©The Author(s) 2025.
World J Diabetes. Jul 15, 2025; 16(7): 108789
Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.108789
Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.108789
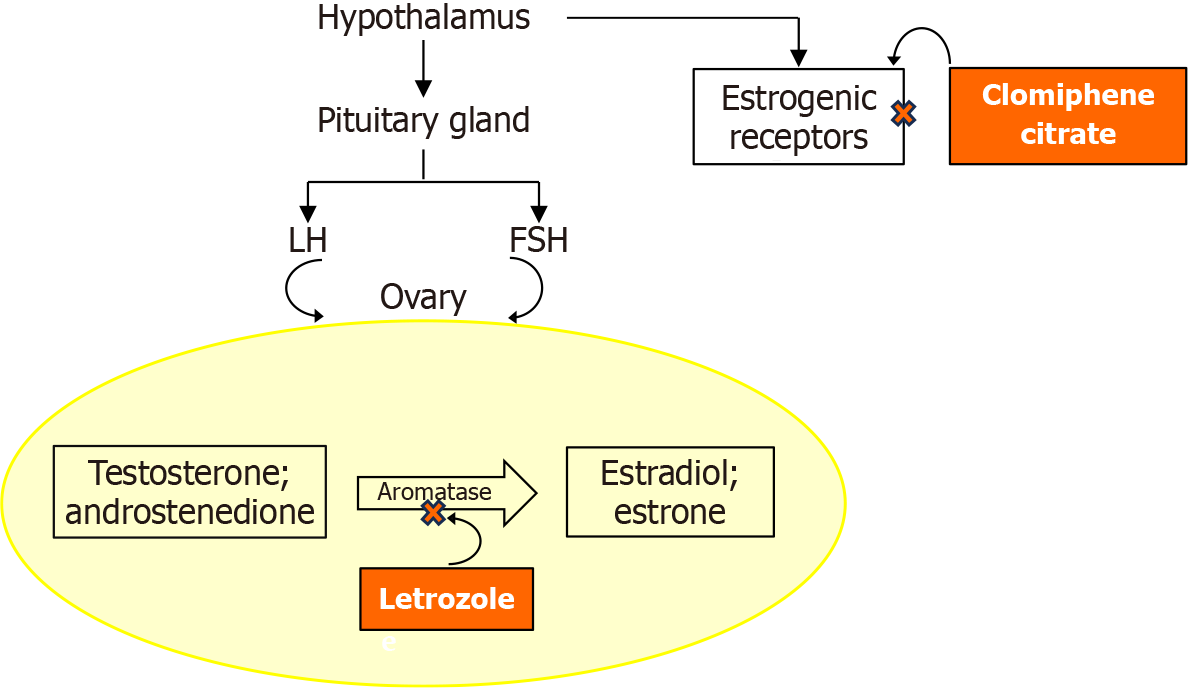
Figure 1 Illustration depicting the mode of action of clomiphene citrate in comparison to letrozole in ovulation induction.
Clomiphene citrate acts as a non-steroidal selective estrogen receptor modulator, i.e., it binds to estrogen receptors in the hypothalamus, thereby inhibiting the negative estrogen feedback to the hypothalamus. In turn, this leads to a change in the pulsatile release of gonadotropin-releasing hormone that drives an increase in the secretion of follicle-stimulating hormone, promoting follicle development. Letrozole, an aromatase inhibitor, reduces estrogen production by blocking the conversion of androgens to estrogens, i.e., letrozole inhibits the cytochrome P450 aromatase enzyme complex. This creates negative feedback to the hypothalamus, stimulating the pituitary gland to produce more follicle-stimulating hormone, leading to folliculogenesis. Letrozole has no adverse effects on the endometrium and cervical mucus and is preferred to clomiphene citrate to induce ovulation. LH: Luteinizing hormone; FSH: Follicle-stimulating hormone.
