Published online Nov 15, 2020. doi: 10.4239/wjd.v11.i11.489
Peer-review started: July 27, 2020
First decision: August 9, 2020
Revised: August 21, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 15, 2020
Processing time: 108 Days and 20.1 Hours
Time in range (TIR), as a novel metric for glycemic control, has robust relevance with diabetic complications. Diabetic peripheral neuropathy (DPN) is characterized by sudomotor dysfunction.
To explore the relationship between TIR obtained from continuous glucose monitoring (CGM) and sudomotor function detected by SUDOSCAN in subjects with type 2 diabetes.
The research enrolled 466 inpatients with type 2 diabetes. All subjects underwent 3-d CGM and SUDOSCAN. SUDOSCAN was assessed with electrochemical skin conductance in hands (HESC) and feet (FESC). Average feet ESC < 60 µS was defined as sudomotor dysfunction (+), otherwise it was sudomotor dysfunction (-). TIR refers to the percentage of time when blood glucose is between 3.9-10 mmol/L during 1 d period.
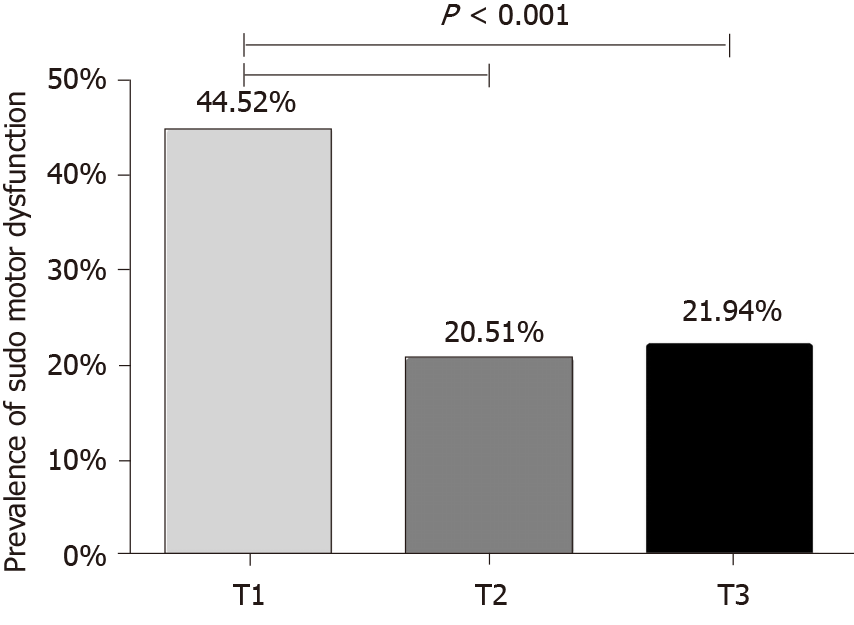
Among the enrolled subjects, 135 (28.97%) presented with sudomotor dysfunction. Patients with sudomotor dysfunction (+) showed a decreased level of TIR (P < 0.001). Compared to the lowest tertile of TIR, the middle and the highest tertiles of TIR was associated with an obviously lower prevalence of sudomotor dysfunction (20.51% and 21.94% vs 44.52%) (P < 0.001). In addition, with the increase of TIR, HESC and FESC increased (P < 0.001). Regression analysis demonstrated that TIR was inversely and independently linked with the prevalence of sudomotor dysfunction after adjusting for confounding values (odds ratio = 0.979, 95%CI: 0.971-0.987, P < 0.001).
The tight glycemic control assessed by TIR is of vitally protective value for sudomotor dysfunction in type 2 diabetes mellitus.
Core Tip: Diabetic peripheral neuropathy (DPN) has posed a serious threat for the economy and development of society. SUDOSCAN is an emerging technique for the detection of DPN through detecting sudomotor function of the sweat gland. Glycemic control is an independent contributor to DPN. Time in range (TIR), as a continuous glucose monitoring-derived pivotal and emerging metric, has been proved to assess short-lived glycemic control. We preliminarily explored the relationship between TIR and sudomotor function, in order to provide a basis for future large-sample, multi-center research.
- Citation: Guo QY, Lu B, Guo ZH, Feng ZQ, Yuan YY, Jin XG, Zang P, Gu P, Shao JQ. Continuous glucose monitoring defined time-in-range is associated with sudomotor dysfunction in type 2 diabetes. World J Diabetes 2020; 11(11): 489-500
- URL: https://www.wjgnet.com/1948-9358/full/v11/i11/489.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i11.489
Diabetic peripheral neuropathy (DPN), as a kind of diabetes-related complications, has posed a serious threat for the economy and development of society. Previous research showed that up to 50% of diabetic individuals were affected DPN[1]. And it is a significant risk element for diabetic foot, so it can greatly increase the mortality and disability of subjects with type 2 diabetes mellitus (T2DM)[2]. DPN often affects distal diminutive nerve fibers and manifests as aching neuropathy, however, upon most occasions, symptoms of DPN are insidious[3]. At present, the gold standard of small fiber assessment detecting DPN is skin biopsy, but it is invasive, which impedes it as a widely used method to screen DPN in a wide range[4]. SUDOSCAN (Impeto Medical, Paris, France) is an emerging technique for the detection of DPN by detecting sudomotor function of the sweat gland[5]. The measurement of SUDOSCAN includes electrochemical skin conductance in hands (HESC, measured in μS) and feet (FESC), and symmetry ratio in hands (HASYM) and feet (FASYM)[6]. Sudomotor dysfunction, derived by SUDOSCAN, could detect DPN in the inchoate stage. Compared with other detection methods, this detection is more sensitive, hence, more and more doctors tend to use it for the inchoate detection of DPN[7].
