Published online Feb 15, 2021. doi: 10.4239/wjd.v12.i2.149
Peer-review started: November 19, 2020
First decision: November 30, 2020
Revised: December 10, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 15, 2021
Processing time: 64 Days and 19 Hours
Metabolic memory is important for the diagnosis and treatment of diabetes in the early stage, and in maintaining blood glucose concentrations within the normal range. The clinical diagnosis of diabetes mellitus is currently made using fasting plasma glucose, 2 h-plasma glucose (2h-PG) during a 75 g oral glucose tolerance test, and hemoglobin A1c (HbA1c) level. However, the fasting plasma glucose test requires fasting, which is a barrier to screening, and reproducibility of the 2h-PG level is poor. HbA1c is affected by a shortened red blood cell lifespan. In patients with anemia and hemoglobinopathies, the measured HbA1c levels may be inaccurate. Compared with HbA1c, glycated albumin (GA) is characterized by more rapid and greater changes, and can be used to diagnose new-onset diabetes especially if urgent early treatment is required, for example in gestational diabetes. In this study, we provided cutoff values for GA and evaluated its utility as a screening and diagnostic tool for diabetes in a large high-risk group study.
To evaluate the utility of GA in identifying subjects with diabetes in northeast China, and to assess the diagnostic accuracy of the proposed GA cutoff in the diagnosis of diabetes mellitus.
This cross-sectional study included 1935 subjects, with suspected diabetes or in high-risk groups, from 2014 to 2015 in the Second Affiliated Hospital of Harbin Medical University (Harbin, China). The use of GA to identify diabetes was investigated using the area under the receiver operating characteristic curve (AUC). The GA cutoffs were derived from different 2h-PG values with hemoglobin A1c cutoffs used as a calibration curve.
The GA cutoff for the diagnosis of diabetes mellitus was 15.15% from the receiver operating characteristic (ROC) curve. ROC analysis demonstrated that GA was an efficient marker for detecting diabetes, with an AUC of 90.3%.
Our study supports the use of GA as a biomarker for the diagnosis of diabetes.
Core Tip: Our study supports the use of glycated albumin (GA) as a biomarker for the diagnosis of diabetes. The GA cutoff for the diagnosis of diabetes mellitus was 15.15% from the receiver operating characteristic (ROC) curve. ROC analysis demonstrated that GA was an efficient marker for detecting diabetes, with an area under the ROC curve of 90.3%.
- Citation: Li GY, Li HY, Li Q. Use of glycated albumin for the identification of diabetes in subjects from northeast China. World J Diabetes 2021; 12(2): 149-157
- URL: https://www.wjgnet.com/1948-9358/full/v12/i2/149.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i2.149
Diabetes mellitus has become a worldwide health problem in both developed and developing countries, even in the least developed countries[1]. Hyperglycemia is a major risk factor for heart disease, kidney disease, stroke, and blindness, which all reduce the quality of life of patients with diabetes[2]. “Metabolic memory” is important for the diagnosis and treatment of diabetes in the early stage and in maintaining blood glucose concentrations within the normal range[3]. The clinical diagnosis of diabetes mellitus is currently made using fasting plasma glucose (FPG), 2 h-plasma glucose (2h-PG) during a 75 g oral glucose tolerance test (OGTT) and hemoglobin A1c (HbA1c) level[4]. However, the FPG test requires fasting, which is a barrier to screening, and reproducibility of the 2h-PG level is poor[5]. HbA1c is the standard for monitoring mean PG concentrations over 2-3 mo, and has been used in many clinical studies[6,7]. The guidelines from the American Diabetes Association and the World Health Organization propose the measurement of HbA1c as a diagnostic criterion for diabetes, suggesting a diagnostic cut-off of ≥ 6.5% (48 mmol/mol). The use of HbA1c for the diagnosis of diabetes is a complement to other measures. However, HbA1c is affected by a shortened red blood cell lifespan. In patients with anemia and hemoglobinopathies, the measured HbA1c levels may be inaccurate[8].
Glycated albumin (GA) as an additional clinical marker for average blood glucose level, reflects mean glycemia over approximately 2-3 wk[9]. Compared with HbA1c, GA is characterized by more rapid and greater changes, and can be used to diagnose new-onset diabetes especially if urgent early treatment is required, for example in gestational diabetes[10]. An ongoing investigation has shown that GA is a potential diagnostic tool for diabetes[11].