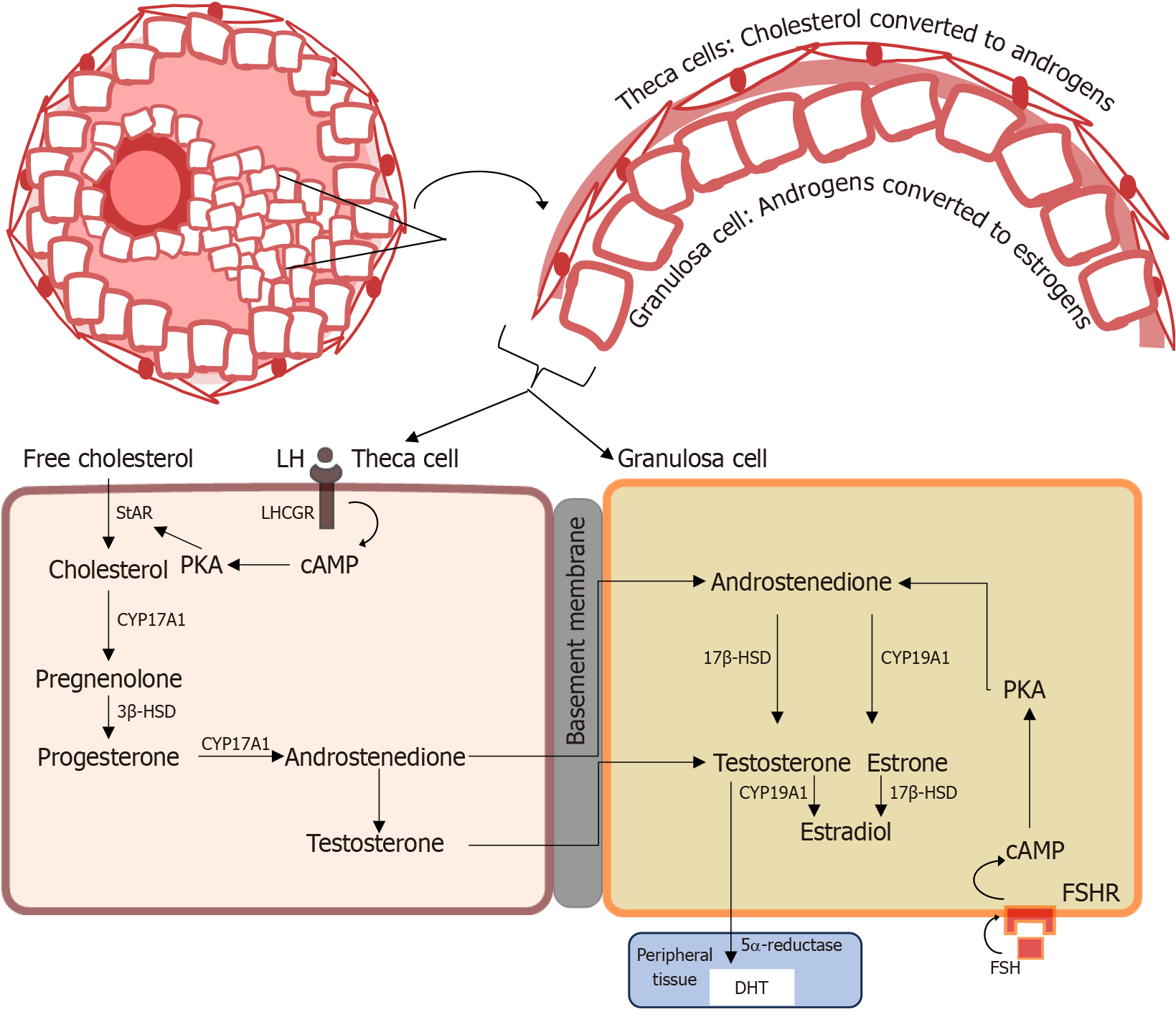
Figure 2 Steroid biosynthesis in the ovary shows the two-cell, two-gonadotropin model, where androgens synthesized in the theca cells are aromatized to estrogens in the granulosa cells, under normal physiological conditions.
In the initial synthesis of androgen, under the control of luteinizing hormone, the side chain of cholesterol is cleaved by the P450 side chain cleavage enzyme, cytochrome P450 17A1, to pregnenolone, a precursor to all steroid hormones. Testosterone is a precursor converted to dihydrotestosterone or estradiol in target tissue by 5α-reductase and the aromatase enzyme, respectively. LH: Luteinizing hormone; LHCGR: Luteinizing hormone/chorionic gonadotropin receptor; cAMP: Cyclic adenosine monophosphate; PKA: Protein kinase A; StAR: Steroidogenic acute regulatory protein; CYP17A1: Cytochrome P450 17A1; 3β-HSD: 3β-hydroxysteroid dehydrogenase; 17β-HSD: 17β-hydroxysteroid dehydrogenase; CYP19A1: Cytochrome P450 19A1; FSHR: Follicle-stimulating hormone receptor; FSH: Follicle-stimulating hormone; DHT: 5α-dihydrotestosterone.
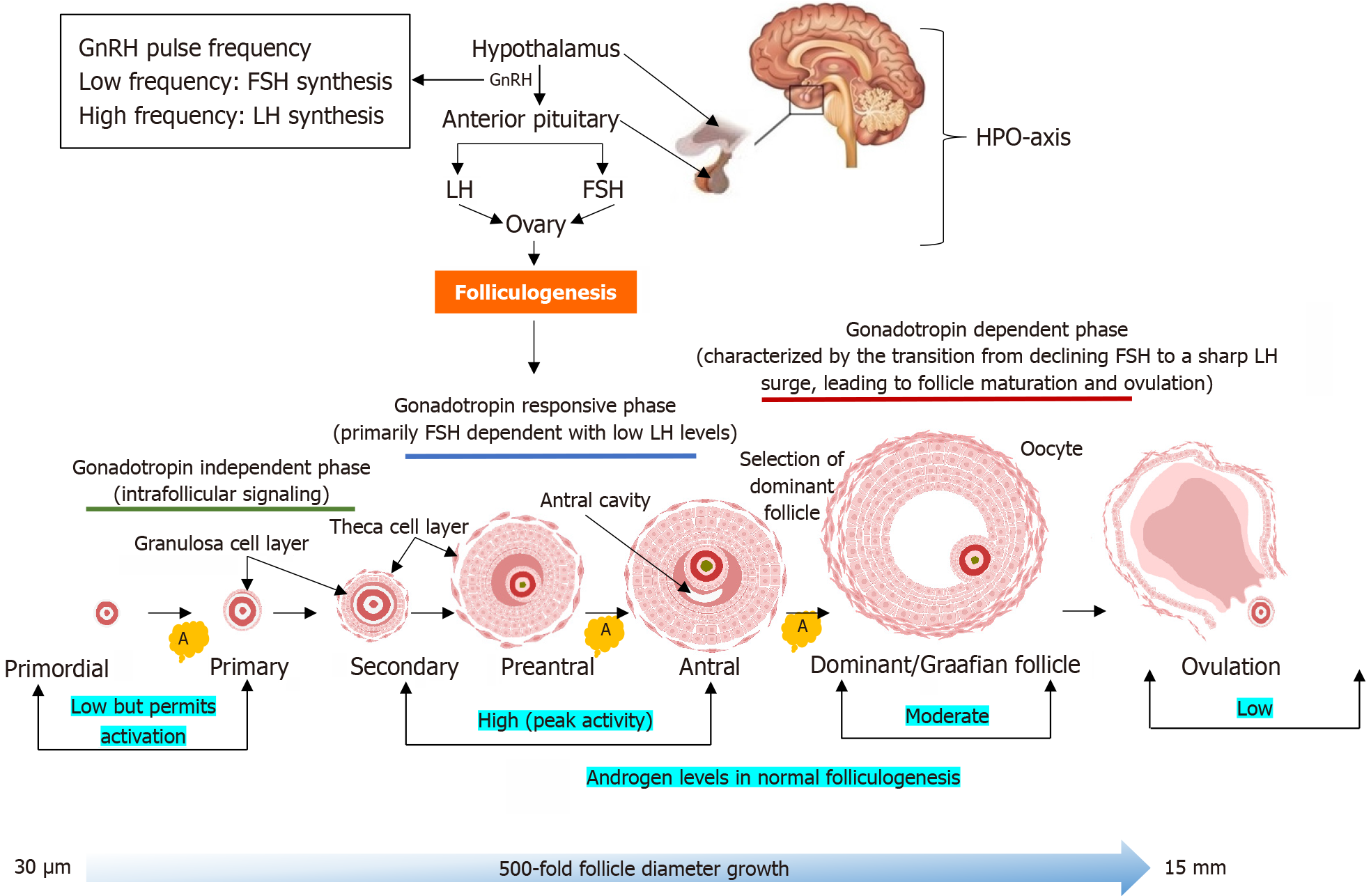
Figure 3 Different stages of normal folliculogenesis from the primordial follicle to the dominant (Graafian) follicle and ovulation.
Gonadotropin-releasing hormone pulsatility controls the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary gland. Rapid gonadotropin-releasing hormone pulse frequency favors LH secretion, while slower pulses enhance FSH secretion. A balanced LH/FSH ratio is critical for coordinated follicular development, i.e., FSH promotes granulosa cell proliferation, estradiol synthesis, and follicle maturation, while LH stimulates theca cells to produce androgens, which serve as substrates for aromatization. Androgens begin to exert effects from the primordial stage by supporting the initial activation of dormant primordial follicles. Androgens exert their most