Published online May 15, 2019. doi: 10.4239/wjd.v10.i5.269
Peer-review started: March 19, 2019
First decision: May 8, 2019
Revised: May 13, 2019
Accepted: May 13, 2019
Article in press: May 14, 2019
Published online: May 15, 2019
Processing time: 58 Days and 10 Hours
Diabetes remains an important health issue as more patients with chronic and uncontrolled diabetes develop diabetic nephropathy (DN), which classically presents with proteinuria followed by a progressive decrease in renal function. However, an increasing proportion of DN patients have a decline in kidney function and vascular complications without proteinuria, known as non-proteinuric DN (NP-DN). Despite the increased incidence of NP-DN, few clinical or experimental studies have thoroughly investigated the pathophysiological mechanisms and targeted treatment for this form of DN. In this review, we will examine the differences between conventional DN and NP-DN and consider potential pathophysiological mechanisms, diagnostic markers, and treatment for both DN and NP-DN. The investigation of the pathophysiology of NP-DN should provide additional insight into the cardiovascular factors influencing renal function and disease and provide novel treatments for the vascular complications seen in diabetic patients.
Core tip: Diabetes remains an important health issue as more patients with chronic and uncontrolled diabetes develop diabetic nephropathy (DN). In recent years, an increasing proportion of DN patients have a decline in kidney function and vascular complications without proteinuria, known as non-proteinuric DN (NP-DN). This manuscript advances this discussion by examining the potential pathophysiological mechanisms, diagnostic markers, and treatments relevant to NP-DN. Furthermore, it illustrates the significance of renal microhemodynamics in the development of NP-DN.
- Citation: Kopel J, Pena-Hernandez C, Nugent K. Evolving spectrum of diabetic nephropathy. World J Diabetes 2019; 10(5): 269-279
- URL: https://www.wjgnet.com/1948-9358/full/v10/i5/269.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i5.269
Diabetes remains an important health issue as an increasing number of patients with chronic and poorly controlled diabetes develop diabetic nephropathy (DN)[1-4]. The main risk factors associated with the development of DN include hypertension, poor glycemic control, smoking, and dyslipidemia[5]. Among several ethnicities, Native Americans have the highest incidence of DN followed by Asians, Hispanics,African-Americans, and Caucasians[6]. Several genetic polymorphisms are also associated with development of DN, including angiotensin type 2 receptor and angiotensin converting enzyme (ACE)[7-10]. In recent years, the number of patients seeking dialysis for kidney-related disorders has increased with the rise in DN[11]. Specifically, DN remains the leading cause of all excess mortality among type I and II diabetic patients with microalbuminuria, macroalbuminuria, or end-stage kidney disease[12,13]. Although kidney transplantation is an option, many DN patients have frequent post-operative complications associated with kidney transplant procedures, including cerebrovascular disease events and graft rejection[14,15]. As a result, clinical studies examining the pathophysiology and therapeutic interventions for DN remain an important public health concern for reducing DN-associated end-stage renal disease and mortality.
DN begins with glomerular hyperperfusion and renal hyperfiltration and then progresses to microalbuminuria and a lowered glomerular filtration rate (GFR). Current guidelines define DN using four main criteria: a decline in renal function, diabetic retinopathy, proteinuria, and a reduction in GFR[16]. Specifically, “Overt nephropathy is characterized by persistent proteinuria (> 500 mg/24 h) that usually precedes a fall in glomerular filtration rate (GFR) significant proteinuria has therefore long been regarded as the hallmark of DN”[17]. DN is diagnosed by urinalysis and confirmed, if necessary, by a kidney biopsy, and its progression is monitored through regular measurements of microalbuminuria, serum creatinine, and calculated GFR[1,18]. With advanced cases of DN, the kidney biopsy shows mesangial hypercellularity and expansion, thickening of the basement membranes, arteriolar hyalinosis, and interstitial fibrosis. In some cases, Kimmelstiel-Wilson lesion seen in DN kidney biopsies correlate with an increased risk of worsening renal function and retinopathy[19]. However, several studies have reported substantial variability in patients with DN that deviates from accepted guidelines, which has encouraged clinicians to incorporate routine biopsy of DN patients[20,21]. As a result, DN is now viewed as a spectrum of presentations with many authorities arguing for expanding the current pathological classification of DN to improve treatment strategies and outcomes[16,22,23].
Among the parameters used to identify DN patients, the presence of proteinuria represents an important prognostic factor reflecting damage to the glomerular filtration barrier[24]. However, several studies have described DN without significant proteinuria (> 500 mg/24 h) in over 50% of diabetic patients[25-32]. Among the 15773 Type 2 diabetic patients with varying severity of renal insufficiency examined in the Renal Insufficiency and Cardiovascular Events Italian Multicenter Study, 56.6% were normoalbuminuric, 30.8% were microalbuminuric (30 to 300 mg/24 h), and 12.6% were macroalbuminuric (> 300 mg/24 h)[33]. In some cases, the proteinuria vanishes with patients having normal albuminuria levels[34-36]. For example, a six-year longitudinal study conducted by the Joslin Clinic showed that 58 percent of the 386 patients who had microalbuminuria eventually had normal albuminuria levels[34].
Compared with patients with type II diabetes and DN, patients with type 1 diabetes and DN with normoalbuminuria had more of glomerular lesions, such as increased glomerular basement membrane thickness and more Kimmelstiel-Wilson nodules, and more frequent progression of DN[28]. As shown in Table 1, a new classification was created to characterize DN patients with a decline in kidney function and vascular complications without proteinuria, known as non-proteinuric DN (NP-DN)[37,38]. Robles summarized these recent studies with this observation, “There have now been reports that in both type 1 and type 2 diabetes mellitus, a proportion of patients may have renal impairment without significant proteinuria or albuminuria, with a variable percentage of patients in these reports having advanced (stage 3–5) kidney disease. It could be interpreted as an accelerated kidney sclerosis due to the interaction of diabetes with other cardiovascular risk factors”[17]. Furthermore, a recent clinical study reported NP-DN is an increasing cause of chronic kidney disease globally[17]. At present, increasing age, repeated cardiovascular injury, such as hypertension, cardiovascular disease, and dyslipidemia, to the kidney, and an over-suppressed renal-angiotensin system have been proposed as potential mechanisms for NP-DN[17].
| Clinical parameter | Diabetic nephropathy | Non-proteinuric diabetic nephropathy |
| Proteinuria | Present | Absent |
| Regression of proteinuria | Present | Absent |
| Histology | Abnormal | Normal or abnormal |
| Glomerular filtration rate | Decreased | Decreased |
| Increased risk of chronic kidney disease | Present | Present |
Despite the increased incidence of NP-DN, few clinical or experimental studies have thoroughly investigated the pathophysiological mechanisms and targeted treatment of NP-DN. As the nephrologist Jean Halimi summarized, “it is not clear why some patients develop the ‘classical’ deiabetic nephropathy with significant proteinuria, while others have impaired renal function associated with very low levels of proteinuria that sometimes persist as late as end-stage renal disease”[38]. Furthermore, a clinical review published by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative showed that “there is insufficient evidence to assume that interventions that prevent or reverse microalbuminuria will necessarily lead to improvement in clinical outcomes and conversely that failure to reduce microalbuminuria precludes a beneficial effect of treatment on diabetic kidney disease”[39]. In this review, we will discuss the differences between DN and NP-DN and consider potential pathophysiological mechanisms, diagnostic markers, and treatment for NP-DN.
Current research suggests that increased vascular resistance in renal interlobar arteries can damage glomerular and non-glomerular nephron structures and contribute to the onset and progression of NP-DN[37,40]. A recent study reported an elevation in arterial stiffness, measured using aortic and brachial-ankle pulse wave velocity, in NP-DN patients, which was strongly associated with increased atherosclerosis and cardiovascular morbidity and mortality and decreased renal function[40-47]. Thus, NP-DN patients likely have more atherosclerosis and increased vascular resistance which reduce glomerular function and damage glomerular-tubular structures.
Several studies examining NP-DN found elevated serum uric acid levels, which were strongly associated with the development of kidney disease[48-50]. Although an antioxidant in the blood, uric acid is also a potent pro-oxidant and damages mitochondria through the stimulation of NADPH oxidases[51]. Elevated uric acid levels could also damage vascular elements and induce endothelial dysfunction through various mechanisms, including activation of Toll-like receptor pathways[51,52]. Furthermore, uric acid induces renal inflammation, vascular smooth muscle cell proliferation, and activation of the renin-angiotensin system[53-56]. Prolonged elevation of uric acid levels in NP-DN patients can produce significant vascular changes that impair renal function leading to NP-DN[57]. Therefore, elevated uric acid levels in NP-DN patients can produce more vascular damage than in DN patients. In recent years, sodium-glucose 2 (SGLT2) inhibitors were shown to increas uric acid excretion through the proximal tubule transporter, SLC2A9 (GLUT9), which improved glycemia control, weight loss, and blood pressure control among DN patients[58-60]. Future clinical studies should include serial measurements of uric acid and uric excretion between DN and NP-DN patients prescribed SGLT2 inhibitors to investigate this mechanism.
Patients with NP-DN have elevated concentrations of serum tumor necrosis factor alpha (TNFα) and Fas-pathways[61]. TNFα is a key mediator of inflammation through the induction of chemokines, IFN-γ inducible protein-10, intercellular adhesion molecule-1, and vascular adhesion molecule-1, which increase glomerular vasoconstriction and albumin permeability[61]. Furthermore, TNFα is involved in the acute kidney injury, regulation of blood pressure, blood flow, and inflammation within the renal vasculature[62-64]. TNFα and Fas also have important roles in apoptosis[61]. The FasL-Fas system regulates renal cell apoptosis during immune and inflammatory responses through the activation of renal cell Fas receptors[65]. In addition, murine models that block the FasL-Fas system prevent renal and tubular cell injury during ischemia-reperfusion experiments[65]. Thus, increased levels of TNFα and Fas in NP-DN can alter renal vasculature and damage the kidney.
NP-DN patients also have elevated levels of osteoprotegerin and vascular endothelial growth factor (VEGF), which function in inflammation and angiogenesis, respectively[66]. Interestingly, VEGF levels are inversely related to proteinuria levels in DN patients[67]. In the presence of TGF-β, VEGF signaling leads to apoptosis and potentially cause glomerular vascular atrophy[68]. Elevated serum VEGF levels in murine models initiate a feedback inhibition of VEGF production by podocytes leading to glomerular injury[69]. In addition, osteoprotegrin is associated with chronic kidney disease in diabetic patients, leading to calcification of vascular tissue, glomerular damage, and proteinuria[70,71].
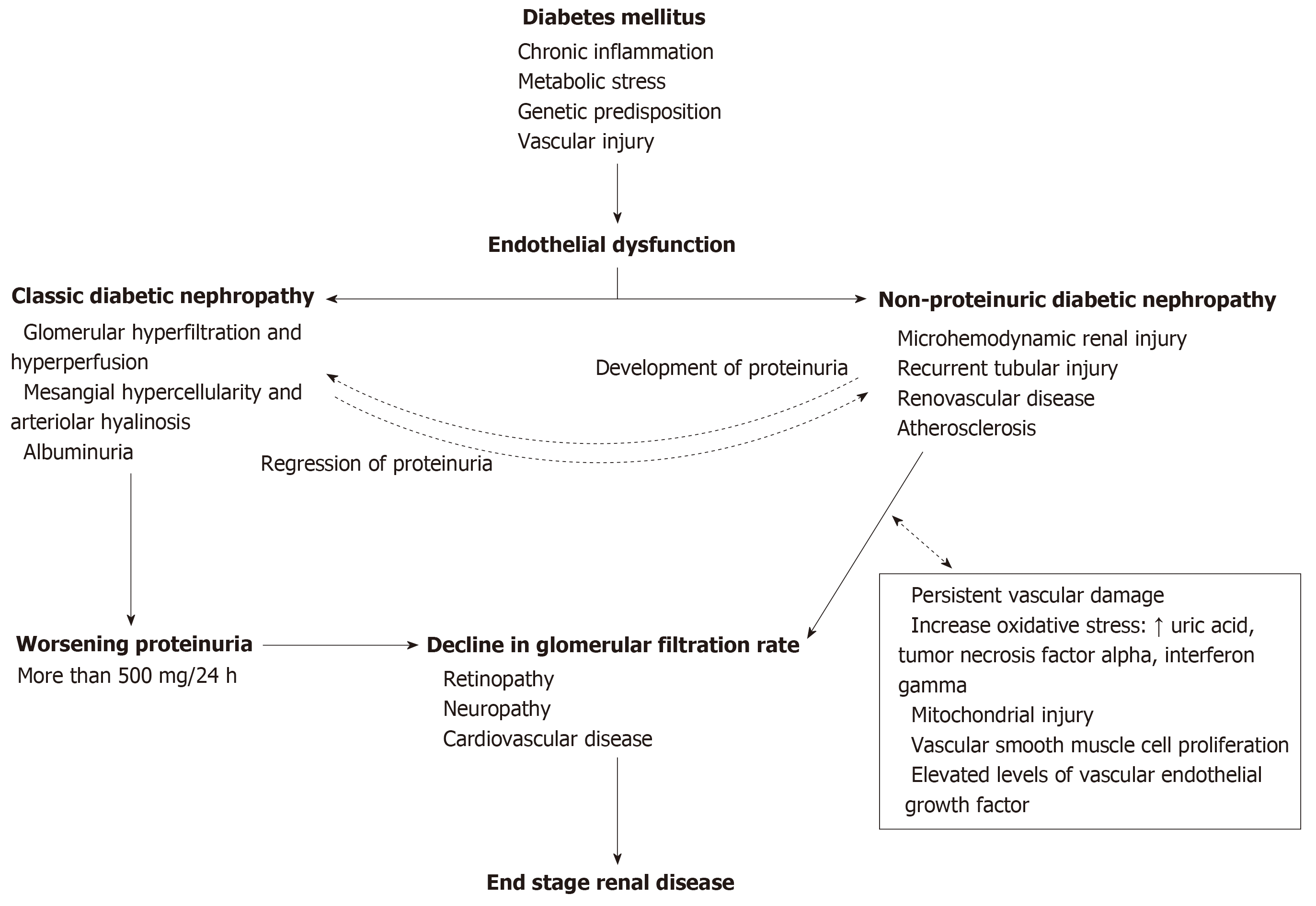
In summary, the pathogenesis of NP-DN appears to involve vascular and soluble elements circulating in the blood, as shown in Figure 1. Comparisons between DN and NP-DN patients should provide insight into the functions of these receptors and other inflammatory responses occurring within the kidney. Furthermore, additional studies investigating non-enzymatic glycation of proteins, metabolic stress, hypertension, N-terminal fragment of pro brain natriuretic peptide and glomerular vascular injury can provide additional insight into the pathogenesis of NP-DN[72]. This information may provide unique insights and possibilities for developing novel treatment for DN and NP-DN.
Given the recent identification of NP-DN, current guidelines should be expanded to include NP-DN and other forms of DN. Kidney biopsies are readily available and provide a detailed analysis of a patient’s renal disease. However, complications, such as infection, bleeding, and other vascular injuries, limit its wider use by physicians[73]. Furthermore, kidney biopsies may not fully detect the vascular changes occurring in NP-DN and DN patients. As a result, the development of safer and accessible diagnostic markers is critical for improving early diagnosis and treatment of conventional DN and NP-DN patients.
Ultrasound technology is one alternative which has provided opportunities for diagnosing and monitoring the progression of DN. Unlike renal biopsies, ultrasound represents an inexpensive and non-invasive method for examining and grading the progression of DN and other related renal pathologies, such as renal cysts or stones[74]. Ultrasound technology can provide measurements on renal anatomy and function associated with DN, acute renal failure, and cirrhosis[75]. Recent studies using ultrasound have provided an additional method for evaluating renal function in DN patients at various stages of the disease[75-79]. Specifically, an increase in the Renal Resistive Index (RRI), which measures renal vascular resistance, has been shown to reliably detect and monitor the progression of DN and NP-DN[75,80,81]. For example, a study in diabetic patients showed that RRI values were elevated in diabetic patients without overt proteinuria or renal atherosclerosis[82]. Therefore, ultrasound sonography provides an effective method to screen, identify, and monitor hemodynamic and morphologic changes in DN patients[82]. Furthermore, diabetic patients identified as high risk for DN could qualify for preventative pharmacologic treatment, which might prevent the onset of DN before the appearance of proteinuria[83]. Recent reports with ultrasound technology and DN strongly suggest that this technology could be used to differentiate DN and NP-DN for diagnostic and screening purposes[82]. In addition, only a few studies have systematically compared the renal function, prognosis, and various blood and urine components in conventional DN and NP-DN patients. More studies examining changes in the levels of TNFα, TGF-β, endothelin, and other interleukins in the blood and urine of DN and NP-DN might provide additional diagnostic criteria and potential insight into the pathophysiological mechanisms of NP-DN. More analysis comparing both groups should help clarify distinct pathological and diagnostic criteria for DN and NP-DN.
Uncontrolled hypertension produces hemodynamic stress that causes fibrinoid necrosis of small blood vessels leading to acute renal failure. The current pharmacological treatment for hypertensive disorders and glomerular vascular syndromes includes thiazide diuretics, beta blockers, ACE inhibitors, angiotensin II receptor blockers, calcium antagonists, and α1 blockers. However, these anti-hypertensive drugs fail to prevent progressive declines in GFR and renal disease[84]. As a result, the development of new pharmaceutical regimens for managing DN and NP-DN are needed[85,86]. Several studies have found ACE-inhibitors lisinopril and enalapril and the angiotensin II receptor antagonist losartan were effective in treating patients with normoalbuminuric type II diabetes through reductions in albuminuria excretion, blood pressure, creatine clearance[87-89]. In recent years, pharmacological alternatives for DN, such as heparin and antibody therapy, have been proposed for treating glomerular vascular syndromes.
Heparin is a potent glycosaminoglycan and anticoagulant used to treat and prevent deep vein thrombosis, pulmonary embolism, and arterial thromboembolism. Patients with diabetes have abnormal metabolism and catabolism of glycosaminoglycans[90]. Diabetic mouse models treated with heparin sulfate and glycosaminoglycan had significant improvement in morphological and functional renal abnormalities[90]. Unlike antihypertensive drugs, heparin reduces proteinuria and improves GFR without interacting with the renin-angiotensin-aldosterone system[91]. Similarly, sulodexide, a heparin derivative, reduced proteinuria and improved renal function in murine models when given orally, intramuscularly, or intravenously[92,93]. Clinical trials with long-term low-dose sulodexide have reported reduced proteinuria and renoprotective properties in DN, chronic kidney disease, hypertensive nephropathy, and primary glomerulonephritis[93,94]. Thus, heparin could provide an effective additive for reducing proteinuria and GFR in conventional DN and NP-DN patients on conventional antihypertensive therapy.