The aims of this study were to provide cutoff values for GA and to evaluate its utility as a screening and diagnostic tool for diabetes in a large high-risk group study.
This cross-sectional, high-risk based, large sample study evaluated the GA cut-off for the diagnosis of diabetes mellitus. A total of 1935 subjects aged 18-79 years took part in a comprehensive assessment, including a 75-g oral glucose tolerance test (OGTT), and the measurement of HbA1c and GA[12,13].
Following 8-12 h overnight fasting, a 75 g OGTT was conducted. Blood samples were obtained at 0, 30, 60, and 120 min after the glucose load. Glucose concentrations were measured using the hexokinase glucose-6-phosphate dehydrogenase method and measured by an automatic biochemical analyzer (Cs400B; Dirui Industrial Co., Ltd., Changchun, China). HbA1c levels in fresh whole blood samples were determined using the automated high-performance liquid chromatography (HPLC) method (Variant II; Bio-Rad, Hercules, CA, United States)[14]. GA levels were measured with the Lucica GA-L Kit (Asahi Kasei Pharma, Tokyo, Japan) and by an automatic biochemical analyzer (Cs400B; Dirui)[11]. Within and between-run coefficients of variation for the GA assay were 1.43% and 2.15%, and for the HbA1c assay were 0.99% and 1.48%, respectively. Serum triglycerides, high-density lipoprotein cholesterol, and uric acid concentrations were measured by enzymatic methods.
Diabetes was diagnosed according to the American Diabetes Association guidelines. In participants with no history of diabetes or treatment for diabetes, or criteria for asymptomatic diabetes, a new diagnosis of diabetes mellitus was made if FPG was ≥ 7.0 mmol/L and/or 2h-PG was ≥ 11.1 mmol/L and/or HbA1c was ≥ 6.5% (48 mmol/mol)[4].
All continuous variables are presented as the mean ± standard deviation. A linear relationship between variables was determined using the Pearson correlation coefficient. P < 0.05 was considered statistically significant. A receiver operating characteristic (ROC) curve was drawn to determine diagnostic sensitivity and specificity. The cut-off value of GA for newly diagnosed diabetes using the OGTT was calculated by ROC analysis using the Youden index (Y = sensitivity + specificity-1). Statistical analyses were performed using SPSS version 17.0 software.
The clinical characteristics of the study population are shown in Table 1. The mean age of the 1935 study subjects was 37.63 ± 10.56 years, and the mean body mass index was 22.8 ± 3.43 kg/m2. The participants were allocated to each group according to the OGTT results. Of these subjects, 376 were newly diagnosed diabetics, 816 had pre-diabetes, and 743 had normal glucose tolerance (NGT). Serum GA levels were 18.36%, 13.69%, 12.36% in subjects with newly diagnosed diabetes, pre-diabetes, and NGT, respectively. In addition, HbA1c increased from 5.2% to 7.3% from NGT to pre-diabetes and then diabetes.
| Characteristics | NGT | Pre-diabetes | Newly diagnosed diabetes |
| Sample number | 743 | 816 | 376 |
| Age (yr) | 28.11 ± 5.44 | 37.15 ± 12.81 | 47.63 ± 13.44 |
| Height (cm) | 162.04 ± 4.42 | 162.90 ± 6.85 | 164.15 ± 8.54 |
| Weight (kg) | 51.96 ± 5.53 | 61.17 ± 10.70 | 68.82 ± 12.32 |
| BMI (kg/m2) | 19.83 ± 2.39 | 23.07 ± 3.92 | 25.51 ± 3.99 |
| SBP (mmHg) | 109.68 ± 10.05 | 121.13 ± 17.15 | 132.24 ± 19.09 |
| DBP (mmHg) | 70.82 ± 7.30 | 77.53 ± 12.55 | 84.02 ± 13.14 |
| TC (mmol/L) | 3.65 ± 0.69 | 4.58 ± 0.91 | 5.25 ± 0.97 |
| TG (mmol/L) | 1.03 ± 0.44 | 1.65 ± 0.74 | 2.33 ± 0.99 |
| GA (%) | 12.36 ± 0.81 | 13.69 ± 1.45 | 18.35 ± 5.00 |
| HbA1c (%) | 5.22 ± 0.20 | 5.77 ± 0.49 | 7.31 ± 1.49 |
| GLU 0 (mmol/L) | 4.85 ± 0.44 | 5.77 ± 0.63 | 8.48 ± 2.30 |
| GLU 30 (mmol/L) | 7.76 ± 1.35 | 9.71 ± 1.65 | 13.50 ± 3.01 |
| GLU 60 (mmol/L) | 7.27 ± 1.65 | 10.71 ± 2.43 | 16.62 ± 3.80 |
| GLU 120 (mmol/L) | 6.28 ± 0.91 | 8.83 ± 1.37 | 15.34 ± 5.89 |
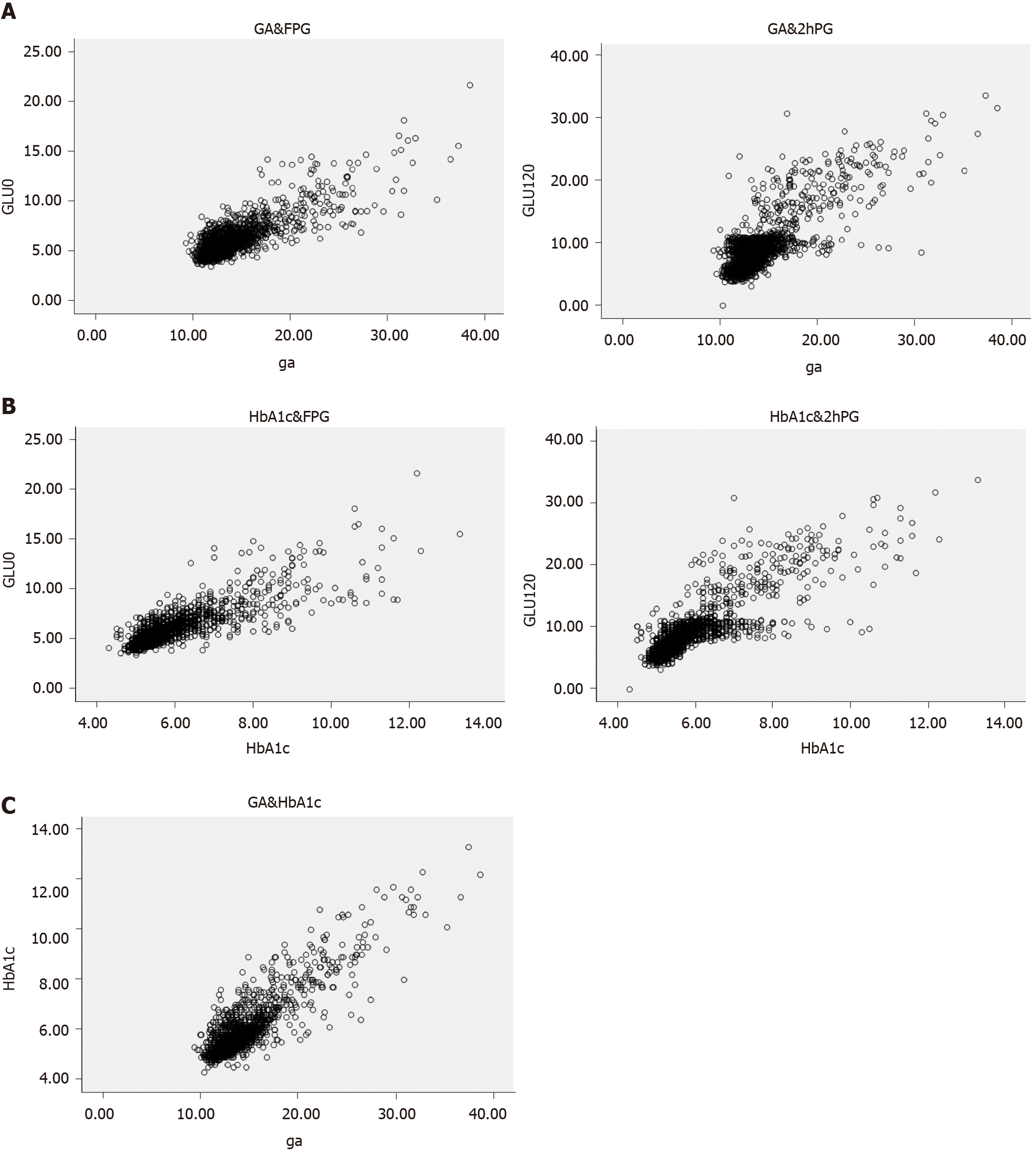
The Pearson correlation coefficient between the OGTT and GA showed a significant association at 0, 30, 60, and 120 min. The OGTT was also correlated with GA and HbA1c (Table 2). Correlations between the OGTT and both GA and HbA1c are shown in Figure 1. GA concentration was significantly and positively correlated with HbA1c level (r = 0.872, P < 0.001). The 2 h-PG levels were positively correlated with GA (r = 0.793, P < 0.001).
| GLU 0 min (mmol/L) | GLU 30 min (mmol/L) | GLU 60 min (mmol/L) | GLU 120 min (mmol/L) | GA (%) | HbA1c (%) | |
| GLU 0 min(mmol/L) | 1 | |||||
| GLU 30 min(mmol/L) | 0.840 | 1 | ||||
| Pearson correlation | P < 0.001 | |||||
| GLU 60 min(mmol/L) | 0.835 | 0.888 | 1 | |||
| Pearson correlation | P < 0.001 | P < 0.001 | ||||
| GLU 120 min(mmol/L) | 0.824 | 0.764 | 0.854 | 1 | ||
| Pearson correlation | P < 0.001 | P < 0.001 | P < 0.001 | |||
| GA (%) | 0.809 | 0.717 | 0.735 | 0.793 | 1 | |
| Pearson correlation | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||
| HbA1c (%) | 0.834 | 0.747 | 0.800 | 0.842 | 0.872 | 1 |
| Pearson correlation | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
A ROC curve (Figure 2) was plotted to determine the sensitivity and specificity of GA and HbA1c in detecting diabetes. The area under the ROC curve (AUC) of the ROC curve of HbA1c to detect diabetes was 0.939 (95% confidence interval [CI] 0.924-0.954). The AUC of the ROC curve of GA to detect diabetes was 0.903 (95%CI 0.879-0.927), indicating that GA is a useful marker for predicting diabetes. From these curves, the cutoff for predicting the diagnosis of diabetes was 15.15% for GA and 6.15% for HbA1c. Using a GA cutoff value ≥ 15.15% to diagnose diabetes resulted in a specificity of 78.9% and a sensitivity of 90.7%.
The OGTT is still the “gold standard” for the diagnosis of diabetes in clinical practice as it has appropriate sensitivity and specificity[4]. However, it is a time-consuming process, is poorly tolerated and there is a growing number of high-risk groups who require testing. Thus, the OGTT cannot be used in all patients[5]. GA and HbA1c are glycated proteins that can be used as glycemic control indicators. GA and HbA1c levels can be obtained at any time of the day irrespective of recent food intake. HbA1c has been introduced for the diagnosis of diabetes. However, HbA1c does not accurately reflect glycemic status in patients with anemia, variant hemoglobin and so on[15,16]. On the other hand, GA reflects short–term glycemic control and is not influenced by the erythrocyte lifespan[17]. GA level is well correlated with the severity of diabetic complications[18]. Therefore, it is feasible to predict diabetes using GA level.
According to recent studies, GA measurement has become a more accurate and automated test for diabetes screening. However, the diagnostic cut-offs reported in different studies are inconsistent[11,19,20]. Thus, it is necessary to determine the cutoff for GA in different populations. This study showed that the best cutoff for GA as a diagnostic tool in northeast Chinese subjects with diabetes was 15.15%. The sensitivity and specificity of GA were found to be 78.9% and 90.7%, respectively[21]. Several studies using GA to diagnose diabetes have been reported. Wu et al[22], reported that the GA cutoff point for diabetes in Taiwan was 15%, with a sensitivity of 74% and a specificity of 85% in 1559 subjects. Hwang et al[23] reported that a GA cutoff of > 14.3% was optimal for the diagnosis of diabetes in Korean adults. Furusyo et al[11] reported that the measurement of GA was a useful marker for the screening of diabetes in a Japanese population and the cut-off level of GA to diagnose diabetes was 15.5%. In addition, Ma et al[20] reported that in Chinese subjects the GA cutoff for diagnosing diabetes was 15.7%. It seems that these differences in GA cutoff values were due to differences in environmental and genetic factors. When taken together, these findings suggest an optimal GA cutoff of 14%-16% for the Asian population. In a recent study, Chiara et al[24] reported that at a cutoff of 13.5%, GA showed high sensitivity of 88.9% and good specificity of 60.4% for the diagnosis of diabetes in a European population. The differences in GA cutoff points may reflect differences in the study population.
In this study, the diagnostic cutoff was based on 2h-PG. In other diseases, e.g., retinopathy, 2h-PG is appropriate for standardization. In addition, 2h-PG eliminates errors from other sources, and a calibration curve of HbA1c cutoff values was included in this study to verify the validity of GA. Previous trials were based on a single value, and HbA1c was used for the calibration curve. In particular, the HbA1c cutoff showed properties in three phases, similar to current diagnostic criteria. The HbA1c cutoff is consistent with the results from other east Asian studies[25,26], and this further confirmed the effectiveness of the GA cutoff. This study used 15.15% as the cutoff for GA and 6.15% for HbA1c. This value was derived from the results of different 2h-PG tests, with the HbA1c cutoffs used in the calibration curve. Therefore, we have confidence in the GA cutoff as a reference for the diagnosis of diabetes.
Due to the heavy financial burden and required clinical care after the diagnosis of diabetes, current diabetes diagnostic criteria using HbA1c and FPG prefer specificity to sensitivity. Diabetes can cause many serious complications[27]. To prevent the development of diabetic complications and disability, diabetic patients should be diagnosed early; therefore, the diagnostic criteria for GA in diabetes should moderate the sensitivity to overemphasize specificity. In terms of “metabolic memory,” early intervention prolongs the benefits of good blood glucose control, and any newly diagnosed diabetic patients will be confirmed by another test or the presence of classic hyperglycemia symptoms. There is considerable controversy regarding the diabetes diagnostic criteria for HbA1c[25,26], and several studies in east Asia have suggested lowering the HbA1c cutoff for diabetes diagnosis. GA has moderate sensitivity and specificity in monitoring blood glucose[27]. Our study has added information on the GA cutoff for diabetes diagnosis in northern China.
Our study had some limitations. First, it was based on a cross-sectional design. Furthermore, the diagnostic criteria should be improved and validated in prospective studies as most previous studies were cross-sectional or retrospective[24]. Second, the GA cut-off of 15.15% was derived from the HbA1c cutoff of 6.15%[28]. This reference was not directly selected to optimize cutoff values with regard to the highest ratio of false-positive and false-negative results. Given the economic and social burden associated with diabetes, the reference should include diagnostic specificity over sensitivity. Third, the study was performed in a single center, and the results should be confirmed in multiple centers. Caution is needed when extrapolating the study results to other ethnic groups.
Our study supports the use of GA as a biomarker for the diagnosis of diabetes.
The use of hemoglobin A1c (HbA1c) for the diagnosis of diabetes is a complement to other measures. However, HbA1c is affected by a shortened red blood cell lifespan. In patients with anemia and hemoglobinopathies, the measured HbA1c levels may be inaccurate. Compared with HbA1c, glycated albumin (GA) is more rapid to diagnose new-onset diabetes.
To provide cutoff values for GA and to evaluate its utility as a screening and diagnostic tool for diabetes in a large high-risk group study.
This cross-sectional, high-risk based, large sample study evaluated the GA cut-off for the diagnosis of diabetes mellitus. A total of 1935 subjects aged 18-79 years took part in a comprehensive assessment, including a 75-g oral glucose tolerance test (OGTT), and the measurement of HbA1c and GA.
A linear relationship between variables was determined using the Pearson correlation coefficient. P < 0.05 was considered statistically significant. A receiver operating characteristic (ROC) curve was drawn to determine diagnostic sensitivity and specificity. The cut-off value of GA for newly diagnosed diabetes using the OGTT was calculated by ROC analysis using the Youden index.
A significant association at 0, 30, 60, and 120 min. The OGTT was also correlated with GA and HbA1cCorrelations between the OGTT and both GA and HbA1c. GA concentration was significantly and positively correlated with HbA1c level (r = 0.872, P < 0.001). The 2 h-PG levels were positively correlated with GA.
Our study supports the use of GA as a biomarker for the diagnosis of diabetes.
The study should be confirmed in multiple centers and extrapolating the study results to other ethnic groups.
We thank the staff of Laboratory of Endocrinology and Metabolism, The Second Affiliated Hospital of Harbin Medical University, China.
| 1. | Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, Pervjakova N, Pers TH, Johnson AD, Eicher JD, Jackson AU, Ferreira T, Lee Y, Ma C, Steinthorsdottir V, Thorleifsson G, Qi L, Van Zuydam NR, Mahajan A, Chen H, Almgren P, Voight BF, Grallert H, Müller-Nurasyid M, Ried JS, Rayner NW, Robertson N, Karssen LC, van Leeuwen EM, Willems SM, Fuchsberger C, Kwan P, Teslovich TM, Chanda P, Li M, Lu Y, Dina C, Thuillier D, Yengo L, Jiang L, Sparso T, Kestler HA, Chheda H, Eisele L, Gustafsson S, Frånberg M, Strawbridge RJ, Benediktsson R, Hreidarsson AB, Kong A, Sigurðsson G, Kerrison ND, Luan J, Liang L, Meitinger T, Roden M, Thorand B, Esko T, Mihailov E, Fox C, Liu CT, Rybin D, Isomaa B, Lyssenko V, Tuomi T, Couper DJ, Pankow JS, Grarup N, Have CT, Jørgensen ME, Jørgensen T, Linneberg A, Cornelis MC, van Dam RM, Hunter DJ, Kraft P, Sun Q, Edkins S, Owen KR, Perry JRB, Wood AR, Zeggini E, Tajes-Fernandes J, Abecasis GR, Bonnycastle LL, Chines PS, Stringham HM, Koistinen HA, Kinnunen L, Sennblad B, Mühleisen TW, Nöthen MM, Pechlivanis S, Baldassarre D, Gertow K, Humphries SE, Tremoli E, Klopp N, Meyer J, Steinbach G, Wennauer R, Eriksson JG, Mӓnnistö S, Peltonen L, Tikkanen E, Charpentier G, Eury E, Lobbens S, Gigante B, Leander K, McLeod O, Bottinger EP, Gottesman O, Ruderfer D, Blüher M, Kovacs P, Tonjes A, Maruthur NM, Scapoli C, Erbel R, Jöckel KH, Moebus S, de Faire U, Hamsten A, Stumvoll M, Deloukas P, Donnelly PJ, Frayling TM, Hattersley AT, Ripatti S, Salomaa V, Pedersen NL, Boehm BO, Bergman RN, Collins FS, Mohlke KL, Tuomilehto J, Hansen T, Pedersen O, Barroso I, Lannfelt L, Ingelsson E, Lind L, Lindgren CM, Cauchi S, Froguel P, Loos RJF, Balkau B, Boeing H, Franks PW, Barricarte Gurrea A, Palli D, van der Schouw YT, Altshuler D, Groop LC, Langenberg C, Wareham NJ, Sijbrands E, van Duijn CM, Florez JC, Meigs JB, Boerwinkle E, Gieger C, Strauch K, Metspalu A, Morris AD, Palmer CNA, Hu FB, Thorsteinsdottir U, Stefansson K, Dupuis J, Morris AP, Boehnke M, McCarthy MI, Prokopenko I; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes. 2017;66:2888-2902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 533] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 2. | Kuniss N, Freyer M, Müller N, Kielstein V, Müller UA. Expectations and fear of diabetes-related long-term complications in people with type 2 diabetes at primary care level. Acta Diabetol. 2019;56:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | LeRoith D, Fonseca V, Vinik A. Metabolic memory in diabetes--focus on insulin. Diabetes Metab Res Rev. 2005;21:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association. Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin Diabetes. 2018;36:14-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 5. | Bartoli E, Fra GP, Carnevale Schianca GP. The oral glucose tolerance test (OGTT) revisited. Eur J Intern Med. 2011;22:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 6. | Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Li TC, Yang CP, Tseng ST, Li CI, Liu CS, Lin WY, Hwang KL, Yang SY, Chiang JH, Lin CC. Visit-to-Visit Variations in Fasting Plasma Glucose and HbA1c Associated With an Increased Risk of Alzheimer Disease: Taiwan Diabetes Study. Diabetes Care. 2017;40:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 8. | Rafat D, Rabbani TK, Ahmad J, Ansari MA. Influence of iron metabolism indices on HbA1c in non-diabetic pregnant women with and without iron-deficiency anemia: effect of iron supplementation. Diabetes Metab Syndr. 2012;6:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 266] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Koga M, Inada S, Nakao T, Kawamori R, Kasayama S. The Glycated Albumin (GA) to HbA1c Ratio Reflects Shorter-Term Glycemic Control than GA: Analysis of Patients with Fulminant Type 1 Diabetes. J Clin Lab Anal. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, Schaefer EJ, Hayashi J. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia. 2011;54:3028-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care. 1989;12:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 297] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J; China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 2329] [Article Influence: 145.6] [Reference Citation Analysis (2)] |
| 14. | Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1068] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 15. | Takahashi S, Uchino H, Shimizu T, Kanazawa A, Tamura Y, Sakai K, Watada H, Hirose T, Kawamori R, Tanaka Y. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007;54:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Miyamoto H, Tao X, Kohzuma T, Ohnishi A. Influences of Anemia, Kidney Disease, Thyroid Dysfunction, and Liver Disease on the Ratio of Glycated Albumin to Hemoglobin A1c. J Diabetes Sci Technol. 2018;12:1082-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, Umayahara Y, Kosugi K, Kaneto H, Yamasaki Y, Hori M. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J. 2008;55:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Huh JH, Lee M, Park SY, Kim JH, Lee BW. Glycated Albumin Is a More Useful Glycation Index than HbA1c for Reflecting Renal Tubulopathy in Subjects with Early Diabetic Kidney Disease. Diabetes Metab J. 2018;42:215-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Ma XJ, Pan JM, Bao YQ, Zhou J, Tang JL, Li Q, Xiang KS, Jia WP. Combined assessment of glycated albumin and fasting plasma glucose improves the detection of diabetes in Chinese subjects. Clin Exp Pharmacol Physiol. 2010;37:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Yang C, Li H, Wang Z, Zhang W, Zhou K, Meng J, Zhao Y, Pan J, Lv X, Liang H, Jiang X. Glycated albumin is a potential diagnostic tool for diabetes mellitus. Clin Med (Lond). 2012;12:568-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Wu WC, Ma WY, Wei JN, Yu TY, Lin MS, Shih SR, Hua CH, Liao YJ, Chuang LM, Li HY. Serum Glycated Albumin to Guide the Diagnosis of Diabetes Mellitus. PLoS One. 2016;11:e0146780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Hwang YC, Jung CH, Ahn HY, Jeon WS, Jin SM, Woo JT, Cha BS, Kim JH, Park CY, Lee BW. Optimal glycated albumin cutoff value to diagnose diabetes in Korean adults: a retrospective study based on the oral glucose tolerance test. Clin Chim Acta. 2014;437:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 24. | Giorda C, Boemi M, Borzì V, Chiaramonte F, Mattei P, Tribulato A. The IMPROVE study--a multinational, multicentre, observational study in type 2 diabetes: results from the Italian cohort. Acta Biomed. 2010;81:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 25. | Heianza Y, Hara S, Arase Y, Saito K, Fujiwara K, Tsuji H, Kodama S, Hsieh SD, Mori Y, Shimano H, Yamada N, Kosaka K, Sone H. HbA1c 5·7-6·4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet. 2011;378:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Mukai N, Yasuda M, Ninomiya T, Hata J, Hirakawa Y, Ikeda F, Fukuhara M, Hotta T, Koga M, Nakamura U, Kang D, Kitazono T, Kiyohara Y. Thresholds of various glycemic measures for diagnosing diabetes based on prevalence of retinopathy in community-dwelling Japanese subjects: the Hisayama Study. Cardiovasc Diabetol. 2014;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Redant S, Hussein H, Mugisha A, Attou R, De Bels D, Honore PM, De Laet CC. Differentiating Hyperlactatemia Type A From Type B: How Does the Lactate/pyruvate Ratio Help? J Transl Int Med. 2019;7:43-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Sehgal V, Ulmer B. Clinical Conundrums in the Management of Diabetic Ketoacidosis in the Elderly. J Transl Int Med. 2019;7:10-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dyson J, Trauner M S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