Published online Oct 15, 2019. doi: 10.4239/wjd.v10.i10.490
Peer-review started: May 20, 2019
First decision: May 31, 2019
Revised: September 25, 2019
Accepted: September 25, 2019
Article in press: September 25, 2019
Published online: October 15, 2019
Processing time: 154 Days and 10.8 Hours
The prevalence of type 2 diabetes (T2D) has increased worldwide and doubled over the last two decades. It features among the top 10 causes of mortality and morbidity in the world. Cardiovascular disease is the leading cause of complications in diabetes and within this, heart failure has been shown to be the leading cause of emergency admissions in the United Kingdom. There are many hypotheses and well-evidenced mechanisms by which diabetic cardiomyopathy as an entity develops. This review aims to give an overview of these mechanisms, with particular emphasis on metabolic inflexibility. T2D is associated with inefficient substrate utilisation, an inability to increase glucose metabolism and dependence on fatty acid oxidation within the diabetic heart resulting in mitochondrial uncoupling, glucotoxicity, lipotoxicity and initially subclinical cardiac dysfunction and finally in overt heart failure. The review also gives a concise update on developments within clinical imaging, specifically cardiac magnetic resonance studies to characterise and phenotype early cardiac dysfunction in T2D. A better understanding of the pathophysiology involved provides a platform for targeted therapy in diabetes to prevent the development of early heart failure with preserved ejection fraction.
Core tip: Altered myocardial metabolism and impaired metabolic flexibility are key mechanisms implicated in diabetic cardiomyopathy. Glucotoxicity, lipotoxicity, coronary microvascular dysfunction and suboptimal substrate utilisation are examined in detail. The mechanisms implicated and the impact on myocardial structure and function have been scrutinised within this review.
- Citation: Athithan L, Gulsin GS, McCann GP, Levelt E. Diabetic cardiomyopathy: Pathophysiology, theories and evidence to date. World J Diabetes 2019; 10(10): 490-510
- URL: https://www.wjgnet.com/1948-9358/full/v10/i10/490.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i10.490
Type 2 diabetes (T2D) is now a global pandemic. The disease is characterised by insulin resistance, relative impairment of insulin secretion and increased hepatic glucose output resulting in high blood glucose levels. It is now among the top 10 causes of death and represents a major cause of mortality and morbidity in the world[1]. Globally there were 422 million patients with diabetes in 2014, with growing prevalence from 4.7% in 1980 to 8.5% in 2014[2]. Heart failure (HF) has emerged as the most common initial cardiovascular complication of diabetes[2,3]. T2D is likely to contribute to the development of HF through a variety of mechanisms, including disease specific myocardial structural, functional and metabolic changes. In the 2015-16 England and Wales National Diabetes Audit, there were 115695 emergency admissions for patients with diabetes and HF compared to 21399 with myocardial infarction and 29392 with stroke[4]. Once HF diagnosis is established in T2D patients over the age of 65, mortality risk increases tenfold, and five-year survival reduces to 12.5%[5].
The diabetic population has been shown to pose a marked preponderance to developing HF following a myocardial infarction[6-8]. In addition to this, systolic and diastolic left ventricular dysfunction has also been described unrelated to the presence of macrovascular coronary disease[9-11]. The prevalence of HF in the general population has been estimated to be 11.8%[12], whereas in clinical trials of cardiovascular outcomes in T2D patients, the prevalence of HF at baseline has varied between approximately 10% and 30% encompassing both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF) with a doubling of the risk of developing HF in those aged 75-84[13-23].
Diabetic cardiomyopathy is defined as cardiac dysfunction encompassing structural, functional and metabolic changes in the absence of coronary artery disease (CAD)[24-26]. This distinct clinical entity was first proposed by Lundbaek[27] in 1954 as diabetic heart disease independent of hypertension and CAD that commonly co-exist with T2D. Rubler et al[9] in 1972 went on to confirm these observations, describing diabetic-related post-mortem findings in four patients with T2D, glomerulosclerosis and HFrEF with normal epicardial coronary arteries the absence of hypertension, CAD, valvular or congenital heart disease. A large United States nationwide case-control study by Bertoni et al[28] in 1995 further confirmed an association between non-ischaemic idiopathic cardiomyopathy and diabetes. Another recent large population study showed that despite good control of all cardiovascular risk factors, the risk of HF hospitalisation in T2D was still consistently higher although there was little or no increase in risk of mortality, myocardial infarction, or stroke in comparison to the general population[29].
Experimental studies have shown that functional and structural alterations within the diabetic heart may be caused by a significant difference in underlying metabolic mechanisms and substrate utilisation. These have been supported by multiple hypotheses on how T2D disease processes affect the structure and function of the myocardium resulting in a HF-like phenotype[30]. The aetiology of diabetic cardiomyopathy is multifactorial. Despite decades of research aiming to determine the causes of this distinct pathology, more research is needed. Current hypotheses include insulin resistance, endothelial dysfunction, fibrosis, cardiac lipotoxicity and energetic impairment. In particular, cardiac metabolism may play a central role in diabetic cardiomyopathy.
Type 1 diabetes (T1D) is a condition of absolute insulin deficiency due to T-cell–mediated autoimmune destruction of pancreatic β-cells. Cardiovascular disease is again a major long-term sequelae of the disease with an impact on healthcare resources. This encompasses coronary artery disease, cerebrovascular disease, peripheral artery disease, heart failure and cardiomyopathy. The pathophysiology of these processes vary and most of the data from population studies and large databases are focused on T2D. Studies looking at atherectomy samples have previously shown that the pathology of atherosclerosis did not differ between diabetes type[31]. Angiographic evidence showed that T1D caused more multivessel, distal and severe stenosis[32]. T1D appear to be affected more by hypoglycaemia and inflammation. Inflammatory markers such as C reactive protein, interleukin receptors and CD4 ligands are higher in T1D[33,34]. Excess adiposity and altered fat distribution have been shown to contribute to diabetic cardiomyopathy in T1D similar to T2D. For the purposes of this review, we will be focusing on the evidence base behind diabetic cardiomyopathy in T2D.
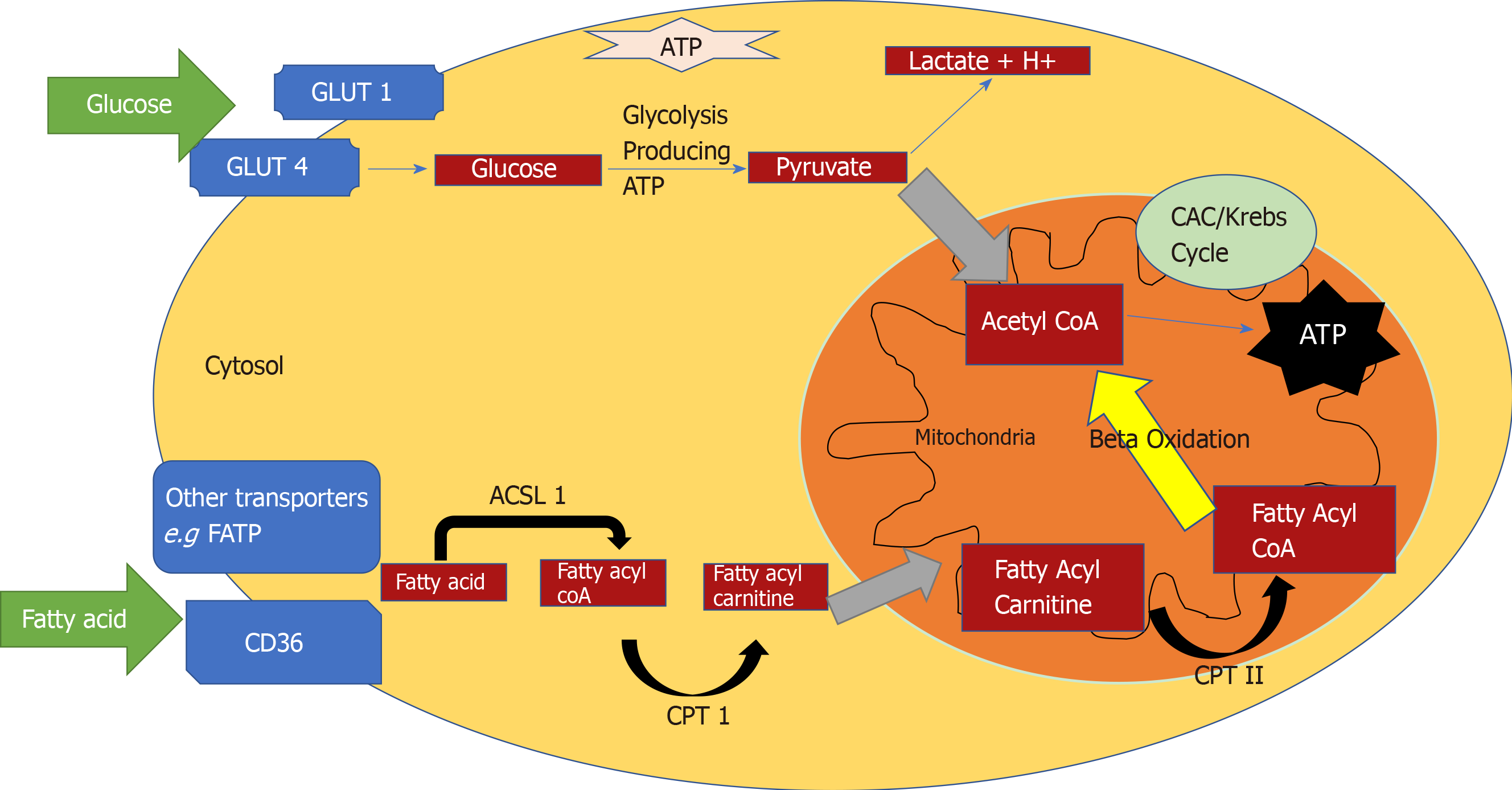
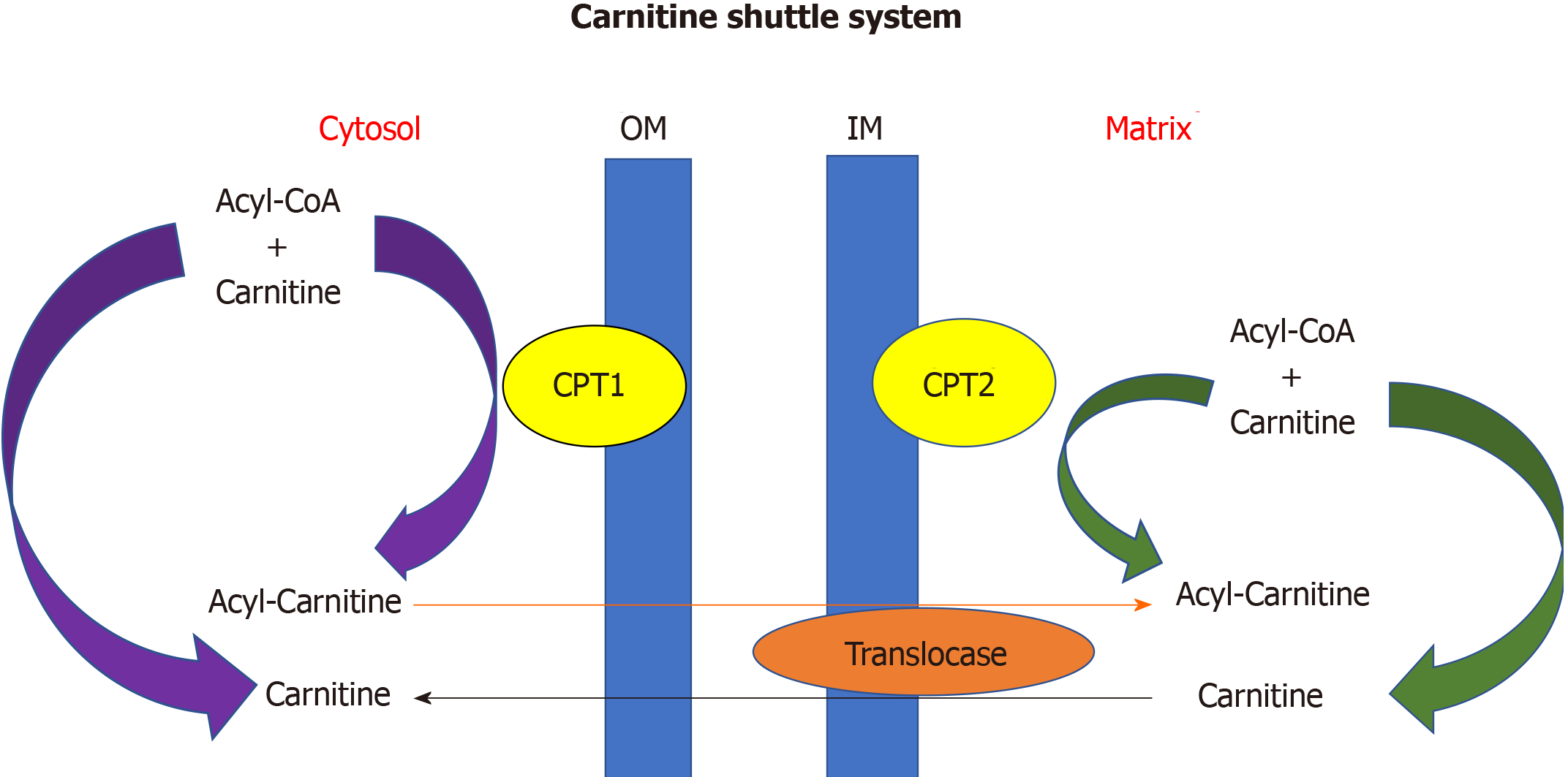
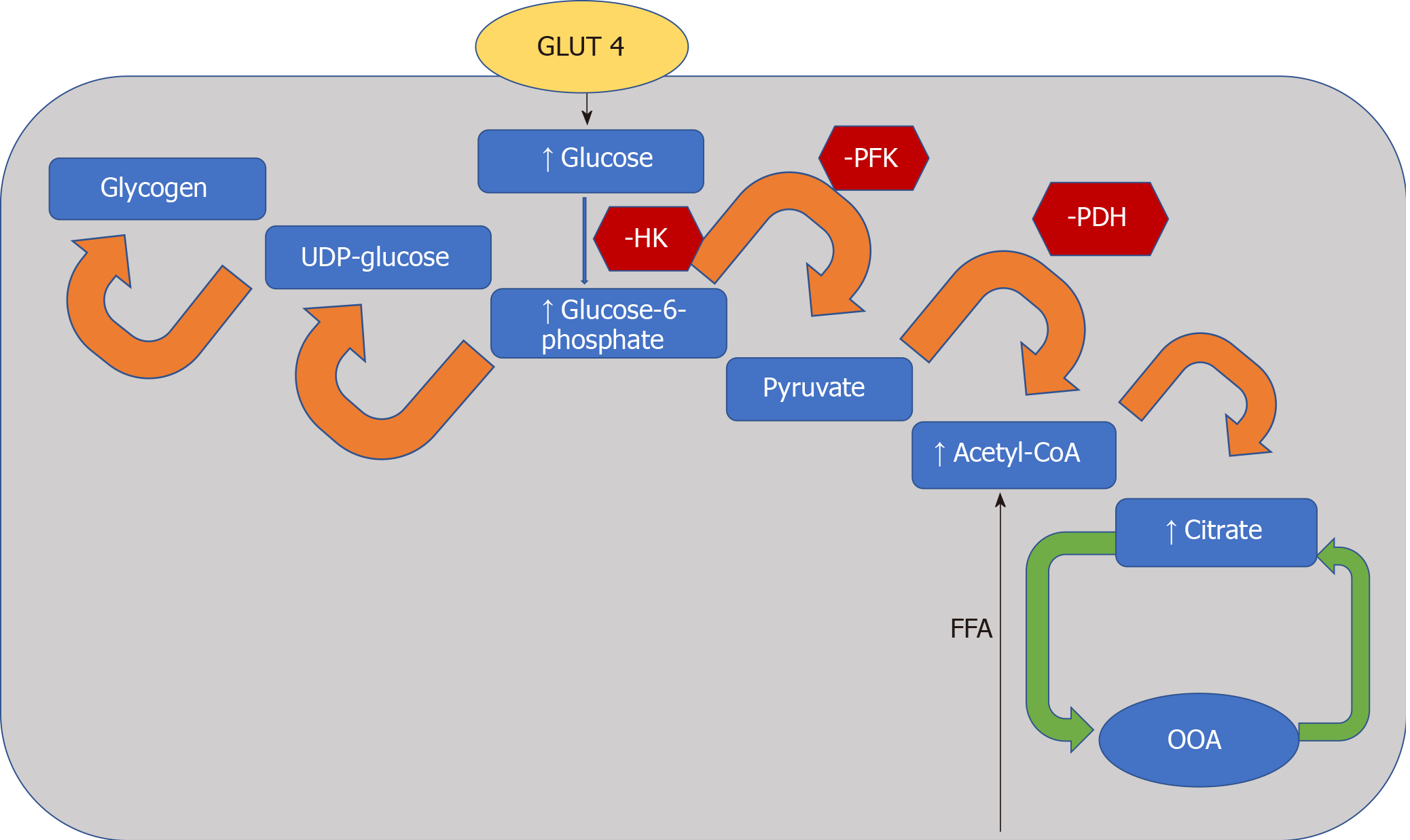
The heart converts chemical energy present in the form of substrates and oxygen to mechanical energy and heat[35,36]. Maintenance of adequate levels of cardiac high-energy phosphate metabolites, adenosine triphosphate (ATP), the energy source for contraction, and phosphocreatine (PCr), the major energy storage compound, are of vital importance for normal heart function[37]. A healthy heart is capable of metabolising a range of substrates, including fatty acid (FA), glucose, amino acids, ketones and lactate, to produce ATP[38,39]. In the normal heart, the main substrates for acetyl-CoA and therefore ATP formation are long chain FAs (60%-90%) and glucose and pyruvate oxidation (10%-40%, formed from glycolysis). The normal cardiac metabolic process involves energy transfer initially from substrate to the high energy phosphate metabolite ATP. This occurs through the generation of acetyl-CoA, that then enters the tricarboxylic acid cycle (TCA), also known as Krebs or citric acid cycle, followed by oxidative phosphorylation[40] resulting in the production of ATP (Figure 1). This is then transferred through facilitated diffusion to the areas requiring the high energy released through ATP hydrolysis, i.e., myosin and sarcoplasmic reticulum[37,41-43]. Figure 2 shows the role of carnitine in the mitochondrial oxidation of fatty acids contained within Figure 1. Figure 3 details the Randle cycle, also referred to as the glucose-fatty acid cycle which helps regulate uptake and utilisation of glucose within the muscle dependent on the rate of fatty acid oxidation.
Altered myocardial substrate metabolism may play a central role in cardiac dysfunction in T2D patients[40,44,45], by affecting myocardial oxygen demand which in turn results in reduced metabolic flexibility[39,46,47]. Metabolic flexibility describes the ability of an organism to respond to changes in metabolic or energy demand as well as the prevailing conditions or activity[38,39]. There are no myocardial ATP reserves[35,48,49]. Energy in the heart is stored in three forms. The first form of stored energy is phosphocreatine, which can rapidly donate its high energy phosphates to produce ATP from ADP. The second endogenous form of energy is glycogen. The third form of stored energy is represented by triglycerides.
Myocardial substrate metabolism can be measured directly by cardiac catheterization and transmyocardial blood sampling. Cardiac metabolic flexibility can be evaluated by coronary sinus (CS) studies invasively and positron emission tomography (PET) studies. Substrate concentrations in arterial and CS blood, in combination with the measured CS flow, yield information about the myocardial use of substrates and oxygen[50]. Simultaneous CS and coronary artery blood sampling allow detailed analyses of myocardial substrate metabolism obtained by measuring the arteriovenous extraction of carbohydrates, fat, ketones, and amino acids. The flow in the CS correlates with cardiac output, mean arterial pressure, and the flow through the coronary arteries and varies with myocardial oxygen demand[51]. The technique has been used in healthy individuals[52], in patients with dilated cardiomyopathy[53], and syndrome X[54] during incremental pacing. These studies looked into FA, glucose and lactate metabolism in the heart as key sources of energy production as well as myocardial oxygen consumption at rest and during periods of increased cardiac work. They confirmed that in healthy individuals, cardiac metabolism relies predominantly on oxidation of FAs, but during increased cardiac workload with maximal pacing stress, glucose uptake increases by a factor of two, while FA metabolism does not change. In summary these studies confirmed that, although FAs are the predominant fuel for energy provision in the human heart in the resting state, carbohydrates are the fuel for the heart in a state of exercise or stress. In T2D, cardiomyocytes have been shown to exhibit insulin resistance, decreased glucose utilisation and increased fatty acid oxidation in response to stress, insulin or variability in FA availability confirming that cardiac metabolic reserve is impaired in diabetes[55].
Stress metabolism can also be assessed non-invasively by PET. There have been only a few studies to date using PET to assess cardiac metabolism in patients with T2D. However, there has been conflicting outcomes from these studies[56] regarding the changes in myocardial glucose uptake and insulin resistance in T2D patients. While one study showed reduced myocardial glucose uptake and myocardial insulin resistance, another study showed preserved myocardial insulin responsiveness in patients with T2D[46,57]. One small study using PET imaging to characterize myocardial glucose metabolism in patients with T2D evaluated the influence of chronic hypertriglyceridemia on myocardial glucose uptake. They compared five T2D patients with high triglyceride (TG) levels to 11 T2D patients with normal TG. Patients were matched by age, gender, blood pressure and glycaemic control. They showed 40 and 65% lower myocardial glucose uptake in the high TG group compared normal TG group, respectively, and evidence of myocardial insulin resistance in T2D. Another small PET study assessing skeletal muscle and myocardial glucose uptake in 10 patients with T2D compared to nine age and weight-matched healthy male controls under normoglycaemic hyperinsulinaemic conditions, showed preserved myocardial glucose uptake[57]. A third PET study compared glucose utilization in diabetic (n = 19) and nondiabetic CAD patients (n = 35) undergoing coronary artery bypass graft surgery grafting surgery (CABG) also showed lower myocardial glucose uptake in diabetic cohort, but this study did not assess myocardial insulin resistance[56].
The phosphocreatine to ATP (PCr/ATP) ratio is a sensitive measure of myocardial energetic status. 31-Phosphorus magnetic resonance spectroscopy (31P-MRS) provides a non-invasive quantification of myocardial PCr/ATP. Normally, the body is able to increase phosphotransferase reactions, glycolysis and glycogenolysis to function under higher energy demands[43,58,59]. Scheuermann-Freestone et al[60] pioneered the study of myocardial energetics in 2003 by studying cardiac and skeletal muscle energetics in T2D. Twenty one diabetics and 15 controls were studied to determine normal energy metabolism and the effect of T2D on circulating glucose and free fatty acid concentrations. They showed that, in the presence of normal cardiac structure, function and morphology, patients with T2D had 35% lower PCr/ATP ratios. There was a significant negative correlation with fasting plasma FA concentration as well as a significant positive correlation with plasma glucose concentration in diabetics compared to controls. In skeletal muscle, energetics was normal at rest, but the decrease in PCr was faster during exercise in those with diabetes and the recovery was slower after exercise. This lends credibility to the theory that changes in myocardial and skeletal muscle energetics is part of the initial disease process in T2D and this results in changes in substrate use and availability. Cardiac muscle is more affected by the energetic alterations and substrate availability[60].
Shivu et al[61] the looked at 25 asymptomatic type 1 diabetics vs 26 healthy controls who underwent 31P-MRS and adenosine stress cardiovascular magnetic resonance (CMR) and again this showed significantly reduced PCr/ATP ratio in diabetics, both long-term and newly-diagnosed, compared to healthy volunteers (2.23 ± 0.56 vs 1.49 ± 0.44, respectively, P < 0.001). Mean myocardial perfusion reserve index (MPRI) correlated negatively with duration of diabetes, with a significant reduction in long-term diabetes (1.7 ± 0.6) compared to newly diagnosed subjects (2.1 ± 0.2, P < 0.05) and controls (2.3 ± 0.3, P = 0.05). However, there was no significant correlation between PCr/ATP and MPRI implying the myocardial energetic alteration was independent of any coronary microvascular dysfunction primarily results from metabolic dysfunction[61].
Most recently, Levelt et al[62] have shown significant correlation between myocardial systolic strain and rest and stress PCr/ATP in 31 T2D compared to 16 healthy controls. Subjects underwent 31P-MRS performed both at rest and exercise and adenosine stress CMR for assessment of perfusion by MPRI and oxygenation. The PCr/ATP was 17% lower in T2D compared to controls (1.74 ± 0.26 vs 2.07 ± 0.35, respectively, P = 0.001) at rest, with a further 12% reduction in PCr/ATP during exercise in T2Ds. However, there was no change in PCr/ATP in the control group during exercise. Similarly, MPRI was lower in diabetes (1.61 ± 0.43 vs 2.11 ± 0.68 in controls, P = 0.002). At rest, no correlation was observed between PCr/ATP and MPRI, but a significant correlation was noted during exercise. These findings support the notion that under stress microvascular dysfunction may exacerbate energetic derangement[62].
Cardiac metabolic reserve is impaired in diabetes. Lower metabolic reserve is likely to be the consequence of impaired metabolic flexibility and may be associated with increased mortality, as has been shown in a small study of patients with dilated cardiomyopathy[63].
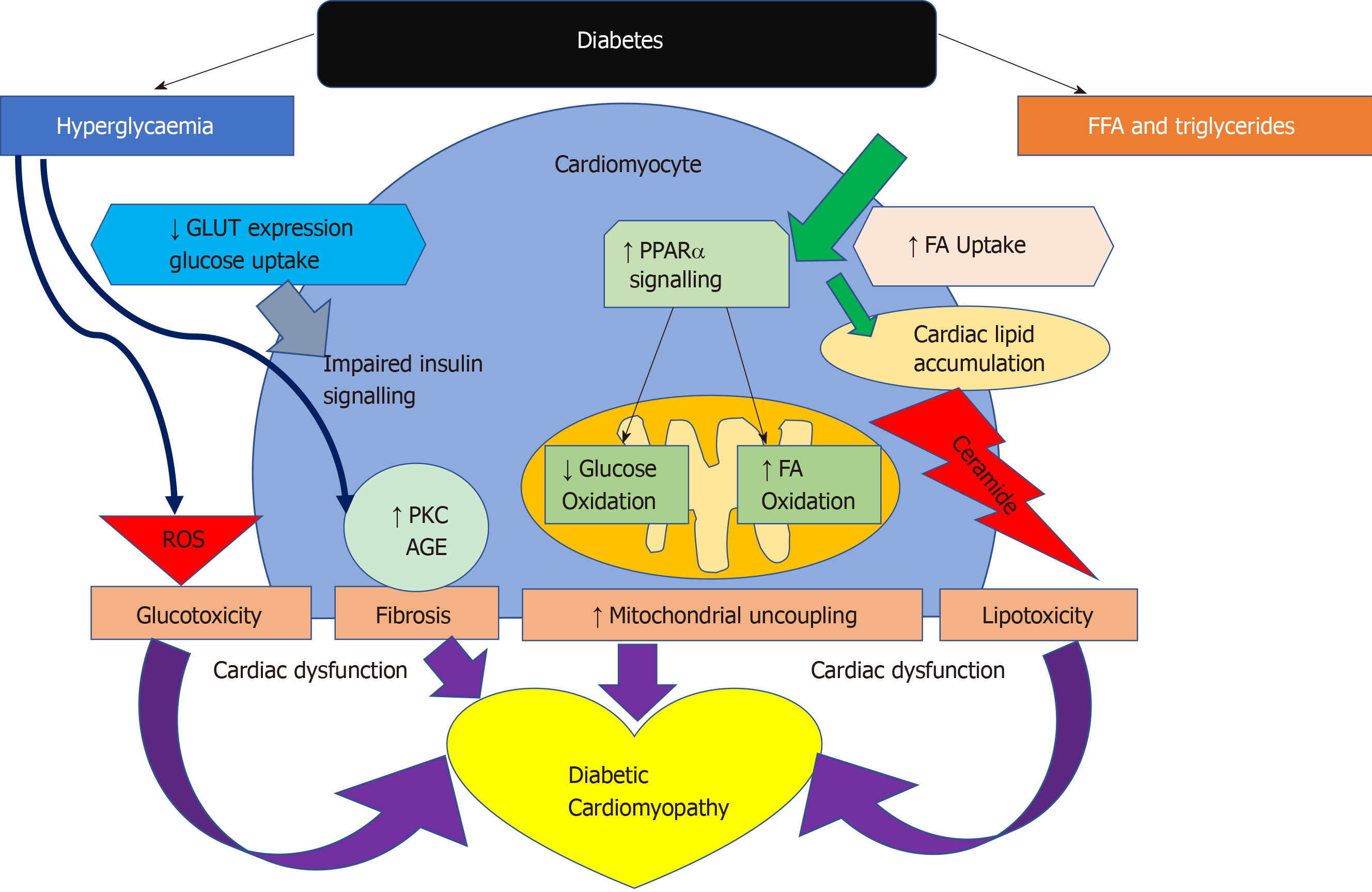
Activity of pyruvate dehydrogenase (PDH), which is a key enzyme that regulates the balance between carbohydrate and fat metabolism in the heart, is decreased in diabetes and pyruvate oxidation impaired[64,65]. Inhibition of PDH limits pyruvate oxidation[66]. The dissociation of glycolysis and pyruvate oxidation in the diabetic heart results in the accumulation of glycolytic intermediates[67]. Failure to adequately control intracellular glucose levels has also been implicated in the development of insulin resistance and in the generation of reactive oxygen species (ROS). These glycolytic intermediates activate glucose sensing transcription factors[68,69]. It has been previously postulated that accumulation of glycolytic intermediates decreases the sarcoplasmic reticulum calcium ATPase 2a (SERCA2a) expression[70], an essential enzyme in calcium homeostasis, which results in diastolic dysfunction[71]. Confirming this, SERCA2a expression in the heart was shown to be decreased in response to diabetes[71,72].
In diabetes, a decrease in insulin sensitivity leads to an inability to suppress lipase within adipose tissue and very low-density lipoprotein within the liver. This results in high levels of circulating FAs and as a consequence peroxisome proliferator activated receptor-α (PPARα) activation, while decreasing glucose-transporter-4 activity[47,73]. PPARα is an essential component in cardiac substrate switching. Decreased PPARα expression (due to pressure overload and/or prolonged exposure to hyperglycemia and/or hyperlipidemia) will limit the FA oxidative capacity of the heart[74]. The discordance between the rates of FA availability and/or uptake with that of FA oxidation results in increased intracellular long chain fatty acyl-CoA concentrations[46] Since cardiomyocytes are not specialised to store lipid, this finding suggests a deleterious effect, and cellular lipid overloading underlies the concept of “lipotoxicity” as a potential mechanism for impaired cardiac function[75-78]. The excess long chain fatty acyl-CoA is then diverted towards non oxidative processes with the production of lipotoxic intermediates such as ceramide and diacyl-glycerol[47]. This is postulated to be a potential mechanism for impaired cardiac structure and function[76-79]. The molecular mechanisms described above that have been implicated in the pathophysiology of diabetic cardiomyopathy are detailed in Figure 4.
Proton (1H)-MRS offers a non-invasive method to measure cardiac triglyceride (TG) content. Using this methodology, studies have shown myocardial TG content to be significantly raised in T2D (Table 1). The studies are all consistent in reporting an increased hepatic triglyceride content in the diabetic population. Levelt et al[62] has also recently shown that myocardial lipid level is a predictor of concentric LV remodelling independent of BMI, systolic and diastolic blood pressure, and circulating FA, and is associated with subclinical contractile dysfunction in T2D. The study recruited 46 non-hypertensive T2D patients and 20 matched controls. Seventy four percent of the diabetic patients were on statin therapy resulting in lower total cholesterol and low-density lipoprotein cholesterol levels in patients compared to controls. This provides another avenue for potential therapy in T2D as myocardial steatosis has been found to be reversible with cholesterol and triglyceride control in addition to glucose control[80,81].
| Study details (Author,Year) | Sample population | Exclusion | Results |
| Jankovic et al[92], 2012 | n = 18 | Previous MI, CAD, HF, digitalis use or thiazolidinediones, previous insulin use or T1D | Baseline IT myocardial lipid content 0.42% ± 0.12% vs 0.80% ± 0.11% water signal, (aP = 0.034). |
| IT (n = 10) | |||
| Mean age 56 ± 2 | Patients on insulin must have had insufficient control on oral, HbA1C > 8% and on oral therapy | Myocardial lipid content decreased by 80% after 10 d IT (P = 0.008). No significant change in hepatic lipid content. After 181 ± 49 d, myocardial lipid content returned to baseline (0.37 ± 0.06, P = 0.692) Hepatic lipid content decreased by 31% (bP < 0.001) | |
| DM duration 9 ± 2 yr | |||
| 6 males | |||
| HbA1c 11.1 ± 0.4 | |||
| Oral therapy (OT) (n = 8) | |||
| Mean age 53 ± 2 | |||
| DM duration 3 ± 1 yr | |||
| 4 males | |||
| HbA1c 9.8% ± 0.7% | |||
| Korosoglou et al[119], 2012 | n = 58 | Unstable condition, clinical signs of heart failure or angina contraindications for CMR, insulin use | Significant association between myocardial triglyceride content and mean diastolic strain rate (r = -0.71, cP < 0.001) and no association was found between triglyceride content and perfusion reserve (r = -0.08, P = NS) |
| T2D (n = 42) | |||
| Mean age 62 ± 6 yr | |||
| 26 male | |||
| Mean BMI 31.6 ± 4.8 kg/m2 | |||
| HV (n = 16) | |||
| Mean age 62 ± 3 yr | |||
| 10 male | |||
| Mean age 62 ± 3 yr | |||
| Mean BMI 23.9 ± 2.5 | |||
| Van der Meer et al[101,155], 2009 | n = 72 T2D | BP > 150/85 mmHg, previous insulin or thiazolidinedione use, previous positive stress echo or arrhythmia, diabetes related complications or significant medical problems | No significant change in myocardial fatty acid uptake at follow up on either arm. Metformin arm showed a significant decrease in fatty acid oxidation and myocardial glucose uptake. No significant change in myocardial triglyceride content in Pioglitazone or Metformin arm after therapy however there was a decrease in hepatic triglyceride content in the Pioglitazone arm |
| All males | |||
| Pioglitazone (n = 39) | |||
| Metformin (n = 39) | |||
| Baseline age 45-65 | |||
| HbA1C 6.5%-8.5% | |||
| BMI 25-32 | |||
| Rijzewijk et al[83], 2008 | n = 66 | Females, HbA1C > 8.5%, BP > 150/80, hepatic impairment or history of liver disease, substance abuse, known CVD, DM complications, contraindication to MRI, use of lipid lowering therapy. | Myocardial triglyceride content in T2D vs controls (0.96% ± 0.07% vs 0.65% ± 0.05%). Hepatic triglyceride content in T2D vs controls (8.6% vs 2.2%). Both cases, dP < 0.05 On univariate analysis, myocardial triglyceride content correlated with age, visceral adipose tissue, cholesterol, plasma glucose and insulin and hepatic triglyceride content (eP < 0.05 for all). E/A independently associated with myocardial triglyceride content on multivariate analysis (inverse correlation) |
| T2D (n = 38) | |||
| All males | |||
| mean age 57 ± 1 yr | |||
| BMI: 28.1 ± 0.6 | |||
| Controls (n = 28) | |||
| All males | |||
| Mean Age: 54 ± 1 | |||
| BMI: 26.9 ± 0.5 | |||
| McGavock[82] 2007 | n = 134 | Age > 70 yr, known CAD, Previous MI, contraindications to MRI, thiazolidinedione treatment | ↑Subcutaneous, visceral fat and hepatic triglyceride in O,I and T2D vs L, ↑myocardial triglyceride content in I and DM vs L (0.95 ± 0.60 vs 1.06 ± 0.62 vs 0.46 ± 0.30 fat/water content, fP < 0.05), this remained significant after adjusted for serum triglyceride, BMI, age and gender. In multiple regression model, Subcutaneous and visceral fat both independent determinants of myocardial triglyceride content (gP < 0.05) however myocardial triclyceride unrelated to hepatic triglyceride or diastolic function |
| Lean(L) (n = 15) | |||
| Age 35 ± 3 yr, 47% males | |||
| BMI 23 ± 2, non T2D | |||
| Overweight/Obese(O): (n = 21) Age 36 ± 12, BMI 32 ± 5, 48% males , non T2D | |||
| Impaired glucose tolerance(I): (n = 20) | |||
| Age 49 ± 9, 25% males, BMI 31 ± 6, | |||
| T2D (n = 78), | |||
| Age 47 ± 10, 47% males BMI 34 ± 7 |
Increased myocardial TG content has been shown in T2D. Compared with lean subjects, myocardial TG content was elevated 2.3-fold in impaired glucose tolerance and 2.1-fold in T2D. This was evidenced in a cross sectional study looking at both lean and increased BMI participants with and without T2D[82]. Myocardial TG has also been shown to be an independent predictor of left ventricular diastolic function in multivariable analysis accounting for BMI, heart rate, visceral fat and diastolic blood pressure. In a study of 38 T2D and 28 matched controls, myocardial TG measured by (1H)-MRS was significantly higher in T2D. Although there was no significant difference in systolic function, all indices of diastolic function were significantly impaired in T2D[83]. Interestingly, calorific restriction in obese T2D patients on a very low calorie diet of 450 kcal/d was been shown to decrease myocardial TG content from 0.88 +/- 0.12% to 0.64 +/- 0.14%, respectively (P = 0.019) over a 16 wk period, and this was associated with better diastolic function (E/A ratio from 1.02 +/- 0.08 to 1.18 +/- 0.06)[84]. Myocardial TG level is a predictor of concentric LV remodelling independent of BMI, systolic and diastolic BP, circulating FA and is associated with subclinical contractile dysfunction in T2D[62].
Pathological evidence characterised by myofibrillar hypertrophy with fibrotic strands extending between muscle fibres to cause diffuse myocardial fibrosis first helped distinguish cardiomyocyte damage in diabetes[9]. The advancement in non-invasive imaging techniques has facilitated further delineation and phenotyping of the diabetic heart. Alterations to left ventricular geometry lead to concentric remodelling, hypertrophy and eventually increased mass. Left ventricular hypertrophy in diabetes includes both concentric and eccentric hypertrophy. Left ventricular concentric remodelling has been shown to have a higher association with cardiovascular mortality than eccentric remodelling on both echocardiographic and CMR studies[62,85-87].
Increased left ventricular mass had initially been shown to be associated with a rise in HbA1c levels in a HF population[88]. Studies using both echocardiography and MRI have been successful in showing an increase in left ventricular mass associated with diabetes independently of other factors[10,62,85,87,89-94]. Table 2 details studies that have examined left ventricular mass and concentric remodelling on MRI in T2D. In summary, there is a general trend that shows an increase in left ventricular mass. However, when corrected for body surface area, this does not persist. In two studies, there remained a higher left ventricular mass/volume ratio, but due to a general increase in end diastolic volume this difference is obliterated when corrected for volume. A decrease in stroke volume is as expected in concentric remodelling. Increased left ventricular mass is a known predictor of cardiovascular mortality and morbidity[95,96].
| Study details (Author, Year) | Sample population | Exclusion criteria | Main findings |
| Ng et al[91], 2012 | n = 69 | Age < 18 yr, arrhythmia, CAD, MI, RWMA, segmental LGE, EF < 50%, valve disease | No difference between groups for LVEDVI, LVESVI, LVMI, LVEF. |
| DMs (n = 50, 35 T1DM) Mean age 51 ± 10 yr, 54% males. BMI 26.3 ± 3.7 | |||
| Controls (n = 19), matched for age (45 ± 15), sex (63.2% males) an BMI 26.1 ± 4.4 | |||
| Wilmot et al[93], 2014 | T2D n = 20, mean age 31.8 ± 6.6, BMI 33.9 ± 5.8 kg/m2 | Weight > 150 kg, contraindications to MRI. In diabetic group BMI > 30 (> 27.5 in South Asians) | ↑ LVM (85.2 vs 80.8 g, jP = 0.002) and LVM/Volume (0.54 vs 0.45 g/m2, kP = 0.029) in participants with diabetes compared to lean controls. No significant difference in LVMI |
| Lean Controls: n = 10, Mean age 30.9 ± 5.6, Mean BMI 33.4 ± 2.4, 60% males | |||
| Obese Controls: n = 10, Mean age 30.0 ± 6.7, Mean BMI 21.9 ± 1.7, 50% males | |||
| Larghat et al[10], 2014 | T2DM: n = 19 Mean age 59 ± 6, 68% males, BMI 30.81 ± 4.6 | Coronary artery Stenosis > 30% luminal narrowing on angiography, previous MI, significant heart disease, contraindications to MRI or adenosine | ↑ LVM (112.8 ± 39.7 vs 91.5 ± 21.3 g, lP = 0.01) in participants with diabetes. Participants with diabetes also showed an increase in LVEDV and SV, but non indexed |
| Pre-DM: n = 30 Mean age 57 ± 8, 43% males, BMI 30.1 ± 5.0 | |||
| Non DM: n = 46, Mean age 57 ± 7, 41% male, BMI 29 ± 4.9 | |||
| Levelt et al[62], 2016 | T2DM: n = 39, Mean age 55 ± 9, 58% males, BMI 28.7 ± 5.6 | History of CVD, chest pain, smoker, uncontrolled hypertension, contraindications to MRI, ischaemia on ECG, renal dysfunction, insulin use, significant CAD on CTCA | EF, LVM, LVMI, no significant difference between groups. |
| Controls: n = 17, Mean age 50 ± 14 yr | ← LVM/Volume (0.98 ± 0.21 vs 0.70 ± 0.12, mP < 0.001), LVDEV (125 ± 30 mL vs 161 ± 39 mL, nP = 0.001) and lower SV in diabetes | ||
| 53% males, BMI 27.1 ± 5.0 |
CMR T1 mapping pre and post-contrast allows calculation of myocardial extracellular volume (ECV) which is a surrogate for diffuse interstitial fibrosis and correlates with pathology samples obtained in patients with heart failure[97]. Levelt et al[62] used similar techniques and concluded that although there was concentric remodelling and evidence of diastolic dysfunction, there was no evidence of expansion of extracellular matrix in people with well-controlled T2D. However, this is contrary to most existing literature on ECV and T1 mapping to date, which has shown increased ECV and native T1 values in T2D compared to healthy controls[98,99]. Swoboda et al[100] also showed increased ECV and native T1 relaxation at baseline in T2D and demonstrated that treatment with renin-angiotensin-aldosterone system inhibition led to an improvement in left ventricular ejection fraction and a decrease in myocardial ECV. The differences in literature on extracellular matrix may be related to patient selection for the controls. For example, young and healthy controls rather than age-matched non-diabetics. Sensitivity of different T1 mapping techniques may also play a small role.
Diabetic cardiomyopathy has mainly been linked with features of diastolic dysfunction. This is especially apparent in asymptomatic individuals as the earliest sign of HF. Most of the evidence in imaging of patients with T2D have not shown a significant decrease in ejection fraction/systolic dysfunction[88,101-103] with the exception of the Strong Heart Study where a direct correlation of ejection fraction was seen associated with HbA1c levels[87]. Diastolic dysfunction is now regarded as the first functional change occurring in diabetic cardiomyopathy.
Strain is a measure of tissue deformation. As the ventricle contracts, muscle shortens longitudinally and circumferentially and thickens radially. The application of strain to measure deformation is constrained by a number of complexities when the parameter is measured by echocardiography[104]. Tissue Doppler imaging (TDI) in echocardiography improved the identification of early diastolic dysfunction and recent developments in strain imaging on CMR has very much improved the sensitivity and accuracy of identifying subclinical diastolic dysfunction in the T2D population. Measurement of strain using CMR is now considered the gold standard. Strain rate measures the time course of deformation and this can be derived from speckle tracking echocardiography or measured directly from MRI cine images with dedicated software programmes of which several are available.
Both longitudinal and circumferential strain have been shown to be impaired in T2D. Table 3 identifies the larger and more recent studies that have shown early changes in strain in people with diabetes[11,88,91,94,105-107]. Different studies have studied and reported different measures of strain making the literature not directly comparable. Global longitudinal strain (GLS) has been shown to be decreased in the diabetic population compared to healthy volunteers in all the studies described across tissue Doppler, speckle tracking echocardiography and CMR. Additionally, some studies have shown a decrease in radial strain. One study has shown that peak early diastolic strain rate (PEDSR) and peak systolic strain rate are both decreased in diabetes even when compared to obese/overweight non-diabetic controls[11]. Strain and strain rate, especially PEDSR is a good measure contractility and contractile reserve. Its application in clinical practice remains in infancy to be used to predict potential development of heart failure with preserved ejection fraction in the form of a contractility index. It is widely regarded as a precursor to the onset of heart failure in T2D and a predictor of cardiovascular mortality and morbidity even in the absence of symptoms[108,109].
| Publication and imaging modality | Group and baseline characteristics | Exclusion | Main findings |
| Ernande et al[107], 2010 | T2DM: n = 119, 69 males | LVEF < 56%, age < 35 or > 65, signs, symptoms or history of heart disease, no RWMA, valve disease, renal disease, T1DM, poor DM control (HbA1C > 12%) | ↓GLS (-19.3% ± 3% vs -22% ± 2%) and GRS (50% ± 16% vs 56% ± 12%, nP < 0.003) in participants with diabetes vs participants without diabetes |
| Echocardiography | Controls: n = 39, 30 males | Multivariate analysis showed DM (t = 3.9, P < 0.001) and gender (t = 3.4, P = 0.001) independent determinants of GLS, DM only independent determinant of GRS. | |
| Ng et al[91], 2012 | n = 69 | Age < 18 yr, arrhythmia, CAD, MI, RWMA, segmental LGE, EF < 50%, valve disease | ↓GLS DM vs controls (-16.1% ± 1.4% vs 20.2% ± 1.0% pP < 0.001) |
| MRI | DMs (n = 50, 35 T1DM) Mean age 51 ± 10 yr, 54% males. BMI 26.3 ± 3.7 | ↓GLS DM T2DM vs T1DM (-15.3% ± 1.2% vs 16.4% ± 1.4%, qP = 0.009) | |
| Controls (n = 19), matched for age (45 ± 15), sex (63.2% males) an BMI 26.1 ± 4.4 | |||
| Khan et al[11], 2014 | T2D n = 20, Mean age 31.8 ± 6.6, BMI 33.9 ± 5.8 kg/m2 | Weight > 150 kg, contraindications to MRI. In diabetic group BMI > 30 (> 27.5 in South Asians) | ↓PEDSR in DMs vs Lean controls vs obese controls (1.51 ± 0.24 vs 1.97 ± 0.34 vs 1.78 ± 0.39 s-1) and PSSR (-21.20 ± 2.75 vs -23.48 ± 2.36 vs -23.3 ± 2.62s-1) |
| MRI | Lean Controls: n = 10, Mean age 30.9 ± 5.6, Mean BMI 33.4 ± 2.4, 60% males. | ||
| Obese controls: n = 10, Mean age 30.0 ± 6.7, Mean BMI 21.9 ± 1.7, 50% males | |||
| Levelt et al[62], 2016 | T2D: n = 39, Mean age 55 ± 9, 58% males. | History of CVD, chest pain, smoker, uncontrolled hypertension, contraindications to MRI, ischaemia on ECG, renal dysfunction, insulin use, significant CAD on CTCA | ↓GLS (-9.6 ± 2.9 vs -11.4 ± 2.8 rP = 0.049) and mid ventricular (-14.2 ± 2 vs -19.3, sP < 0.001) systolic strain (PCr/ATP ratio at rest and exercise, correlated with reduced systolic strain results; there was also a negative association between the myocardial lipid levels and systolic strain in this cohort) |
| MRI | Controls: n = 17, Mean age 50 ± 14 yr |
Coronary microvascular dysfunction in diabetes is a complex pathophysiological process, which involves structural, functional and metabolic alterations. It is likely to be a multifactorial phenomenon, related to changes in perivascular and interstitial fibrosis[110], myocardial hypertrophy[111], reduced capillary density, and autonomic neuropathy[112]. Coronary microvascular dysfunction has emerged among the potential mechanisms leading to increased incidence of heart failure[113] and risk of cardiovascular mortality[3,114] in patients with diabetes. Both hyperglycaemia and dyslipidaemia have been shown to cause abnormal structure and function within the endothelium. High circulating glucose concentration causes downregulation of nitric oxide resulting in increased vasoconstriction[115]. This in turn results in increased vascular tone, permeability, thinning of vascular endothelium, weakening of intercellular junctions, altered protein synthesis and ultimately causes remodelling[115].
Using CMR[10,94,116] and PET[114,117] myocardial perfusion during vasodilator stress has been shown to be impaired in patients with diabetes[10,94,116] and in the absence of epicardial coronary artery stenosis, this finding is indicative of coronary microvascular dysfunction. Importantly, coronary microvascular dysfunction is an early precursor of cardiovascular events and was shown to be associated with a 2.5% annual major adverse event rate that includes cardiovascular mortality, nonfatal myocardial infarction, nonfatal stroke, and congestive HF even among patients without epicardial coronary artery stenosis[118]. Consequently, early identification of coronary microvascular disease may be beneficial in prognosis evaluation and patient stratification for optimal medical therapy
Using adenosine stress perfusion CMR Larghat et al[10] have assessed myocardial perfusion reserve in 65 patients with no significant epicardial coronary artery stenosis on angiography (< 30% coronary luminal stenosis). Left ventricular mass was shown to be higher in diabetics than non-diabetics (112.8 ± 39.7 g vs 91.5 ± 21.3 g, P = 0.01) and in diabetics than prediabetics (112.8 ± 39.7 g vs 90.3 ± 18.7 g, P = 0.02). MPR was lower in diabetics than non-diabetics (2.10 ± 0.76 vs 2.84 ± 1.25, respectively, P = 0.01)[10]. Khan et al[11] have also studied 40 young patients (mean age 30 years), including 20 diabetics and 10 lean and 10 obese controls who underwent CMR. This study, however, did not show any significant correlation between left ventricular intracardiac measures, including mass, MPR and perfusion and the presence of T2D[11]. Diastolic dysfunction was found to be associated with TG content but not with impaired myocardial perfusion reserve in another study with patients with T2D with preserved systolic function[119].
Another study looking at the interplay between metabolic and ischaemic changes in the hearts of people with well-controlled T2D, even in the absence of arterial hypertension or significant CAD on computed tomography coronary angiography, showed there were significant reductions in MPRI and evidence of blunted oxygenation response compared with controls. These findings support the concept that hypoperfusion as a result of microvascular dysfunction plays a role in the impaired ability to increase and/or maintain myocardial oxygenation during vasodilator stress in diabetes[94].
In a large study of 2783 consecutive patients (1172 diabetics and 1611 non-diabetics) measuring coronary flow reserve (CFR) by PET[120], they showed that diabetic patients with preserved CFR have similar rates of cardiac events to nondiabetic patients, whereas impaired CFR was associated with an adjusted 3.2- and 4.9-fold increase in the rate of cardiac death for both diabetics and nondiabetics. This raises the question of whether CFR should be included in a cardiac risk model for patients with diabetes. However, there is selection bias due to patients recruited consecutively, some had regional perfusion defects and the results of angiography were not known therefore the impaired flow reserve cannot be attributed directly to microvascular dysfunction.
In a small (n = 64) randomised trial comparing the effects of spironolactone, hydrochlorothiazide or placebo on coronary flow reserve in T2D assessed by cardiac positron emission tomography, spironolactone was associated with modest improvements in coronary flow reserve (from 2.77 ± 0.82 to 3.10 ± 0.74) compared to the other two treatment arms (P = 0.01) after six months[117]. The mechanism for this is thought to be due to expression of the mineralocorticoid receptor in endothelium, vascular smooth muscle cells, cardiomyocytes and circulating leukocytes[117]. Mineralocorticoid receptor activation causes vascular inflammation with increased ROS production and increased vascular damage, vascular dysfunction, and perivascular fibrosis. The findings of this study support the potential role of mineralocorticoid use in reversing coronary microvascular dysfunction in T2D. However, there was no reporting of cardiovascular outcomes, relatively small sample size and limited follow up period.
The evidence of the role of coronary microvascular dysfunction in T2D is conflicting. This suggests that the metabolic dysfunction caused by diabetes likely may predispose to some degree of microvascular dysfunction however these may not be the main mechanisms contributing to cardiac dysfunction in diabetes.
The evidence for pharmacological therapy for heart failure targeted at the diabetic population thus far has been limited to studies of both patients with and without diabetes. ACE-inhibitors are recommended within the ESC/EASD guidance for diabetes and impaired glucose tolerance. The CHARM, ATLAS and HEAAL trials have all shown the beneficial effects of ACE in HF in terms of morbidity and mortality, however subgroup analyses showed no difference with and without diabetes[121-124]. This is similarly the case with the use of mineralocorticoid antagonists[125,126], nitrates and hydralazine[127], ivabradine[128] as well as more recently sacubitril/valsartan[129].
The impact of better glycaemic control on left ventricular systolic and[130] diastolic function remains debatable due to varying evidence. Previous large studies including United KingdomPDS[131], ADVANCE[130] , ACCORD[132] and VADT[15] have not shown an improvement in cardiovascular outcomes with good glycaemic control. A meta-analysis of 37229 people with T2D data from multiple randomized trials concluded there was no observed benefit of intensive glycaemic control on HF related outcomes[133]. Patients with T2D who received intensive glucose lowering therapy had a reduced risk of major adverse cardiovascular events, but there was no effect on total mortality, cardiac death, stroke, and heart failure[133].
There is also a large body of evidence to suggest T2D therapies may increase the risk of developing heart failure. This has been particularly the case with dipeptidyl peptidase-4 (DPP4) inhibitor saxagliptin and thiazolidinediones (TZDs). In SAVOR-TIMI 53 looking at saxagliptin vs placebo there was a significantly increased risk of heart failure hospitalisation[134,135]. These patients then went on to have a high rate of subsequent death. The latter result was also found in the RECORD trial[136]. The TECOs trial looking at sitagliptin[17] and EXAMINE for alogliptin[18], both against placebo there was no increase in heart failure. Further studies are required to consolidate the effects of DPP4 inhibitors on heart failure in T2D. There are current ongoing studies, CARMELINA (Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients with Type 2 Diabetes Mellitus; NCT01897532) and CAROLINA (Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes; NCT01243424), looking at Linagliptin in T2D that may provide better insight.
More recent studies have shown favourable cardiovascular outcomes with newer glucose lowering agents. There have recently been numerous non-inferiority cardiovascular outcome trials using newer glucose lowering therapies in T2D, namely GLP1 – Receptor Agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors.
Multiple glucagon-like peptide-1 (GLP-1) receptor agonists in the form of once weekly exenatide, dulaglutide, albiglutide and semaglutide have been recently developed. GLP-1 agonists exert their effects by stimulating insulin secretion, suppressing appetite, decreasing circulating glucagon levels and gastric emptying[137]. They have also been associated with weight loss. The cardiovascular benefits of GLP-1 is multifaceted. The first is glycaemic control. Long acting agonists have been shown to be better at reducing HBA1c than short term[138]. Despite the failure to show an improvement in cardiovascular outcomes with some large studies in the past[15,139,140], the United Kingdom PDS study that looked at tight glycaemic control at diagnosis showed a reduction in cardiovascular events after a decade even with similar HbA1c levels[131]. The second is through blood pressure control. None of the GLP-1 studies conducted thus far have been specifically to look at its’ effects on blood pressure however both studies as well as reviews and meta analyses have shown a decrease in BP of both exenatide and liraglutide compared with placebo and other antidiabetic drugs[141]. It has also been shown to have favourable effects on the vascular endothelium in that Liraglutide has been shown to improve nitric oxide synthase (NOS) activity as well as attenuates plasminogen activator inhibitor type 1 (PAI-1) and vascular adhesion molecule (VAM) within the vascular endothelium. These apply a protective effect against endothelial dysfunction[142,143]. GLP-1 agonists also appear to have a cardioprotective effect on atherosclerosis progression and myocardial ischaemia, and this has been shown with the use of exenatide as an adjunct therapy in STEMI and thrombolysis[144,145]. GLP-1 agonist use has not been associated with a significant effect on heart failure hospitalisation.
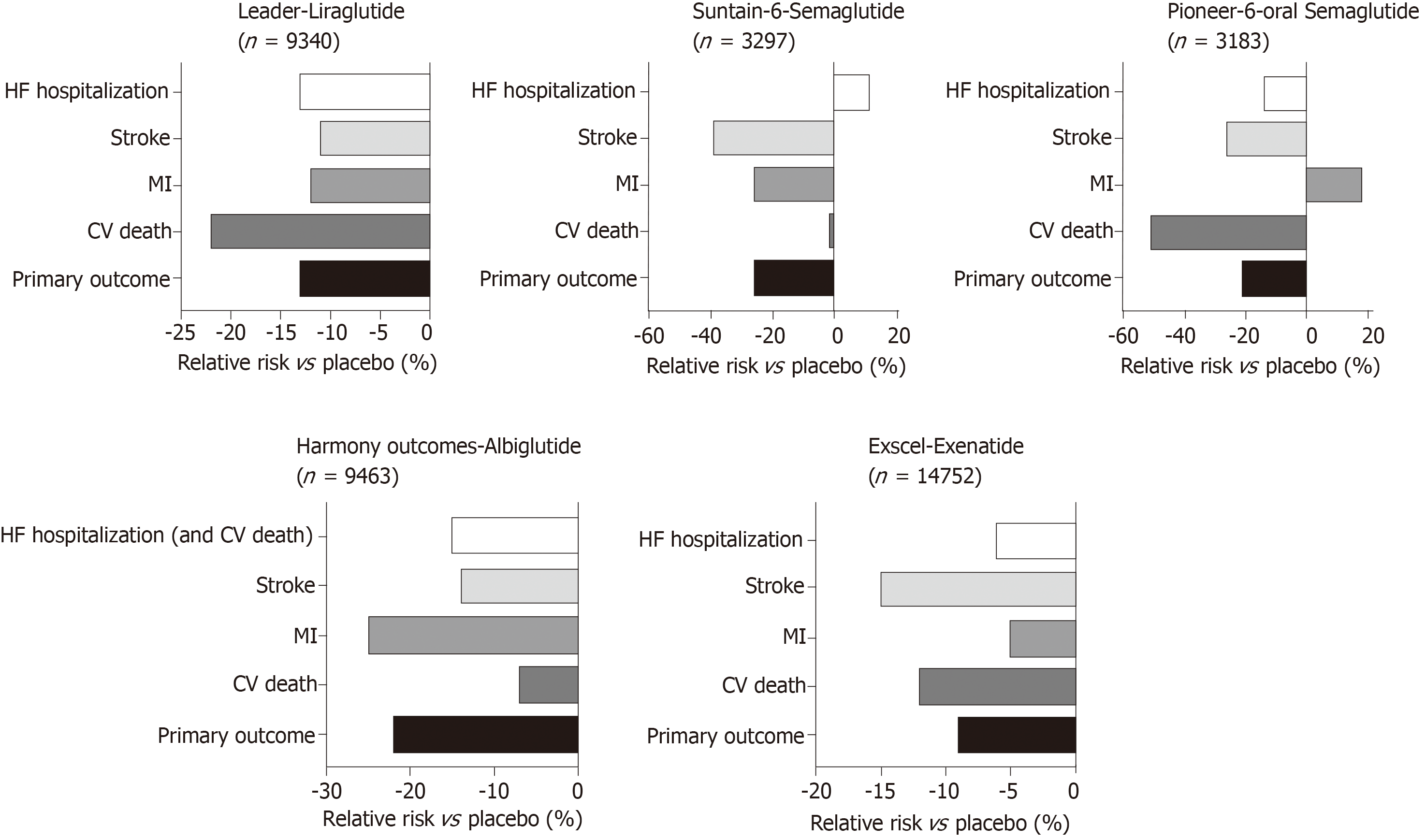
Large studies examining the impact of GLP-1 agonists on cardiovascular outcomes in T2D have been summarised in Figure 5. In the LEADER trial, patients with T2D on Liraglutide had lower rates of cardiovascular death[146]. Similarly SUSTAIN-6 also looked at GLP-1 agonists with Semaglutide being an injectable version with longer half-life have also shown improved cardiovascular outcomes. However, in the EXSCEL[23], Exenatide did not show overall cardiovascular risk benefit compared to placebo. Most recently, the PIONEER 6 trial has studied the non-inferiority of oral daily semaglutide against placebo for cardiovascular outcomes[147]. It has been shown to have a significant reduction in the composite primary outcome of first occurrence of death from cardiovascular causes, nonfatal myocardial infarction (including silent), or nonfatal stroke, i.e., 8.6% in the semaglutide group vs 6.6% in placebo, with a hazard ratio of 0.79, 95%CI: 0.57-1.11, P < 0.001 for non-inferiority, P = 0.02 for superiority. However, when the components of the primary outcome are looked at, non-fatal MI and death from cardiovascular causes comes up non-significant. However, this study was relatively underpowered compared to the other cardiovascular outcome trials and this may explain why the reduction was not statistically significant. Increasing evidence for oral GLP-1 agonists has marked benefits for those who have reservations regarding all previous GLP-1 agonists that have been in the form of a subcutaneous injection and will allow for better compliance with the new ADA/EASD guidance to use a GLP-1 agonist as second line therapy in diabetes. There is a larger phase three cardiovascular outcome trial underway, the Heart Disease Study of Semaglutide in patients with Type 2 Diabetes (SOUL; NCT03914326), looking at oral semaglutide against placebo and its effects on lowering MACE in over 9000 patients. The results of this is eagerly awaited.
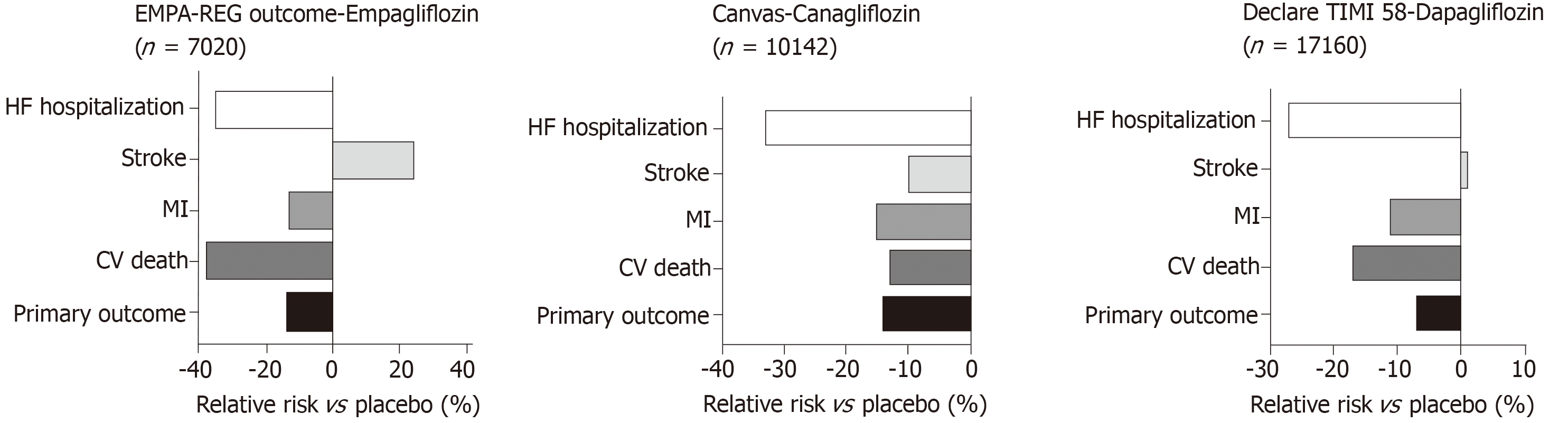
In both the EMPA-REG OUTCOME[19] and CANVAS[20] studies (Figure 6) there was a relative risk reduction in cardiovascular mortality and hospitalisation for HF in patients with T2D. The hazard ratio for HF hospitalisation in the EMPA-REG OUTCOME trial was 0.65, (0.57, 0.82, P = 0.002) for empagliflozin vs placebo[21]. A reduction in hospitalization for HF was also observed with Sodium glucose co-transporter 2 (SGLT2) inhibitor Dapagliflozin in DECLARE-TIMI 58 trial[148]. An important consideration in interpretation of these results is that the patients in these studies either had established cardiovascular disease or multiple risk factors for atherosclerotic cardiovascular disease risk factors and it is uncertain whether the results can be translated in to lower risk groups with prevention of the development of HF.
The mechanisms by which SGLT2 inhibitors lower HF hospitalisation rates in T2D are still under scrutiny. However, it is hypothesised to be related to the urinary sodium and glucose excretion that leads to increased fluid losses resulting in reduced pre-load and afterload[149]. Secondary effects include fluid loss and blood pressure lowering. There is also suggestion that SGLT2 inhibitors shift myocardial metabolism towards ketones, resulting in this having a favourable effect on cardiac energetics[150].
Diabetic heart disease is multi-faceted and spans metabolic, structural and functional changes. Recent advancements in imaging has aided significantly in understanding the pathophysiology as well as the remodelling and functional changes within the heart. Further research into the degree of dependence on fatty acid metabolism instead of glucose in the presence of diabetes is required. The relationship between the metabolic changes within the heart and functional measures such as myocardial strain rates as well as triglyceride content will help us better understand how to treat this disease process. This will also add towards increasing the mechanistic accuracy of new therapeutic targets.
| 1. | WHO. Diabetes Fact Sheet. World Health Organisation, Geneva. Available from: https://www.who.int/en/news-room/fact-sheets/detail/diabetes. |
| 2. | WHO. The top 10 causes of death [updated May 2018]. World Health Organization, Geneva. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. |
| 3. | Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2337] [Cited by in RCA: 2181] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 4. | NHS Trusts. National Diabetes Audit Complications and Mortality 2015-2016. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/national-diabetesaudit-complications-and-mortality-2015-2016. |
| 5. | Hippisley-Cox J, Coupland C. Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. BMJ. 2016;354:i3477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Kannel WB. Framingham study insights on diabetes and cardiovascular disease. Clin Chem. 2011;57:338-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1041] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 599] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 9. | Rubler S, Yuceoglu YZ, Kumral T, Grishman A, Branwood AW, Dlugash J. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1296] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 10. | Larghat AM, Swoboda PP, Biglands JD, Kearney MT, Greenwood JP, Plein S. The microvascular effects of insulin resistance and diabetes on cardiac structure, function, and perfusion: a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2014;15:1368-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Khan JN, Wilmot EG, Leggate M, Singh A, Yates T, Nimmo M, Khunti K, Horsfield MA, Biglands J, Clarysse P, Croisille P, Davies M, McCann GP. Subclinical diastolic dysfunction in young adults with Type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging. 2014;15:1263-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016;18:242-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 565] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 13. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12805] [Article Influence: 457.3] [Reference Citation Analysis (0)] |
| 14. | Patel A; ADVANCE Collaborative Group, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1449] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 15. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3361] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 16. | Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg G, Bhatt DL. Response to Letter Regarding Article, "Heart Failure, Saxagliptin and Diabetes Mellitus: Observations From the SAVOR-TIMI 53 Randomized Trial". Circulation. 2015;132:e121-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1831] [Cited by in RCA: 1924] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 18. | White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1917] [Article Influence: 147.5] [Reference Citation Analysis (6)] |
| 19. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8728] [Article Influence: 793.5] [Reference Citation Analysis (2)] |
| 20. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5658] [Article Influence: 628.7] [Reference Citation Analysis (0)] |
| 21. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 5290] [Article Influence: 529.0] [Reference Citation Analysis (0)] |
| 22. | Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 1808] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 23. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1601] [Article Influence: 177.9] [Reference Citation Analysis (0)] |
| 24. | Lee WS, Kim J. Diabetic cardiomyopathy: where we are and where we are going. Korean J Intern Med. 2017;32:404-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Karnik AA, Fields AV, Shannon RP. Diabetic cardiomyopathy. Curr Hypertens Rep. 2007;9:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond). 2004;107:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Lundbaek K. Diabetic angiopathy: a specific vascular disease. Lancet. 1954;266:377-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Bertoni AG, Tsai A, Kasper EK, Brancati FL. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care. 2003;26:2791-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjörnsdottir S. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379:633-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 996] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 30. | Seferović PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718-1727, 1727a-1727c. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 31. | Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 291] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Pajunen P, Taskinen MR, Nieminen MS, Syvänne M. Angiographic severity and extent of coronary artery disease in patients with type 1 diabetes mellitus. Am J Cardiol. 2000;86:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Snell-Bergeon JK, West NA, Mayer-Davis EJ, Liese AD, Marcovina SM, D'Agostino RB, Hamman RF, Dabelea D. Inflammatory markers are increased in youth with type 1 diabetes: the SEARCH Case-Control study. J Clin Endocrinol Metab. 2010;95:2868-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Cipollone F, Chiarelli F, Davì G, Ferri C, Desideri G, Fazia M, Iezzi A, Santilli F, Pini B, Cuccurullo C, Tumini S, Del Ponte A, Santucci A, Cuccurullo F, Mezzetti A. Enhanced soluble CD40 ligand contributes to endothelial cell dysfunction in vitro and monocyte activation in patients with diabetes mellitus: effect of improved metabolic control. Diabetologia. 2005;48:1216-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 36. | Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter HG, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ; American Heart Association Council on Basic Cardiovascular Sciences. Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association. Circ Res. 2016;118:1659-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 37. | Peterzan MA, Lygate CA, Neubauer S, Rider OJ. Metabolic remodeling in hypertrophied and failing myocardium: a review. Am J Physiol Heart Circ Physiol. 2017;313:H597-H616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Heather LC, Clarke K. Metabolism, hypoxia and the diabetic heart. J Mol Cell Cardiol. 2011;50:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation. 2004;110:894-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Harmancey R, Taegtmeyer H. The complexities of diabetic cardiomyopathy: lessons from patients and animal models. Curr Diab Rep. 2008;8:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1315] [Cited by in RCA: 1532] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 42. | Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1652] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 43. | Zhang J, Duncker DJ, Ya X, Zhang Y, Pavek T, Wei H, Merkle H, Uğurbil K, From AH, Bache RJ. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation. 1995;92:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, Carr CA, Heather LC, Tyler DJ. Increasing Pyruvate Dehydrogenase Flux as a Treatment for Diabetic Cardiomyopathy: A Combined 13C Hyperpolarized Magnetic Resonance and Echocardiography Study. Diabetes. 2015;64:2735-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C, Trent J 2nd, Champion JC, Durand JB, Lenihan DJ. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112:2500-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 442] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 47. | Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 376] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 48. | Kassiotis C, Rajabi M, Taegtmeyer H. Metabolic reserve of the heart: the forgotten link between contraction and coronary flow. Prog Cardiovasc Dis. 2008;51:74-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1640] [Cited by in RCA: 1818] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 50. | Langenberg CJ, Pietersen HG, Geskes G, Wagenmakers AJ, Soeters PB, Durieux M. Coronary sinus catheter placement: assessment of placement criteria and cardiac complications. Chest. 2003;124:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Rothman MT, Baim DS, Simpson JB, Harrison DC. Coronary hemodynamics during percutaneous transluminal coronary angioplasty. Am J Cardiol. 1982;49:1615-1622. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 57] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Camici P, Marraccini P, Marzilli M, Lorenzoni R, Buzzigoli G, Puntoni R, Boni C, Bellina CR, Klassen GA, L'Abbate A. Coronary hemodynamics and myocardial metabolism during and after pacing stress in normal humans. Am J Physiol. 1989;257:E309-E317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L'Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270-H3278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Camici PG, Marraccini P, Lorenzoni R, Buzzigoli G, Pecori N, Perissinotto A, Ferrannini E, L'Abbate A, Marzilli M. Coronary hemodynamics and myocardial metabolism in patients with syndrome X: Response to pacing stress. J Am Coll Cardiol. 1991;17:1461-1470. [RCA] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 129] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 56. | Dutka DP, Pitt M, Pagano D, Mongillo M, Gathercole D, Bonser RS, Camici PG. Myocardial glucose transport and utilization in patients with type 2 diabetes mellitus, left ventricular dysfunction, and coronary artery disease. J Am Coll Cardiol. 2006;48:2225-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Utriainen T, Takala T, Luotolahti M, Rönnemaa T, Laine H, Ruotsalainen U, Haaparanta M, Nuutila P, Yki-Järvinen H. Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia. 1998;41:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Carley AN, Taegtmeyer H, Lewandowski ED. Matrix revisited: mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 2014;114:717-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 59. | Taegtmeyer H, Wilson CR, Razeghi P, Sharma S. Metabolic energetics and genetics in the heart. Ann N Y Acad Sci. 2005;1047:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 399] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 61. | Shivu GN, Phan TT, Abozguia K, Ahmed I, Wagenmakers A, Henning A, Narendran P, Stevens M, Frenneaux M. Relationship between coronary microvascular dysfunction and cardiac energetics impairment in type 1 diabetes mellitus. Circulation. 2010;121:1209-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Levelt E, Mahmod M, Piechnik SK, Ariga R, Francis JM, Rodgers CT, Clarke WT, Sabharwal N, Schneider JE, Karamitsos TD, Clarke K, Rider OJ, Neubauer S. Relationship Between Left Ventricular Structural and Metabolic Remodeling in Type 2 Diabetes. Diabetes. 2016;65:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 63. | Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 510] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 64. | Hall JL, Stanley WC, Lopaschuk GD, Wisneski JA, Pizzurro RD, Hamilton CD, McCormack JG. Impaired pyruvate oxidation but normal glucose uptake in diabetic pig heart during dobutamine-induced work. Am J Physiol. 1996;271:H2320-H2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Seymour AM, Chatham JC. The effects of hypertrophy and diabetes on cardiac pyruvate dehydrogenase activity. J Mol Cell Cardiol. 1997;29:2771-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes. 1999;48:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 230] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 350] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 68. | Doiron B, Cuif MH, Chen R, Kahn A. Transcriptional glucose signaling through the glucose response element is mediated by the pentose phosphate pathway. J Biol Chem. 1996;271:5321-5324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Foretz M, Carling D, Guichard C, Ferré P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273:14767-14771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 187] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105:1861-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 71. | Zhong Y, Ahmed S, Grupp IL, Matlib MA. Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol Heart Circ Physiol. 2001;281:H1137-H1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Lacombe VA, Viatchenko-Karpinski S, Terentyev D, Sridhar A, Emani S, Bonagura JD, Feldman DS, Györke S, Carnes CA. Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1787-R1797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Gulick T, Cresci S, Caira T, Moore DD, Kelly DP. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc Natl Acad Sci U S A. 1994;91:11012-11016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 411] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 74. | Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001;276:44390-44395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Park TS, Yamashita H, Blaner WS, Goldberg IJ. Lipids in the heart: a source of fuel and a source of toxins. Curr Opin Lipidol. 2007;18:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 422] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 77. | Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 758] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 79. | Park TS, Yamashita H, Blaner WS, Goldberg IJ. Lipids in the heart: a source of fuel and a source of toxins. Curr Opin Lipidol. 2007;18:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Monji A, Mitsui T, Bando YK, Aoyama M, Shigeta T, Murohara T. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;305:H295-H304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Ramírez E, Klett-Mingo M, Ares-Carrasco S, Picatoste B, Ferrarini A, Rupérez FJ, Caro-Vadillo A, Barbas C, Egido J, Tuñón J, Lorenzo Ó. Eplerenone attenuated cardiac steatosis, apoptosis and diastolic dysfunction in experimental type-II diabetes. Cardiovasc Diabetol. 2013;12:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 82. | McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 494] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 83. | Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 84. | Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, Meinders EA, Romijn JA, de Roos A, Smit JW. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 85. | Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, Kitzman DW, Hopkins PN, Morgan D, Rao DC, Devereux RB. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 86. | Bella JN, Devereux RB, Roman MJ, Palmieri V, Liu JE, Paranicas M, Welty TK, Lee ET, Fabsitz RR, Howard BV. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study). Am J Cardiol. 2001;87:1260-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 652] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 88. | Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J, Bello N, Aguilar D, Vardeny O, Matsushita K, Selvin E, Solomon S. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ Heart Fail. 2015;8:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Celentano A, Galderisi M, Tammaro P, Mureddu GF, Garofalo M, de Simone G, de Divitiis O. Slow-release isradipine in mild to moderate hypertension: hemodynamic and antihypertensive effects. Int J Clin Pharmacol Ther Toxicol. 1991;29:494-498. [PubMed] |
| 90. | Lee M, Gardin JM, Lynch JC, Smith VE, Tracy RP, Savage PJ, Szklo M, Ward BJ. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am Heart J. 1997;133:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Ng DS, Saw NM. The role of HDL and its modulators in the development of diabetes. Curr Opin Lipidol. 2012;23:167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Jankovic D, Winhofer Y, Promintzer-Schifferl M, Wohlschläger-Krenn E, Anderwald CH, Wolf P, Scherer T, Reiter G, Trattnig S, Luger A, Krebs M, Krssak M. Effects of insulin therapy on myocardial lipid content and cardiac geometry in patients with type-2 diabetes mellitus. PLoS One. 2012;7:e50077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Wilmot EG, Leggate M, Khan JN, Yates T, Gorely T, Bodicoat DH, Khunti K, Kuijer JP, Gray LJ, Singh A, Clarysse P, Croisille P, Nimmo MA, McCann GP, Davies MJ. Type 2 diabetes mellitus and obesity in young adults: the extreme phenotype with early cardiovascular dysfunction. Diabet Med. 2014;31:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Levelt E, Rodgers CT, Clarke WT, Mahmod M, Ariga R, Francis JM, Liu A, Wijesurendra RS, Dass S, Sabharwal N, Robson MD, Holloway CJ, Rider OJ, Clarke K, Karamitsos TD, Neubauer S. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J. 2016;37:3461-3469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 95. | Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 647] [Article Influence: 24.9] [Reference Citation Analysis (1)] |
| 96. | Savage DD, Levy D, Dannenberg AL, Garrison RJ, Castelli WP. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study). Am J Cardiol. 1990;65:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 153] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, Piechnik SK, Robson MD, Hausenloy DJ, Sheikh AM, Hawkins PN, Moon JC. T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging. 2013;6:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 98. | Jellis CL, Kwon DH. Myocardial T1 mapping: modalities and clinical applications. Cardiovasc Diagn Ther. 2014;4:126-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 99. | van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbély A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 563] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 100. | Swoboda PP, McDiarmid AK, Erhayiem B, Ripley DP, Dobson LE, Garg P, Musa TA, Witte KK, Kearney MT, Barth JH, Ajjan R, Greenwood JP, Plein S. Diabetes Mellitus, Microalbuminuria, and Subclinical Cardiac Disease: Identification and Monitoring of Individuals at Risk of Heart Failure. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 101. | Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 102. | Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 103. | Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2001;87:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 104. | Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 425] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 105. | Roos CJ, Scholte AJ, Kharagjitsingh AV, Bax JJ, Delgado V. Changes in multidirectional LV strain in asymptomatic patients with type 2 diabetes mellitus: a 2-year follow-up study. Eur Heart J Cardiovasc Imaging. 2014;15:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 106. | Karagöz E, Tanoglu A, Ozalper V. Mean platelet volume: a novel diagnostic factor of systemic lupus erythematosus? Clin Rheumatol. 2015;34:1655-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 107. | Ernande L, Rietzschel ER, Bergerot C, De Buyzere ML, Schnell F, Groisne L, Ovize M, Croisille P, Moulin P, Gillebert TC, Derumeaux G. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle-tracking imaging study. J Am Soc Echocardiogr. 2010;23:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 108. | Liu JH, Chen Y, Yuen M, Zhen Z, Chan CW, Lam KS, Tse HF, Yiu KH. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 109. | Jørgensen PG, Biering-Sørensen T, Mogelvang R, Fritz-Hansen T, Vilsbøll T, Rossing P, Jensen MT. Predictive value of echocardiography in Type 2 diabetes. Eur Heart J Cardiovasc Imaging. 2019;20:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 110. | Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 375] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 111. | Eguchi K, Boden-Albala B, Jin Z, Rundek T, Sacco RL, Homma S, Di Tullio MR. Association between diabetes mellitus and left ventricular hypertrophy in a multiethnic population. Am J Cardiol. 2008;101:1787-1791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Taskiran M, Fritz-Hansen T, Rasmussen V, Larsson HB, Hilsted J. Decreased myocardial perfusion reserve in diabetic autonomic neuropathy. Diabetes. 2002;51:3306-3310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Sucato V, Novo G, Evola S, Novo S. Coronary microvascular dysfunction in patients with diabetes, hypertension and metabolic syndrome. Int J Cardiol. 2015;186:96-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 114. | Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 424] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 115. | Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 295] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 116. | Bratis K, Child N, Terrovitis J, Nanas J, Felekos I, Aggeli C, Stefanadis C, Mastorakos G, Chiribiri A, Nagel E, Mavrogeni S. Coronary microvascular dysfunction in overt diabetic cardiomyopathy. IJC Metabolic & Endocrine. 2014;5:19-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, Jerosch-Herold M, Kwong RY, Di Carli MF, Adler GK. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2015;64:236-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 118. | Rubinshtein R, Yang EH, Rihal CS, Prasad A, Lennon RJ, Best PJ, Lerman LO, Lerman A. Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. Eur Heart J. 2010;31:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 119. | Korosoglou G, Humpert PM, Ahrens J, Oikonomou D, Osman NF, Gitsioudis G, Buss SJ, Steen H, Schnackenburg B, Bierhaus A, Nawroth PP, Katus HA. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging. 2012;35:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 120. | Dorbala S, Murthy VL. Coronary artery calcification and vascular function. J Nucl Cardiol. 2012;19:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 121. | Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100:2312-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 819] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 122. | Rydén L, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA. Efficacy and safety of high-dose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J. 2000;21:1967-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 123. | MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ; CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 521] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 124. | Konstam MA, Neaton JD, Dickstein K. Effects of high-dose vs low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1888-1888. [RCA] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 508] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 125. | Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6409] [Cited by in RCA: 6266] [Article Influence: 232.1] [Reference Citation Analysis (0)] |
| 126. | Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2034] [Cited by in RCA: 2233] [Article Influence: 148.9] [Reference Citation Analysis (0)] |
| 127. | Taylor AL, Ziesche S, Yancy CW, Carson P, Ferdinand K, Taylor M, Adams K, Olukotun AY, Ofili E, Tam SW, Sabolinski ML, Worcel M, Cohn JN; African-American Heart Failure Trial Investigators. Early and sustained benefit on event-free survival and heart failure hospitalization from fixed-dose combination of isosorbide dinitrate/hydralazine: consistency across subgroups in the African-American Heart Failure Trial. Circulation. 2007;115:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Komajda M, Tavazzi L, Francq BG, Böhm M, Borer JS, Ford I, Swedberg K; SHIFT Investigators. Efficacy and safety of ivabradine in patients with chronic systolic heart failure and diabetes: an analysis from the SHIFT trial. Eur J Heart Fail. 2015;17:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 129. | Kristensen SL, Martinez F, Jhund PS, Arango JL, Bĕlohlávek J, Boytsov S, Cabrera W, Gomez E, Hagège AA, Huang J, Kiatchoosakun S, Kim KS, Mendoza I, Senni M, Squire IB, Vinereanu D, Wong RC, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJ. Geographic variations in the PARADIGM-HF heart failure trial. Eur Heart J. 2016;37:3167-3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 130. | ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4759] [Cited by in RCA: 4956] [Article Influence: 275.3] [Reference Citation Analysis (0)] |
| 131. | UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5771] [Cited by in RCA: 5338] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 132. | Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6292] [Cited by in RCA: 5684] [Article Influence: 315.8] [Reference Citation Analysis (0)] |
| 133. | Castagno D, Baird-Gunning J, Jhund PS, Biondi-Zoccai G, MacDonald MR, Petrie MC, Gaita F, McMurray JJ. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J. 2011;162:938-948.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 134. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2804] [Cited by in RCA: 2642] [Article Influence: 203.2] [Reference Citation Analysis (7)] |
| 135. | Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL; SAVOR-TIMI 53 Steering Committee and Investigators. Heart Failure, Saxagliptin, and Diabetes Mellitus: Observations from the SAVOR-TIMI 53 Randomized Trial. Circulation. 2015;132:e198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 136. | Komajda M, McMurray JJ, Beck-Nielsen H, Gomis R, Hanefeld M, Pocock SJ, Curtis PS, Jones NP, Home PD. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J. 2010;31:824-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 137. | MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51 Suppl 3:S434-S442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 424] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 138. | Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L; LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1125] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 139. | Zoungas S, de Galan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, MacMahon S, Marre M, Neal B, Patel A, Woodward M, Chalmers J; ADVANCE Collaborative Group, Cass A, Glasziou P, Harrap S, Lisheng L, Mancia G, Pillai A, Poulter N, Perkovic V, Travert F. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: New results from the ADVANCE trial. Diabetes Care. 2009;32:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 140. | Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:844-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 141. | Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, Zhang Y, Quan X, Ji L, Zhan S. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Res Clin Pract. 2015;110:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 142. | Gaspari T, Liu H, Welungoda I, Hu Y, Widdop RE, Knudsen LB, Simpson RW, Dear AE. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE-/- mouse model. Diab Vasc Dis Res. 2011;8:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 143. | Del Olmo-Garcia MI, Merino-Torres JF. GLP-1 Receptor Agonists and Cardiovascular Disease in Patients with Type 2 Diabetes. J Diabetes Res. 2018;2018:4020492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 144. | Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Køber L, Treiman M, Holst JJ, Engstrøm T. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 145. | Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim WS, Seon HJ, Kim KS. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 146. | Buse JB; the LEADER Steering Committee. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:1798-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 147. | Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC; PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1334] [Article Influence: 190.6] [Reference Citation Analysis (0)] |
| 148. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4523] [Article Influence: 646.1] [Reference Citation Analysis (0)] |
| 149. | Sattar N, Petrie MC, Zinman B, Januzzi JL. Novel Diabetes Drugs and the Cardiovascular Specialist. J Am Coll Cardiol. 2017;69:2646-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 150. | Mudaliar S, Alloju S, Henry RR. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care. 2016;39:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 474] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 151. | Kim TT, Dyck JR. The role of CD36 in the regulation of myocardial lipid metabolism. Biochim Biophys Acta. 2016;1861:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 152. | Kathirvel E, Morgan K, French SW, Morgan TR. Acetyl-L-carnitine and lipoic acid improve mitochondrial abnormalities and serum levels of liver enzymes in a mouse model of nonalcoholic fatty liver disease. Nutr Res. 2013;33:932-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 153. | Hales CN, Randle PJ. Effects of low-carbohydrate diet and diabetes mellitus on plasma concentrations of glucose, non-esterified fatty acid, and insulin during oral glucose-tolerance tests. Lancet. 1963;1:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 252] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 154. | Matshela M. Second in a series on diabetes and the heart: diabetic cardiomyopathy-mechanisms and mode of diagnosis. E-Journal of Cardiology Practice. 2016;14. |
| 155. | van der Meer P, Groenveld HF, Januzzi JL, van Veldhuisen DJ. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart. 2009;95:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saisho Y, Surani S S-Editor: Ma RY L-Editor: A E-Editor: Wu YXJ