Published online Jan 15, 2021. doi: 10.4239/wjd.v12.i1.56
Peer-review started: September 9, 2020
First decision: October 21, 2020
Revised: November 3, 2020
Accepted: November 18, 2020
Article in press: November 18, 2020
Published online: January 15, 2021
Processing time: 119 Days and 19.7 Hours
Type 1 diabetes (T1D) contributes to altered lipid profiles and increases the risk of cardiovascular disease (CVD). Youth with T1D may have additional CVD risk factors within the first decade of diagnosis.
To examine risk factors for dyslipidemia in young subjects with T1D.
Longitudinal and cross-sectional retrospective study of 170 young subjects with T1D (86 males; baseline mean age 12.2 ± 5.6 years and hemoglobin A1c 8.4% ± 1.4%) were followed in a single tertiary diabetes center for a median duration of 15 years. Predictors for outcomes of lipid profiles at last visit (total cholesterol [TC], triglycerides [TGs], low-density lipoprotein-cholesterol [LDL-c], and high-density lipoprotein-cholesterol [HDL-c]) were analyzed by stepwise linear regression models.
At baseline, 79.5% of the patients had at least one additional CVD risk factor (borderline dyslipidemia/dyslipidemia [37.5%], pre-hypertension/hypertension [27.6%], and overweight/obesity [16.5%]) and 41.6% had multiple (≥ 2) CVD risk factors. A positive family history of at least one CVD risk factor in a first-degree relative was reported in 54.1% of the cohort. Predictors of elevated TC: family history of CVD (β[SE] = 23.1[8.3], P = 0.006); of elevated LDL-c: baseline diastolic blood pressure (DBP) (β[SE] = 11.4[4.7], P = 0.003) and family history of CVD (β[SE] = 20.7[6.8], P = 0.017); of elevated TGs: baseline DBP (β[SE] = 23.8[9.1], P = 0.010) and family history of CVD (β[SE] = 31.0[13.1], P = 0.020); and of low HDL-c levels: baseline DBP (β[SE] = 4.8[2.1], P = 0.022]).
Our findings suggest that elevated lipid profiles are associated with DBP and a positive family history of CVD. It is of utmost importance to prevent and control modifiable risk factors such as these, as early as childhood, given that inadequate glycemic control and elevation in blood pressure intensify the risk of dyslipidemia.
Core Tip: Co-occurrence of type 1 diabetes (T1D) and cardiovascular disease (CVD) risk factor clustering (overweight/obesity, hypertension, family history of CVD and dyslipidemia) may contribute to early-onset CVD. Our findings demonstrated that most T1D patients already had at least one CVD risk factor during childhood, with dyslipidemia being the most prevalent. It is noteworthy that clustering of CVD risk factors was observed in approximately one-half of the cohort and that there was a positive family history of at least one CVD risk factor in more than 50% of the patients. The number and distribution of CVD risk factors were similar for males and females.
- Citation: Krepel Volsky S, Shalitin S, Fridman E, Yackobovitch-Gavan M, Lazar L, Bello R, Oron T, Tenenbaum A, de Vries L, Lebenthal Y. Dyslipidemia and cardiovascular disease risk factors in patients with type 1 diabetes: A single-center experience. World J Diabetes 2021; 12(1): 56-68
- URL: https://www.wjgnet.com/1948-9358/full/v12/i1/56.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i1.56
Type 1 diabetes (T1D) is a chronic disease in children and adolescents, with a steady global increase in the number of diagnosed children[1-3]. This chronic disease is associated with well-documented, life-long increases in morbidity and mortality. Over the past several decades, there has been a marked increase in the available data on T1D, resulting in a broad understanding of many aspects of the disease including its genetics, epidemiology, and disease burden. Although a number of methods to improve clinical disease management have been assessed, wide gaps still exist in the ability to standardize clinical care and decrease disease-associated complications and burdens.
One study of the natural history of the development of atherosclerosis clearly showed a possible origin of the lesions in childhood and adolescence[4]. In recent decades, numerous studies have shown that children and adolescents with T1D exhibit subclinical cardiovascular disease (CVD) abnormalities after 10 years of disease duration[5-10]. CVD is a major complication among subjects with T1D, which may lead to a higher incidence of mortality and morbidity than found in the general population. Abnormalities in serum lipid concentrations and composition are commonly associated with both T1D and type 2 diabetes (T2D) and are believed to contribute to excess CVD risk in adults[11].
Childhood obesity and overweight have significantly increased during the last 20 years and have become a major worldwide health concern[12-15]. This trend towards increased body weight is also apparent in the T1D population[16,17] and it has recently been reported that being overweight has a significant effect on the glycemic control of T1D patients[18]. Moreover, dyslipidemia has been documented among T1D youth aged 10-22 years, with a substantial proportion found to have lipid levels outside the recommended target range[19]. Dyslipidemia is a significant and modifiable risk factor contributing to the increased risk of atherosclerotic CVD in diabetes[20-22]. Epi-demiological data on the prevalence of dyslipidemia and phenotype distribution in youth with T1D are scarce, with only limited longitudinal data on serum lipids in this population[23,24].
Co-occurrence of T1D and CVD risk factor clustering (overweight/obesity, hypertension, family history of CVD and dyslipidemia) may contribute to early-onset CVD. In this retrospective longitudinal study, we examined the association between CVD risk factors in childhood and dyslipidemia in young adulthood, and determined the prevalence of CVD risk factor clustering among T1D patients.