The values linked with DPN are also relevant to sudomotor dysfunction[8]. The exact pathogenesis of DPN is not fully clarified. The individuals who have these risk factors like a long diabetic duration, high blood pressure, high blood glucose, lipid metabolic disorders, and smoking are prone to have sudomotor dysfunction or DPN[4,8]. Among them, the link between persistent hyperglycemia, determined by hemoglobin A1c (HbA1c), with the progression of DPN has been established. What’s more, in T2DM patients with well-controlled HbA1c, the researchers identified that glycemic variability evaluated by mean amplitude of glucose excursions (MAGE) was an independent contributor to DPN[9].
Time in range (TIR), as a CGM-derived pivotal and emerging metric, has been proved to assess short-lived glycemic control. TIR not only provides more comprehensive and sensitive results, but is also unsusceptible to clinical conditions like anemia and uremia[10]. A lower level of TIR had an adverse effect in patients who were diagnosed with diabetes mellitus with diabetic microvascular complications, including microalbuminuria and retinopathy[11]. Our previous study also documented that patients with combined diabetic cardiovascular autonomic neuropathy had a lower level of TIR. TIR was reversely associated with the presence of diabetic cardiovascular autonomic neuropathy independent of HbA1c in Chinese T2DM patients[12]. However, the association between TIR and sudomotor dysfunction has not been explored clearly yet.
In this study, we aimed to explore the correlation between TIR calculated by continuous glucose monitoring (CGM) and sudomotor dysfunction detected by SUDOSCAN in Chinese subjects with (T2DM).
This study included 466 inpatients with T2DM diagnosed according to the 1999 WHO diagnostic criteria for diabetes[13], all of whom were hospitalized at the endocrinology department of Jinling Hospital, Nanjing University from October 2017 to May 2019. The exclusion criteria included: (1) Individuals with severe illness or acute stress such as heart failure, liver failure, acute or chronic inflammatory disorders, malignant diseases, and surgery; (2) Subjects who had a history of using oral medications that may affect the nervous system and a recent history of alcoholism; and (3) Subjects with other metabolic disorders like the lack of vitamin B12. The study was approved by the local ethics committee.
Medical information including age, gender, diabetes duration, height, weight, and smoking was collected through the medical records system. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured, and body mass index (BMI) was calculated. Blood samples were collected after overnight fasting for measuring hemoglobin A1C (HbA1c), uric acid (UA), triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), fasting blood glucose (FBG), 2-h postprandial blood glucose (PBG), serum urea nitrogen (BUN), and serum creatinine (SCr). Diabetic nephropathy (DN) was defined on the basis of persistent microalbuminuria detected during at least two examinations with a high urine albumin-to-creatinine ratio (ACR > 30 mg/g). Diabetic retinopathy (DR) was diagnosed when non-proliferative or proliferative retinopathy was observed during fundus examination and stereofundus photography after pupillary dilation by the same ophthalmologist. The treatment for diabetes was recorded from the medical record system.
Seventy-two-hour dynamic blood glucose was monitored with the assistance of the CGMS (Meiqi Corporation). During this period, the nurse must input a minimum of four times of capillary blood glucose measurements per day to check monitoring procedures. In addition, all of the subjects were informed to forbid strenuous activities. All data produced every 3 s were recorded, stored, and downloaded, thus making it possible to calculate the parameters of blood glucose variability. In this work, we applied EasyGV Version 9.0R2 from Oxford University to calculate the indicators of blood glucose variability, including standard deviation (SD), MAGE, means of daily differences (MODD), average daily risk range (ADDR), M value, and TIR. TIR refers to the percentage of time when blood glucose is between 3.9-10 mmol/L during a 1-d period. TBR refers to the percentage of time when blood glucose is below 3.9 mmol/L during a 1-d period. Time above range (TAR) refers to the percentage of time when blood glucose is above 10 mmol/L during a 1-d period[10]. In our study, all subjects underwent 3-d CGM, and we used the average time percentage of blood glucose within the target range over a 3-d period as the final value of TIR, TBR, or TAR.
