Published online Apr 15, 2021. doi: 10.4239/wjd.v12.i4.499
Peer-review started: December 11, 2020
First decision: January 11, 2021
Revised: January 12, 2021
Accepted: March 7, 2021
Article in press: March 7, 2021
Published online: April 15, 2021
Processing time: 118 Days and 2.1 Hours
Type 2 diabetes mellitus (T2DM) has been strongly associated with an increased risk of developing cognitive dysfunction and dementia. The mechanisms of diabetes-associated cognitive dysfunction (DACD) have not been fully elucidated to date. Some studies proved lower cerebral blood flow (CBF) in the hippocampus was associated with poor executive function and memory in T2DM. Increasing evidence showed that diabetes leads to abnormal vascular endothelial growth factor (VEGF) expression and CBF changes in humans and animal models. In this study, we hypothesized that DACD was correlated with CBF alteration as measured by three-dimensional (3D) arterial spin labeling (3D-ASL) and VEGF expression in the hippocampus.
To assess the correlation between CBF (measured by 3D-ASL and VEGF expression) and DACD in a rat model of T2DM.
Forty Sprague-Dawley male rats were divided into control and T2DM groups. The T2DM group was established by feeding rats a high-fat diet and glucose to induce impaired glucose tolerance and then injecting them with streptozotocin to induce T2DM. Cognitive function was assessed using the Morris water maze experiment. The CBF changes were measured by 3D-ASL magnetic resonance imaging. VEGF expression was determined using immunofluorescence.
The escape latency time significantly reduced 15 wk after streptozotocin injection in the T2DM group. The total distance traveled was longer in the T2DM group; also, the platform was crossed fewer times. The percentage of distance in the target zone significantly decreased. CBF decreased in the bilateral hippocampus in the T2DM group. No difference was found between the right CBF value and the left CBF value in the T2DM group. The VEGF expression level in the hippocampus was lower in the T2DM group and correlated with the CBF value. The escape latency negatively correlated with the CBF value. The number of rats crossing the platform positively correlated with the CBF value.
Low CBF in the hippocampus and decreased VEGF expression might be crucial in DACD. CBF measured by 3D-ASL might serve as a noninvasive imaging biomarker for cognitive impairment associated with T2DM.
Core Tip: This study aimed to assess the correlation between cerebral blood flow measured by three-dimensional arterial spin labeling, vascular endothelial growth factor expression, and diabetes-associated cognitive dysfunction in a rat model of type 2 diabetes (T2D). Our results showed low cerebral blood flow in the hippocampus and decreased vascular endothelial growth factor expression might be crucial in diabetes-associated cognitive dysfunction. Cerebral blood flow measured by three-dimensional arterial spin labeling might serve as a noninvasive imaging biomarker for cognitive impairment associated with T2D. This study would help in the early detection of diabetes-associated cognitive dysfunction and guide treatment.
- Citation: Shao JW, Wang JD, He Q, Yang Y, Zou YY, Su W, Xiang ST, Li JB, Fang J. Three-dimensional-arterial spin labeling perfusion correlation with diabetes-associated cognitive dysfunction and vascular endothelial growth factor in type 2 diabetes mellitus rat. World J Diabetes 2021; 12(4): 499-513
- URL: https://www.wjgnet.com/1948-9358/full/v12/i4/499.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i4.499
Type 2 diabetes mellitus (T2DM) is an endocrine and chronic metabolic disorder characterized by insulin resistance and insulin deficiency caused by pancreatic b-cell dysfunction[1]. The number of people with diagnosed or undiagnosed diabetes, aged 20-64 years, was 351.7 million in 2019, and T2DM accounted for more than 90% of all cases. Approximately 116.4 million adults aged 20-79 years have diabetes in China, which ranked first worldwide. The United States has the third most affected adults (31.0 million)[2]. T2DM is one of the largest public health problems and represents a global public health challenge due to a gradual increase in its incidence.
The major complications of T2DM are stroke, dementia, and depression. It is associated with a 1.5 times increased risk of dementia[3]. Dementia is a complex condition marked by diminished cognitive performance (i.e. language, memory, visuospatial, and executive functions), affecting the quality of patients’ everyday lives[4,5]. T2DM has been strongly associated with an increased risk of developing Alzheimer’s disease and vascular dementia, accounting for 95% of all dementia cases[6,7]. The mechanisms of diabetes-associated cognitive dysfunction (DACD) have not been fully elucidated to date. The white matter disease of vascular origin, inflammation, cerebral insulin resistance, axonal loss, and vascular endothelial dysfunction may be the mechanisms underlying the dementia risk in diabetes[8-10].
Vascular endothelial growth factor (VEGF; also known as VEGF-A) is involved in microvascular structure and function and the development of axon branching[11,12]. Many studies have shown that enhancing VEGF signaling or VEGF restoration can ameliorate cognitive function[13,14]. Most of these studies have focused on the relationship between serum VEGF level and cognitive performance. Few studies explored how the differences in VEGF expression in the hippocampus influenced cognitive ability.
The hippocampus is a part of the limbic system. It has multimodal roles in the integration of information from short-term memory with that from long-term memory[15-17]. Perception and memory impairment have resulted from damage to the hippocampus, in turn contributing to cognitive dysfunction[18]. Most previous brain studies on T2DM have revealed the whole brain and hippocampal macrostructural changes in diabetes using magnetic resonance imaging (MRI), including cerebral perfusion changes, atrophy, and decreased white matter fiber connection linked to poor cognitive performance[19-22]. Previous findings showed that lower cerebral blood flow (CBF) in the hippocampus was associated with poor executive function and memory in T2DM[23,24].
Three-dimensional pseudo-continuous arterial spin labeling (3D-ASL) provides an intrinsically high signal-to-noise ratio and precise detection of CBF[25]. It is noninvasive MRI technique that involves no contrast agent or ionizing radiation. In this study, the 3D-ASL approach was applied to analyze CBF in the hippocampus. Despite increasing evidence that diabetes leads to abnormal VEGF expression and CBF changes in humans and animal models, no study explored whether VEGF signaling in the hippocampus in the T2DM animal model was related to CBF measured by 3D-ASL or aimed to assess the relationship between VEGF expression in the hippocampus and DACD, which became the objectives of the present study.
Sprague-Dawley rats have been used as a model of type 2 diabetes in many experiments. In this study, T2DM Sprague-Dawley rats were used to conduct the experiments. The study found that diabetes led to a reduction of VEGF expression in the hippocampus of rats with T2DM. Decreased VEGF expression in the hippocampus and reduction of hippocampal CBF positively correlated with poor cognitive function.
The experimental procedures were carried out according to the National Institutes of Health guidelines for the care of experimental animals with approval from the institutional animal ethics committee of the Kunming Medical University. All efforts were made to minimize suffering or animal discomfort and the number of animals used.
An online power and sample size calculator (Power and Sample Size.com) was used to calculate the sample size. Forty specific-pathogen-free grade Sprague-Dawley male rats (aged 4-5 wk and weighing 200-220 g) from the Experimental Animal Centre of Kunming Medical University (certificate o. SCXK 2015-0002; Kunming, China) were used in this study. Before initiating the protocol, all animals were acclimatized in the institutional animal house for 2 wk. The animals were randomly divided into two groups: normal control (n = 20) and T2DM (n = 20). Weight and fasting blood glucose (FBG) were measured before the beginning of the experimental procedure. All animals were allowed free access to water with a normal day/night cycle (12 h/12 h), a relative humidity of 40%-50%, and a temperature of 20-25 °C. The normal control group was fed standard chow, whereas the T2DM group was kept on a high-fat diet (41% carbohydrate, 24% protein, and 24% fat; 4.73 kcal/g; Beijing HFK Bioscience Co., Ltd. Beijing, China) for 3 wk (certificate no. SCXK 2019-0008; Beijing, China).
Streptozotocin (STZ) was purchased from Sigma (MO, United States). After overnight fasting, STZ was administered by intraperitoneal injections on the first, third, and fifth days in the first round, which was repeated with the same dose of STZ on the 21st, 23rd, and 25th days in the second round[26]. The elevated glucose levels in T2DM were evaluated on day 7 (24 h after the last administration). The blood glucose level was measured using an Accu-Chek glucometer (Roche, Mannheim, Germany) in tail-tip blood samples from overnight fasted animals. The rats with FBG > 16.7 mmol/L were considered as the T2DM model[27].
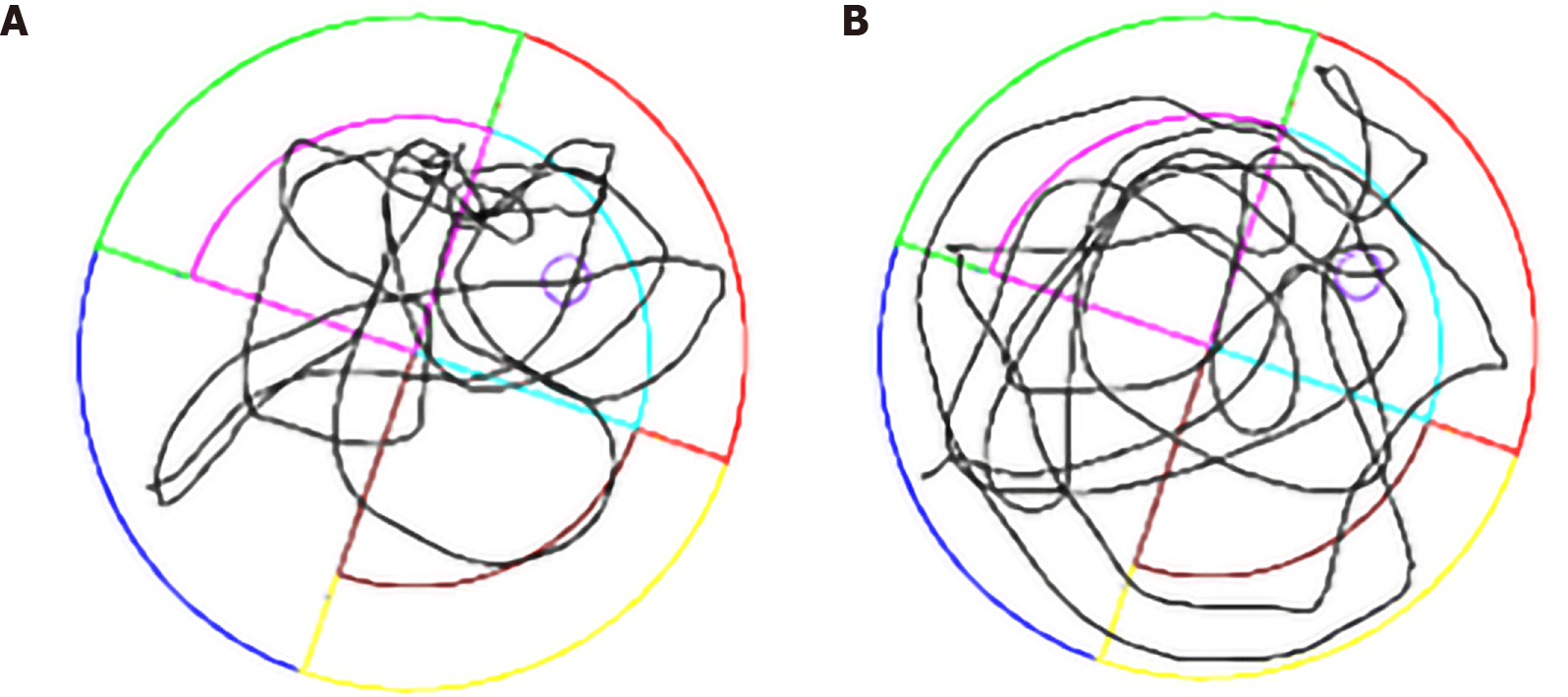
The spatial learning and reference memory of each rat were tested in a Morris water maze (MWM) after the model was successfully established (15 wk after STZ injection). MWM testing was conducted for 6 d, including place navigation for 5 d and spatial probe for 1 d[28]. All behavioral tests were performed under controlled environmental conditions; the water temperature was kept at 22 °C with silence and dim illumination[29]. MWM testing was conducted in a black circular pool, 2 m in diameter and 0.2 m deep filled with water. The water was made opaque with black ink. The position of the hidden platform was kept constant during all the trials involving spatial navigation, and the amount of water exceeded 1 cm of the platform. Each rat was placed at a fixed starting position in every quadrant in spatial navigation and was allowed to swim until the rat reached the platform. The swimming time was 60 s. If it exceeded this time, the rat was guided to the platform. In this procedure, the escape latency and traveled distance were recorded using a video tracking system (ANY-Maze, San Diego Instrument, CA, United States). The interval for each rat between the same tests was 15 min. The platform was removed in the spatial probe test to assess memory consolidation (Figure 1). The swimming trajectory within 60 s and the number of crossings of the platform location were recorded.
After MWM experiment, the rats were anesthetized with 3.6% chloral hydrate (360 mg/kg, intraperitoneal injection). MRI was performed using a 1.5T scanner (Signa Excite TwinSpeed HDx, GE Healthcare, WI, United States) with a special coil for rats (Shanghai Chenguang Medical Technologies Co., Shanghai, China). The MRI protocol included T1-weighted imaging, T2-weighted imaging, and 3D-ASL. T1-weighted imaging included brain sagittal images, whereas T2-weighted imaging and 3D-ASL included brain coronal images of the hippocampus. MRI scans were acquired in the supine position of the rat. The parameters used were as follows: T1-weighted imaging: time of repetition/time of echo = 260 ms/10.4 ms; scanning time = 138 s; field of view = 100 mm × 100 mm; slice thickness = 2.0 mm; and interslice gap = 0. T2-weighted imaging: time of repetition/time of echo = 2200 ms/85 ms; slice thickness = 1.0 mm; interslice gap = 0; number of excitations = 4; number of slices = 16; matrix size = 224 × 192; field of view = 100 mm × 100 mm; and scanning time = 331 s. 3D-ASL: time of repetition/time of echo = 4132 ms/11 ms; slice thickness = 2.0 mm; no interslice gap; number of excitations = 5; field of view = 80 mm × 80 mm; bandwidth = 62.5 kHz; matrix = 512 point × 12 arms; post-label delay = 1025 ms; and scanning time = 595 s.
3D-ASL image preprocessing and analysis were implemented using the Advantage Workstation (Advantage Workstation version 4.7, GE Medical Systems, United States), with the Function Tool software package. The threshold was adjusted, and the region of imaging in the bilateral hippocampal area of rats was chosen to measure CBF. Every position was repeated three times and averaged.
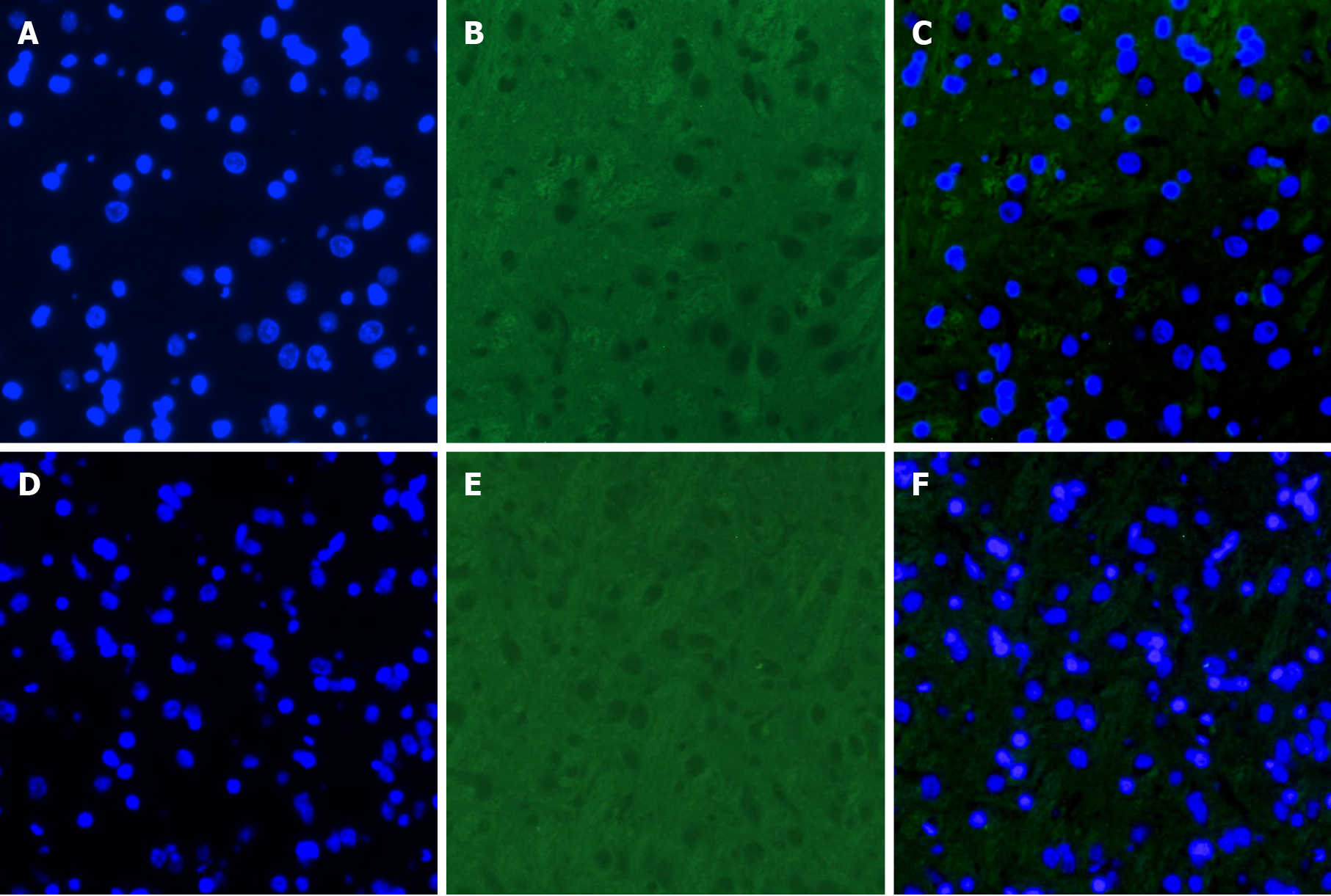
The rats were sacrificed with an overdose of chloral hydrate. They were given intracardial perfusion of 4% paraformaldehyde, and the brain tissues (hippocampus) of the mice were harvested. The hippocampal tissue samples were frozen in liquid nitrogen and stored at -80 °C prior to proteomic analysis. The hippocampus was dehydrated and then embedded in paraffin. One 10-μm-thick and four 20-μm-thick sections were cut, and 4’, 6-diamidino-2-phenylindole staining was applied for morphological assessment and immunofluorescence staining. All examinations were performed on the bilateral hippocampus. Paraffin sections of the hippocampal tissues were rehydrated and boiled in ethylene diamine tetraacetic acid buffer for 10 min to induce antigen retrieval. Immunofluorescence of VEGF was performed using commercial kits following the manufacturer’s protocols. The sections (40 μm) were incubated with the primary VEGF antibody (Abcam Biotech Co., Ltd., MA, United States). Subsequently, the mixture was incubated with secondary antibodies (Beyotime Biotech Co., Ltd., Nanjing, China). Immunofluorescence-stained sections were observed under a Zeiss Pascal laser scanning confocal microscope (Carl Zeiss International, Jena, Germany).
Total RNA was isolated from the hippocampal tissue of rats in the control and T2DM groups using RNAiso Plus reagent (Takara, Dalian, China) and then reverse transcribed into cDNA using HiScript III RT SuperMix for quantitative PCR (qPCR) (+g DNA wiper) (Vazyme Biotech Co., Ltd., Nanjing, China). Real-time qPCR was performed using miRNA Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.) on a BioRad qPCR system with the following primer pair: 5’-TGCATGGTGACTGCTACCTTCTC-3’, 5’-AAATCACAGCAGCCTACCCACTC-3’. The PCR conditions started with a denaturation step at 94 °C for 5 min, followed by 30 cycles of denaturation at 94°C for 25 s, annealing at 50.5 °C for 40 s, and extension at 72 °C for 30 s, ending with a final extension step at 72 °C for 5 min. RNA relative expression was calculated by the 2-ΔΔCt method with reduced glyceraldehyde-phosphate dehydrogenase as the control.
The data were analyzed using the SPSS version 23.0 statistical package (IBM Corp., NY, United States). Normal distribution within samples was assessed using the Kolmogorov-Smirnov test for normal distribution. Normally distributed data were presented as the mean ± SD. CBF value-derived 3D-ASL, target crossing times, distant in zone (%) platform, and total distance traveled were analyzed using the unpaired-sample Student t test between the normal and T2DM groups. Correlation coefficients between CBF and VEGF expression were calculated with the Spearman rank test. Multivariate analysis of variance was conducted for the time of escape latency for each group of rats. For behavior data, Sidak’s multiple comparisons test was conducted to correct multiple comparisons using a two-way ANOVA. For behavioral parameters and to compare VEGF levels, statistical analyses were carried out using GraphPad Prism 8.0 (GraphPad Software, Inc., CA, United States). In addition, a P value < 0.05 was considered significant.
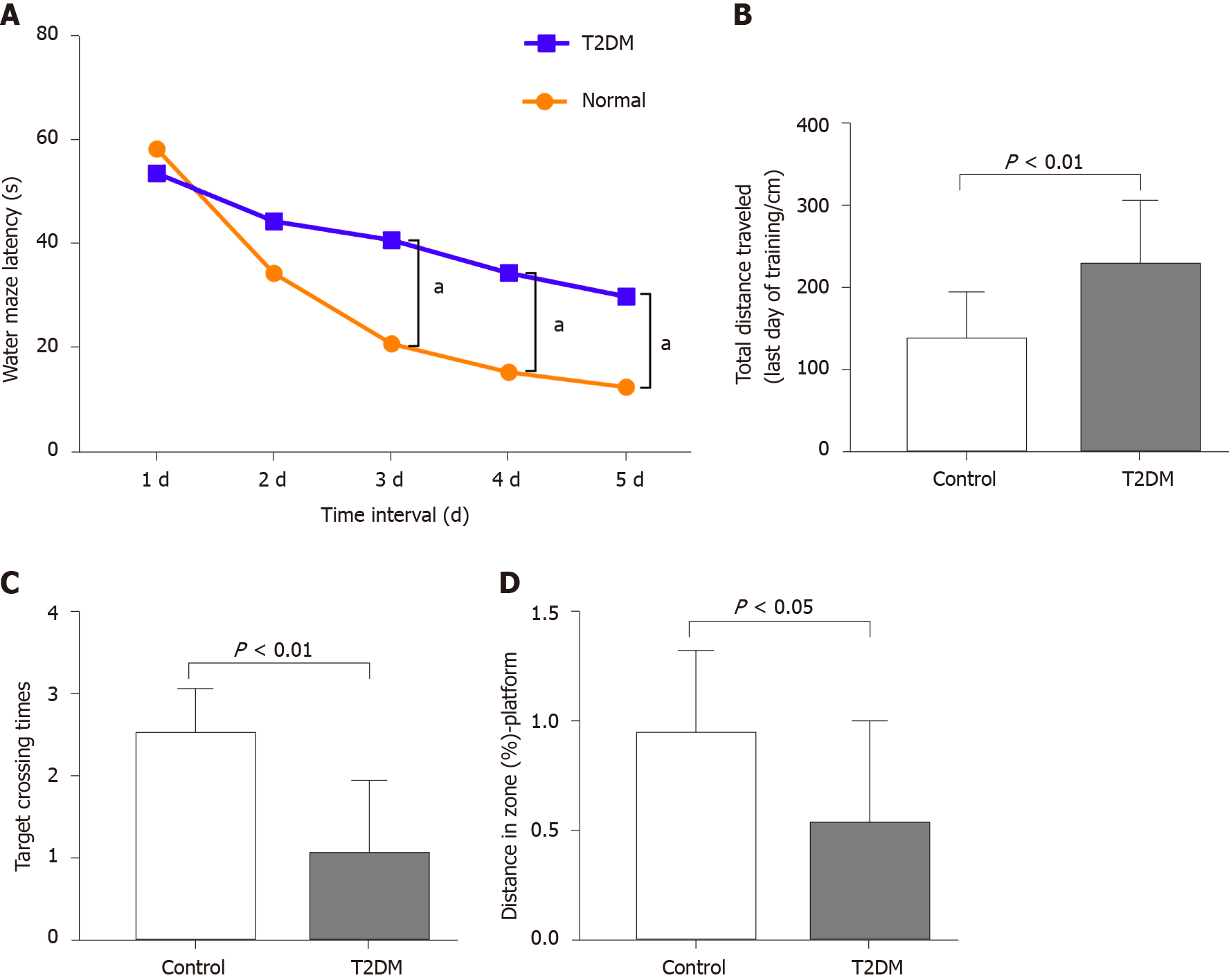
The MWM test was conducted to explore the learning performance and spatial memory ability of experimental rats. The positioning cruise experiments showed that the swimming trajectory of the rats in the T2DM group was more chaotic (Figure 1). It was reflected as a significant increase in the time of escape latency compared with the normal group (F = 21.07, P < 0.0001) (Table 1). The total distance traveled was longer in the T2DM group than in the control group (t = 2.053, P = 0.003), and the platform was crossed fewer times (t = 2.491, P = 0.006). The percentage of distance in the zone target significantly decreased (t = 1.447, P = 0.020) (Figure 2). In the present study, greater time spent in the T2DM group was also reflected in the probe testing on day 5 of the experiment. These data indicated that the spatial learning memory ability in the T2DM group significantly decreased (Table 2).
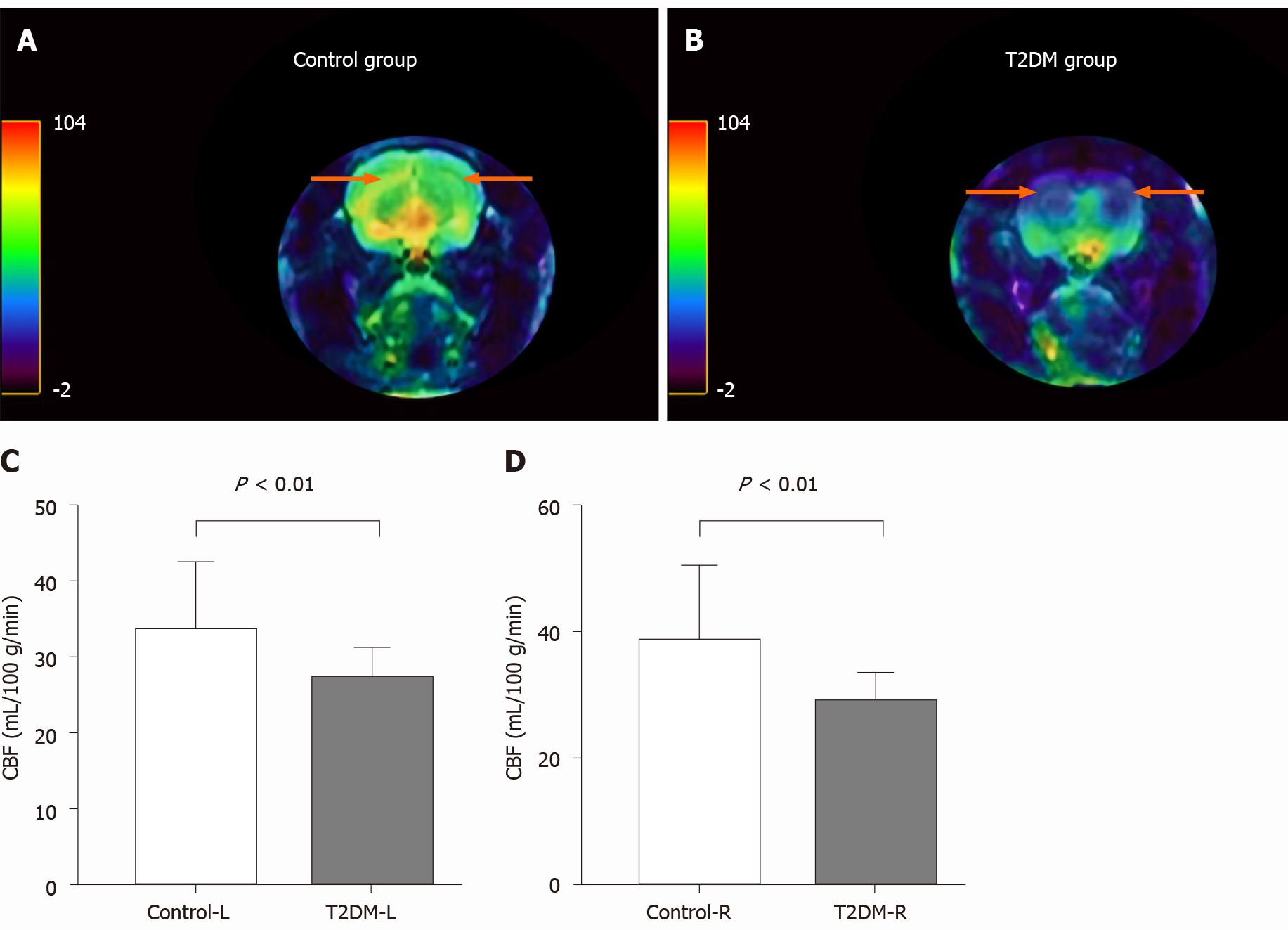
CBF decreased in the bilateral hippocampal area in rats in the T2DM group. The left CBF value in the control and T2DM groups was 33.58 ± 2.91 mL/100 g/min and 27.20 ± 0.87 mL/100 g/min (t = 2.772, P = 0.0093), respectively. The right CBF value in the control and T2DM groups was 38.62 ± 3.76 mL/100 g/min and 29.0 ± 0.98 mL/100g/min (t = 3.373, P = 0.0020), respectively (Figure 3). No difference was observed between the right CBF value and the left CBF value in the T2DM group (P = 0.173).
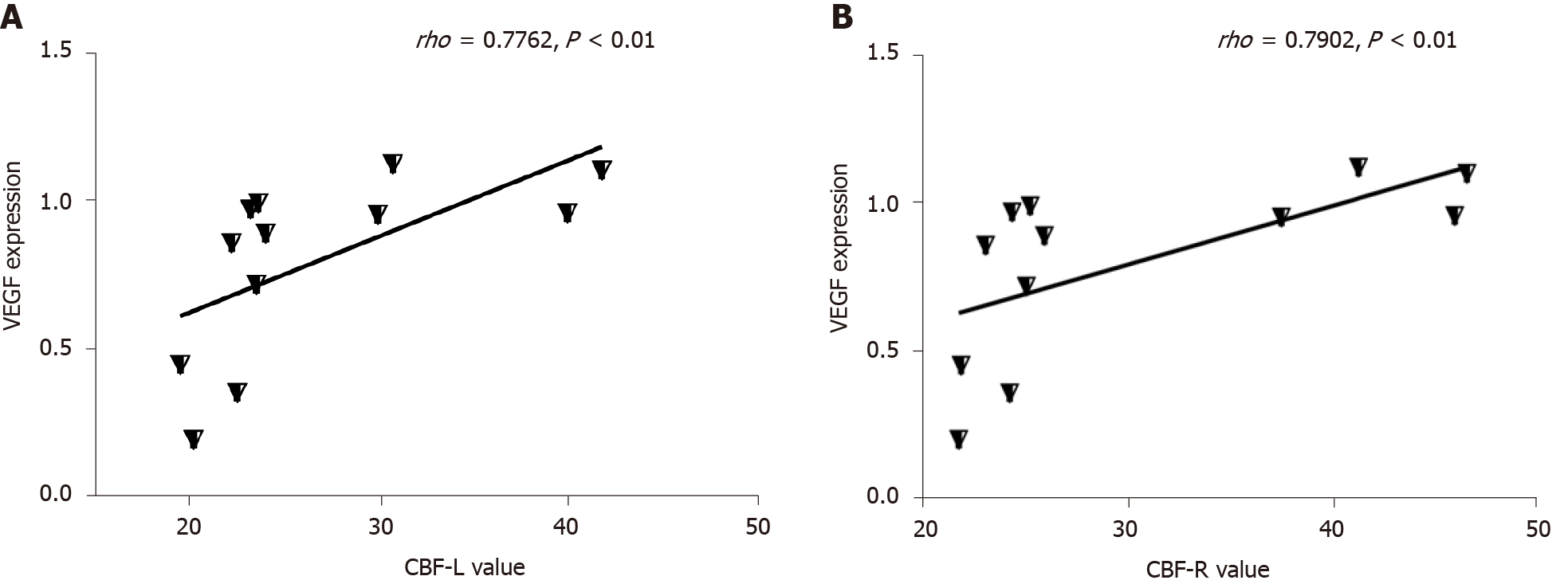
The expression of VEGF was lower in the T2DM group than in the control group (t = 2.768, P = 0.0325) (Figures 4 and 5). VEGF expression positively correlated with the left CBF value (rho = 0.776, P < 0.01) and the right CBF value (rho = 0.790, P < 0.01) (Figure 6). These data suggested that CBF in rats with T2DM might develop, at least partially, due to the decreased expression of VEGF.