The study population included 170 young patients with T1D (86 males) followed in the National Center for Childhood Diabetes, Schneider Children’s Medical Center of Israel, during the years 1998-2013. Inclusion criteria were: children/adolescents aged less than 18 years at diabetes onset, T1D diagnosis prior to 1998, and regular clinical follow-up at our diabetes center. Exclusion criteria were: Patients lost to follow-up, those with a lipid profile not available at the predetermined time points, and those with concomitant diseases likely to interfere with lipid metabolism.
The study was approved by the ethics committee of our institution, which waived the need to obtain informed consent.
This longitudinal and cross-sectional retrospective cohort study was based on data collected from medical records of patients treated in our national diabetes center in accordance with the principles of Good Clinical Practice. The following data were retrieved from medical files: Sociodemographic parameters (date of birth, sex, ethnicity), anthropometric measurements (height, weight, calculated body mass index [BMI]) pubertal stage, blood pressure measurements, and diabetes-related parameters (age at diagnosis of T1D, diabetes duration, glycemic control as expressed by levels of glycosylated hemoglobin [HbA1c]), and serum lipid profile. The clinical and laboratory data were extracted from the medical files at four points in time (1998, 2003, 2008 and 2013) and a medical interview was conducted in 2017.
The routine clinical practice followed for T1D patients in our center has been quarterly clinic visits, with follow-up every 3-6 mo for weight (in light clothing using a standard calibrated scale) and height (using a commercial Harpenden-Holtain stadiometer, until adult height). BMI was calculated as weight in kilograms divided by height in meters squared. BMI-standard deviation scores (SDS) were calculated according to the recommendations of the Centers for Disease Control and Pre-vention[25].
The medical team (nurses and physicians) routinely questioned patients about cigarette smoking and alcohol consumption, and self-reported responses were documented in the medical files. Regular smoking was defined as smoking at least one cigarette once a week and regular alcohol consumption as drinking at least one alcoholic beverage once a week. Female patients were routinely questioned about their menstrual cycle (whether menses were absent or present and whether the cycle was regular or irregular) as well as their use of oral contraceptives.
HbA1c was routinely tested at each visit at 3-4 mo intervals. Capillary HbA1c values were measured by an automated immunochemical technique (DCA 2000; Siemens Medical Solutions Diagnostics, Tarrytown, NY, United States; 95% confidence interval [CI] 4.3%-5.7%). Our routine policy is to screen T1D patients for autoimmune thyroid disease, celiac disease, and pernicious anemia at diagnosis and annually thereafter and to screen for dyslipidemia annually. Routine screening for microvascular com-plications was generally initiated in pubertal patients during the first year after diagnosis, with subsequent annual assessment[26]. Screening for microvascular complications included an ophthalmologic examination, testing of urine for albumin secretion, and neurological examination (bedside Neuropathy Disability Score) to screen for distal polyneuropathy[27].
BMI was calculated using the anthropometric measurements documented in the medical files. In childhood and adolescence, BMI values were converted to age- and sex-specific percentiles according to the CDC2000[25]. In adulthood, BMI values were converted according to the reference data of the National Health and Nutrition Examination Survey and National Center for Health Statistics in 2003-2006[28]. Weight status was categorized as: Obese, ≥ 95th percentile; overweight, ≥ 85th to < 95th percentiles; normal weight, ≥ 5th to < 85th percentiles and underweight, < 5th percentile[29]. Blood pressure was measured according to the recommendations of the National High Blood Pressure Education Program (NHBPEP)[30]. In childhood, percentiles for systolic blood pressure (BP) and diastolic BP were calculated according to height, sex, and age[31]. Normal BP, prehypertension, and hypertension, were de-fined according to the NHBPEP[30].
Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and triglycerides (TGs) were converted to age- and sex-specific percentiles according to American Academy of Pediatrics (AAP) criteria for children aged 5-19 years[32]. In adults, hypercholesterolemia was defined when: TC levels were > 240 mg/dL; LDL-c levels were > 130 mg/dL; TG levels were > 150 mg/dL and HDL-c levels were < 40 mg/dL in males and < 50 mg/dL in females. CVD risk factors in T1D included: Overweight/obesity, pre-hypertension/hypertension, dyslipidemia (elevated LDL-c/elevated TG/low HDL-c) and positive family history for cardiometabolic diseases. Patients under 5 years of age were not included in the CVD risk factor analysis since lipid level reference values are less established.
In accordance with IRB approval, former T1D patients no longer treated in our center were each sent a letter explaining the general goal of the study and asking permission for a detailed phone-call interview. In 2017, a structured telephone interview was carried out by a pediatric endocrinology fellow, who explained the purposes of the study, the anonymity of responses, and participants’ rights. The interview contained questions pertaining to current medical status and updated family history in first-degree relatives (parents and/or siblings), as follows: (1) Habitual behavior - smoking, and if yes, age at initiation and number of cigarettes; and regular physical activity, type (aerobic/muscle-strengthening) and duration (weekly hours); (2) Autoimmune co-morbidities (thyroid disease, celiac disease, pernicious anemia), age at diagnosis; (3) Diabetes complications (retinopathy, microalbuminuria, nephropathy, neuropathy); (4) CVD risk factors (hypertension, dyslipidemia); (5) Current and past medications; (6) In females - age at menarche, regularity of menses, polycystic ovary disease, and oral contraceptive use, and if yes, indication; and (7) family history of cardio-metabolic diseases (diabetes, hypertension, dyslipidemia, CVD, and cerebrovascular episodes).