SUDOSCAN (Impeto Medical, Paris, France), as a quick, non-intrusive, and quantitative device, was applied to detect DPN on the basis of the sudomotor function of small C fibers. The appliance had two parts of electrodes for hands and feet, both of which are connected to a computer. Patients placed their limbs on the electrodes only about 2 or 3 min and an incremental low voltage (< 4 V) was applied to the electrodes automatically. The measurements of SUDOSCAN including ESC (measured in μS) and symmetry ratio in hands (HASYM) and feet (FASYM). In our study, we applied 60 µS of average FESC as the diagnostic threshold for sudomotor dysfunction on the basis of previous research[5]. Average FESC ≥ 60 µS was regarded as sudomotor dysfunction (-), otherwise sudomotor dysfunction (+). Four hundred and sixty-six patients were classified into 331 diabetic patients without sudomotor dysfunction, and 135 diabetic patients complicated with sudomotor dysfunction. What’s more, the quantitative results are unaffected by room temperature[5].
SPSS 22.0 software was applied for statistical analyses. All data are presented as the mean ± SD, median (upper and lower quartiles), or proportions according to different data types. For continuous variables, Student’s t-test was used to compare the difference between two groups, and one way ANOVA was applied for comparisons among more than two samples. Wilcoxon rank-sum test was applied for abnormal distributions. A Chi-square test was used for categorical variables. Spearman’s rank correlation was carried out to evaluate the association of TBR, TIR, or TAR and SUDOSCAN metrics. And binary logistic regression analysis was applied to explore the link between TIR (as a continuous or categorical variable) and sudomotor dysfunction after adjusting for clinical conditions including age, diabetes duration, sex, BMI, SBP, DBP, smoking, TG, TC, HbA1c, and glycemic variability metrics. A P value < 0.01 was considered statistically significant.
Of the 466 T2DM subjects, 135 (28.97%) presented with sudomotor dysfunction. The mean age of all participants was 54.50 ± 8.76 years, and the median duration of diabetes was 8 (range, 3-13) years. Table 1 presents the baseline information between the sudomotor dysfunction (-) and sudomotor dysfunction (+) groups. Compared with patients with sudomotor dysfunction (-), patients with sudomotor dysfunction (+) had an older age (P = 0.001), a longer duration of diabetes, a bigger possibility of smoking and having diabetic nephropathy (DN) and diabetic retinopathy (P < 0.001), and a higher level of BUN (P < 0.01). However, the percentage of males, SBP, DBP, SCr, UA, TC, TG, HDL, LDL, HbA1c, BMI, FBG, PBG, SD, MODD, ADDR, MAGE, and TBR were comparable between the two groups (P > 0.05). Of note, patients with sudomotor dysfunction (+) showed increased levels of M value and TAR and a decreased level of TIR (P < 0.001).
| Sudomotor dysfunction (-) | Sudomotor dysfunction (+) | χ2/t/z | P value | |
| n | 331 | 135 | - | - |
| Male, n (%) | 234 (70.69) | 92 (68.15) | 0.296 | 0.586 |
| Age (yr) | 53.30 ± 11.91 | 57.45 ± 13.03 | -3.318 | 0.001 |
| Diabetes duration (yr) | 7 (2, 11) | 10 (6, 15) | -4.266 | < 0.001 |
| Smoking, n (%) | 84 (25.38) | 65 (48.15) | 22.86 | < 0.001 |
| SBP (mmHg) | 132.12 ± 15.82 | 135.30 ± 20.02 | -1.646 | 0.101 |
| DBP (mmHg) | 79.46 ± 9.69 | 79.13 ± 10.57 | 0.322 | 0.748 |
| BUN (mmol/L) | 5.40 (4.50, 6.63) | 5.90 (4.80, 7.40) | -2.845 | 0.004 |
| SCr (µmol/L) | 56.00 (48.00, 67.00) | 60.00 (48.00, 79.00) | -1.703 | 0.089 |
| UA (µmol/L) | 328.18 ± 88.92 | 324.04 ± 88.30 | 0.456 | 0.648 |
| TC (mmol/L) | 4.35 (3.77, 5.11) | 4.30 (3.55, 5.31) | -0.567 | 0.571 |
| TG (mmol/L) | 1.51 (1.04, 2.40) | 1.67 (1.12, 2.52) | -0.856 | 0.392 |
| HDL (mmol/L) | 1.03 (0.90, 1.24) | 1.09 (0.91, 1.27) | -1.067 | 0.286 |
| LDL (mmol/L) | 2.56 (2.10, 3.25) | 2.48 (1.82, 3.19) | -1.297 | 0.195 |
| HbA1c (%) | 8.60 ± 2.01 | 8.94 ± 2.28 | -1.564 | 0.118 |
| BMI (kg/m2) | 25.61 ± 3.53 | 25.27 ± 3.82 | 0.899 | 0.369 |
| FBG (mmol/L) | 7.23 ± 2.35 | 7.74 ± 2.71 | -1.770 | 0.079 |
| PBG (mmol/L) | 15.62 ± 3.63 | 15.92 ± 3.68 | -0.710 | 0.478 |
| SD (mmol/L) | 2.25 (1.80, 2.85) | 2.46 (1.89, 3.06) | -2.234 | 0.025 |
| MAGE (mmol/L) | 4.38 (3.47, 5.50) | 4.08 (3.62, 5.56) | -0.168 | 0.866 |
| MODD (mmol/L) | 1.95 (1.40, 2.67) | 2.28 (1.63, 3.08) | -2.562 | 0.010 |