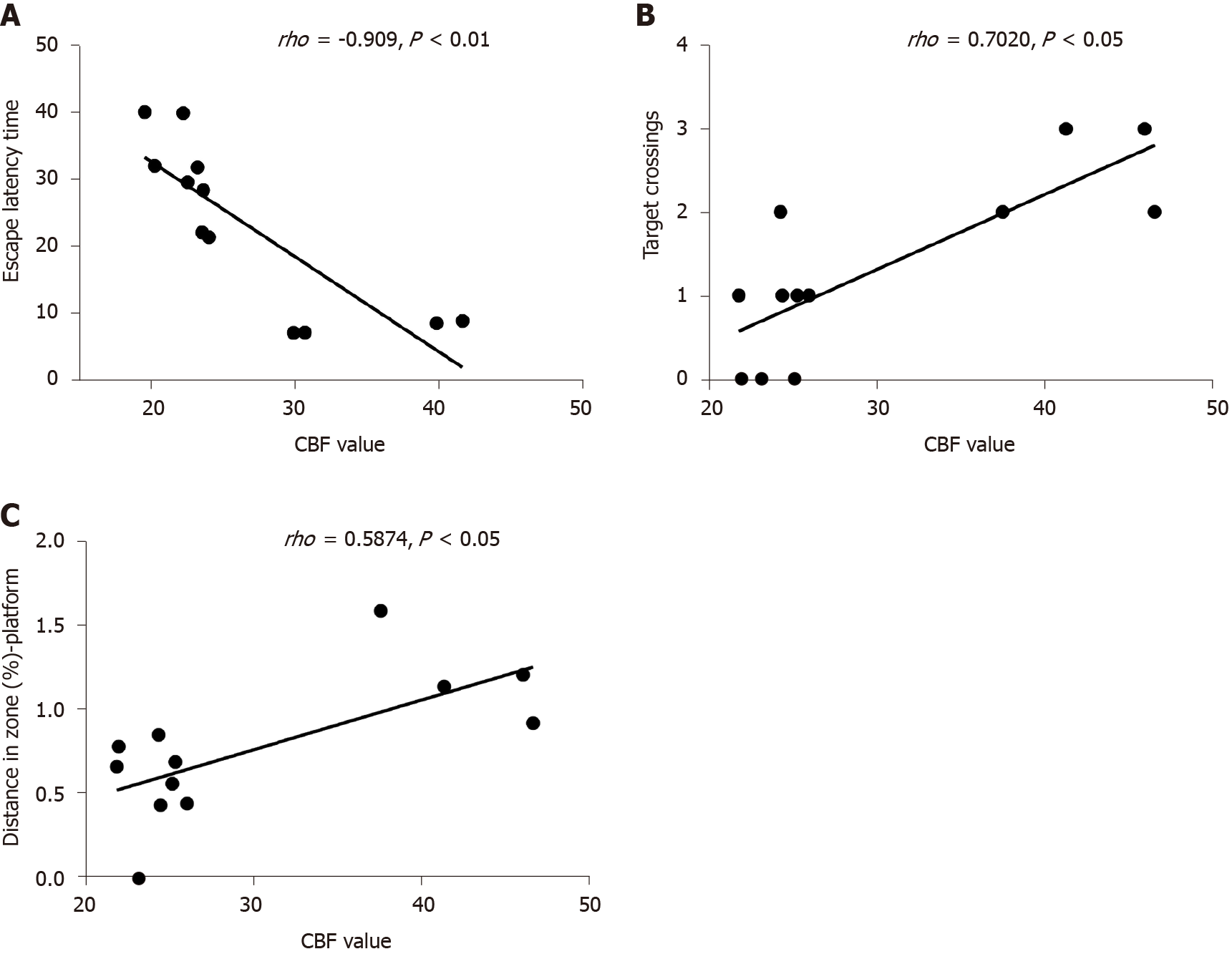
The escape latency negatively correlated with the CBF value (rho = -0.909, P < 0.01). The number of rats crossing the platform positively correlated with the CBF value (rho = 0.702, P < 0.05). A significant positive correlation was found between CBF and distance in the zone target (rho = 0.587, P < 0.05) (Figure 7).
Behavioral methods, including the MWM test, were used in this study to detect the changes in cognitive function. The changes in CBF in a rat model of T2DM were observed compared with those in the control group. In addition, the relationship between VEGF expression, CBF, and DACD was further explored. Several studies confirmed that T2DM led to cognitive impairment[10,30], but the mechanisms were unclear. The present study showed that the spatial memory and the reference memory in the MWM test were impaired in the T2DM group compared with the control group. CBF in the hippocampus of rats in the T2DM group was significantly reduced and was positively correlated with the data obtained from MWM, suggesting that low perfusion in the hippocampus was indeed associated with DACD. The correlation analysis also supported the conclusion that hypoperfusion in the hippocampus was indeed a risk factor for DACD[31,32]. However, no difference was found between the right CBF value and the left CBF value in the T2DM group. The study confirmed that decreased CBF in the hippocampus could be considered as an imaging biomarker to predict the risk of DACD, which was consistent with previous findings[24,33].
The hippocampus is an important anatomical structure related to cognition, particularly memory function. The hippocampal region is prone to suffer from cerebral microvascular disease due to its thin blood vessels and the relative lack of capillary anastomoses[15,34]. Therefore, it was speculated that the pathological changes in small vessels caused by a hyperglycemic environment could easily lead to a reduction of hippocampal CBF. Decreases in regional blood flow in the hippocampus can contribute to an ischemic and hypoxic environment, leading to neuronal damage in the hippocampus and cognitive impairment[35,36]. Previous studies focused mainly on the effect of hypoperfusion on cognition. No experimental study explored how VEGF expression in the hippocampus affected CBF based on 3D-ASL under the influence of long-term hyperglycemia in a T2DM rat model. In this study, the effect of VEGF on hippocampal perfusion in diabetes was studied by immunofluorescence detection of VEGF. The results showed that the expression of VEGF in the hippocampus was significantly lower in the T2DM group compared with the control group, and positively correlated with CBF. Nevertheless, previous experimental findings on the changes in VEGF expression in the hippocampus in diabetes were controversial[37-39].
Previous data indicated that different VEGF levels might be due to the different stages of diabetes. High VEGF expression mainly existed in the early stage of diabetes, while low levels were seen in the late stage[40]. In the present study, the T2DM rat model (15 wk after STZ injection) already developed diabetic complications, which usually occurred in the late stage[41]. Thus, it was speculated that the low level of VEGF expression might be related to long-term hyperglycemia and late stage of the disease. However, this needs further investigation. A positive correlation was observed between decreased VEGF signaling and low CBF in the present study. Abnormal VEGF signaling caused by diabetes can lead to vascular dysfunction and pathological vessel remodeling, leading to vascular occlusion and insufficient blood supply[42-45]. Meanwhile, some studies found that the inhibition of VEGF signaling affected hippocampal dentate gyrus microvasculature and caused impairment in spatial memory[46]. Therefore, the decreased expression of VEGF in the late stage of diabetes led to hypoperfusion in the hippocampus, as found in the present study, leading to cognitive abnormalities.