Given the inflammatory activities associated with diabetes, some anti-inflammatory drugs, such as pentoxifylline, have been studied in the treatment of DN[95,96]. Pentoxifylline is a methylxanthine derivative and a non-specific phosphodiesterase inhibitor of TNF-α. Several studies with pentoxifylline have shown a decrease or stabilization in the progression of DN with additional reno-protective effects, such as decreased C-reactive protein, TNF-α, and risk for long-term dialysis[97-100]. In addition, pentoxifylline attenuates the progression of glomerular crescents, sclerosis, mesangial expansion, and interstitial fibrosis seen in DN patients[101]. Patients with NP-DN have elevated levels of TNF-α and other cytokines and could respond to pentoxifylline with improvement in renal vasculature and glomerular structures. Additional studies investigating other anti-inflammatory drugs in DN and NP-DN patients would provide an alternative first-line treatment in conjunction with current anti-hypertensive therapy. Table 2 shows the summary of NP-DN literature.
| Field | Summary of non-proteinuric diabetic nephropathy literature |
| Prevalence | 57% of diabetic nephropathy patients |
| Pathogenesis | Vascular and soluble elements, such as uric acid, TNFα, and VEGF, affecting renal microhemodynamics |
| Diagnosis | (1) Increased renal resistive index; (2) Alterations in TNFα, TGF-β, endothelin, and other interleukins |
| Treatment | (1) Enalapril; (2) Losartan; (3) Heparin; (4) Pentoxifylline |
In summary, inflammation remains a central factor involved in the onset and pathogenesis of diabetes and diabetes-related complications. With the increase in NP-DN cases, new treatment and diagnostic markers are needed to understand the pathogenesis of both DN and NP-DN. New therapies beyond current anti-hypertensive therapy regimens hold promise in providing an effective measure for the prevention and treatment of DN and NP-DN. More clinical studies are needed to examine the differences between DN and NP-DN in pathogenesis, diagnosis, and treatment. Specifically, additional studies examining the use of allopurinol to reduce uric acid levels among NP-DN patients would provide a readily accessible treatment for both clinicians and patients. Furthermore, studies examining RRI can yield additional anatomical and pathophysiological data distinguishing NP-DN and DN. Despite these challenges, investigation of the pathophysiology of NP-DN requires further analysis into the cardiovascular factors influencing renal function and disease and identify novel treatment for the vascular complications seen in diabetic patients.
We thank Dr. Ali Roghani and Dr. Sharma Prabhkar for their help crafting this manuscript.
| 1. | Fineberg D, Jandeleit-Dahm KA, Cooper ME. Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol. 2013;9:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Espinel E, Agraz I, Ibernon M, Ramos N, Fort J, Serón D. Renal Biopsy in Type 2 Diabetic Patients. J Clin Med. 2015;4:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Lytvyn Y, Bjornstad P, Pun N, Cherney DZ. New and old agents in the management of diabetic nephropathy. Curr Opin Nephrol Hypertens. 2016;25:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Guideline development group. Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant. 2015;30 Suppl 2:ii1-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Lim AKh. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 413] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 6. | Prabhakar SS. Advances in Pathogenesis of Diabetic Nephropathy. New York: Nova Biomedical/Nova Science 2012; . |
| 7. | Pettersson-Fernholm K, Fröjdö S, Fagerudd J, Thomas MC, Forsblom C, Wessman M, Groop PH; FinnDiane Study Group. The AT2 gene may have a gender-specific effect on kidney function and pulse pressure in type I diabetic patients. Kidney Int. 2006;69:1880-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Dellamea BS, Pinto LC, Leitão CB, Santos KG, Canani LH. Endothelial nitric oxide synthase gene polymorphisms and risk of diabetic nephropathy: a systematic review and meta-analysis. BMC Med Genet. 2014;15:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Lin Z, Huang G, Zhang J, Lin X. Adiponectin gene polymorphisms and susceptibility to diabetic nephropathy: a meta-analysis. Ren Fail. 2014;36:478-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 10. | Zhou TB, Guo XF, Yin SS. Association of peroxisome proliferator-activated receptor γ Pro12Ala gene polymorphism with type 2 diabetic nephropathy risk in Caucasian population. J Recept Signal Transduct Res. 2014;34:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16:1859-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Groop PH, Thomas MC, Moran JL, Wadèn J, Thorn LM, Mäkinen VP, Rosengård-Bärlund M, Saraheimo M, Hietala K, Heikkilä O, Forsblom C; FinnDiane Study Group. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 462] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 13. | Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 962] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 14. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3961] [Article Influence: 146.7] [Reference Citation Analysis (2)] |
| 15. | Lentine KL, Rocca Rey LA, Kolli S, Bacchi G, Schnitzler MA, Abbott KC, Xiao H, Brennan DC. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin J Am Soc Nephrol. 2008;3:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Bermejo S, Pascual J, Soler MJ. The large spectrum of renal disease in diabetic patients. Clin Kidney J. 2017;10:255-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Robles NR, Villa J, Felix FJ, Fernandez-Berges D, Lozano L. Non-proteinuric diabetic nephropathy is the main cause of chronic kidney disease: Results of a general population survey in Spain. Diabetes Metab Syndr. 2017;11 Suppl 2:S777-S781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Tan R, Warraich T. Histopathologic Aspects of Diabetic Nephropathy. Advances in Pathogenesis of Diabetic Nephropathy. New York: Nova Biomedical/Nova Science 2012; . |