| ADDR (mmol/L) | 21.46 (14.55, 28.02) | 27.37 (15.11, 34.82) | -2.411 | 0.016 |
| M value (mmol/L) | 5.99 (3.01, 11.25) | 11.30 (3.85, 24.67) | -4.096 | < 0.001 |
| TBR (%) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | -1.093 | 0.274 |
| TIR (%) | 72.82 (53.06, 86.79) | 53.12 (28.36, 79.69) | -4.446 | < 0.001 |
| TAR (%) | 26.38 (12.65, 42.97) | 44.73 (19.28, 71.20) | -4.602 | < 0.001 |
| Microvascular complications | ||||
| DN, n (%) | 51 (15.41) | 53 (39.26) | 31.465 | < 0.001 |
| DR, n (%) | 69 (20.85) | 62 (45.93) | 29.846 | < 0.001 |
| Treatment, n (%) | ||||
| No treatment | 27 (8.16) | 7 (5.19) | 7.107 | 0.069 |
| OHA | 104 (31.42) | 29 (21.48) | ||
| Insulin | 44 (13.29) | 20 (14.81) | ||
| OHA and insulin | 156 (47.13) | 79 (58.52) |
In order to perform more in-depth analyses of the association between TIR and sudomotor dysfunction, we further stratified all participants based on tertiles of TIR. Supplementary Table 1 shows the detailed results. Participants with the highest tertile of TIR had a shorter duration of diabetes and lower levels of HbA1c, FBG, PBG, SD, MAGE, MODD, ADDR, and M value (P < 0.01). The three groups were comparable with regard to age, smoking, SBP, DBP, BUN, SCr, UA, TC, TG, HDL, LDL, and BMI. Of note, patients with the highest tertile of TIR had higher HESC, FESC (P < 0.001) and lower HASYM, FASYM (P < 0.01).
As shown in Figure 1, participants were divided into three groups on the basis of tertiles of TIR. Compared with the lowest tertile of TIR (T1 group), the middle tertile of TIR (T2 group) and the highest tertile of TIR (T3 group) were associated with an obviously lower prevalence of sudomotor dysfunction (20.51% and 21.94% vs 44.52%) (P < 0.001).
The association between TBR, TIR, or TAR and indicators of SUDOSCAN were examined by Spearman’s rank correlation analysis. As shown in Table 2. TIR was positively associated with HESC and FESC, but negatively associated with HASYM and FASYM (P < 0.001). TAR was inversely associated with HESC and FESC, and positively associated with HASYM and FASYM (P < 0.001). It should be noted that there was no relationship between TBR and parameters of SUDOSCAN (Table 2).
| TBR | TIR | TAR | ||||
| r | P value | r | P value | r | P value | |
| HESC | -0.032 | 0.497 | 0.195 | < 0.001 | -0.207 | < 0.001 |
| FESC | -0.035 | 0.452 | 0.164 | < 0.001 | -0.164 | < 0.001 |
| HASYM | -0.014 | 0.766 | -0.175 | < 0.001 | 0.183 | < 0.001 |
| FASYM | 0.046 | 0.320 | -0.180 | < 0.001 | 0.169 | < 0.001 |
On binary logistic regression analysis, the results demonstrated that TIR was reversely and independently linked with the prevalence of sudomotor dysfunction after adjusting for confounding factors including age, diabetes duration, sex, BMI, SBP, DBP, smoking, TG, and TC (Model1) [odds ratio (OR) = 0.979, 95% confidence interval (CI): 0.971-0.987, P < 0.001]. After adding HbA1c as a confounding variable (Model2), the association between TIR and sudomotor dysfunction remained (OR = 0.976, 95%CI: 0.967-0.986, P < 0.001), but the association between HbA1c and sudomotor dysfunction did not exist (P > 0.05). When the blood glucose fluctuation parameters were added to confounding factors, the role of TIR was weakened. And there was a robust and independent link between M value and sudomotor dysfunction. On the basis of these data, it could be concluded that a higher level of M value and lower level of TIR were significant independent contributors to sudomotor dysfunction (Table 3).
| OR | 95%CI | P value | ||
| Model 1 | ||||
| TIR | -0.021 | 0.979 | 0.971-0.987 | < 0.001 |
| Age | 0.023 | 1.024 | 1.003-1.045 | 0.027 |
| Smoking | 0.632 | 1.881 | 1.115-3.174 | 0.018 |
| Model 2 | ||||
| TIR | -0.024 | 0.976 | 0.967-0.986 | < 0.001 |
| Age | 0.024 | 1.024 | 1.003-1.045 | 0.027 |
| Smoking | 0.632 | 1.881 | 1.113-3.177 | 0.018 |
| Model 3 | ||||
| TIR | -0.025 | 0.975 | 0.957-0.994 | 0.010 |
| Age | 0.031 | 1.031 | 1.008-1.055 | 0.008 |
| Smoking | 0.717 | 2.047 | 1.146-3.659 | 0.016 |
| M value | 0.093 | 1.097 | 1.040-1.158 | 0.001 |
Further statistics were applied to ascertain the link between TIR (as a categorical variable) and sudomotor dysfunction. The data demonstrated that the highest tertile of TIR was connected with a lower prevalence of sudomotor dysfunction compared with the lowest tertile of TIR in all models. But the connection was weakened after adjusting glycemic variability metrics (Table 4).
