Published online Jun 15, 2019. doi: 10.4239/wjd.v10.i6.350
Peer-review started: February 26, 2019
First decision: March 11, 2019
Revised: May 10, 2019
Accepted: May 14, 2019
Article in press: May 14, 2019
Published online: June 15, 2019
Processing time: 111 Days and 2 Hours
The risk of developing prediabetes based on the metabolic/obesity phenotypes has been poorly investigated.
To examine the association of baseline metabolic/obesity phenotypes and their changes over time with the risk of prediabetes development.
In a population-based cohort study, 1741 adults (aged > 19 years) with normal blood glucose were followed for 14 years. Anthropometric and biochemical measures were evaluated regularly during the follow-up period. According to body mass index and metabolic health status, participants were categorized into four groups: Metabolically healthy normal weight (MHNW), metabolically healthy obese (MHO), metabolically unhealthy normal weight (MUNW) and metabolically unhealthy obese (MUO). Multivariable Cox regression analysis was used to measure the risk of prediabetes according to the baseline metabolic/obesity phenotype and their changes during the follow-up.
In the whole population with a mean (95CCI for mean) follow up duration of 12.7 years (12.6-12.9), all three MUNW, MHO, MUO groups were at higher risk for developing prediabetes compared to the MHNW group (P = 0.022). The MUNW group had the highest risk for developing prediabetes (hazard ratio (HR): 3.84, 95%CI: 1.20, 12.27). In stratified analysis by sex, no significant association was found in men, while women in the MUNW group were at the greatest risk for prediabetes (HR: 6.74, 95%CI: 1.53, 29.66). Transforming from each phenotype to MHNW or MHO was not related to the risk of prediabetes development, whereas transforming from each phenotype to MUO was associated with an increased risk of prediabetes (HR > 1; P < 0.05).
Our findings indicate that MHO is not a high risk, unless it transforms into MUO over time. However, people in the MUNW group have the greatest risk for developing prediabetes, and therefore, they should be screened and treated.
Core tip: The risk of developing prediabetes based on metabolic/obesity phenotypes has been poorly investigated. In a 14-year follow-up cohort study, we observed that metabolically unhealthy normal weight, metabolically healthy obese (MHO), and metabolically unhealthy obese (MUO) were at higher risk for developing prediabetes compared to metabolically healthy normal weight (MHNW) subjects. The results stratified by sex demonstrated no significant association in men, while the risk of prediabetes development was significantly higher in all metabolic/obesity phenotypes in women compared to MHNW. Transforming from each phenotype to MHNW or MHO was not related to an increased risk of prediabetes development, whereas transforming from each phenotype to MUO was associated with an increased risk of prediabetes.
- Citation: Haghighatdoost F, Amini M, Aminorroaya A, Abyar M, Feizi A. Different metabolic/obesity phenotypes are differentially associated with development of prediabetes in adults: Results from a 14-year cohort study. World J Diabetes 2019; 10(6): 350-361
- URL: https://www.wjgnet.com/1948-9358/full/v10/i6/350.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i6.350
Type 2 diabetes mellitus (T2DM) is a public health concern worldwide[1]. The preva-lence and burden of diabetes has increased faster in low-income and developing countries than in high-income countries[2]. Prediabetic subjects are at a 3-12 times higher risk for developing diabetes compared to the general population[3]. In addition, the prevalence of cardiovascular and renal diseases has increased in American prediabetic patients over the last decades[3]. Therefore, identification of effective measures to prevent prediabetes risk might be useful for reducing the risk of T2DM, cardiovascular and renal diseases.
Although body mass index (BMI), as a measure of obesity, is positively correlated with the risk of various non-communicable diseases[4,5], approximately 35% of obese individuals are metabolically healthy[6]. In contrast, many normal weight subjects may suffer from a variety of metabolic abnormalities, such as insulin resistance, hyper-tension, dyslipidemia and hyperglycemia[7,8]. However, metabolic abnormalities are more common amongst metabolically healthy obese (MHO) than metabolically healthy normal weight (MHNW) individuals[6]. Consistently, a recent meta-analysis showed that MHO subjects with or without fatty liver had a greater risk for developing T2DM compared to MHNW subjects without fatty liver[9]. In a 10-year follow up study among Koreans, the incident diabetes risk was higher in both metabolically unhealthy normal weight (MUNW) and metabolically unhealthy obese (MUO) individuals than MHNW individuals. Nevertheless, in MHO subjects in this population, the incidence of T2DM was significantly higher in subjects younger than 45 years, but not in older adults[10]. Although the association between metabolic/ obesity phenotypes and T2DM have been investigated in various populations[10-14], few studies have been conducted to evaluate such association not only in Iran where diabetes mellitus is one of the main causes of years lived with disability, but also worldwide[15]. In our previous publication, MHO and MUOW subjects were at considerably greater risk for developing T2DM compared to MHNW subjects[12]. Nevertheless, the risk of developing prediabetes based on metabolic/obesity phenotypes has been poorly investigated. In a retrospective Japanese population cohort study, the prevalence of prediabetes was remarkably higher in obese individuals compared to normal weight subjects (60% vs 34%)[16]. Another longitudinal study revealed no association between general adiposity and diabetes or prediabetes risk, while dysfunctional adiposity, determined by excess visceral fat and insulin resistance, was associated with the occurrence of diabetes or prediabetes[17]. Due to our limited knowledge regarding prediabetes risk, in the current study, we aimed to: (1) Estimate the prevalence of different metabolic/obesity phenotypes in an Iranian population and (2) Determine the association of baseline metabolic/obesity phenotypes and their interchanges during follow-up with the risk of prediabetes development in a prospective cohort study.