The data were analyzed using IBM SPSS statistical software (release 25.0; IBM SPSS Statistics for Windows, Armonk, NY, United States). All statistical tests were performed as two-sided. The Kolmogorov-Smirnov z-test was performed to test the null hypothesis that the variable has a normal distribution. Data are expressed as the mean and standard deviation (SD) for normal distribution median, interquartile range for skewed distribution, and number and percent for discrete variables. Pearson’s chi-square test was used for analysis of between-group differences in discrete variables. Independent-samples t-tests or Mann–Whitney U test were used to compare between groups for continuous variables, with normal or skewed distributions, respectively. Predictors for long-term outcomes of lipid profile (TC, TGs, LDL-c, and HDL-c) were analyzed by a stepwise linear regression model. The independent variables included in all four linear model analyses were potential predictors and confounders (sex, ethnicity, Tanner stage, BMI-SDS, systolic and diastolic BP, and HbA1c levels, age at diagnosis, T1D duration, and family history of cardiometabolic diseases). P ≤ 0.05 was considered statistically significant.
The baseline characteristics of the 170 young subjects with T1D (86 males) at a mean age 12.2 ± 5.6 years are presented in Table 1. At the first evaluation, 46.5% (79) patients were prepubertal (Tanner stage 1), while 24.7% (42) were in puberty (Tanner stage 2-4) and 28.8% (49) were fully pubertal. At baseline, mean HbA1c was 8.4% ± 1.4%, 61.2% were treated by multiple daily insulin injections (the rest treated with insulin pump), and 14.1% (24/170) had co-existent autoimmune thyroid disease.
| All, n = 170 | Males, n = 86 | Females, n = 84 | P value | |
| Age in yr | 12.6 ± 5.6 | 12.4 ± 6.0 | 12.6 ± 5.1 | 0.854 |
| Age at diabetes diagnosis | 8.1 ± 4.4 | 8.0 ± 4.7 | 8.2 ± 4.1 | 0.735 |
| Diabetes duration | 4.4 ± 4.0 | 4.4 ± 4.2 | 4.3 ± 3.8 | 0.828 |
| HbA1c, % | 8.4 ± 1.4 | 8.6 ± 1.4 | 8.2 ± 1.4 | 0.064 |
| HbA1c in mmol/L | 68.3 | 70.5 | 66.1 | |
| Pubertal stage, n (%) | ||||
| Tanner 1 | 79 (46.5) | 47 (54.7) | 32 (38.1) | 0.291 |
| Tanner 2 | 11 (6.5) | 4 (4.7) | 7 (8.3) | |
| Tanner 3 | 19 (11.2) | 8 (9.3) | 11 (13.1) | |
| Tanner 4 | 12 (7.1) | 5 (5.8) | 7 (8.3) | |
| Tanner 5 | 49 (28.8) | 22 (25.6) | 27 (32.1) | |
| Menarche | NA | 48 (57.1) | ||
| Age at menarche | NA | 13.3 ± 1.3 | ||
| Ethnicity, n (%) | ||||
| Ashkenazi Jew | 102 (60.0) | 47 (54.7) | 55 (65.5) | 0.120 |
| North African Jew | 19 (11.2) | 7 (8.1) | 12 (14.3) | |
| Oriental Jew | 19 (11.2) | 10 (11.6) | 9 (10.7) | |
| Yemenite Jew | 16 (9.4) | 12 (14) | 4 (4.8) | |
| Ethiopian Jew | 4 (2.4) | 2 (2.3) | 2 (2.4) | |
| Arab | 10 (5.9) | 8 (9.3) | 2 (2.4) | |
| Cardiovascular disease risk factors in patients, n (%) | ||||
| Overweight | 20 (11.8) | 9 (10.5) | 11 (13.1) | 0.864 |
| Obesity | 8 (4.7) | 4 (4.7) | 4 (4.8) | |
| Systolic pre-hypertension, ≥ 90th to the 95th percentile | 15 (8.8) | 8 (9.3) | 7 (8.3) | 0.336 |
| Systolic stage 1 hypertension, ≥ 95th to the < | 19 (11.2) | 12 (14) | 7 (8.3) | |
| Systolic stage 2 hypertension, ≥ 99th percentile | 8 (4.7) | 5 (5.8) | 3 (3.6) | |
| Diastolic pre-hypertension, ≥ 90th to the 95th percentile | 3 (1.8) | 2 (2.3) | 1 (1.2) | 0.670 |
| Diastolic stage 1 hypertension, ≥ 95th to the < 99th percentile | 7 (4.1) | 3 (3.5) | 4 (4.8) | |
| Diastolic stage 2 hypertension, ≥ 99th percentile | 1 (0.6) | 1 (1.2) | 0 (0) | |
| Pre-hypertension, systolic and/or diastolic ≥ 90th to the 95th percentile | 15 (8.8) | 9 (10.5) | 6 (7.1) | 0.426 |
| Stage 1 hypertension, systolic and/or diastolic ≥ 95th to the < 99th percentile | 23 (13.5) | 12 (14) | 11 (13.1) | |
| Stage 2 hypertension, systolic and/or diastolic ≥ 99th percentile | 9 (5.3) | 6 (7) | 3 (3.6) | |
| Lipid profile1 | n = 144 | n = 71 | n = 73 | |
| LDL-c, n (%) | 0.049 | |||
| < 75th percentile | 63 (43.8) | 27 (38) | 36 (49.3) | |
| 75th-90th percentile | 47 (32.6) | 21 (29.6) | 26 (35.6) | |
| Borderline elevated 90th-95th percentile | 10 (6.9) | 7 (8.1) | 3 (4.1) | |
| Elevated > 95th | 24 (16.7) | 16 (18.6) | 8 (11) | |
| Triglycerides, n (%) | 0.393 | |||
| < 75th percentile | 31 (21.5) | 12 (16.9) | 19 (26) | |
| 75th-90th percentile | 59 (41) | 30 (42.3) | 29 (39.7) | |
| Borderline elevated 90th-95th percentile | 16 (11.1) | 7 (9.9) | 9 (12.3) | |
| Elevated > 95th | 38 (26.3) | 22 (30.9) | 16 (21.9) | |
| HDL-c, n (%) | 0.728 | |||
| Normal level > 10th percentile | 127 (88.2) | 62 (87.3) | 65 (89%) | |
| Borderline low 5th -10th percentile | 9 (6.2) | 4 (5.6) | 5 (6.8) | |
| Low level < 5th percentile | 8 (5.5) | 5 (6.9) | 3 (4.1) | |
| Positive family history of cardiovascular risk factors in a first-degree relative, n (%) | ||||
| Cardiovascular disease risk factors | ||||
| Type 2 diabetes | 51 (30) | 23 (26.7) | 28 (33.3) | 0.349 |
| Premature coronary artery disease | 24 (14.1) | 10 (11.6) | 14 (16.7) | 0.346 |
| Dyslipidemia | 32 (18.8) | 17 (19.8) | 15 (17.9) | 0.750 |
| Hypertension | 33 (19.4) | 16 (18.6) | 17 (20.2) | 0.778 |
| Cerebrovascular accident | 4 (2.4) | 4 (4.7) | 0 (0) | 0.035 |
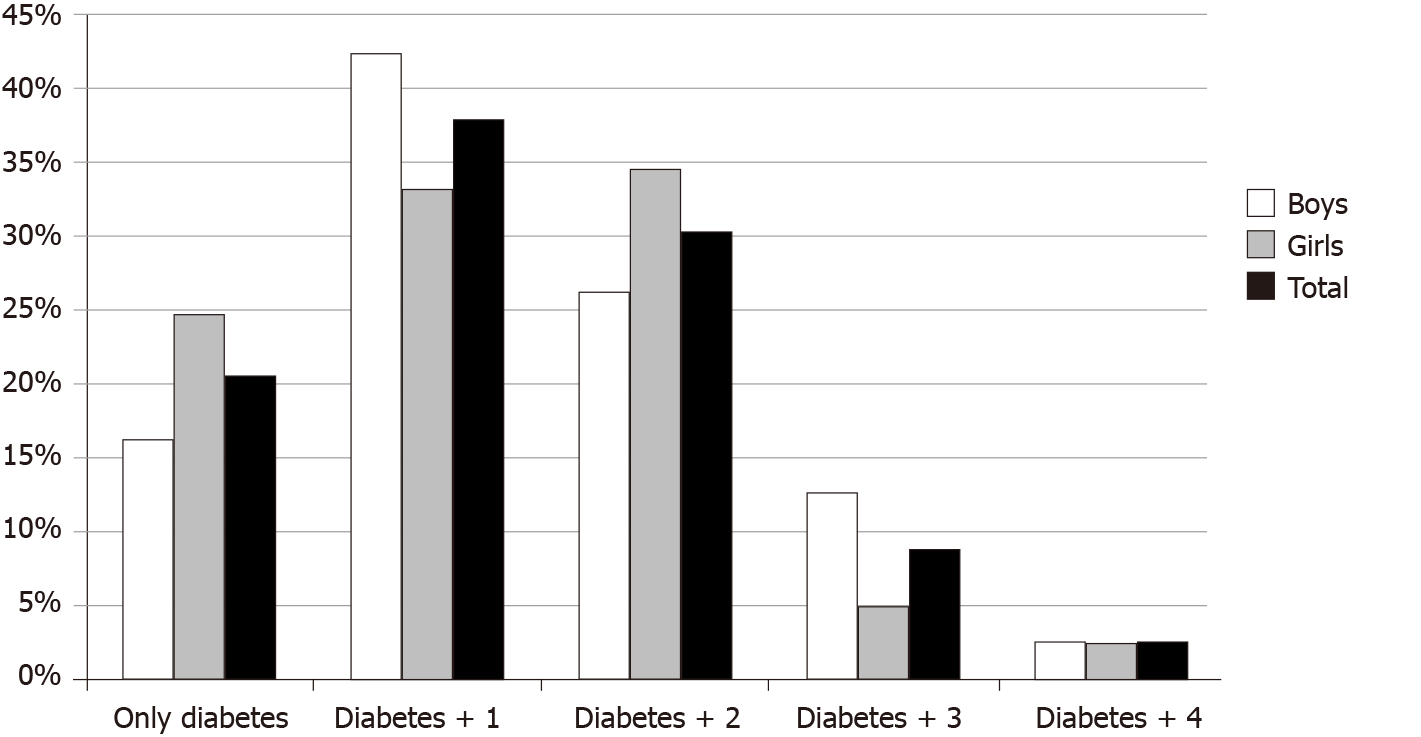
Positive family history of at least one CVD risk factor (T2D, premature coronary artery disease, dyslipidemia, hypertension or cerebrovascular accident) in a first-degree relative was reported in 54.1% (92/170) of the cohort. At baseline 128/161 patients (79.5%) already had an additional CVD risk factor in addition to the diabetes. Occurrence of multiple CVD risk factors (2 or more) was found in 67/161 patients (41.6%). The number and distribution of multiple CVD risk factors is presented in Figure 1, with no significant differences between males and females (P = 0.210). CVD risk factors in descending order of frequency were borderline dyslipidemia/ dyslipidemia (37.5%), pre-hypertension/hypertension (27.6%), and overweight/ obesity (16.5%), with no significant differences between males and females.
The characteristics of the study cohort at last visit at a mean age of 26.3 ± 5.7 years are presented in Table 2. Smoking was reported in 7.6% (13/170) of the cohort. 13.1% (11/84) of females reported oral contraceptive use. The number and distribution of multiple CVD risk factors at young adulthood is presented in Figure 2, with no significant differences between males and females (P = 0.275). CVD risk factors in descending order of frequency were borderline dyslipidemia/dyslipidemia (66.5%), overweight/obesity (39.9%), and pre-hypertension/hypertension (24.3%), with no significant differences between males and females.
| All, n = 170 | Males, n = 86 | Females, n = 84 | P value | |
| Age in yr | 26.3 ± 5.7 | 26.5 ± 6.0 | 26.0 ± 5.5 | 0.540 |
| Diabetes duration in yr | 18.2 ± 5.6 | 18.6 ± 5.5 | 17.8 ± 5.8 | 0.375 |
| Duration follow-up in yr | 13.7 ± 3.7 | 14.1 ± 3.6 | 13.4 ± 3.9 | 0.222 |
| HbA1c, % | 8.0 ± 1.4 | 8.2 ± 1.6 | 7.9 ± 1.1 | 0.164 |
| HbA1c in mmol/L | 63.9 | 66.1 | 62.8 | |
| Smoker, n (%) | 13 (7.6) | 7 (8.1) | 6 (7.1) | 0.807 |
| Oral contraceptive use, n (%) | 11 (13.1) | |||
| Cardiovascular disease risk factors in patients1, n (%) | All, n = 149 | Males, n = 74 | Females, n = 75 | |
| Diabetes only | 13 (8.7) | 5 (6.8) | 8 (10.7) | 0.578 |
| + 1 | 41 (27.5) | 21 (28.4) | 20 (26.7) | |
| + 2 | 62 (41.6) | 28 (37.8) | 34 (45.3) | |
| + 3 | 27 (18.1) | 16 (21.6) | 11 (14.7) | |
| + 4 | 6 (4.0) | 4 (5.4) | 2 (2.7) | |
| Cardiovascular disease risk factors in patients, n (%) | ||||
| Overweight/obesity, n = 158 | 63/158 (39.9) | 32 (40) | 31 (39.7) | 0.974 |
| Hypertension, n = 152 | 37 (24.3) | 26 (35.1) | 11 (14.1) | 0.003 |
| Systolic BP > 130 mmHg | 28 (18.4) | 22 (29.7) | 6 (7.7) | < 0.001 |
| Diastolic BP > 80 mmHg | 21 (13.8) | 16 (21.6) | 5 (6.4) | 0.007 |
| Dyslipidemia | 113 (66.5) | 57 (66.3) | 56 (66.7) | 0.957 |
| LDL-c > 100 mg/dL | 83 (48.8) | 43 (50.0) | 40 (47.6) | 0.641 |
| Triglycerides > 150 mg/dL | 27 (15.9) | 11 (12.7) | 16 (19.0) | 0.279 |
| HDL-c < 40 mg/dL (males) and < 50 mg/dL (females) | 39 (22.9) | 15 (17.4) | 24 (28.5) | 0.099 |
| Statin use2 | 29 (18.2) | 18 (22.5) | 11 (13.9) | 0.316 |
Predictors for dyslipidemia are presented in Table 3. Predictors for elevated TC: Family history of CVD (β[SE] = 23.1[8.3], P = 0.006); elevated LDL-c: baseline diastolic blood pressure (DBP) (β[SE] = 11.4[4.7], P = 0.003) and family history of CVD (β[SE] = 20.7[6.8], P = 0.017); elevated TGs: baseline DBP (β[SE] = 23.8[9.1], P = 0.010) and family history of CVD (β[SE] = 31.0[13.1], P = 0.020); low HDL-c levels: baseline DBP (β[SE] = 4.8[2.1], P = 0.022).
| β (SE) | P value | 95%CI | |
| Low-density lipoprotein cholesterol | |||
| Diastolic blood pressure 1998 | 11.4 (4.7) | 0.003 | 2.0, 20.7 |
| Positive family history of cardiovascular disease | 20.7 (6.8) | 0.017 | 7.2, 34.1 |
| Triglycerides | |||
| Diastolic blood pressure 1998 | 23.8 (9.1) | 0.01 | 5.8, 41.8 |
| Positive family history of cardiovascular disease | 31.0 (13.1) | 0.02 | 5.0, 57.0 |
| High-density lipoprotein cholesterol | |||
| Diastolic blood pressure 1998 | -4.8 (2.1) | 0.022 | -8.9, -0.7 |
| Total cholesterol | |||
| Positive family history of CVD | 23.1 (8.3) | 0.006 | 6.6, 39.5 |
CVD is a leading cause of increased morbidity and mortality in subjects with T1D[33]. Co-occurrence of T1D and CVD risk factor clustering (overweight/obesity, hypertension, family history of CVD and dyslipidemia) may contribute to early-onset CVD. Goldberg et al[34] recently reported that clustering of cardiometabolic risk factors was more prominent in young adults diagnosed with T1D in early childhood, thus placing them at risk for premature cardiovascular morbidity and mortality. Our findings demonstrate that most T1D patients already had at least one CVD risk factor during childhood, with dyslipidemia being the most prevalent. It is noteworthy that clustering of CVD risk factors was observed in approximately one-half of the cohort and that there was a positive family history of at least one CVD risk factor in many patients. Number and distribution of CVD risk factors were similar for males and females.
Weight gain is a clinical concern in patients with T1D. The insulin resistance in overweight and obese individuals with T1D may be associated with an increased risk of vascular complications[35,36]. Over the 15-year observation period of this study, we found a marked increase in the percentage of T1D individuals with over-weight/obesity, from 16.5% to 40% of the cohort. This increase in BMI may mirror the increased prevalence of overweight/obesity in the general Israeli population with progression of age[37,38]. Our findings are in line with other reports from the United States, Europe, and Australia[16,39,40]. Since all T1D individuals followed in our institute receive medical nutrition therapy, the high prevalence of overweight/obesity is surprising. The excessive weight gain may perhaps be partially attributable to the intensive insulin therapy[41,42] or reflect the increase in overweight/obesity in the general Israeli population[37]. Although BMI is a good predictor of weight status, it is not a direct measure of adiposity and may slightly overestimate weight status in individuals with a relatively high muscle mass. It is therefore plausible that the rate of overweight/obesity is overestimated in our cohort. Unfortunately, body composition analysis was not available.
Hypertension is a co-morbid condition of T1D that contributes to the onset and progression of both microvascular and macrovascular complications of the disease. Studies in adults with T1D also have increased mortality rates when systolic and DBPs are elevated[43,44]. Moreover, elevated BP is independently associated with an increased risk of stroke in individuals with T1D[45]. In children with T1D, the prevalence of elevated BP is reportedly as high as 4%-16%[23,46,47]. Our data show a prevalence of hypertension in approximately 25% of the cohort, both at first evaluation and at last visit, which is higher than previously reported. However, one should keep in mind that there is a known under-diagnosis of hypertension in children with T1D.
T1D and dyslipidemia are both risk factors for CVD. International guidelines recommend lifestyle modifications and then consideration of statin pharmacotherapy, depending on an individual’s age and the severity of CVD risk based on LDL-c level and other risk factors[22,48,49]. Previous studies have reported a high frequency of dyslipidemia among pediatric and young adult patients with T1D[19,50,51], with a prevalence rate between 26%-72% and the highest prevalence (72%) in a Brazilian study[52]. Similarly, we found a relatively high prevalence of dyslipidemia already during childhood in slightly more than one-third of our study population, rising to about 60% at adulthood. In 2008 the American Academy of Pediatrics endorsed pharmacologic intervention for children with diabetes when LDL concentration is > 130 mg/dL[33]. Our findings suggest that, although statin therapy is recommended from the age of 8 years, physicians and patients are reluctant to initiate therapy in childhood and adolescence.
BP and cholesterol are major modifiable CVD risk factors and key components of risk prediction algorithms[53]. We found that elevated lipid levels were associated with DBP in childhood and a positive family history of CVD. An atherogenic lipid profile (specifically, elevated LDL-c in adulthood) was associated with both a positive family history of CVD and DBP in childhood; low HDL-c in adulthood was associated with DBP in childhood. In a recent report on pooled data from six large prospective United States cohort studies (of over 36000 participants), young adult exposures to elevated DBP and LDL-c were associated with incident congestive heart disease, and young adult exposure to elevated SBP and DBP was associated with incident heart failure, independent of later adult exposures[52]. These findings suggest that intervention to control modifiable risk factors during childhood, adolescence and young adulthood may reduce the future burden of CVD. Furthermore, since a family background of CVD risk factors plays such a pivotal role in the cardiometabolic health of patients, it is important to update medical files over time.
The strengths of this study lie in the fact that all the patients in our cohort received a similar standard of clinical care, as provided by the same team in a tertiary care center, and the relatively long follow-up period (median of 15 years) from childhood through adolescence to young adulthood. It should be noted that young T1D patients are referred to our center from all over the country and thus serve as a representative sample of all sectors of the Israeli population, including patients of various ethnic origins and socioeconomic status. This study had some limitations, including the single-center experience, the small sample size, and importantly, the lack of an intermediate outcome measure of CV risk/damage (i.e. cardiovascular risk score, intimal media thickness). Although patients were advised to perform fasting prior to lipid profile testing, there was no guarantee that the lipid profile was taken after fasting. In the study population, there was an underrepresentation of the Arab population. Another limitation was the lack of precise data on lifestyle, including physical activity levels and dietary habits. Finally, there may be limits to the generalization of our findings, which are based on T1D patients in our country, and may differ from T1D patients in other countries. Despite these limitations, this study provides important data regarding which factors are associated with elevated CVD risk in young T1D patients.
In conclusion, our findings suggest that an elevated lipid profile is associated with DBP and positive family history of CVD. It is of utmost importance to prevent and control these types of modifiable risk factors as early as childhood, given that inadequate glycemic control and elevation in blood pressure intensify the risk for dyslipidemia. The clustering of CVD risk factors is recognized as being more prominent in patients whose TID is poorly controlled, further emphasizing the importance of rapid and intense intervention when required.
Type 1 diabetes (T1D) contributes to altered lipid profiles and increased cardi-ovascular disease (CVD) risk.
Co-occurrence of T1D and CVD risk factor clustering (overweight/obesity, hypertension, family history of CVD and dyslipidemia) may contribute to early-onset CVD.
We examined the association between CVD risk factors in childhood and dyslipidemia in young adulthood and determined the prevalence of CVD risk factor clustering among T1D patients.
Longitudinal and cross-sectional retrospective study of 170 young subjects with T1D followed in a single tertiary diabetes center for a median duration of 15 years.
Our findings demonstrate that most T1D patients already had at least one CVD risk factor during childhood, with dyslipidemia being the most prevalent. It is noteworthy that clustering of CVD risk factors was observed in approximately one-half of the cohort and that there was a positive family history of at least one CVD risk factor in many patients. The number and distribution of CVD risk factors were similar for males and females.
Our findings suggest that an elevated lipid profile is associated with diastolic blood pressure and positive family history of CVD.
It is of utmost importance to prevent and control modifiable risk factors as early as childhood, given that inadequate glycemic control and elevation in blood pressure intensify the risk for dyslipidemia.
| 1. | Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, Rami-Merhar B, Soltesz G, Svensson J, Parslow RC, Castell C, Schoenle EJ, Bingley PJ, Dahlquist G, Jarosz-Chobot PK, Marčiulionytė D, Roche EF, Rothe U, Bratina N, Ionescu-Tirgoviste C, Weets I, Kocova M, Cherubini V, Rojnic Putarek N, deBeaufort CE, Samardzic M, Green A. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 2. | DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 840] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 3. | Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L; SEARCH for Diabetes in Youth Study. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;376:1419-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1095] [Article Influence: 121.7] [Reference Citation Analysis (0)] |
| 4. | Berenson GS, Wattigney WA, Tracy RE, Newman WP 3rd, Srinivasan SR, Webber LS, Dalferes ER Jr, Strong JP. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (The Bogalusa Heart Study). Am J Cardiol. 1992;70:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 352] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 2003;41:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Järvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, Lehtimäki T, Rönnemaa T, Viikari J, Raitakari OT. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | Costacou T, Lopes-Virella MF, Zgibor JC, Virella G, Otvos J, Walsh M, Orchard TJ. Markers of endothelial dysfunction in the prediction of coronary artery disease in type 1 diabetes. The Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Complications. 2005;19:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 832] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 9. | Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jørgensen K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care. 2010;33:2043-2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Urbina EM, Isom S, Bell RA, Bowlby DA, D'Agostino R Jr, Daniels SR, Dolan LM, Imperatore G, Marcovina SM, Merchant AT, Reynolds K, Shah AS, Wadwa RP, Dabelea D; SEARCH for Diabetes in Youth Study Group. Burden of Cardiovascular Risk Factors Over Time and Arterial Stiffness in Youth With Type 1 Diabetes Mellitus: The SEARCH for Diabetes in Youth Study. J Am Heart Assoc. 2019;8:e010150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63:1469-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 381] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 12. | Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv Exp Med Biol. 2017;960:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 763] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 13. | Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2774] [Cited by in RCA: 2681] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 14. | Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2121] [Article Influence: 117.8] [Reference Citation Analysis (2)] |
| 15. | Jackson-Leach R, Lobstein T. Estimated burden of paediatric obesity and co-morbidities in Europe. Part 1. The increase in the prevalence of child obesity in Europe is itself increasing. Int J Pediatr Obes. 2006;1:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Maffeis C, Birkebaek NH, Konstantinova M, Schwandt A, Vazeou A, Casteels K, Jali S, Limbert C, Pundziute-Lycka A, Toth-Heyn P, de Beaufort C, Sumnik Z, Cherubini V, Svensson J, Pacaud D, Kanaka-Gantenbein C, Shalitin S, Bratina N, Hanas R, Alonso GT, Poran L, Pereira AL, Marigliano M; SWEET Study Group. Prevalence of underweight, overweight, and obesity in children and adolescents with type 1 diabetes: Data from the international SWEET registry. Pediatr Diabetes. 2018;19:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, Dabelea D, Hamman R, Waitzfelder B, Kahn HS; SEARCH for Diabetes in Youth Study Group. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 18. | Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycemic control in patients with diabetes mellitus: Analysis of physician electronic health records in the US from 2009-2011. J Diabetes Complications. 2016;30:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Kershnar AK, Daniels SR, Imperatore G, Palla SL, Petitti DB, Pettitt DJ, Marcovina S, Dolan LM, Hamman RF, Liese AD, Pihoker C, Rodriguez BL. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2006;149:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Shah AS, Maahs DM, Stafford JM, Dolan LM, Lang W, Imperatore G, Bell RA, Liese AD, Reynolds K, Pihoker C, Marcovina S, D'Agostino RB Jr, Dabelea D. Predictors of Dyslipidemia Over Time in Youth With Type 1 Diabetes: For the SEARCH for Diabetes in Youth Study. Diabetes Care. 2017;40:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, Kelly AS, Nadeau KJ, Martyn-Nemeth P, Osganian SK, Quinn L, Shah AS, Urbina E; American Heart Association Atherosclerosis; Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council for High Blood Pressure Research; and Council on Lifestyle and Cardiometabolic Health. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130:1532-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, Zinman B, Eckel RH. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37:2843-2863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 292] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 23. | Schwab KO, Doerfer J, Hecker W, Grulich-Henn J, Wiemann D, Kordonouri O, Beyer P, Holl RW; DPV Initiative of the German Working Group for Pediatric Diabetology. Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: cross-sectional data from the German diabetes documentation and quality management system (DPV). Diabetes Care. 2006;29:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Polak M, Souchon PF, Benali K, Tubiana-Rufi N, Czernichow P. Type 1 diabetic children have abnormal lipid profiles during pubertal years. Pediatr Diabetes. 2000;1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;1-190. [PubMed] |
| 26. | Lovshin JA, Bjornstad P, Lovblom LE, Bai JW, Lytvyn Y, Boulet G, Farooqi MA, Santiago S, Orszag A, Scarr D, Weisman A, Keenan HA, Brent MH, Paul N, Bril V, Perkins BA, Cherney DZI. Atherosclerosis and Microvascular Complications: Results From the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Care. 2018;41:2570-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 953] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 28. | McDowell MA, Fryar CD, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2003–2006. Natl Health Stat Report. 2008;1-48. [PubMed] |
| 29. | Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010;1-5. [PubMed] |
| 30. | National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555-576. [PubMed] |
| 31. | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart; Lung; and Blood Institute Joint National Committee on Prevention; Detection; Evaluation; and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572. [PubMed] |
| 32. | Daniels SR, Greer FR; Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 795] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 33. | Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992-1999. Diabetologia. 2006;49:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Goldberg T, Brener A, Levy S, Interator H, Laurian I, Dorfman A, Chorna E, Oren A, Eyal O, Lebenthal Y. Association between age at type 1 diabetes diagnosis and metabolic outcome at young adulthood: a real-life observational study. Diabetes Metab Res Rev. 2020;e3356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 1438] [Article Influence: 179.8] [Reference Citation Analysis (0)] |
| 36. | Šimonienė D, Platūkiene A, Prakapienė E, Radzevičienė L, Veličkiene D. Insulin Resistance in Type 1 Diabetes Mellitus and Its Association with Patient's Micro- and Macrovascular Complications, Sex Hormones, and Other Clinical Data. Diabetes Ther. 2020;11:161-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Ministry of Health. Prevention and Treatment of Obesity A Subcommittee of the Health Behaviors Committee. 2011 https://www.health.gov.il/PublicationsFiles/Obesity-prof_en.pdf. |
| 38. | Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011-2014. NCHS Data Brief. 2015;1-8. [PubMed] |
| 39. | Flechtner-Mors M, Schwab KO, Fröhlich-Reiterer EE, Kapellen TM, Meissner T, Rosenbauer J, Stachow R, Holl RW. Overweight and Obesity Based on Four Reference Systems in 18,382 Paediatric Patients with Type 1 Diabetes from Germany and Austria. J Diabetes Res. 2015;2015:370753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Minges KE, Whittemore R, Weinzimer SA, Irwin ML, Redeker NS, Grey M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: The T1D Exchange Clinic Registry. Diabetes Res Clin Pract. 2017;126:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 41. | Baskaran C, Volkening LK, Diaz M, Laffel LM. A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the Diabetes Control and Complications Trial. Pediatr Diabetes. 2015;16:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Purnell JQ, Zinman B, Brunzell JD; DCCT/EDIC Research Group. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 43. | Rawshani A, Rawshani A, Sattar N, Franzén S, McGuire DK, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, Rosengren A, Gudbjörnsdottir S. Relative Prognostic Importance and Optimal Levels of Risk Factors for Mortality and Cardiovascular Outcomes in Type 1 Diabetes Mellitus. Circulation. 2019;139:1900-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 44. | Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH; EURODIAB Prospective Complications Study Group. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care. 2008;31:1360-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Hägg-Holmberg S, Dahlström EH, Forsblom CM, Harjutsalo V, Liebkind R, Putaala J, Tatlisumak T, Groop PH, Thorn LM; FinnDiane Study Group. The role of blood pressure in risk of ischemic and hemorrhagic stroke in type 1 diabetes. Cardiovasc Diabetol. 2019;18:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Margeirsdottir HD, Larsen JR, Brunborg C, Overby NC, Dahl-Jørgensen K; Norwegian Study Group for Childhood Diabetes. High prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes: a population-based study. Diabetologia. 2008;51:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | Rodriguez BL, Dabelea D, Liese AD, Fujimoto W, Waitzfelder B, Liu L, Bell R, Talton J, Snively BM, Kershnar A, Urbina E, Daniels S, Imperatore G; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr 2010; 157: 245-251. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | American Diabetes Association. 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S86-S104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 371] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 49. | Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart; Lung; and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213-S256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1658] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 50. | Stankute I, Dobrovolskiene R, Danyte E, Razanskaite-Virbickiene D, Jasinskiene E, Mockeviciene G, Marciulionyte D, Schwitzgebel VM, Verkauskiene R. Factors Affecting Cardiovascular Risk in Children, Adolescents, and Young Adults with Type 1 Diabetes. J Diabetes Res. 2019;2019:9134280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Bulut T, Demirel F, Metin A. The prevalence of dyslipidemia and associated factors in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2017;30:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Homma TK, Endo CM, Saruhashi T, Mori AP, Noronha RM, Monte O, Calliari LE. Dyslipidemia in young patients with type 1 diabetes mellitus. Arch Endocrinol Metab. 2015;59:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, Balte PP, Alonso A, Newman AB, Ives DG, Rana JS, Lloyd-Jones D, Vasan RS, Bibbins-Domingo K, Gooding HC, de Ferranti SD, Oelsner EC, Moran AE. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood With Later Cardiovascular Events. J Am Coll Cardiol. 2019;74:330-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Israel
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ishizawa K, Li SY S-Editor: Huang P L-Editor: Filipodia P-Editor: Ma YJ