| OR | 95%CI | P value | ||
| Model 1 | ||||
| TIR T1 | - | - | - | - |
| TIR T2 | -1.175 | 0.309 | 0.183-0.521 | < 0.001 |
| TIR T3 | -0.961 | 0.383 | 0.225-0.649 | < 0.001 |
| Age | 0.022 | 1.022 | 1.002-1.044 | 0.035 |
| Smoking | 0.600 | 1.823 | 1.085-3.063 | 0.023 |
| Model 2 | ||||
| TIR T1 | - | - | - | - |
| TIR T2 | -1.241 | 0.289 | 0.164-0.509 | < 0.001 |
| TIR T3 | -1.032 | 0.356 | 0.189-0.673 | 0.001 |
| Age | 0.022 | 1.022 | 1.001-1.044 | 0.036 |
| Smoking | 0.608 | 1.837 | 1.092-3.091 | 0.022 |
| Model 3 | ||||
| TIR T1 | - | - | - | - |
| TIR T2 | -0.809 | 0.445 | 0.209-0.948 | 0.036 |
| TIR T3 | -1.141 | 0.320 | 0.123-0.831 | 0.019 |
| Age | 0.029 | 1.030 | 1.007-1.053 | 0.011 |
| Smoking | 0.670 | 1.953 | 1.100-3.468 | 0.022 |
| M value | 0.111 | 1.117 | 1.057-1.180 | < 0.001 |
In conclusion, in our study, we enrolled a relatively large cohort of participants with T2DM who underwent SUDOSCAN testing and 3-d CGM monitoring. We found that TIR was inversely and independently associated with the prevalence of sudomotor dysfunction detected by SUDOSCAN. Patients who suffered from sudomotor dysfunction had a lower level of TIR (P < 0.001). Compared with the lowest tertile of TIR (T1 group), the middle tertile of TIR (T2 group) and the highest tertile of TIR (T3 group) were associated with an obviously lower prevalence of sudomotor dysfunction, which was less than half of that in the T1 group (P < 0.001).
As is known to all, DPN is a vital contributing factor increasing the mortality and disability of individuals with T2DM. Previous research showed that up to 50% of diabetic individuals suffered from DPN[1]. The most common variety of DPN is distal symmetric polyneuropathy (DSPN). As one of the most vital risk factors for diabetic foot, DSPN can greatly increase the mortality and disability of individuals with T2DM[2]. The main manifestation of DSPN at the very early stage is the destruction of small unmyelinated sympathetic C nerve fibers, further resulting impairment of sudomotor function, so it is difficult to detect DSPN at an early stage[3]. On the one hand, conventional screening ways for DPN covered a variety of questionnaires, for example, Neuropathy Disability Score (NDS), Neurological Symptom Score (NSS) and so on[4]. However, not only does it require the active cooperation of patients, but it is also highly subjective. On the other hand, the traditional and common methods to detect small C fiber neuropathies include skin biopsy, quantitative sudomotor axon reflex test (QSART), and neuropad[4]. Although skin biopsy is the most authoritative assessment for small fiber, it is invasive, and also requires good skills of the pathologist[14]. QSART is a recommended method, but it is time-consuming. Also, neuropad is not a quantitative test, and it has a low sensitivity and specificity[4].
Sudomotor function measurement by SUSOSCAN is an emerging method in recent years[15]. The American Association of Clinical Endocrinologists guidelines in 2015 mentioned that SUDOSCAN can be used to detect early neuropathy[16]. SUDOSCAN test can be used in a wide range because it is fast, impersonally quantitative, and uncomplicated, and does not require patients’ preparation or full attention. Hence, more and more clinicians tend to use it for the inchoate diagnosis of DPN. In recent years, several researchers have documented the accuracy and availability of SUDOSCAN for detecting DPN. Yajnik et al[17] compared different DPN assessments and SUDOSCAN, and the results revealed that a lower level of ESC was obviously linked with more severe symptoms on the Michigan Neuropathy Scoring Instrument. Mayaudon et al[18] also documented that a set of data from SUDOSCAN had robust relevance to clinical questionnaires and pain scores. The sensitivity and specificity of SUDOSCAN in the diagnosis of DPN were 78% and 92%, separately[18].