Subjects in the present study were from the Isfahan Diabetes Prevention Study (IDPS). Details regarding the IDPS population and study design have been described elsewhere[18]. In brief, the IDPS is an ongoing prospective cohort study that began in 2003, and participants were selected from a consecutive sample of patients who attended the clinics of Isfahan Endocrine and Metabolism Research Center. This study was conducted to evaluate the role of lifestyle factors in developing prediabetes and T2DM in the immediate family of T2DM patients. A total of 1741 subjects (439 men and 1302 women) without prediabetes or T2DM aged from 30 to 70 years and with complete data were included in the current cohort study to identify metabolic status and metabolic/obesity phenotypes. Subjects were followed for 14 years (2003 to 2017). Information regarding health status and lifestyle risk factors for T2DM, like physical activity and dietary intakes and demographic variables were collected using validated questionnaires and updated according to a medical care standard in diabetes[19]. Accordingly, participants were tested for the diagnosis of new-onset prediabetes or diabetes in at least at 3-year intervals. Informed written consent was obtained from each participant at baseline. The Ethical Committee of Isfahan University of Medical Sciences approved the study protocol.
All measurements were acquired by well-trained examiners at baseline. Weight was determined using a balanced scale while participants were minimally clothed and recorded to the nearest 0.1 kg. Height was measured using a wall-fixed tape measure while shoulders were in the normal position and participants were without footwear, and recorded to the nearest 0.5 cm. Waist circumference (WC) and hip circumference (HC) were measured using a metal tape measure without imposing any pressure to body surface and were recorded to the nearest 0.5 cm. WC was considered as the narrowest level between the lowest rib and iliac crest, and HC was considered as the largest level[20]. BMI was calculated by dividing body weight in kg by height in m2. Waist to hip ratio (WHR) was calculated as dividing WC by HC.
A 10-h overnight fasting blood sample was gathered to measure serum lipids [total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C) and triglyceride (TG) and fasting plasma glucose (FPG)]. Postprandial plasma glucose levels were determined in venous blood sample at 30, 60, and 120 min after oral glucose administration. Plasma glucose and lipid profile concentrations were measured using the oxidase method (Pars Azmoon, Tehran, Iran) adapted to a Selectra-2 auto-analyzer (Vital Scientific, Spankeren, The Netherlands). Serum LDL-C levels were calculated using the Friedwald equation when serum TG levels were < 400 mg/dL[21]. Whole blood samples were used to determine HbA1c concentrations through the pink reagent kit on a DS5 analyzer. For all markers, intra- and inter-assay coefficients of variability (CVs) were < 2.2%.
To measure blood pressure, subjects were asked to rest for 15 min, and then while subjects were sitting, blood pressure was measured twice with a 30 s interval between the two measurements using a Mercury sphygmomanometer. The mean of the two measurements was recorded as the blood pressure value.
Prediabetes was defined according to the definition of the American Diabetes Association. Accordingly, subjects with 100 ≤ FPG < 126 mg/dL or HbA1C ≥ 6.5% or 2-h oral glucose test tolerance (2h-OGTT) ≥ 200 mg/dL were defined as being prediabetic[19]. Normal weight and overweight/obese were defined as BMI < 25 and ≥ 25 kg/m2, respectively. Metabolic unhealthy was defined as the presence of at least one component of the following criteria: (1) Elevated blood pressure (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg); (2) Low HDL-C concentration (< 50 mg/dL in women and < 40 mg/dL in men); and (3) High serum TG (≥ 150 mg/dL)[22].
Participants were categorized into four metabolic/obesity phenotypes categories. Normal distribution of quantitative data was tested using the Kolmogrov-Smirnov test and Q-Q plot. Data were reported as mean ± SE or percentage for continuous and categorical variables, respectively. The association between categorical variables was examined using the chi-square test. Between groups differences for quantitative variables were evaluated using Analysis of variance (ANOVA).
Event-free rates were estimated using the Kaplan-Meier method, and the diffe-rences between survival curves for all metabolic/obesity phenotypes at the end of follow-up were compared by using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for developing prediabetes were calculated using univariate and multivariate Cox proportional hazards regression models. The crude model included only the metabolic/obesity phenotypes, and model 1 was adjusted for age, sex, smoking and physical activity as possible confounding factors. Statistical analyses were performed using statistical package for social science (SPSS version 16, SPSS, Inc., IL, United States).
Baseline characteristics of the study population across the metabolic/obesity pheno-types are shown in Table 1. Of the 1741 subjects with normal glucose tolerance at baseline, 274 persons (15.7%) were MHNW. The most and least prevalent phenotypes were MHO (48.4%) and MUNW (4.1%), respectively. Normal weight groups, either metabolically healthy or unhealthy, were more likely to be male and highly educated. In both normal weight and overweight/obese groups, the means of age, weight, BMI, WC, HC and WHR were higher in metabolically unhealthy subjects than metabolically healthy subjects. Similar results were also observed for biochemical tests, including blood sugar-30 -60 and-120 min, lipid profile (TG, TC, HDL-C, LDL-C), SBP and DBP. FPG and HbA1c were not significantly different across the metabolic/obesity phenotypes. Physical activity level and smoking were not significantly different across the metabolic/obesity phenotypes.
