Published online Nov 15, 2020. doi: 10.4239/wjd.v11.i11.540
Peer-review started: August 13, 2020
First decision: September 16, 2020
Revised: September 29, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: November 15, 2020
Processing time: 91 Days and 12.8 Hours
The sodium/glucose cotransporter-2 inhibitors (SGLT-2i) and glucagon-like-1 receptor agonists (GLP-1RA) are antidiabetic agents effective both in hemoglobin A1c (HbA1c) reduction (with a low risk of hypoglycemia) and cardiovascular event prevention. In patients with type 2 diabetes, the add-on value of combination therapy of GLP-1RA and an SGLT-2i seems promising.
To investigate whether the efficacy of GLP-1RA and SGLT-2i combination observed in randomized controlled trials translates into therapeutic benefits in the Croatian population during routine clinical practice and follow-up.
We included 200 type 2 diabetes patients with poor glycemic control and analyzed the effects of treatment intensification with (1) GLP-1RA on top of SGLT-2i, (2) SGLT-2i on top of GLP-1RA compared to (3) simultaneous addition of both agents. The primary study endpoint was the proportion of participants with HbA1c < 7.0% and/or 5% bodyweight reduction. Secondary outcomes included changes in fasting plasma glucose (FPG), prandial plasma glucose, low-density lipoprotein cholesterol, estimated glomerular filtration rate (eGFR), and cardiovascular (CV) incidents assessment over a follow-up period of 12 mo.
The majority of patients were over 65-years-old, had diabetes duration for more than 10 years. The initial body mass index was 39.41 ± 5.49 kg/m2 and HbA1c 8.32 ± 1.26%. Around half of the patients in all three groups achieved target HbA1c below 7%. A more pronounced decrease in the HbA1c seen with simultaneous SGLT-2i and GLP-1RA therapy was a result of higher baseline HbA1c and not the effect of initiating combination therapy. The number of patients achieving FPG below 7.0 mmol/L was significantly higher in the SGLT-2i group (P = 0.021), and 5% weight loss was dominantly achieved in the simultaneous therapy group (P = 0.044). A composite outcome (reduction of HbA1c below 7% (53 mmol/mol) with 5% weight loss) was achieved in 32.3% of total patients included in the study. Only 18.2% of patients attained composite outcome defined as HbA1c below 7% (53 mmol/mol) with 5% weight loss and low-density lipoprotein cholesterol < 2.5 mmol/L. There were no significant differences between treatment groups. No differences were observed regarding CV incidents or eGFR according to treatment group over a follow-up period.
Combination therapy with GLP-1RA and SGLT-2i is effective in terms of metabolic control, although it remains to be determined whether simultaneous or sequential intensification is better.
Core Tip: Both glucagon-like-1 receptor agonists and sodium/glucose cotransporter-2 inhibitors when added sequentially to the other or simultaneously decrease the hemoglobin A1c (HbA1c) and reduce the body mass index, but the weight loss and glucose-lowering potential is not additive in case of their simultaneous use. Half of the patients achieved target HbA1c below 7%, irrespective of treatment group (sequential or simultaneous addition). Five percent weight loss was dominantly achieved in the simultaneous therapy group. Composite outcome (reduction of HbA1c below 7% with 5% weight loss) was achieved in 32.3%, while 18.2% of total patients attained composite outcome defined as HbA1c below 7% with 5% weight loss and low-density lipoprotein cholesterol < 2.5 mmol/L.
- Citation: Cigrovski Berkovic M, Bilic-Curcic I, Bozek T, Herman Mahecic D, Klobucar Majanovic S, Canecki-Varzic S, Andric J, Marusic S, Mrzljak A. Glucagon-like-1 receptor agonists and sodium/glucose cotransporter-2 inhibitors combination—are we exploiting their full potential in a real life setting? World J Diabetes 2020; 11(11): 540-552
- URL: https://www.wjgnet.com/1948-9358/full/v11/i11/540.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i11.540
Treating type 2 diabetes (T2DM) in the era of new anti-diabetic drugs has not lessened the challenge of choosing the appropriate agents for the specific phase in the disease pathophysiology.
Both glucagon-like-1 receptor agonists (GLP-1RA) and sodium glucose cotransporter-2 inhibitors (SGLT-2i) have recently been shown to reduce cardiovascular (CV) events when used in either primary or secondary prevention in the treatment of patients with T2DM. SGLT-2 inhibitors exert CV protective actions by primarily hemodynamic effects, while GLP-1 RAs mainly act via anti-atherogenic mechanisms. Both drug classes reduce microalbuminuria and slow down the development/progression of end-stage renal disease. In terms of managing overweight or obese T2DM patients with inadequately controlled glycemia, the GLP-1RA/SGLT-2i combination might be particularly useful in achieving both glycemic and body weight targets[1].
The possibility of the additive value of treatment intensification with GLP-1RA in uncontrolled T2DM on SGLT-2i was assessed in five randomized controlled trials (RCTs) (AWARD-10, DUAL IX, DURATION-8, SUSTAIN 9 and PIONEER-4), including altogether 1793 T2DM patients. Combination therapy with the addition of dulaglutide, insulin degludec/liraglutide, exenatide once weekly, and subcutaneous and oral semaglutide or liraglutide, respectively, was more efficacious in lowering hemoglobin A1c (HbA1c), weight loss, lowering systolic blood pressure and decreasing LDL-cholesterol[2-6] compared to sole SGLT-2 inhibition.
Literature also suggests the benefits of the drug combinations in the real-world setting, and the question remains if the sequence in which drugs are introduced is relevant for the management of diabetes and the long-term safety and efficacy results of such treatment modality[7,8].
The use of some glucose-lowering drugs is related to the risk of hypoglycemia and additional weight gain, so adding agents with pleiotropic effects, especially CV benefits, such as GLP-1RA and SGLT-2i seems promising. Findings from the dedicated Cardiovascular Outcome Trials had an effect on the newer guidelines for the management of T2DM recommending the addition of GLP-1RA or SGLT-2i in patients with increased CV risk. In case of heart failure and renal disease, SGLT-2i appears more protective, but otherwise, due to lack of sufficient data from comparative trials, clinicians can choose between the two drug classes in a personalized approach.
Data from RCTs suggest GLP-1RA/SGLT-2i combinations are more effective than SGLT-2i on HbA1c, with additional benefits seen through weight-losing potential and low risk of hypoglycemia and positive effects on systolic blood pressure and LDL-cholesterol, which are the known markers of CV disease[9]. Additionally, data from everyday clinical practice support the benefits of the mentioned combination but still leave the open question of the sequence of drug introduction and long term safety and efficacy of the combination[10].
According to the national health insurance policy, GLP-1 RAs are reimbursed in Croatia when added to individuals with T2DM failing glycemic targets on at least two anti-diabetic agents, whose body mass index (BMI) exceeds 35 kg/m2.
The aim was to investigate whether the efficacy of GLP-1RA and SGLT-2i combination observed in RCTs translates into therapeutic benefits in the Croatian population during routine clinical practice and follow-up. In addition, the efficacy of simultaneous vs sequential initiation approach was also compared. Therefore, we performed the multi-centric electronic medical records data analysis for patients with T2DM treated with the SGLT-2i/GLP-1RA combination in Croatia's everyday clinical practice.
This was a retrospective and prospective multi-centric cohort study involving five tertiary diabetes centers in Croatia. A sample of adult T2DM Caucasian patients with poorly controlled T2DM who were prescribed both SGLT-2i (empagliflozin, dapagliflozin) and GLP-1RA (liraglutide, dulaglutide) between January 2016 and April 2019 was identified from electronic medical records and served as a source population. Mutual patients of the included sites were evaluated to prevent duplicated data. The index visit was the one where either SGLT-2i was prescribed to already present GLP-1RA (1), GLP-1RA was prescribed on top of already present SGLT-2i therapy (2) or both agents were prescribed simultaneously on top of background glucose-lowering agents (3). Included were overall 200 patients for whom demographic data, data on glycemic control (fasting plasma glucose (FPG), prandial plasma glucose (PPG), glycosylated HbA1c, data on renal function [serum urea, creatinine, estimated glomerular filtration rate (eGFR) albuminuria] and lipid profile were known. Patients were excluded if they did not have HbA1c, weight and background therapy documented at the baseline and follow-up appointments, underwent bariatric surgery during or before the study period, were prescribed weight-loss medication (including high-dose liraglutide > 1.8 mg/d), had complex insulin regiments (more than one dose) and/or had documented nonadherence to either GLP-1 RA or SGLT-2i therapy. All patients received treatment of other comorbidities according to standards of care.
The study was approved by the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Patients who adhered to prescribed therapy were followed for up to 1 year.
The primary study endpoint was the proportion of participants with HbA1c < 7.0% and/or 5% bodyweight reduction. Secondary outcomes included changes in FPG, PPG, LDL cholesterol, eGFR and assessment of CV incidents over a period.
Data were analyzed using SPSS software (IBM SPSS Statistics for Windows, Version 24.0., IBM Corp., Armonk, NY, United States). Descriptive statistics were used to describe the baseline characteristics of study subjects (proportions for categorical data and mean ± standard deviation for normally distributed continuous variables or median and interquartile range for variables deviating from a normal distribution). The normality of distribution was verified using Shapiro-Wilk test. One-way analysis of variance was used to calculate differences among the three therapy groups at baseline (in case Levene's test showed a significant deviation in homogeneity of variances, Kruskal-Wallis was used). Also, repeated-measures two-way analysis of covariance for three points of measurements (with Bonferroni correction for multiple comparisons) was used, with the therapy group as an independent factor. In addition, the same analysis was performed after adjustment for age, sex, duration of diabetes and duration of treatment. Chi-square test was used for analyzing differences in outcome variables during a follow-up period.
We included 200 T2DM patients with inadequate glycemic control. The majority of patients were over 60 years of age (63.5%) at the time of inclusion in the study, and over half (50.7%) had diabetes for more than 10 years. CV disease was present in 44 (22.1%) prior to inclusion, and the majority of patients had hypertension (85.5%) and hyperlipidemia (77.4%) with no difference between therapy groups. Initial BMI was 39.41 ± 5.49 kg/m2, HbA1c 8.32 ± 1.26%; additional data are shown in Table 1.
| Mean | SD | Median | Minimum | Maximum | n | |
| Age, yr | 62.12 | 9.49 | 63 | 37 | 86 | 200 |
| Duration of diabetes, yr | 11.67 | 6.18 | 11 | 1 | 43 | 196 |
| HbA1c, % | 8.32 | 1.26 | 8.20 | 5.60 | 13.60 | 200 |
| FPG, mmol/L | 9.82 | 2.88 | 9.60 | 3.40 | 20.30 | 126 |
| PPG, mmol/L | 11.17 | 3.23 | 10.90 | 4.50 | 24.30 | 145 |
| Urea, mmol/L | 6.38 | 1.83 | 6.10 | 2.80 | 11.80 | 89 |
| Creatinine, mmol/L | 73.82 | 20.41 | 71.00 | 29.00 | 163.00 | 159 |
| eGFR, mL/min per 1.73 m2 | 85.88 | 19.27 | 90.00 | 28.00 | 125.00 | 128 |
| Albuminuria, mg/L | 35.52 | 62.49 | 13.60 | 2 | 418 | 62 |
| Cholesterol, mmol/L | 4.89 | 1.21 | 4.80 | 2.60 | 9.50 | 144 |
| HDL, mmol/L | 1.24 | 0.51 | 1.15 | 0.60 | 5.60 | 151 |
| LDL, mmol/L | 2.60 | 0.97 | 2.50 | 0.80 | 5.30 | 146 |
| Triglycerides | 2.41 | 1.38 | 2.00 | 0.70 | 8.60 | 159 |
| BMI | 39.41 | 5.49 | 37.90 | 30.00 | 69.00 | 193 |
At the time of inclusion, patients were divided into three groups according to the background therapy; one group had background SGLT-2i at the time of inclusion and GLP-1RA was added (24% of patients), another group had GLP-1RA as a background therapy (38% of patients) and SGLT-2i was added and the third group lacked both drugs before inclusion and agents (GLP-1RA and SGLT-2i) were added simultaneously (38% of patients).
The groups differed in some parameters at baseline. There was a significant difference in HbA1c between groups of patients who started therapy with SGLT-2i or GLP-1RA alone and simultaneously (P = 0.002), with HbA1c being significantly higher in the latter. In addition, FPG and BMI were higher in the group on simultaneous therapy than the other two groups (P = 0.001, P = 0.045, respectively), while there was no difference in other observed parameters (Table 2).
| Mean | SD | n | F ratio/Kruskal-Wallis | P value | |
| HbA1c, % | |||||
| SGLT-2i | 8.09 | 1.07 | 48 | 6.436 | 0.002 |
| GLP-1RA | 8.07 | 1.08 | 76 | ||
| Simultaneous | 8.72 | 1.45 | 76 | ||
| Total | 8.32 | 1.26 | 200 | ||
| FPG, mmol/L | |||||
| SGLT-2i | 8.75 | 2.17 | 35 | 0.001 | |
| GLP-1RA | 9.44 | 2.43 | 55 | ||
| Simultaneous | 11.43 | 3.45 | 36 | ||
| Total | 11.17 | 3.23 | 145 | ||
| BMI, kg/m2 | |||||
| SGLT-2i | 38.18 | 3.60 | 47 | 0.045 | |
| GLP-1RA | 38.74 | 4.75 | 70 | ||
| Simultaneous | 40.80 | 6.72 | 76 | ||
| Total | 39.41 | 5.49 | 193 |
The average duration of diabetes was over 10 years, and most patients were treated with metformin, however insulin therapy comes into second place (36% of patients), while only around 15% of patients were treated with sulphonylurea and other oral antihyperglycemic drugs (Table 3).
| Therapy group | |||
| SGLT-2i, n (%) | GLP-1RA, n (%) | Simultaneous, n (%) | |
| Insulin | 11 (32.4) | 28 (45.2) | 11 (26.2) |
| Metformin | 32 (94.1) | 53 (85.5) | 33 (78.6) |
| SU | 4 (11.8) | 12 (19.4) | 6 (14.3) |
| Other OAD | 3 (8.8) | 10 (16.1) | 8 (19.0) |
Interestingly, the group treated with GLP-1RA had the highest number of insulin users compared to other therapy groups (P = 0.006). For sulphonylurea and other therapies, differences were not statistically significant (P > 0.05).
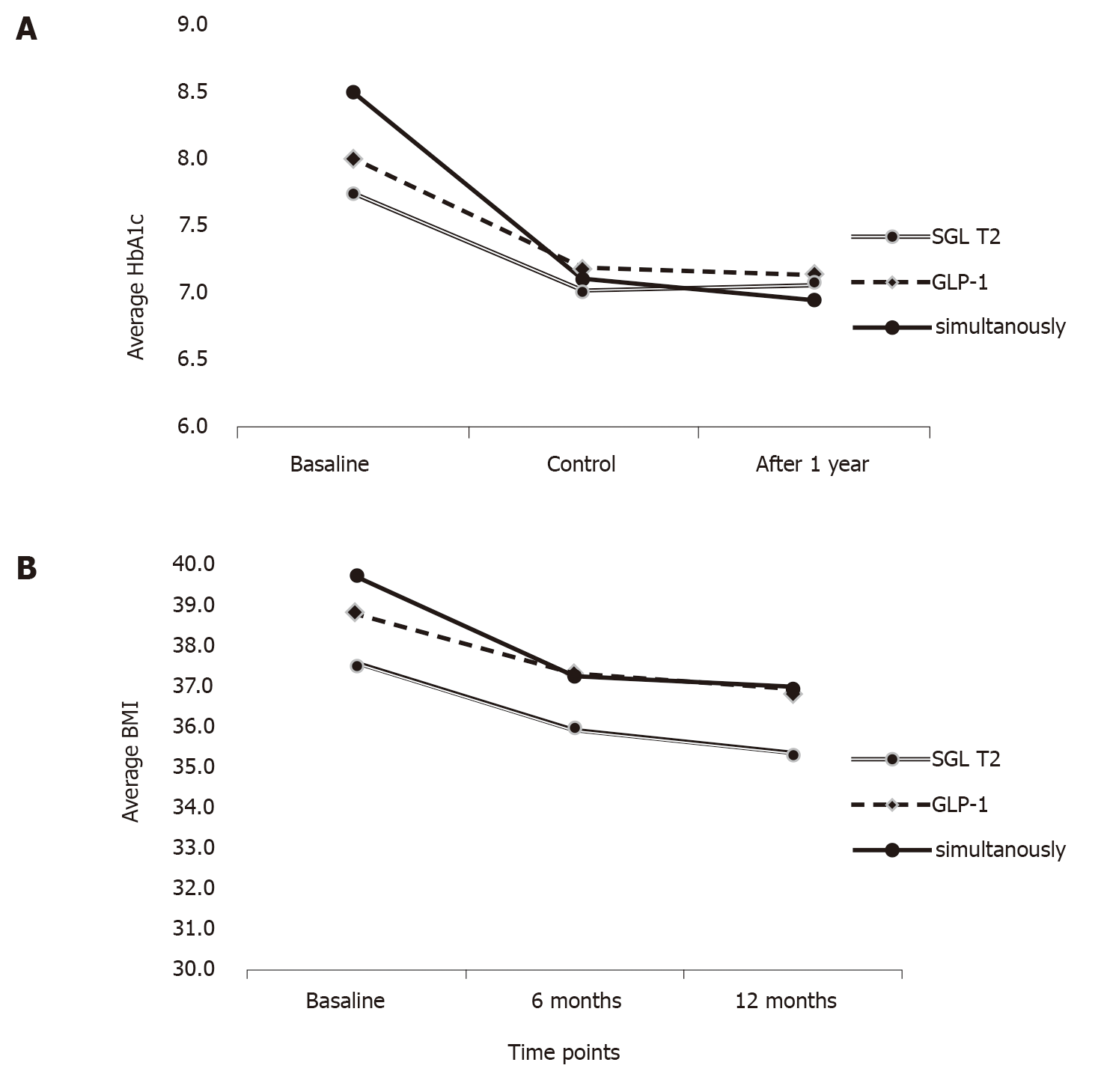
Two-way analysis of variance with repeated measurements was performed in order to establish effect of measurement point, the interaction of therapy and time point and the effect of therapy alone. There were no differences prior and after adjustment for age, sex and duration of diabetes (Table 4). There was a significant reduction of HbA1c over time [F(1.6, 201) = 7.035, P = 0.002], with a significant interaction of therapy and measurement point [F(3.3, 201) = 4.389, P = 0.004], but the effect of specific therapy was not significant [F(2, 120) = 0.432, P = 0.650]. The post hoc analysis demonstrated that more pronounced decrease in the group treated with simultaneous therapy was a result of higher baseline values of HbA1c and not the effect of initiating combination therapy (Table 4 and Figure 1).
| Parameter | Therapy | Baseline | 6 mo | 1 yr | Two-way repeated measures ANCOVA | F value | P value | |||
| Mean | SD | Mean | SD | Mean | SD | |||||
| HbA1c | SGLT-2i | 7.74 | 0.78 | 7.03 | 0.72 | 7.09 | 0.83 | Measurement point effect | 7.035 | 0.002 |
| GLP-1RA | 8.03 | 1.10 | 7.22 | 1.00 | 7.16 | 0.85 | Therapy and measurement point | 4.389 | 0.004 | |
| Simultaneous | 8.49 | 1.34 | 7.11 | 0.96 | 6.96 | 0.85 | Therapy effect | 0.432 | 0.650 | |
| FPG | SGLT-2i | 7.98 | 1.40 | 7.11 | 2.41 | 6.84 | 1.26 | Measurement point effect | 2.647 | 0.091 |
| GLP-1RA | 9.90 | 2.33 | 7.48 | 1.67 | 7.99 | 1.68 | Therapy and measurement point | 4.333 | 0.007 | |
| simultaneous | 10.86 | 3.53 | 7.53 | 1.64 | 7.33 | 1.31 | Therapy effect | 4.227 | 0.020 | |
| BMI | SGLT-2i | 37.54 | 3.18 | 35.92 | 3.01 | 35.32 | 3.05 | Measurement point effect | 4.867 | 0.013 |
| GLP-1RA | 38.76 | 4.60 | 37.27 | 4.44 | 36.80 | 4.43 | Therapy and measurement point | 2.123 | 0.092 | |
| Simultaneous | 39.66 | 5.59 | 37.22 | 5.41 | 36.94 | 5.42 | Therapy effect | 1.473 | 0.234 | |
In addition, measurement point effect on FPG values was not significant [F(1.5, 85.4) = 2.647, P = 0.091], but there was a significant interaction of therapy and measurement point [F(3.0, 85.4) = 4.333, P = 0.007] and therapy effect [F(2, 56) = 4.227, P = 0.020]. Group treated with SGLT-2i had significantly lower FPG values compared to GLP-1 RA (P = 0.029) as well as compared to simultaneous therapy (P = 0.024). Interactions indicate that the decrease was not equal among groups, which is expected given the initial differences in FPG being lowest in SGLT-2i group (Table 5).
| SGLT-2i | GLP-1RA | Simultaneous | Chi-square | P value | |
| HbA1c | |||||
| < 7% | 51.7 | 47.9 | 47.4 | 0.156 | 0.925 |
| > 7% | 48.3 | 52.1 | 52.6 | ||
| Total | 100.0 | 100.0 | 100.0 | ||
| FPG | |||||
| < 7 mmol | 70.6 | 30.0 | 54.5 | 7.748 | 0.021 |
| > 7 mmol | 29.4 | 70.0 | 45.5 | ||
| Total | 100.0 | 100.0 | 100.0 | ||
| PPG | |||||
| < 10 mmol | 90.0 | 84.4 | 72.7 | 2.775 | 0.250 |
| > 10 mmol | 10.0 | 15.6 | 27.3 | ||
| Total | 100.0 | 100.0 | 100.0 | ||
| eGFR | |||||
| > 60 mL/min per 1.73 m2 | 90.9 | 80.8 | 86.0 | 1.008 | 0.604 |
| < 60 mL/min per 1.73 m2 | 9.1 | 19.2 | 14.0 | ||
| Total | 100.0 | 100.0 | 100.0 | ||
| LDL | |||||
| < 1.8 mmol | 16.7 | 21.6 | 29.5 | 1.571 | 0.456 |
| > 1.8 mmol | 83.3 | 78.4 | 70.5 | ||
| Total | 100.0 | 100.0 | 100.0 | ||
| LDL | |||||
| < 2.5 mmol | 66.7 | 54.1 | 52.3 | 1.413 | 0.493 |
| > 2.5 mmol | 33.3 | 45.9 | 47.7 | ||
| Total | 100.0 | 100.0 | |||
| Weight loss > 5% | |||||
| No | 58.6 | 52.3 | 33.3 | 6.232 | 0.044 |
| Yes | 41.4 | 47.7 | 66.7 | ||
| Total | 100.0 | 100.0 | 100.0 |
On the other hand, a significant main effect of BMI reduction over time [F(1.6, 188.1) = 4.867, P = 0.013] was observed, however there was no interaction of therapy and measurement point [F(3.3, 188.1) = 2.123, P = 0.092] nor a significant effect of therapeutic group [F(2, 113) = 1.473, P = 0.234] (Table 4 and Figure 1). No significant differences were observed for other parameters (data not shown).
There were no differences in CV incidents or eGFR during a follow-up period compared to baseline or with respect to specific treatment groups. Only three patients (2.2%) had a CV incident during a follow-up period of 12 mo.
Around half of the patients in all three groups achieved target HbA1c below 7% with no differences between groups, while the number of patients achieving FPG below 7.0 mmol/L was significantly higher in the SGLT-2i group (P = 0.021), and 5% weight loss was dominantly achieved in the simultaneous therapy group (P = 0.044) (Table 5).
A composite outcome [reduction of HbA1c below 7% (53 mmol/mol) with 5% weight loss] was achieved in 32.3% of total patients included in the study. In addition, only 18.2% of patients attained composite outcome defined as HbA1c below 7% (53 mmol/mol) with 5% weight loss and LDL cholesterol < 2.5 mmol/L. There were no significant differences between treatment groups (Table 6).
| n | (%) | ||
| HbA1c < 7% and 5% weight loss | No | 88 | (67.7) |
| Yes | 42 | (32.3) | |
| Total | 130 | (100.0) | |
| HbA1c < 7%, 5% weight loss and LDL < 2.5 mmol/L | No | 72 | (81.8) |
| Yes | 16 | (18.2) | |
| Total | 88 | (100.0) | |
Current guidelines recommend assessing and regularly pursuing HbA1c goal on an individual basis (for the majority of individuals < 7%). In everyday clinical practice, this mostly transcribes to the need to add other anti-diabetic agents to metformin in the background while taking into account compatibility of drug actions, stage in the disease pathophysiology and patients' comorbidities, complications as well as preferences.
Patients who started simultaneous therapy had worse glycemic control and higher FPG and BMI values than the other two groups, emphasizing the problem of clinical inertia. According to some studies, it takes several years on average instead of the recommended 6 mo before initiating another drug, either oral or injectable, in order to improve glycemia[11,12]. Obviously, in a real-world setting, we hesitate to use a more aggressive approach while waiting for patients to reach worse control of diabetes. The reasons could be numerous, for example; patient-related since not all of the patients are interested in the initiation of multiple drugs at the same time; physician-related as a result of lack of time or even fear of potential side effects; and finally health system-related, mainly due to reimbursement policy[13]. Later is specific for Croatia since GLP-1RAs until a couple of weeks ago were reimbursed in patients with BMI above 35 kg/m2 and after the failure of two anti-diabetic agents, while SGLT-2i prescription was possible in case of BMI above 27 kg/m2 with obligatory co-payment.
There was a statistical significance in the reduction of HbA1c in all three groups, but no difference was achieved in the reduction depending on the therapy group, i.e. the largest decrease in HbA1c was in the simultaneous group, but this was expected given the highest HbA1c at baseline. This is in line with the results of published studies so far, showing a lack of expected additive effect of GLP-1RA and SGLT-2i[2], which could be explained by the opposing actions of SGLT-2i and GLP-1RA on endogenous glucose production[14]. However, a recently published meta-analysis[10,15] supports quite the opposite; the combination of those two drug classes does indeed improve glycemic control compared to each agent alone. Our study concluded that the simultaneous approach was not more effective than a sequential approach.
Nevertheless, in real-life clinical settings, conditions are somewhat different. For example, an observational study in older adults demonstrated a significant reduction of HbA1c and body weight, the highest being in patients who started both drugs simultaneously. Nonetheless, in the milieu of our health system, this can be a reflection of reimbursement policy, since SGLT-2 inhibitors are initiated earlier, given the light restrictions compared to those for GLP-1RAs. When the criteria are met, we add GLP-1RA. On the other hand, in patients who meet the criteria, we initiate GLP-1RA first and wait with SGLT-2i initiation considering they are not fully reimbursed, and co-payment is necessary. A successful concept known as a step-down approach or early initiation of two agents affecting different but complementary pathophysiological mechanisms was demonstrated in the VERIFY study, where concomitant administration of metformin and dipeptidyl peptidase 4 inhibitors in newly diagnosed patients delayed the progression of diabetes relative to sequential drug addition[16,17]. Our patients had long-standing diabetes, and perhaps the greatest benefits could be expected in patients who are newly diagnosed or in the earlier stages of the disease.
In addition, a group in which treatment was started with SGLT-2i had a statistically significantly greater reduction in FPG, which is understandable given that in those subjects, FPG was initially lower compared to the other two groups. However, there was no significant effect of time in all three groups, meaning that the change occurred in the first few months, with no change occurring during 1 year. The explanation for this finding is also a consequence related to our health care system because SGLT-2i can be prescribed immediately after metformin, in the earlier stage of the disease when FPG values are lower compared to the later stage of the disease.
In addition to glycemic control, an important treatment outcome is a loss of body weight. In all three patient groups, a significant reduction in BMI was achieved, but without the effect of a specific treatment group, thus the simultaneous approach did not impose a more substantial effect on body weight compared to sequential addition supporting findings from a recently published meta-analysis[15]. However, a significant reduction of body weight, defined as a loss of 5% or more of initial body weight, was predominantly achieved in the simultaneous therapy group, which is in agreement with RCTs findings[3,18-20].
Still, the greatest value of these drug classes or their combination is the effect on the cardiometabolic outcomes of diabetic patients demonstrated in Cardiovascular Outcome Trials[20-25]. The complementary mechanism of action makes them the perfect pair, while SGLT-2i primarily act through hemodynamic effects on heart failure, GLP-1RA has an effect on atherosclerotic disease[26]. No difference was observed in our patients in the incidence of CV events, whether it was three points mild autonomous cortisol excess or heart failure, which may be a reflection of short follow-up time or a small number of events. Again, it must be emphasized that one of the main criteria for prescribing these drugs should be the presence of CVD as required by the guidelines, which has only recently been implemented in the health care system of our country, otherwise we are too late in terms of metabolic control and prevention of CV incidents.
Also, no differences in eGFR were noted, which was expected given that SGLT-2i are still indicated in patients with eGFR over 60 mL/min; thus, all of our patients had normal kidney function. Combination therapy in this subset of patients could primarily affect the onset or progression of micro/macroalbuminuria due to the mechanism of action of GLP 1RA and SGLT-2i[27]; however, only a small number of patients had available data on albuminuria, which prevented adequate analysis.
A particularly disturbing fact is the number of patients (32.3%) who achieved a composite outcome defined as HbA1c < 7% and 5% weight loss, which deviates significantly from the results of available RCTs promising more favorable results[3,18,19,23] substantiated by recently published meta-analysis[10,15]. However, this is in line with a recently published observational study in older patients receiving combination therapy where 34.3% achieved the combined endpoint of A1C levels < 7% and weight loss ≥ 5% without hypoglycemia[28]. This discrepancy between RCTs results compared to the results of observational studies in real-world settings has long been recognized. Seemingly, the main culprit is the lack of patients’ adherence, especially those treated with the GLP-1RA class related to gastrointestinal side effects[29]. The same problem could be present with SGLT-2i and their safety profile in terms of the frequent urinary tract and genital infections. Obviously, there are several reasons for such poor results of GLP-1RA and SGLT-2i combination drug treatment, which presently have an advantage as second-line therapy[9] and possibly as a first-line in patients with established CV disease[30], but we cannot escape the impression that this is mostly the result of our health insurance reimbursement policy.
Moreover, only 18.2% of patients attained composite outcomes defined as HbA1c below 7% with 5% weight loss and less stringent target of LDL cholesterol < 2.5 mmol/L, highlighting a significant problem of nonadherence and sub dosage of statin therapy[31,32].
The main limitation of this study is the non-interventional observational design and disposal of routine data from everyday clinical settings. Information was collected from electronic medical registries; therefore, hypoglycemic incidents, changes in blood pressure and adverse effects of drugs were underreported, and data were insufficient for us to perform the analysis. In addition, it is possible that some clinical events, as well as clinical data, were omitted, which could influence the interpretation of results. In order to establish CV benefits, longer follow-up studies are needed with a larger sample size.
Thus, we can conclude that the combination of those two drug classes has positive effects in terms of metabolic control, although it is not clear whether simultaneous or sequential intensification makes a difference. Simultaneous use was mainly reserved for patients with the highest degree of dysglycemia. SGLT-2 inhibitors were used more commonly early in the disease course when FPG was still in range or only slightly elevated. In these obese, poorly regulated patients with long-standing diabetes, both options seem to be equally effective, although the real question is whether the results would have been different if we had the opportunity to start the simultaneous therapy earlier. It was also not possible to assess the most significant benefit of this combination and its effects on CV complications and kidney function. The next step would be to monitor patients treated with this particular combination in earlier stages of the disease and longer. According to a new reimbursement policy, we are presently able to initiate GLP-1RA in patients with a BMI over 30 kg/m2 or 28 kg/m2 if they have proven CV disease after failure of dual oral therapy and SGLT-2i irrespective of patient's BMI, thus enabling us to establish the actual value of GLP-1RA and SGLT-2i combination treatment.
Currently, not much is known on the efficacy, safety and potential cardiometabolic benefits of combination therapy, including sodium/glucose cotransporter-2 inhibitors (SGLT-2i) and glucagon-like-1 receptor agonists (GLP-1RA), in the treatment of patients with type 2 diabetes (T2DM) with poorly controlled glycemia. Separately, both drug classes have proven cardiovascular (CV) benefits, mainly in secondary and some also in primary prevention. Moreover, both drug classes improve glycemic control by not enhancing the risk of hypoglycemia and having additional beneficial effects on body weight, making them a preferred option for treating obese/overweight patients with T2DM.
Data on using combination therapy are still modest. The analyses of results coming from randomized controlled trials (RCTs) suggest improvements of glycemic control as well as in CV surrogate markers when GLP-1RAs are added on top of SGLT-2i. Interestingly, glycemic improvements when both drugs are given simultaneously might not be additive. Moreover, data from real-life clinical practice with mentioned drug classes not always follow the results of RCTs. The question of the right timing for the mentioned agents in the course of the disease and if their effect is durable still remains open.
The aim was to investigate whether the efficacy of GLP-1RA and SGLT2i combination observed in randomized controlled trials translates into therapeutic benefits during routine clinical practice and 12 mo follow-up. In addition, the efficacy of simultaneous vs sequential initiation approach was also compared. The primary study endpoint was the proportion of participants with hemoglobin A1c (HbA1c) < 7.0% and/or 5% bodyweight reduction. Secondary outcomes included changes in fasting plasma glucose (FPG), prandial plasma glucose (PPG), low-density lipoprotein cholesterol (LDL) cholesterol, estimated glomerular filtration rate (eGFR) and CV incidents assessment over a follow-up period.
This retrospective and prospective multi-centric cohort study included 200 T2DM Caucasian patients with long-standing, poorly controlled T2DM prescribed both SGLT-2i (empagliflozin, dapagliflozin) and GLP-1RA (liraglutide, dulaglutide) between January 2016 and April 2019. Patients were identified from electronic medical records and served as a source population. The index visit was the one where either SGLT-2i was prescribed to already present GLP-1RA (1), GLP-1RA was prescribed on top of already present SGLT-2i therapy (2), or both agents were prescribed simultaneously on top of background glucose-lowering agents (3).
Patients who started simultaneous therapy had poorer glycemic control, higher FPG and body mass index (BMI) values than the other two groups. There was a statistical significance in the reduction of HbA1c in all three groups. However, no difference was achieved in the reduction depending on the specific therapy group, meaning that simultaneous or sequential intensification does not make a difference in this population of patients. A significant reduction of body weight, defined as a loss of 5% or more, was predominantly achieved in the simultaneous therapy group. No difference was observed in our patients in the incidence of CV events, whether it was three points mild autonomous cortisol excess or heart failure, which may be a reflection of short follow-up time or a small number of events. No differences in eGFR were noted, which was expected given that SGLT-2i are still indicated in patients with eGFR over 60 mL/min; thus, all our patients had normal kidney function. Only small proportion of patients (32.3%) achieved composite outcomes defined as HbA1c < 7% and 5% weight loss, which deviates significantly from the results of available RCTs promising more favorable results, which emphasizes the problem of clinical inertia and poor adherence, especially related to side effects. However, these results could be mainly a reflection of Croatian health reimbursement policy since GLP-1RAs were reimbursed in patients with BMI above 35 kg/m2 and after the failure of two anti-diabetic agents, while SGLT-2i prescription was possible in case of BMI above 27 kg/m2 with obligatory co-payment preventing physicians from initiating therapy in the earlier course of the disease. Only 18.2% of patients attained composite outcome defined as HbA1c below 7% with 5% weight loss and less stringent target of LDL cholesterol < 2.5 mmol/L highlighting a significant problem of nonadherence and sub dosage of statin therapy.
Simultaneous use of both drugs in everyday clinical practice is mainly reserved for patients with the highest degree of dysglycemia (including HbA1c, FPG, and PPG). SGLT-2i are commonly used early in the disease course when FPG is still in range or only slightly elevated. GLP-1RA and SGLT-2i, when added sequentially to the other or simultaneously, decrease the HbA1c and reduce the BMI, but weight loss and glucose-lowering potential are not additive in case of their simultaneous use. In terms of metabolic control, the combination of these two drugs has positive effects, although it is not clear whether simultaneous or sequential intensification makes a difference. In these obese, poorly regulated patients with long-standing diabetes, both options seem to be equally effective, although the real question is whether the results would have been different if we had the opportunity to start the therapy earlier. It was also not possible to assess the most significant benefit of this combination and its effects on CV complications and kidney function.
The next step would be to monitor patients treated with this particular combination in earlier stages of the disease and through a longer period. According to a new reimbursement policy, we are presently able to initiate GLP-1RA in patients with a BMI over 30 kg/m2 or 28 kg/m2 if they have proven CV disease after failure of dual oral therapy and SGLT-2i irrespective of patient's BMI, thus enabling us to establish the actual value of GLP-1RA and SGLT-2i combination treatment.
| 1. | DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 2. | Frías JP, Guja C, Hardy E, Ahmed A, Dong F, Öhman P, Jabbour SA. Exenatide once weekly plus dapagliflozin once daily vs exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 wk, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 312] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 3. | Ludvik B, Frías JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, García-Pérez LE, Woodward DB, Milicevic Z. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 4. | Philis-Tsimikas A, Billings LK, Busch R, Portillo CM, Sahay R, Halladin N, Eggert S, Begtrup K, Harris S. Superior efficacy of insulin degludec/Liraglutide vs insulin glargine U100 as add-on to sodium-glucose co-transporter-2 inhibitor therapy: A randomized clinical trial in people with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ; PIONEER 4 investigators. Oral semaglutide vs subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 404] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 6. | Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, Thrasher J, Woo V, Philis-Tsimikas A. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (1)] |
| 7. | Scheen AJ. SGLT2 inhibitor or GLP-1 receptor agonist in type 2 diabetes? Lancet Diabetes Endocrinol. 2019;7:818-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Castellana M, Cignarelli A, Brescia F, Perrini S, Natalicchio A, Laviola L, Giorgino F. Efficacy and safety of GLP-1 receptor agonists as add-on to SGLT2 inhibitors in type 2 diabetes mellitus: A meta-analysis. Sci Rep. 2019;9:19351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 10. | Guo M, Gu J, Teng F, Chen J, Ma X, Chen Q, Pu Y, Jiang Z, Long Y, Xu Y. The efficacy and safety of combinations of SGLT2 inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes or obese adults: a systematic review and meta-analysis. Endocrine. 2020;67:294-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 11. | Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411-3417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 12. | Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 13. | Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017;43:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 14. | Ali AM, Martinez R, Al-Jobori H, Adams J, Triplitt C, DeFronzo R, Cersosimo E, Abdul-Ghani M. Combination Therapy With Canagliflozin Plus Liraglutide Exerts Additive Effect on Weight Loss, but Not on HbA1c, in Patients With Type 2 Diabetes. Diabetes Care. 2020;43:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Mantsiou C, Karagiannis T, Kakotrichi P, Malandris K, Avgerinos I, Liakos A, Tsapas A, Bekiari E. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2020;22:1857-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Cersosimo E, Johnson EL, Chovanes C, Skolnik N. Initiating therapy in patients newly diagnosed with type 2 diabetes: Combination therapy vs a stepwise approach. Diabetes Obes Metab. 2018;20:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Matthews DR, Paldánius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S; VERIFY study group. Glycaemic durability of an early combination therapy with vildagliptin and metformin vs sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019;394:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 18. | Jabbour SA, Frías JP, Hardy E, Ahmed A, Wang H, Öhman P, Guja C. Safety and Efficacy of Exenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients With Type 2 Diabetes Inadequately Controlled With Metformin Monotherapy: 52-Week Results of the DURATION-8 Randomized Controlled Trial. Diabetes Care. 2018;41:2136-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Blonde L, Belousova L, Fainberg U, Garcia-Hernandez PA, Jain SM, Kaltoft MS, Mosenzon O, Nafach J, Palle MS, Rea R. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22:929-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 5297] [Article Influence: 529.7] [Reference Citation Analysis (0)] |
| 21. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5185] [Cited by in RCA: 4533] [Article Influence: 453.3] [Reference Citation Analysis (1)] |
| 22. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1921] [Cited by in RCA: 2037] [Article Influence: 291.0] [Reference Citation Analysis (1)] |
| 23. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8737] [Article Influence: 794.3] [Reference Citation Analysis (2)] |
| 24. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5664] [Article Influence: 629.3] [Reference Citation Analysis (0)] |
| 25. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4530] [Article Influence: 647.1] [Reference Citation Analysis (0)] |
| 26. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019;139:2022-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 577] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 27. | Ninčević V, Omanović Kolarić T, Roguljić H, Kizivat T, Smolić M, Bilić Ćurčić I. Renal Benefits of SGLT 2 Inhibitors and GLP-1 Receptor Agonists: Evidence Supporting a Paradigm Shift in the Medical Management of Type 2 Diabetes. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Carretero Gómez J, Arévalo Lorido JC, Gómez Huelgas R, García de Lucas D, Mateos Polo L, Varela Aguilar JM, Seguí Ripoll JM, Ena J; Diabetes; Obesity; and Nutrition Spanish Working Group. Combination Therapy With Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors in Older Patients With Type 2 Diabetes: A Real-World Evidence Study. Can J Diabetes. 2019;43:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Edelman SV, Polonsky WH. Type 2 Diabetes in the Real World: The Elusive Nature of Glycemic Control. Diabetes Care. 2017;40:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2772] [Article Influence: 554.4] [Reference Citation Analysis (0)] |
| 31. | Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 32. | Gislason GH, Rasmussen JN, Abildstrøm SZ, Gadsbøll N, Buch P, Friberg J, Rasmussen S, Køber L, Stender S, Madsen M, Torp-Pedersen C. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang LL S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LYT