As far as we know, the values linked with DPN are also relevant to sudomotor dysfunction[8]. The individuals who had these risk factors like a long diabetic duration, high blood pressure, high blood glucose, lipid metabolic disorders, and smoking were prone to have combined sudomotor dysfunction or DPN[4,8]. The prevalence and onset of DPN arise from persistent hyperglycemia[8]. HbA1c, as a gold criterion for diabetic control and management, can reflect average blood glucose during the previous 3 mo. Previous studies have documented that hyperglycemia determined by HbA1c is a necessary contributor to the presence of DPN. The Diabetes Control and Complications Trial study with a large-scale sample of type 1 diabetic participants revealed that, compared to the conventional insulin therapy group, the intensively treated group had a lower level of HbA1c (7.4% vs 9.1%), in which an evident risk decline for DPN (64%) was found[19]. Besides, a 6-year follow-up longitudinal trial concluded that intensive glucose management determined by HbA1c played an important role in preventing DPN in T2DM[20]. The Wisconsin epidemiologic study estimated that the prevalence of DPN declined by 20% for an every 2% increase in HbA1c[21]. Shivaprasad et al[8] also concluded that there was a robust link between hyperglycemia and sudomotor dysfunction. Otherwise, some studies reported diverse outcomes. The United Kingdom Prospective Diabetes Study (UKPDS) included more than 3000 type 2 diabetic subjects with a short diabetic duration and found that rigorous HbA1c management did not play an obvious role in DPN[22]. Thus, many researchers stressed that there were other coexisting confounders such as glycemic variability that may affect or in turn effect DPN. In 2014, a small sample study first demonstrated that glycemic variability assessed by MAGE was an independent risk factor for DPN[23]. Another cross-sectional study with 982 type 2 diabetic individuals added evidence for this relevance in 2018[9].
TIR, as a CGM-derived pivotal and emerging metric, has been proved to assess short-lived glycemic control. TIR can reflect the percentage of time when blood glucose is between 3.9-10 mmol/L during a 1-d period. TBR can reflect the percentage of time when blood glucose is below 3.9 mmol/L during a 1-d period. Otherwise, TAR mirrors the percentage of time when blood glucose is above 10 mmol/L during a 1-d period. TIR not only provides a more comprehensive and sensitive profile for blood glucose, but is also unsusceptible to clinical conditions like anemia, uremia, and so on[10]. Recent few studies put forward that patients who had a lower level of TIR were easy to suffer from different diabetic-related complications. Of a sample of more than 3000 subjects combined with T2DM, Lu et al[24] documented that patients in advanced diabetic retinopathy groups had a lower level of TIR. Besides, the prevalence of any stage of diabetic retinopathy declined significantly with the increase of TIR. The authors concluded that TIR had a significant effect on diabetic retinopathy independent of glycemic variability metrics. Beck et al[11] demonstrated that TIR correlated with the progression of retinopathy and microalbuminuria. What’s more, another research documented that TIR correlated with carotid intima-media thickness in T2DM, a predictive factor for cardiovascular events[25]. Finally, our previous study also documented that patients who had combined diabetic cardiovascular autonomic neuropathy had a lower level of TIR. TIR was reversely associated with the presence of diabetic cardiovascular autonomic neuropathy in Chinese T2DM patients[12].
In our work, the SUDOSCAN device was used to detect sudomotor dysfunction. Among 466 T2DM subjects, 135 (28.97%) presented with sudomotor dysfunction. First, like the data in the table, patients who had combined sudomotor dysfunction had more obvious glucose excursions, such as higher M value and TAR, and lower TIR, indicating a relevance between glycemic variability and sudomotor dysfunction. But the two groups were fully comparable with regard to TBR, and the reason may be that all of the subjects in our research were monitored with a CGM system at the inpatient department, therefore a timely intervention could be achieved when the blood glucose was less than 3.9 mmol/L. Next, compared to the lowest tertile of TIR (T1 group), the middle tertile of TIR (T2 group) and the highest tertile of TIR (T3 group) were associated with an obviously lower prevalence of sudomotor dysfunction, which was less than half of that in the T1 group. In addition, TIR was linked with SUDOSCAN indicators like FESC, HESC, HASYM, and FASYM. With the increase of TIR, HESC and FESC increased, HASYM and FASYM declined. Finally, the regression analysis demonstrated that TIR was inversely and independently linked with the prevalence of sudomotor dysfunction after adjusting for confounding factors including age, diabetes duration, sex, BMI, SBP, DBP, smoking, TG, TC, HbA1c, and glycemic variability metrics. Of note, our study found that M value rather than MAGE was an independent contributor to sudomotor dysfunction. Besides, the difference of HbA1c between the sudomotor dysfunction (-) and sudomotor dysfunction (+) groups was not significant and the regression analysis revealed there was no relevance between HbA1c and sudomotor dysfunction. These may because, as a cross-sectional study, we could only get the preliminary correlation between HbA1c level and sudomotor function, so the data from our study may only provide a basis for future longitudinal studies. And in the future study, we will focus on the relationship between the long-term variation of HbA1c and sudomotor function.
The relevant mechanism between glycemic variability and sudomotor dysfunction is not fully clarified, which may involve oxidative stress, inflammatory reaction, and destruction of vascular endothelium[26]. Persistent hyperglycemia had an adaptive effect on endothelial cells, but blood glucose fluctuation could over-activate oxidative stress and cause toxic effects[27]. In addition, Quagliaro et al[28] found that endothelial cells exposed to hyperglycemia can undergo apoptosis too quickly due to the production of reactive oxygen. Blood glucose fluctuation could also upregulate the expression of inflammatory regulatory factors, like interleukin-6 and intercellular adhesion molecule-1, thus aggravating oxidative stress and inflammatory response further[29]. Yang et al[30] also found that glycemic variability could disrupt the structure of both myelin sheath and axons.