| Metabolically healthy and normal weight | Metabolically healthy and overweight or obese | Metabolically unhealthy and normal weight | Metabolically unhealthy and overweight or obese | P value2 | |
| Number (%) | 274 (15.7) | 843 (48.4) | 71 (4.1) | 553 (31.8) | |
| Age in yr | 41.05 ± 0.42 | 42.33 ± 0.22 | 43.38 ± 0.76 | 43.31 ± 0.27 | < 0.0001 |
| Weight in kg | 59.22 ± 0.46 | 74.77 ± 0.38 | 61.64 ± 0.99 | 77.92 ± 0.50 | < 0.0001 |
| BMI in kg/m2 | 22.75 ± 0.11 | 29.47 ± 0.12 | 23.61 ± 0.16 | 30.35 ± 0.16 | < 0.0001 |
| WC in cm | 76.79 ± 0.42 | 88.67 ± 0.29 | 81.19 ± 0.80 | 92.62 ± 0.38 | < 0.0001 |
| HC in cm | 97.39 ± 0.30 | 108.71 ± 0.27 | 98.22 ± 0.57 | 109.51 ± 0.35 | < 0.0001 |
| WHR | 0.79 ± 0.004 | 0.82 ± 0.002 | 0.83 ± 0.008 | 0.85 ± 0.003 | < 0.0001 |
| FBS in mg/dL | 88.04 ± 0.42 | 88.33 ± 0.24 | 87.13 ± 1.12 | 88.54 ± 0.34 | 0.446 |
| Blood sugar 30 min in mg/dL | 124.91 ± 1.53 | 129.96 ± 0.94 | 132.30 ± 3.32 | 133.48 ± 1.13 | < 0.0001 |
| Blood sugar 60 min in mg/dL | 118.09 ± 1.94 | 125.60 ± 1.12 | 127.76 ± 4.49 | 135.30 ± 1.38 | < 0.0001 |
| Blood sugar 120 min in mg/dL | 95.74 ± 1.31 | 101.32 ± 0.74 | 102.16 ± 2.30 | 101.35 ± 0.95 | 0.001 |
| HbA1c as % | 4.92 ± 0.04 | 4.98 ± 0.03 | 4.84 ± 0.08 | 5.04 ± 0.04 | 0.070 |
| Triglyceride, mg/dL | 110.16 ± 3.14 | 124.47 ± 2.39 | 190.99 ± 7.46 | 218.15 ± 4.59 | < 0.0001 |
| Total cholesterol, mg/dL | 182.54 ± 2.17 | 194.29 ± 1.39 | 192.34 ± 3.30 | 198.68 ± 1.69 | < 0.0001 |
| LDL-C, mg/dL | 110.17 ± 1.92 | 120.04 ± 1.30 | 115.51 ± 3.28 | 118.31 ± 1.58 | 0.001 |
| HDL-C, mg/dL | 49.89 ± 0.83 | 49.93 ± 0.44 | 38.97 ± 1.0 | 39.03 ± 0.35 | < 0.0001 |
| Systolic blood pressure, mmHg | 100.50 ± 0.08 | 110.01 ± 0.05 | 110.82 ± 0.17 | 120.22 ± 0.07 | < 0.0001 |