This study was novel in investigating the correlation between CBF based on 3D-ASL, VEGF expression, and cognition in a T2DM rat model. However, it had certain limitations: (1) Previous studies revealed that the effect of VEGF expression on memory was primarily a result of axonal loss, demyelination, or changing plasticity of mature neurons[12,39,47]. The possibility that the decreased VEGF level might cause DACD via hippocampal neural injury was not examined in the present study; and (2) Previous findings indicated that each division of the hippocampus had different functions[48]. In the present study, CBF and VEGF expression in each hippocampal region were not examined. Thus, future studies are needed for detailed investigation.
Low perfusion of the hippocampus was associated with DACD. VEGF expression decreased in the hippocampal area of rats in the T2DM group in long-term hyperglycemia. Positive correlations were observed between CBF and VEGF expression in the hippocampus of rats with T2DM. Decreased CBF and low VEGF levels in the hippocampus might be risk factors of DACD. CBF measured by 3D-ASL might serve as a noninvasive imaging biomarker for detecting cognitive impairment associated with T2DM.
The mechanisms of diabetes-associated cognitive dysfunction (DACD) have not been fully elucidated to date. Some studies proved that lower cerebral blood flow (CBF) in the hippocampus was associated with poor executive function and memory in type 2 diabetes mellitus (T2DM). Increasing evidence showed that diabetes leads to abnormal vascular endothelial growth factor (VEGF) expression and CBF changes in humans and animal models. This study explored whether DACD was correlated with CBF alteration and VEGF expression in the hippocampus.
Our study aimed to assess the relationship among CBF alteration, VEGF expression in the hippocampus, and DACD. Our findings may help reveal the mechanisms of DACD. This study would help in the detection of DACD and guide treatment.
This study aimed to explore whether VEGF signaling in the hippocampus in the T2DM rat model was related to CBF (measured by three dimensional arterial spin labeling) and DACD.
Forty specific-pathogen-free grade Sprague-Dawley male rats were randomly divided into normal control and T2DM groups. The T2DM group was kept on a high-fat diet and then streptozotocin was administered by intraperitoneal injections to induce diabetes. The Morris water maze test was conducted to explore the learning performance and spatial memory ability of experimental rats. CBF measured by three dimensional arterial spin labeling was detected in the bilateral hippocampus. Immunofluorescence of VEGF in the bilateral hippocampus was performed, and VEGF expression was quantified with quantitative real-time PCR.
Our data indicated that the spatial learning memory ability in the T2DM group significantly decreased. An obvious reduction in CBF in rats with T2DM in the bilateral hippocampal area was observed. The expression of VEGF was lower in the T2DM group than in the control group. VEGF expression positively correlated with the CBF value in the hippocampus. A significant correlation was found between CBF and the spatial learning memory ability in the T2DM group.
The new theories of this study was low perfusion of the hippocampus was associated with DACD and decreased VEGF expression in the hippocampal area of rats in the T2DM group in long-term hyperglycemia. To the best of our knowledge, this was the first study to explore the relationship of DACD, VEGF expression, and CBF of the hippocampus.
Decreased CBF and low VEGF levels in the hippocampus might be risk factors for DACD. CBF measured by three dimensional arterial spin labeling might serve as a noninvasive imaging biomarker for detecting cognitive impairment associated with T2DM.
We would like to thank each author, especially Zou YY, MD, for advice on diabetic mouse models and the histopathologic procedures.
| 1. | Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1811] [Article Influence: 201.2] [Reference Citation Analysis (1)] |
| 2. | International Diabetes Federation. International Diabetes Federation Diabetes Atlas. 9th ed. Brussels: The Institute; 2019. |
| 3. | Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 723] [Article Influence: 55.6] [Reference Citation Analysis (6)] |
| 4. | McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 5. | Biessels GJ, Whitmer RA. Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia. 2020;63:3-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 6. | Kubis-Kubiak A, Dyba A, Piwowar A. The Interplay between Diabetes and Alzheimer's Disease-In the Hunt for Biomarkers. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Albai O, Frandes M, Timar R, Roman D, Timar B. Risk factors for developing dementia in type 2 diabetes mellitus patients with mild cognitive impairment. Neuropsychiatr Dis Treat. 2019;15:167-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 962] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 9. | van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 480] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 10. | Moulton CD, Stewart R, Amiel SA, Laake JP, Ismail K. Factors associated with cognitive impairment in patients with newly diagnosed type 2 diabetes: a cross-sectional study. Aging Ment Health. 2016;20:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Gerber HP, Wu X, Yu L, Wiesmann C, Liang XH, Lee CV, Fuh G, Olsson C, Damico L, Xie D, Meng YG, Gutierrez J, Corpuz R, Li B, Hall L, Rangell L, Ferrando R, Lowman H, Peale F, Ferrara N. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc Natl Acad Sci USA. 2007;104:3478-3483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Luck R, Urban S, Karakatsani A, Harde E, Sambandan S, Nicholson L, Haverkamp S, Mann R, Martin-Villalba A, Schuman EM, Acker-Palmer A, Ruiz de Almodóvar C. VEGF/VEGFR2 signaling regulates hippocampal axon branching during development. Elife. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Ortuzar N, Rico-Barrio I, Bengoetxea H, Argandoña EG, Lafuente JV. VEGF reverts the cognitive impairment induced by a focal traumatic brain injury during the development of rats raised under environmental enrichment. Behav Brain Res. 2013;246:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Zhao Q, Niu Y, Matsumoto K, Tsuneyama K, Tanaka K, Miyata T, Yokozawa T. Chotosan ameliorates cognitive and emotional deficits in an animal model of type 2 diabetes: possible involvement of cholinergic and VEGF/PDGF mechanisms in the brain. BMC Complement Altern Med. 2012;12:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, Brown T, DeCarli C, Barnes CA, Mayeux R, Vannucci SJ, Small SA. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol. 2008;64:698-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Robin J, Rai Y, Valli M, Olsen RK. Category specificity in the medial temporal lobe: A systematic review. Hippocampus. 2019;29:313-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Fotowat H, Lee C, Jun JJ, Maler L. Neural activity in a hippocampus-like region of the teleost pallium is associated with active sensing and navigation. Elife. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Gros A, Wang SH. Behavioral tagging and capture: long-term memory decline in middle-aged rats. Neurobiol Aging. 