| 19. | Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. The Collaborative Study Group. Nephrol Dial Transplant. 1998;13:2547-2552. [PubMed] |
| 20. | Yuan CM, Nee R, Ceckowski KA, Knight KR, Abbott KC. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin Kidney J. 2017;10:257-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A. Diabetic nephropathy: Is it time yet for routine kidney biopsy? World J Diabetes. 2013;4:245-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 22. | Piccoli GB, Grassi G, Cabiddu G, Nazha M, Roggero S, Capizzi I, De Pascale A, Priola AM, Di Vico C, Maxia S, Loi V, Asunis AM, Pani A, Veltri A. Diabetic Kidney Disease: A Syndrome Rather Than a Single Disease. Rev Diabet Stud. 2015;12:87-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Pugliese G. Updating the natural history of diabetic nephropathy. Acta Diabetol. 2014;51:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Drakopoulos A, Goumenos DS, Vlachojannis JG, Tsakas S. Endothelin receptors in the kidney of patients with proteinuric and non-proteinuric nephropathies. Ren Fail. 2006;28:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Watanabe Y, Fujii H, Aoki K, Kanazawa Y, Miyakawa T. A cross-sectional survey of chronic kidney disease and diabetic kidney disease in Japanese type 2 diabetic patients at four urban diabetes clinics. Intern Med. 2009;48:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Budhiraja P, Thajudeen B, Popovtzer M. Absence of albuminuria in type 2 diabetics with classical diabetic nephropathy: Clinical pathological study. J of Biomed Sci and Engineering. 2013;06:20-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Kramer CK, Leitão CB, Pinto LC, Silveiro SP, Gross JL, Canani LH. Clinical and laboratory profile of patients with type 2 diabetes with low glomerular filtration rate and normoalbuminuria. Diabetes Care. 2007;30:1998-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Hobeika L, Hunt KJ, Neely BA, Arthur JM. Comparison of the Rate of Renal Function Decline in NonProteinuric Patients With and Without Diabetes. Am J Med Sci. 2015;350:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Laranjinha I, Matias P, Mateus S, Aguiar F, Pereira P, Perneta Santos M, Costa R, Lourenço A, Guia J, Barata JD, Campos L. Diabetic kidney disease: Is there a non-albuminuric phenotype in type 2 diabetic patients? Nefrologia. 2016;36:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Robles NR, Villa J, Gallego RH. Non-Proteinuric Diabetic Nephropathy. J Clin Med. 2015;4:1761-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Porrini E, Ruggenenti P, Mogensen CE, Barlovic DP, Praga M, Cruzado JM, Hojs R, Abbate M, de Vries AP; ERA-EDTA diabesity working group. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 33. | Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Cavalot F, Cignarelli M, Laviola L, Morano S, Nicolucci A, Pugliese G; Renal Insufficiency And Cardiovascular Events (RIACE) Study Group. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011;29:1802-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 528] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 35. | Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, Koya D. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. 2005;54:2983-2987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 787] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 37. | Chawla V, Roshan B. Non-proteinuric diabetic nephropathy. Curr Diab Rep. 2014;14:529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Halimi JM. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab. 2012;38:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol. 2010;21:2020-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Nakao K, Uzu T, Araki S, Kume S, Deji N, Chin-Kanasaki M, Araki H, Isshiki K, Sugimoto T, Kawai H, Nishio Y, Kashiwagi A, Maegawa H. Arterial stiffness and renal impairment in non-proteinuric type 2 diabetic patients. J Diabetes Investig. 2012;3:86-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2551] [Cited by in RCA: 2673] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 42. | Madero M, Wassel CL, Peralta CA, Najjar SS, Sutton-Tyrrell K, Fried L, Canada R, Newman A, Shlipak MG, Sarnak MJ; Health ABC Study. Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol. 2009;20:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Smith A, Karalliedde J, De Angelis L, Goldsmith D, Viberti G. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Tetzner F, Scholze A, Wittstock A, Zidek W, Tepel M. Impaired vascular reactivity in patients with chronic kidney disease. Am J Nephrol. 2008;28:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Ratto E, Leoncini G, Viazzi F, Vaccaro V, Falqui V, Parodi A, Conti N, Tomolillo C, Deferrari G, Pontremoli R. Ambulatory arterial stiffness index and renal abnormalities in primary hypertension. J Hypertens. 2006;24:2033-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 46. | Mulè G, Cottone S, Cusimano P, Incalcaterra F, Giandalia M, Costanzo M, Nardi E, Palermo A, Geraci C, Costa R, Cerasola G. Inverse relationship between ambulatory arterial stiffness index and glomerular filtration rate in arterial hypertension. Am J Hypertens. 2008;21:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | García-García A, Gómez-Marcos MA, Recio-Rodriguez JI, González-Elena LJ, Parra-Sanchez J, Fe Muñoz-Moreno M, Alonso CP, Gude F, García-Ortiz L. Relationship between ambulatory arterial stiffness index and subclinical target organ damage in hypertensive patients. Hypertens Res. 2011;34:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Rosolowsky ET, Ficociello LH, Maselli NJ, Niewczas MA, Binns AL, Roshan B, Warram JH, Krolewski AS. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 49. | Mohandas R, Johnson RJ. Uric acid levels increase risk for new-onset kidney disease. J Am Soc Nephrol. 2008;19:2251-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Giordano C, Karasik O, King-Morris K, Asmar A. Uric Acid as a Marker of Kidney Disease: Review of the Current Literature. Dis Markers. 2015;2015:382918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121:e71-e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 52. | Rabadi MM, Kuo MC, Ghaly T, Rabadi SM, Weber M, Goligorsky MS, Ratliff BB. Interaction between uric acid and HMGB1 translocation and release from endothelial cells. Am J Physiol Renal Physiol. 2012;302:F730-F741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553-3562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 684] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 54. | Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 560] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 55. | Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, Dai C, Yang J. Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PLoS One. 2012;7:e39738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 56. | Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, Johnson RJ, Kang DH. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 2013;304:F471-F480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 57. | Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811-1821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1874] [Cited by in RCA: 1922] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 58. | Xin Y, Guo Y, Li Y, Ma Y, Li L, Jiang H. Effects of sodium glucose cotransporter-2 inhibitors on serum uric acid in type 2 diabetes mellitus: A systematic review with an indirect comparison meta-analysis. Saudi J Biol Sci. 2019;26:421-426. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, Bull S, Cannon CP, Charytan DM, de Zeeuw D, Edwards R, Greene T, Heerspink HJL, Levin A, Pollock C, Wheeler DC, Xie J, Zhang H, Zinman B, Desai M, Perkovic V; CREDENCE study investigators. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) Study Rationale, Design, and Baseline Characteristics. Am J Nephrol. 2017;46:462-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 60. | Rastogi A, Bhansali A. SGLT2 Inhibitors Through the Windows of EMPA-REG and CANVAS Trials: A Review. Diabetes Ther. 2017;8:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Niewczas MA, Ficociello LH, Johnson AC, Walker W, Rosolowsky ET, Roshan B, Warram JH, Krolewski AS. Serum concentrations of markers of TNFalpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2009;4:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 63. | Vielhauer V, Mayadas TN. Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol. 2007;27:286-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76:262-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 65. | Ortiz A, Lorz C, Egido J. The Fas ligand/Fas system in renal injury. Nephrol Dial Transplant. 1999;14:1831-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Esteghamati A, Arefzadeh A, Zandieh A, Salehi Sadaghiani M, Noshad S, Nakhjavani M. Comparison of osteoprotegerin and vascular endothelial growth factor in normoalbuminuric Type 1 diabetic and control subjects. J Endocrinol Invest. 2013;36:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Bortoloso E, Del Prete D, Dalla Vestra M, Gambaro G, Saller A, Antonucci F, Baggio B, Anglani F, Fioretto P. Quantitave and qualitative changes in vascular endothelial growth factor gene expression in glomeruli of patients with type 2 diabetes. Eur J Endocrinol. 2004;150:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P. VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2006;103:17260-17265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Hakroush S, Moeller MJ, Theilig F, Kaissling B, Sijmonsma TP, Jugold M, Akeson AL, Traykova-Brauch M, Hosser H, Hähnel B, Gröne HJ, Koesters R, Kriz W. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am J Pathol. 2009;175:1883-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 70. | Montañez-Barragán A, Gómez-Barrera I, Sanchez-Niño MD, Ucero AC, González-Espinoza L, Ortiz A. Osteoprotegerin and kidney disease. J Nephrol. 2014;27:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Lewis JR, Lim WH, Ueland T, Wong G, Zhu K, Lim EM, Bollerslev J, Prince RL. Elevated Circulating Osteoprotegerin and Renal Dysfunction Predict 15-Year Cardiovascular and All-Cause Mortality: A Prospective Study of Elderly Women. PLoS One. 2015;10:e0134266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Danis R, Ozmen S, Arikan S, Gokalp D, Alyan O. Predictive value of serum NT-proBNP levels in type 2 diabetic people with diabetic nephropathy. Diabetes Res Clin Pract. 2012;95:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | Brachemi S, Bollée G. Renal biopsy practice: What is the gold standard? World J Nephrol. 2014;3:287-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Jastaniah SM, Alsayed NA, Awad IR, Fida HH, Elniel H. Evaluation of renal disorders in type 2 diabetic patients using ultrsonography. Open J of Med Imaging. 2013;03:165-170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Fiorini F, Barozzi L. The role of ultrasonography in the study of medical nephropathy. J Ultrasound. 2007;10:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Leong-Poi H. Contrast ultrasound and targeted microbubbles: diagnostic and therapeutic applications in progressive diabetic nephropathy. Semin Nephrol. 2012;32:494-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Chong YB, Keng TC, Tan LP, Ng KP, Kong WY, Wong CM, Cheah PL, Looi LM, Tan SY. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail. 2012;34:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Goya C, Kilinc F, Hamidi C, Yavuz A, Yildirim Y, Cetincakmak MG, Hattapoglu S. Acoustic radiation force impulse imaging for evaluation of renal parenchyma elasticity in diabetic nephropathy. AJR Am J Roentgenol. 2015;204:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 79. | Lin HY, Lee YL, Lin KD, Chiu YW, Shin SJ, Hwang SJ, Chen HC, Hung CC. Association of Renal Elasticity and Renal Function Progression in Patients with Chronic Kidney Disease Evaluated by Real-Time Ultrasound Elastography. Sci Rep. 2017;7:43303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Abd El Dayem S, El Bohy Ael M, El Shehaby A. Value of the intrarenal arterial resistivity indices and different renal biomarkers for early identification of diabetic nephropathy in type 1 diabetic patients. J Pediatr Endocrinol Metab. 2016;29:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Spatola L, Andrulli S. Doppler ultrasound in kidney diseases: a key parameter in clinical long-term follow-up. J Ultrasound. 2016;19:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Mancini M, Masulli M, Liuzzi R, Mainenti PP, Ragucci M, Maurea S, Riccardi G, Vaccaro O. Renal duplex sonographic evaluation of type 2 diabetic patients. J Ultrasound Med. 2013;32:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G; Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 84. | Kasper DL, Hauser SL, Jameson JL, Fauci AS, Longo DL, Loscalzo J. Harrison's Principles of Internal Medicine. 19th ed. New York City: McGraw-Hill 2015; 2424-2426. |
| 85. | Usuelli V, La Rocca E. Novel therapeutic approaches for diabetic nephropathy and retinopathy. Pharmacol Res. 2015;98:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Arora MK, Singh UK. Molecular mechanisms in the pathogenesis of diabetic nephropathy: an update. Vascul Pharmacol. 2013;58:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 87. | Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet. 1997;349:1787-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 333] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 88. | Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;128:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 89. | Crespo A, Cedillo A, Fernández R. Nephroprotection in normotensive normoalbuminuria diabetic patients. Pinnacel Journal Publication. 2016;3. |
| 90. | Gambaro G, Baggio B. Role of glycosaminoglycans in diabetic nephropathy. Acta Diabetologica. 1992;29:149-155. [DOI] [Full Text] |
| 91. | Benck U, Haeckel S, Clorius JH, van der Woude FJ. Proteinuria-lowering effect of heparin therapy in diabetic nephropathy without affecting the renin-angiotensin-aldosterone system. Clin J Am Soc Nephrol. 2007;2:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Yung S, Chau MK, Zhang Q, Zhang CZ, Chan TM. Sulodexide decreases albuminuria and regulates matrix protein accumulation in C57BL/6 mice with streptozotocin-induced type I diabetic nephropathy. PLoS One. 2013;8:e54501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Gaddi AV, Cicero AF, Gambaro G. Nephroprotective action of glycosaminoglycans: why the pharmacological properties of sulodexide might be reconsidered. Int J Nephrol Renovasc Dis. 2010;3:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Zilişteanu DS, Atasie T, Voiculescu M. Efficacy of long-term low-dose sulodexide in diabetic and non-diabetic nephropathies. Rom J Intern Med. 2015;53:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Eren Z, Günal MY, Bakir EA, Coban J, Çağlayan B, Ekimci N, Ethemoglu S, Albayrak O, Akdeniz T, Demirel GY, Kiliç E, Kantarci G. Effects of paricalcitol and aliskiren combination therapy on experimental diabetic nephropathy model in rats. Kidney Blood Press Res. 2014;39:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Rodriguez-Morán M, González-González G, Bermúdez-Barba MV, Medina de la Garza CE, Tamez-Pérez HE, Martínez-Martínez FJ, Guerrero-Romero F. Effects of pentoxifylline on the urinary protein excretion profile of type 2 diabetic patients with microproteinuria: a double-blind, placebo-controlled randomized trial. Clin Nephrol. 2006;66:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, Macía M, del Castillo N, Rivero A, Getino MA, García P, Jarque A, García J. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015;26:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 98. | Goicoechea M, García de Vinuesa S, Quiroga B, Verdalles U, Barraca D, Yuste C, Panizo N, Verde E, Muñoz MA, Luño J. Effects of pentoxifylline on inflammatory parameters in chronic kidney disease patients: a randomized trial. J Nephrol. 2012;25:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Sun HK, Lee YM, Han KH, Kim HS, Ahn SH, Han SY. Phosphodiesterase inhibitor improves renal tubulointerstitial hypoxia of the diabetic rat kidney. Korean J Intern Med. 2012;27:163-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Kuo KL, Hung SC, Liu JS, Chang YK, Hsu CC, Tarng DC. Add-on Protective Effect of Pentoxifylline in Advanced Chronic Kidney Disease Treated with Renin-Angiotensin-Aldosterone System Blockade - A Nationwide Database Analysis. Sci Rep. 2015;5:17150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Chen YM, Chiang WC, Yang Y, Lai CF, Wu KD, Lin SL. Pentoxifylline Attenuates Proteinuria in Anti-Thy1 Glomerulonephritis via Downregulation of Nuclear Factor-κB and Smad2/3 Signaling. Mol Med. 2015;21:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Saeki K S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