| Diastolic blood pressure in mmHg | 60.79 ± 0.06 | 70.15 ± 0.04 | 70.96 ± 0.12 | 80.03 ± 0.05 | < 0.0001 |
| Physical activity , MET-hr/wk | 19.73 ± 4.25 | 16.61 ± 3.03 | 26.63 ± 11.83 | 19.10 ± 3.11 | 0.742 |
| Male as % | 33.6 | 19.7 | 33.8 | 28.4 | < 0.0001 |
| Educational level as % | 0.006 | ||||
| Illiterate | 2.6 | 3.4 | 5.6 | 5.5 | |
| < 12 yr | 38.4 | 48.1 | 42.3 | 50.3 | |
| = 12 yr | 36.6 | 32.9 | 39.4 | 29.7 | |
| > 12 yr | 22.4 | 15.6 | 12.7 | 14.5 | |
| Current smoker as % | 16.8 | 6.9 | 13.0 | 11.2 | 0.073 |
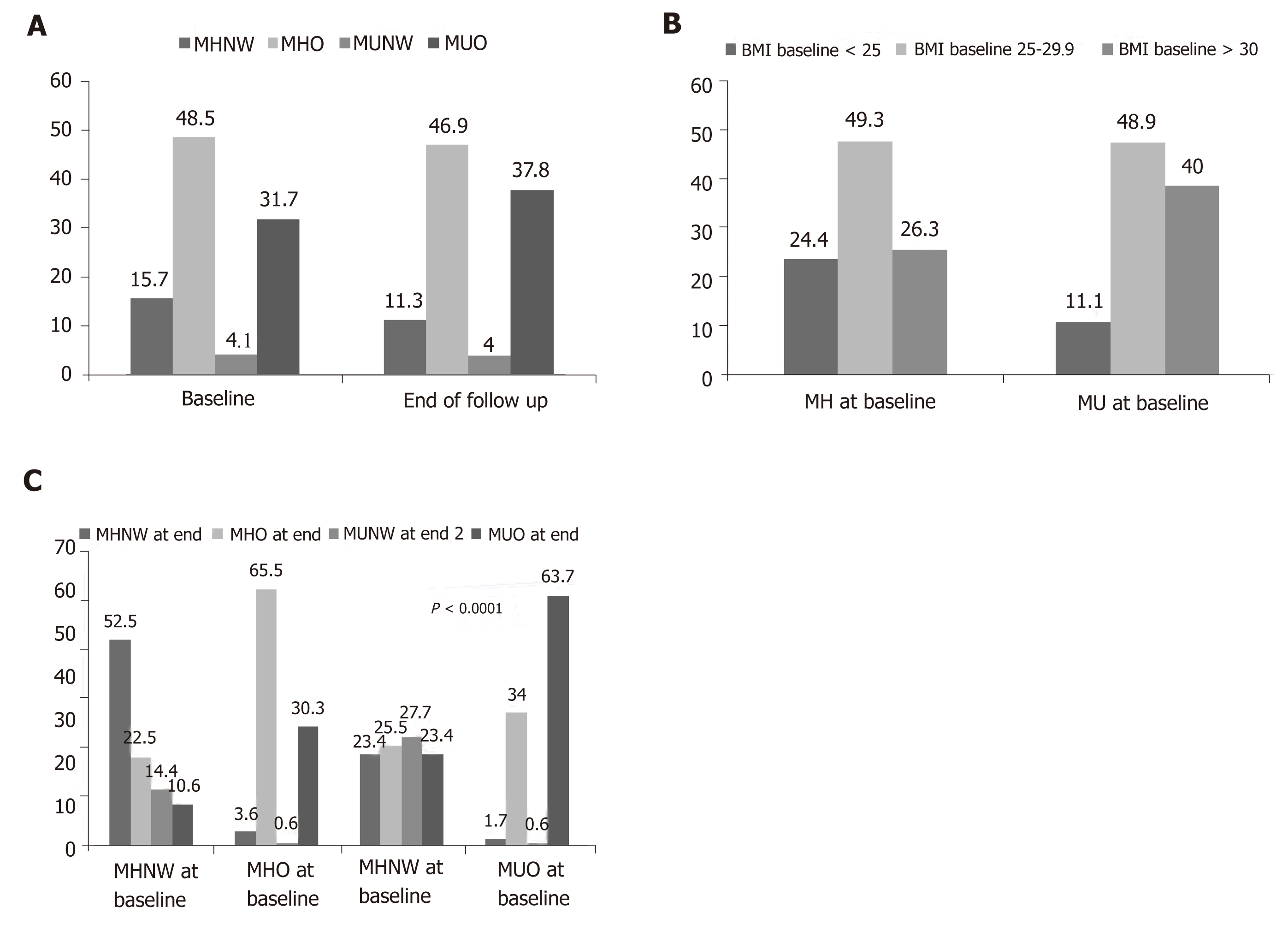
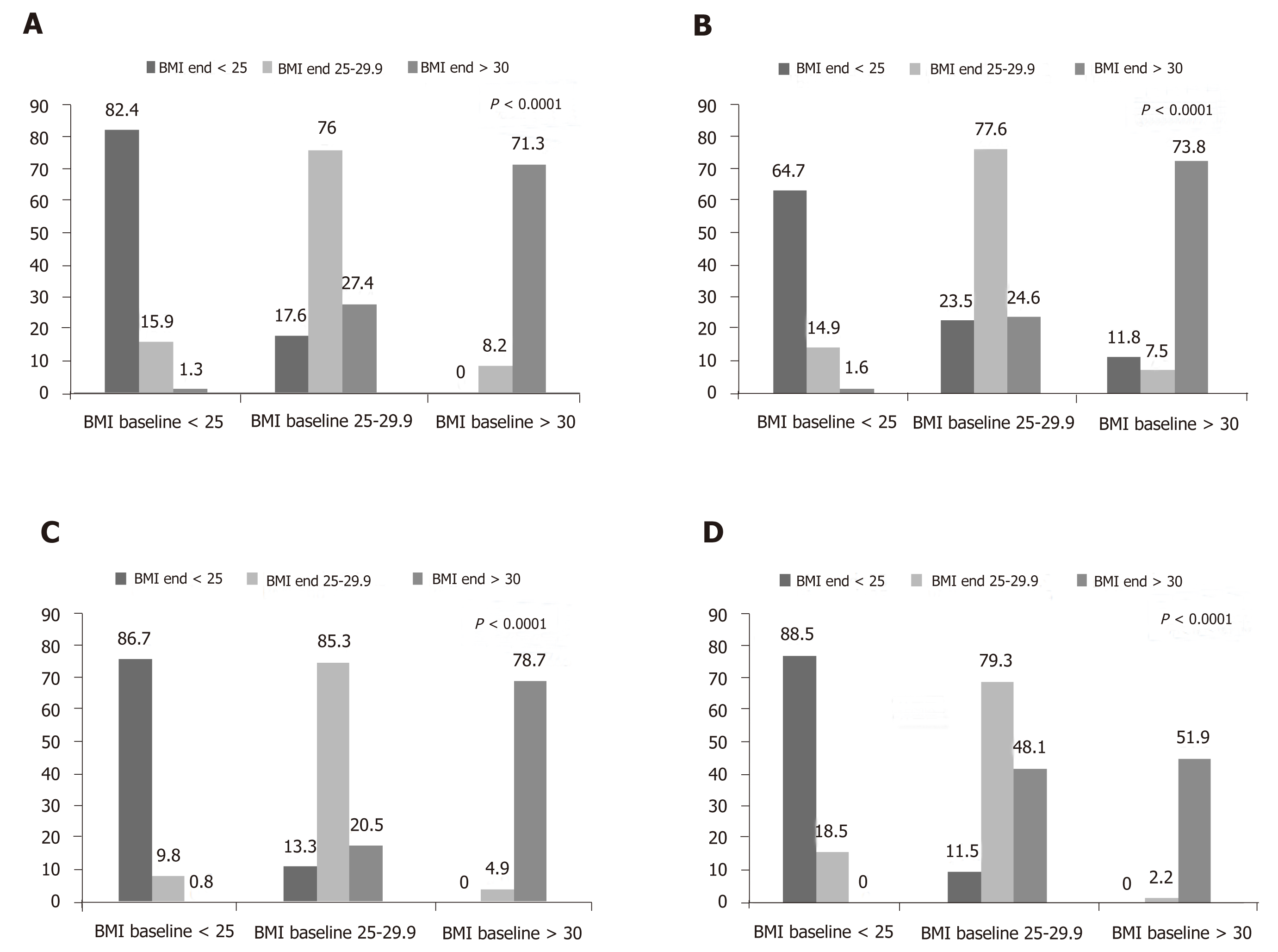
Person-years follow up (incidence rate per 1000-person years) for MHNW and overweight/obese subjects were 3007 (14.96) and 9501 (18), respectively. Corres-ponding values in MUNW subjects were 736 (29.89), and 6204 overweight/obese (20.9), respectively. In total, person-years follow up (incidence rate per 1000-person years) in metabolically healthy and unhealthy subjects were 12508 (17.34) and 6940 (22.33), respectively. The prevalence of different metabolic/obesity phenotypes at baseline and the end of study is illustrated in Figure 1. The most common phenotype at baseline or at the end of the study was related to MHO (baseline: 48.5% and end of follow-up: 46.9%), whilst the least common phenotype was MUNW (baseline: 4.1% and end of follow-up: 4.0%). At baseline, 24% of metabolically healthy subjects and 11% of metabolically unhealthy subjects had normal weights (Figure 1). Changing in the prevalence of metabolic/obesity phenotypes was statistically significant over the study follow-up (Figure 1). Figure 2 shows how the prevalence of overweight and obesity based on metabolic health change changed during the follow-up. In all four groups, BMI status significantly changed (all P -values < 0.0001).