2018;67:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Sun Q, Chen GQ, Wang XB, Yu Y, Hu YC, Yan LF, Zhang X, Yang Y, Zhang J, Liu B, Wang CC, Ma Y, Wang W, Han Y, Cui GB. Alterations of White Matter Integrity and Hippocampal Functional Connectivity in Type 2 Diabetes Without Mild Cognitive Impairment. Front Neuroanat. 2018;12:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Li J, Guo Y, Li Q, Miao K, Wang C, Zhang D, Tian C, Zhang S. Presence of White Matter Lesions Associated with Diabetes-Associated Cognitive Decline in Male Rat Models of Pre-Type 2 Diabetes. Med Sci Monit. 2019;25:9679-9689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Münch G, Wood AG, Forbes J, Greenaway TM, Pearson S, Srikanth V. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36:4036-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 22. | Brundel M, van den Berg E, Reijmer YD, de Bresser J, Kappelle LJ, Biessels GJ; Utrecht Diabetic Encephalopathy Study group. Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. J Diabetes Complications. 2012;26:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Bangen KJ, Werhane ML, Weigand AJ, Edmonds EC, Delano-Wood L, Thomas KR, Nation DA, Evangelista ND, Clark AL, Liu TT, Bondi MW. Reduced Regional Cerebral Blood Flow Relates to Poorer Cognition in Older Adults With Type 2 Diabetes. Front Aging Neurosci. 2018;10:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Hu B, Yan LF, Sun Q, Yu Y, Zhang J, Dai YJ, Yang Y, Hu YC, Nan HY, Zhang X, Heng CN, Hou JF, Liu QQ, Shao CH, Li F, Zhou KX, Guo H, Cui GB, Wang W. Disturbed neurovascular coupling in type 2 diabetes mellitus patients: Evidence from a comprehensive fMRI analysis. Neuroimage Clin. 2019;22:101802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X. Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology. 2016;281:337-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 394] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 26. | Sharma G, Ashhar MU, Aeri V, Katare DP. Development and characterization of late-stage diabetes mellitus and -associated vascular complications. Life Sci. 2019;216:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, Gong Z, Gao J, Mu Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. 2019;10:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3541] [Cited by in RCA: 3555] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 29. | Cassano V, Leo A, Tallarico M, Nesci V, Cimellaro A, Fiorentino TV, Citraro R, Hribal ML, De Sarro G, Perticone F, Sesti G, Russo E, Sciacqua A. Metabolic and Cognitive Effects of Ranolazine in Type 2 Diabetes Mellitus: Data from an in vivo Model. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Biessels GJ, Verhagen C, Janssen J, van den Berg E, Zinman B, Rosenstock J, George JT, Passera A, Schnaidt S, Johansen OE; CARMELINA Investigators. Effect of Linagliptin on Cognitive Performance in Patients With Type 2 Diabetes and Cardiorenal Comorbidities: The CARMELINA Randomized Trial. Diabetes Care. 2019;42:1930-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Ogoh S. Relationship between cognitive function and regulation of cerebral blood flow. J Physiol Sci. 2017;67:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Heo S, Prakash RS, Voss MW, Erickson KI, Ouyang C, Sutton BP, Kramer AF. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 2010;1315:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Yu Y, Yan LF, Sun Q, Hu B, Zhang J, Yang Y, Dai YJ, Cui WX, Xiu SJ, Hu YC, Heng CN, Liu QQ, Hou JF, Pan YY, Zhai LH, Han TH, Cui GB, Wang W. Neurovascular decoupling in type 2 diabetes mellitus without mild cognitive impairment: Potential biomarker for early cognitive impairment. Neuroimage. 2019;200:644-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Perosa V, Priester A, Ziegler G, Cardenas-Blanco A, Dobisch L, Spallazzi M, Assmann A, Maass A, Speck O, Oltmer J, Heinze HJ, Schreiber S, Düzel E. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain. 2020;143:622-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 35. | Woo MA, Ogren JA, Abouzeid CM, Macey PM, Sairafian KG, Saharan PS, Thompson PM, Fonarow GC, Hamilton MA, Harper RM, Kumar R. Regional hippocampal damage in heart failure. Eur J Heart Fail. 2015;17:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Rossini PM, Caltagirone C, Silvestrini M. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke. 2001;32:1552-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Oliveira WH, Nunes AK, França ME, Santos LA, Lós DB, Rocha SW, Barbosa KP, Rodrigues GB, Peixoto CA. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016;1644:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 38. | Zhang T, Jia W, Sun X. 3-n-Butylphthalide (NBP) reduces apoptosis and enhances vascular endothelial growth factor (VEGF) up-regulation in diabetic rats. Neurol Res. 2010;32:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Taylor SL, Trudeau D, Arnold B, Wang J, Gerrow K, Summerfeldt K, Holmes A, Zamani A, Brocardo PS, Brown CE. VEGF can protect against blood brain barrier dysfunction, dendritic spine loss and spatial memory impairment in an experimental model of diabetes. Neurobiol Dis. 2015;78:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Jerić M, Vukojević K, Vuica A, Filipović N. Diabetes mellitus influences the expression of NPY and VEGF in neurons of rat trigeminal ganglion. Neuropeptides. 2017;62:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Wang-Fischer Y, Garyantes T. Improving the Reliability and Utility of Streptozotocin-Induced Rat Diabetic Model. J Diabetes Res. 2018;2018:8054073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Warren CM, Ziyad S, Briot A, Der A, Iruela-Arispe ML. A ligand-independent VEGFR2 signaling pathway limits angiogenic responses in diabetes. Sci Signal. 2014;7:ra1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | Mu ZH, Jiang Z, Lin XJ, Wang LP, Xi Y, Zhang ZJ, Wang YT, Yang GY. Vessel Dilation Attenuates Endothelial Dysfunction Following Middle Cerebral Artery Occlusion in Hyperglycemic Rats. CNS Neurosci Ther. 2016;22:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, Johnson M, Ergul A. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther. 2012;342:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Jenkins MJ, Edgley AJ, Sonobe T, Umetani K, Schwenke DO, Fujii Y, Brown RD, Kelly DJ, Shirai M, Pearson JT. Dynamic synchrotron imaging of diabetic rat coronary microcirculation in vivo. Arterioscler Thromb Vasc Biol. 2012;32:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 46. | Koester-Hegmann C, Bengoetxea H, Kosenkov D, Thiersch M, Haider T, Gassmann M, Schneider Gasser EM. High-Altitude Cognitive Impairment Is Prevented by Enriched Environment Including Exercise via VEGF Signaling. Front Cell Neurosci. 2018;12:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA. 2011;108:5081-5086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 48. | Pang CC, Kiecker C, O'Brien JT, Noble W, Chang RC. Ammon's Horn 2 (CA2) of the Hippocampus: A Long-Known Region with a New Potential Role in Neurodegeneration. Neuroscientist. 2019;25:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papazafiropoulou A S-Editor: Zhang H L-Editor: Filipodia P-Editor: Ma YJ