Published online Dec 15, 2021. doi: 10.4239/wjd.v12.i12.2011
Peer-review started: February 22, 2021
First decision: June 5, 2021
Revised: July 13, 2021
Accepted: October 31, 2021
Article in press: October 31, 2021
Published online: December 15, 2021
Processing time: 296 Days and 14.4 Hours
As the global burden of diabetes is rapidly increasing, the incidence of diabetic foot ulcers is continuously increasing as the mean age of the world population increases and the obesity epidemic advances. A significant percentage of diabetic foot ulcers are caused by mixed micro and macro-vascular dysfunction leading to impaired perfusion of foot tissue. Left untreated, chronic limb-threatening ischemia has a poor prognosis and is correlated with limb loss and increased mortality; prompt treatment is required. In this review, the diagnostic challenges in diabetic foot disease are discussed and available data on minimally invasive treatment options such as endovascular revascularization, stem cells, and gene therapy are examined.
Core Tip: Recognizing and promptly treating ischemia in patients with diabetic foot ulcers is essential for wound healing and limb salvage. A plethora of novel minimally invasive technologies and techniques are currently available, including dedicated peripheral angioplasty balloon catheters, drug-eluting stents, drug-coated balloons, angiosome-guided angioplasty, pedal arch angioplasty, and percutaneous deep vein arterialization, while research on gene and stem cell therapies is ongoing and initial data are deemed positive. Large, multicenter randomized trials specifically focused on optimizing endovascular treatment options for diabetic foot ulcers remain limited, and more high-quality, long-term, data are expected.
- Citation: Spiliopoulos S, Festas G, Paraskevopoulos I, Mariappan M, Brountzos E. Overcoming ischemia in the diabetic foot: Minimally invasive treatment options. World J Diabetes 2021; 12(12): 2011-2026
- URL: https://www.wjgnet.com/1948-9358/full/v12/i12/2011.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i12.2011
The global burden of diabetes mellitus (DM) has rapidly increased over the past decade, and many international scientific organizations now consider DM as the upcoming public health emergency of the 21st century, while health professionals and patients are becoming gradually aware of the gravity of diabetes-related complications[1]. Diabetes is the foremost cause of lower-limb loss worldwide. Every year, more than one million patients with DM suffer a lower limb amputation, and nearly every 20 s, an amputation is performed due to diabetic complications. Diabetic foot (DF) ulcers (DFU) are continuously becoming more frequent, and the incidence will further increase as the mean age of the world population increases and the obesity epidemic advances[1].
Moreover, diabetic patients are twice as likely to suffer from peripheral artery disease (PAD) in comparison with the non-diabetic population[2]. It has been also estimated that in middle and high-income countries, nearly half of patients with diabetes and foot ulceration suffer from underlying PAD and present with mixed neuroischemic type ulcers. On the contrary, neuropathic ulcers are less common and usually more frequent in lower-income countries[3,4]. Interestingly, in subjects with diabetes, PAD may remain undiagnosed before tissue loss, as patients may not experience any preceding clinical symptoms of PAD such as claudication or rest pain[5]. The pathophysiology of critical limb ischemia (CLI) involves chronic atheroma development, epithelial injury, and thrombus formation. This entity results in both lower limb micro and macro-vascular disease.
Established treatment options include open surgical and percutaneous endovascular revascularization techniques, while the experience gained from coronary interventions has also broadened peripheral endovascular capabilities with the use of drug-eluting stents (DES) and drug-coated balloons (DCB)[6]. For very small vessel disease, novel therapeutic options, at present under investigation, include gene and stem cell therapy aimed at local, targeted drug delivery triggering angiogenesis and vasculogenesis.
The purpose of this review is to present currently available minimally invasive interventions, for the management of ischemia in the diabetic foot.
The first step in the treatment of DF-related ischemia is prompt differentiation between purely neuropathic and neuroischemic DFU. DFU is defined as a complicated pathology of infection, ulceration, or destruction of tissues of the foot linked to neuropathy and/or peripheral artery disease in the lower extremity of a patient with a history of DM[7]. The key components of diabetic foot pathophysiology are a triad: Neuropathy, angiopathy, and structural and/or gait abnormalities. Peripheral neuropathy is one of the major factors correlated to diabetic ulcerations. Due to the loss of this nociceptive mechanism, patients are incapable of appreciating local foot trauma. As a result, the foot is at high risk of trauma and ulceration, which could lead to amputation[9].
Concomitant lower-limb arterial, atherosclerotic, steno-occlusive disease, PAD, is common in individuals with long-standing diabetes. Many mechanisms contribute to the development of PAD, particularly, endothelium dysfunction, arterial stiffness, thrombotic abnormalities, low-grade inflammation, advanced glycation end-products, and oxidative stress[10]. DFU are classified according to the underlying pathology in namely three categories: Neuropathic, ischemic, and neuroischemic. The ischemic component (PAD) is considered a form of macro-vascular complication and is positively associated with age, smoking, and other forms of macro-vascular complications, including hypertension and myocardial infarction, which increase the risk of cardiovascular death. On the other hand, peripheral neuropathy is a form of micro-vascular complication of diabetes. A mixed micro- and macro-vascular dysfunction results in neuroischemic disturbances where micro-vascular abnormalities impair perfusion of DF[3].
Physical examination of the foot is essential. Meticulous inspection is advised for the identification of neuropathic changes such as dry skin, cracks, malformations, callus, foot structure abnormalities, ulceration, and nail lesions. Major ischemia can be suspected in the presence of hair loss on the foot’s dorsal aspect and should be assessed by careful examination of peripheral pulses (common femoral, popliteal, distal foot arteries). Sensory neuropathy can be tested using monofilaments, biothesiometry or cotton wool, pinprick, and vibration sense for light touch[11]. A portable pocket doppler device can confirm the presence of pulses and quantify the arterial supply of the foot. The ankle-brachial index (ABI) should also be measured. However, ABI is not a reliable test for subjects with diabetes as they may present incompressible calf vessels due to significant Monckeberg medial calcific sclerosis, and therefore false-negative results are very common. The American Diabetes Association recommends that all people with diabetes and a foot wound should have pedal perfusion assessed by ABI and either toe-brachial index (TBI) or transcutaneous oxygen pressure (TcPO2)[12]. The 2019 Global vascular guidelines on the management of chronic limb-threatening ischemia, endorsed by the Society for Vascular Surgery, European Society for Vascular Surgery, and World Federation of Vascular Societies, does not suggest computed tomography angiography for the detailed visualization of infrapopliteal disease and recommends that patients with suspected chronic limb-threatening ischemia who are suitable candidates for limb salvage should not be denied revascularization without first undergoing complete diagnostic angiography including the ankle and foot[13].
Neuroischemic wounds are more arduous to heal than nonischemic and are correlated with higher rates of amputation and mortality. Thus, prompt revascularization for the treatment of mixed-neuroischemic DFU is today considered a medical emergency and should be performed using surgical and/or endovascular techniques, following a multidisciplinary team, case-sensitive decision[13,14].
The Fontaine and Rutherford-Becker classification systems have become obsolete for everyday clinical practice, as a wide spectrum of underlying factors such as degree of arterial disease, ulcer type, anatomical location and extent, presence, and severity of infection have been highly correlated to limb salvage in patients suffering from DFU. To address the need for a more accurate wound description, the Society for Vascular Surgery Lower Extremity Guidelines Committee recommended in 2014 a new classification system, based on three major factors: Wound, ischemia, and foot infection (WIfI). The WIfI classification system epitomizes a synthesis of many formerly published classification schemes and merges systems focused only on DFU or pure ischemia models. A brief description of the WIfI classification is presented in Table 1[15].
| Score | Wound | Ischemia (Toe pressure TcPO2) | Foot infection |
| 0 | No ulcer and no gangrene | 60 mmHg | Uninfected |
| 1 | Small ulcer no gangrene | 40-59 mmHg | Mild (< 2 cm cellulitis) |
| 2 | Deep ulcer and gangrene limited to toes | 30-39 mmHg | Moderate (> 2 cm cellulitis/purulence) |
| 3 | Extensive ulcer or extensive gangrene | < 30 mmHg | Severe (systematic response/sepsis) |
Due to very limited level Ia evidence comparing open vs endovascular revascularization, in subjects with DM the decision to proceed with open surgical bypass or endovascular treatment is case-sensitive and should be discussed in the ambit of a multi-disciplinary team meeting involving vascular surgeons, interventional radiologists, and diabetologists. Factors influencing treatment choice include patient’s age, comorbidities, surgical risk, location, extent of arterial disease, DFU characteristics, the availability of healthy vein for distal bypass, and local expertise. Endovascular treatment is often preferred over surgery (debridement, lower limb amputation, skin drafting, incision-drainage, sequestrectomy) in the presence of severe comorbidities and disease of the pedal outflow vessels. On the other hand, endovascular treatment should be conducted in large-volume dedicated centers by experienced hands[16].
In subjects with DM, atherosclerosis is more prevalent in the infrapopliteal arteries; however, concomitant femoropopliteal arterial disease is also common, while iliac artery disease, especially isolated, is less frequent, but not rare[17]. Therefore, multi-level revascularization of various arterial segments, with variable lumen diameters and different histology, is commonly required.
Significant advantages of minimal invasive treatment options over open surgical repair include less hospital stay and decreased periprocedural morbidity and mortality, especially in critically ill patients and those at high surgical risk. In the vast majority of cases general anesthesia is not required, and revascularization can be obtained using local anesthesia and mild conscious sedation. Moreover, endovascular methods represent the only option in patients with significant pedal arch arterial disease in which surgery is not an option for technical reasons[13,16,17].
Plain balloon angioplasty (POBA) remains the first-line endovascular treatment option in long infrapopliteal lesions, typically noted in patients with DM, although studies investigating POBA in exclusively diabetic populations are scarce. Starting from 2000, several studies have documented a high immediate technical success rate ranging between 80%-100% and a satisfactory (up to 80%) limb salvage rate at the 2-year follow-up in mixed diabetic and non-diabetic populations. Since 2005, the BASIL randomized trial remains the only randomized comparison of open surgical bypass vs POBA. Of 452 patients (42% with DM) presenting to 27 United Kingdom hospitals, 228 were randomly assigned to a bypass surgery-first and 224 to a balloon angioplasty-first revascularization treatment. Follow-up period was set for at least 3 years. Patients with infrapopliteal disease (with or without femoropopliteal disease) demonstrated similar amputation-free and overall survival, following vein bypass surgery or endovascular treatment[18]. Notable to mention, Faglia et al[19] published a population based cohort study with 292 diabetic patients with CLI according to the TransAtlantic Inter-Society Consensus II recommendations. Researchers report that angioplasty for diabetic patients with type D and/or long infrapopliteal lesions without good run-off at the foot and/or high surgical risk achieved high revascularization rates as well as less amputation rates[19].
In 2008, Romiti et al[20] published a meta-analysis of 30 studies (2557 patients, 2.653 limbs) to assess the mid-term outcomes of infrapopliteal POBA in patients with CLI and compared results with a meta-analysis of popliteal-to-distal vein bypass. Although both treatment modalities resulted in similar limb salvage rates, significantly lower 1-year patency rates were noted for POBA (48.6% ± 8.0% vs 72.3% ± 2.7%). The 30-d mortality and complication rates were significantly higher for infrainguinal bypass[20]. However, it should be noted that only two studies included in this meta-analysis investigated only diabetic patients, while the conclusions may not be reliable due to the methodological limitations of this review.
According to the existing literature data, the main disadvantage of POBA remains the development of neointimal hyperplasia, resulting in short-term restenosis, low patency rates, and clinical relapse, requiring more reintervention to sustain clinical outcomes. To overcome the limitation of restenosis following POBA, several new technologies were investigated. In 2010, Cryoplasty was compared with POBA in a single-center randomized trial that included 50 diabetic patients with femoropopliteal disease. The Cryoplasty balloon catheter (Boston Scientific, Boston, MA, United States) uses low-temperature angioplasty to induce smooth muscle cell apoptosis and reduce neointimal hyperplasia. However, at the 3-year follow-up there were no significant differences to patients’ survival and lower limb salvage, while lower primary patency and more repeat procedures due to clinical relapse were observed in the Cryoplasty subgroup[21].
The latest comparative studies also suggest that endovascular treatment demonstrates a similar limb salvage rate to open bypass. Specifically, in 2016 Patel et al[22] reported outcomes of a large retrospective, controlled study using propensity score matching to compare POBA with distal bypass surgery for the treatment of CLI. The study included 243 patients (with DM: 48.8% in the surgical group and 55.2% in the endovascular group in the propensity score-matched groups), and similar limb salvage rates were noted at 1-year follow-up (94.2% endovascular vs 90.4% surgery). However, at 1-year, primary (54.4% vs 51.4%), assisted (77.5 vs 62.7%), and secondary (84.4% vs 65.8%) patency rates were significantly better following open surgery. On the other hand, overall complications and length of hospital stay were significantly lower following endovascular treatment. Interestingly, according to binary logistic regression analysis, DM was identified as a preoperative factor favoring bypass surgery as the treatment choice[22].
Following the establishment of the long-term efficacy of DES in the treatment of coronary disease, the use of infrapopliteal DES has been investigated in retrospective analysis providing optimistic initial results that were further validated by multicenter randomized clinical trials (RCTs). Scheinert et al[23] published the “ACHILLES” multicenter RCT (200 patients; 64% with DM), which was the first-ever designed to investigate the efficacy and safety of a balloon-expandable, sirolimus-eluting stent compared to POBA in patients with symptomatic infrapopliteal arterial disease up to 90 mm in length. At 1-year follow-up, lower angiographic restenosis rates (22.4% vs 41.9%, P = 0.019), as well as superior vessel patency (75.0% vs 57.1%, P = 0.025), were noted in the sirolimus-eluting stent group, while similar death, repeat revascularization, and index-limb amputation rates were reported[23]. Additionally, two multicenter RCTs produced similar outcomes favoring DES over bare-metal stents in short- to medium-length infrapopliteal lesions, and one multicenter RCT demonstrated the long-term safety and efficacy of infrapopliteal paclitaxel-eluting stents over POBA[24,25].
Long-term, 10-year clinical results of infrapopliteal DES in an exclusively diabetic population are also available from a single-center retrospective study published in 2015. In total 214 patients (311 limbs, 562 arteries, 679 lesions) with DM and CLI were treated. At the 1-, 5-, and 10-year follow-up, survival and amputation-free rates were 90.8%, 55.5%, and 36.2%, and 94.9%,90.4%, and 90.4%, respectively, while target limb reintervention-free rates were 79.7%, 55.2%, and 49.7%, at 1, 5, and 10 years. Long-standing diabetes, concomitant coronary artery disease, and dialysis were identified as independent predictors of decreased survival[26]. However, limitations of infrapopliteal DES use include the presence of a continuous mechanical stimulus of the vessel wall eventually leading to restenosis, even in the long-term, the increased cost for the treatment of long lesions where multiple stents are required, and stent fractures occurring in specific various locations such as the distal below ankle arterial segments and the pedal arch[27].
To overcome such issues, DCB have emerged as a promising technology developed to overcome the limitations of standard balloon angioplasty and stenting. Specially designed paclitaxel-coatings have been developed to deliver a single dose of the cytotoxic agent paclitaxel to inhibit neointimal growth of vascular smooth muscle cells and prevent restenosis. The majority of multicenter RCTs investigated patients with femoropopliteal artery lesions suffering from intermittent claudication without tissue loss have proven the superiority of paclitaxel-coated balloons (PCBs) in late lumen loss, binary restenosis, and freedom from target lesion revascularization (TLR), providing a sufficient level of evidence to support equivalent or favorable mid-to-long-term outcomes for PCBs in comparison to POBA[28].
In a meta-analysis of randomized trials published in 2016 including 1609 patients (1403 subjects with claudication and 206 with CLI), high-quality evidence demonstrated a significant superiority of PCBs in reducing late lumen loss (LLL) [mean difference -0.89 mm; 95% confidence interval (CI): -1.14 to -0.64], less binary restenosis (relative risk 0.47; 95%CI: 0.37 to 0.61), and re-interventions (relative risk 0.33; 95%CI: 0.22 to 0.49)[29].
In 2019, long-term 5-year outcomes from a multicenter RCT investigating a 3 μm/mm2 PCB for femoropopliteal lesions demonstrated a sustained treatment effect with less re-interventions due to clinical relapse compared to POBA (target lesion revascularization 74.5% vs 65.3%, P = 0.02)[30]. However, in terms of clinical endpoints more specific for DF disease and ischemia such as wound healing, time to wound healing, and limb salvage, the superiority of PCBs over standard POBA has not been proven, as data remain limited and contradictive, especially for patients suffering from infrapopliteal disease.
More recently, data from three more RCTs investigating two different PCBs were made available with contradictive outcomes. The ACOART-BTK single-center RCT randomized 105 patients (nearly all diabetics) with CLI, and outcomes in the PCB group were superior to those in the POBA group for LLL, restenosis, and re-interventions at 6 mo follow-up. Most importantly, healing time, which is a highly significant clinical endpoint for the diabetic population under investigation, was also significantly improved in the PCB group (5.2 ± 2.7 mo vs 7.7 ± 3.9 mo, P = 0.005), while complete wound healing rate at 1 year was nearly significant in the PCB group (89.4% vs 74.5%, P = 0.05). Moreover, no major amputations were noted at the 1-year follow-up in both groups[31].
Lately, Del Giudice et al[31] conducted a prospective single-center cohort study that assessed the safety and efficacy of a new generation low-dose DCB with a reduced crystalline structure to treat below the knee (BTK) lesions in patients with CLI. To be more specific, immediate technical success was 97% (29/30), and primary safety outcome parameter was 94% (28/30). Angiographic follow-up was available in 20 patients. Results demonstrated primary angiographic patency 57% (12/21 lesions) and LLL 0.99 ± 0.68 mm at 6 mo. Moreover, freedom from TLR was 89% at 12 mo, and the rate of ulcer healing was 76% at 12 mo. Thus, ranger DCB balloons documented a positive trend with good safety outcome parameters for the treatment of CLI patients[31]. On the other hand, data from the larger multicenter Lutonix BTK RCT that randomized 442 patients (287 in group PCB and 155 group POBA) were not analogous, as the PCB under investigation failed to demonstrate superiority compared to POBA[32]. Similarly, outcomes of the multicenter, IN.PACT BTK randomized study to assess safety and efficacy of IN.PACT 014 vs PTA (50 CLI patients; 74% diabetics) reported no significant difference in LLL and re-intervention rate at 9-mo follow-up, although LLL was numerically lower in the PCB group[33].
However, a wide range of variability in study design, eligibility criteria, and outcome endpoints among RCTs was noted. Therefore, currently, there is no up-to-date available high-quality evidence to support the superiority of PCBs over POBA in reducing major amputations, and long-term randomized data are still in scarcity[34].
Moreover, significant safety issues have been raised following the publication of two meta-analyses of RCT that have reported an increased risk of death following the use of paclitaxel-eluting stents and PCBs in femoropopliteal lesions and decreased amputation-free survival following PCB use in the infrapopliteal arteries[35].
Nevertheless, the subject remains controversial, as following these findings, several large retrospective “real-life” studies have not confirmed these results. As available RCTs are contradictive and safety issues have been raised, the use of PCBs in infrapopliteal disease remains controversial, and further multicenter RCTs are required to support their use and safety in every-day clinical practice.
A significant subgroup of diabetic patients with advanced PAD, especially those with concomitant end-stage renal disease, suffer from a diffuse steno-occlusive disease of the infrapopliteal and distal plantar vessels[36]. The main technical advantages of endovascular treatment over open bypass surgery include the possibility of revascularizing more than one infrapopliteal artery and, most importantly, treating outflow plantar artery disease reconstituting the pedal arch (arch-plasty), which is not amenable to surgical reconstruction[37]. Following revascularization of blood flow to the ischemic tissue, adequate blood reperfusion is established, relieving ischemic symptoms and promoting wound healing[38]. A 2009 landmark study by Manzi et al[39] compared infrapopliteal angioplasty with or without pedal arch angioplasty. This study referred to the pedal-plantar loop technique. The authors retrospectively analyzed outcomes following the recanalization of the pedal and plantar arteries and their anatomical anastomosis in 135 patients, aimed at the restoration of a direct arterial in-flow from both anterior and posterior tibial vessels (the pedal-plantar loop technique; first reported by Fusaro et al[40], in 2007). The acute success of the technique was 85%, clinical improvement was maintained after a mean follow-up of 12 mo, while a significant improvement of TCpO2 at 15 d was noted in the group with successful plantar arteries revascularization[39].
In 2017, the first large-scale multicenter retrospective analysis was published. The Retrospective Analysis for the Clinical Impact of Pedal Artery Revascularization Versus Non-Revascularization Strategy for Patients With Critical Limb Ischemia retrospective registry investigated a total of 257 CLI patients (with 187 or 72.8% diabetic patients) separated into two groups based on additional pedal angioplasty (n = 140) or not (n = 117). Wound healing (57.5% vs 37.3%, P = 0.003) and time to wound healing (211 d vs 365 d, P = 0.008) were notably better in the pedal angioplasty group compared to the no-pedal angioplasty group[41].
In 2019, a meta-analysis of below-the-ankle angioplasty (BTA) (10 studies, with 478 patients and 524 legs) was published by Huizing et al[42]. Pooled 1-year limb salvage and amputation-free survival rates were 92% and 78%, respectively, while no statistically significant difference was detected in these clinical endpoints following additional BTA angioplasty compared to standard infrapopliteal angioplasty only. However, the wound healing rate was superior when additional BTA angioplasty was performed, while for more severe pedal artery disease, wound healing results were also superior after BTA angioplasty. Notably, complete wound healing and time-to-wound healing are highly significant endpoints for the specific DFU population, as these correlate with the quality of life, hospitalization time, frequency of hospital visits, and, eventually, long-term limb salvage extending beyond 1 year, which is more frequently reported[42].
Further developments on tissue reperfusion techniques were initially published in 2011 by Alexandrescu et al[43], who reported the first results of the angiosome-guided infrapopliteal angioplasty. The fundamentals of angiosome theory are based on a wound-adjusted revascularization strategy, aiming to enhance wound healing and limb salvage. Despite the limitations of this initial study (a small number of participants, short-term follow-up, limitations in angiography interpretation, selection bias), additional pedal and plantar artery angioplasty of the branch directly supplying blood to the wound seemed to result in excellent limb salvage rate. Thus, angiosome-based revascularization improves wound perfusion and decreases time to wound healing, but there is a lack of solid evidence regarding limb salvage improvement[43].
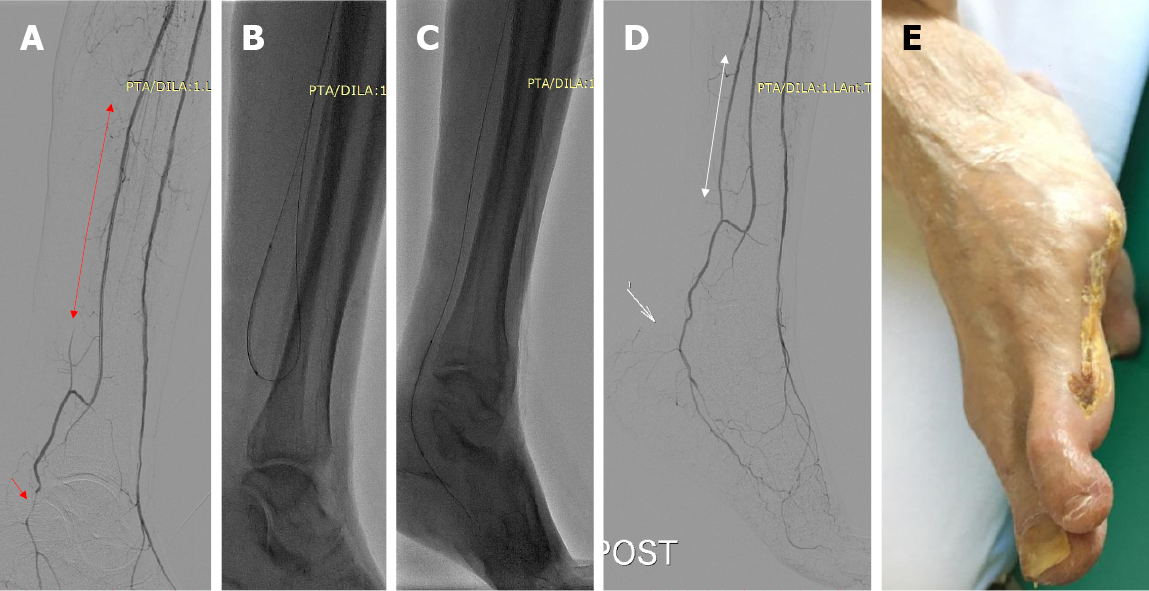
Only recently, Ma et al[44] reported outcomes of a prospective single-center observational cohort study investigating the intraoperative quantification of parenchymal blood volume (BV) in different foot regions assessed by C-arm computed tomography before and after revascularization in 27 patients. Interestingly, direct revascularization, according to the angiosome approach, resulted in a 197% BV increase compared to a 39% increase following indirect revascularization (P = 0.028). The authors concluded that direct revascularization of the ischemic area results in superior tissue perfusion than indirect revascularization[44]. Despite the widespread use of the plantar arch and angiosome-guided angioplasty, triggered by clinical experience and available results (Figure 1), the current level of evidence regarding the clinical superiority of these techniques remains low, and their effect on overall amputation-free survival remains unclear[41]. Larger, carefully designed RCTs are required to determine the optimal endovascular treatment algorithm in diabetic patients with CLI.
Percutaneous deep venous arterialization (pDVA) has been recently introduced as a novel technique to overcome ischemia in “no option” patients who lack a viable target vessel for either surgery or endovascular treatment. The technique is based on the concept that arterialization of the venous system could be considered as an alternative source of perfusion of the distal foot. In 2020, Schmidt et al[45] published the mid-term results of the largest available series, revealing a promising potential for this complex group of “no option patients”. Specifically, investigators reported outcomes of a retrospective study of 32 consecutive patients (66% with type 2 DM) treated with pDVA using the LimFlow device in four vascular centers in Alkmaar (Netherlands), Leipzig (Germany), Paris (France), and Singapore (ALPS). The procedure aimed to create a fistula between a tibial artery and a tibial vein and provide pressurized arterial flow to the venous system of the foot. Considering the stage, extent, and prognosis of CLI in this group of patients, pDVA using the LimFlow device resulted in a high technical success rate (96.9%), very satisfactory limb salvage (79.8%), and complete wound healing (72.7%) at the 2-year follow-up. Therefore, according to currently available initial data, pDVA could provide an option in selected “no-option” CLI patients[45].
Hepatocyte growth factor: Hepatocyte growth factor (HGF), also known as scatter factor, is a paracrine cellular growth, motility, and morphogenic factor. It is a mesenchyme-derived pleiotropic angiogenetic growth factor that targets and acts primarily upon epithelial and endothelial cells and secondarily acts on hemopoietic progenitor cells and T cells. Recently, a novel therapeutic strategy for ischemic diseases using angiogenic growth factors to augment collateral artery development has been proposed[46].
HGF is implemented in the regulation of angiogenesis and has been shown to induce the formation of collateral vessels in rabbits. The first attempt to examine the pro-angiogenetic properties of HGF for limb ischemia in real-world patients was conducted by Nakagami and his colleagues[47] in 2005. Investigators performed intramuscular injection of naked plasmid DNA in the ischemic limbs of 6 patients, which demonstrated great potential. Although the study was designed to demonstrate the safety in a phase I/early phase IIa trial, these initial clinical outcomes using HGF gene transfer regarding its effectiveness as the sole therapy for CLI are optimistic[47].
Following the same concept, Gu et al[48] conducted a phase I clinical trial to evaluate the safety, tolerability, and preliminary efficacy of naked DNA therapy expressing two isoforms of hepatocyte growth factor (pCK-HGF-X7) in CLI patients. Improvement in wound healing was observed in 66.6% of patients with a baseline foot ulcer. These results supported the design of phase II RCT with pCK-HGF-X7[48,49].
Shigematsu and his colleagues[50], conducted a multicenter, randomized, double-blind, placebo-controlled trial in order to measure safety and efficacy of HFG plasmid DNA in patients with CLI. Efficacy was evaluated after 12 wk. Researchers report that overall improvement rate of the primary end point (improvement of rest pain or reduction of ulcer size) was 70.4% (19/27) in HGF group and 30.8% (4/13) in placebo group, showing a significant difference (P = 0.014). Furthermore HGF plasmid also improved quality of life. Thus, intramuscular injection of naked hepatocyte growth factor plasmid is safe and feasible for patients with CLI[50].
Two years later, the same group published a second multi-center study (the HGF-0205 trial). Powell et al[51] tried to explore further the safety and efficacy of HGF using a modified delivery technique. Patients classified as Rutherford-Becker categories 5 and 6 were enrolled. There was a significant improvement in the primary endpoint of the TBI and the secondary endpoint of rest pain assessment at 6 mo. Nonetheless, no significant difference was observed regarding wound healing and amputation rates. Recently, in a randomized, double-blinded, placebo-controlled phase II study of HGF published in 2019 by Yongquan et al[52], the NL003 DNA plasmid (pCK-HGF-X7) was investigated. This DNA plasmid encodes a genomic complementary DNA hybrid human HGF sequence designed to express simultaneously two wild-type isoforms of HGF: HGF723and HGF728. According to the study design, 200 patients (Rutherford scale 4-5) were randomly assigned: Placebo (n = 50), low-dose (n = 50), middle-dose (n = 50), or high-dose NL003 (n = 50). The drug was administered to the affected limb on days 0, 14, and 28. No significant differences in the incidence of adverse events or serious adverse effects were found among the groups. Even though there were no statistically significant differences in TcPO2, ABI, or TBI values between the four groups, this study reported the first effective evidence of significant improvement in complete ulcer healing, complete pain relief without analgesics, and safety for NL003 in patients with Rutherford stage 4–5[52].
Vascular endothelial growth factor: Vascular endothelial growth factor (VEGF) is a key factor in angiogenesis, stimulating the proliferation and migration of endothelial cells, which leads to the formation of new vessels. Today, several members of the VEGF family have been identified[53]. The VEGF-A is the main VEGF investigated in several clinical trials, as it has been recognized as a strong inducer of vascular permeability, with high angiogenic efficacy.
Another landmark study, published in 2002 by Mäkinen et al[54], first reported the possibility of VEGF transfer using an adenoviral vector. The authors conducted a phase II randomized, placebo-controlled, double-blind study evaluating local intra-arterial catheter-mediated AdVEGF165 gene therapy after percutaneous transluminal angioplasty. At 3 mo follow-up, DSA indicated increased vascularity in the VEGF-treated groups. There was also a numerical improvement in the Rutherford class and ABI values compared to the baseline, but this improvement was not significantly different from that observed in the placebo arm[54].
In 2003, Shyu et al[54] investigated the safety and efficacy of intramuscular gene therapy with vascular endothelial growth factor (VEGF 165) in patients with chronic CLI. Magnetic resonance angiography revealed qualitative evidence of improved distal flow in 19 limbs (79%). Ischemic ulcers healed or improved markedly in 12 limbs (75%). Rest pain was relieved or improved markedly in 20 limbs (83%). Complications were limited to transient leg edema in six limbs. As a result, this landmark study was among the first to show safety, efficacy, and feasibility of intramuscular gene therapy with VEGF (165) for patients with CLI[54].
In the Regional Angiogenesis with VEGF, also known as RAVE, trial published in 2003 by Rajagopalan et al[55], the intramuscular administration of VEGF was tested using different dose regimes vs placebo. In total, 105 patients with unilateral exercise-limiting intermittent claudication were enrolled to receive a low or high dose of AdVEGF121 or placebo by 20 intramuscular injections in a single session. However, patients receiving VEGF did not demonstrate significant improvement in the primary endpoint of peak walking time nor the secondary endpoints of ABI and quality of life measures compared to placebo. Furthermore, patients treated with VEGF developed peripheral edema, which may indicate its potential bioactivity[55].
Hypoxia-inducible factor 1a: Hypoxia-inducible factor (HIF) 1a is a transcriptional regulatory factor that orchestrates cellular responses to hypoxia. This agent has demonstrated an ability to increase collateral blood vessels, capillary density, and neovascularization in pre-clinical animal studies[56,57]. In a phase II prospective, randomized, double-blind, placebo-controlled, parallel-group, multicenter study conducted in 35 sites (27 in the United States, four in the United Kingdom, and four in Germany), a total of 289 patients with claudication were randomized in a double-blind manner to one of three doses of Ad2/HIF-1α/VP16 or placebo administered by 20 intramuscular injections to each leg. Unfortunately, HIF 1a failed to achieve significantly superior outcomes compared to placebo in the primary endpoint of peak walking time at 6 mo follow-up and in all secondary endpoints. Complementary studies to evaluate the potential usefulness of HIF-1a in CLI treatment are needed[58].
Stromal derived factor-1: Stromal-derived factor-1 (SDF-1), also known as CXC motif chemokine 12 (CXCL12), is a chemokine protein that in humans is encoded by the CXCL12 gene on chromosome 10. SDF-1 has a pivotal role in the retention and homing of hematopoietic stem/progenitor cells into the bone marrow microenvironment[59,60]. Non-viral DNA plasmid encoding human stromal derived factor-1 (pSDF-1) enhance neovascularization at the micro-vascular level. In 2014 a promising phase IIa randomized double-blind placebo-controlled trial to Evaluate Plasmid Stromal Cell-Derived Factor-1 for Treatment of Critical Limb Ischemia (The STOP-CLI trial) was published that aimed to compare the effect of a biological agent vs placebo in the progression of CLI. Forty-eight CLI (Rutherford 4 or 5) patients who were poor candidates for surgical revascularization on stable medical therapy with ankle systolic pressure ≤ 70 mmHg or toe systolic pressure ≤ 50 mmHg were enrolled into four cohorts (n = 12 each). The study aimed to evaluate the safety and tolerability of escalating doses of 1 mg/mL pSDF-1 (4 mg, 8 mg, 8 mg, or 16 mg) delivered via direct intramuscular injection (8 mg or 16 mg) to the ischemic limb. Interim results indicated the safety of SDF-1 and suggested its use for improving the clinical status of poor candidates for revascularization[61].
Recently, in 2019, Shishehbor et al[62] conducted a double-blind, placebo-controlled, phase IIB trial. Investigators randomized 109 patients (86 with DM) with CLI (Rutherford class V or VI) to 8 mg or 16 mg intramuscular injections of placebo vs a non-viral gene therapy that stimulates endogenous regenerative repair mechanisms known as JVS-100. Investigators set primary efficacy end point as a 3-mo wound healing score estimated by an independent wound core laboratory. The primary safety end point was major adverse limb events[62].
However, results from the three groups (placebo, 8 mg and 16 mg injections) revealed only 26% of wounds completely healed at 3 mo. Surprisingly, no variations among the three groups (26.5%, 26.5%, and 25%, respectively) were documented. Correspondingly, researchers notice no significant changes to TBI after 3 mo. Notable to mention that rates of major adverse limb events at 3 mo were 8.8%, 20%, and 8.3%, respectively[62].
Thus, while being safe, JVS-100 missed to improve wound healing or hemodynamic calibrations at 3 mo. A quarter, alone of CLI wounds was treated at 3 mo despite successful revascularization. These results point out the necessity for further research considering microcirculation augmentation therapies for PAD patients[62].
Basic fibroblast growth factor: Basic fibroblast growth factor (bFGF) (also known as FGF-β or FGF-2) is a growth factor and signaling protein that triggers a harmonized arteriogenic effect, activating several downstream signals such as VEGF and monocyte chemoattractant protein-1[63,64]. Until today, the level of evidence remains low as very few clinical trials have examined the role of bFGF in patients with PAD, while some clinical trials were prematurely terminated[65].
In 2009, Hashimoto et al[66] investigated the safety of selective and sustained delivery of bFGF using acidic gelatin hydrogel microspheres (AGHMs) for the treatment PAD in a single-arm prospective observational study in 8 patients with PAD. AGHM suspension containing 100 mg bFGF was infused into the artery of the affected limb. Evaluation of safety and changes in clinical symptoms, resting ABI measurement, and TcPO2 as well as angiography was conducted at baseline and at various time points. No serious adverse events were observed. All cases demonstrated improvement of symptoms, although this was frequently temporary. The authors concluded that selective sustained delivery of bFGF by AGHMs was safe and well-tolerated in PAD[66].
Following the same concept, Kumagai et al[67] conducted an open-label, single-dose, phase I-IIa study that included 10 CLI patients to investigate the safety and efficacy of a sustained-release system of bFGF using biodegradable gelatin hydrogel. A single dose of 200 μg of bFGF-incorporated gelatin hydrogel microspheres was injected into the ischemic limb gastrocnemius. No serious procedure-related adverse events were recorded, while TcO2 was significantly improved at 6 mo follow-up. Secondary endpoints (6 min walk, Rutherford class, rest pain, cyanotic scales) were also significantly improved. The authors concluded that a sustained release of bFGF from biodegradable gelatin hydrogel seems to induce angiogenesis and provide an effective alternative treatment option for CLI patients. However, more appropriately powered clinical investigations, especially in dose escalation, are needed[67].
Over the last 2 decades, stem cell therapy (SCT) has emerged as a favorable alternative to traditional surgical and/or endovascular revascularization to treat ischemia in the DF. The primary benefit of SCT is to induce therapeutic neovascularization and generate collateral vessel formation to increase blood flow in the ischemic limb and soft tissue. Reported literature provides a solid rationale for ongoing in-depth studies aimed at advancing current SCT that may change the way PAD/CLI patients are treated.
The first landmark study was performed in 2002 by Tateishi-Yuyama et al[68]. The researchers recruited a mixed population of bone-marrow-derived CD34+ and CD34- cells for no-option CLI patients. They conducted a pilot study and a subsequent larger, blinded RTC. Cells were only sorted and concentrated before limb implantation. Investigators reported a marginal increase in ABI values in treated limbs compared with untreated limbs (approximately +0.1). Nonetheless, a noteworthy increase in TcPO2 was reported. Notably, MR-angiography demonstrated an increased number of collateral vessels in the treatment group compared to the control group, and this was also correlated with clinical improvement[68].
The abovementioned promising results were partly reproduced in 2005 by Huang et al[69] This time, researchers focused completely on diabetic patients (both type 1 and type 2 DM). Authors selected peripheral blood mononuclear cells (MNCs) after mobilization via administration of granulocyte colony-stimulating factor. At the end of the 3-mo follow-up, lower limb pain and ulcers were significantly improved in patients included in the transplant group. Nevertheless, this was an open-label non-randomized trial, without a predetermined sample size[69]. Moreover, in both trials, mixed bone-marrow cell populations were used and whether mesenchymal stem cells or mixed MNCs contributed to the reported clinical benefit is not clear.
In 2011, Lu et al[70] conducted a single-center, three-arm, randomized, double-blind study to assess the effectiveness of stem cells therapy in CLI and to evaluate further the relative benefit of mixed bone marrow population and mesenchymal stem cells (MSCs), by comparing a mixed population of bone-marrow-derived MNCs and sorted bone-marrow MSCs with a placebo group of limbs, in which only normal saline was injected. Clinical benefit was reported over the control group for both treatments, with a more marked increase noted in limbs receiving MSCs. This benefit included a notable 100% ulcer healing and no amputation in the treated limbs. This study represents a milestone trial in cell therapy as it provided a high-level of evidence regarding the safety and effectiveness of MSCs therapy over bone marrow MNCs in increasing perfusion and promoting ulcer healing in diabetic patients with CLI[70]. Several other smaller studies have also confirmed these results[71-73].
The RESTORE-CLI, a multicenter, sponsor-initiated, double-blinded phase II RCT published in 2012, investigated a cellular product named Ixymielocel-T, crafted from each patient’s bone marrow stem cells. Ixymielocel-T was a mixed population of MSCs and HSCs that underwent expansion by a proprietary procedure[74]. The study randomized 77 patients (Ixmyelocel-T: 48 patients and placebo 24 patients). Safety was demonstrated, and the efficacy endpoint of time to first occurrence of treatment failure was significantly longer for patients treated with Ixmyelocel-T (P = 0.0032). Moreover, a trend vs superior amputation-free survival was also noted in patients in the investigational group; however, this result did not reach statistical significance[74].
Summarizing the above-mentioned results, stem cell based therapies have proven to be safe and efficient to promote angiogenesis and blood flow in patients with CLI and especially those with no other options. Although initial results seem positive, variability between clinical trials is huge. As a result, there is an unresolved consensus regarding crucial factors such as cell doses, cell types or sources, administration routes, the parameters to define outcome efficacy, or the cohorts themselves. A lot of work needs to be done in order to translate the clinical benefits of SCT to a widely accepted model[75].
Hyperbaric oxygen therapy (HBOT) for diabetic ulcers involves intermittent administration of 100% oxygen, usually in daily sessions of 90 min each, at pressures of 1.5 to 3.0 atmospheres absolute (ATA) in an airtight cabin. By increasing the blood oxygen content, HBOT creates a favorable gradient for the diffusion of oxygen into the tissues. In hypoxic tissues, the enhanced oxygen supply has multiple effects that may benefit wound healing. Additionally, by increasing the expression of VEGF and FGF, HBOT may enhance angiogenesis and fibroblast proliferation. Moreover, the resulting hyperoxia may cause vasoconstriction, thereby decreasing tissue edema. By reducing the expression of pro-inflammatory cytokines, HBOT reduces inflammation while simultaneously enhancing the bactericidal activity of leukocytes.
In 2014, Stoekenbroek et al[76] conducted a systematic review of RCTs to assess the additional value of HBOT in promoting the healing of DFU and preventing amputations. According to these results, some evidence of the effectiveness of HBOT in improving the healing of diabetic leg ulcers in patients with concomitant ischemia was reported.
Considering the low quality of current evidence, high costs of HBOT, and the burdensome nature of a full HBOT regimen, there is insufficient evidence to support the routine use of HBOT as an adjunct to standard wound care in diabetic patients with foot ulcers and more data are awaited[76].
Minimally invasive preventive surgery at an early stage can be used to reduce focal points of pressure (off-loading) and to correct deformities (hammer and mallet toe) that may increase the risk for ulceration. Specifically, surgical off-loading is carried out by minimal percutaneous surgery, with specific well-established interventions such as distal metatarsal and phalanx osteotomies, tenotomies, and capsulotomies. The main objective of minimally invasive corrective foot surgery is to restore a stable foot during stance, which suggests that the head of the first and the fifth metatarsal as well as calcaneus are on the same plane. In addition, the aim is to minimize trauma without osteosynthesis, possibly decreasing the risk of infection and vascular and healing complications in diabetic patients. As a result, a subsequent more extensive surgery could be avoided. Similarly to endovascular techniques, minimally invasive surgery for DFU requires specific equipment (blades, high-speed burrs, high power machines) with fluoroscopy control and a far-reaching learning curve for devoted surgeons[77,78].
DF is a challenging pathology with a broad spectrum of pathophysiological mechanisms and clinical manifestations. Prompt diagnosis of ischemia is crucial for timely treatment and rapid wound healing and should include detailed arterial assessment. Treatment of ischemia should be considered a medical emergency and decided in multidisciplinary team meetings. Open surgical, minimally invasive, or combined endovascular/surgical revascularization procedures should be readily available, and the choice of the optimal revascularization plan should be individualized. Both minimally invasive and surgical revascularization options have been reported to achieve satisfactory mid-to-long-term limb-salvage rates. Recently, highly specialized, large-volume vascular centers have endorsed the “endovascular-first” approach, which achieves similar limb salvage rates with open bypass, without precluding future surgical treatment options.
Various endovascular devices, mainly DES and DCB, have been used to reduce restenosis after endovascular treatment and minimize the need for reinterventions due to clinical relapse, while new revascularization techniques such as angiosome-guided angioplasty, pedal arch angioplasty, and pDVA have been endorsed by endovascular experts in everyday clinical practice in an attempt to optimize wound healing, time to wound healing, and limb salvage.
Multicenter randomized trials specifically focused on optimizing endovascular treatment options for DFU remain limited, and more high-quality data are expected. Gene and stem cell therapies have also been investigated mainly in “no option” CLI patients, not amenable to revascularization, and while initial data have been deemed positive, more evidence is required to justify their use. The authors speculate that soon these therapies combined with continuously improving endovascular revascularization techniques will optimize outcomes of DFU treatment.
| 1. | Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1590] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 2. | Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L; 1999-2000 national health and nutrition examination survey. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 411] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 3. | Yotsu RR, Pham NM, Oe M, Nagase T, Sanada H, Hara H, Fukuda S, Fujitani J, Yamamoto-Honda R, Kajio H, Noda M, Tamaki T. Comparison of characteristics and healing course of diabetic foot ulcers by etiological classification: neuropathic, ischemic, and neuro-ischemic type. J Diabetes Complications. 2014;28:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchlife RJ; Lipsky BA on behalf of the International Working Group on the Diabetic Foot (IWGDF) Part of the 2019 IWGDF Guidelines on the Prevention and Management of Diabetic Foot Disease. [cited 22 February 2021]. Available from: https://iwgdfguidelines.org/wp-content/uploads/2019/05/04-IWGDF-PAD-guideline-2019.pdf. |
| 5. | Davies MG. Criticial limb ischemia: epidemiology. Methodist Debakey Cardiovasc J. 2012;8:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Wickramarachchi U, Eccleshall S. Drug-coated Balloon-only Angioplasty for Native Coronary Disease Instead of Stents. Interv Cardiol. 2016;11:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | van Netten JJ, Bus SA, Apelqvist J, Lipsky BA, Hinchliffe RJ, Game F, Rayman G, Lazzarini PA, Forsythe RO, Peters EJG, Senneville É, Vas P, Monteiro-Soares M, Schaper NC; International Working Group on the Diabetic Foot. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 8. | Armstrong DG, Fisher TK, Lepow B, White ML, Mills JL. Pathophysiology and Principles of Management of the Diabetic Foot. In: Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]. Adelaide (AU): University of Adelaide Press; 2011. [PubMed] |
| 9. | Nativel M, Potier L, Alexandre L, Baillet-Blanco L, Ducasse E, Velho G, Marre M, Roussel R, Rigalleau V, Mohammedi K. Lower extremity arterial disease in patients with diabetes: a contemporary narrative review. Cardiovasc Diabetol. 2018;17:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 10. | Pendsey SP. Understanding diabetic foot. Int J Diabetes Dev Ctries. 2010;30:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Bandyk DF. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin Vasc Surg. 2018;31:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 12. | Abouhamda A, Alturkstani M, Jan Y. Lower sensitivity of ankle-brachial index measurements among people suffering with diabetes-associated vascular disorders: A systematic review. SAGE Open Med. 2019;7:2050312119835038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Ndip A, Jude EB. Emerging evidence for neuroischemic diabetic foot ulcers: model of care and how to adapt practice. Int J Low Extrem Wounds. 2009;8:82-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. J Diabetes Sci Technol. 2011;5:1591-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Mills JL Sr. Update and validation of the Society for Vascular Surgery wound, ischemia, and foot infection threatened limb classification system. Semin Vasc Surg. 2014;27:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Reekers JA, Lammer J. Diabetic foot and PAD: the endovascular approach. Diabetes Metab Res Rev. 2012;28 Suppl 1:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 358] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 18. | Popplewell MA, Davies H, Jarrett H, Bate G, Grant M, Patel S, Mehta S, Andronis L, Roberts T, Deeks J, Bradbury A; BASIL-2 Trial Investigators. Bypass versus angio plasty in severe ischaemia of the leg - 2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials. 2016;17:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Faglia E, Clerici G, Airoldi F, Tavano D, Caminiti M, Curci V, Mantero M, Morabito A, Edmonds M. Revascularization by angioplasty of type D femoropopliteal and long infrapopliteal lesion in diabetic patients with critical limb ischemia: are TASC II recommendations suitable? Int J Low Extrem Wounds. 2012;11:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Spiliopoulos S, Katsanos K, Karnabatidis D, Diamantopoulos A, Kagadis GC, Christeas N, Siablis D. Cryoplasty vs conventional balloon angioplasty of the femoropopliteal artery in diabetic patients: long-term results from a prospective randomized single-center controlled trial. Cardiovasc Intervent Radiol. 2010;33:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Patel SD, Biasi L, Paraskevopoulos I, Silickas J, Lea T, Diamantopoulos A, Katsanos K, Zayed H. Comparison of angioplasty and bypass surgery for critical limb ischaemia in patients with infrapopliteal peripheral artery disease. Br J Surg. 2016;103:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Scheinert D, Katsanos K, Zeller T, Koppensteiner R, Commeau P, Bosiers M, Krankenberg H, Baumgartner I, Siablis D, Lammer J, Van Ransbeeck M, Qureshi AC, Stoll HP; ACHILLES Investigators. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 24. | Rastan A, Tepe G, Krankenberg H, Zahorsky R, Beschorner U, Noory E, Sixt S, Schwarz T, Brechtel K, Böhme C, Neumann FJ, Zeller T. Sirolimus-eluting stents vs bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011;32:2274-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Spreen MI, Martens JM, Knippenberg B, van Dijk LC, de Vries JPM, Vos JA, de Borst GJ, Vonken EPA, Bijlstra OD, Wever JJ, Statius van Eps RG, Mali WPTM, van Overhagen H. Long-Term Follow-up of the PADI Trial: Percutaneous Transluminal Angioplasty Versus Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Spiliopoulos S, Theodosiadou V, Katsanos K, Kitrou P, Kagadis GC, Siablis D, Karnabatidis D. Long-Term Clinical Outcomes of Infrapopliteal Drug-Eluting Stent Placement for Critical Limb Ischemia in Diabetic Patients. J Vasc Interv Radiol. 2015;26:1423-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Puranik AS, Dawson ER, Peppas NA. Recent advances in drug eluting stents. Int J Pharm. 2013;441:665-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Barkat M, Torella F, Antoniou GA. Drug-eluting balloon catheters for lower limb peripheral arterial disease: the evidence to date. Vasc Health Risk Manag. 2016;12:199-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Katsanos K, Spiliopoulos S, Paraskevopoulos I, Diamantopoulos A, Karnabatidis D. Systematic Review and Meta-analysis of Randomized Controlled Trials of Paclitaxel-Coated Balloon Angioplasty in the Femoropopliteal Arteries: Role of Paclitaxel Dose and Bioavailability. J Endovasc Ther. 2016;23:356-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Laird JA, Schneider PA, Jaff MR, Brodmann M, Zeller T, Metzger DC, Krishnan P, Scheinert D, Micari A, Wang H, Masters M, Tepe G. Long-Term Clinical Effectiveness of a Drug-Coated Balloon for the Treatment of Femoropopliteal Lesions. Circ Cardiovasc Interv. 2019;12:e007702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 31. | Del Giudice C, Galloula A, Tiercelin C, Vilfaillot A, Alsac JM, Messas E, Déan CL, Larger E, Sapoval M. "Ranger BTK" a Prospective Single-Centre Cohort Study on a New Drug-Coated Balloon for Below the Knee Lesions in Patients with Critical Limb Ischemia. Cardiovasc Intervent Radiol. 44:1017-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Geraghty P. Lutonix BTK Trial. A Prospective, Multicenter, Single Blind, Randomized, Controlled Trial Comparing the Lutonix Drug Coated Balloon Versus Standard Balloon Angioplasty for Treatment of Below-the-Knee (BTK) Arteries. ClinicalTrials.gov. [cited 29 January 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT01870401. ClinicalTrials.gov Identifier: NCT01870401. |
| 33. | Barvy AA. IN.PACT BTK Trial. [cited 29 January 2020]. Available from: https://www.acc.org/Latest-in-cardiology/clinical-trials/2020/10/17/19/04/inpact-btk#.YBUauT1MUTA.gmail. ClinicalTrials.gov Indentifinder NCT02963649. |
| 34. | Spiliopoulos S, Reppas L. Is There Still Hope for Infrapopliteal PCB Angioplasty? JACC Cardiovasc Interv. 2020;13:2287-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2018;7:e011245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 711] [Cited by in RCA: 729] [Article Influence: 91.1] [Reference Citation Analysis (17)] |
| 36. | Leskinen Y, Salenius JP, Lehtimäki T, Huhtala H, Saha H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis. 2002;40:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 200] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Spiliopoulos S, Reppas L, Palialexis K, Brountzos E. Below-the-ankle Angioplasty: Current Evidence and Future Perspectives. Vascular Endovascular Review. 2019;2:6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Diehm N, Rohrer S, Baumgartner I, Keo H, Do D, Kalka C. Distribution pattern of infrageniculate arterial obstructions in patients with diabetes mellitus and renal insufficiency - implications for revascularization. Vasa. 2008;37:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Manzi M, Fusaro M, Ceccacci T, Erente G, Dalla Paola L, Brocco E. Clinical results of below-the knee intervention using pedal-plantar loop technique for the revascularization of foot arteries. J Cardiovasc Surg (Torino). 2009;50:331-337. [PubMed] |
| 40. | Fusaro M, Dalla Paola L, Biondi-Zoccai G. Pedal-plantar loop technique for a challenging below-the-knee chronic total occlusion: a novel approach to percutaneous revascularization in critical lower limb ischemia. J Invasive Cardiol. 2007;19:E34-E37. [PubMed] |
| 41. | Nakama T, Watanabe N, Haraguchi T, Sakamoto H, Kamoi D, Tsubakimoto Y, Ogata K, Satoh K, Urasawa K, Andoh H, Fujita H, Shibata Y. Clinical Outcomes of Pedal Artery Angioplasty for Patients With Ischemic Wounds: Results From the Multicenter RENDEZVOUS Registry. JACC Cardiovasc Interv. 2017;10:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Huizing E, Schreve MA, de Vries JPM, Ferraresi R, Kum S, Ünlü Ç. Below-the-Ankle Angioplasty in Patients with Critical Limb Ischemia: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol. 2019;30:1361-1368.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Jeon EY, Cho YK, Yoon DY, Kim DJ, Woo JJ. Clinical outcome of angiosome-oriented infrapopliteal percutaneous transluminal angioplasty for isolated infrapopliteal lesions in patients with critical limb ischemia. Diagn Interv Radiol. 2016;22:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Ma J, Lai Z, Shao J, Lei J, Li K, Wang J, Xu L, Fang L, Yu X, Qi W, Wang C, Cao W, Liu X, Yuan J, Liu B. Infrapopliteal endovascular intervention and the angiosome concept: intraoperative real-time assessment of foot regions' blood volume guides and improves direct revascularization. Eur Radiol. 2021;31:2144-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Schmidt A, Schreve MA, Huizing E, Del Giudice C, Branzan D, Ünlü Ç, Varcoe RL, Ferraresi R, Kum S. Midterm Outcomes of Percutaneous Deep Venous Arterialization With a Dedicated System for Patients With No-Option Chronic Limb-Threatening Ischemia: The ALPS Multicenter Study. J Endovasc Ther. 2020;27:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 46. | Gallagher JT. Heparan sulfate: growth control with a restricted sequence menu. J Clin Invest. 2001;108:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 240] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 47. | Nakagami H, Kaneda Y, Ogihara T, Morishita R. Hepatocyte growth factor as potential cardiovascular therapy. Expert Rev Cardiovasc Ther. 2005;3:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Gu Y, Zhang J, Guo L, Cui S, Li X, Ding D, Kim JM, Ho SH, Hahn W, Kim S. A phase I clinical study of naked DNA expressing two isoforms of hepatocyte growth factor to treat patients with critical limb ischemia. J Gene Med. 2011;13:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Zhang J, Hu W, Diao Q, Wang Z, Miao J, Chen X, Xue Z. Therapeutic effect of the epidermal growth factor on diabetic foot ulcer and the underlying mechanisms. Exp Ther Med. 2019;17:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, Yamaguchi T, Ogihara T, Morishita R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Powell RJ, Goodney P, Mendelsohn FO, Moen EK, Annex BH; HGF-0205 Trial Investigators. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J Vasc Surg. 2010;52:1525-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 52. | Gu Y, Cui S, Wang Q, Liu C, Jin B, Guo W, Chu T, Shu C, Zhang F, Han C, Liu Y. A Randomized, Double-Blind, Placebo-Controlled Phase II Study of Hepatocyte Growth Factor in the Treatment of Critical Limb Ischemia. Mol Ther. 2019;27:2158-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 7049] [Article Influence: 306.5] [Reference Citation Analysis (0)] |
| 54. | Shyu KG, Chang H, Wang BW, Kuan P. Intramuscular vascular endothelial growth factor gene therapy in patients with chronic critical leg ischemia. Am J Med. 2003;114:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Rajagopalan S, Mohler ER 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 417] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 56. | Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marcé M, Semenza GL. Adenoviral transfer of HIF-1alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci USA. 2009;106:18769-18774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Giménez CS, Castillo MG, Simonin JA, Núñez Pedrozo CN, Pascuali N, Bauzá MDR, Locatelli P, López AE, Belaich MN, Mendiz AO, Crottogini AJ, Cuniberti LA, Olea FD. Effect of intramuscular baculovirus encoding mutant hypoxia-inducible factor 1-alpha on neovasculogenesis and ischemic muscle protection in rabbits with peripheral arterial disease. Cytotherapy. 2020;22:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Creager MA, Olin JW, Belch JJ, Moneta GL, Henry TD, Rajagopalan S, Annex BH, Hiatt WR. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 59. | Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2814] [Cited by in RCA: 2845] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 60. | Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N, Margalit R, Zsak M, Nagler A, Hardan I, Resnick I, Rot A, Lapidot T. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 275] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 61. | Kibbe MR. A Phase IIa Randomized Double-Blind, Placebo Controlled Study to Evaluate Plasmid Stromal Cell-Derived Factor-1 for Treatment of Critical Limb Ischemia- The STOP-CLI Trial. Circulation. 2014;130:A19419. [DOI] [Full Text] |
| 62. | Shishehbor MH, Rundback J, Bunte M, Hammad TA, Miller L, Patel PD, Sadanandan S, Fitzgerald M, Pastore J, Kashyap V, Henry TD. SDF-1 plasmid treatment for patients with peripheral artery disease (STOP-PAD): Randomized, double-blind, placebo-controlled clinical trial. Vasc Med. 2019;24:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Deindl E, Hoefer IE, Fernandez B, Barancik M, Heil M, Strniskova M, Schaper W. Involvement of the fibroblast growth factor system in adaptive and chemokine-induced arteriogenesis. Circ Res. 2003;92:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Fujii T, Yonemitsu Y, Onimaru M, Tanii M, Nakano T, Egashira K, Takehara T, Inoue M, Hasegawa M, Kuwano H, Sueishi K. Nonendothelial mesenchymal cell-derived MCP-1 is required for FGF-2-mediated therapeutic neovascularization: critical role of the inflammatory/arteriogenic pathway. Arterioscler Thromb Vasc Biol. 2006;26:2483-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Cooper LT Jr, Hiatt WR, Creager MA, Regensteiner JG, Casscells W, Isner JM, Cooke JP, Hirsch AT. Proteinuria in a placebo-controlled study of basic fibroblast growth factor for intermittent claudication. Vasc Med. 2001;6:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Hashimoto T, Koyama H, Miyata T, Hosaka A, Tabata Y, Takato T, Nagawa H. Selective and sustained delivery of basic fibroblast growth factor (bFGF) for treatment of peripheral arterial disease: results of a phase I trial. Eur J Vasc Endovasc Surg. 2009;38:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Kumagai M, Marui A, Tabata Y, Takeda T, Yamamoto M, Yonezawa A, Tanaka S, Yanagi S, Ito-Ihara T, Ikeda T, Murayama T, Teramukai S, Katsura T, Matsubara K, Kawakami K, Yokode M, Shimizu A, Sakata R. Safety and efficacy of sustained release of basic fibroblast growth factor using gelatin hydrogel in patients with critical limb ischemia. Heart Vessels. 2016;31:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T; Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1234] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 69. | Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 70. | Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 332] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 71. | Li M, Zhou H, Jin X, Wang M, Zhang S, Xu L. Autologous bone marrow mononuclear cells transplant in patients with critical leg ischemia: preliminary clinical results. Exp Clin Transplant. 2013;11:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Procházka V, Gumulec J, Jalůvka F, Salounová D, Jonszta T, Czerný D, Krajča J, Urbanec R, Klement P, Martinek J, Klement GL. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010;19:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 73. | Ozturk A, Kucukardali Y, Tangi F, Erikci A, Uzun G, Bashekim C, Sen H, Terekeci H, Narin Y, Ozyurt M, Ozkan S, Sayan O, Rodop O, Nalbant S, Sıldıroglu O, Yalnız FF, Senkal IV, Sabuncu H, Oktenli C. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J Diabetes Complications. 2012;26:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, Stern TP, Watling S, Bartel RL. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther. 2012;20:1280-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Beltrán-Camacho L, Rojas-Torres M, Durán-Ruiz MC. Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 76. | Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, van den Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J Vasc Endovasc Surg. 2014;47:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 77. | Botezatu I, Laptoiu D. Minimally invasive surgery of diabetic foot-review of current techniques. J Med Life. 2016;9:249-254. [PubMed] |
| 78. | Chalya PL, Mabula JB, Dass RM, Kabangila R, Jaka H, McHembe MD, Kataraihya JB, Mbelenge N, Gilyoma JM. Surgical management of Diabetic foot ulcers: A Tanzanian university teaching hospital experience. BMC Res Notes. 2011;4:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Peripheral vascular disease
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Primadhi RA, Zhang LL S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