INTRODUCTION
Ketogenesis, consisting of the production by perivenous hepatocytes of the ketone bodies β-hydroxybutyrate (BHB) and acetoacetate (AcAc) using fatty acid-derived acetyl CoA, is a physiologically important process to produce an alternative metabolic source of energy to glucose during the neonatal period, starvation, or prolonged physical effort (figure with ketogenesis pathway in hepatocyte mitochondria). Spontaneous decarboxylation of AcAc yields acetone, the third and quantitatively minor ketone body[1].
The biosynthesis of ketone bodies is connected to multiple metabolic pathways, including the tricarboxylic acid cycle, β-oxidation of fatty acids, de novo lipogenesis, sterol biosynthesis, glucose metabolism, and the mitochondrial electron transport chain. Moreover, hormonal signaling and intracellular signal transduction pathways are also affected by ketone bodies.
In extrahepatic tissues, AcAc- and BHB-derived AcAc serve as a substrate to produce AcAc-CoA via the enzymatic activity of succinyl-CoA:3-oxoacid-CoA transferase (coded by the gene OXCT1)[1]. In turn, AcAc-CoA generates two molecules of AcCoA. The metabolic necessity of physiological ketogenesis is highlighted by the fact that whole-body OXCT1 gene ablation induces postnatal mortality in mice[2]. BHB is the most abundant ketone body and has been reported to serve as an alternative energy source for the heart in diabetic patients[3] and to be slightly elevated in diabetic patients treated with the sodium-glucose co-transporter 2 inhibitor dapagliflozin, with this low-ketonemic state being correlated to improved insulin sensitivity[4].
On the other hand, diabetic ketoacidosis, in which concentrations of ketone bodies are extremely high, is a serious complication of diabetes mellitus, whereby serum concentrations of ketone bodies attain high levels and serve as nutriment to compensate for the organ’s failure to utilize glucose[4]. Therefore, ketone bodies can be either a necessary nutrient or the reflection of a pathological status depending on their plasmatic concentration.
The understanding of the physiological role(s) of ketone bodies, and in particular BHB, recently took an unexpected turn when it was demonstrated that BHB acts as a histone deacetylase inhibitor, thereby favoring histone hyperacetylation. Such histone hyperacetylation has been associated with the antioxidant properties of BHB in a mouse model[5]. At approximately the same time, BHB has been proposed to act as an anti-inflammatory molecule by targeting the inflammasome[6]. In addition, a novel histone post-translational modification (PTM) termed β-hydroxybutyrylation, which consists of the formation of an amide bond between the BHB carboxyl group and the histone lysine ε amino group, has been reported. This discovery expanded the repertoire of histone PTMs and identified BHB as a molecule involved in epigenetic control of transcription[7].
Earlier studies analyzed the genome-wide histone 3 lysine 9 and lysine 14 (H3K9/K14) hyperacetylation and DNA methylation in response to elevated glucose concentrations in primary human vascular cells. Therein, a number of specific hyperacetylation and CpG methylation signatures associated with the transcriptional upregulation of genes involved in metabolic and cardiovascular disease were identified[8]. Chronic exposure of vascular endothelial cells (ECs) to high glucose concentrations: (1) Recapitulated hyperglycemia-mediated induction of gene ontology classes and cellular pathways via the modulation of acetylated H3K9/K14 in multiple genomic loci[9]; and (2) Induced the upregulation of several proinflammatory and proatherosclerotic genes[8]. These initial observations linking prolonged exposure of ECs to high-glucose media and epigenetic alterations have been validated in clinical samples (circulating blood mononuclear cells) from participants in the DCCT/EDIC trial[10].
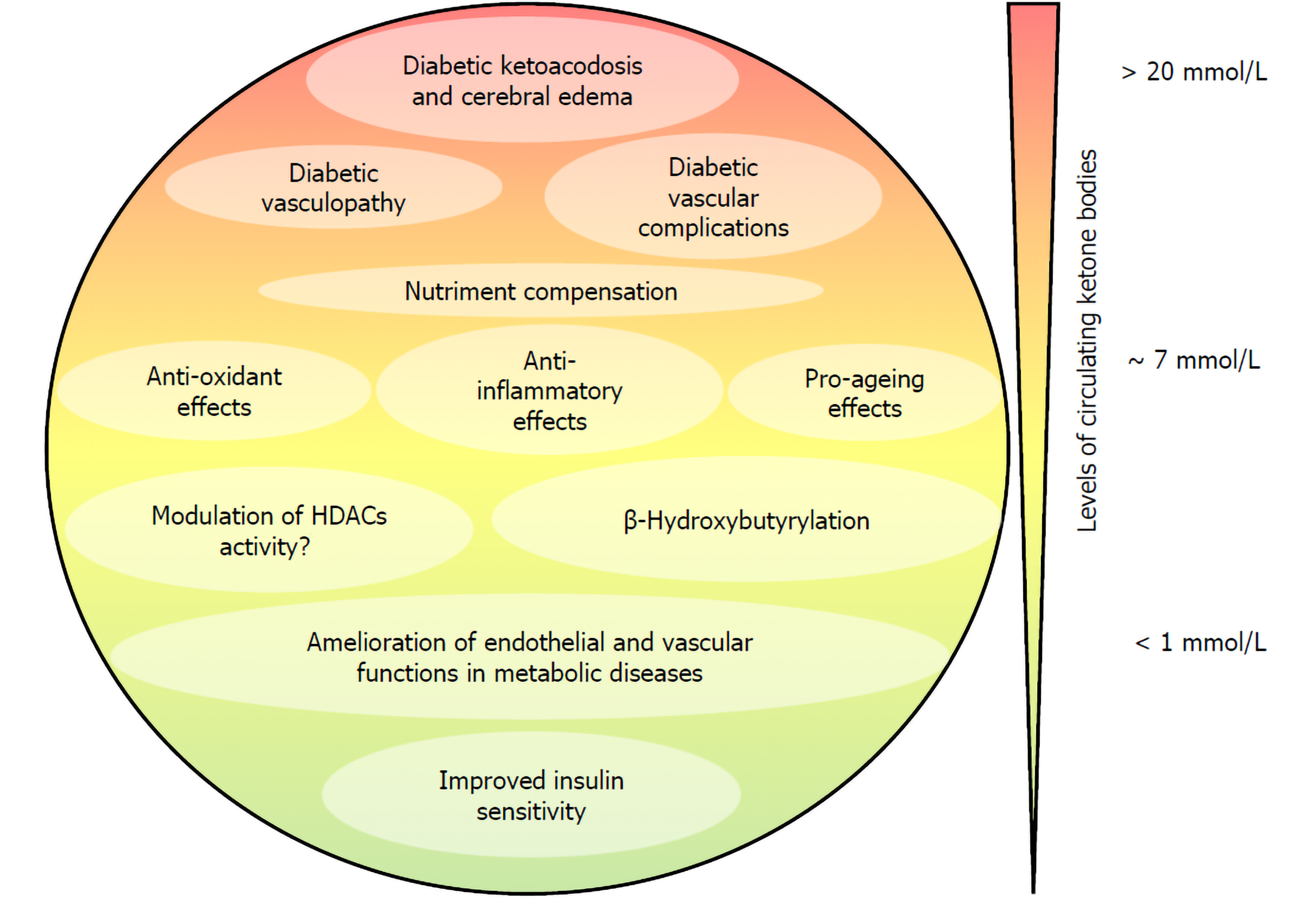
Collectively, these data hint at the possibility that the endothelium may be amenable to pharmacological interventions targeting the epigenome to reverse epigenetic changes occurring in diabetic endothelial dysfunction and diabetes-induced endothelial proliferation[11]. Given that the endothelium is the first-line tissue exposed to circulating ketone bodies and considering that BHB has been shown to purport/support/mediate anti-inflammatory and antioxidative features, a strong interest has been directed in the last few years towards understanding the effect of BHB on the physiology of ECs. We review here the evidence indicating that ketone bodies, and in particular BHB, at low concentrations potentially contribute to ameliorating endothelial and vascular function in metabolic disease, while elevated concentrations of ketone bodies as observed in diabetic ketoacidosis contribute to the diabetic vasculopathy and diabetic vascular complications (Figure 1).
Figure 1 Concentration dependency of the biochemical and physiological responses to ketone bodies.
Low to medium concentrations of ketone bodies, attained through fasting, ketogenic diet, or physical effort, convey physiologically beneficial effects. Conversely, pathological ketone body concentrations, observed in diabetic ketoacidosis, contribute to the disease morbidity and can be life threatening. HDACs: Histone deacetylases.
DETRIMENTAL VASCULAR EFFECTS OF HIGH KETONE BODIES AND BHB CONCENTRATIONS IN DIABETIC KETOACIDOSIS
The morbidity and mortality burden of type 1 diabetes (T1D) can be largely attributed to vascular inflammation and cardiovascular disease (CVD) that are promoted by the installment of a chronic inflammatory state of the endothelium[12]. Furthermore, diabetic patients experiencing frequent episodes of diabetic ketoacidosis have an increased incidence of morbidity and mortality due to vascular complications and cerebral edema[13,14]. In T1D, concentrations of ketone bodies can reach 25 mmol/L[4], as compared to low millimolar concentrations (< 7 mmol/L) induced by prolonged starving on obese volunteers[15] or during postexercise ketosis[16].
Cerebral edema is the most serious complication linked to diabetic ketoacidosis and is the first cause of mortality in type 1 diabetic children[17]. Pharmacological studies demonstrated that activation of the Na-K-Cl cotransporter expressed in the ECs of the blood-brain barrier and astrocytes mediates cerebral edema formation and inhibition of the cotransporter by bumetanide reduced edema formation in the cerebral artery occlusion models of stroke in rats. In this study, in which diabetic ketoacidosis was induced by streptozotocin and BHB and reached 3.7 mmol/L, bumetanide efficiently reduced cerebral edema formation[18]. Furthermore, in cultured bovine cerebral microvascular ECs, both AcAc and BHB stimulated the activity of the Na-K-Cl cotransporter[18].
In a model of traumatic brain injury in rats, affecting the blood-brain barrier (BBB) integrity, BHB did not display a protective effect and, furthermore, caused a slight damage in the BBB integrity in healthy animals infused with BHB[19].
LOW DOSE BHB PROTECTS THE ENDOTHELIUM FROM CELL SENESCENCE
Cellular senescence is a process that results from a variety of stress and with time leads to a state of irreversible growth cessation. Senescent cells accumulate during aging and have been involved in the promotion of various age-related diseases. Cellular senescence can play an important role in tumor suppression, wound healing, and tissue fibrosis protection. However, there is also growing evidence that senescent cells can cause adverse effects in vivo and contribute to tissue remodeling, aging of the body, and vascular diseases associated with inflammation and dysfunction of ECs and smooth muscles[20,21]. Although many studies have focused on the beneficial effects of calorie restriction, which can prolong life and delay aging in various species[22], the effect and mechanism of ketone bodies on the quiescence of vascular cells and senescence have been less investigated[20,23]. An early study indicated that BHB supplementation extends the lifespan of Caenorhabditis elegans by 20% through the DAF-16/FOXO and SKN-1/Nrf pathways[24]. In mammals, BHB decreases the secretory phenotype associated with senescence and vascular cell senescence[25]. In addition, the ketogenic diet (KD) significantly extended the median lifespan of mice and preserved the physical function of older mice[26].
Treatment of human umbilical vein ECs (HUVEC) and human aortic smooth muscle cells treated with BHB suppressed oxidant-induced elevation of senescence markers. ΒHB has been shown to prevent both replicative and stress-induced senescence in vascular cells and to reduce in vivo serum levels of the interleukin-1α prosenescence marker. Indeed, senescence by BHB is dependent on Oct4A and independent of p53. ΒHB is involved in Oct4A elevation by direct binding to heterogeneous ribonuclear particle A1 (hnRNP A1), which induces quiescence of vascular cells and thus prevents cell senescence.
As aging is one of the major risk factors for cardiovascular disease, delaying or preventing vascular aging can protect against cardiovascular dysfunction. Caloric restriction induced the production of ketones that delay vascular aging by preventing both replication and stress that induce senescence of vascular cells. This prevention of senescence by BHB is done through its direct binding of hnRNP A1 resulting in the stabilization of the mRNA of Oct4A and the expression of Oct4A. Targeting of Oct4A by BHB or BHB-like compounds appears to be effective in preventing or delaying the progression of EC senescence. BHB has an important role in the positive regulation of Oct4A in the vascular system, which provides a new strategy to prevent vascular aging associated with senescence by accumulating or maintaining vascular cells at rest[27].
KETOGENIC DIET AND LOW BHB LEVELS POSITIVELY AFFECT CARDIOVASCULAR FUNCTION
Obesity is a predisposing factor to cardiovascular pathologies including coronary heart disease and hypertension[28,29]. As a KD could decrease body weight, indirect beneficial effects on the cardiovascular function may ensue. In a 1-year multicenter clinical study aimed at body weight (BW) reduction through a very low carbohydrate KD, BW reduction at 4 wk was 7 kg, and at week 12 was 5 kg; a reduction that was maintained until the end of the study period. At weeks 4 and 12, body fat was reduced by 3.8% and 3.4%, respectively[30]. After a 4-wk nutritional trial consisting of a KD supplemented with n-3 polyunsaturated fatty acids on overweight but otherwise healthy subjects, total body weight was reduced by an average of 4.70 kg, and body fat was reduced by 5.41 kg. In parallel, a decrease of glucose (-18.2 mg/L), total cholesterol (-16 mg/L), triglyceride (-40.5 mg/L), and low-density lipoprotein cholesterol (-9.8 mg/L) was observed in blood samples[31]. In another 6-mo study, KD, while not lowering the levels of total cholesterol, induced a shift from small and dense low-density lipoprotein to large and buoyant low-density lipoprotein, which is associated with a lowering of cardiovascular disease risk[32].
KD has an impact on the synthesis of endogenous cholesterol. 3-hydroxy3-methylglutaryl–CoA reductase 2, an enzyme transcriptionally promoted by insulin, leads to the synthesis of β-hydroxy-β-methylglutaryl-CoA, which is a precursor for hepatic ketone body production as well as endogenous cholesterol synthesis[33]. Saturated fatty acids, which promote CVD, decreased in plasma after administration of a low-carbohydrate diet[34]. Volek et al[35] showed that women are more sensitive to a KD because they have a more significant increase of HDL than men. However, upon administration of a KD, levels of fasting triglycerides are decreased in both sexes[36].
KD has also been shown to exert some effect on changes in blood pressure. A 48-wk study reported an improvement in systolic and diastolic blood pressure in overweight participants on a KD compared to a control group on a low-fat diet with the addition of orlistat[37]. Decreased systolic blood pressure was observed after 3-mo of a KD, and the decrease persisted even after a year[30]. As a decrease of systolic blood pressure and diastolic blood pressure is also occurring upon a weight loss of 5%[38], a convergence between body weight loss, KD, and blood pressure can be hypothesized.
EPIGENETIC ALTERATIONS INDUCED BY KETONE BODIES IN THE CARDIOVASCULAR SYSTEM
Epigenetics is the field of study about persistent or inherited changes in phenotypes and gene expression without any change in the DNA sequence. Epigenetic mechanisms to control gene expression involve methylation of DNA, histone PTMs, and noncoding RNAs.
Methylation of DNA
Several epigenome-wide association studies led to the identification of several loci potentially associated with the development of incident CVD[39]. By analyzing DNA methylation data from three independent cohorts, a methylation-based risk score associated to CVD could be defined, suggesting that CVD-specific DNA methylation patterns may identify subjects at-risk for CVD[40]. Similarly, a global decrease in DNA methylation was observed in the blood of patients with essential hypertension[41]. In atherosclerosis, global hypomethylation of DNA was observed in mouse, rabbit, and human atherosclerotic lesions[39]. DNA hypomethylation could thus mediate an expanded transcriptional activity and activation of vascular cell proliferation[42]. A decrease of the DNA methylation in CVD may also affect the inflammatory functions of leukocytes, which correlate with CV risk via the modulation of adhesion and migration molecules as well as soluble molecules[43]. At present, however, it is unclear whether ketone bodies affect DNA methylation patterns to control cardiovascular function (Figure 2).
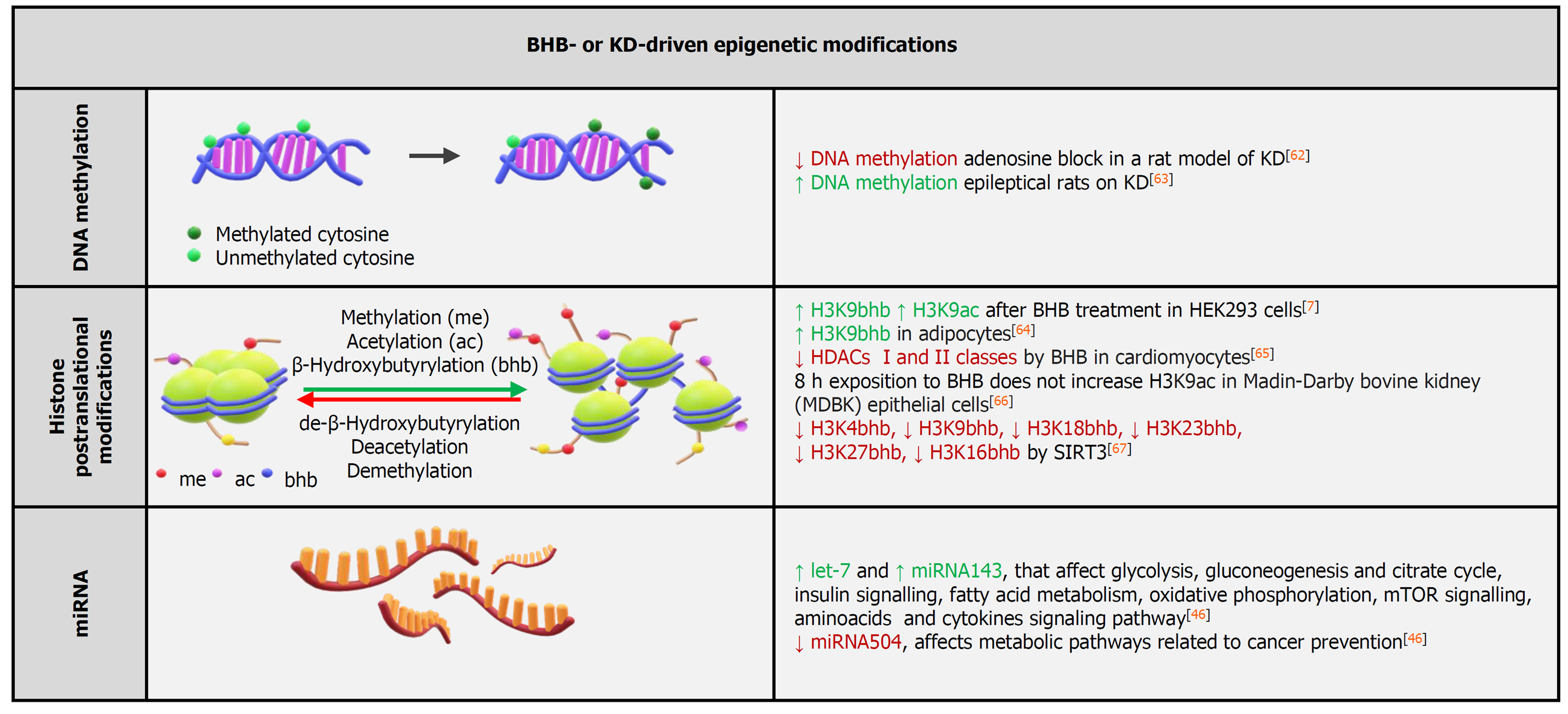
Figure 2 Schematic diagram summarizing the main epigenetic alterations induced by β-hydroxybutyrate or a ketogenic diet[7,46,62-67].
BHB: β-Hydroxybutyrate; HDACs: Histone deacetylases; KD: Ketogenic diet.
Histone PTMs
β-hydroxybutyrate is reportedly an endogenous inhibitor of class I histone deacetylases[5]. Furthermore, BHB induces a novel histone PTM, lysine β-hydroxybutyrylation, that was identified as a new type of histone mark on multiple lysine residues of histones H1, H2A, and H3. Increased histone 3 lysine 9 β-hydroxybutyrylation levels led to the upregulation of starvation-responsive genes[7]. β-hydroxybutyrylation was also shown to occur on lysine residues on p53, simultaneously affecting p53 acetylation[44]. When directly tested on human microvascular ECs (HMEC-1), BHB administration did not increase histone acetylation, while increasing histone lysine β-hydroxybutyrylation, and BHB administration to crude nuclear extracts did not inhibit histone deacetylases catalytic activity[45]. Overall, these results further support the notion that BHB induces β-hydroxybutyrylation while producing negligible changes in the acetylation patterns[7] (Figure 2).
miRNA
In a study in human volunteers, miRNA expression profiles after a 6-wk regimen on a KD was significantly different from baseline and displayed sex-specific differences. In females, overexpression of miR-148, miR-26, miR-30, miR-502, miR-520, miR-548, miR-590, and miR-644 were observed, while in males the increment was amongst the following miRNAs: miR-30, miR-502, miR-548, miR-590, and miR-644. Overall, the volunteers on a KD displayed regulation of miRNAs targeting specific genes linked to nutrient metabolism as well as mTOR, PPARs, insulin, and cytokine signaling pathways[46].
Interestingly, only 18 miRNA families constitute up to 90% of the cardiac miRNAs pool, with miR-1 being the most expressed miRNA in cardiomyocytes. Together with miR-1, miR-133 (its expression is lower) generates from the same bicistronic transcript. miR-1 and miR-133 jointly promote mesoderm differentiation in embryonic stem cells. In later development, miR-1 favors and mi-R133 inhibits the differentiation of the mesoderm into cardiomyocytes. Cardiogenesis is influenced by miR-1 through the control of transcriptional factors Irx5 and Hand2, with the latter contributing to the development of the right ventricle[47] (Figure 2).
THE EFFECTS OF BHB ON THE INFLAMMATORY STATUS OF THE ENDOTHELIUM ARE DOSE-DEPENDENT
Inflammation is a major driving mechanism for the development of vascular complications related to atherosclerosis and diabetes. Hyperketonemia can contribute to establishing an inflammatory environment in the EC layer and circulating monocytes. The secretion of cytokines and chemokines generates a gradient favoring the migration of the monocytes at the endothelium and promotes the adhesion of monocytes to the EC layer. Thus, hyperketonemia contributes to the inflammatory state that potentiates the adhesion of monocytes to ECs and by inducing endothelial dysfunction amplifies the risk of CVD in diabetes.
In vitro experiments demonstrated that BHB and AcAc at concentrations up to 10 mmol/L induced adhesion of the monocyte cell lines THP-1 and U937 as well as of isolated human monocytes to HUVEC cells by induction of endothelial intercellular-1 adhesion molecule (ICAM-1) and lymphocyte function-associated antigen-1 in monocytes[48]. In addition, AcAc induced an increase in monocyte chemotactic protein-1 and interleukin-8 secretion in both HUVEC cells and THP-1 and U937 monocytes[48]. AcAc, but not BHB, also induced overexpression of ICAM-1 in human brain microvascular ECs[49].
According to a study by Kanikarla-Marie et al[50], high ketone levels can positively regulate NOX4, leading to an increase in reactive oxygen species in HUVEC. This increase was much greater when ketone bodies were coadministered to a high-glucose containing medium. ICAM-1 is a cell surface glycoprotein expressed on cells, and the induction of ICAM-1 promotes the recruitment of monocytes/macrophages allowing macrophages to adhere. ICAM-1 levels are predictors of endothelial dysfunction and are elevated in patients with type 1 diabetes. Reactive oxygen species (ROS) derived from NADPH oxidase play a physiological role in the regulation of endothelial function and the inflammation underlying the vascular remodeling of diabetes. Various isoforms of NADPH oxidase (NOX) have now been identified and characterized as having an important function in the mediation of oxidative stress and thus the regulation of cellular functions. Several reports indicated that NOX contributes to oxidative stress in diabetes. It was also proven that activation of these enzymes can be caused by various stimuli such as cytokines, growth factors, hyperglycemia, and lipids. A high level of glucose is known to activate NOX.
Indeed, NOX4 upregulation causes an increase in ROS, which can activate various signaling pathways leading to ICAM-1 overexpression and increased adhesion of monocytes to ECs, a surrogate biomarker for vascular dysfunction. The expression of ICAM-1 on the endothelial surface plays an essential role because it facilitates recruitment, attachment, and intravasation of monocytes, which can lead to initiation and progression of plaque formation in the walls of vessels contributing to the development of atherosclerosis. The adhesion between monocytes and ECs increased in response to both AcAc and BHB. It has been shown that several markers of vascular inflammation are influenced by the presence of hyperketonemia, which can influence the progression of CVD in diabetes by increasing monocyte adherence to ECs.
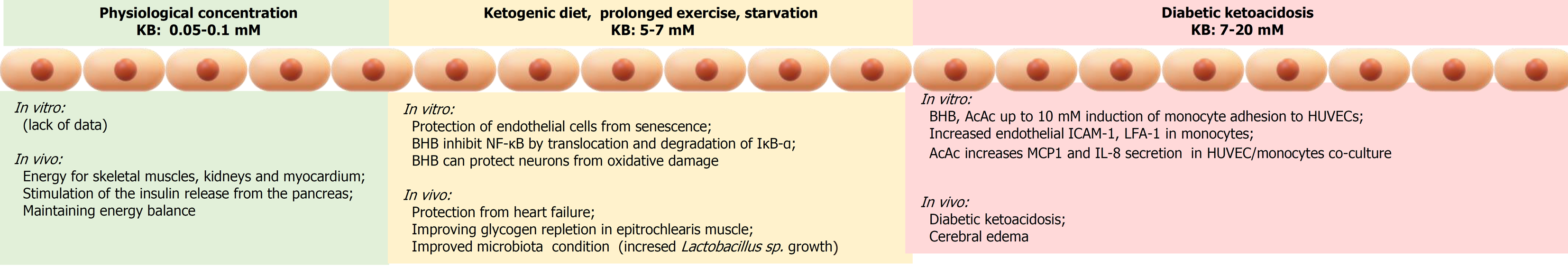
Cardiovascular disease consists of a number of different diseases that affect the heart and blood vessels, most of which have similar causes, mechanisms, and treatments. Atherogenesis is a common denominator of most vascular diseases, and it is well-recognized that inflammation is the driving force behind this process. In this context, the anti-inflammatory action of a low concentration of ketone bodies may help alleviate the inflammatory burden occurring in the vasculature of individuals with CVD (Figure 3).
Figure 3 In vitro and in vivo responses to ketone bodies are dependent of their concentrations, with low to moderate concentrations exerting beneficial effects on the endothelium and the cardiovascular system and high concentrations typical of diabetic ketoacidosis being detrimental.
AcAc: Acetoacetate; BHB: β-hydroxybutyrate; HUVECs: Human umbilical vein endothelial cells; ICAM-1: Intercellular-1 adhesion molecule; IL: Interleukin; KB: Ketone bodies; LFA-1: Lymphocyte function-associated antigen-1; MCP1: Monocyte chemotactic protein-1.
THE INFLUENCE OF KETONE BODIES ON THE MYOCARDIUM
Cardiomyocytes are structural units of the heart that similarly to oxidative skeletal muscle have a high density of mitochondria. With such an abundance of mitochondria, the myocardium is capable of oxidizing various substrates to produce ATP. Acetyl-CoA from glucose (via glycolysis) or lipids (via β-oxidation) enters the Krebs cycle. Ketone bodies, generated by the liver, also constitute major acetyl-CoA precursors for the heart[51].
As ketone bodies are not able to complement the intermediates of the Krebs cycle, these intermediates are constantly lost. Thus ketone body oxidation is cataplerotic as it leads to depletion of the Krebs cycle intermediates and impairment of the metabolic efficiency[16]. This cataplerotic effect must be balanced by anaplerotic substances such as circulating glucose, glycogen, or glucogenic amino providing pyruvate[51] with heart pyruvate carboxylase being the key anaplerotic enzyme in the heart[52].
BHB, as the quantitatively major ketone body, has been studied on myocardial tissue from patients with severe heart failure (HF) resulting in increased myocardial utilization in these patients[53]. Nielsen et al[54] demonstrated that BHB infusion in patients with HF reduced the ejection fraction and improved cardiac output by 2.0 L/min and left ventricular ejection fraction by 8%, simultaneously reducing systemic vascular resistance by 30% in comparison to placebo infusion. The observed effect was dose-dependent and reached significant hemodynamic effects at a circulating BHB concentration in the physiological range and exerted a beneficial hemodynamic effect on control healthy volunteers.
Early studies demonstrated that the isolated perfused rat heart at high concentrations of BHB resulted in decreased cardiac output when ketone bodies were administrated as the sole energy substrate, and the addition of glucose to the perfusate reversed this detrimental effect[55]. In accordance to these data, the coadministration of BHB (4 mmol/L) and glucose increased cardiac work[56] and cardiac output in comparison to glucose alone in an isolated rat heart model[57]. However, pathological remodeling and cardiac dysfunction in HF has been observed as a result of a heart-specific knock-out of Oxct-1, the gene responsible for control of flux through the enzymes of cardiac ketone metabolism[58]. This suggested a crucial role for sustained ketone oxidation in HF[59]. Hence, increased myocardial ketone utilization has been demonstrated in explanted hearts from patients with severe HF[53]. Collectively, these data indicated that circulating ketone bodies derived from a KD may improve myocardium functioning and can contribute to the treatment of patients with impaired functions of the cardiovascular system.
Diabetic patients suffering from HF have been observed to have an increased cardiac uptake of ketone bodies as compared to those without diabetes[3]. Chronic insulin resistance in the diabetic heart results in alterations of fuel availability and change of affinity and utilization abilities of myocytes for different substrates[60] with free fatty acids becoming the preferred substrate, which leads to significant reduction of energy efficiency and accumulation of toxic byproducts that exacerbate HF and insulin resistance[61]. Mizuno et al[3] demonstrated that in diabetic HF the uptake of total ketone bodies and BHB is higher in comparison to nondiabetic HF. This suggests that ketone bodies serve as a partial energy source replacement in the human diabetic heart.
CONCLUSION
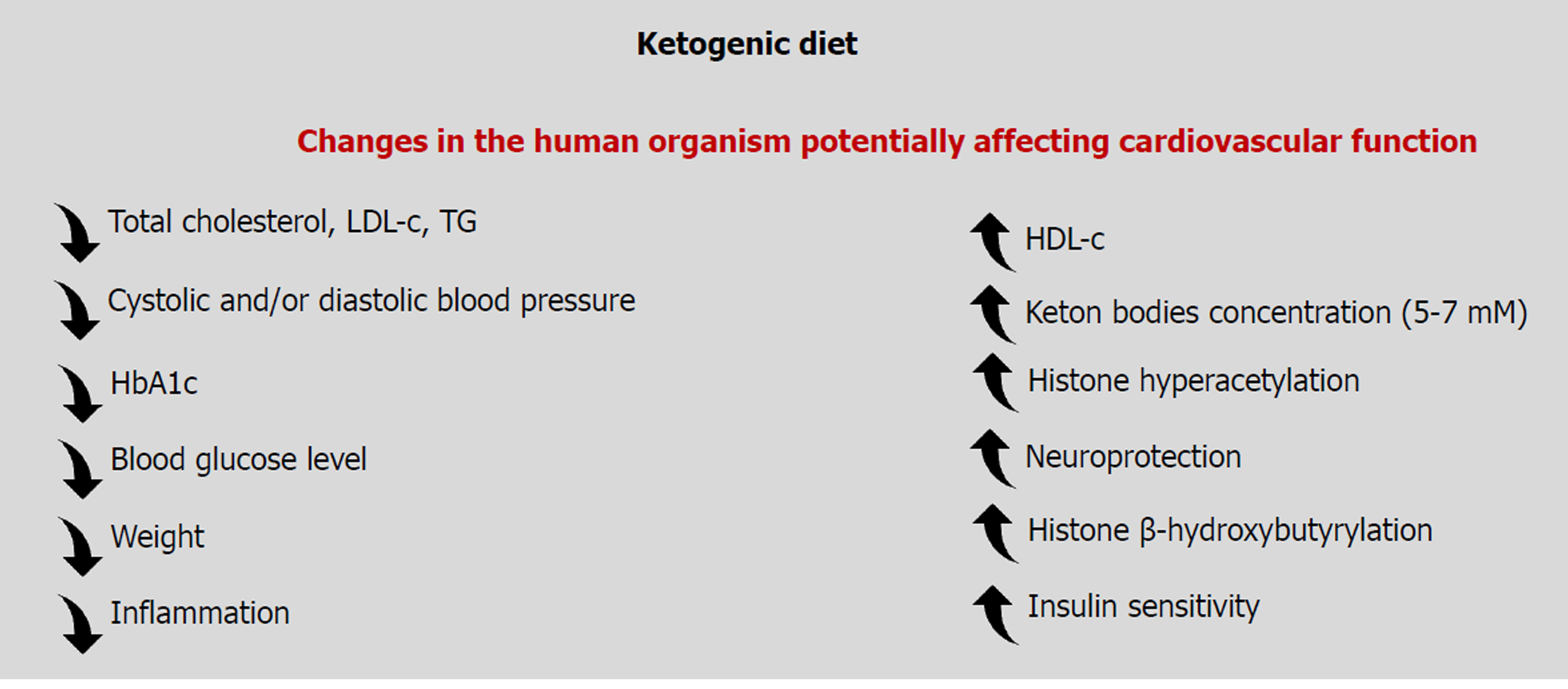
As discussed in this review, the cardiovascular effects of ketone bodies are context-dependent and concentration-dependent (Figure 4). It is clear and undisputed because for decades the high concentrations of ketone bodies attained in diabetic ketoacidosis have severe detrimental vascular effects, worsening the morbidity and mortality of diabetic patients. On the contrary, it has emerged in more recent years that lower concentrations of circulating ketone bodies, following dietary restriction, physical effort or a medically controlled KD can exert beneficial effects on the endothelium and the cardiovascular system. The circulating concentrations of ketone bodies are of primary importance on determining the final physiological effects on the cardiovascular system.
Figure 4 Summary of the physiological changes occurring upon assumption of a ketogenic diet that affects cardiovascular function.
HbA1c: Hemoglobin A1c; HDL-c: High-density lipoprotein cholesterol; LDL-c: Low-density lipoprotein cholesterol; TG: Triglycerides.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