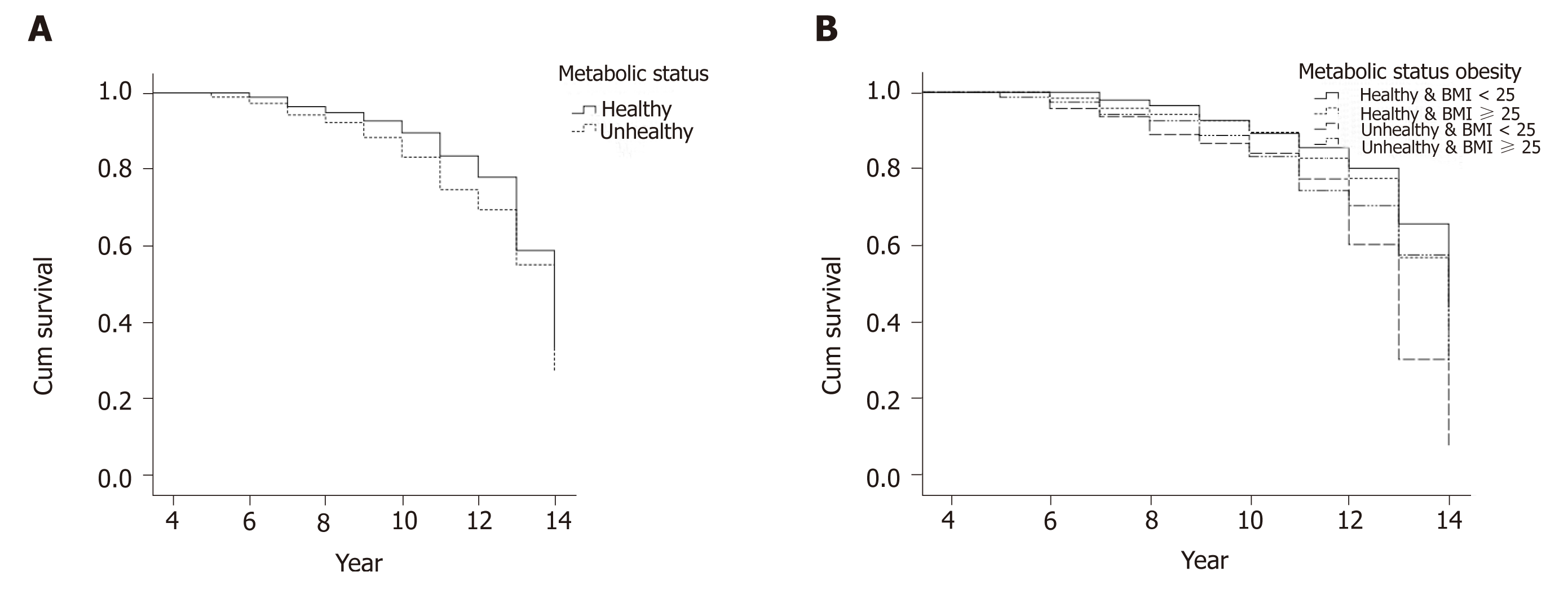
Figure 3A and B show the Kaplan-Meier survival curves of prediabetes incidence, comparing the two (metabolically healthy and unhealthy) and four groups (MHNW MUNW, MHO, MUO). The results of log rank tests showed significant difference between groups, indicating a significantly different probability of incidence rate of prediabetes between study groups. Metabolic unhealthy people had a higher probability (Chi-square = 5.71; P = 0.023), and the MUNW, MHO, MUO groups had higher event-rate rates compared to MHNW subjects (Chi-square = 12.49; P = 0.006).
Table 2 shows the risk of prediabetes development across different metabolic/ obesity phenotypes. In the whole population, all three MUNW, MHO, MUO groups were at higher risk for developing prediabetes compared to the MHNW group. Although this association was marginally significant in the crude model (P = 0.058), adjustment for potential confounders strengthened the associations (P = 0.022). In the crude model, the MUNW group was at the greatest risk for developing prediabetes compared to other groups (HR: 2.05, 95%CI: 1.05, 4.02), and this association became stronger after adjustment for confounders (HR: 3.84, 95%CI: 1.20, 12.27).