Our study had some major limitations. First of all, all subjects in this study underwent 3 d of CGM, therefore, the results of this study may not be generalizable. Second, the study had a cross-sectional design, thus it is difficult to observe the relationship between 5-10 years of A1c and sudomotor function longitudinally. Third, this is a retrospective study and hospital-based study, and therefore unmeasured bias cannot be ruled out. Therefore, based on these limitations, large-sample, multi-center research is required in the future.
In general, our study found that patients who have combined sudomotor dysfunction have a lower value of TIR. TIR is linked with SUDOSCAN indicators like FESC, HESC, HASYM, and FASYM robustly. TIR is inversely associated with the prevalence of sudomotor dysfunction independent of HbA1c. Further prospective research is needed to identify the significant role of TIR in sudomotor dysfunction.
Previous research showed that up to 50% of diabetic individuals were affected by diabetic peripheral neuropathy (DPN). SUDOSCAN (Impeto Medical, Paris, France) is an emerging technique for the detection of DPN through detecting sudomotor function of the sweat gland. Sudomotor dysfunction, derived by SUDOSCAN, could detect DPN in the inchoate stage. The measurement of SUDOSCAN includes electrochemical skin conductance in hands (HESC, measured in μS) and feet (FESC). The exact pathogenesis of DPN is not fully clarified. The researchers identified glycemic variability as an independent contributor to DPN. Time in range (TIR), as a CGM-derived pivotal metric, has been proved to assess short-lived glycemic control. A lower level of TIR had an adverse effect in patients who were diagnosed with diabetes mellitus with diabetic microvascular complications, including microalbuminuria and retinopathy. But the association between TIR and sudomotor dysfunction has not explored clearly yet.
In this study, we aimed to explore the correlation between TIR calculated by continuous glucose monitoring (CGM) and sudomotor dysfunction detected by SUDOSCAN in Chinese subjects with type 2 diabetes mellitus (T2DM).
Our aim was to provide a novel and objective metric to monitor glycemic control in patients with T2DM, especially those who have combined complications.
This study enrolled 466 inpatients with type 2 diabetes. All subjects underwent 3-d CGM and SUDOSCAN. SUDOSCAN was assessed with electrochemical skin conductance in hands (HESC) and feet (FESC). Average feet ESC < 60 µS was defined as sudomotor dysfunction (+), otherwise it was sudomotor dysfunction (-). TIR refers to the percentage of time when blood glucose is between 3.9-10 mmol/L during a 1-d period. First, we compared clinical variables between the sudomotor dysfunction (-) and sudomotor dysfunction (+) groups. Next, in order to perform more in-depth analyses of the association between TIR and sudomotor dysfunction, we further stratified all participants based on tertiles of TIR. And the prevalence of sudomotor dysfunction in different tertiles of TIR was compared. Next, Spearman’s rank correlation was carried out to evaluate the association of TBR, TIR, or TAR and SUDOSCAN metrics. And binary logistic regression analysis was applied to explore the link between TIR (as a continuous or categorical variable) and sudomotor dysfunction after adjusting for clinical factors including age, diabetes duration, sex, BMI, SBP, DBP, smoking, TG, TC, HbA1c, as well as glycemic variability metrics. A P value < 0.01 was considered statistically significant.
Among 466 T2DM subjects, 135 (28.97%) presented with sudomotor dysfunction. Patients who had combined sudomotor dysfunction had a lower value of TIR (P < 0.001). Compared with the lowest tertile of TIR (T1 group), the middle tertile of TIR (T2 group) and the highest tertile of TIR (T3 group) were associated with an obviously lower prevalence of sudomotor dysfunction (20.51%, 21.94% vs 44.52%, P < 0.001). In addition, TIR was linked with SUDOSCAN indicators like FESC and HESC. With the increase of TIR, HESC and FESC increased (P < 0.001). The regression analysis demonstrated that TIR was inversely and independently linked with the prevalence of sudomotor dysfunction after adjusting for confounding factors (odds ratio = 0.979, 95%CI: 0.971-0.987, P < 0.001).
Patients who have combined sudomotor dysfunction have a lower value of TIR. TIR is linked with SUDOSCAN indicators like FESC, HESC, HASYM, and FASYM robustly. TIR is inversely associated with the prevalence of sudomotor dysfunction independent of HbA1c.
This is the first study to investigate the association between TIR and sudomotor dysfunction assessed by SUDOSCAN. These results will interest researchers in the prevalence and management of DPN. The preliminary data from our study may provide a basis for future large-sample, multi-center research.
| 1. | Vinik A, Ullal J, Parson HK, Casellini CM. Diabetic neuropathies: clinical manifestations and current treatment options. Nat Clin Pract Endocrinol Metab. 2006;2:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 288] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 3. | Ragé M, Van Acker N, Knaapen MW, Timmers M, Streffer J, Hermans MP, Sindic C, Meert T, Plaghki L. Asymptomatic small fiber neuropathy in diabetes mellitus: investigations with intraepidermal nerve fiber density, quantitative sensory testing and laser-evoked potentials. J Neurol. 2011;258:1852-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 787] [Article Influence: 56.2] [Reference Citation Analysis (1)] |
| 5. | Vinik AI, Nevoret ML, Casellini C. The New Age of Sudomotor Function Testing: A Sensitive and Specific Biomarker for Diagnosis, Estimation of Severity, Monitoring Progression, and Regression in Response to Intervention. Front Endocrinol (Lausanne). 2015;6:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Smith AG, Lessard M, Reyna S, Doudova M, Singleton JR. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications. 2014;28:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Novak P. Electrochemical Skin Conductance Correlates with Skin Nerve Fiber Density. Front Aging Neurosci. 2016;8:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Shivaprasad C, Amit G, Anish K, Rakesh B, Anupam B, Aiswarya Y. Clinical correlates of sudomotor dysfunction in patients with type 2 diabetes and peripheral neuropathy. Diabetes Res Clin Pract. 2018;139:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Hu YM, Zhao LH, Zhang XL, Cai HL, Huang HY, Xu F, Chen T, Wang XQ, Guo AS, Li JA, Su JB. Association of glycaemic variability evaluated by continuous glucose monitoring with diabetic peripheral neuropathy in type 2 diabetic patients. Endocrine. 2018;60:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R, Bosi E, Buckingham B, Cobelli C, Dassau E, Doyle FJ, Heller S, Hovorka R, Jia W, Jones T, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Maahs D, Murphy HR, Nørgaard K, Parkin CG, Renard E, Saboo B, Scharf M, Tamborlane WV, Weinzimer SA, Phillip M. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1717] [Cited by in RCA: 1459] [Article Influence: 162.1] [Reference Citation Analysis (0)] |
| 11. | Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, Close KL. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care. 2019;42:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 563] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 12. | Guo Q, Zang P, Xu S, Song W, Zhang Z, Liu C, Guo Z, Chen J, Lu B, Gu P, Shao J. Time in Range, as a Novel Metric of Glycemic Control, Is Reversely Associated with Presence of Diabetic Cardiovascular Autonomic Neuropathy Independent of HbA1c in Chinese Type 2 Diabetes. J Diabetes Res. 2020;2020:5817074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 125] [Reference Citation Analysis (0)] |
| 14. | Lauria G, Devigili G. Skin biopsy as a diagnostic tool in peripheral neuropathy. Nat Clin Pract Neurol. 2007;3:546-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther. 2013;15:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, Blonde L, Bray GA, Cohen AJ, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda OP, Garber AJ, Garvey WT, Henry RR, Hirsch IB, Horton ES, Hurley DL, Jellinger PS, Jovanovič L, Lebovitz HE, LeRoith D, Levy P, McGill JB, Mechanick JI, Mestman JH, Moghissi ES, Orzeck EA, Pessah-Pollack R, Rosenblit PD, Vinik AI, Wyne K, Zangeneh F. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21 Suppl 1:1-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 17. | Yajnik CS, Kantikar VV, Pande AJ, Deslypere JP. Quick and simple evaluation of sudomotor function for screening of diabetic neuropathy. ISRN Endocrinol. 2012;2012:103714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Mayaudon H, Miloche PO, Bauduceau B. A new simple method for assessing sudomotor function: relevance in type 2 diabetes. Diabetes Metab. 2010;36:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 20. | Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2132] [Cited by in RCA: 1946] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 21. | Klein R, Klein BE, Moss SE. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med. 1996;124:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 162] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [PubMed] |
| 23. | Xu F, Zhao LH, Su JB, Chen T, Wang XQ, Chen JF, Wu G, Jin Y, Wang XH. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W. Association of Time in Range, as Assessed by Continuous Glucose Monitoring, With Diabetic Retinopathy in Type 2 Diabetes. Diabetes Care. 2018;41:2370-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 25. | Lu J, Ma X, Shen Y, Wu Q, Wang R, Zhang L, Mo Y, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W, Zhou J. Time in Range Is Associated with Carotid Intima-Media Thickness in Type 2 Diabetes. Diabetes Technol Ther. 2020;22:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 26. | Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 978] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 27. | Giannini S, Benvenuti S, Luciani P, Manuelli C, Cellai I, Deledda C, Pezzatini A, Vannelli GB, Maneschi E, Rotella CM, Serio M, Peri A. Intermittent high glucose concentrations reduce neuronal precursor survival by altering the IGF system: the involvement of the neuroprotective factor DHCR24 (Seladin-1). J Endocrinol. 2008;198:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD (P)H-oxidase activation. Diabetes. 2003;52:2795-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 635] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 29. | Abdelzaher LA, Imaizumi T, Suzuki T, Tomita K, Takashina M, Hattori Y. Astaxanthin alleviates oxidative stress insults-related derangements in human vascular endothelial cells exposed to glucose fluctuations. Life Sci. 2016;150:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Yang J, Zhao Z, Yuan H, Ma X, Li Y, Wang H, Ma X, Qin G. The mechanisms of glycemic variability accelerate diabetic central neuropathy and diabetic peripheral neuropathy in diabetic rats. Biochem Biophys Res Commun. 2019;510:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Papazafiropoulou A, Serhiyenko VA, Shah V S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Ma YJ