prominent effects during the preantral and early antral stages, enhancing granulosa cell proliferation, FSH receptor expression and aromatase activity for estrogen biosynthesis. In the antral and dominant (Graafian) stages, androgens support follicle maturation and upregulate LH receptor expression. While androgens do not directly mediate ovulation, their prior actions facilitate the follicle’s response to the LH surge. Follicular atresia, which eliminates non-dominant follicles, is indirectly influenced by androgen. HPO: Hypothalamic-pituitary-ovarian; GnRH: Gonadotropin-releasing hormone; LH: Luteinizing hormone; FSH: Follicle-stimulating hormone; A: Atresia.
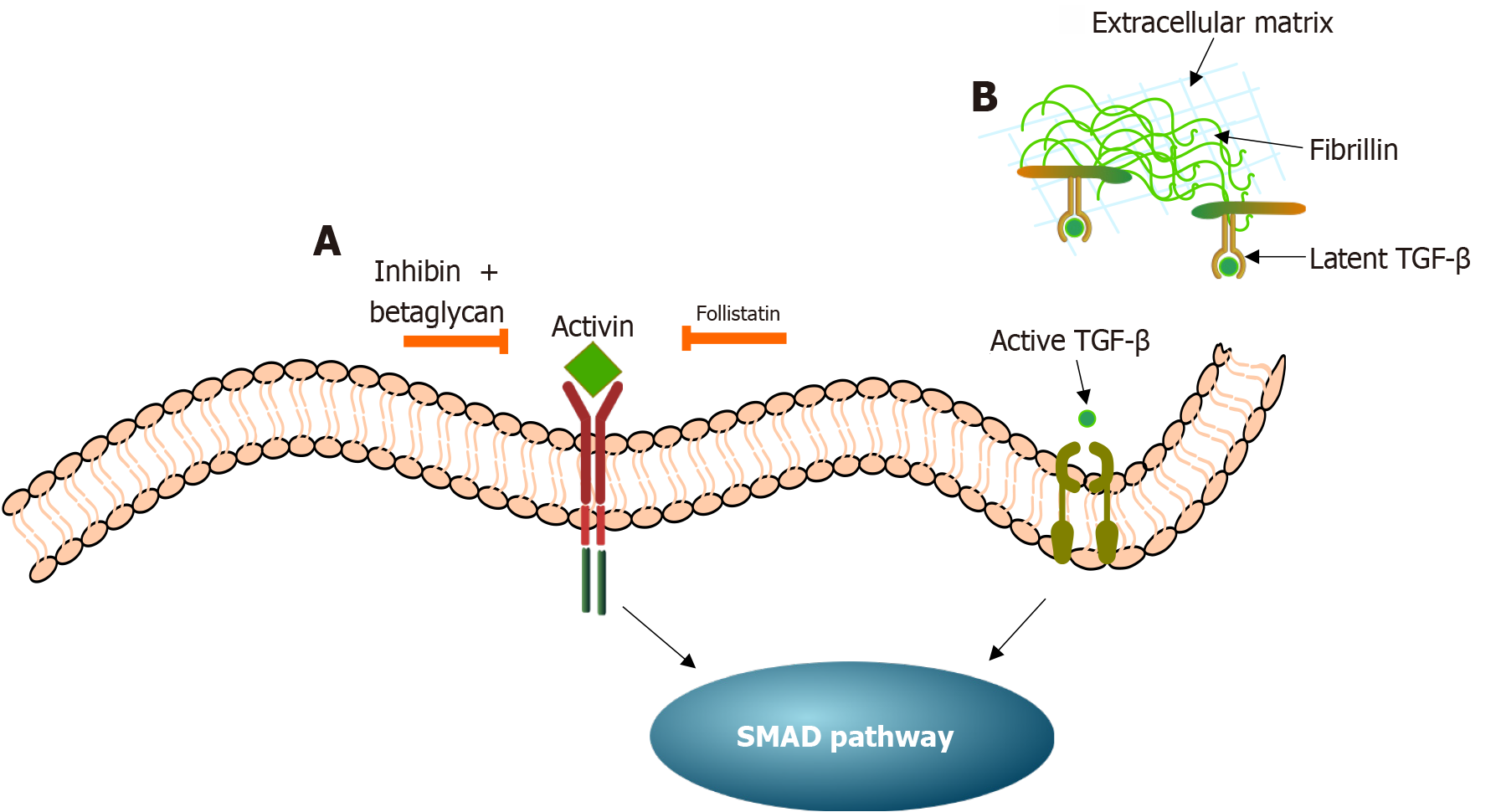
Figure 4 Mechanism diagram of activin, transforming growth factor-β signaling regulation, and suppressor of mothers against de
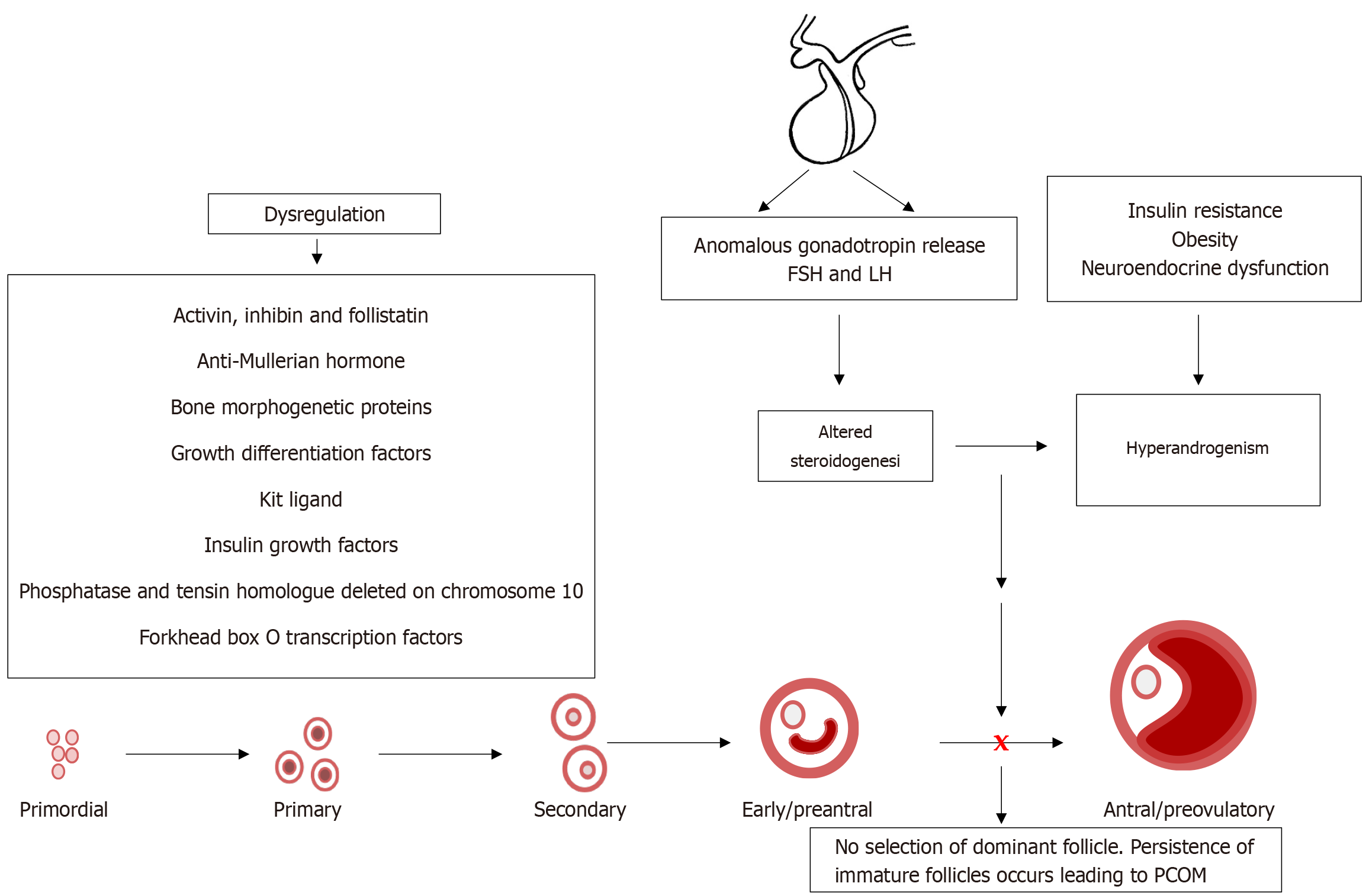
Figure 5 Intraovarian and systemic factors play a role in disrupting folliculogenesis in polycystic ovary syndrome, resulting in altered steroidogenesis.
Heightened luteinizing hormone, insulin resistance, and obesity stimulate theca cell androgen production, while granulosa cell dysfunction impairs aromatization of androgens to estrogens. This hormonal imbalance leads to irregular or absent selection of a dominant follicle, resulting in the persistence of small antral follicles, leading to the characteristic polycystic ovarian morphology. Hyperandrogenism further amplifies the arrest of follicular development, resulting in a vicious cycle of anovulation and hormonal imbalance. LH: Luteinizing hormone; FSH: Follicle-stimulating hormone; PCOM: Polycystic ovarian morphology.
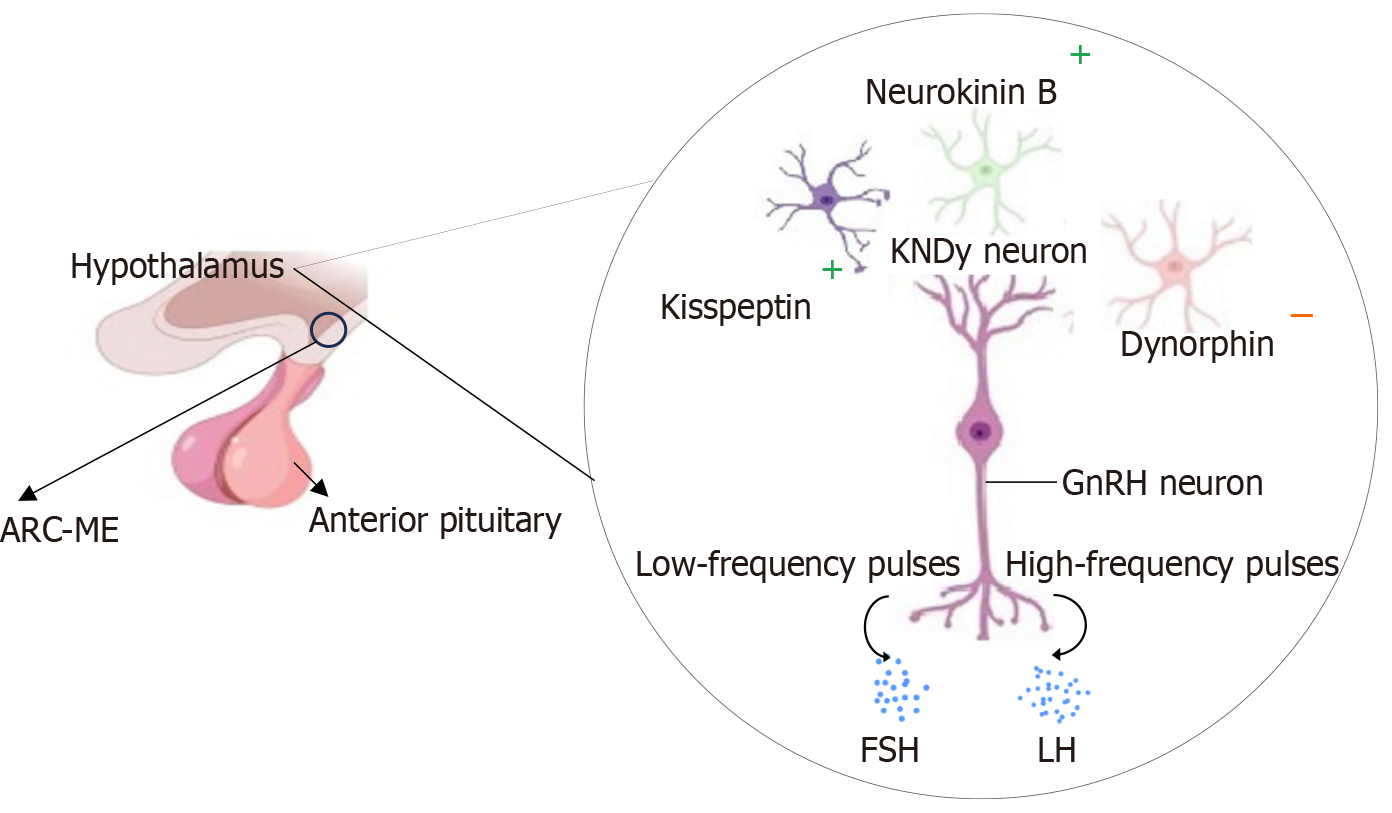
Figure 6 Schematic illustration of the role of kisspeptin/neurokinin B/dynorphin neurons in regulating the rhythmic release of go
- Citation: Rambaran N, Islam MS. Decoding androgen excess in polycystic ovary syndrome: Roles of insulin resistance and other key intraovarian and systemic factors. World J Diabetes 2025; 16(7): 108789
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/108789.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.108789