharmacol. 2019;10:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Norouzirad R, González-Muniesa P, Ghasemi A. Hypoxia in Obesity and Diabetes: Potential Therapeutic Effects of Hyperoxia and Nitrate. Oxid Med Cell Longev. 2017;2017:5350267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Sakagami H, Makino Y, Mizumoto K, Isoe T, Takeda Y, Watanabe J, Fujita Y, Takiyama Y, Abiko A, Haneda M. Loss of HIF-1α impairs GLUT4 translocation and glucose uptake by the skeletal muscle cells. Am J Physiol Endocrinol Metab. 2014;306:E1065-E1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Bento CF, Pereira P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia. 2011;54:1946-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53:3226-3232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 29. | Nomoto H, Pei L, Montemurro C, Rosenberger M, Furterer A, Coppola G, Nadel B, Pellegrini M, Gurlo T, Butler PC, Tudzarova S. Activation of the HIF1α/PFKFB3 stress response pathway in beta cells in type 1 diabetes. Diabetologia. 2020;63:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Ekberg NR, Eliasson S, Li YW, Zheng X, Chatzidionysiou K, Falhammar H, Gu HF, Catrina SB. Protective Effect of the HIF-1A Pro582Ser Polymorphism on Severe Diabetic Retinopathy. J Diabetes Res. 2019;2019:2936962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, Januszyk M, Brownlee M, Gurtner GC. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A. 2009;106:13505-13510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 355] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 32. | Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975-27981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 417] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 33. | Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu M, Le YZ, Ge J, Johnson RS, Ma JX. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells. Diabetologia. 2011;54:1554-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (16)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alsubai J, Okada S S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX