Published online Dec 15, 2021. doi: 10.4239/wjd.v12.i12.2058
Peer-review started: August 24, 2021
First decision: September 5, 2021
Revised: September 7, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: December 15, 2021
Processing time: 114 Days and 5.8 Hours
Kallmann syndrome (KS) is a hypogonadotropic hypogonadism accompanied by anosmia or hyposmia. It is associated with the low secretion of gonadotropins which can lead to other abnormal endocrine metabolism disorders such as diabetes. Through genetic and molecular biological methods, more than 10 KS pathogenic genes have been found.
To identify the existing mutation sites of KS with diabetes and reveal the relationship between genotype and phenotype.
We studied KS pathogenesis through high-throughput exome sequencing on four diabetes’ patients with KS for screening the potential pathogenic sites and exploring the genotype-phenotype correlation. Clinical data and peripheral blood samples were collected from the patients. White blood cells were separated and genomic DNA was extracted. High-throughput sequencing of all exons in the candidate pathogenic genes of probands was performed, and the results obtained were analyzed.
Sequencing revealed mutations in the KLB p.T313M, ANOS1 p.C172F, and IGSF10 gene (p.Lys1819Arg and p.Arg1035Thr) at different sites, which may have been associated with disease onset.
The diagnosis of KS is challenging, especially in early puberty, and the clinical manifestations reflect physical delays in development and puberty. Timely diagnosis and treatment can induce puberty, thereby improving sexual, bone, metabolic and mental health.
Core Tip: Kallmann syndrome is associated with low secretion of gonadotropins, which can also lead to other abnormal endocrine metabolism, such as diabetes. Sequencing revealed mutations in the KLB p.T313M, ANOS1 p.C172F, and IGSF10 gene (p.Lys1819Arg and p.Arg1035Thr) at different sites, which may have been associated with disease onset. Timely diagnosis and treatment can induce puberty, thereby improving sexual, bone, metabolic and mental health.
- Citation: Sun SS, Wang RX. Molecular diagnosis of Kallmann syndrome with diabetes by whole exome sequencing and bioinformatic approaches. World J Diabetes 2021; 12(12): 2058-2072
- URL: https://www.wjgnet.com/1948-9358/full/v12/i12/2058.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i12.2058
Idiopathic hypogonadotropic hypogonadism (IHH) is caused by congenital hypothalamic gonadotropin-releasing hormone (GnRH) neuron deficiency or dysfunction in GnRH synthesis or secretion, which leads to a reduction in gonadotropin secretion by the pituitary[1]. Low levels of gonadotropin secretion may consequently lead to insufficient gonadal function. This can manifest in the form of underdeveloped secondary sex characteristics, gamete synthesis disorders, and delayed bone closure, among other conditions. According to the clinical symptoms, there are two types: those with impaired sense of smell are called Kallmann syndrome (KS); those with normal sense of smell are called normosmic hypogonadotropic hypogonadism (nIHH) with normal sense of smell. Measurement of the levels of gonadotropins (luteinizing hormone, LH; follicle-stimulating hormone, FSH) and imaging examinations can help confirm the diagnosis of IHH and hormone replacement therapy can be used as a suitable therapeutic strategy.
KS is a hypogonadotropic hypogonadism accompanied by anosmia (cannot recognize any odor) or hyposmia(recognizable part of a strong pungent odor). It is a disease with clinical and genetic heterogeneity. KS can be familial or sporadic. There are three ways of inheritance: X-linked recessive inheritance, autosomal dominant inheritance, and autosomal recessive inheritance. The pathogenesis of KS is not fully understood. It is currently believed that GnRH neurons originating from the olfactory substrate cannot migrate normally and locate in the hypothalamus due to various reasons, resulting in complete or partial loss of the ability to synthesize and secrete GnRH, cause hypothalamic-pituitary-gonadal axis dysfunction and failure to activate puberty.
Long-term lack of sex hormones can also lead to other abnormal endocrine metabolism, such as calcium malabsorption, osteoporosis; abnormal glucose and lipid metabolism, insulin resistance, diabetes, hyperlipidemia, hypertension, etc.
The diagnosis of KS is challenging, especially in early puberty, and the clinical manifestations reflect physical delays in development and puberty. Timely diagnosis and treatment can induce puberty, thereby improving development in sexual, bone, metabolic and mental health.
In this study, we reported four cases of clinically confirmed KS with diabetes by investigating the mutation sites in these patients. The aim is to identify the existing mutation sites and reveal the relationship between genotype and phenotype.
The diagnosis of KS is based on: (1) Males > 18-years-old (selecting 18-years-old can exclude some cases that did not enter puberty at the age of 14-18); (2) Clinical manifestations of hypogonadism; (3) LH, FSH, T Levels are low; (4) Thyroid axis function, adrenal axis function, growth hormone axis function and prolactin are normal; (5) Sellar MRI shows no organic abnormalities of the hypothalamus and pituitary; (6) Olfactory bulb/olfactory tract MRI: olfactory bulb, olfactory bundle dysplasia or underdevelopment; (7) Lagging bone age; and (8) Delayed response in GnRH excitability test, 9) normal chromosome karyotype.
Patient 1: A 35-year-old woman, diagnosed with diabetes, suffering from amenorrhea for 17 years was hospitalized in March 2018. The patient had menstrual cramps, underdeveloped breasts, sparse armpit and pubic hair, female secondary sexual characteristics, hyposmia, normal intelligence, pubic hair – Tanner II, and breast Tanner – V. Hospitalization was recommended for further diagnosis and treatment. Patient 2: A 24-year-old man diagnosed with diabetes and sexual underdevelopment 11 years prior was hospitalized in May 2018. The patient first observed at 13 years of age that his penis and testicles were underdeveloped. The patient suffered from azoospermia. Secondary sexual characteristics, such as pubic hair, axillary hair, laryngeal knot, and voice change, among others, were absent. No secondary sexual characteristics were observed. Bilateral breast enlargement was a feminine characteristic observed. Other characteristics included pubic hair – Tanner II, testicular development – Tanner II, and loss sense of smell. Patient 3: A 22-year-old man diagnosed with diabetes, who had hyposmia and found penis and testicles were underdeveloped, Secondary sexual characteristics, such as pubic hair, axillary hair, laryngeal knot, among others, were absent. Patient 4: An 18-year-old man diagnosed with diabetes, also found penis and testicles were underdeveloped, Secondary sexual characteristics, such as pubic hair, axillary hair, laryngeal knot, among others, were absent. and with loss of the sense of smell.
Data on the medical history of the patients and their family members were collected in detail and analyzed. Physical examination, routine electrocardiogram, and echocardiography were performed and the results were analyzed. Laboratory tests were performed on chemiluminescence instrument (MAGLUMI 4000 PLUS, China).
We also completed the GnRH stimulation test. Initial blood is drawn to check the basic values of LH and FSH. Next, the patient is injected with GnRH intravenously and the LH and FSH values are checked at 15 min, 30 min, 1 h, and 120 min later. The dynamic changes of these two hormones are observed.
DNA extraction and whole-exome sequencing: For DNA extraction, 2 mL of a venous blood sample was collected from each patient and their parents, which were treated with heparin to prevent coagulation. Genomic DNA was extracted according to the instruction provided by the Se Blood DNA kits (Omega Bio-Tek, Inc.), and the patients’ DNA sample was dispatched to Aiji Taikang for whole-exome sequencing (WES).
Analysis of biological information of KS and nIHH related pathogenic genes: Search for KS and nIHH related pathogenic genes from published papers[2-5], analyze the intersection of the two related genes, and use kobas[6] to perform Gene Ontology and KEGG pathway enrichment analysis on the two related genes. The intersection and enrichment analysis graphs are drawn on the hiplot (https://hiplot.com.cn) platform. We then use STRING[7] to analyze the protein-related effects of KS and nIHH-related pathogenic genes.
The raw sequence data obtained was subjected to quality control using FastQC[8] and the clean reads were aligned with the human reference genome (hg19) using bwa[9]. The duplicate reads were labeled using SAMBLASTER[10]. We used the GATK HaplotypeCaller[11] for variant calling, dbNSFP[12] databases were used to annotate the variant sites.
The following were filtered from the data: population with a mutation frequency greater than 1% (gnomAD data from dbNSFP), mutation in the dbSNP database, and nonsense mutation sites (intron regions, synonymous mutations, and other mutations that do not affect protein function). To predict the effect of variation on protein function(MutationTaster, SIFT_pred, Polyphen2_HDIV methods from dbNSFP).
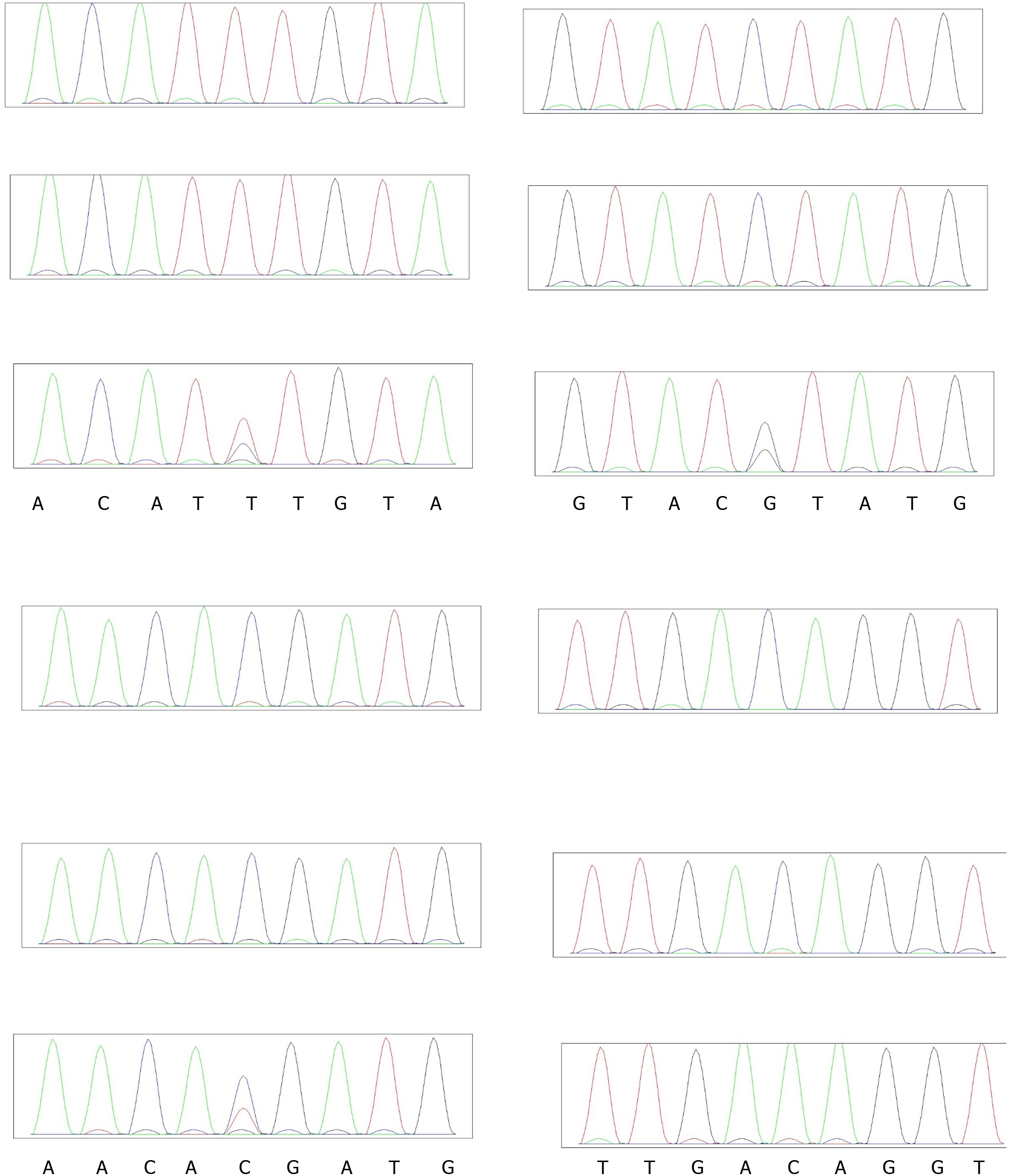
In order to verify the variants of candidate genes, we extracted DNA from the blood of their parents and verified the genotype of these variants with Sanger sequencing, the primer pairs used for PCR see Table 1.
| Patient | Gene | Template ID | Forward Primer | Reverse Primer | Amp Size (bp) |
| Patient 1 | IGSF10 | chr4:39435930-39435950 | ACATTTCCGCCCACATCAGAAG | TCAGCTGTGCCTCTCATCTCAT | 246 |
| Patient 2 | IGSF10 | chr3:151161270-151161285 | TAACAGGTGGTGCTGCAATGAC | AAGCACTGTGGAACTGAAGTGC | 251 |
| Patient 3 | KLB | chr3:151164660-151164670 | AGCAATGTCAGCTTTGGGGAAG | GCTTTGGGAGGCAGAGGAAAAT | 260 |
| Patient 4 | ANOS1 | chrX:8565100-8565108 | TGTGACACTGCATGTGTCTTCAC | TGACCAGCTGTGAGTTCCTCAA | 236 |
In patient 1, the level of 25-hydroxyvitamin D was found to be 11.78 ng/mL. Liver function, renal function, erythrocyte sedimentation rate, and the levels of electrolytes, calcium phosphate, parathyroid hormone, and C-reactive protein were normal. Blood osmotic pressure was 297 mOsm/kg·H20 and urine osmotic pressure was 620 mOsm/kg·H20. The growth hormone level was found to be 0.895 ng/mL. No abnormalities were observed in the thyroid function test. The cortisol circadian rhythm pattern was as follows: 08:00 120 ng/mL; 16:00 106.6 ng/mL; 00:00 13.93 ng/mL. The 24-h urine-free cortisol level was 188.40 µg/24 h. Color Doppler ultrasound examination revealed that the uterus size was small. The levels of the following sex hormones were measured: blood prolactin 94.67 µIU/mL; estradiol 13.7 pg/mL; progesterone 0.303 ng/mL; testosterone 0.13 ng/mL; LH 0.66 mIU/mL; FSH 0.88 mIU/mL.
The GnRH stimulation test revealed that the peak values of LH and FSH exceeded 1 mIU/mL, which indicated stimulation, see Table 2. MRI scan of the pituitary and CT scan of the adrenal glands revealed no abnormalities. No abnormal lesions were observed in the uterus and breasts.
| Dose (mIU/ml) | 0 | 15 min | 30 min | 60 min | 120 min | |
| Patient 1 | FSH | 0.88 | 1.33 | 1.72 | 2.47 | 3.07 |
| LH | 0.66 | 0.98 | 1.22 | 2.31 | 2.67 | |
| Patient 2 | FSH | 0.69 | 1.19 | 1.26 | 1.61 | 3.07 |
| LH | 0.23 | 0.32 | 0.38 | 0.64 | 2.61 | |
| Patient 3 | FSH | 0.85 | 1.29 | 1.64 | 1.88 | 2.42 |
| LH | 0.63 | 0.84 | 1.02 | 1.36 | 1.53 | |
| Patient 4 | FSH | 0.78 | 1.30 | 1.44 | 1.84 | 2.02 |
| LH | 0.68 | 0.94 | 1.29 | 1.33 | 1.63 |
The following observations were made in patient 2: ACTH 08:00 91.32 pg/mL; alanine aminotransferase 12.58 IU/L; aspartate aminotransferase 12.81 IU/L; blood creatinine 43.75 μmol/L; urine osmotic pressure 898 mOsm/kg·H20; blood osmotic pressure 291 mOsm/kg·H20.
The GnRH stimulation test revealed that the basal values of LH and FSH secretion were low. Both peaked at 120 min, see Table 2. The levels of the following sex hormones were tested: blood prolactin, 201.1 µIU/mL; estradiol 14.12 pg/mL; progesterone 0.479 ng/mL; testosterone 0.361 ng/mL; LH 0.23 mIU/mL; FSH 0.69 mIU/mL. The cortisol rhythm was found to be normal, and MRI scan of the pituitary and CT scan of the bilateral adrenal glands revealed no obvious abnormalities.
In patient 3, Urine protein 2+; urine glucose 3+, ketone body 3+, blood ketone 2.8mmol/L, the growth hormone level was found to be 0.301 ng/mL. No abnormalities were observed in the thyroid function test. The cortisol circadian rhythm pattern was as follows: 08:00 103.1 ng/mL; 16:00 29.67 ng/mL; 00:00 37.39 ng/mL. The levels of the following sex hormones were measured: blood prolactin 350.6 µIU/mL; estradiol 21.17 pg/mL; progesterone 1.07 ng/mL; testosterone 0.46 ng/mL; LH 0.63 mIU/mL; FSH 0.85 mIU/mL.
The GnRH stimulation test revealed that the peak values of LH and FSH exceeded 1 mIU/mL, which indicated stimulation, see Table 2.
In patient 4, The growth hormone level was found to be 0.175 ng/mL. No abnormalities were observed in the thyroid function test. The cortisol circadian rhythm pattern was normal. The levels of the following sex hormones were measured: blood prolactin157.2 µIU/mL; estradiol 5 pg/mL; progesterone 0.13 ng/mL; testosterone 0.46 ng/mL; LH 0.68 mIU/mL; FSH 0.78 mIU/mL.
The GnRH stimulation test revealed that the peak values of LH and FSH exceeded 1 mIU/mL, which indicated stimulation, see Table 2.
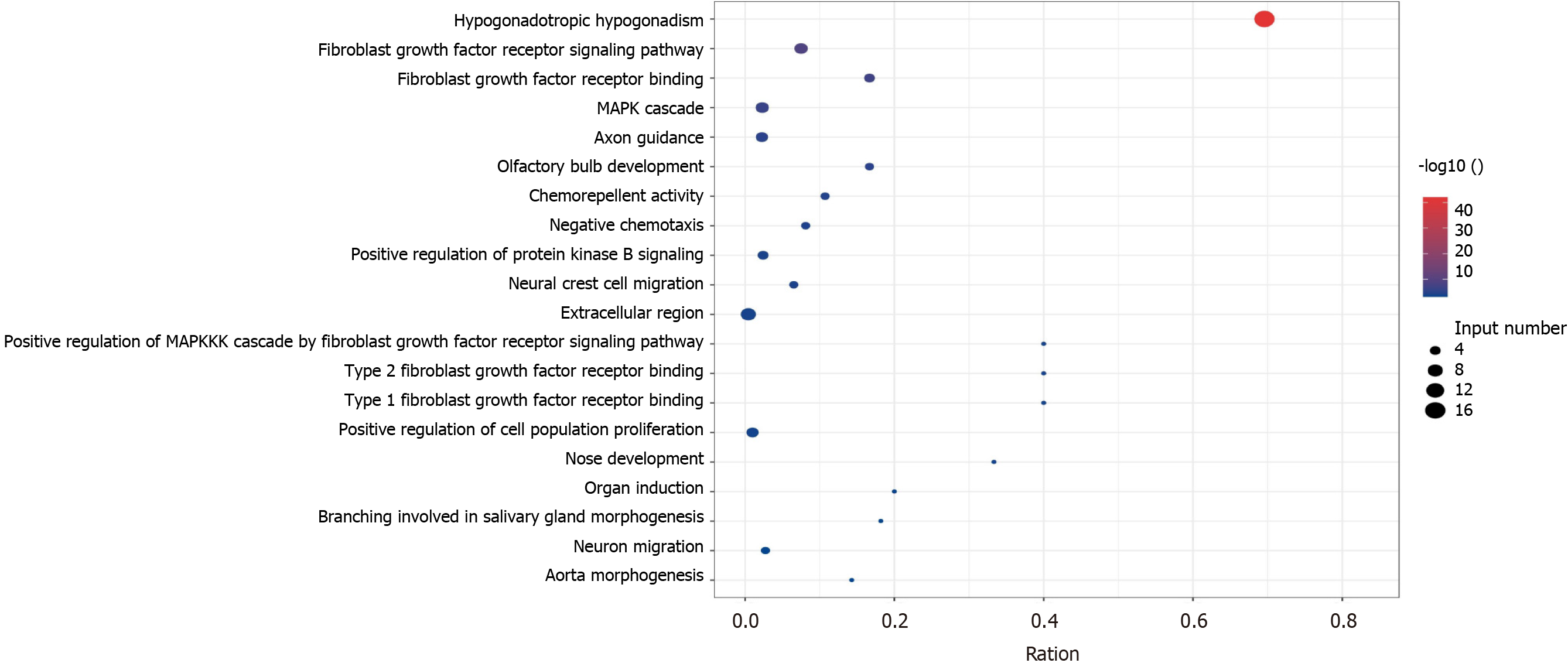
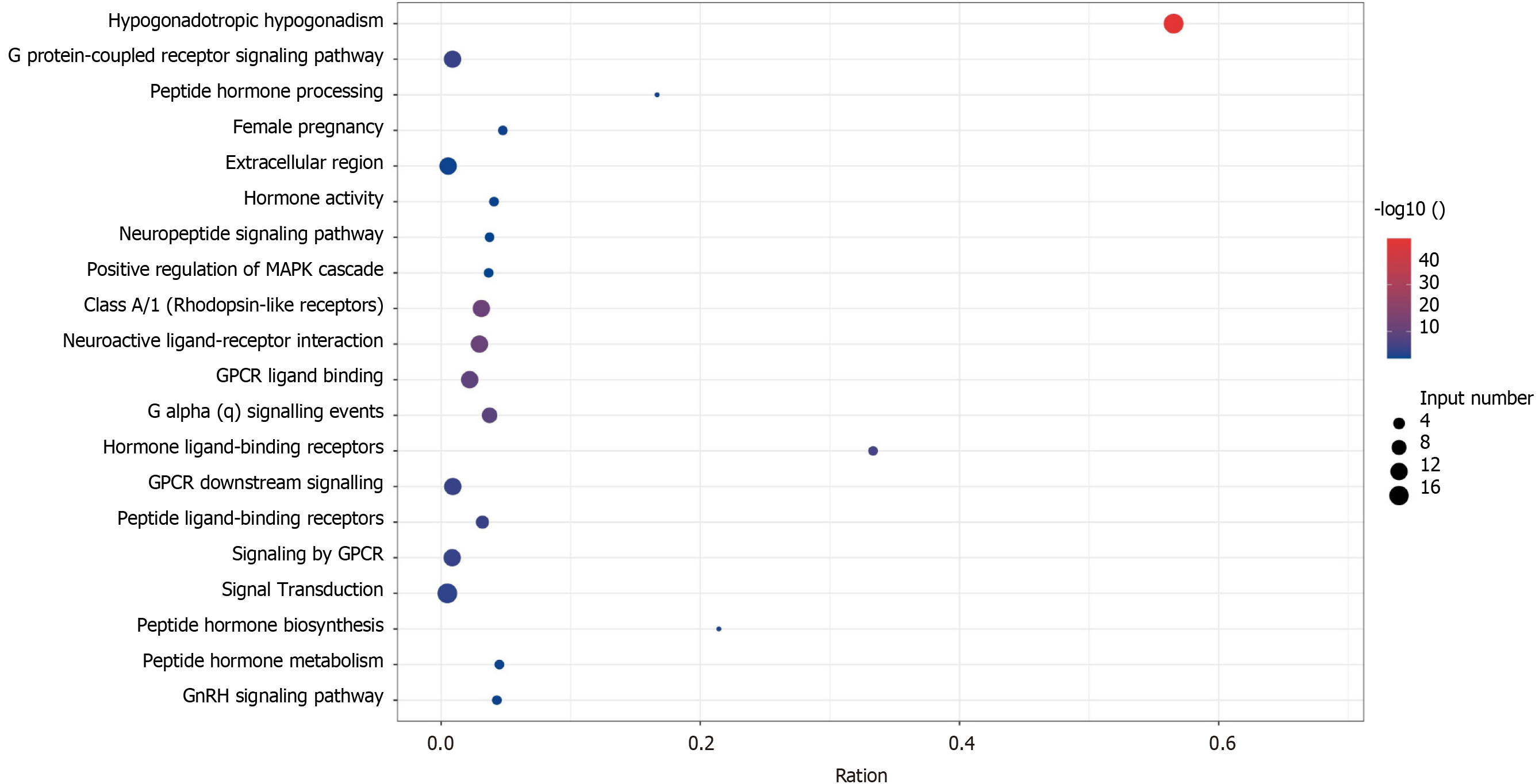
KS and nIHH related genes can be seen in Table 3. There were 6 genes both in the KS and nIHH. There were 31 genes only in nIHH, however, there were 17 genes in KS (Figure 1). KS related genes were enrichment in Hypogonadotropic hypogonadism, hypothalamus and pituitary gland diseases, endocrine and metabolic diseases, which may be related to fibroblast growth factor receptor signaling pathway[13] (Figure 2). However, nIHH related genes were enrichment with Neuroactive ligand-receptor interaction, GnRH signaling pathway, RNA polymerase, ovarian steroidogenesis, Cytosolic DNA-sensing pathway (Figure 3).
| Disease | Gene (Phenotype MIM number) | Count |
| Kallmann syndrome and normosmic hypogonadotropic hypogonadism | FGFR1(147950), FGF8 (612702), PROK2 (610628), CHD7 (612370), WDR11 (614858) | 5 |
| Normosmic hypogonadotropic hypogonadism | LEP (614962), LEPR (614963), NR0B1 (300200), SRA1, GNRHR (146110), GNRH1 (614841),KISS1R (614837), KISS1 (614842), TACR3 (614840), TAC3 (614839), NR5A1, HESX-1, LHX3, SOX2, FSHB (229070), LHB (228300), PC1, PNPLA6 (215470), RNF216, OTUD4, STUB1, POLR3A (607694), POLR3B (614381), RAB3GAP1, RAB3GAP2, RAB18, TBCID20, DMXL2, KISS1R(614837), NDNF (618841) | 30 |
| Kallmann syndrome | ANOS1 (308700), FGF17 (615270), IL17RD (615267), DUSP6 (615269), SPRY4 (615266), FLRT3 (615271),, KLB, PROKR2 (244200), SEMA3A (614897), SEMA3E, SOX10, HS6ST1 (614880), CCDC141, FEZF1 (616030), IGSF10, SMCHD1, NELF (614838), SOX3 | 18 |
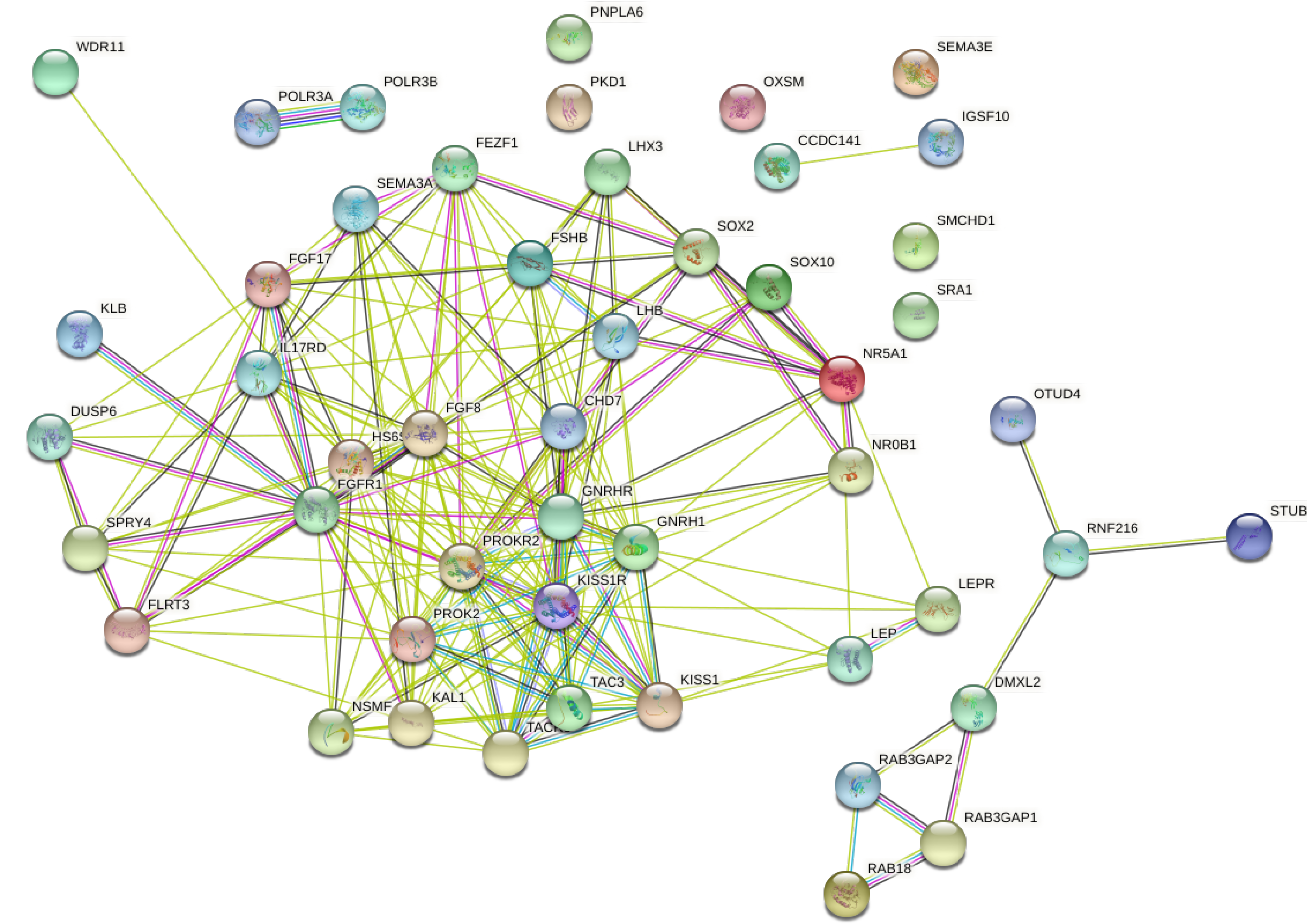
FGF8, CHD7, GNRH1 genes were located in the center of the protein interaction network of KS and nIHH related genes (Figure 4).
Quality control of raw WES data: Quality control analysis of the raw WES data (using FastQC) of the four samples is illustrated in Table 2. The average quality of bases was greater than 30 (accuracy greater than 99.9%), and the sequence quality was satisfactory. See Figure 5.
Sequence alignment and sequencing depth: Exome sequencing of the four samples yielded 39M paired-end reads, of which 99% (mapped reads) sequences could be matched to the human reference genome, and the proportion of duplicate reads was approximately 15%. The average sequencing depth (mean depth) exceeded 130X. See Table 4.
| Sample | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
| Raw reads (PEM) | 36.83373 | 39.8752 | 39.856 | 39.975 |
| reads mapping rate (%) | 99.59 | 99.58 | 99.64 | 99.65 |
| Target duplication rate (%) | 14.15 | 14.32 | 14.59 | 14.75 |
| Target mean depth | 130.55 | 139.46 | 151.43 | 145.43 |
| T 10X coverage rate (%) | 99.57 | 99.6 | 99.45 | 99.27 |
| T 20X coverage rate (%) | 99.04 | 99.13 | 99.26 | 99.04 |
| T 30X coverage rate (%) | 97.98 | 98.23 | 98.57 | 98.35 |
Extent of variation: The bioinformatics analysis revealed that the sample from patient 1 had 82,986 SNPs and 13,495 INDELs. Through dbSNP annotation, 99.06% of the SNPs and 91.15% of the INDELs could be annotated (Tables 5-6).
| Sample | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
| Total | 82986 | 84748 | 84579 | 84731 |
| dbsnp, n (%) | 82205 (99.06) | 83887 (98.98) | 83752 (99.02) | 83835 (98.94) |
| 1000g_EAS, n (%) | 77404 (93.27) | 78717 (92.88) | 78737 (93.09) | 78824 (93.03) |
| ExAC_EAS, n (%) | 45558 (54.90) | 45630 (53.84) | 45773 (54.12) | 45936 (54.21) |
| GnomAD_exome_EAS, n (%) | 45617 (54.97) | 45698 (53.92) | 45866 (54.23) | 46025 (54.32) |
| GnomAD_genome_EAS, n (%) | 81924 (98.72) | 83549 (98.59) | 83441 (98.65) | 83536 (98.59) |
| Exonic, n (%) | 22847 (27.53) | 23016 (27.16) | 23100 (27.31) | 22921 (27.05) |
| Splicing, n (%) | 239 (0.29) | 246 (0.29) | 251 (0.30) | 259 (0.31) |
| UTR3, n (%) | 3103 (3.74) | 3161 (3.73) | 3152 (3.73) | 3084 (3.64) |
| UTR5, n (%) | 2234 (2.69) | 2310 (2.73) | 2305 (2.73) | 2333 (2.75) |
| Intronic, n (%) | 48754 (58.75) | 50084 (59.10) | 49683 (58.74) | 50121 (59.15) |
| Intergenic, n (%) | 1902 (2.29) | 2108 (2.49) | 2184 (2.58) | 2145 (2.53) |
| Upstream, n (%) | 816 (0.98) | 881 (1.04) | 882 (1.04) | 827 (0.98) |
| Downstream, n (%) | 371 (0.45) | 379 (0.45) | 379 (0.45) | 366 (0.43) |
| Ncrna_exonic, n (%) | 764 (0.92) | 726 (0.86) | 730 (0.86) | 790 (0.93) |
| Ncrna_splicing, n (%) | 6 (0.01) | 8 (0.01) | 4 (0.00) | 3 (0.00) |
| Ncrna_intronic, n (%) | 1888 (2.28) | 1764 (2.08) | 1848 (2.18) | 1823 (2.15) |
| Synonymous SNV, n (%) | 11529 (13.89) | 11613 (13.70) | 11559 (13.67) | 11518 (13.59) |
| Nonsynonymous SNV, n (%) | 10755 (12.96) | 10727 (12.66) | 10815 (12.79) | 10749 (12.69) |
| Stopgain, n (%) | 85 (0.10) | 92 (0.11) | 94 (0.11) | 84 (0.10) |
| Stoploss, n (%) | 10 (0.01) | 10 (0.01) | 11 (0.01) | 8 (0.01) |
| Unknown, n (%) | 483 (0.58) | 589 (0.70) | 635 (0.75) | 576 (0.68) |
| Sample | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
| Total | 13495 | 13931 | 13760 | 13794 |
| dbsnp, n (%) | 12301 (91.15) | 12675 (90.98) | 12538 (91.12) | 12551 (90.99) |
| 1000g_EAS, n (%) | 8269 (61.27) | 8454 (60.68) | 8378 (60.89) | 8410 (60.97) |
| ExAC_EAS, n (%) | 5560 (41.20) | 5547 (39.82) | 5562 (40.42) | 5637 (40.87) |
| GnomAD_exome_EAS, n (%) | 5306 (39.32) | 5284 (37.93) | 5286 (38.42) | 5355 (38.82) |
| GnomAD_genome_EAS, n (%) | 12567 (93.12) | 12991 (93.25) | 12760 (92.73) | 12812 (92.88) |
| Exonic, n (%) | 708 (5.25) | 733 (5.26) | 707 (5.14) | 694 (5.03) |
| Splicing, n (%) | 199 (1.47) | 182 (1.31) | 201 (1.46) | 192 (1.39) |
| UTR3, n (%) | 669 (4.96) | 676 (4.85) | 667 (4.85) | 661 (4.79) |
| UTR5, n (%) | 375 (2.78) | 384 (2.76) | 364 (2.65) | 373 (2.70) |
| Intronic, n (%) | 10469 (77.58) | 10845 (77.85) | 10712 (77.85) | 10767 (78.06) |
| Intergenic, n (%) | 297 (2.20) | 312 (2.24) | 309 (2.25) | 307 (2.23) |
| Upstream, n (%) | 160 (1.19) | 187 (1.34) | 170 (1.24) | 184 (1.33) |
| Downstream, n (%) | 51 (0.38) | 65 (0.47) | 66 (0.48) | 68 (0.49) |
| Ncrna_exonic, n (%) | 103 (0.76) | 93 (0.67) | 102 (0.74) | 99 (0.72) |
| Ncrna_splicing, n (%) | 0 (0.00) | 2 (0.01) | 4 (0.03) | 1 (0.01) |
| Ncrna_intronic, n (%) | 407 (3.02) | 398 (2.86) | 400 (2.91) | 391 (2.83) |
| Frameshift insertion, n (%) | 94 (0.70) | 103 (0.74) | 96 (0.70) | 100 (0.72) |
| Frameshift deletion, n (%) | 135 (1.00) | 124 (0.89) | 132 (0.96) | 137 (0.99) |
| Nonframeshift insertion, n (%) | 198 (1.47) | 216 (1.55) | 200 (1.45) | 181 (1.31) |
| Nonframeshift deletion, n (%) | 217 (1.61) | 215 (1.54) | 202 (1.47) | 203 (1.47) |
| Stopgain, n (%) | 7 (0.05) | 8 (0.06) | 9 (0.07) | 10 (0.07) |
| Stoploss, n (%) | 1 (0.01) | 1 (0.01) | 0 (0.00) | 1 (0.01) |
| Unknown, n (%) | 99 (0.73) | 106 (0.76) | 111 (0.81) | 103 (0.75) |
Patient 2 had 84,748 SNPs, and 13,931 INDELs. Through dbSNP annotation, 98.98% of SNPs and 90.98% of the INDELs could be annotated (Tables 5-6).
Patient 3 had 84, 579 SNPs, and 13, 760 INDELs. Through dbSNP annotation, 99.02% of SNPs and 90.99% of the INDELs could be annotated (Tables 5-6).
Patient 4 had 84,731 SNPs, and 13,794 INDELs. Through dbSNP annotation, 98.94% of SNPs and 90.99% of the INDELs could be annotated (Tables 5-6).
Analysis of candidate gene mutations: The gene mutations were filtered according to the following criteria: (1) The mutation should be located in the exon; (2) The mutation should not be synonymous; (3) Population frequency should be greater than 0.001; and (4) Gene in the KS and nIHH related genes list.
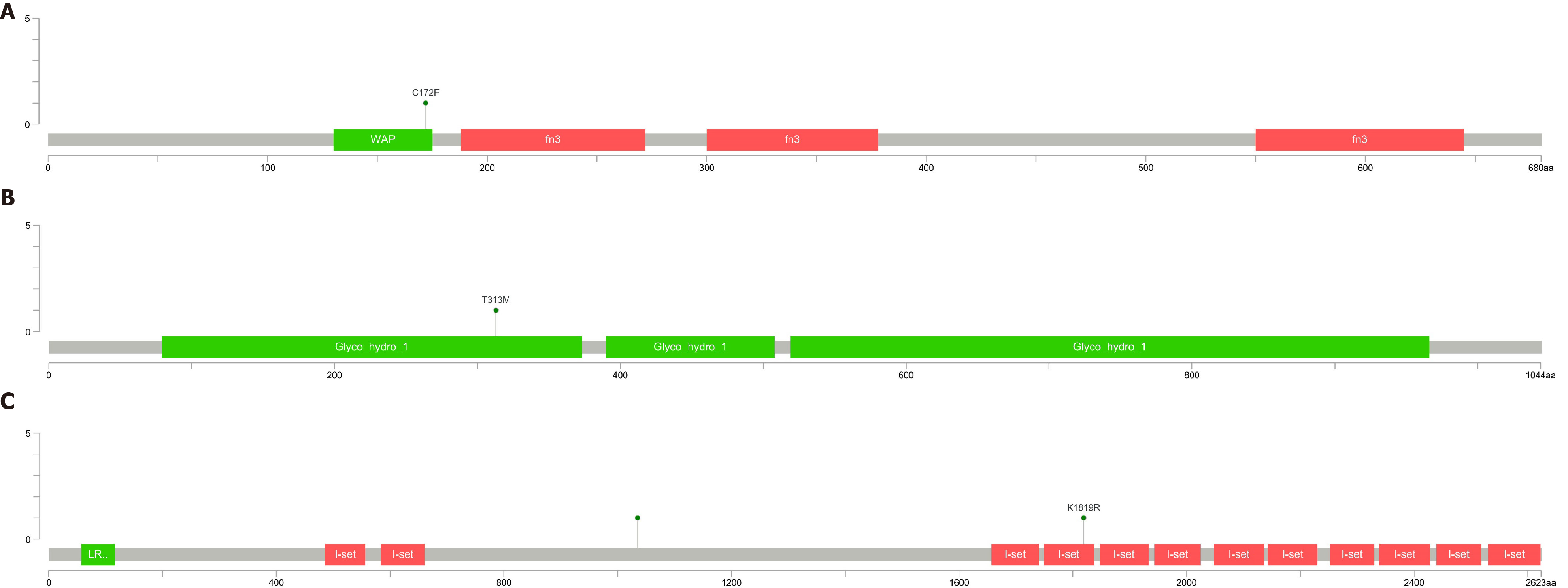
After filtering, only two variants were detected (one each in IGSF10 and CHD7 genes) for patient 1, whereas four variants were detected (one each in DMXL2, IGSF10, and ANOS1 genes) for patient 2, one variant was detected in the KLB for patient 3, one variant were detected in the ANOS1 for patient 4. Variants in the IGSF10 gene were common to both patients, see Table 7 and Figure 6. The mutations of ANOS1 are located in the region encoding the WAP domain(Figure 6A), The mutations of KLB are located in the region encoding the Glyco_hydro_1 domain (Figure 6B). The two mutations of IGSF10 are located in the region encoding the immunoglobulin I-set domain and in the non-domain region (Figure 6C). Furthermore, the literature search revealed that the genes CHD7, ANOS1, IGSF10, and DMXL2 were also related to IHH.
| Type | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
| Chr.Start.End | chr3.151161279.151161279 | chr3.151164665.151164665 | chr4.39435942.39435942 | chrX.8565101.8565101 |
| Vcf_mut | T/C | C/G | C/T | C/A |
| GT | 0/1 | 0/1 | 0/1 | 1/1 |
| AD | 51/53 | 30/31 | 34/47 | 0/86 |
| AAChange.HGVS | IGSF10:NM_178822.4:5/6:c.5456A>G:p.(Lys1819Arg) | IGSF10:NM_178822.4:4/6:c.3104G>C:p.(Arg1035Thr) | KLB:NM_175737:exon2:c.C938T:p.T313M | ANOS1:NM_000216:exon4:c.G515T:p.C172F |
| cytoBand | 3q25.1 | 3q25.1 | 4p14 | Xp22.31 |
| InterVar_automated | Uncertain significance | Uncertain significance | Uncertain significance | Uncertain significance |
| ACMG(missense only) | PM1,PM2,BP4 | . | PM1,BP4 | PM1,PM2,PP3 |
| gnomAD_exome_ALL | . | 0.0004 | 0.0001 | 0 |
| SIFT_pred | T | D | T | D |
| Polyphen2_HDIV_score | 0.011 | 0.981 | 0.036 | 1 |
| Polyphen2_HDIV_pred | B | D | B | D |
| Polyphen2_HVAR_score | 0.056 | 0.69 | 0.016 | 1 |
| Polyphen2_HVAR_pred | B | P | B | D |
| LRT_score | 0.039 | 0 | 0.59 | 0 |
| LRT_pred | N | D | N | D |
| MutationTaster_score | 0.808 | 1 | 1 | 1 |
| MutationTaster_pred | D | N | N | D |
| MutationAssessor_score | 0.15 | 2.47 | 2.535 | 4.455 |
| MutationAssessor_pred | N | M | M | H |
| FATHMM_score | -0.27 | -0.65 | 1.43 | -5.61 |
| FATHMM_pred | T | T | T | D |
| CADD_raw | 0.585 | 1.94 | -0.287 | 6.358 |
| CADD_phred | 8.054 | 15.84 | 0.711 | 29.4 |
| fathmm-MKL_coding_score | 0.039 | 0.257 | 0.068 | 0.967 |
| fathmm-MKL_coding_pred | N | N | N | D |
| GERP++_RS | 0.193 | 5.46 | -7.48 | 4.51 |
Verification of candidate sites: To verify the pathogenic sites in the four patients, we compared the parental genotypes and found that IGSF10 (p.Lys1819Arg), KLB p.T313M and ANOS1 p.C172F may harbor the pathogenic site, see Figure 7. Population data did not reveal the presence of a mutation at this site, and the mutation frequency of p. Arg1035Thr in the gnomAD database (from dbNSFP) was found to be 0.0004. and the mutation frequency of p.T313M in the gnomAD database was found to be 0.0001. and the mutation frequency of p.C172F in the gnomAD database was found to be 0.
MutationTaster (from dbNSFP) predicted Lys1819Arg to be a harmful mutation, whereas SIFT_pred (from dbNSFP) and Polyphen2_HDIV (from dbNSFP) predicted Arg1035Thr to be a harmful mutation. Whereas SIFT_pred predicted p.C172F to be a harmful mutation.
In this study, the mutant genes and loci of 4 KS patients were analyzed by WES, and four potential pathogenic loci of IGSF10 gene (p.lys1819Arg and p.arg1035Thr) , KLB p.T313M and ANOS1 p.C172F were identified. These loci are new loci that have not been reported.
With the further research on KS genetics, some genes related to KS pathogenesis have been found, such as KAL1, FGFRI, FGF8, PROKR2, PROK2. The function of these genes may be related to the normal migration of GnRH neurons and the development of the olfactory bulb. However, only 30% of the incidence of Kallmann syndrome is related to the above genes, suggesting that there are other disease-related genes of KS that have not been found.
In this study, WES was performed to analyze the mutant genes and loci in four patients with KS and two potential pathogenic loci of the IGSF10 gene (p. Lys1819Arg and p. Arg1035Thr) were identified. According to the analysis of the IGSF10 gene mutations in the two patients, the variations included alteration of the amino acid at the 1819th position from lysine to arginine and at the 1035th position from arginine to threonine. The Lys1819Arg site is located in the I-set domain of the protein which is primarily associated with immune function and angiogenesis. The discovery of this site can improve our understanding of KS pathogenesis and serve as a novel target in further studies on KS.
IGSF10 is a member of the immunoglobulin superfamily[14]. While its exact function is yet to be clarified, studies have shown that IGSF10 expression is associated with combined pituitary hormone deficiency. It is also considered a novel prognostic biomarker for breast and lung cancers and has been associated with various diseases, such as primary ovarian insufficiency and endometrial cancer. Mutations in the IGSF10 gene are reportedly associated with abnormal regulation of the migration of GnRH neurons, which may delay puberty and other developmental processes.
Among KS patients, about 90% of the patients, lack pubic and armpit hair. Bone age lagged behind chronological age in some patients. Some patients have anosmia or hyposmia. Some males have breast hyperplasia, a small penis, cryptorchidism and a lack of vas deferens. Some patients can also be accompanied by other body or organ abnormalities, such as facial cranial midline deformity, nervous system abnormalities, musculoskeletal system abnormalities and other systemic abnormalities.
KLB and KL genes have homology. It is highly expressed in metabolic tissues and especially in fat tissues. FGF21 is an endocrine FGF that is mainly secreted by the liver, which regulates the main metabolic processes such as glucose and lipid metabolism. Endogenous FGF21 regulates the physiological response of starvation and various other metabolic stresses. FGF21 signals through the KLB/FGFR1c receptor complex in a tissue-specific manner. KLB enhances the binding of FGF21-FGFR1c, thereby promoting FGF21 signal transduction by binding FGF21 and FGFR1c to itself through two different sites at the same time. In addition, the competitive binding of FGF8 and b-Klotho to the same site of FGFR1 will facilitate the binding to endocrine FGF21 and inhibit the binding and signal transduction of paracrine FGF8. Most patients with KLB mutations exhibit KS and metabolic defects, such as overweight, diabetes, and dyslipidemia and are consistent with the metabolic effects of this pathway[15-20]. Patient 3 was diagnosed with Kallmann syndrome with diabetes, which belongs to the KLB gene mutation, which also confirmed the above view. It suggests that Kallmann syndrome with KLB gene mutation should pay attention to the change of blood sugar and the occurrence of diabetes
ANOS1 gene is the pathogenic gene found to cause x-linked KS. It is located on the X chromosome (Xp22.3), contains 14 exons, adjacent to the pseudo-autosomal 1 region (PAR1), which is a highly variable and unstable region on the chromosome. ANOS1 encodes anosmin-1, an extracellular matrix protein. Anosmin-1 consists of a cysteine-rich region (CR domain), a whey acidic protein (WAP)-like domain similar, four consecutive fibronectin type III domains and a C-terminal region rich in basic histidines and prolines. Anosmin-1 promotes neuronal cell adhesion, neurite outgrowth, axon guidance and CNS projection neuron branching. In addition, it is also involved in the migration of many types of neural precursors, including GnRH-producing neurons and oligodendrocyte precursors. The ANOS1 mutation is found in patients with familial and sporadic KS. The ANOS1 gene mutation has a low incidence in patients with sporadic KS, but a high incidence in patients with familial KS. In KS patients with ANOS1 mutations, the loss or dysplasia of cryptorchidism and olfactory bulb is high.
In our study, another two potential pathogenic loci of the KLB p.T313M and ANOS1 p.C172F were identified, It suggests that KS disease with KLB mutation should be alert to the risk of diabetes, and KS disease with ANOS1 mutation is related to X-linked recessive inheritance. Although our analysis is limited to 4 patients with KS, it supports the previous view and found new mutation sites to facilitate follow-up research.
Because KS hyposmia can be manifested in different degrees, sometimes it is not easy to distinguish KS and nIHH, especially in patients with hypogonadism, often without careful evaluation of olfactory function. There is genetic evidence that the genes encoding GnRH and Kisspeptin receptors are related to nIHH, but not related to the migration of GnRH neuroendocrine cells (KS patients may have abnormal migration of GnRH neuroendocrine cells), suggesting that KS and nIHH may have different inheritance background and pathogenesis.
Exon sequencing can be used for studying various diseases. It is useful as a diagnostic tool owing to its low cost and high throughput. The method can be used to detect all mutations in human exons simultaneously. With technological advancements, exon capture has emerged as a useful method. Currently, the chip used has been up to 60M, which can include multiple introns and untranslated regions, and provides valuable information for the study of disease-causing sites.
In four Kallmann syndrome patients with diabetes, sequencing revealed mutations in the KLB p.T313M, ANOS1 p.C172F,and IGSF10 gene (p.Lys1819Arg and p.Arg1035Thr) at different sites, which may have been associated with disease onset. The diagnosis of KS is challenging, especially in early puberty, and the clinical manifestations reflect physical delays in development and puberty. Timely diagnosis and treatment can induce puberty, thereby improving sexual, bone, metabolic and mental health.
Kallmann syndrome is a hypogonadotropic hypogonadism accompanied by anosmia or hyposmia. Through genetic and molecular biological methods, more than 10 KS pathogenic genes have been found.
The diagnosis of KS is challenging, especially in early puberty, and the clinical manifestations reflect physical delays in development and puberty.
To identify the existing mutation sites of Kallmann syndrome with Diabetes and reveal the relationship between genotype and phenotype.
We studied KS pathogenesis through high-throughput exome sequencing on four diabetes’ patients with KS for screening the potential pathogenic sites and exploring the genotype-phenotype correlation. The results obtained were analyzed.
Sequencing revealed mutations in the KLB p.T313M, ANOS1 p.C172F,and IGSF10 gene (p.Lys1819Arg and p.Arg1035Thr) at different sites, which may have been associated with disease onset.
The diagnosis of KS is challenging. Timely diagnosis and treatment can induce puberty, thereby improving sexual, bone, metabolic and mental health.
Exon sequencing can be used for studying various diseases. It is useful as a diagnostic tool owing to its low cost and high throughput and it is very helpful for the diagnosis and treatment of KS.
| 1. | Chen K, Wang H, Lai Y. Kallmann Syndrome Due to Heterozygous Mutation in SOX10 Coexisting With Waardenburg Syndrome Type II: Case Report and Review of Literature. Front Endocrinol (Lausanne). 2020;11:592831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Men M, Wu J, Zhao Y, Xing X, Jiang F, Zheng R, Li JD. Genotypic and phenotypic spectra of FGFR1, FGF8, and FGF17 mutations in a Chinese cohort with idiopathic hypogonadotropic hypogonadism. Fertil Steril. 2020;113:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Wit JM, Oostdijk W, Losekoot M, van Duyvenvoorde HA, Ruivenkamp CA, Kant SG. MECHANISMS IN ENDOCRINOLOGY: Novel genetic causes of short stature. Eur J Endocrinol. 2016;174:R145-R173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Beate K, Joseph N, Nicolas de R, Wolfram K. Genetics of isolated hypogonadotropic hypogonadism: role of GnRH receptor and other genes. Int J Endocrinol. 2012;2012:147893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Topaloğlu AK. Update on the Genetics of Idiopathic Hypogonadotropic Hypogonadism. J Clin Res Pediatr Endocrinol. 2017;9:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY, Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316-W322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2658] [Cited by in RCA: 3624] [Article Influence: 241.6] [Reference Citation Analysis (0)] |
| 7. | Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447-D452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6477] [Cited by in RCA: 7835] [Article Influence: 652.9] [Reference Citation Analysis (0)] |
| 8. | de Sena Brandine G, Smith AD. Falco: high-speed FastQC emulation for quality control of sequencing data. F1000Res. 2019;8:1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754-1760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29052] [Cited by in RCA: 36569] [Article Influence: 2151.1] [Reference Citation Analysis (0)] |
| 10. | Faust GG, Hall IM. SAMBLASTER: fast duplicate marking and structural variant read extraction. Bioinformatics. 2014;30:2503-2505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 650] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 11. | McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16037] [Cited by in RCA: 20073] [Article Influence: 1254.6] [Reference Citation Analysis (0)] |
| 12. | Liu X, Wu C, Li C, Boerwinkle E. dbNSFP v3.0: A One-Stop Database of Functional Predictions and Annotations for Human Nonsynonymous and Splice-Site SNVs. Hum Mutat. 2016;37:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 756] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 13. | Messina A, Pulli K, Santini S, Acierno J, Känsäkoski J, Cassatella D, Xu C, Casoni F, Malone SA, Ternier G, Conte D, Sidis Y, Tommiska J, Vaaralahti K, Dwyer A, Gothilf Y, Merlo GR, Santoni F, Niederländer NJ, Giacobini P, Raivio T, Pitteloud N. Neuron-Derived Neurotrophic Factor Is Mutated in Congenital Hypogonadotropic Hypogonadism. Am J Hum Genet. 2020;106:58-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Kim JH, Seo GH, Kim GH, Huh J, Hwang IT, Jang JH, Yoo HW, Choi JH. Targeted Gene Panel Sequencing for Molecular Diagnosis of Kallmann Syndrome and Normosmic Idiopathic Hypogonadotropic Hypogonadism. Exp Clin Endocrinol Diabetes. 2019;127:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Zhang Q, He HH, Janjua MU, Wang F, Yang YB, Mo ZH, Liu J, Jin P. Identification of two novel mutations in three Chinese families with Kallmann syndrome using whole exome sequencing. Andrologia. 2020;52:e13594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Hamada J, Ochi F, Sei Y, Takemoto K, Hirai H, Honda M, Shibata H, Hasegawa T, Eguchi M. A novel SOX10 variant in a Japanese girl with Waardenburg syndrome type 4C and Kallmann syndrome. Hum Genome Var. 2020;7:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Thakker S, Persily J, Najari BB. Kallman syndrome and central non-obstructive azoospermia. Best Pract Res Clin Endocrinol Metab. 2020;34:101475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Erbaş İM, Paketçi A, Acar S, Kotan LD, Demir K, Abacı A, Böber E. A nonsense variant in FGFR1: a rare cause of combined pituitary hormone deficiency. J Pediatr Endocrinol Metab. 2020;33:1613-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Gach A, Pinkier I, Sałacińska K, Szarras-Czapnik M, Salachna D, Kucińska A, Rybak-Krzyszkowska M, Sakowicz A. Identification of gene variants in a cohort of hypogonadotropic hypogonadism: Diagnostic utility of custom NGS panel and WES in unravelling genetic complexity of the disease. Mol Cell Endocrinol. 2020;517:110968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Howard SR, Guasti L, Ruiz-Babot G, Mancini A, David A, Storr HL, Metherell LA, Sternberg MJ, Cabrera CP, Warren HR, Barnes MR, Quinton R, de Roux N, Young J, Guiochon-Mantel A, Wehkalampi K, André V, Gothilf Y, Cariboni A, Dunkel L. IGSF10 mutations dysregulate gonadotropin-releasing hormone neuronal migration resulting in delayed puberty. EMBO Mol Med. 2016;8:626-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papadopoulos VP S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL