Published online Oct 15, 2021. doi: 10.4239/wjd.v12.i10.1750
Peer-review started: April 14, 2021
First decision: May 12, 2021
Revised: June 7, 2021
Accepted: September 6, 2021
Article in press: September 6, 2021
Published online: October 15, 2021
Processing time: 181 Days and 12.5 Hours
Antagonists of cannabinoid type 1 receptor (CB1) have been shown to promote body weight loss and improve insulin sensitivity. Cannabinoids decrease adiponectin, and CB1 blocker increase adiponectin. However, the mediators of CB1 actions are not well defined.
To investigate whether the beneficial effects of CB1 inhibition are, at least in part, mediated by adiponectin.
We compared metabolic and inflammatory phenotypes of wild-type (WT) mice, CB1-null (CB1-/-) and CB1/adiponectin double-knockout (DKO) mice. We assessed the insulin sensitivity using insulin tolerance test and glucose tolerance test, and inflammation using flow cytometry analysis of macrophages.
CB1-/- mice exhibited significantly reduced body weight and fat mass when compared to WT mice. While no significance was found in total daily food intake and locomotor activity, CB1-/- mice showed increased energy expenditure, enhanced thermogenesis in brown adipose tissue (BAT), and improved insulin sensitivity compared to WT mice. DKO showed no difference in body weight, adiposity, nor insulin sensitivity; only showed a modestly elevated thermogenesis in BAT compared to CB1-/- mice. The metabolic phenotype of DKO is largely similar to CB1-/- mice, suggesting that adiponectin is not a key mediator of the metabolic effects of CB1. Interestingly, CB1-/- mice showed reduced pro-inflammatory macrophage polarization in both peritoneal macrophages and adipose tissue macrophages compared to WT mice; in contrast, DKO mice exhibited increased pro-inflammatory macrophage polarization in these macrophages compared to CB1-/- mice, suggesting that adiponectin is an important mediator of the inflammatory effect of CB1.
Our findings reveal that CB1 functions through both adiponectin-dependent and adiponectin-independent mechanisms: CB1 regulates energy metabolism in an adiponectin-independent manner, and inflammation in an adiponectin-dependent manner. The differential effects of adiponectin on CB1-mediated metabolic and inflammatory functions should be taken into consideration in CB1 antagonist utilization.
Core Tip: Antagonists of cannabinoid type 1 receptor (CB1) have been shown to promote body weight loss and improve insulin sensitivity. Cannabinoids have been shown to regulate adiponectin. However, it is unclear whether adiponectin is a key mediator of the functions of CB1. We compared metabolic and inflammatory phenotypes of CB1-null vs CB1/adiponectin double-knockout mice. Our findings reveal that CB1 functions through both adiponectin-dependent and adiponectin-independent mechanisms: CB1 regulates energy metabolism in an adiponectin-independent manner, and inflammation in an adiponectin-dependent manner.
- Citation: Wei Q, Lee JH, Wu CS, Zang QS, Guo S, Lu HC, Sun Y. Metabolic and inflammatory functions of cannabinoid receptor type 1 are differentially modulated by adiponectin. World J Diabetes 2021; 12(10): 1750-1764
- URL: https://www.wjgnet.com/1948-9358/full/v12/i10/1750.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i10.1750
The incidence of obesity has increased rapidly during recent decades, particularly in developed/industrialized countries. Obesity increases the incidences of hyperinsulinemia, insulin resistance, type 2 diabetes, dyslipidemia, atherosclerosis, hyperten-sion, inflammation, and cancer[1,2]. Endocannabinoids are key regulators of food intake and energy metabolism, and the effects are mediated through the activation of the cannabinoid type 1 receptor 1 (CB1)[3,4]. Recent studies have demonstrated that blocking the activity of the endogenous cannabinoid system might be a strategy for the treatment of obesity and metabolic syndrome[5-7].
Previous study demonstrated that CB1 knockout mice consume less food and have reduced body weight[4,8]. Rimonabant, a specific antagonist of CB1, reduces food intake by blocking the orexigenic effect of cannabinoids[9]. There is also evidence that endogenous cannabinoids regulate energy expenditure[10]. It has been shown that virally-induced hypothalamic CB1 knockout mice showed no change in food intake, but did show less body weight gain over time due to increased energy expenditure; and the mRNA expression of β3-adrenergic receptor and uncoupling protein-1 (UCP-1) was elevated in the brown adipose tissue (BAT)[10]. There are also data showing that rimonabant alleviates dyslipidemia and obesity via a BAT thermogenesis-mediated increase of energy expenditure[9]. It has been shown that peripheral CB1 blockade is effective in activating thermogenesis in BAT to mitigate dyslipidemia and obesity[11], which suggests that the function of CB1 in BAT can be peripherally mediated and is not necessarily dependent on its central action. These results suggest that endocannabinoids may regulate energy metabolism by binding to CB1 expressing cells in peripheral white adipose tissue (WAT)[8] and/or BAT[9].
Adiponectin, an adipokine with insulin-sensitizing functions, has been reported to be relevant in many metabolic diseases such as obesity, and with associated complications such as diabetes, hyperinsulinemia, insulin resistance, dyslipidemia, hypertension, and inflammation[12]. Adiponectin treatment reduces body weight, improves hyperglycemia, ameliorates hyperinsulinemia and insulin resistance, and increases fatty acid oxidation and lipid clearance, in animal models of obesity and diabetes[13,14]. One of the most intriguing consequences of rimonabant treatment is increased adiponectin gene expression in adipose tissue of diet-induced obese (DIO) mice[9] and in cultured adipocytes[15]. However, the rimonabant-treated adiponectin- and leptin-deficient mice exhibit significantly ameliorated insulin resistance, which suggests that rimonabant reduces insulin resistance via both adiponectin-dependent and adiponectin-independent mechanisms[16]. These results suggest that rimonabant may regulate adiponectin expression in adipocytes, and the metabolic effects of rimonabant, at least in part, could be due to enhanced adiponectin secretion.
To determine whether adiponectin is indeed required for the peripheral functions of CB1, we used a genetic approach by breeding CB1-/- mice with adiponectin-deficient mice to generate a mouse model lacking both CB1 and adiponectin, aka double KO (DKO). We studied metabolic regulation such as thermogenesis and insulin sensitivity in these mice. The link between inflammation and obesity is now increasingly recognized and inflammation is considered a culprit of insulin resistance. Thus, we also characterized macrophage polarization in peritoneal macrophages and adipose tissue macrophages to elucidate whether CB1 acts through adiponectin to modulate CB-1 mediated inflammation.
All procedures using animal experiments were approved by the Institution of Animal Care and Use Committee at Baylor College of Medicine. All mice used in this study were congenic male mice. All mice were on a pure C57/6J background. To generate mice lacking both CB1 and adiponectin, CB1-/- mice and adiponectin-/- mice were bred to each other to create compound heterozygotes that were CB1+/-/adiponectin+/-. In the second cross, compound heterozygotes were further bred to each other to yield homozygous CB1-/-/adiponectin-/- (aka double-knockout DKO mice); CB1-/- adiponectin+/+ mice (aka CB1-/-), and CB1+/+ adiponectin+/+ (aka WT mice). Age-matched male WT, CB1-/- and DKO were used in the studies. There were three groups of mice used in the study: (WT) control group, CB1-/- group, DKO group. Animals were housed under controlled temperature and lighting (75 ± 1 ℉; 12 h light-dark cycle). The diet was from Harlan-Teklad (2920X) and the diet compositions are as follows: 16% of calories from fat, 60% from carbohydrates, and 24% from protein. All experiments were approved by the Animal Care Research Committee of the Baylor College of Medicine.
Magnetic Resonance Imaging analysis of body composition was also carried out using an EchoMRI Whole Body Composition Analyzer (Echo MRI®, United States). Metabolic parameters were obtained using an Oxymax open-circuit indirect calorimetry Comprehensive Lab Animal Monitoring System (CLAMS) from Columbus Instruments (Columbus, OH, United States). Energy expenditure (EE) was calculated as the product of the value of oxygen (3.815 + 1.232 × RQ) and the volume of O2 consumed. Respiratory quotient (RQ) ratio of VCO2/VO2 was then calculated[17]. Energy expenditure was normalized to both body weight and lean mass. Locomotor activity was measured using infrared beams to count the number of beam breaks during the recording period. The CLAMS data was the average of 3 d of data that were collected after 3 d of acclimation.
The Insulin tolerance test (ITT) and glucose tolerance test (GTT) were carried out on WT, CB1-/- and DKO mice. For ITT, after being fasted for 6 h, glucose of mouse tail blood was measured using One Touch Ultra glucose meter (lifeScan, New Brunswick, NJ, United States). It can detect glucose concentrations from 20 to 600 mg/dl using an electrochemical biosensor technology based on glucose oxidase chemistry. Mice then received an i.p. injection of human insulin (Eli Lilly Indianapolis, IN, United States) at a dose of 1.0 U kg-1 of body weight. Tail blood glucose concentration was measured at 0, 30, 60, 90 and 120 min after i.p. insulin injection. The GTT was carried out after the mice were fasted for 18 h overnight. The mice received i.p. injection of glucose (Sigma-Aldrich, St. Louis, MO, United States) at a dose of 2.0 g kg-1 body weight. The tail blood glucose was measured at 0, 15, 30, 60 and 120 min after glucose injection, and blood was collected for ELISA insulin analysis at 0, 15, 30 and 120 min after glucose injection.
Peritoneal macrophage and stromal vascular (SV) cells of epididymal adipose tissues were fractionated as described[18,19]. Briefly, to get peritoneal macrophage, 5 ml of cold phosphate buffer saline (PBS) was injected into mouse peritoneal cavities immediately after anesthesia. After shaking the mice for 2-3 min, peritoneal fluid was harvested and spun down for peritoneal macrophages at 500 g for 5 min at 4 °C. The stromal vascular cells were isolated from the equal mass of epididymal adipose tissues using the collagenase digestion method. For flow cytometry analysis, same quantity cells (1 × 106) were subsequently re-suspended and stained with appropriate antibodies (F4/80 and CD11c for M1 type macrophage, or F4/80 and CD206 for M2 type macrophage) as described in our previous study[20]. Antibody information used in flow cytometry analysis is as follows: PE anti-mouse F4/80 antigen (eBioscience, San Diego, CA), FITC anti-mouse CD11c antigen (BD Bioscience, San Jose, CA), purified CD16/CD32 antigen (BD Bioscience, San Jose, CA), and APC anti-mouse CD206 antigen (BD Bioscience, San Jose, CA). All data were collected using FACScan and analyzed using CellQuest software (BD Biosciences, San Jose, CA).
BAT and WAT were snap-frozen in liquid nitrogen and stored at -80 ℃. Total RNA was extracted from frozen tissue samples using TRIzol Reagent (Invitrogen, Carlsbad, CA). RNA was subsequently treated with DNase (Ambion, Austin, TX). RNA quality was assessed on 1.5% agarose gel electrophoresis in the presence of formaldehyde, and RNA concentration was determined by NanoDrop. The cDNA was synthesized from 1g RNA using the Superscript Ⅲ First-Strand Synthesis system for reverse transcription-polymerase chain reaction (RT-PCR) (Invitrogen). Quantitative real-time RT-PCR was performed on an ABI7900 using the SYBR Green PCR Master Mix or the Taqman gene expression Master Mix (Applied Biosystems, Carlsbad, CA, United States). After amplification, the PCR product was subjected to 2% agarose gel electrophoresis. 18S RNA and -actin were used as internal controls. The primer sequences of quantitative RT-PCR are listed in Table 1 below.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| UCP-1 | GTGAAGGTCAGAATGCAAGC | AGGGCCCCCTTCATGAGGTC |
| PGC-1α | CATTTGATGCACTGACAGATGGA | CCGTCAGGCATGGAGGAA |
| IR | CAAAAGCACAATCAGAGTGAGTATGAC | ACCACGTTGTGCAGGTAATCC |
| IRS1 | GCCTGGAGTATTATGAGAACGAGAA | GGGGATCGAGCGTTTGG |
| PPARγ2 | GCCTATGAGCACTTCACAAGAAATT | TGCGAGTGGTCTTCCATCAC |
| GLUT4 | GCCTTGGGAACACTCAACCA | CACCTGGGCAACCAGAATG |
| F4/80 | CTTTGGCTATGGGCTTCCAGTC | GCAAGGAGGACAGAGTTTATCGTG |
| CD11C | CTGGATAGCCTTTCTTCTGCTG | GCACACTGTGTCCGAACTC |
| CD206 | TGATTACGAGCAGTGGAAGC | GTTCACCGTAAGCCCAATTT |
| TNFα | GAGAAAGTCAACCTCCTCTCTG | GAAGACTCCTCCCAGGTATATG |
| IL-1β | TGTTCTTTGAAGTTGACGGACCC | TCATCTCGGAGCCTGTAGTGC |
| IL-6 | CCAGAGATACAAAGAAATGATGG | ACTCCAGAAGACCAGAGGAAAT |
Data are expressed as means ± SEM. Two groups were compared by t-test. P < 0.05 was considered statistically significant. All statistical analyses were carried out with SPSS 23.0 statistical software (IBM, Armonk, NY, United States).
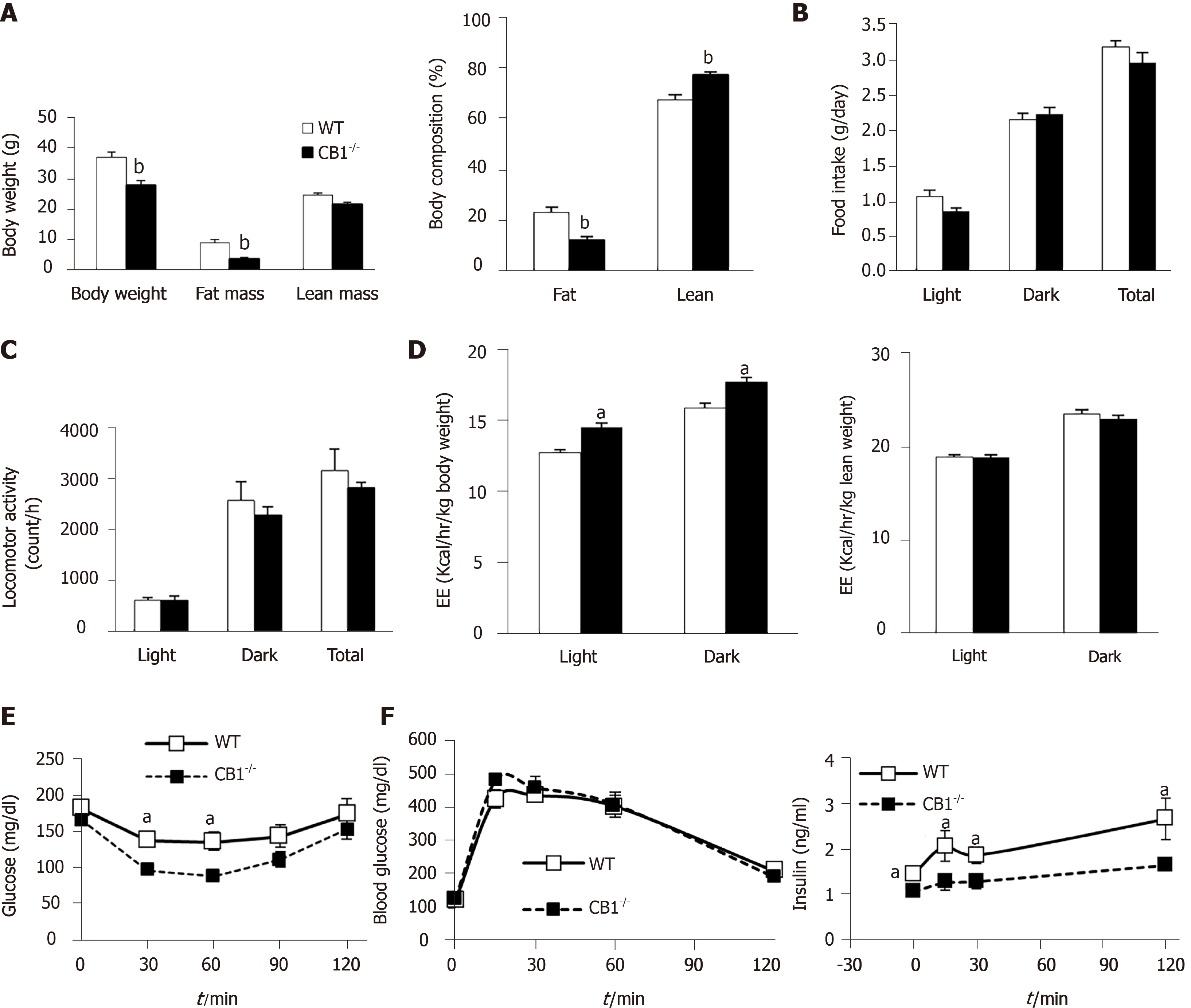
The body weights of CB1-/- mice were significantly lower than WT littermates; the analysis of body composition showed a markedly decreased percentage of fat mass in CB1-/- mice compared to WT mice (Figure 1A). We then assessed food intake, locomotor activity, and energy expenditure using CLAMS. Our data showed there was a trend of reduction but no significant difference in total daily food intake by CB1-/-mice compared to WT mice (Figure 1B). To further determine whether there is a difference in locomotor activity, we analyzed spontaneous locomotor activity of these mice. Neither total daily locomotor activity nor the locomotor activity during light or dark cycles was altered (Figure 1C). We next calculated energy expenditure and found that energy expenditure of CB1-/- mice was higher compared to their WT counterparts when normalized to body weight but no difference when normalized to lean mass (Figure 1D). Together, the results indicate that while CB1 ablation reduces body weight and fat deposition, it may be a due to a combination of changes in food intake and exergy expenditure.
Next, we assessed the glycemic phenotype. ITT showed that CB1-/- mice were more responsive to insulin challenge than WT mice (Figure 1E). During GTT, there was no difference in glucose clearance following a glucose load in WT and CB1-/- mice; but remarkably, the insulin levels of CB1-/- mice were significantly lower, indicative of increased insulin sensitivity (Figure 1F). These results indicate that CB1-/- mice have improved insulin sensitivity, which is in line with reduced body weight and body fat.
Collectively, these data suggest that CB1 is an important regulator of energy homeostasis and insulin sensitivity.
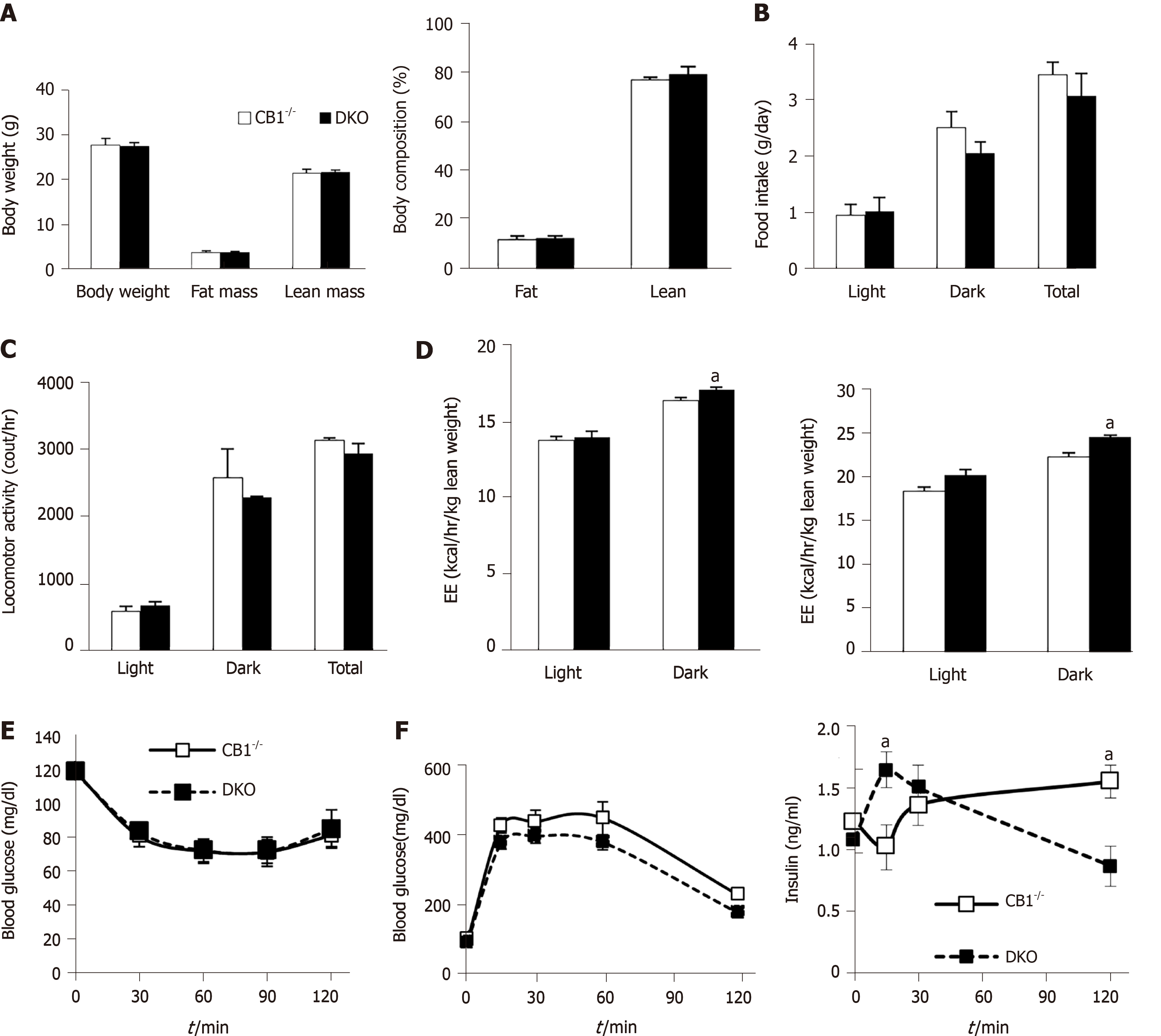
To determine whether the metabolic effects of CB1 are mediated by adiponectin, we compared the metabolic phenotypes of CB1-/- and DKO mice. The body weights of CB1-/- mice were similar to their age-matched DKO (Figure 2A). There were also no differences in fat mass and lean mass between CB1-/- and DKO mice. Indirect calorimetry analysis showed similar total food intake (Figure 2B) and locomotor activity (Figure 2C) between DKO and CB1-/- mice. Interestingly, compared to CB1-/- mice, DKO mice had increased energy expenditure when corrected either by total body weight or by lean weight (Figure 2D).
Furthermore, there was no difference in insulin sensitivity assessment of ITT between CB1-/- and DKO mice (Figure 2E). We further assessed glucose response during GTT: No difference was detected in glucose response, but interestingly, the insulin of DKO was lower at 15 min but higher at 120 min as compared to that of CB1-/- mice (Figure 2F). These data suggest that DKO mice have mostly similar metabolic profile, insulin sensitivity and glycemic response as CB1-/- mice, despite there are some varied insulin responses to glucose. Taken together, the effects of CB1 on metabolism are dominant; adiponectin is not essential in mediating the metabolic effects of CB1.
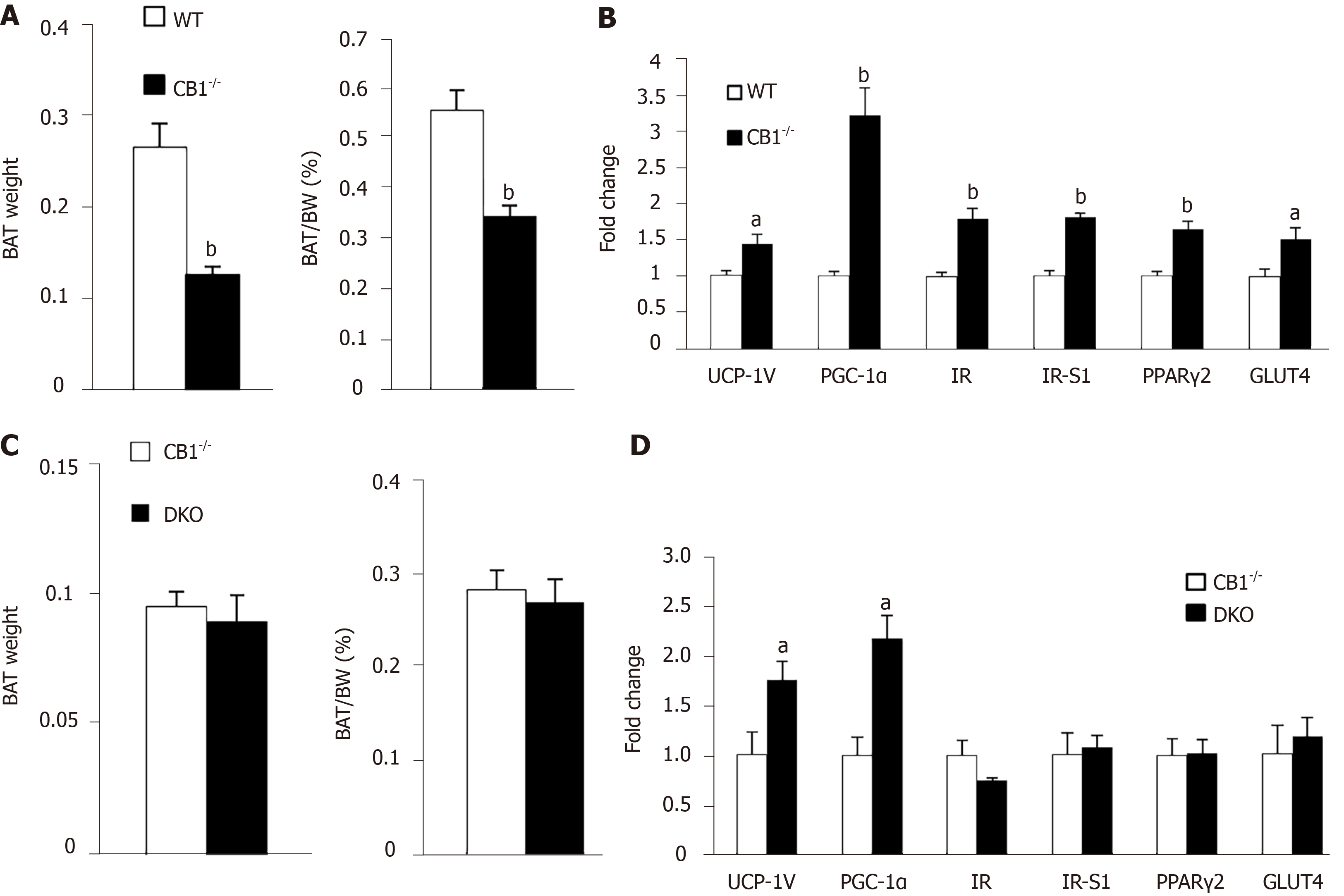
To determine the underlying mechanisms of the increased energy expenditure observed in CB1-/- mice, we subsequently analyzed BAT collected from the mice. CB1-/- mice showed a decreased ratio of BAT: Body weight as compared to WT mice (Figure 3A). Mitochondrial uncoupling protein 1 (UCP1) is the hallmark regulator of mitochondrial biogenesis and thermogenesis; when activated, UCP1 dissipates the transmembrane proton gradient and generates heat[21]. UCP1 mRNA was increased in CB1-/-mice as compared to WT controls (Figure 3B). Peroxisome proliferator-activated receptorγcoactivator-1 (PGC-1) is an upstream regulator of UCP1[22]. Indeed, PGC-1 expression was also increased in the CB1-/- mice when compared to that of WT mice (Figure 3B).
Our result in Figure 1 showed CB1-/- mice have higher insulin sensitivity compared to WT mice. Consistently, the gene expression of insulin receptor (IR) and insulin receptor substrate 1 (IRS-1) were increased in BAT of CB1-/- mice. Peroxisome proliferator-activated receptorsγ2 (PPARγ2) is an important master adipogenic regulator[11]. Here we found that PPARγ2 was higher in BAT of CB1-/- mice (Figure 3B). Glucose transporter type 4 (GLUT4) is a key mediator of glucose uptake in the adipose tissues[23]. As expected, GLUT4 expression in BAT of CB1-/- mice was increased (Figure 3B), supporting increased glucose uptake and consistent with increased heat production. Together, ablation of CB1 increased BAT thermogenic activity, likely by modulating mitochondrial function, insulin signaling, adipogenesis, and glycose uptake signaling pathways in BAT.
We have reported that adiponectin has an important role in body temperature maintenance and thermogenesis. Here, we compared the weight of BAT depots in CB1-/- and DKO mice. There was also no difference in total weight or BAT percentage between CB1-/- and DKO mice (Figure 3C). The expression of thermogenic regulators UCP1 and PGC-1 was increased in BAT of DKO mice compared to that of CB1-/- mice, while the expression of IR and IRS-1, GLUT4, and PPARγ2 were unchanged (Figure 3D). These results suggest that while adiponectin may be an important mediator for the effect of CB1 on mitochondrial genes in BAT, it is not critical for the regulation of CB1 in insulin signaling, adipogenesis, and glucose uptake in BAT.
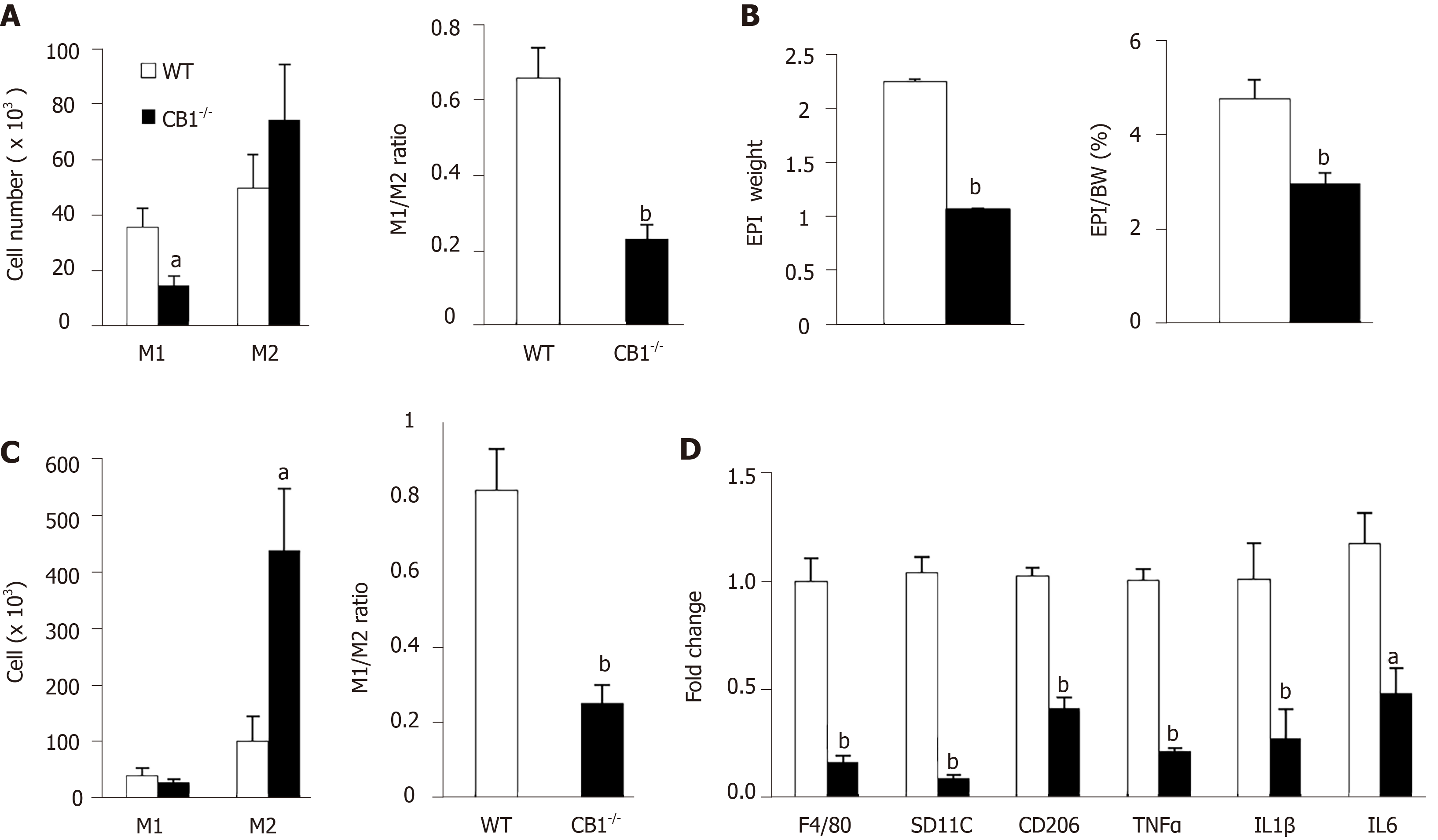
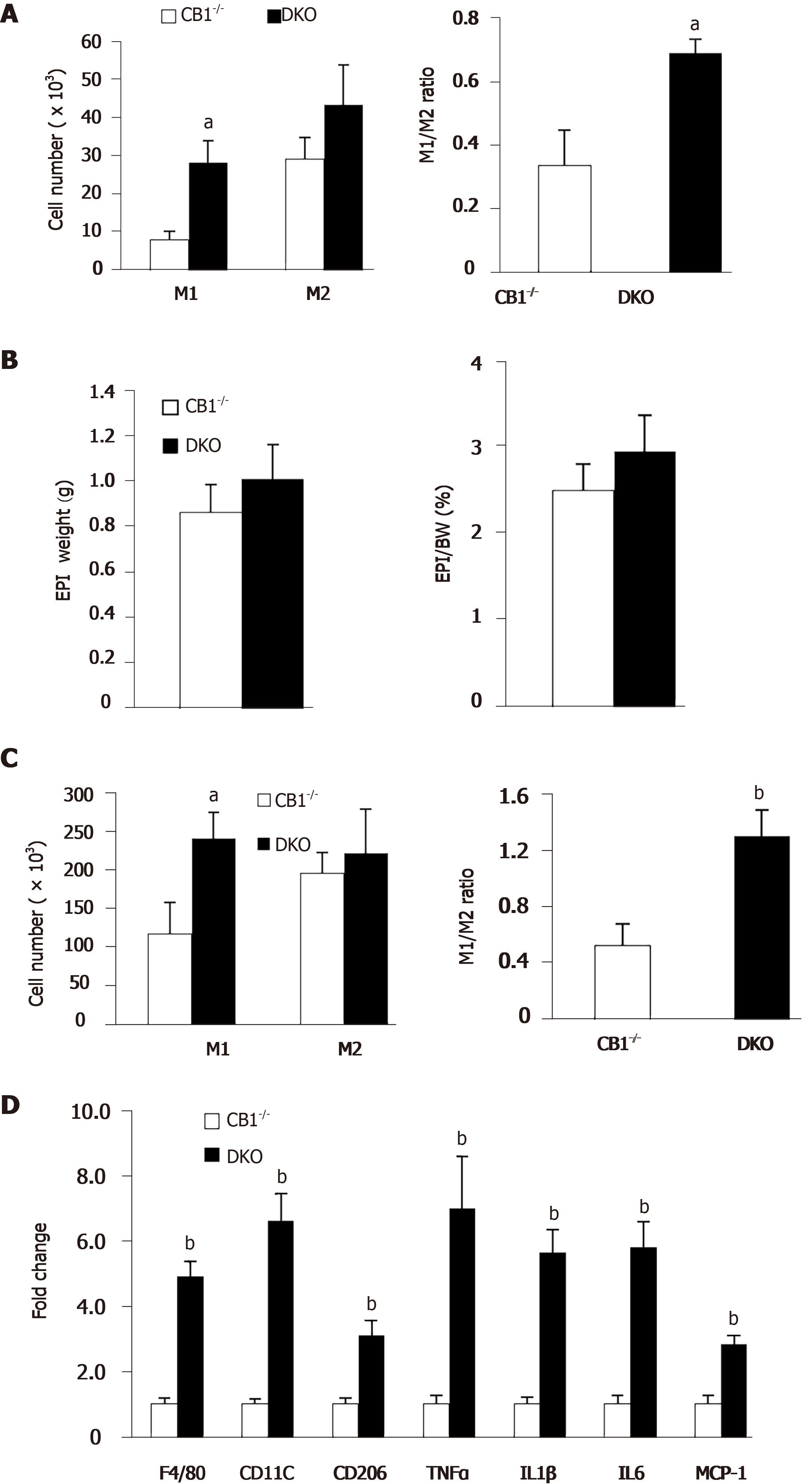
Macrophages have an important role in inflammation and insulin resistance[20]. To determine the underlying mechanisms of improved insulin sensitivity in CB1-/- mice, we conducted flow cytometry analysis on peritoneal macrophages and adipose tissue macrophages. M1-like macrophages are pro-inflammatory and M2-like macrophages are anti-inflammatory[20]. Peritoneal M1-like macrophages, as well as the M1/M2 ratio as a readout of inflammation, were significantly decreased in CB1-/-mice compared to WT mice; this suggests that CB1 ablation reduces systemic inflammation (Figure 4A). Since insulin resistance is closely linked to adipose tissue mass and adipose macrophages (ATM), we next assessed epididymal white adipose tissue. As expected, both the weight and the ratio of epididymal fat/body weight was lower in CB1-/- mice (Figure 4B). To assess the effect of CB1 on ATM polarization, we isolated the stromal vascular fraction from epididymal adipose tissues. Our flow cytometry studies revealed that while M1 was slightly decreased, M2 was significantly increased in epididymal fat of CB1-/- mice (Figure 4C). The M1/M2 ratio of ATM was decreased in epididymal fat of CB1-/- mice (Figure 4C). Next, we studied the gene expression of macrophage markers of F4/80, CD11c, CD206, as well as proinflammatory cytokines of tumor necrosis factor-a (TNF-a), interelukin-1 (IL-1), and interelukin-6 (IL-6) in epididymal fat. The expression levels of F4/80, CD11c, CD206, TNF, IL-1 , and IL-6 were significantly decreased in the epididymal fat of CB1-/- mice compared to WT mice (Figure 4D), which is in agreement with the reduced inflammation revealed by flow cytometry analysis of ATM.
Adipose tissue releases adiponectin, which plays an important role in the regulation of energy metabolism and inflammation[24]. Intriguingly, our flow cytometry analysis showed increased pro-inflammatory peritoneal M1 macrophages and increased ratio of M1/M2 in DKO mice; this suggests that the adiponectin deletion abolishes the anti-inflammatory effect of CB1 knockout (Figure 5A). Subsequently, we analyzed epididymal white adipose tissues of CB1-/- and DKO mice. There was no difference in the percentage of fat depot: Body weight between CB1-/- and DKO mice (Figure 5B). Our flow cytometry studies further revealed that the M1/M2 ratio of ATM was increased in epididymal fat of DKO mice compared to CB1-/- mice (Figure 5C). To investigate the effect of adiponectin ablation of CB1 on ATM-mediated inflammation, gene expressions of proinflammatory cytokines were evaluated in epididymal fat. The expression levels of F4/80, CD11c, CD206, TNF-a, IL-1, IL-6 and MCP-1 were significantly increased in epididymal fat of DKO mice as compared to CB1-/- mice (Figure 5D), in line with increased inflammation observed by flow cytometry.
Collectively, the data indicate that the CB1 deficiency-induced anti-inflammatory effect on macrophage polarization is adiponectin-dependent, suggesting that adiponectin is a key mediator for the effect of CB1 on inflammation.
The CB1 blockade has been shown to ameliorate metabolic abnormalities of obese animals and to promote weight loss and improved insulin sensitivity[25]. Adipokine adiponectin is an insulin-sensitizer, and it has many beneficial effects that phenocopy CB1 antagonists[12]. It has been shown that cannabinoids decrease adiponectin[26]. Moreover, CB1 blocker rimonabant has been reported to increase the plasma adiponectin levels in obese and diabetic animal models[6,27,28]. Thus, adiponectin is thought to be a mediator of the effects of CB1 antagonists such as rimonabant. However, the functional relationship between adiponectin and the endocannabinoid system is not fully defined. To determine whether CB1 and adiponectin are functionally dependent on each other, we conducted a comparative study of the CB1-/- and DKO mice to investigate whether the adiponectin deletion abolishes the healthy phenotype of CB1-/- in metabolism and inflammation.
As expected in CB1-/- mice, we observed decreased body weight:fat mass, increased thermogenic activation in BAT, and improved whole-body insulin sensitivity. Interestingly, DKO mice showed changes similar to CB1-/- mice in the body weight:fat mass ratio, BAT thermogenic regulation, and insulin sensitivity. These results suggest that the beneficial metabolic effects of CB1 blockage are not mediated by adiponectin. Our findings are mostly consistent with previous reports in literature, but with some differences which could be due to models of choice and/or diet variations. Watanabe et al[16] reported that rimonabant improved hepatic insulin resistance in both ob/ob and adiponectin-/-ob/ob mice. Migrenne et al[29] reported that adiponectin is not required for body weight loss in diet-induced obese mice, but is required in rimonabant-induced improvement of insulin sensitivity. Our experiment was conducted with a genetic approach of loss-of-function with CB1 knockout, not with CB1 antagonist; under regular diet-feeding, not diet-induced obesity. It is possible that the impact of adiponectin on CB1 metabolic regulation differs under different metabolic states. Indeed, Tam et al[30] reported a reversal of the HFD-induced hepatic steatosis and fibrosis by chronic administration of CB1 blocker or adiponectin, but the reduction of adiposity and improved glycemic control are not affected by adiponectin, which is similar to our results.
The findings from our current study and others[4,8] support the idea of increased energy expenditure induced by CB1 suppression, either by CB1 blocker such as rimonabant or by CB1 gene ablation. It is well known that BAT plays an important role in adaptive thermogenesis, and that thermogenic activation of BAT can directly affect metabolic rate through the function of mitochondrial protein UCP1. UCP1 is a key regulator of thermogenesis; it recruits free fatty acid into the mitochondrial matrix to dissipate as heat, depleting circulating lipids and increasing energy expenditure[31]. Previous studies demonstrated that rimonabant treatment increased the expression of UCP1 mRNA in BAT[32]. In metabolic profiling, DKO mice showed even higher energy expenditure than CB1-/- mice. Similarly, UCP-1 expression in BAT was higher in DKO mice than in CB1-/- mice. These results suggest that adiponectin deletion not diminishes the CB1 deficiency-induced thermogenic activation in BAT. In the current study, we found that insulin signaling IR and IRS-1 gene expression in BAT was increased in CB1-/- mice, and the expression of these genes was no different between DKO and CB1-/- mice. Our thermogenic gene expression data in DKO showed that adiponectin deletion further enhanced the thermogenic activation compared to CB1-/- mice, implying that the effect of CB1 on thermogenesis is largely independent of adiponectin. The effect of adiponectin on thermogenesis is an area of ongoing debate currently. Qiao et al[33] reported that adiponectin suppresses thermogenic action in BAT to reduce energy expenditure. We reported that the core body temperature of adiponectin-null mice was not affected under normal housing temperature but reduced under cold temperature, supporting that adiponectin is required for maintaining body temperature in cold[24]. Different from our previous report, our current study was conducted under room temperature, so it is not surprising that the effect of adiponectin on thermogenic activation of CB1-/- mice is minimal.
Since metabolism and insulin sensitivity are closely linked to inflammation, we further studied the role of CB1 deficiency in macrophages. Remarkably, both systemic (peritoneal macrophages) and tissue macrophages (ATM) showed an anti-inflammatory polarization shift, supporting reduced inflammation in CB1-/- mice. Especially, CB1-/- mice exhibited decreased pro-inflammatory M1 macrophages in peritoneal macrophages, less epididymal fat mass, and reduced M1/M2 ratio and pro-inflammatory cytokine expression in the epididymal fat as compared to WT mice. The results indicate that CB1-/- mice have reduced adiposity and adipose inflammation, which is consistent with improved systemic insulin sensitivity. Intriguingly, our study further revealed that DKO mice had an opposite profile of increased inflammation compared to CB1-/- mice, which suggested that adiponectin deletion reversed the anti-inflammatory effect of CB1 deletion. The DKO mice exhibited an increase in pro-inflammatory M1 macrophages and M1/M2 ratio for both peritoneal macrophages and ATM, as well as elevated pro-inflammatory cytokine expression in epididymal fat compared to CB1-/- mice. The anti-inflammatory effect on CB1-/- mice was reversed in the DKO mice clearly demonstrates that adiponectin is required for the anti-inflammatory benefit of CB1 antagonism, and the inflammation phenotype of CB1 is adiponectin-dependent. These exciting results suggest that adiponectin counters the pro-inflammatory effect of cannabinoids, and the beneficial anti-inflammatory effect of CB1 antagonists is dependent on adiponectin. Indeed, data from a mouse model of adipocyte-specific deletion of the CB1 gene lends support to our conclusion[34]. Plasma adiponectin levels were significantly increased in the adipocyte-specific CB1-deleted mice, and adipocyte-specific deletion of CB1 was shown to be sufficient to protect against diet-induced obesity and promote anti-inflammatory polarization towards alternatively-activated M2 macrophages.
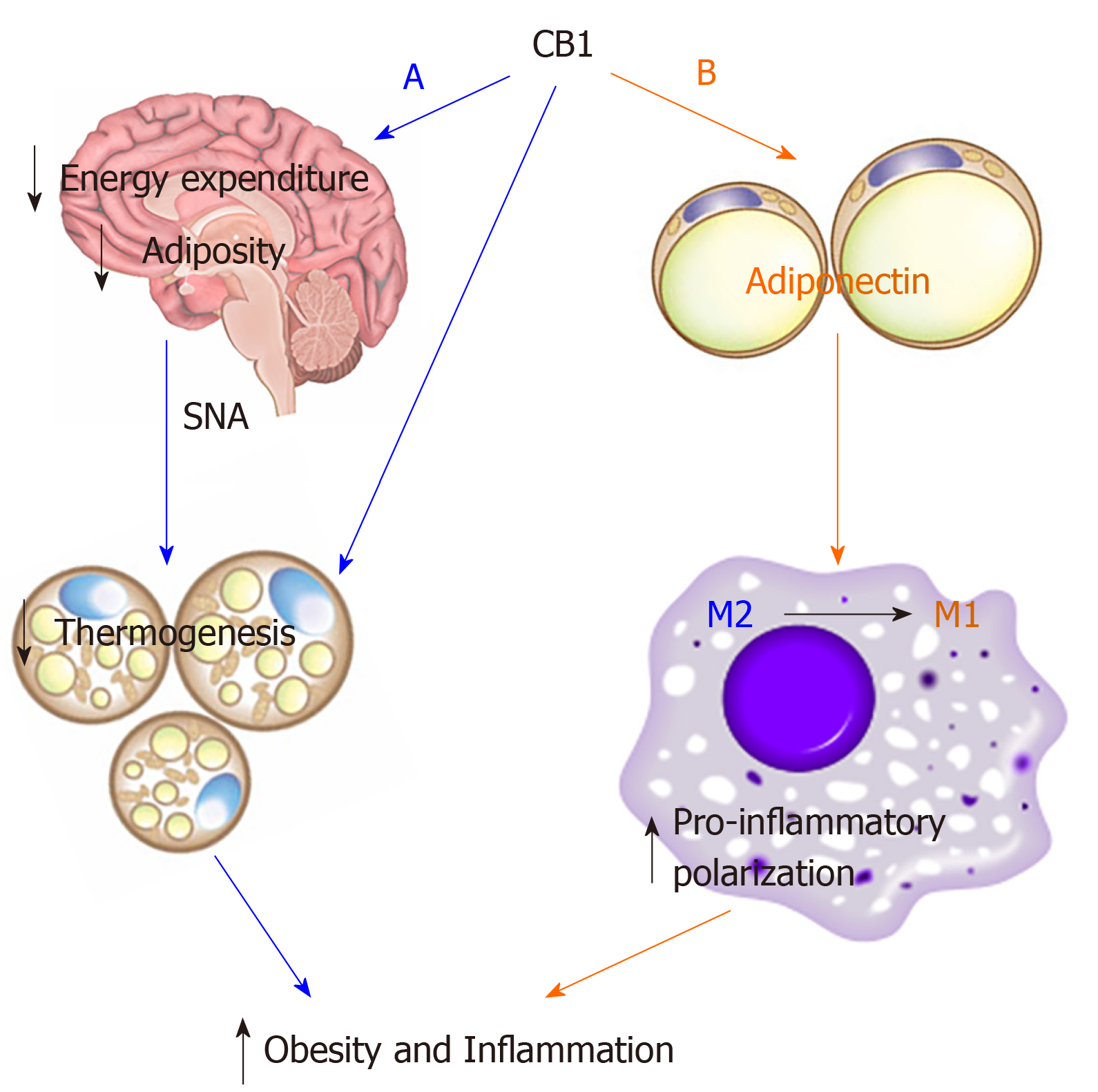
In conclusion, our study demonstrates that CB1 deletion activates thermogenesis and suppresses inflammation via adiponectin-independent and adiponectin-dependent pathways, respectively (Figure 6). Based on our findings, we conclude that there are differential pathways and mechanisms by which CB1 utilizes to regulate metabolism and inflammation; that the effects on metabolism are adiponectin-independent and the effects on inflammation are adiponectin-dependent. CB1 deletion increases plasma adiponectin[30,35], which promotes anti-inflammatory polarization of macrophages, thereby promoting the beneficial anti-inflammatory effect. Adiponectin is not required for CB1-mediated metabolism, but is required for CB1-mediated inflammation. A better understanding of the signaling crosstalk between CB1 and adiponectin would facilitate further therapeutic development of CB1 antagonists. Our study provides new insights to the comprehensive connection between CB1 and adiponectin for regulation of energy homeostasis, insulin sensitivity and inflammation.
Antagonists of cannabinoid type 1 receptor (CB1) have been shown to promote body weight loss and improve insulin sensitivity.
Cannabinoids is implicated in regulation of adiponectin. However, the mediators of CB1 actions are not fully defined, specifically in regard to adiponectin signaling in vivo.
To determine whether adiponectin is indeed required for the peripheral functions of CB1.
We compared metabolic and inflammatory phenotypes of CB1-null (CB1-/-) vs. CB1/Adiponectin double-knockout (DKO) mice. We investigated the insulin sensitivity using insulin tolerance test and glucose tolerance test, and inflammation using flow cytometry analysis of macrophages.
CB1-/- mice significantly reduced body weight and fat mass without change of total daily food intake and locomotor activity compared to wild-type (WT) mice. CB1-/- mice showed increased energy expenditure and improved insulin sensitivity compared to WT mice. DKO showed no difference in body weight, adiposity, or insulin sensitivity, only showed a modestly elevated thermogenesis in BAT compared to CB1-/- mice. CB1-/- mice showed reduced pro-inflammatory macrophage polarization in both peritoneal macrophages and adipose tissue macrophages compared to WT mice; in contrast, DKO mice exhibited elevated pro-inflammatory macrophage polarization in these macrophages compared to that of CB1-/- mice.
Our findings reveal that CB1 functions through both adiponectin-dependent and adiponectin-independent mechanisms: CB1 regulates energy metabolism in an adiponectin-independent manner, and inflammation in an adiponectin-dependent manner.
Adiponectin is not required for CB1-mediated metabolism but is required for CB1-mediated inflammation. To fully understand the direct interactions and regulatory mechanisms between CB1 and adiponectin, further dissemination in co-culture system to might be beneficial.
Metabolic analysis was performed in the Mouse Metabolic Research Unit at the USDA/ARS Children’s Nutrition Research Center, Baylor College of Medicine. The authors are very grateful to Michael R. Honig at Houston’s Community Public Radio Station KPFT for his excellent editorial assistance.
| 1. | Blaha MJ, Bansal S, Rouf R, Golden SH, Blumenthal RS, Defilippis AP. A practical "ABCDE" approach to the metabolic syndrome. Mayo Clin Proc. 2008;83:932-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Tota-Maharaj R, Defilippis AP, Blumenthal RS, Blaha MJ. A practical approach to the metabolic syndrome: review of current concepts and management. Curr Opin Cardiol. 2010;25:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl). 1999;143:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 380] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1173] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 5. | Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S; RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1050] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 6. | Després JP, Golay A, Sjöström L; Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 971] [Article Influence: 46.2] [Reference Citation Analysis (5)] |
| 7. | Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohórquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano IN 3rd, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depré M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 854] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 9. | Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Péleraux A, Pénarier G, Soubrié P, Le Fur G, Galiègue S, Casellas P. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Cardinal P, Bellocchio L, Clark S, Cannich A, Klugmann M, Lutz B, Marsicano G, Cota D. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology. 2012;153:4136-4143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Boon MR, Kooijman S, van Dam AD, Pelgrom LR, Berbée JF, Visseren CA, van Aggele RC, van den Hoek AM, Sips HC, Lombès M, Havekes LM, Tamsma JT, Guigas B, Meijer OC, Jukema JW, Rensen PC. Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J. 2014;28:5361-5375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3510] [Cited by in RCA: 3540] [Article Influence: 141.6] [Reference Citation Analysis (9)] |
| 14. | Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 614] [Article Influence: 24.6] [Reference Citation Analysis (8)] |
| 15. | Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrié P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 452] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 16. | Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, Awazawa M, Katsuyama H, Hasegawa C, Tokuyama K, Moroi M, Sugi K, Yamauchi T, Noda T, Nagai R, Terauchi Y, Tobe K, Ueki K, Kadowaki T. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem. 2009;284:1803-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Obici S, Wang J, Chowdury R, Feng Z, Siddhanta U, Morgan K, Rossetti L. Identification of a biochemical link between energy intake and energy expenditure. J Clin Invest. 2002;109:1599-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 18. | Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Robker RL, Collins RG, Beaudet AL, Mersmann HJ, Smith CW. Leukocyte migration in adipose tissue of mice null for ICAM-1 and Mac-1 adhesion receptors. Obes Res. 2004;12:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Ma X, Lin L, Yue J, Pradhan G, Qin G, Minze LJ, Wu H, Sheikh-Hamad D, Smith CW, Sun Y. Ghrelin receptor regulates HFCS-induced adipose inflammation and insulin resistance. Nutr Diabetes. 2013;3:e99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1231] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 22. | Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes. 2005;54:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Kawashita NH, Brito MN, Brito SR, Moura MA, Festuccia WT, Garofalo MA, Machado UF, Kettelhut IC, Migliorini RH. Glucose uptake, glucose transporter GLUT4, and glycolytic enzymes in brown adipose tissue from rats adapted to a high-protein diet. Metabolism. 2002;51:1501-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Wei Q, Lee JH, Wang H, Bongmba OYN, Wu CS, Pradhan G, Sun Z, Chew L, Bajaj M, Chan L, Chapkin RS, Chen MH, Sun Y. Adiponectin is required for maintaining normal body temperature in a cold environment. BMC Physiol. 2017;17:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschöp J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschöp MH. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977-2991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Engeli S. Peripheral metabolic effects of endocannabinoids and cannabinoid receptor blockade. Obes Facts. 2008;1:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O'Connor SE, Janiak P, Herbert JM. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, Croci T, Soubrié P, Oury-Donat F, Maffrand JP, Scatton B, Lacheretz F, Le Fur G, Herbert JM, Bensaid M. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 241] [Article Influence: 12.7] [Reference Citation Analysis (8)] |
| 29. | Migrenne S, Lacombe A, Lefèvre AL, Pruniaux MP, Guillot E, Galzin AM, Magnan C. Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R929-R935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Tam J, Godlewski G, Earley BJ, Zhou L, Jourdan T, Szanda G, Cinar R, Kunos G. Role of adiponectin in the metabolic effects of cannabinoid type 1 receptor blockade in mice with diet-induced obesity. Am J Physiol Endocrinol Metab. 2014;306:E457-E468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4296] [Cited by in RCA: 4915] [Article Influence: 223.4] [Reference Citation Analysis (0)] |
| 32. | Perwitz N, Wenzel J, Wagner I, Büning J, Drenckhan M, Zarse K, Ristow M, Lilienthal W, Lehnert H, Klein J. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes Metab. 2010;12:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Qiao L, Yoo Hs, Bosco C, Lee B, Feng GS, Schaack J, Chi NW, Shao J. Adiponectin reduces thermogenesis by inhibiting brown adipose tissue activation in mice. Diabetologia. 2014;57:1027-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Ruiz de Azua I, Mancini G, Srivastava RK, Rey AA, Cardinal P, Tedesco L, Zingaretti CM, Sassmann A, Quarta C, Schwitter C, Conrad A, Wettschureck N, Vemuri VK, Makriyannis A, Hartwig J, Mendez-Lago M, Bindila L, Monory K, Giordano A, Cinti S, Marsicano G, Offermanns S, Nisoli E, Pagotto U, Cota D, Lutz B. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J Clin Invest. 2017;127:4148-4162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 35. | Perwitz N, Fasshauer M, Klein J. Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Horm Metab Res. 2006;38:356-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Asadzade Aghdaei H, Prysyazhnyuk V S-Editor: Wang LL L-Editor: A P-Editor: Yu HG