| Metabolically healthy and normal weight | Metabolically healthy and overweight or obese | Metabolically unhealthy and normal weight | Metabolically unhealthy and overweight or obese | P value1 | |
| Whole population | |||||
| Crude | 1 | 1.38 (0.92, 2.05) | 2.05 (1.05, 4.02) | 1.67 (1.10, 2.52) | 0.058 |
| Model 1 | 1 | 1.43 (0.69, 2.94) | 3.84 (1.20, 12.27) | 2.50 (1.19, 5.25) | 0.022 |
| Male | |||||
| Crude | 1 | 1.06 (0.54, 2.08) | 0.95 (0.30, 3.00) | 1.39 (0.71, 2.73) | 0.715 |
| Model 1 | 1 | 0.94 (0.27, 3.27) | 0.69 (0.08, 6.25) | 1.10 (0.32, 3.75) | 0.976 |
| Female | |||||
| Crude | 1 | 1.74 (1.04, 2.93) | 3.25 (1.39, 7.58) | 2.0 (1.16, 3.44) | 0.026 |
| Model 1 | 1 | 1.67 (0.63, 4.49) | 6.74 (1.53, 29.66) | 3.45 (1.23, 9.68) | 0.014 |
In stratified analysis by sex (Table 2), a non-statistically significant increase was observed in MUO men but attenuated after adjustment for potential confounders. However, consistent with the whole population, the risk of incident prediabetes in women was greater in the MHO, MUNW and MUO groups compared to the MHNW group. The greatest risk was found in the MUNW group (HR: 6.74, 95%CI: 1.53, 29.66; P = 0.014). When participants were categorized into six groups (metabolically healthy/unhealthy-normal weight/overweight/obese) and considering metabolically healthy-normal weight group as the reference, the greatest risk of developing prediabetes was observed in metabolically unhealthy-obese subjects in both the crude (HR: 2.09, 95%CI: 1.30, 3.38) and adjusted models (HR: 2.16, 95%CI: 1.32, 3.53) (data not shown).
Table 3 shows the risk of developing prediabetes based on changing metabolic/ obesity phenotypes during the follow-up. Transforming from each phenotype at baseline to MHNW or MHO was not significantly related to the risk of prediabetes incidence, whereas transforming from each phenotype to MUO was significantly associated with an increased risk of prediabetes compared with stable MHNW. Although there was no significant increment in the risk of prediabetes by transforming from MHNW and MHO to MUNW, stable MUNW was associated with a significantly higher risk for developing prediabetes (HR: 5.22, 95%CI: 1.53, 17.86; P < 0.0001).
| Baseline metabolic/obesity phenotype | Last metabolic/obesity phenotype | HR (95%CI) |
| MHNW | MHNW | 1 (reference) |
| MHO | 1.04 (0.40, 2.69) | |
| MUNW | 2.67 (0.96, 7.40) | |
| MUO | 5.87 (1.75, 19.66) | |
| MHO | MHNW | 1.25 (0.40, 3.97) |
| MHO | 1.10 (0.62, 1.95) | |
| MUNW | 1.63 (0.14, 19.00) | |
| MUO | 6.68 (3.56, 12.54) | |
| MUNW | MHNW | 2.17 (0.55, 8.52) |
| MHO | 0.65 (0.13, 3.24) | |
| MUNW | 5.22 (1.53, 17.86) | |
| MUO | 5.71 (1.51, 21.63) | |
| MUO | MHNW | 1.63 (0.28, 9.61) |
| MHO | 0.82 (0.41, 1.63) | |
| MUNW | 0.00 (0.0, 0.0) | |
| MUO | 3.98 (2.21, 7.15) | |
In this prospective cohort study on the immediate family of patients with T2DM, we found that MHO was the most prevalent metabolic/obesity phenotype in this population. Although the risk of prediabetes increased in all individuals who were MUNW, MHO and MUO at baseline, individuals in the MUNW group had the greatest risk compared with other phenotypes. Moreover, transition from any phenotype into MUO and stable MUNW were associated with significantly increased risk of prediabetes by the end of follow-up. In the stratified analysis by sex, the effect of metabolic/obesity phenotype on prediabetes incidence was significant in females but not males and in line with the findings in the whole population, as the greatest risk was found in MUNW category. In the whole population and women, metabolic status was a strong predictor for prediabetes incidence rather than obesity status.
Thus far, several studies have examined the effect of metabolic/obesity phenotypes on diabetes incidence. In a 6-yr follow-up study among Chinese, Wang et al[11] found that MUNW, MHO and MUO were at increased risk for developing T2DM. They also observed that transition from the MHO category at baseline into the MUO category at the end of follow-up was associated with an increased risk of T2DM compared to stable MHNW, but not compared to stable MHO. On the other hand, obesity at baseline, regardless of changes in metabolic status, increased the risk of incident T2DM. Nevertheless, in MUNW, transformation to MHNW was not associated with an increased risk of T2DM compared with stable MHNW[11]. Similar results were found in a 10-year follow-up study among Korean subjects[10]. They found that MUNW and MUO were at higher risk for developing diabetes and cardiovascular diseases compared to MHNW subjects, whilst the association in MHO was statistically significant only in younger individuals. Compared to stable MHNW, those with persistent MHO had a higher risk of incident T2DM after ten years follow-up[10]. In our earlier study, we found that regardless of BMI, metabolically unhealthy subjects were more likely to develop T2DM. In spite of an increased risk of T2DM in MHO, it was considerably lower than MUO, suggesting that metabolic abnormality is a more relevant risk factor for developing T2DM than obesity[12]. This finding is consistent with results of the present study showing that metabolically unhealthy subjects, even those with normal weight, are more likely to develop prediabetes compared to metabolically healthy counterparts. Further analysis according to changes in metabolic/obesity phenotypes also confirmed that metabolic health status is a better predictor of prediabetes incidence than BMI status. We observed that transition from MUNW or MUO into metabolically healthy status, regardless of changes in BMI, was not associated with an increased risk of prediabetes incidence. However, in participants with baseline metabolically healthy status, risk of prediabetes only increased when they were affected by both metabolic abnormality and obesity during the follow-up period.
The finding that MUNW subjects had the highest risk for prediabetes development is in line with the results of the English Longitudinal Study of Ageing (ELSA)[13]. They found that despite the increased risk of T2DM in MHO individuals, they are at lower risk for T2DM compared to metabolically unhealthy subjects in any BMI category. For example, the risk of developing T2DM in MHO was 8.6 times higher than MHNW subjects whilst the corresponding value in MUNW subjects was 9.9 times higher[13]. In an Iranian population-based cohort study among the elderly, Mirbolouk et al[23] demonstrated that the MUNW phenotype was associated with the greatest risk of developing cardiovascular disease (CVD), CVD mortality and all-causes mortality. However, the incident risk of CVD in MUNW and MUO was similar[23]. Therefore, greater attention should be paid to MUNW subjects, as they may be less targeted for preventive interventions.
The reason for the greater risk of incident prediabetes among MUNW might be attributed to an aspect of the participants’ body composition that was not measured. It has been shown that dysfunctional adiposity but not general adiposity is associated with an increased incidence of diabetes and prediabetes in obese adults[17]. Moreover, in general, normal weight diabetic subjects have greater abdominal and total fat compared to obese diabetic individuals, which adversely affect insulin sensitivity[24]. Sarcopenic obesity, a medical condition determined by low muscle mass accompanied by high fat mass, frequently occurs in older ages[25] and is significantly correlated with insulin resistance[26]. Therefore, BMI as an obesity index, which only consists of body weight and height, cannot reflect fat distribution. It is possible that, MUNW in our study population, who were older than other metabolic/obesity phenotypes, had more fat but less muscle masses compared to other categories. However, WC as a central adiposity measure was not greater in MUNW compared to MHO and MUO. Therefore, abdominal fat distribution might not explain our findings per se. Given that higher gluteofemoral fat mass is associated with lower risk of insulin resistance and diabetes[27,28], it is possible that increased HC in MHO and MUO led to a lower risk of incident prediabetes compared to MUNW.
Several studies have suggested that reductions in visceral fat mass increase insulin sensitivity in MHO subjects, and consequently decrease diabetes risk[29]. However, standard weight-reduction interventions may adversely affect appetite, mood and energy expenditure[30] without any favorable effect on metabolic status in MHO subjects[31-33]. These changes may promote weight regain. Therefore, regarding the relevance of favorable fat distribution in MHO, which is determined by lower visceral fat and higher subcutaneous fat, interventions targeting fat-loss rather than weight-loss might be more successful in reducing T2DM risk in MHO.
Although this is the first longitudinal study to predict the risk of prediabetes incidence according to metabolic/obesity phenotypes, it has several limitations that must be kept in mind. The study population was not a representative sample of Iranians, and therefore, our findings might not be generalizable to other populations of Iranians. Moreover, we used BMI as an anthropometric measure to determine obesity status, which does not consider fat distribution and does not differentiate between fat mass and lean mass. Finally, our study population mainly consisted of females, and therefore, the limited number of males may not allow us to identify true associations.
The strengths of our study are long-term follow-up and enough incident predia-betic cases that enhance the statistical power of analyses. To our knowledge, this is the first study that evaluated the association of metabolic/obesity phenotype with the development of prediabetes among Iranians. For diagnosing new cases of prediabetes, HbA1c, oral glucose test tolerance and fasting blood sugar were available so that new cases were not missed. Moreover, sex-specific associations were reported in the current analysis, and the confounding effects of various factors were controlled.
In conclusion, our study showed that MHO is not a high risk unless it progresses to MUO. However, the MUNW group has the greatest risk for developing prediabetes, and they should therefore be screened and treated. During the follow-up, changes to the phenotype status were significantly related to the risk of prediabetes develop-ment. In stratified analysis by sex, this association was evident among females but not males. Given that various metabolic/obesity phenotypes can increase the risk of prediabetes incidence, developing appropriate guidelines to care for various meta-bolic/obesity phenotypes to reduce prediabetes occurrence is necessary.
The risk of developing prediabetes based on the metabolic/obesity phenotypes has been poorly investigated.
Due to the potential association between various metabolic/obesity phenotypes and the risk of prediabetes incidence, developing appropriate guidelines to care for various metabolic/obesity phenotypes to reduce prediabetes occurrence is necessary.
This study aimed to (1) estimate the prevalence of different metabolic/obesity phenotypes in an Iranian population and (2) determine the association of baseline metabolic/obesity phenotypes and their interchanges during follow-up with the risk of prediabetes development in a pros-pective cohort study.
In a population-based cohort study, 1741 adults (aged > 19 years) with normal blood glucose were followed for 14 years. According to body mass index and metabolic health status, participants were categorized into four groups: metabolically healthy normal weight (MHNW), metabolically healthy obese (MHO), metabolically unhealthy normal weight (MUNW) and metabolically unhealthy obese (MUO). Multivariable Cox regression analysis was used to measure the risk of prediabetes according to the baseline metabolic/obesity phenotype and their changes during the follow-up.
In the whole population, all three MUNW, MHO, MUO groups were at higher risk for developing prediabetes compared to MHNW. The MUNW group was at the greatest risk for developing prediabetes (HR: 3.84). In stratified analysis by sex, no significant association was found in men, while women in the MUNW group were at the greatest risk for prediabetes (HR: 6.74). Transforming from each phenotype to MHNW or MHO was not related to the risk of prediabetes development, whereas transforming from each phenotype to MUO was associated with an increased risk of prediabetes.
Our findings indicate that MHO is not a high risk unless it progresses to MUO. However, individuals in the MUNW group have the greatest risk for developing prediabetes, and therefore need to be screened and treated.
Given that various metabolic/obesity phenotypes can boost the risk of prediabetes incidence, clinical trials need to be developed with appropriate guidelines to care for various metabolic/ obesity phenotypes to reduce prediabetes occurrence.
| 1. | Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011;8:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1467] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 2. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2594] [Article Influence: 259.4] [Reference Citation Analysis (0)] |
| 3. | Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol. 2018;6:392-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Kodama S, Horikawa C, Fujihara K, Heianza Y, Hirasawa R, Yachi Y, Sugawara A, Tanaka S, Shimano H, Iida KT, Saito K, Sone H. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. Am J Epidemiol. 2012;176:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Guo Y, Yue XJ, Li HH, Song ZX, Yan HQ, Zhang P, Gui YK, Chang L, Li T. Overweight and Obesity in Young Adulthood and the Risk of Stroke: a Meta-analysis. J Stroke Cerebrovasc Dis. 2016;25:2995-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Lin H, Zhang L, Zheng R, Zheng Y. The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity: a systematic review and meta-analysis: A PRISMA-compliant article. Medicine (Baltimore). 2017;96:e8838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 738] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 8. | Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 489] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 9. | Hashimoto Y, Hamaguchi M, Tanaka M, Obora A, Kojima T, Fukui M. Metabolically healthy obesity without fatty liver and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes Res Clin Pract. 2018;12:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kim NH, Seo JA, Cho H, Seo JH, Yu JH, Yoo HJ, Kim SG, Choi KM, Baik SH, Choi DS, Shin C, Cho NH. Risk of the Development of Diabetes and Cardiovascular Disease in Metabolically Healthy Obese People: The Korean Genome and Epidemiology Study. Medicine (Baltimore). 2016;95:e3384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Wang B, Zhang M, Wang S, Wang C, Wang J, Li L, Zhang L, Ren Y, Han C, Zhao Y, Zhou J, Wang G, Shen Y, Wu D, Pang C, Yin L, Feng T, Zhao J, Luo X, Hu D. Dynamic status of metabolically healthy overweight/obesity and metabolically unhealthy and normal weight and the risk of type 2 diabetes mellitus: A cohort study of a rural adult Chinese population. Obes Res Clin Pract. 2018;12:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Janghorbani M, Salamat MR, Amini M, Aminorroaya A. Risk of diabetes according to the metabolic health status and degree of obesity. Diabetes Metab Syndr. 2017;11 Suppl 1:S439-S444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev. 2014;15:504-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 14. | Latifi SM, Karandish M, Shahbazian H, Taha JM, Cheraghian B, Moradi M. Prevalence of Metabolically Healthy Obesity (MHO) and its relation with incidence of metabolic syndrome, hypertension and type 2 Diabetes amongst individuals aged over 20 years in Ahvaz: A 5 Year cohort Study (2009-2014). Diabetes Metab Syndr. 2017;11 Suppl 2:S1037-S1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Djalalinia S, Saeedi Moghaddam S, Moradi-Lakeh M, Shahraz S, Naghavi M, Murray CJL, Vos T, Mokhdad AH, Krohn K, Danaei G, Afshin A, Sepanlou SG, Bazargan-Hejazi S, Peykari N, Rezaei N, Roshandel G, Karimkhani C, Moazen B, Pourmalek F, Esteghamati AR, Hafezi-Nejad N, Sheikhbahaei S, Katibeh M, Ahmadieh H, Safi S, Qorbani M, Islami F, Khosravi A, Hasanvand MS, Mahdavi M, Kiadaliri AA, Farvid MS, Karimi SM, Mohammadi A, Asayesh H, Assadi R, Khubchandani J, Heydarpour P, Fereshtehnejad SM, Safiri S, Kasaeian A, Larijani B, Malekzadeh R, Farzadfar F. Prevalence and Years Lived with Disability of 310 Diseases and Injuries in Iran and its Neighboring Countries, 1990-2015: Findings from Global Burden of Disease Study 2015. Arch Iran Med. 2017;20:392-402. [PubMed] |
| 16. | Fujihara K, Matsubayashi Y, Yamamoto M, Osawa T, Ishizawa M, Kaneko M, Matsunaga S, Kato K, Seida H, Yamanaka N, Kodama S, Sone H. Impact of body mass index and metabolic phenotypes on coronary artery disease according to glucose tolerance status. Diabetes Metab. 2017;43:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 491] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 18. | Amini M, Janghorbani M. Diabetes and impaired glucose regulation in first-degree relatives of patients with type 2 diabetes in isfahan, iran: prevalence and risk factors. Rev Diabet Stud. 2007;4:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | American Diabetes Association. Executive summary: Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 20. | World Health Organization. Measuring obesity—classification and description of anthropometric data. Report on a WHO consultation of the epidemiology of obesity. Warsaw 21-23 October 1987. Copenhagen: WHO, 1989. Nutrition Unit document, EUR/ICP/NUT 1987; 123 Available from: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1673058. |
| 21. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [PubMed] |
| 22. | Ko SH, Baeg MK, Ko SY, Jung HS, Kim P, Choi MG. Obesity and Metabolic Unhealthiness Have Different Effects on Colorectal Neoplasms. J Clin Endocrinol Metab. 2017;102:2762-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Mirbolouk M, Asgari S, Sheikholeslami F, Mirbolouk F, Azizi F, Hadaegh F. Different obesity phenotypes, and incident cardiovascular disease and mortality events in elderly Iranians: Tehran lipid and glucose study. Geriatr Gerontol Int. 2015;15:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Vaag A, Lund SS. Non-obese patients with type 2 diabetes and prediabetic subjects: distinct phenotypes requiring special diabetes treatment and (or) prevention? Appl Physiol Nutr Metab. 2007;32:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 873] [Cited by in RCA: 794] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 26. | Levine ME, Crimmins EM. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring). 2012;20:2101-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond). 2010;34:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 598] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 28. | Esmaillzadeh A, Mirmiran P, Moeini SH, Azizi F. Larger hip circumference independently contributed to reduced metabolic risks in Tehranian adult women. Int J Cardiol. 2006;108:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care. 2010;33:1957-1959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Chaput JP, Arguin H, Gagnon C, Tremblay A. Increase in depression symptoms with weight loss: association with glucose homeostasis and thyroid function. Appl Physiol Nutr Metab. 2008;33:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond). 2006;30:1529-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Kantartzis K, Machann J, Schick F, Rittig K, Machicao F, Fritsche A, Häring HU, Stefan N. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia. 2011;54:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia. 2008;51:1752-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fatima SS, Hussain SAR S-Editor: Ji FF L-Editor: Filipodia E-Editor: Wang J